Introduction
Pancreatic cancer is one of the most serious
diseases, which lacks specific symptoms and progresses rapidly. It
ranks the fourth in all cancer mortality rates. Despite decades of
effort, the 5-year survival rate remains at only ~5% (1). Therefore, it is urgent to take a deep
look of its biological characteristics, to insure that we can
detect and diagnose pancreatic cancer in the early stage.
Epithelial-mesenchymal transition (EMT) is a
developmental process where cells lose their epithelial features
including loss of their sheet-like architecture, loss of polarity
and develop a mesenchymal phenotype. As cells undergo EMT, they
start expressing mesenchymal markers, such as N-cadherin, Vimentin
and downregulating E-cadherin (2–5). EMT
was identified to be regulated by YAP through modulation of
TGFβ-Smad signaling (6). It is
suggested that YAP/Smad pathway potentially plays an important role
in EMT.
Furin, a member of proprotein convertases (PCs),
which belongs to a family of serine proteases capable of cleaving
carboxyl-terminal of specific basic amino acid motifs and
activating various precursor proteins (7,8).
These precursor proteins include growth factors and differentiation
factors, receptors, adhesion molecules and enzymes like matrix
metal-loproteases (MMPs), which have been associated with different
stages of tumor development, progression, vascularization and
metastasis (9,10). Previously, it has been reported
that Furin is highly linked to various human primary tumors
(11,12) including skin tumor (13), colon tumor (14), head and neck (15) and breast (16) and ovarian cancer (17). Moreover, Furin can correctly cleave
the TGF-β precursor, which has been identified as a key regulator
of EMT (18), indicating that
Furin may link to EMT of tumor cells. However, the role of Furin in
pancreatic cancer cells remains to be clarified.
In the present study, we found that Furin was
critical for the growth and EMT of pancreatic cancer cells. Also,
Furin upregulated total YAP protein level and downregulated YAP
phosphoration level, indicating that Furin was involved in the YAP
activation. Therefore, we speculate that Furin promotes
epithelial-mesenchymal transition in pancreatic cancer cells
probably via Hippo-YAP pathway. All these findings prove that Furin
promotes EMT and may play a crucial role in pancreatic cancer
progression.
Materials and methods
Cell culture
The pancreatic cancer cells PaTu8988, BxPC3, PANC1
and SW1990 were obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA) and the Cancer Cell Repository (Shanghai,
China). Cells were maintained in Dulbecco's modified Eagle's medium
(DMEM) with 10% fetal bovine serum (FBS) at standard cell culture
conditions (37°C, 5% CO2 in humidified incubator). DMEM,
FBS and trypsin were purchased from Gibco (Carlsbad, CA, USA).
Plasmid construction
The construction of sh-Furin: the oligonucleotide
sequences were inserted into the EcoRI and AgeI sites
of the pLKO.1-TRC plasmid and ligated into the vector
(Sigma-Aldrich, St. Louis, MO, USA). The targeting sequences for
Furin was searched from Sigma-Aldrich and produced by Sangon
Biotech Co., Ltd. (Shanghai, China). The oligo sequences of Furin
shRNA included: Furin shRNA (F), CCG GGT GGC AAA GCG ACG GAC TAA
ACT CGA GTT TAG TCC GTC GCT TTG CCA CTT TTT G and Furin shRNA (R),
AAT TCA AAA AGT GGC AAA GCG ACG GAC TAA ACT CGA GTT TAG TCC GTC GCT
TTG CCA C.
The construction of Flag-Furin: the full-length
complementary DNA (cDNA) for human Furin was obtained from a cDNA
library via polymerase chain reaction (PCR) amplification using
primers Furin-all-F (5′-CCCAAGCTTATGGAGCTGAGGCCCTGGTTGC-3′) and
Furin-all-R (5′-CCGGAATTCGAGGGCGCTCTGGTCTTTGATAAA-3′) and cloned
into the EcoRI/HindIII site of p3xFLAG-Myc-CMV-24.
The sequence was confirmed by DNA sequencing.
Transfection of the cell line
psPAx2 and pMD2.G were co-transfected with sh-EGFP
or sh-Furin into HEK293T cells using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA). After 48 h the supernatants were
collected and concentrated. BxPC3 and SW1990 cells were stably
transfected with either sh-EGFP or sh-Furin plasmid. Cells were
infected with 1×106 recombinant lentivirus transduction
units in the presence of 8 mg/ml polybrene (Sigma-Aldrich). Cells
were then cultured with puromycin (1:10,000 dilution) and the cells
in blank group all became unviable.
PANC1 and PaTu8988 cells at 60–80% confluence were
transfected with Lipofectamine 2000 reagent according to the
manufacturer's instructions. The amount of vector and Flag-Furin
DNA used for transfection was 2 µg/well in a 6-well plate,
after cultured for 48–72 h, the cells transfected were washed and
harvested for further study.
D6R treatment
The pancreatic cancer cells BxPC3 were seeded at a
final density of 100,000 cells/well on a 6-well culture plates and
the cells were plated at 30–50% confluence after 8 h. Then, we
added different doses of Furin inhibitor D6R. After treatment with
D6R for 48 h, cells were collected for different assays. The total
cellular proteins were collected after treated with D6R for 72
h.
Cell proliferation assay
To analyze the cell proliferation and the viability,
we performed Cell Counting kit-8 (CCK-8). The pancreatic cancer
cells were collected by trypsinization, and incubated in a 96-well
plate at a final density of 2×103 cells/well for
counting. A CCK-8 kit was added to assess the cells viability at
24, 48, 72, 96 and 120 h and the absorbance was finally determined
at 490 nm.
Colony formation assay
The colony formation assay was used to detect the
anchorage-independent growth of the pancreatic cancer cells. The
cells were plated at a final density of 500 cells/well. Each
transfection group was seeded on 6-well culture plates. After the
cells were incubated for 10–14 days, the cell colonies with >50
cells were counted then fixed with 4% paraformaldehyde and stained
with crystal violet. Following the colony count a graph was
prepared.
Scratch wound healing assay
To detect the ability of migration we incubated the
cells at the density of 1×105 cells/well in a 24-well
culture plate, and disrupted the confluent monolayer with a
10-µl pipette tip and then washed with phosphate-buffered
saline (PBS) three times. The wounded monolayer was photographed
over the following 24 h. The migration ability of the cells was
calculated by the ratio of the healing width at 24 h to the wound
width at 0 h.
Cell invasion and migration assay
The cells were incubated in the Transwell chambers
which were coated with 4 µl/well Matrigel (for an invasion
assay; BD Biosciences) or without Matrigel (for a migration assay)
in a serum-free DMEM according to the manufacturer's instruction
and in the lower chambers 10% FBS WAS added. At 24 h, the cells
that remained on the top of the filter were wiped off and the
invasive cells on the lower chamber were stained and counted.
Real-time PCR
Total RNA was extracted using RNAiso Plus (Takara).
Reverse transcription was performed using RevertAid First Strand
cDNA Synthesis kit (Thermo Fisher Scientific) according to the
manufacturer's specification. Real-time PCR was performed in
triplicate in 20 µl reactions with iQ SYBR®
Premix Ex Taq™ Perfect Real-Time (Bio-Rad Laboratories, Hercules,
CA, USA), 50 ng first strand cDNA and 0.2 µg each primer.
The primer pair used for the amplification of the human Furin gene
was as follows: forward primer, 5′-CCAAAGACATCGGGAAACG-3′ and
reverse primer, 5′-TTAAACCCATCTGCGGAGTAG-3′; and GAPDH primer:
forward, 5′-GGTGAAGGTCGGTGTGAACG-3′ and reverse,
5′-CTCGCTCCTGGAAGATGGTG-3′. Samples were cycled once at 95°C for 2
min, subjected to 35 cycles of 95°C, 56°C and 72°C for 30 sec each.
The relative mRNA content was calculated using the
2−ΔΔCT method with GAPDH as an endogenous control.
Western blot analysis
Cells were washed with PBS three times and lysed in
2X loading buffer on ice, then collected. Total cellular protein
were boiling for 5 min to denature the fractions, and then
separated equally on 10% SDS-PAGE gels, transferred onto PVDF
membranes. The membranes were blocked with 5% skimmed milk for 1 h
at room temperature. The membranes were incubated in the primary
antibodies overnight at 4°C, and the secondary horseradish
peroxidase-conjugated antibodies for 1 h at room temperature. The
bands were detected by enhanced chemiluminescence. The antibodies
were rabbit anti-Furin (18413-1-AP; Proteintech, Rosemont, IL,
USA), mouse anti-β-Tubulin (cat. no. 6181; Cell Signaling
Technology, Danvers, MA, USA), mouse anti-Flag (cat. no. F1804;
Sigma-Aldrich), rabbit anti-N-cadherin (cat. no. 13116; Cell
Signaling Technology), rabbit anti-E-cadherin (cat. no. 3195; Cell
Signaling Technology), rabbit anti-Vimentin (cat. no. 5741; Cell
Signaling Technology), rabbit anti-YAP (cat. no. 8418; Cell
Signaling Technology), rabbit anti-p-YAP (cat. no. 13619; Cell
Signaling Technology), rabbit anti-Mob1 (cat. no. 13730; Cell
Signaling Technology), rabbit anti-p-Mob1 (cat. no. 8699; Cell
Signaling Technology).
Results
Furin expression varies in pancreatic
cancer cells
We first used real-time PCR and western blotting to
detect the expression of Furin of pancreatic cancer cells. The data
showed that the Furin mRNA and protein levels remained highly
abundant in BxPC3 and SW1990 cells, while had weak expression in
PANC1 and PaTu8988 cells (Fig. 1A and
B). Next, we transfected BxPC3 and SW1990 cells with sh-EGFP or
sh-Furin, the mRNA level of Furin was decreased by at least 70% in
sh-Furin group compared with sh-EGFP group (Fig. 1C), as well as its protein level
(Fig. 1D). Furin overexpression
was also examined, and both mRNA and protein levels of Furin were
significant upregulated in Flag-Furin group compared with vector
group (Fig. 1E and F).
Furin promotes the growth of pancreatic
cancer cells
To assess the effect of Furin on the cell growth, we
used CCK-8 assays to determine the relative proliferation rates in
human pancreatic cancer cells. As demonstrated by Fig. 2A and B, Furin knockdown decreased
the relative rates of proliferation in BxPC3 and SW1990 cells,
while Furin overexpression increased in PANC1 and PaTu8988 cells
(Fig. 2C and D), indicating that
Furin promoted the proliferation of pancreatic cancer cells. Colony
forming assay provided additional support, and the data showed that
the number of colonies were 635.5±17.5 and 390±11 in sh-EGFP and
sh-Furin SW1990 cells, and 410±13 and 192±14 in sh-EGFP and
sh-Furin BxPC3 cells (Fig. 2E),
while the numbers of colonies were 64±5 and 165±12 in vector and
Flag-Furin PANC1 cells, and 140±17 and 304±18 in vector and
Flag-Furin PaTu8988 cells (Fig.
2F), suggesting that Furin promoted the ability of colony
formation. All these data suggested that Furin promoted
proliferation in pancreatic cancer cells.
Furin enhances migration of pancreatic
cancer cells
To determine the roles of Furin in progress of
pancreatic cancer cells, we used Transwell migration assays and
wound healing assays to examine the ability of migration of
pancreatic cancer cells. As showed in Fig. 3A and B, the numbers of migrated
cells was 551±20 and 450±13 in sh-EGFP and sh-Furin SW1990 cells,
and 354±13 and 540±16 in sh-EGFP and sh-Furin BxPC3 cells, while
309±15 and 463±12 in vector and Flag-Furin PANC1 cells, and 344±21
and 211±12 in vector and Flag-Furin PaTu8988 cells. Consistently,
the migration rate was 0.27±0.02 and 0.225±0.005 in sh-EGFP and
sh-Furin SW1990 cells, and 0.255±0.025 and 0.145±0.015 in sh-EGFP
and sh-Furin BxPC3 cells, while 0.2455±0.0115 and 0.384±0.011 in
vector and Flag-Furin PANC1 cells, and 0.186±0.007 and
0.3095±0.0125 in vector and Flag-Furin PaTu8988 cells (Fig. 3C). The data suggested that Furin
promoted migration of pancreatic cancer cells.
Furin activates the ability of invasion
of pancreatic cancer cells
Then, we examined the effect of Furin on the
invasive abilities of the pancreatic cancer cells using Transwell
invasion assay. As shown in Fig. 4A
and B, the number of invaded cells was 474±15 and 238.5±12.5 in
sh-EGFP and sh-Furin SW1990 cells, and 320.5±14.5 and 204±8 in
sh-EGFP and sh-Furin BxPC3 cells, while the number of invaded cells
was 262±17 and 362±13 in vector and Flag-Furin PANC1 cells, and
157±8 and 212.5±11.5 in vector and Flag-Furin PaTu8988 cells
suggesting that Furin promoted the invasion ability of pancreatic
cancer cells.
As MMP-2 and MMP-9 possess the ability to hydrolyze
components of the basement membrane and regulate various aspects of
tumor growth and metastasis, we determined the effects of Furin on
expression of MMP-2 and MMP-9. The results indicated that Furin
overexpression led to the increase of the protein level of MMP-2
and MMP-9, and Furin knockdown resulted in the opposite effects
(Fig. 4C). The above data
suggested that Furin promoted the invasive ability of pancreatic
cancer cells.
Furin induces EMT in pancreatic cancer
cells
EMT is thought to be a key mechanism in which
primary tumor cells are capable of metastasizing (19). To determine whether EMT is involved
in Furin-induced migration and invasion, we first detected the
expression of EMT markers at protein levels. The data showed that
Furin knockdown resulted in the downregulation of N-cadherin and
Vimentin and in the upregulation of E-cadherin in SW1990 and BxPC3
cells. In PANC1 and PaTu8988 cells, Furin overexpression remarkably
led to the opposite effects (Fig.
5A). Our data confirmed that Furin promoted EMT in pancreatic
cancer cells.
Furin affects the Hippo-YAP pathway in
pancreatic cancer cells
Previous studies identified that EMT can be
regulated via Hippo-YAP signaling (20–22).
To explore whether YAP is functional in Furin driving EMT in
pancreatic cancer cells, we detected the expression of the relevant
proteins in classic Hippo-YAP pathway, such as Mob1, p-Mob1, YAP
and p-YAP. Our results revealed that Furin knockdown suppressed the
expression of total YAP and p-Mob1, and upregulated p-YAP and Mob1
level, while Furin overexpression resulted in the opposite effects
(Fig. 5B). These data suggested
that Furin affected Hippo-YAP pathway in pancreatic cancer
cells.
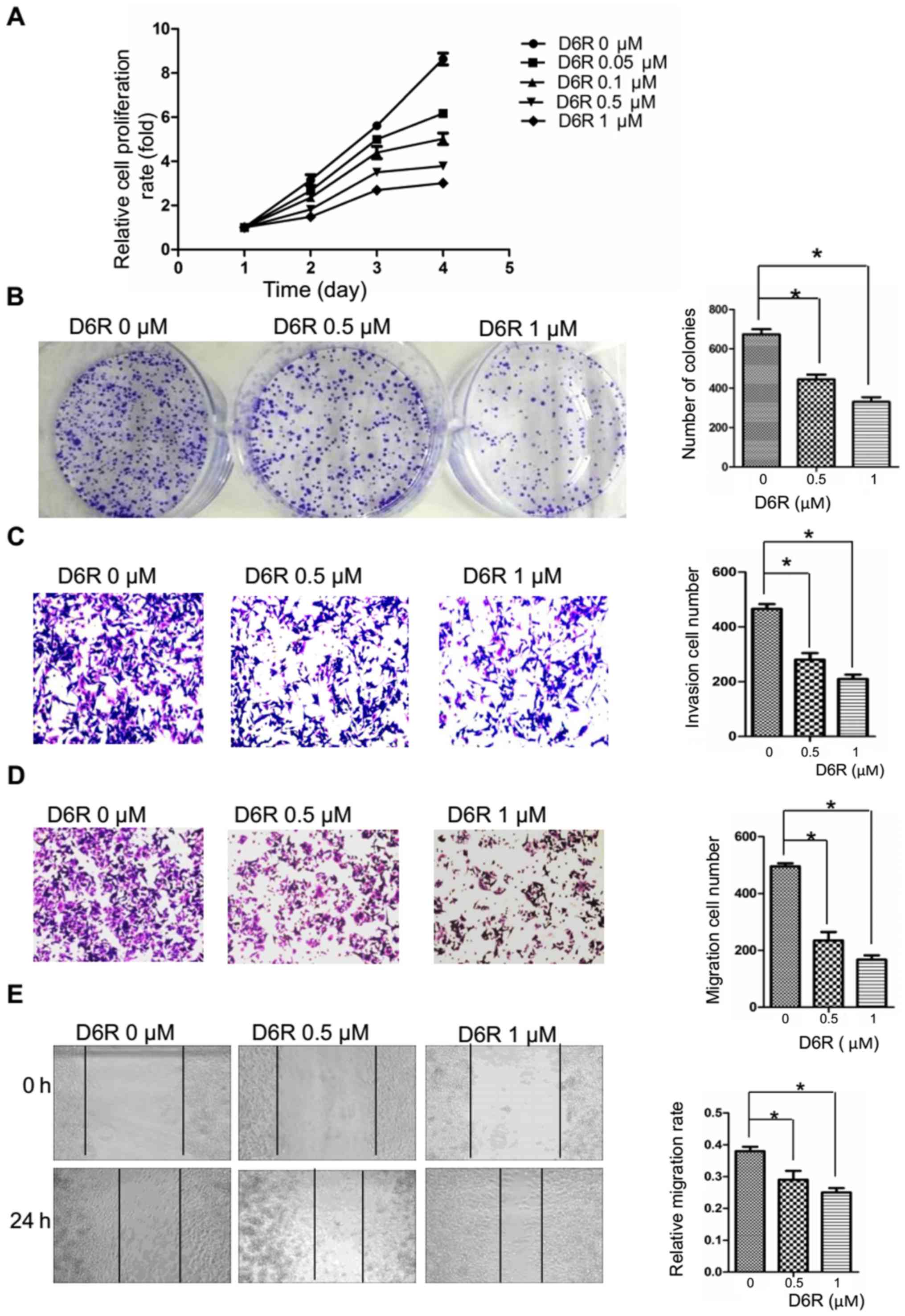
Furin inhibitor D6R suppresses the
proliferation, migration and invasion of BxPC3 cells
Furin inhibition seems to be a logical route to
inhibiting the activation of its substrates, many of which are
essential components of the invasive/metastatic cascade (e.g. TGF-β
and MT-MMPs). The above data confirm that the expression levels of
Furin affected EMT probably via Hippo-YAP pathway in pancreatic
cancer cells. It is worth assessing whether the activity of Furin
similarly affects their growth and EMT. Therefore, we first treated
BxPC3 cells with different doses of Furin inhibitor D6R (0, 0.05,
0.1, 0.5 and 1 µM). The data indicated that D6R
significantly inhibited the cell proliferation in dose-dependent
manner (Fig. 6A), and the half
maximal inhibitory concentration (IC50) of D6R is
between 0.5 and 0.6 µM. Therefore, the concentration of 0,
0.5 and 1 µM was selected for further study. We found that
compared to the D6R-free group, the abilities of proliferation,
migration and invasion were significantly suppressed in the D6R
groups (Fig. 6B–E), indicating
that the activity of Furin actually affects the biological behavior
of pancreatic cancer cells.
Furin inhibitor D6R affects EMT and
Hippo-YAP pathway in pancreatic cancer cells
Next, we examined the effects of D6R on EMT and
Hippo-YAP pathway. As shown in Fig.
7A, D6R resulted in the downregulation of N-cadherin, Vimentin
and in the upregulation of E-cadherin. Also, D6R resulted in the
downregulation of total YAP, p-Mob1 and in the upregulation of
p-YAP, Mob1 (Fig. 7B). It is
suggested that Furin inhibitor D6R affects EMT and Hippo-YAP
pathway in pancreatic cancer cells.
Discussion
We identified that Furin functions as an oncogene in
pancreatic cancer cells. Furin promotes proliferation, migration
and invasion in pancreatic cancer cells. Importantly, Furin
promotes EMT probably via the Hippo-YAP signal pathway.
There is evidence connecting Furin with
tumorigenicity of a wide spectrum of human tumors. Furin promotes
cell proliferation, tumorigenicity and invasiveness of head and
neck squamous cell carcinoma (HNSCC) cells in vitro and
in vivo (11,15). Increasing Furin expression enhanced
skin tumor development and growth (13). Inhibition of Furin suppressed the
IGF-1 receptor which affected IRS-1 and Akt phosphorylation and
showed a significantly reduced ability to form liver metastases
in vivo (14). In this
study, we find that Furin activates oncogenic activities such as
the proliferation and the ability of colony formation in pancreatic
cancer cells are consistent with previous studies in other tumors
and further support its oncogenic potential.
Furthermore, Furin can process a group of notorious
molecules involved directly or indirectly in tumor growth and
progression, such as vascular endothelial growth factor (VEGF),
insulin-like growth factor-1 receptor (IGF-1R), transforming growth
factor-β (TGF-β), insulin-like growth factor 2 (IGF-2) and membrane
type 1 matrix metalloproteinase (MT1-MMP), which contributes to
aggression and metastatic potential of cancer cells (23–27).
Accumulating evidence reveal that Furin-processed substrate
molecules, including TGF-β and MT1-MMP, are critical for enhancing
invasion metastasis and promoting EMT (24,25).
Hence, it would be logical to conclude that Furin played a central
role in EMT. In the present study, we proved that Furin knockdown
resulted in downregulation of N-cadherin and Vimentin, and
upregulation of E-cadherin while Furin overexpression remarkably
led to the opposite effects. Moreover, the fact that Furin enhanced
the ability of migration and invasion in pancreatic cancer cells,
was consistent with previous studies and further supported the
significant role of Furin in EMT. As a precursors cleaved by Furin,
some investigations showed that N-cadherin rendered a substantial
decrease in cell migration (28),
whereas other studies linked the NCAD activated by Furin to
intestinal tumorigenesis (29).
These conflicting data show that Furin function and/or expression
in cancer cells varies in a tumor-specific fashion. It is reported
that Furin activated NCAD at site RQKR↓DW161 and a second putative
PC-processing site RIRSDR↓DK189 located in the first extracellular
domain. Cleavage at the second site would inactive NCAD because of
the loss of the critical Trp161 (30). We surmised that the
activated/inactivated NCAD exist in a balance. In our model, we
speculated that Furin may mainly activate NCAD in pancreatic cancer
cells. Furthermore, Furin activated precursors implicated in
epithelial to mesenchymal transition (31), an ECAD-to-NCAD transition, such as
TGF-β (25).
YAP, as a direct downstream effector of the Hippo
pathway, is found to play an important role in EMT (20). A previous study confirms that YAP
and KRAS converge on the FOS to regulate EMT (21). YAP and TAZ likely together with the
co-factor Tead2 provoke the induction of EMT (20,22).
These observations suggested that YAP1 interacted with specific
transcription factors to regulate EMT. Our results demonstrated
conclusively that Furin-induced regulation of EMT accompanied with
the alterations of YAP phosphoration level and total YAP protein
level. This effect strongly suggests that Furin might have a role
in cleaving the upstream proteins of Hippo-YAP pathway. For
example, Furin is probably involved in the activation of growth
factors and adhesion molecules which may influence the subcellular
localization of YAP, such as TGF-β and E-cadherin (28,32).
The possible divergence in function of Furin leads to a link with
YAP. However, further investigations are required to elucidate the
role of Furin processing in the regulation of EMT via Hippo-YAP
pathway.
In conclusion, inhibition or depletion of Furin
results in a marked reduction of proliferation, migration and
invasiveness of pancreatic cancer cells. Our results indicate that
Furin promotes EMT in pancreatic cancer cells may be through
affecting the Hippo-YAP pathway. Further study is needed on the
mechanisms of Furin. Taken together, this information indicates
that inhibition or depletion of Furin may be a viable route to
ameliorate the malignant phenotype of pancreatic cancer cells.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81672402,
81472333 and 81372718) and the Natural Science Foundation of
Jiangsu Province (BK20131247).
References
|
1
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rasheed ZA, Yang J, Wang Q, Kowalski J,
Freed I, Murter C, Hong SM, Koorstra JB, Rajeshkumar NV, He X, et
al: Prognostic significance of tumorigenic cells with mesenchymal
features in pancreatic adenocarcinoma. J Natl Cancer Inst.
102:340–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dangi-Garimella S, Krantz SB, Shields MA,
Grippo PJ and Munshi HG: Epithelial-mesenchymal transition and
pancreatic cancer progression. Pancreatic Cancer and Tumor
Microenvironment. Grippo PJ and Munshi HG: Trivandrum (India):
Transworld Research Network; 2012, Chapter 5 Available from:
https://www.ncbi.nlm.nih.gov/books/NBK98932/.
|
|
6
|
Zhang H, von Gise A, Liu Q, Hu T, Tian X,
He L, Pu W, Huang X, He L, Cai CL, et al: Yap1 is required for
endothelial to mesenchymal transition of the atrioventricular
cushion. J Biol Chem. 289:18681–18692. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molloy SS, Bresnahan PA, Leppla SH,
Klimpel KR and Thomas G: Human furin is a calcium-dependent serine
endopro-tease that recognizes the sequence Arg-X-X-Arg and
efficiently cleaves anthrax toxin protective antigen. J Biol Chem.
267:16396–16402. 1992.PubMed/NCBI
|
|
8
|
Walker JA, Molloy SS, Thomas G, Sakaguchi
T, Yoshida T, Chambers TM and Kawaoka Y: Sequence specificity of
furin, a proprotein-processing endoprotease, for the hemagglutinin
of a virulent avian influenza virus. J Virol. 68:1213–1218.
1994.PubMed/NCBI
|
|
9
|
Seidah NG, Mayer G, Zaid A, Rousselet E,
Nassoury N, Poirier S, Essalmani R and Prat A: The activation and
physiological functions of the proprotein convertases. Int J
Biochem Cell Biol. 40:1111–1125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Artenstein AW and Opal SM: Proprotein
convertases in health and disease. N Engl J Med. 365:2507–2518.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
López de Cicco R, Bassi DE, Zucker S,
Seidah NG and Klein-Szanto AJ: Human carcinoma cell growth and
invasiveness is impaired by the propeptide of the ubiquitous
proprotein convertase furin. Cancer Res. 65:4162–4171. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scamuffa N, Sfaxi F, Ma J, Lalou C, Seidah
N, Calvo F and Khatib AM: Prodomain of the proprotein convertase
subtilisin/kexin Furin (ppFurin) protects from tumor progression
and metastasis. Carcinogenesis. 35:528–536. 2014. View Article : Google Scholar
|
|
13
|
Fu J, Bassi DE, Zhang J, Li T, Nicolas E
and Klein-Szanto AJ: Transgenic overexpression of the proprotein
convertase furin enhances skin tumor growth. Neoplasia. 14:271–282.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scamuffa N, Siegfried G, Bontemps Y, Ma L,
Basak A, Cherel G, Calvo F, Seidah NG and Khatib AM: Selective
inhibition of proprotein convertases represses the metastatic
potential of human colorectal tumor cells. J Clin Invest.
118:352–363. 2008. View
Article : Google Scholar
|
|
15
|
Bassi DE, Mahloogi H, Lopez De Cicco R and
Klein-Szanto A: Increased furin activity enhances the malignant
phenotype of human head and neck cancer cells. Am J Pathol.
162:439–447. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng M, Watson PH, Paterson JA, Seidah N,
Chrétien M and Shiu RP: Pro-protein convertase gene expression in
human breast cancer. Int J Cancer. 71:966–971. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Page RE, Klein-Szanto AJ, Litwin S,
Nicolas E, Al-Jumaily R, Alexander P, Godwin AK, Ross EA, Schilder
RJ and Bassi DE: Increased expression of the pro-protein convertase
furin predicts decreased survival in ovarian cancer. Cell Oncol.
29:289–299. 2007.PubMed/NCBI
|
|
18
|
Bax NA, van Oorschot AA, Maas S, Braun J,
van Tuyn J, de Vries AA, Groot AC and Goumans MJ: In vitro
epithelial-to-mesenchymal transformation in human adult epicardial
cells is regulated by TGFβ-signaling and WT1. Basic Res Cardiol.
106:829–847. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Overholtzer M, Zhang J, Smolen GA, Muir B,
Li W, Sgroi DC, Deng CX, Brugge JS and Haber DA: Transforming
properties of YAP, a candidate oncogene on the chromosome 11q22
amplicon. Proc Natl Acad Sci USA. 103:12405–12410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shao DD, Xue W, Krall EB, Bhutkar A,
Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW, et
al: KRAS and YAP1 converge to regulate EMT and tumor survival.
Cell. 158:171–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diepenbruck M, Waldmeier L, Ivanek R,
Berninger P, Arnold P, van Nimwegen E and Christofori G: Tead2
expression levels control the subcellular distribution of Yap and
Taz, zyxin expression and epithelial-mesenchymal transition. J Cell
Sci. 127:1523–1536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pei D and Weiss SJ: Furin-dependent
intracellular activation of the human stromelysin-3 zymogen.
Nature. 375:244–247. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yana I and Weiss SJ: Regulation of
membrane type-1 matrix metalloproteinase activation by proprotein
convertases. Mol Biol Cell. 11:2387–2401. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dubois CM, Blanchette F, Laprise MH, Leduc
R, Grondin F and Seidah NG: Evidence that furin is an authentic
transforming growth factor-beta1-converting enzyme. Am J Pathol.
158:305–316. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duguay SJ, Jin Y, Stein J, Duguay AN,
Gardner P and Steiner DF: Post-translational processing of the
insulin-like growth factor-2 precursor. Analysis of O-glycosylation
and endoproteolysis. J Biol Chem. 273:18443–18451. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lopez de Cicco R, Watson JC, Bassi DE,
Litwin S and Klein-Szanto AJ: Simultaneous expression of furin and
vascular endothelial growth factor in human oral tongue squamous
cell carcinoma progression. Clin Cancer Res. 10:4480–4488. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maret D, Gruzglin E, Sadr MS, Siu V, Shan
W, Koch AW, Seidah NG, Del Maestro RF and Colman DR: Surface
expression of precursor N-cadherin promotes tumor cell invasion.
Neoplasia. 12:1066–1080. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun X, Essalmani R, Seidah NG and Prat A:
The proprotein convertase PC5/6 is protective against intestinal
tumorigenesis: In vivo mouse model. Mol Cancer. 8:732009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maret D, Sadr MS, Sadr ES, Colman DR, Del
Maestro RF and Seidah NG: Opposite roles of furin and PC5A in
N-cadherin processing. Neoplasia. 14:880–892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen X, Halberg RB, Burch RP and Dove WF:
Intestinal adenomagenesis involves core molecular signatures of the
epithelial-mesenchymal transition. J Mol Histol. 39:283–294. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bassi DE, Mahloogi H and Klein-Szanto AJ:
The proprotein convertases furin and PACE4 play a significant role
in tumor progression. Mol Carcinog. 28:63–69. 2000. View Article : Google Scholar : PubMed/NCBI
|