Introduction
Osteosarcoma (OS) is the most common malignant bone
tumour, and it mainly affects children and adolescents (1–3). The
progression of this disease is characterized by aggressive tumour
growth, frequent recurrence, and high risk of pulmonary metastasis
(3,4). Although its treatments have advanced
from amputation to complex limb-sparing surgery (LSS) and
incorporated multi-agent chemotherapy (5), the 5-year survival rate is only
60–70% (6). Moreover, the serious
side effects associated with traditional chemotherapy drugs greatly
decrease the patients' quality of life while reducing the
effectiveness of the OS treatments (5). Increasing evidence suggest that OS
may be a differentiation dysfunction disease caused by defects in
the terminal differentiation of osteoblasts (7–13).
Therefore, the promotion and/or circumvention of differentiation
defects may be used as an adjuvant therapy for OS.
Bone morphogenetic proteins (BMPs) are
multifunctional growth factors, which belong to the TGF-β
superfamily (14). BMPs play
important roles in development and cellular physiology processes,
such as proliferation, differentiation, apoptosis, adhesion and
migration (15,16). Yang et al suggested that OS
cells might be maintained in an undifferentiated state secondary to
impaired TGF-β/BMP signalling (13). BMP9, a member of BMPs, is known as
the most potent osteogenic factor compared to the other family
members in mesenchymal stem cells (MSCs) (17,18).
However, Luo et al reported that BMPs (including BMP9) were
unable to induce bone formation in twelve OS cell lines (including
143B cells) and promoted OS cell growth by locking the cells in the
early proliferative phase of the osteogenic pathway (10). Other studies also indicated that
BMP9 failed to induce osteogenic differentiation in OS (8,9,11–13).
Aberrant expression of BMP9 might result in many human tumours,
such as colon cancer (19), breast
cancer (20), ovarian cancer
(21), hepatocellular carcinoma
(22) and gastric cancer (16). These data indicate that the
abnormal osteogenic differentiation related to BMP9 might be one of
the determinants in the pathogenesis of OS. Therefore, restoring
the normal osteogenic ability of BMP9 in OS cells might contribute
to the treatment of OS.
All-trans retinoic acid (ATRA) is a highly
potent derivative of vitamin A, which is required for virtually all
essential physiological processes and functions (23). The biological effects of ATRA are
mediated by two families of nuclear receptors, retinoic acid
receptor (RAR) and retinoid X receptor (RXR), which work as RAR/RXR
heterodimers and bind to retinoic acid response elements in the
promoter regions of retinoid-responsive genes (24). ATRA can induce differentiation in
acute promyelocytic leukaemia (APL) and other tumour types, such as
neuroblastoma, breast cancer and melanoma (25–28).
Additionally, ATRA can induce osteoblastic differentiation of
osteosarcoma cells both in vivo and in vitro
(29,30). Luo et al (31) and Ying et al (32) have reported that ATRA-induced
osteogenic differentiation is mediated by the retinoid-suppressed
phosphorylation of RARα in OS cells. These findings indicate that
RARα plays a key role in ATRA-induced osteogenic differentiation.
It has been reported that ATRA could potentiate BMP9-induced
osteogenesis in 3T3-L1 preadipocytes (33). However, the effect of ATRA on the
osteogenic ability of BMP9 in OS cells is still unclear.
This study investigated the combined effect of BMP9
and ATRA on proliferation and osteogenic differentiation of human
OS 143B cells. The results showed that, in 143B cells, the
osteogenic ability of BMP9 could be exerted in the presence of
ATRA, and the combination of BMP9 and ATRA generated a stronger
anti-proliferative effect than ATRA alone. Additionally, these
effects may originate from the activation of the p38 MAPK
pathway.
Materials and methods
Reagents and antibodies
ATRA was obtained from Sigma-Aldrich (St. Louis, MO,
USA), dissolved in dimethyl sulfoxide (DMSO), divided to aliquots
and stored at −20°C. DMSO was used as a control. Antibodies were
obtained from Santa Cruz Biotechnology. SB203580 was purchased from
Selleckchem (Houston, TX, USA). All other reagents were purchased
form Sigma-Aldrich or Thermo Fisher Scientific, unless otherwise
indicated.
Cell lines and culture
The human 143B (OS) and HEK293 cell lines were
purchased from the American Type Culture Collection (ATCC). 143B
cells or HEK293 cells were maintained in Dulbecco's modified
Eagle's medium (DMEM; Hyclone) and supplemented with 10%
heat-inactivated FBS (Hyclone Laboratories), 100 U/ml
benzylpenicillin, and 100 mg/ml streptomycin at 37°C in 5%
CO2.
Construction of recombinant
adenoviruses
Recombinant adenoviruses expressing BMP9 (AdBMP9)
were generated previously using the AdEasy technology, as described
(34). AdBMP9 also expressed GFP
as a marker to monitor infection efficiency. Adenoviruses
expressing GFP (AdGFP) were used as control.
Crystal violet assay
Cell viability was determined with a crystal violet
assay, conducted as previously described (35). Briefly, 143B cells were plated in a
24-well plate and treated with the indicated concentrations of
ATRA, and/or AdBMP9 and/or SB203580. The cells were washed
carefully 2 times with ice-cold (4°C) phosphate-buffered saline
(PBS); then, the cell viability was assessed upon staining with a
0.2–0.3% crystal violet formalin solution at room temperature for
20 min. For scanning and quantification, 500 µl/well of 20%
acetic acid were added to dissolve the crystal violet, and the
plate was shaken for 20 min at room temperature. The absorbance was
detected at 570 nm.
Cell cycle analysis
143B cells were plated into 6-well plates. Then, the
cells were treated with ATRA and/or AdBMP9 for 48 h. The cells were
washed 3 times with PBS without calcium and magnesium and fixed in
70% ethanol overnight at 4°C. The cells were washed twice with
ice-cold PBS and centrifuged at 300 × g for 10 min, then
resuspended in a staining solution, which contained 0.1% Triton
X-100 and 500 mg/ml propidium iodide (PI). After incubation in the
dark at room temperature for 30 min, the cells were analysed by
fluorescence-activated cell sorting (FACS) analysis.
Reverse transcription and polymerase
chain reaction analysis (RT-PCR)
143B cells were seeded in T25 flasks and treated
with the indicated infection rates of AdBMP9 for 48 h. Total RNA
was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA,
USA) and used to obtain cDNA templates by reverse transcription
(RT). Then, the cDNAs were used as templates for determining the
expression of target genes by semi-quantitative PCR (sqPCR), as
described (36). The primers for
each gene were as follows: GAPDH, F: 5′-CAACGAATTTGGCTACAGCA-3′, R:
5′-AGGGGAGATTCAGTGTGGTG-3′. BMP9, F: 5′-GCTCCGACTCTATGTCTCC TGT-3′,
R: 5′-CCAGCTTATTTTTGCTCTTGGT-3′.
Western blotting
The detailed method was previously described
(36). In brief, subconfluent 143B
cells were plated in 6-well plates and treated with the indicated
concentrations of ATRA and/or AdBMP9 and/or SB203580. At the chosen
time-points, the cells were washed with ice-cold PBS and lysed with
300 µl of lysis buffer. Then, the lysates were boiled for 10
min. Total proteins were separated by SDS-PAGE and then transferred
to polyvinylidene difluoride (PVDF) membranes. Membranes were
blocked with bovine serum albumin (BSA) (5%) at room temperature
for 1 h, and then blotted with primary antibodies. Finally, the
bands corresponding to the targeted proteins were detected with the
enhanced chemiluminescence method (ECL, substrate no. 34095; Thermo
Fisher Scientific, USA).
Alkaline phosphatase (ALP) activity
assay
ALP activity was assessed by a modified Great Escape
SEAP Chemiluminescence assay (BD Clontech, Mountain View, CA, USA)
as described previously (34). For
the bioluminescence assays, each analysis was performed in
triplicate, and the results were repeated in at least three
independent experiments. ALP activity was normalized to total
cellular protein concentration in each sample.
Statistical analysis
All the experiments were performed at least twice
independently and the results were repeated in triplicate.
Statistical analysis was performed using the GraphPad Prism 5
software (La Jolla, CA, USA). All data are represented as the mean
± SD. Statistical significance between two groups was determined
with Student's t-test. A value of p<0.05 was considered to be
statistically significant.
Results
BMP9 promotes the proliferation of OS
cells
First, AdBMP9 infection was identified in human OS
143B cells (Fig. 1A and B). Then,
to investigate the effect of BMP9 on 143B cell proliferation, cells
were infected with AdBMP9 for 24, 48 or 72 h in 24-well plates. The
cell viability was assessed by crystal violet assay: the results
showed that BMP9 promoted the proliferation of 143B cells
(p<0.01) (Fig. 1C and D). We
next examined the cell cycle distribution of 143B cells
overexpressing BMP9 by flow cytometry; the results showed that BMP9
decreased the percentage of cells in G1 phase compared to the
control group (Fig. 1E). The
protein level of proliferating cell nuclear antigen (PCNA), which
plays a key role in the cell cycle (37), was also significantly increased
(Fig. 1F). These results suggested
that BMP9 overexpression promoted the proliferation of 143B cells
in vitro.
BMP9 failed to induce osteogenic
differentiation of OS cells
To investigate the osteogenic differentiation
activity of BMP9 in 143B cells, the expression level of
osteogenesis-related markers was examined by western blotting and
ALP activity assays. The data showed that the protein level of
markers of both early osteogenic differentiation (such as the
transcription factors Dlx-5, Runx-2) and late osteogenesis (such as
OCN and OPN) in 143B cells did not significantly change between the
AdBMP9 and AdGFP groups (Fig. 2A and
B). Similar results were obtained in the analysis of ALP
activity, an early maker of osteogenesis (38) (Fig.
2C). These results demonstrated that BMP9 could not induce
osteogenic differentiation of 143B cells in vitro.
BMP9 inhibits the proliferation of OS
cells in the presence of ATRA
We next investigated the combined effect of BMP9 and
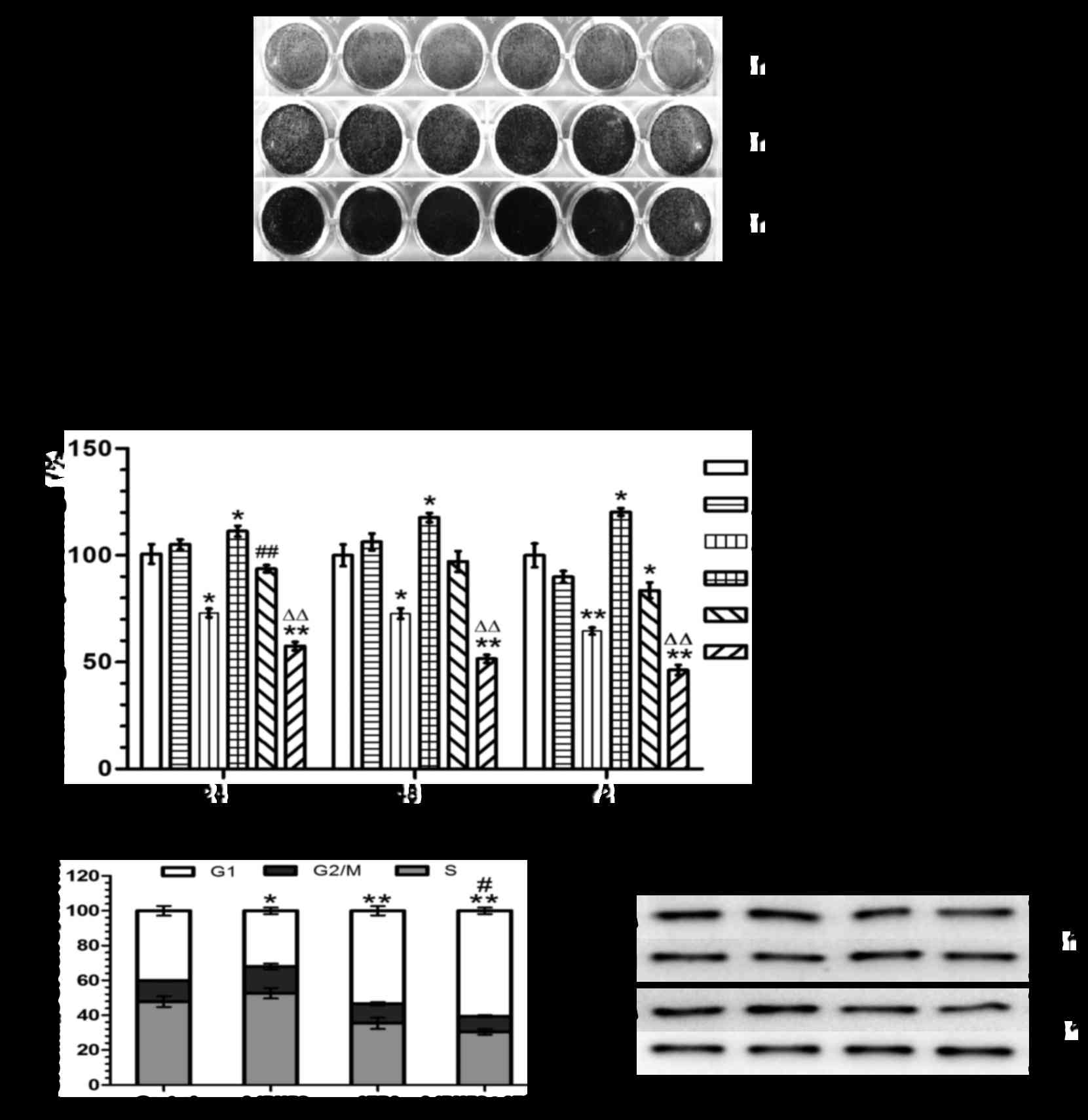
ATRA on the proliferation of 143B cells. It has been reported that
the proliferation of 143B cells is inhibited by ATRA in a
concentration and time-dependent manner; the effective ATRA
concentration may be 5, 10, 20, 40 or 80 µM (29). At first, 20 and 40 µM were
chosen as the candidate concentrations of ATRA: the crystal violet
assay showed that there was no significant change in proliferation
upon treatment with 20 µM ATRA; however, the proliferation
of 143B cells was significantly inhibited upon treatment with 40
µM ATRA (p<0.05) (Fig. 3A
and B). This concentration was therefore used in the next steps
of this study, together with the infection rate of ++ for
AdBMP9.
A crystal violet assay suggested that BMP9
overexpression enhanced the anti-proliferative effect of ATRA
(p<0.01) (Fig. 3A and B).
Likewise, cell cycle analysis showed that BMP9 overexpression
enhanced the effects of ATRA-induced cell cycle arrest in G1 phase
in 143B cells (Fig. 3C). A similar
result was found by western blotting assays detecting the
expression of PCNA (Fig. 3D).
These results suggested that BMP9 could inhibit the proliferation
of 143B cells in the presence of ATRA in vitro.
BMP9 induces the osteogenic
differentiation of OS cells in the presence of ATRA
Then, we studied the osteogenic differentiation
activity of BMP9 on OS cells in the presence of ATRA in
vitro. The expression level of osteogenesis-related markers was
analysed in 143B cells. Osteocalcin (OCN) expression was unchanged
in 143B cells treated with BMP9, increased one-half-fold in cells
treated with ATRA (p<0.01, vs. the control group), and increased
more than three-fold when the cells were treated with BMP9 and ATRA
(p<0.001, vs. the control group) (Fig. 4A and B). Other osteogenesis-related
markers (such as Dlx-5, Runx-2, OPN and ALP activity) showed
similar changes (Fig. 4). These
data indicated that BMP9 could induce the osteogenic
differentiation of 143B cells in the presence of ATRA in
vitro.
BMP9 affects OS cells by activating the
p38 MAPK pathway in the presence of ATRA
Finally, we sought to determine the mechanism
through which BMP9 overexpression effected OS cells in the presence
of ATRA, in vitro. Western blot assays indicated that
treatment with ATRA or ATRA combined with BMP9 showed no difference
on the expression levels of phosphorylated Smad1/5/8 (p-Smad1/5/8),
and phosphorylated Akt1/2/3 (p-Akt1/2/3) (Fig. 5A). However, ATRA could
significantly increase the level of phosphorylated p38 MAPK (p-p38)
in 143B cells, while BMP9 did not have any significant effect.
Interestingly, BMP9 overexpression significantly enhanced the
ability of ATRA to increase the levels of p-p38 (Fig. 5B), and this effect could be partly
reversed by treatment with SB203580 (a p38 MAPK inhibitor)
(Fig. 5C). Furthermore, SB203580
promoted the proliferation of 143B cells and reduced the
anti-proliferative effects of ATRA or ATRA combined with BMP9
(Fig. 6A). Moreover, the
expression of Runx-2 decreased nearly one-fold when cells were
treated with ATRA combined with BMP9 in the presence of SB203580
(p<0.001). Other osteogenesis-related markers (such as Dlx-5 and
ALP activity) showed similar changes (Fig. 6B–D). These results suggested that
the p38 MAPK signalling pathway was involved in the
anti-proliferative and osteogenic differentiation effects induced
by BMP9 in the presence of ATRA in 143B cells in vitro.
Discussion
BMP9 was first identified in the developing mouse
liver as playing an important role in regulating iron metabolism
and the development of cholinergic neurons (39). BMP9 has been implied in
tumourigenesis (7–13,16,19–22).
The proliferative effect of BMP9 on tumours varies considerably.
BMP9 promotes the proliferation of ovarian cancer cells (21) and hepatocellular carcinoma cells
(22), but it inhibits the
proliferation of colon cancer cells (19), breast cancer cells (20) and gastric cancer cells (16). However, the proliferative effect of
BMP9 on OS is unclear. Li et al reported that BMP9 promoted
human OS cell proliferation and tumour growth, possibly through the
Notch signalling pathway (40). In
contrast, Lv et al reported that BMP9 inhibited the growth
of OS cells through the Wnt/β-catenin pathway (41). Our study suggested that BMP9
promoted proliferation (Fig. 1)
and failed to induce osteogenic differentiation in human OS 143B
cells (Fig. 2). It has been
suggested that the tight link between cell proliferation and
differentiation is often compromised in cancer cells, and the
inhibition of proliferation can result from the induction of
differentiation (42–47). The modulation of the activity of
transcription factors can promote differentiation and inhibit
proliferation, and it has been used in the treatment of leukaemia
(46). Similarly, the
overexpression of CDK inhibitors prevents proliferation and
simultaneously induces differentiation in a variety of tumour cells
(42–44). Therefore, the restoration of the
osteogenic ability of BMP9 in OS cells may contribute to the
treatment of OS.
ATRA promotes terminal differentiation of immature
cells, including various types of cancer cells (25–30),
and can promote osteogenic differentiation (29–33,48).
Yang et al reported that ATRA could inhibit proliferation,
induce apotosis and promote osteogenic differentiation in 143B
cells, but the effect of apotosis induced by ATRA is not obvious.
Therefore, they concluded that ATRA inhibits the proliferation of
OS by inducing osteogenesis (29).
In this study, it is comfirmed that BMP9 could not induce
osteogenic differentiation alone in 143B cells (Fig. 2) (8,9,11–13).
However, BMP9 induced osteogenic differentiation in 143B cells in
the presence of ATRA (p<0.001) (Fig. 4). The effect of the combination of
BMP9 and ATRA on the inhibition of proliferation of 143B cells was
more significant than that of ATRA alone (p<0.01) (Fig. 3). This indicates that ATRA can
restore the osteogenic ability of BMP9, thus inhibiting the
proliferation of 143B cells. BMP9 signals through the canonical
BMP/Smad pathway. BMP9 binds to type II or type I BMP receptors
(BMPRII or BMPRI), phosphorylates Smad1/5/8 and forms a complex
with Smad4, followed by translocation to the nucleus and regulation
of downstream targets (18,38).
However, this study suggested that ATRA alone or ATRA combined with
BMP9 groups failed to activate the Smad1/5/8 signalling (Fig. 5A), indicating that the osteogenesis
induced by BMP9 in the presence of ATRA may not be mediated through
the canonical BMP/Smad pathway in 143B cells. The combination of
ATRA and BMP9 also failed to activate the Akt1/2/3 signalling
(Fig. 5A). BMP9 can exert its
function through the non-canonical BMP/Smad pathway, involving p38
MAPK and PI3K/Akt (38,49). Thus, we surmised that p38 MAPK
might play an important role in this phenomenon.
It has been reported that p38 MAPK is involved in
cell differentiation, proliferation, apoptosis, metastasis and
autophagy (19,50–53).
Activation of p38 MAPK is essential for BMP9-induced osteogenesis
in mesenchymal progenitor cells (49,54,55).
However, BMP9 failed to activate p38 MAPK in 143B cells (Fig. 5B) indicating that the inactivation
of p38 MAPK might be the cause for the ineffectiveness of BMP9 to
activate osteogenesis in OS cells. This study further suggests that
ATRA activated p38 MAPK, and the concomitant treatment with ATRA
and BMP9 significantly enhanced this effect (p<0.01) (Fig. 5B). The osteogenic differentiation
and proliferation inhibition effects of ATRA alone or ATRA combined
with BMP9 groups were inhibited by a p38 MAPK inhibitor (SB203580)
(Fig. 6). These results indicate
that ATRA restored osteogenic ability of BMP9 in 143B cells,
probably through the reactivation of p38 MAPK.
In conclusion, this study suggests that the
proliferative effect of BMP9 on human OS 143B cells is related to a
failure in osteogenic differentiation. ATRA could restore the
osteogenic ability of BMP9 in 143B cells, and the combination of
ATRA and BMP9 generated a more significant anti-proliferative
effect than ATRA alone. This result may be due to the reactivation
of the p38 MAPK pathway.
Acknowledgments
We would like to thank Dr T.C. He (University of
Chicago Medical Center, USA) for generously providing all
recombinant adenoviruses. This study was supported by research
grants from the Natural Science Foundation of China (NSFC 81572226
to B.C.H. and 81601895 to R.L.).
References
|
1
|
Helman LJ and Meltzer P: Mechanisms of
sarcoma development. Nat Rev Cancer. 3:685–694. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whelan JS, Bielack SS, Marina N, Smeland
S, Jovic G, Hook JM, Krailo M, Anninga J, Butterfass-Bahloul T,
Böhling T, et al EURAMOS collaborators: EURAMOS-1, an international
randomised study for osteosarcoma: Results from pre-randomisation
treatment. Ann Oncol. 26:407–414. 2015. View Article : Google Scholar :
|
|
4
|
Wang LL: Biology of osteogenic sarcoma.
Cancer J. 11:294–305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dai X, Ma W, He X and Jha RK: Review of
therapeutic strategies for osteosarcoma, chondrosarcoma, and
Ewing's sarcoma. Med Sci Monit. 17:RA177–RA190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thomas D and Kansara M: Epigenetic
modifications in osteogenic differentiation and transformation. J
Cell Biochem. 98:757–769. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haydon RC, Luu HH and He TC: Osteosarcoma
and osteoblastic differentiation: A new perspective on oncogenesis.
Clin Orthop Relat Res. 454:237–246. 2007. View Article : Google Scholar
|
|
9
|
Tang N, Song WX, Luo J, Haydon RC and He
TC: Osteosarcoma development and stem cell differentiation. Clin
Orthop Relat Res. 466:2114–2130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo X, Chen J, Song WX, Tang N, Luo J,
Deng ZL, Sharff KA, He G, Bi Y, He BC, et al: Osteogenic BMPs
promote tumor growth of human osteosarcomas that harbor
differentiation defects. Lab Invest. 88:1264–1277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Zhang H, Zhang W, Huang E, Wang N,
Wu N, Wen S, Chen X, Liao Z, Deng F, et al: Bone morphogenetic
protein-9 effectively induces osteo/odontoblastic differentiation
of the reversibly immortalized stem cells of dental apical papilla.
Stem Cells Dev. 23:1405–1416. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Wagner ER, Yan Z, Wang Z, Luther G,
Jiang W, Ye J, Wei Q, Wang J, Zhao L, et al: The calcium-binding
protein S100A6 accelerates human osteosarcoma growth by promoting
cell proliferation and inhibiting osteogenic differentiation. Cell
Physiol Biochem. 37:2375–2392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang R, Piperdi S, Zhang Y, Zhu Z,
Neophytou N, Hoang BH, Mason G, Geller D, Dorfman H, Meyers PA, et
al: Transcriptional profiling identifies the signaling axes of IGF
and transforming growth factor-β as involved in the pathogenesis of
osteosarcoma. Clin Orthop Relat Res. 474:178–189. 2016. View Article : Google Scholar
|
|
14
|
Sánchez-Duffhues G, Hiepen C, Knaus P and
Ten Dijke P: Bone morphogenetic protein signaling in bone
homeostasis. Bone. 80:43–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen D, Zhao M, Harris SE and Mi Z: Signal
transduction and biological functions of bone morphogenetic
proteins. Front Biosci. 9:349–358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duan L, Ye L, Wu R, Wang H, Li X, Li H,
Yuan S, Zha H, Sun H, Zhang Y, et al: Inactivation of the
phosphatidylinositol 3-kinase/Akt pathway is involved in
BMP9-mediated tumor-suppressive effects in gastric cancer cells. J
Cell Biochem. 116:1080–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiang L, Liang C, Zhen-Yong K, Liang-Jun Y
and Zhong-Liang D: BMP9-induced osteogenetic differentiation and
bone formation of muscle-derived stem cells. J Biomed Biotechnol.
2012:6109522012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang JH, Liu YZ, Yin LJ, Chen L, Huang J,
Liu Y, Zhang RX, Zhou LY, Yang QJ, Luo JY, et al: BMP9 and COX-2
form an important regulatory loop in BMP9-induced osteogenic
differentiation of mesenchymal stem cells. Bone. 57:311–321. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan SX, Wang DX, Wu QX, Ren CM, Li Y,
Chen QZ, Zeng YH, Shao Y, Yang JQ, Bai Y, et al: BMP9/p38 MAPK is
essential for the antiproliferative effect of resveratrol on human
colon cancer. Oncol Rep. 35:939–947. 2016.
|
|
20
|
Holtzhausen A, Golzio C, How T, Lee YH,
Schiemann WP, Katsanis N and Blobe GC: Novel bone morphogenetic
protein signaling through Smad2 and Smad3 to regulate cancer
progression and development. FASEB J. 28:1248–1267. 2014.
View Article : Google Scholar :
|
|
21
|
Herrera B, van Dinther M, Ten Dijke P and
Inman GJ: Autocrine bone morphogenetic protein-9 signals through
activin receptor-like kinase-2/Smad1/Smad4 to promote ovarian
cancer cell proliferation. Cancer Res. 69:9254–9262. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herrera B, García-Álvaro M, Cruz S, Walsh
P, Fernández M, Roncero C, Fabregat I, Sánchez A and Inman GJ: BMP9
isa proliferative and survival factor for human hepatocellular
carcinoma cells. PloS One. 8:e695352013. View Article : Google Scholar
|
|
23
|
Wu LZ, Chaudhary SC, Atigadda VR, Belyaeva
OV, Harville SR, Elmets CA, Muccio DD, Athar M, Kedishvili NY and
Retinoid X: Receptor agonists upregulate genes Responsible for the
biosynthesis of all-trans-retinoic acid in human epidermis. PLoS
One. 11:e015355620162016.
|
|
24
|
Dilworth FJ and Chambon P: Nuclear
receptors coordinate the activities of chromatin remodeling
complexes and coactivators to facilitate initiation of
transcription. Oncogene. 20:3047–3054. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Atashrazm F, Lowenthal RM, Dickinson JL,
Holloway AF and Woods GM: Fucoidan enhances the therapeutic
potential of arsenic trioxide and all-trans retinoic acid in acute
promyelocytic leukemia, in vitro and in vivo. Oncotarget.
7:46028–46041. 2016.PubMed/NCBI
|
|
26
|
Silvis AM, McCormick ML, Spitz DR and
Kiningham KK: Redox balance influences differentiation status of
neuroblastoma in the presence of all-trans retinoic acid. Redox
Biol. 7:88–96. 2016. View Article : Google Scholar :
|
|
27
|
Yan Y, Li Z, Xu X, Chen C, Wei W, Fan M,
Chen X, Li JJ, Wang Y and Huang J: All-trans retinoic acids induce
differentiation and sensitize a radioresistant breast cancer cells
to chemotherapy. BMC Complement Altern Med. 16:113–123. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amann PM, Czaja K, Bazhin AV, Rühl R,
Skazik C, Heise R, Marquardt Y, Eichmüller SB, Merk HF and Baron
JM: Knockdown of lecithin retinol acyltransferase increases
all-trans retinoic acid levels and restores retinoid sensitivity in
malignant melanoma cells. Exp Dermatol. 23:832–837. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang QJ, Zhou LY, Mu YQ, Zhou QX, Luo JY,
Cheng L, Deng ZL, He TC, Haydon RC and He BC: All-trans retinoic
acid inhibits tumor growth of human osteosarcoma by activating Smad
signaling-induced osteogenic differentiation. Int J Oncol.
41:153–160. 2012.PubMed/NCBI
|
|
30
|
Zhang L, Zhou Q, Zhang N, Li W, Ying M,
Ding W, Yang B and He Q: E2F1 impairs all-trans retinoic
acid-induced osteogenic differentiation of osteosarcoma via
promoting ubiquitination-mediated degradation of RARα. Cell Cycle.
13:1277–1287. 2014. View Article : Google Scholar :
|
|
31
|
Luo P, Yang X, Ying M, Chaudhry P, Wang A,
Shimada H, May WA, Adams GB, Mock D, Triche TJ, et al:
Retinoid-suppressed phosphorylation of RARalpha mediates the
differentiation pathway of osteosarcoma cells. Oncogene.
29:2772–2783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ying M, Zhang L, Zhou Q, Shao X, Cao J,
Zhang N, Li W, Zhu H, Yang B and He Q: The E3 ubiquitin protein
ligase MDM2 dictates all-trans retinoic acid-induced osteoblastic
differentiation of osteosarcoma cells by modulating the degradation
of RARα. Oncogene. 35:4358–4367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Liu Y, Zhang R, Wang X, Huang F,
Yan Z, Nie M, Huang J, Wang Y, Wang Y, et al: All-trans retinoic
acid modulates bone morphogenic protein 9-induced osteogenesis and
adipogenesis of preadipocytes through BMP/Smad and Wnt/β-catenin
signaling pathways. Int J Biochem Cell Biol. 47:47–56. 2014.
View Article : Google Scholar
|
|
34
|
Tang N, Song WX, Luo J, Luo X, Chen J,
Sharff KA, Bi Y, He BC, Huang JY, Zhu GH, et al: BMP-9-induced
osteogenic differentiation of mesenchymal progenitors requires
functional canonical Wnt/beta-catenin signalling. J Cell Mol Med.
13B:2448–2464. 2009. View Article : Google Scholar
|
|
35
|
He BC, Chen L, Zuo GW, Zhang W, Bi Y,
Huang J, Wang Y, Jiang W, Luo Q, Shi Q, et al: Synergistic
antitumor effect of the activated PPARgamma and retinoid receptors
on human osteosarcoma. Clin Cancer Res. 16:2235–2245. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meng ZJ, Wu N, Liu Y, Shu KJ, Zou X, Zhang
RX, Pi CJ, He BC, Ke ZY, Chen L, et al: Evodiamine inhibits the
proliferation of human osteosarcoma cells by blocking PI3K/Akt
signaling. Oncol Rep. 34:1388–1396. 2015.PubMed/NCBI
|
|
37
|
Strzalka W and Ziemienowicz A:
Proliferating cell nuclear antigen (PCNA): A key factor in DNA
replication and cell cycle regulation. Ann Bot. 107:1127–1140.
2011. View Article : Google Scholar :
|
|
38
|
Huang J, Yuan SX, Wang DX, Wu QX, Wang X,
Pi CJ, Zou X, Chen L, Ying LJ, Wu K, et al: The role of COX-2 in
mediating the effect of PTEN on BMP9 induced osteogenic
differentiation in mouse embryonic fibroblasts. Biomaterials.
35:9649–9659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Herrera B, Dooley S and Breitkopf-Heinlein
K: Potential roles of bone morphogenetic protein (BMP)-9 in human
liver diseases. Int J Mol Sci. 15:5199–5220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li R, Zhang W, Cui J, Shui W, Yin L, Wang
Y, Zhang H, Wang N, Wu N, Nan G, et al: Targeting BMP9-promoted
human osteosarcoma growth by inactivation of notch signaling. Curr
Cancer Drug Targets. 14:274–285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lv Z, Wang C, Yuan T, Liu Y, Song T, Liu
Y, Chen C, Yang M, Tang Z, Shi Q, et al: Bone morphogenetic protein
9 regulates tumor growth of osteosarcoma cells through the
Wnt/β-catenin pathway. Oncol Rep. 31:989–994. 2014.
|
|
42
|
Kranenburg O, Scharnhorst V, Van der Eb AJ
and Zantema A: Inhibition of cyclin-dependent kinase activity
triggers neuronal differentiation of mouse neuroblastoma cells. J
Cell Biol. 131:227–234. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Adachi M, Roussel MF, Havenith K and Sherr
CJ: Features of macrophage differentiation induced by p19INK4d, a
specific inhibitor of cyclin D-dependent kinases. Blood.
90:126–137. 1997.PubMed/NCBI
|
|
44
|
Matushansky I, Radparvar F and Skoultchi
AI: Reprogramming leukemic cells to terminal differentiation by
inhibiting specific cyclin-dependent kinases in G1. Proc Natl Acad
Sci USA. 97:14317–14322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rosenbauer F and Tenen DG: Transcription
factors in myeloid development: Balancing differentiation with
transformation. Nat Rev Immunol. 7:105–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ruijtenberg S and van den Heuvel S:
Coordinating cell proliferation and differentiation: Antagonism
between cell cycle regulators and cell type-specific gene
expression. Cell Cycle. 15:196–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang W, Deng ZL, Chen L, Zuo GW, Luo Q,
Shi Q, Zhang BQ, Wagner ER, Rastegar F, Kim SH, et al: Retinoic
acids potentiate BMP9-induced osteogenic differentiation of
mesenchymal progenitor cells. PLoS One. 5:e119172010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhao Y, Song T, Wang W, Wang J, He J, Wu
N, Tang M, He B and Luo J: P38 and ERK1/2 MAPKs act in opposition
to regulate BMP9-induced osteogenic differentiation of mesenchymal
progenitor cells. PLoS One. 7:e433832012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signalling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cheng HL, Lin CW, Yang JS, Hsieh MJ, Yang
SF and Lu KH: Zoledronate blocks geranylgeranylation not
farnesylation to suppress human osteosarcoma U2OS cells metastasis
by EMT via Rho A activation and FAK-inhibited JNK and p38 pathways.
Oncotarget. 7:9742–9758. 2016.PubMed/NCBI
|
|
52
|
Lv T, Wu Y, Mu C, Liu G, Yan M, Xu X, Wu
H, Du J, Yu J and Mu J: Insulin-like growth factor 1 promotes the
proliferation and committed differentiation of human dental pulp
stem cells through MAPK pathways. Arch Oral Biol. 72:116–123. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu J, Wang B, Huang P, Wang H, Xu K, Wang
X, Xu L and Guo Z: Microcystin-LR promotes cell proliferation in
the mice liver by activating Akt and p38/ERK/JNK cascades.
Chemosphere. 163:14–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xu DJ, Zhao YZ, Wang J, He JW, Weng YG and
Luo JY: Smads, p38 and ERK1/2 are involved in BMP9-induced
osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. BMB
Rep. 45:247–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ye G, Li C, Xiang X, Chen C, Zhang R, Yang
X, Yu X, Wang J, Wang L, Shi Q, et al: Bone morphogenetic protein-9
induces PDLSCs osteogenic differentiation through the ERK and p38
signal pathways. Int J Med Sci. 11:1065–1072. 2014. View Article : Google Scholar : PubMed/NCBI
|