Introduction
In 2007, the International Agency for Cancer
Research (IACR) announced that human papillomavirus (HPV), in
addition to smoking and alcohol consumption, was a strong risk
factor for oropharyngeal squamous cell carcinoma (OPSCC) where
tonsillar and base of tongue cancer (TSCC and BOTSCC) dominate
(1). Studies performed by others
and us have shown a steady increased incidence of HPV-positive
(HPV+) TSCC and BOTSCC from the 1970s, and the past
decade ~70% of all TSCC and BOTSCC have been HPV+ in
Stockholm, Sweden (2–5). Furthermore, patients with
HPV+ TSCC and BOTSCC have a much better prognosis
compared to patients with HPV-negative (HPV−) tumors,
with ~80 vs. 40–60% 5-year disease-free survival (6–9).
Head and neck cancer (HNSCC) in general has a very
poor clinical outcome and, therefore, over the past decade,
treatment has been intensified with radiation therapy in
combination with chemotherapy, and in some cases with cetuximab,
resulting in more severe side-effects (10,11).
Intensified therapy is most likely not necessary for
most patients with HPV+ TSCC and BOTSCC, that together
e.g. in Sweden make up ~35% of all HNSCC (12). In order to de-escalate therapy for
the former two patient groups, it is important to identify patients
with a very high probability to respond to therapy. By combining
additional prognostic markers with HPV status in TSCC and BOTSCC,
some biomarkers were found to be of particular interest.
High CD8+ tumor infiltrating lymphocyte
(TIL) counts, absent/low human leukocyte antigen (HLA) class I
expression and absent/low CD44 intensity expression were all
positive prognostic markers for HPV+ TSCC and BOTSCC
(13–18). The fact that having high numbers of
CD8+ TILs was favorable for prognosis was expected and
has been shown for several types of tumors (19). However, it was paradoxical that
absent/low HLA class I expression was an advantage, since it is
assumed that foreign antigens, in this case HPV-derived peptide
antigens, are presented to CD8+ TILs by HLA class I
molecules (20). In
HPV+ tumors, however, it has been shown that HPV E5 and
E7 have the ability to suppress HLA class I expression, thereby
assisting the virus to avoid immune recognition (21–24).
In an experiment using mice with HPV+
tumors, it was shown that treatment with radiation therapy and
chemotherapy was only efficient in immunocompetent and not in
immunodeficient mice (25). It is
likely that the immune system also plays an important role for
tumor clearance in humans, and that radiation therapy in
particular, may induce increased HLA class I expression in
HPV+ tumors, this way facilitating immune recognition
after treatment.
In the present study, the hypothesis that radiation
therapy increases HLA class I expression was therefore tested in
vitro in HPV+ and HPV− base of tongue and
mobile tongue squamous cell carcinoma cell lines.
Materials and methods
Cell lines
Three HPV+ cancer cell lines (UM-SCC-47,
UPCI-SCC-154 and UPCI-SCC-090) and one HPV− cancer cell
line (UT-SCC-14) were studied throughout these experiments
(26–28). Cell line characteristics are
summarized in Table I. Cell lines
UM-SCC-47 and UPCI-SCC-090 were cultured in Dulbecco's modified
Eagle's medium (HyClone Laboratories, Inc., Logan, UT, USA)
containing 10% fetal bovine serum (FBS; Gibco, Waltham, MA, USA),
1% L-glutamine (Gibco) and 1% penicillin streptomycin solution
(Gibco). Cell lines UT-SCC-14 and UPCI-SCC-154 were cultured in
minimum essential medium (MEM; Gibco) containing 10% FBS, 1%
L-glutamine, 1% non-essential amino acids (Gibco) and 0.1%
gentamicin (Gibco). Stocks of all cell lines were grown in t75 cell
culture flasks with filter lids (Sarstedt, Nümbrecht, Germany) from
which cells were plated into 6-well cell culture plates (TPP Techno
Plastic Products AG, Trasadingen, Switzerland) for the irradiation
experiments, and kept at 37°C with 0.5% carbon dioxide at 100%
humidity. Medium was changed regularly; usually every 2–3 days and
the cell cultures were split using trypsin EDTA 1x (Gibco) when
~70–100% confluent. All cell lines were tested for mycoplasma using
the Takara mycoplasma detection set (Takara Bio, Shiga, Japan).
 | Table ICell lines characteristics. |
Table I
Cell lines characteristics.
| Cell lines | Origin | HPV status | Stage | Ref. |
|---|
| UM-SCC-47 | Lateral tongue
cancer | HPV16 mRNA
positive | T3N1M0 | (26) |
| UPCI-SCC-154 | Base of tongue
cancer | HPV16 mRNA
positive | T4N2M0 | (27) |
| UPCI-SCC-090 | Recurrence of base
of tongue cancer | HPV16 mRNA
positive | T2N0M1 | (27) |
| UT-SCC-14 | Mobile tongue
cancer | HPV16 mRNA
negative | T3N1M0 | (28) |
Irradiation and experimental setup
A Caesium-137 source was used to treat the cells
with radiation therapy. Initial experiments and testing different
radiation doses, were performed on cell lines UM-SCC-47,
UPCI-SCC-154 and UT-SCC-14. Irradiation was tested with doses of
2–10 Gray (Gy), as well as fractionated doses of 2 Gy given for 5
consecutive days, and as a result of this calibration, one dose of
10 Gy was used for the consecutive experiments. Standardized
experiments were performed on the four cell lines plated in 6-well
plates, with the goal of reaching 75% confluence upon initiation of
experiments. To obtain this, 2.5×105 cells/well were
plated for UM-SCC-47; 8×105 cells/well for UPCI-SCC-154;
2×105 cells/well for UPCI-SCC-090; and
3.5×105 million cells/well for UT-SCC-14. For cell cycle
analysis half the amount of previously indicated cell numbers per
cell line were plated.
Flow cytometry for evaluation of HLA
class I expression, cell cycle analysis and apoptosis
HLA class I analysis
The cells were stained for HLA class I proteins 48 h
after irradiation. The cells were washed, trypsinized and collected
in 5 ml tubes for centrifugation. After discarding the supernatant,
2×105 cells/well were collected for staining,
re-suspended in 2 ml FACS buffer (DPBS, 30% BSA, 2 mM EDTA) and
spun down at 2,000 rpm for 5 min. To distinguish live cells from
dead, cells were incubated at 4°C for 10 min with 0.3 µl
LIVE/DEAD® Fixable Near-IR Dead Cell Stain kit (Thermo
Fisher Scientific) diluted in 40 µl FACS buffer. The cells
were then washed, spun down in FACS buffer and incubated at 4°C for
20 min with 3 µl HLA class I (A, B and C) specific antibody,
clone W6/32, coupled to an Alexa Fluor 488 (anti-human) fluorescent
dye (BioLegend, San Diego, CA, USA). Unstained controls and isotype
controls [1 µl IgG2a (mouse) per control] (BioLegend) were
included. The samples were then washed and within 1-h run on a
NovoCyte™ (ACEA Biosciences, Inc., San Diego, CA, USA) flow
cytometer. HLA class I expression for each sample was acquired and
further analyzed using NovoExpress™ software (ACEA Biosciences).
Initial experiments (Fig. 1) were
run on an LSR II flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA) and analyzed with FlowJo software (FlowJo, LLC, Ashland,
OR, USA).
Cell cycle analysis
Cells were stained for DNA content in order to
analyze their stage in the cell cycle. Floating cells were
collected and pooled with adherent cells after trypsin treatment
and spun down for 5 min at 2,000 rpm at 4°C, this was done 24 h
after irradiation. The cell pellet was then re-suspended in 0.75 ml
cold PBS, followed by drop-wise addition of 1 ml cold 100% ethanol,
while vortexing. Samples were stored overnight at 4°C for fixation.
The cells were then spun down and stained with 100 µl
propidium iodine (PI; Sigma-Aldrich, St. Louis, MO, USA)/RNase A
solution (Sigma-Aldrich) (0.05 mg/ml PI, 0.25 mg/ml RNase A,
diluted in 1X PBS) for 30 min at 37°C in the dark. The cells were
then washed in PBS and spun down twice before being re-suspended in
200 µl PBS and run on the NovoCyte™ flow cytometer. Cell
cycle stage was further analyzed with the software
NovoExpress™.
Apoptosis assay
To distinguish apoptotic from normal cells, cells
were stained with a FITC Annexin V apoptosis detection kit I (BD
Biosciences, San Diego, CA, USA), which binds to phospholipid
phosphatidylserines, a protein that in apoptotic cells becomes more
exposed. This was done 96 h after the irradiation. Floating cells
were collected and pooled with adherent cells after trypsin
treatment, spun down for 5 min at 2,000 rpm at 4°C, washed in cold
PBS containing Mg2+ and Ca2+, then spun down
and re-suspended in Annexin V binding buffer. Cells/well
(105) were then added to FACS tubes and incubated for 15
min at room temperature in the dark with 5 µl Annexin V
coupled to a FITC fluorophore and 5 µl PI. The cells were
then re-suspended in Annexin V buffer and within 1 h run on a
NovoCyte flow cytometer. Each sample was further analyzed for
apoptosis using NovoExpress software.
RNA extraction, cDNA synthesis and
quantitative real-time PCR
Irradiated cells and control cells were collected 48
h after treatment and used immediately, or frozen down at −70°C for
later extraction. RNA was extracted using the RNeasy®
Mini kit (Qiagen, Venlo, The Netherlands). Samples were DNase
treated using the RNase-free DNase set (Qiagen) to ensure DNA free
samples. In total 0.1 µg of RNA was utilized for first
strand cDNA synthesis using a First Strand cDNA Synthesis kit
(Thermo Fisher Scientific). For this, random hexamers were used as
primers. The product of the cDNA synthesis was subsequently taken
for a SYBR-Green based qPCR, as previously described by Lindquist
et al (9). The following
genes were examined: HPV16 E5, E7, with primers as described in
Ramqvist et al (29) and
HLA-A for exon 3 with primers as described by Villabona et
al (30). GUS B was added as
an endogenous control. Triplicates were included from each cell
line, treated and non-treated, for each gene of interest and
endogenous controls, as well as triple-negative controls. Treated
samples were compared to non-treated samples by calculation of ΔΔCt
values.
Statistical analyses
Two-tailed unpaired Student's t-test was used to
examine the difference in means between the treated and the
non-treated groups. A P<0.05 was considered statistically
significant.
Results
Sensitivity to irradiation distributed as
a single or several doses was similar
Preparatory irradiation experiments were performed
in order to calibrate the dosage and administration frequency.
Testing single doses of 2 or 5 Gy compared to untreated cell lines
showed no significant difference in HLA class I expression (data
not shown). However, 10 Gy given either at one time-point or with 2
Gy given daily for 5 consecutive days tended to show an increase of
HLA class I expression for HPV-positive UM-SCC-47, UPCI-SCC-154 and
HPV-negative UT-SCC-14 (Fig. 1),
while HPV-positive UPCI-SCC-090 was not tested in these first
experiments. In addition, since controls and cells irradiated with
2 Gy × 5 were kept for a longer time period, experiments were
performed with cells that were split or not split after 2 days of
irradiation, to identify possible fluctuations in HLA class I
expression depending on culture conditions. HLA class I expression
tended to increase in both split and non-split cell lines (Fig. 1). Similar results were observed
when 10 Gy was administered as a single dose (data not shown). Due
to that no differences were observed in HLA class I expression
after giving 10 Gy at one time-point, or during a time period of 5
days, all additional experiments were performed giving 10 Gy as a
single dose.
In these initial sets of experiments, regardless if
the cells had received irradiation, or not, base line HLA class I
expression differed between cell lines and was lowest for
UPCI-SSC-154 and highest for UT-SCC-14 and UM-SCC-47 (Fig. 1). When UPCI-SCC-090 later was
tested, it was shown that its HLA class I expression was the lowest
of all the cell lines included in this analysis (data not
shown).
Irradiation with 10 Gy tends to increase
HLA class I expression in all cell lines
Three sets of experiments, with duplicate samples,
were performed with 10 Gy administered as a single dose to
HPV-positive cell lines UM-SCC-47, UPCI-SCC-154, UPCI-SCC-090 and
HPV-negative cell line UT-SCC-14 and HLA-class I expression was
compared to non-treated cells. Calculating the mean fluorescence
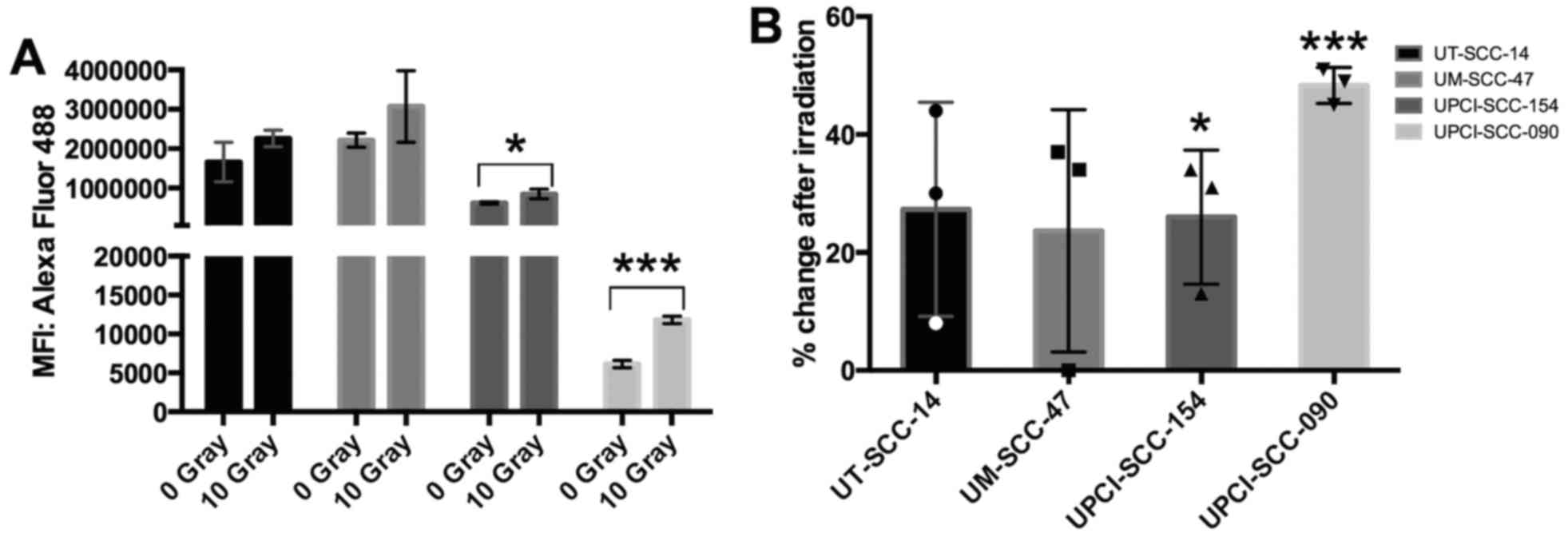
intensity (MFI) values from these three experiments, a significant
increase of HLA class I expression was indicated in cell lines
UPCI-SCC-154 (P=0.035) and UPCI-SCC-090 (P<0.001) (Fig. 2A). In cell lines UM-SCC-47 and
UT-SCC-14 a similar tendency with an increase of HLA class I
expression was observed, although it did not reach statistical
significance (P=0.185 and P=0.129) (Fig. 2A). In addition, the average
increase of HLA class I expression for UM-SCC-47, UPCI-SCC-154 and
UT-SCC-14 was 20–25%, while UPCI-SCC-090 with a very low initial
HLA class I expression disclosed an increased expression of ~50%
(Fig. 2B).
To confirm that the results were specific, an
isotype antibody was included as a control for each cell line and
treatment group. The background MFI of the isotype controls ranged
between 2,000 and 16,000 for all non-treated and 10 Gy treated cell
lines and were relatively low and negligible for UT-SCC-14,
UM-SCC-47 and UPCI-SCC-154 that exhibited high values (MFIs ranging
between 400,000 and 3,000,000) with the HLA class I (A, B and C)
antibody. For UPSCI-SCC-090 the isotype MFI values ranged between
6,000 and 11,000, thus, close to those obtained for the HLA class I
antibody (Fig. 2).
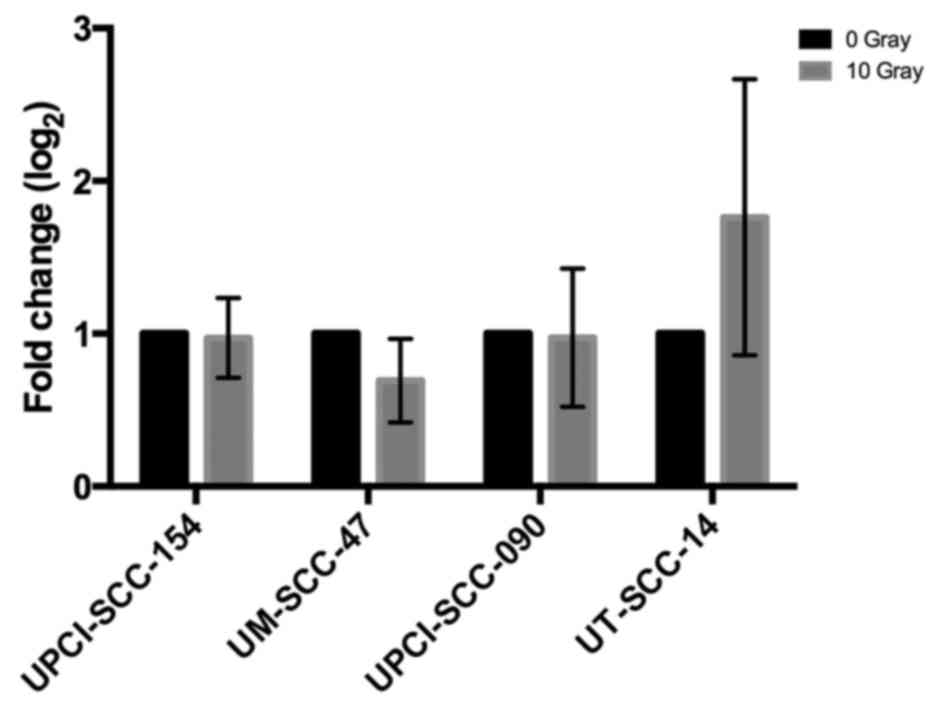
HLA-A mRNA expression was analyzed by quantitative
PCR in three consecutive experiments for all cell lines. HLA-A mRNA
expression did not differ significantly between treated (10 Gy) and
non-treated cells (Fig. 3).
HPV16 E5 and E7 mRNA expression after a
10-Gy radiation dose
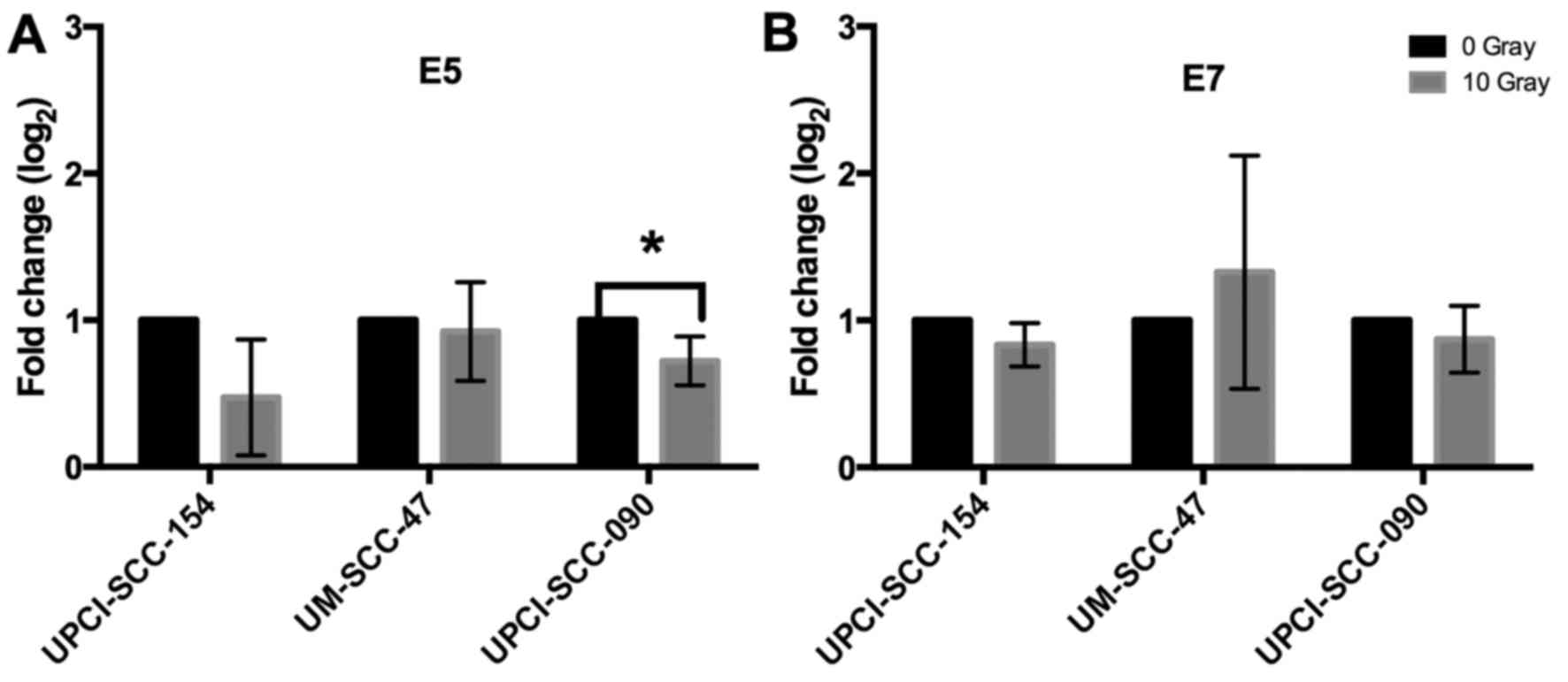
To examine whether presence of HPV E5 or E7 mRNA
expression was correlated to HLA class I expression, mRNA levels of
E5 and E7 were measured by RT-qPCR in all HPV+ cell
lines. Three different experiments were performed with triplicate
samples 48 h after irradiation in treated (10 Gy) and non-treated
cells. E5 mRNA expression was significantly decreased in cell line
UPCI-SCC-090 after irradiation compared to non-treated cells, and a
similar tendency was observed for UPCI-SCC-154, but here there was
a greater variability between experiments and statistical
significance was not reached (Fig.
4). No other significant changes were observed for HPV16 E5 and
E7 mRNA expression (Fig. 4).
Irradiation induces cell cycle
changes
After irradiation with 5 and 10 Gy, compared to
non-treated cells, cell cycle changes were observed in all four
cell lines, where an increasing dose generally lead to G2/M arrest
and a decrease of cells in S-phase with some variation between the
different cell lines. UM-SCC-47 and UPCI-SCC-154 showed a more
drastic increase of cells in G2/M with less cells in G1 compared to
UPCI-SCC-090 and UT-SCC-14 (Fig.
5). P-values are presented in Table II.
 | Table IIP-values from the cell cycle
analysis. |
Table II
P-values from the cell cycle
analysis.
| UM-SCC-47
| UPCI-SCC-154
| UPCI-SCC-090
| UT-SCC-14
|
|---|
| G1 | S | G2/M | G1 | S | G2/M | G1 | S | G2/M | G1 | S | G2/M |
|---|
| 5 Gy | 0.055 | 0.020 | <0.001 | <0.001 | 0.007 | <0.001 | 0.127 | 0.738 | 0.353 | 0.915 | 0.115 | 0.327 |
| 10 Gy | 0.002 | 0.013 | <0.001 | <0.001 | 0.014 | 0.003 | 0.509 | 0.122 | 0.204 | 0.458 | 0.029 | 0.037 |
Irradiation with 10 Gy induces
apoptosis
Apoptosis was estimated using Annexin V staining 96
h after irradiation with 10 Gy and the percentage of cells
undergoing apoptosis was compared to non-treated controls. All cell
lines showed a significant increase of apoptosis in the treated
groups (Fig. 6). However, since a
large proportion of the cells were already fragmented 96 h after
radiation and were not included in this analysis, the amount of
cell death was likely higher.
Discussion
In the present study, the hypothesis that HLA class
I expression is upregulated upon radiation therapy was examined
in vitro in HPV+ cancer cell lines UM-SCC-47,
UPCI-SCC-154 and UPCI-SCC-090, and the HPV− cancer cell
line UT-SCC-14. A single dose of 10 Gy tended to increase HLA class
I expression on a protein level for all four tested cell lines.
This increase was statistically significant for UPCI-SCC-154 and
UPCI-SCC-090, and was accompanied by a significant drop in HPV16 E5
mRNA expression, for UPCI-SCC-090, with a similar tendency for
UPCI-SCC-154. There was, however, no significant change in HLA-A
mRNA expression in any of the cell lines, or in HPV16 E7 mRNA
expression in any of the HPV+ cell lines after
irradiation. A shift from G1 to G2/M and decrease in S-phase after
irradiation was observed in 3/4 cell lines and irradiation-induced
apoptosis in all cell lines.
The obtained data could partially explain why
HPV+ TSCC/BOTSCC with low HLA class I expression still
could have a good clinical response after radiation therapy. An
increase in HLA-class I expression could definitely trigger or
improve a cytotoxic T cell response, and explain the often
excellent tumor clearance observed after radiation therapy
especially of HPV+ TSCC/BOTSCC.
It is possible, that the increase in HLA class I
expression, at least in UPCI-SCC-090 and possibly in UPCI-SCC-154
could in part be due to a decrease in HPV16 E5 mRNA expression,
since it has been postulated that HPV16 E5 can downregulate HLA
class I (21–24). Nevertheless, other mechanisms can
also be involved in the upregulation of HLA class I after
irradiation. This is supported by the fact that a similar tendency
with an increased HLA class I expression was also found in the
HPV− cell line, suggesting that this propensity is not
necessarily unique for HPV+ tumors. Nonetheless, our
data must be considered with caution, especially for
HPV− cancer, since only one HPV− tumor cell
line was included. In addition, although HLA class I expression
tended to be upregulated in the HPV− cancer cell line in
this study, the effect on tumor clearance may not be as great as
for patients with HPV+ cancer, since HPV−
cancer is not virus driven and viral antigens cannot be presented
to the immune system.
One can further speculate as to why HLA class I
expression is upregulated after radiation therapy. The amount of
intracellular peptides is a limiting factor for HLA class I
expression and recently Reits et al (31) have shown, that radiation therapy
increases the intracellular peptide pool, which in turn leads to an
upregulation of MHC class I molecules. For HPV+ cancer
this may be of great benefit since increasing the peptide pool of
e.g. the HPV16 E6 and E7 nucleoproteins and increasing HLA class I
expression can be very useful for potentiating the immune response
against such tumors, with the result of a more efficient rejection
of them.
Nevertheless, it is possible that irradiation also
targets other mechanisms than the above in viral induced tumors,
where downregulation of HLA class I molecules is often induced in
different ways to escape immune recognition (21–24,32).
The decrease, or tendency of a decrease of HPV16 E5 mRNA expression
after irradiation in this study, in two of the HPV+ cell
lines indicates that this could be the case. To resolve this
further, additional specific investigations are necessary.
Although an increase in HLA class I at the protein
level on the cell surface was observed, this was not correlated to
rise in HLA-A mRNA expression. However, HLA class I expression on
the cell surface, does not necessarily reflect HLA-A mRNA
expression and it has been reported that E5 inhibits surface
expression of HLA class I proteins by retaining them in the Golgi
apparatus (21).
The fact that a considerable amount of cells
underwent apoptosis and a G1 phase to G2/M phase transition was
obtained was not unexpected following irradiation with 10 Gy, and
has been reported also by others (33,34).
Still, there could be other intrinsic differences with regard to
radio-sensitivity of the different cell lines irrespective of HPV
status or HLA class I expression, however, this needs to be
investigated further. A study by Gupta et al (35) for example showed that UM-SCC-47 was
several-fold more sensitive to radiation as compared to
UPCI-SCC-090.
There are several limitations in the present study.
First of all the analysis was performed in vitro on cell
lines, thus, lacking the possible cytokine burst accompanied after
irradiation in vivo (36).
Secondly, we only tested a limited number of HPV+ cell
lines and only one HPV− cell line and the data for
UPCI-SCC-090 were due to its very low initial HLA class I
expression problematic with regard to background isotype staining.
Nevertheless, there was a consistent tendency for HLA class I
expression to increase after irradiation in all cell lines, thus,
indicating that indeed HLA class I expression could increase after
radiation therapy. Furthermore, notably for the UPCI-SCC-090 line
E5 mRNA expression dropped significantly, and since E5 has the
capability to decrease HLA class I surface, the observed increase
in HLA class I expression in this cell line could in fact be a
result of this effect.
Another limitation of this study was that at least
one cell line was not derived from the tonsils or base of tongue,
which is commonly where HPV-driven tumors occur. Whether the
HPV-positive cell line, derived from lateral tongue, is from the
mobile or base of tongue is not published, but since it expresses
HPV E6 and E7 mRNA, this indicates that it is HPV-driven.
To conclude, in the present study, irradiation with
10 Gy induced an increased HLA class I expression in two
HPV+ cell lines, with a similar tendency in the
remaining HPV+ and HPV− cell lines. Although
further studies are needed on additional HPV+ cell lines
our findings suggest that radiation may possibly potentiate the
immune response to HPV+ tumors, where viral antigens
contribute to immune recognition.
Abbreviations:
|
BOTSCC
|
base of tongue squamous cell
carcinoma
|
|
FACS
|
flow cytometry
|
|
FBS
|
fetal bovine serum
|
|
Gy
|
gray
|
|
HLA
|
human leukocyte antigen
|
|
HPV
|
human papillomavirus
|
|
MFI
|
mean fluorescence intensity
|
|
OPSCC
|
oropharyngeal squamous cell
carcinoma
|
|
PI
|
propidium iodine
|
|
TIL
|
tumor infiltrating lymphocyte
|
|
TSCC
|
tonsillar squamous cell carcinoma
|
Acknowledgments
We would like to thank Dr Reidar Grénman, Turku
University, for kindly providing us with the cell line UT-SCC-14 in
2003. The cell lines UPCI-SCC-154 and UPCI-SCC-090 were kindly
provided by Dr Susanne Gollin, University of Pittsburgh, in 2012,
and the cell line UM-SCC-47 was provided by Dr John Lee, Sanford
University, in 2012.
References
|
1
|
International Agency for Research on
Cancer: IARC Monographs on the Evaluation of Carcinogenic Risks to
Humans. Human Papillomaviruses. 90. IARC Press; Lyon: 2007
|
|
2
|
Golas SM: Trends in palatine tonsillar
cancer incidence and mortality rates in the United States.
Community Dent Oral Epidemiol. 35:98–108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Syrjänen S: HPV infections and tonsillar
carcinoma. J Clin Pathol. 57:449–455. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Näsman A, Attner P, Hammarstedt L, Du J,
Eriksson M, Giraud G, Ahrlund-Richter S, Marklund L, Romanitan M,
Lindquist D, et al: Incidence of human papillomavirus (HPV)
positive tonsillar carcinoma in Stockholm, Sweden: An epidemic of
viral-induced carcinoma? Int J Cancer. 125:362–366. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Näsman A, Nordfors C, Holzhauser S,
Vlastos A, Tertipis N, Hammar U, Hammarstedt-Nordenvall L, Marklund
L, Munck-Wikland E, Ramqvist T, et al: Incidence of human
papillomavirus positive tonsillar and base of tongue carcinoma: A
stabilisation of an epidemic of viral induced carcinoma? Eur J
Cancer. 51:55–61. 2015. View Article : Google Scholar
|
|
6
|
Gillison ML, Koch WM, Capone RB, Spafford
M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, et
al: Evidence for a causal association between human papillomavirus
and a subset of head and neck cancers. J Natl Cancer Inst.
92:709–720. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mellin H, Friesland S, Lewensohn R,
Dalianis T and Munck-Wikland E: Human papillomavirus (HPV) DNA in
tonsillar cancer: Clinical correlates, risk of relapse, and
survival. Int J Cancer. 89:300–304. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dahlstrand HM and Dalianis T: Presence and
influence of human papillomaviruses (HPV) in tonsillar cancer. Adv
Cancer Res. 93:59–89. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lindquist D, Romanitan M, Hammarstedt L,
Näsman A, Dahlstrand H, Lindholm J, Onelöv L, Ramqvist T, Ye W,
Munck-Wikland E, et al: Human papillomavirus is a favourable
prognostic factor in tonsillar cancer and its oncogenic role is
supported by the expression of E6 and E7. Mol Oncol. 1:350–355.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pryor DI, Solomon B and Porceddu SV: The
emerging era of personalized therapy in squamous cell carcinoma of
the head and neck. Asia Pac J Clin Oncol. 7:236–251. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramqvist T and Dalianis T: An epidemic of
oropharyngeal squamous cell carcinoma (OSCC) due to human
papilloma-virus (HPV) infection and aspects of treatment and
prevention. Anticancer Res. 31:1515–1519. 2011.PubMed/NCBI
|
|
12
|
Cancercentrum R: Nationellt vårdprogram
Huvud- och hals-cancer. 2015, In Swedish.
|
|
13
|
Näsman A, Andersson E, Nordfors C, Grün N,
Johansson H, Munck-Wikland E, Massucci G, Dalianis T and Ramqvist
T: MHC class I expression in HPV positive and negative tonsillar
squamous cell carcinoma in correlation to clinical outcome. Int J
Cancer. 132:72–81. 2013. View Article : Google Scholar
|
|
14
|
Näsman A, Andersson E, Marklund L,
Tertipis N, Hammarstedt-Nordenvall L, Attner P, Nyberg T, Masucci
GV, Munck-Wikland E, Ramqvist T, et al: HLA class I and II
expression in oropharyngeal squamous cell carcinoma in relation to
tumor HPV status and clinical outcome. PLoS One. 8:e770252013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Näsman A, Romanitan M, Nordfors C, Grün N,
Johansson H, Hammarstedt L, Marklund L, Munck-Wikland E, Dalianis T
and Ramqvist T: Tumor infiltrating CD8+ and
Foxp3+ lymphocytes correlate to clinical outcome and
human papillomavirus (HPV) status in tonsillar cancer. PLoS One.
7:e387112012. View Article : Google Scholar
|
|
16
|
Lindquist D, Ahrlund-Richter A, Tarján M,
Tot T and Dalianis T: Intense CD44 expression is a negative
prognostic factor in tonsillar and base of tongue cancer.
Anticancer Res. 32:153–161. 2012.PubMed/NCBI
|
|
17
|
Näsman A, Nordfors C, Grün N,
Munck-Wikland E, Ramqvist T, Marklund L, Lindquist D and Dalianis
T: Absent/weak CD44 intensity and positive human papillomavirus
(HPV) status in oropharyngeal squamous cell carcinoma indicates a
very high survival. Cancer Med. 2:507–518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nordfors C, Grün N, Tertipis N,
Ährlund-Richter A, Haeggblom L, Sivars L, Du J, Nyberg T, Marklund
L, Munck-Wikland E, et al: CD8+ and CD4+
tumour infiltrating lymphocytes in relation to human papillomavirus
status and clinical outcome in tonsillar and base of tongue
squamous cell carcinoma. Eur J Cancer. 49:2522–2530. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gooden MJ, de Bock GH, Leffers N, Daemen T
and Nijman HW: The prognostic influence of tumour-infiltrating
lymphocytes in cancer: A systematic review with meta-analysis. Br J
Cancer. 105:93–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sasagawa T, Takagi H and Makinoda S:
Immune responses against human papillomavirus (HPV) infection and
evasion of host defense in cervical cancer. J Infect Chemother.
18:807–815. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Campo MS, Graham SV, Cortese MS, Ashrafi
GH, Araibi EH, Dornan ES, Miners K, Nunes C and Man S: HPV-16 E5
down-regulates expression of surface HLA class I and reduces
recognition by CD8 T cells. Virology. 407:137–142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ashrafi GH, Haghshenas MR, Marchetti B,
O'Brien PM and Campo MS: E5 protein of human papillomavirus type 16
selectively downregulates surface HLA class I. Int J Cancer.
113:276–283. 2005. View Article : Google Scholar
|
|
23
|
Li W, Deng XM, Wang CX, Zhang X, Zheng GX,
Zhang J and Feng JB: Down-regulation of HLA class I antigen in
human papillomavirus type 16 E7 expressing HaCaT cells: Correlate
with TAP-1 expression. Int J Gynecol Cancer. 20:227–232. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deng XM, Li W, Zhang X, Wang CX, Dong ZG,
Zhang X, Zheng GX, Zhang XH, Zheng N, Wang LL, et al: RNA
interference of human papillomavirus type 16 E7 increases HLA class
I antigen expression in HaCaT-E7 cells. Int J Gynecol Cancer.
21:28–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spanos WC, Nowicki P, Lee DW, Hoover A,
Hostager B, Gupta A, Anderson ME and Lee JH: Immune response during
therapy with cisplatin or radiation for human
papillomavirus-related head and neck cancer. Arch Otolaryngol Head
Neck Surg. 135:1137–1146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brenner JC, Graham MP, Kumar B, Saunders
LM, Kupfer R, Lyons RH, Bradford CR and Carey TE: Genotyping of 73
UM-SCC head and neck squamous cell carcinoma cell lines. Head Neck.
32:417–426. 2010.
|
|
27
|
White JS, Weissfeld JL, Ragin CCR, Rossie
KM, Martin CL, Shuster M, Ishwad CS, Law JC, Myers EN, Johnson JT,
et al: The influence of clinical and demographic risk factors on
the establishment of head and neck squamous cell carcinoma cell
lines. Oral Oncol. 43:701–712. 2007. View Article : Google Scholar
|
|
28
|
Kiuru A, Servomaa K, Grénman R, Pulkkinen
J and Rytömaa T: p53 mutations in human head and neck cancer cell
lines. Acta Otolaryngol Suppl. 529(Suppl): 237–240. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramqvist T, Mints M, Tertipis N, Näsman A,
Romanitan M and Dalianis T: Studies on human papillomavirus (HPV)
16 E2, E5 and E7 mRNA in HPV-positive tonsillar and base of tongue
cancer in relation to clinical outcome and immunological
parameters. Oral Oncol. 51:1126–1131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Villabona L, Leon Rodriguez DA, Andersson
EK, Seliger B, Dalianis T and Masucci GV: A novel approach for
HLA-A typing in formalin-fixed paraffin-embedded-derived DNA. Mod
Pathol. 27:1296–1305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reits EA, Hodge JW, Herberts CA, Groothuis
TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH,
Neijssen J, et al: Radiation modulates the peptide repertoire,
enhances MHC class I expression, and induces successful antitumor
immunotherapy. J Exp Med. 203:1259–1271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Griffin BD, Gram AM, Mulder A, Van Leeuwen
D, Claas FH, Wang F, Ressing ME and Wiertz E: EBV BILF1 evolved to
downregulate cell surface display of a wide range of HLA class I
molecules through their cytoplasmic tail. J Immunol. 190:1672–1684.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee BJ, Chon KM, Kim YS, An WG, Roh HJ,
Goh EK and Wang SG: Effects of cisplatin, 5-fluorouracil, and
radiation on cell cycle regulation and apoptosis in the
hypopharyngeal carcinoma cell line. Chemotherapy. 51:103–110. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 59:928–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gupta AK, Lee JH, Wilke WW, Quon H, Smith
G, Maity A, Buatti JM and Spitz DR: Radiation response in two
HPV-infected head-and-neck cancer cell lines in comparison to a
non-HPV-infected cell line and relationship to signaling through
AKT. Int J Radiat Oncol Biol Phys. 74:928–933. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Di Maggio FM, Minafra L, Forte GI,
Cammarata FP, Lio D, Messa C, Gilardi MC and Bravatà V: Portrait of
inflammatory response to ionizing radiation treatment. J Inflamm
(Lond). 12:142015. View Article : Google Scholar
|