Introduction
Hypoxia-inducible factor-1α (HIF-1α) plays a crucial
role in the adaptation of cancer cells to hypoxia by activating
transcription of target genes that regulate several biological
processes, including cell proliferation, glucose metabolism and pH
regulation (1). Slominski et
al found that HIF-1α was upregulated in advanced malignant
melanoma compared with melanocytic nevi or thin melanomas localized
to the skin (1). High expression
level of HIF-1α is an independent predictor of poor prognosis after
radiotherapy (2,3). 2-Methoxyestradiol (2-MeOE2) is a
special inhibitor that suppresses HIF-1α protein levels and its
transcriptional activity. It was shown to inhibit the expression of
HIF-1α in a dose-dependent manner in cancer cells by depolymerising
microtubules and blocking HIF-1α nuclear accumulation (4). Activation of glycolytic genes by
HIF-1α is considered to be a very important factor for metabolic
adaptation to hypoxia, with increased conversion of glucose to
pyruvate and subsequently to lactate (5). Many studies demonstrated that the
expression and activity of glycolytic enzymes and the lactic acid
concentration were reduced by inhibiting HIF-1α (6,7).
Kim et al found that HIF-1 suppressed glucose
metabolism through the tricarboxylic acid cycle (TCA) by directly
transactivating the gene encoding pyruvate dehydrogenase kinase 1
(PDK1). PDK1 inactivated the TCA cycle enzyme and pyruvate
dehydrogenase (PDH), which converted pyruvate to acetyl-CoA, and
rescued these cells from hypoxia-induced apoptosis (8). HIF-1α causes an increase in pyruvate
dehydrogenase kinase 1 (PDK1), which acts to limit the amount of
pyruvate entering the citric acid cycle, leading to decreased
mitochondrial oxygen consumption. PDK downregulates the activity of
PDH-E1α, decreases the oxidation of pyruvate in mitochondria, and
increases the conversion of pyruvate to lactate in the cytosol.
Dichloroacetate (DCA), as an inhibitor of pyruvate dehydrogenase
kinase (PDK), decreases the glycolysis state of cells by leading to
the reactivation of pyruvate dehydrogenase (PDH) and shifts glucose
metabolism from glycolysis to mitochondrial oxidation (9).
The reprogramming of metabolism, especially the
glucose metabolism is one of the hallmarks of cancer (10). Cancer cells have generally higher
level of glucose uptake and lactate secretion, regardless of oxygen
content. This phenomenon is called 'aerobic glycolysis' or the
'Warburg effect' (11,12). Metabolic studies supported the
metabolic switch toward aerobic glycolysis in melanoma cells
(13,14). Recently, some studies revealed that
elevated glycolysis of cancer cells will not only provide a growth
advantage but also involves in resistance to chemotherapy and
ionizing radiation resistance (15,16).
High glycolytic states of tumor cells are known to correlate
strongly with radioresistance (17–21).
In our previous study, radiosensitive/radioresistant
human melanoma cell model MDA-MB-435/MDA-MB-435R was established
(22). An elevated level of HIF-1α
expression in radioresistant melonoma cells was also demonstrated
in our recent experiments. Therefore, we aimed to investigated the
effect of HIF-1α on glycolysis and radioresistance in the435R
cells. Since PDK1 is a key regulator of glycolysis and it can be
downregulated by inhibition of HIF-1α, DCA was used in the recent
study to elucidate the possible underlying mechanisms of 2-MeOE2
radiosensiting to radioresistant melanoma cells, especially the
HIF-1α/PDK1-mediated glycolysis.
Materials and methods
Cells, cell culture and reagents
Human melanoma cell line MDA-MB-435S was purchased
from the Cell Bank of Type Culture Collection of Chinese Academy of
Sciences (Shanghai, China). Cell lines were cultured in DMEM growth
media (Life Technologies, Carlsbad, CA, USA) which was supplemented
with 10% fetal bovine serum (FBS, Life Technologies) and maintained
at 37°C in a humidified atmosphere at 5% CO2. DCA and
2-MeOE2 were purchased from Sigma-Aldrich (St. Louis, MO, USA).
X-ray irradiation
Radioresistant cell model of MDA-MB-435S were
established by irradiation with X-ray. All the cells were first
grown to approximately 90% confluence then irradiated by a Simens
Primus Accelerator at an average dose rate of 2 Gy/fraction, total
dose was 60 Gy.
Colony formation assay
The standard clonogenic assay was performed as
previously described (23,24). Cells were digested with trypsin
enzyme at room temperature for 30–60 sec and then the clumped cells
were pipetted. The single cell suspension was adjusted and seeded
into typical 6-well plates. Then, cells were left to settle
overnight, and exposed to irradiation at room temperature with the
dose of 0, 2, 4, 6, 8 and 10 Gy, then incubated at 37°C, 5%
CO2 for 14 days. After fixation and staining, colonies
of >50 cells were scored. Surviving rates were evaluated
relative to 0 Gy radiation treated controls. A single-hit
multi-target model was used to analyze the data (25). The survival curve of each group was
plotted as the log of the survival fraction vs. the radiation dose
using GraphPad Prism 5.0 software.
RNA extraction and quantitative reverse
transcription PCR
Total RNA was isolated using TRIzol regent
(Invitrogen) following the manufacturer's instructions.
First-strand cDNA was obtained using the RevertAid™ First-Strand
cDNA Synthesis kit (Fermentas International, Inc., Burlington, ON,
Canada). For the quantitative analysis of HIF-1α mRNA, the human
β-actin gene was used as an internal control. Primer sequences were
designed as follows: HIF-1α sense, TGCAACATGGAAGGTATTGC, and
antisense, TTCACAAATCAGCACCAAGC; β-actin sense,
TGCGTTACACCCTTTCTTGA, and antisense, CACCTTCACCGTTCCAGTTT. The
Threshold cycle (Ct) values were measured by SYBR-Green PCR Master
mix (Takara, Shiga, Japan) with Mx3000P (Stratagene, La Jolla, CA,
USA). The Mx 3000P analysis program was used to analyze the
results.
Cellular ATP content and extracellular
lactate level
Cells (1×106/ml) were seeded into 6-well
plates. The extracellular lactate level was detected by Lactic acid
assay kit (Jiancheng, Nanjing, China) following the manufacturer's
instructions. The cellular ATP content was determined by measuring
the luminescence with an ATP-dependent bioluminescence assay kit
(Jiancheng). Briefly, the cells (1×106/ml) were seeded
into 6-well plates, lysed with lyolysis on ice, collected into 1.5
ml tubes, and centrifuged for 5 min at 10,000 × g; the Absorbance
Microplate Reader was used to determine the OD of the supernatant
(BioTek Instruments Inc., Winooski, VT, USA) at 630 nm (A630). The
ATP content was calculated according to the formula provided by
manufacturer's instructions. The above procedures were performed
three times under the same condition.
Mitochondrial membrane potential and
apoptosis analysis
The mitochondrial membrane potential was detected by
the JC-1 kit (Beyotime Biotechnology, Haimen, China). Cell
suspensions at 1×105/ml were grown on glass coverslips
with/without 2-MeOE2 for 2 h before irradiation treatment. At 30
min after irradiation, the cells were incubated with DPBS with 1
μl JC-1 reagent at 37°C for 15 min. Then, cells were washed
three times. The mitochondrial membrane potential was detected
under the fluorescence microscope. The increase of green
fluorescence suggests the decrease of the mitochondrial membrane
potential.
For detection of apoptosis, 200,000 cells were
seeded in a 6-well plate, pre-incubated with/without 2-MeOE2 for 2
h before irradiation treatment. The cells were harvested 48 h after
irradiation. The cells were re-suspended in 100 μl staining
buffer with 5 μl Annexin V- FITC (BD Pharmingen, San Jose,
CA, USA) and stained at room temperature for 15 min followed by a
quick staining with 1 μg/ml propidium iodide (PI). The
samples were analyzed on LSRII flow cytometer (Cytomics FC 500;
Beckman Coulter, Fullerton, CA, USA), and the data were analyzed
using Flow Jo software (FlowJo, Ashlan, OR, USA). Cells were
stained with Annexin V-fluorescein isothiocyanate and PI and
classified into 4 subpopulations as follows: viable cells (Annexin
V and PI double-negative), apoptotic cells (Annexin V-positive),
early dead cells (Annexin V and PI double-positive) and dead cells
(PI-positive).
CCK-8 assay for cell proliferation
Cell proliferation was determined using a Cell
Counting Kit-8 (CCK-8; Dojindo Kumamoto, Japan). Cells were seeded
in 96-well plates, serum starved, and then treated with the
experimental reagents as described (26). At the end of the incubation, CCK-8
solution was added and 3 h later, the absorbance at 450 nm
(A450 nm) was measured with a microplate reader
(Sunrise). Cell proliferation rates were calculated and normalized
with the OD value of the first day.
Immunofluorescence
The immunofluorescence of γ-H2AX foci was used to
determine the residual DNA double-strand breaks (DSBs). Melanoma
cells were grown on glass coverslips with/without 2-MeOE2 for 2 h
before irradiation, 30 min after irradiation, cells were fixed in
4% paraformaldehyde for 15 min before staining overnight with
rabbit anti-γ-H2AX (ser139, Millipore Corp., Billerica, MA, USA)
diluted in PBS 1:400. After staining for 60 min with goat
anti-rabbit Cy3 (Millipore Corp.) diluted 1:100, the coverslips
were mounted in Prolong Gold mounting medium containing DAPI (Life
Technologies). Fluorescent images were obtained using Zeiss LSM710
confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany)
equipped with Plan-Apochromat X63/1.4 Oil DICII objective and
analyzed using the ZEN2011 software and Adobe Photoshop CS5.
Protein isolation and western blot
analysis
Briefly, 10×105 cells were seeded in 60
mm2 plates and cultured in the presence or absence of 5
μM 2-MeOE2 for 24 h. Following treatment, cells were lysed
on ice with lysis buffer (Cell Signaling Technology, Danvers, MA,
USA) supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF).
Samples were centrifuged at 12,000 × g for 10 min at 4°C, and
supernatant was aliquoted and stored at −80°C for future use.
Total protein (30 μg) was resolved in a 10%
polyacrylamide gel using SDS-PAGE then transferred to a
polyvinylidene difluoride (PVDF) membrane. Following transfer,
membrane was blocked for 2 h at room temperature in 5% non-fat dry
milk diluted in 0.1% Tween-20 in TBS (TBST) followed by an
overnight incubation in blocking solution with primary antibodies.
After washing, membranes were incubated for 2 h at room temperature
with the appropriate peroxidase-conjugated secondary antibody,
washed, and subjected to chemiluminescent substrate (Luminata
Forte; Millipore Corp.). Membranes were imaged using the ChemiDoc
XRS+ system (Bio-Rad, Hercules, CA, USA) and densitometry was
performed using Image Lab software (Bio-Rad). Every target protein
of our experiment was calculated by gray scanning using ImageJ
(free software from NIH website), the relative density of target
proteins were normalized to its marker internal protein. Primary
antibodies used included rabbit anti-HIF1-α, β-actin, GLUT1, LDHA,
PDK1. The quantification of band density was performed using ImageJ
software.
Statistical analyses
All the experiments were repeated at least 3 times.
Data are presented as the means ± standard deviation (SD).
Statistical analyses were performed using two-tailed Student's
t-test (specified in the figure legend when paired analysis was
used) and one way ANOVA using SPSS 19.0 and GraphPad Prism 5.0
software. The threshold for statistical significance was defined as
P≤0.05.
Results
HIF-1α and glycolysis-related proteins
were highly expressed in radioresistant melanoma cells
In this study, we used a radioresistant cell model
of melanoma cells to investigate radioresistance of melanoma cells
more accurately. Radioresistant cell model MDA-MB-435R (435R) was
established from melanoma cell line MDA-MB-435 (435S) through
continuous X-ray radiation (22).
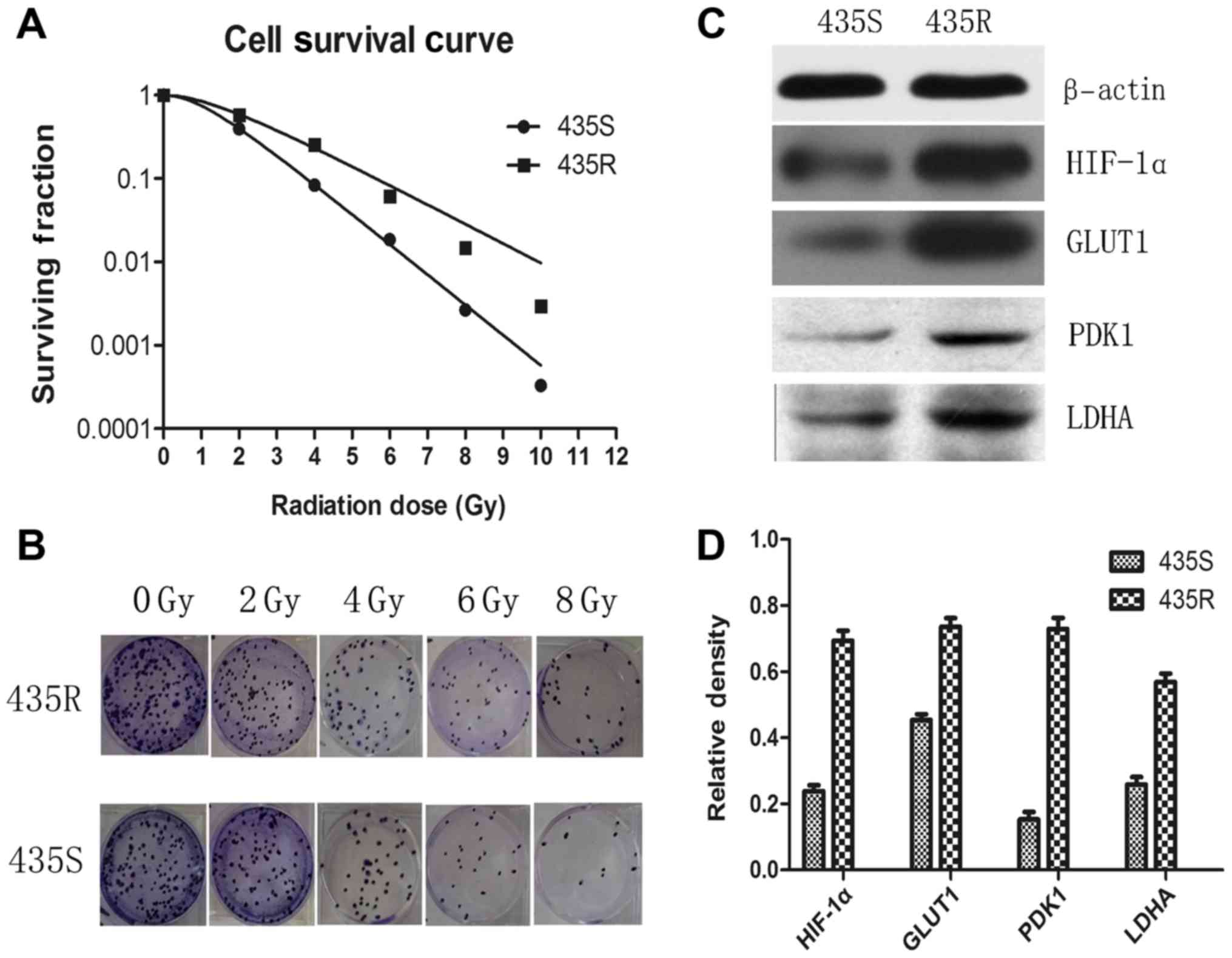
The results of colony formation assay show that 435R cells
displayed stronger radioresistance compared with its parental 435S
cells (Fig. 1A and B), and
demonstrate that we established the radioresistant cell model
successfully.
Our data demonstrated that the expression of HIF-1α
in the 435R cells was higher than the parental cells 435S (Fig. 1C and D). Expression of the
glycolysis-related proteins such as PDK1, GLUT1 and LDHA in the
435R cells were higher than the 435S cells (Fig. 1C and D).
2-MeOE2 decreases radioresistance, PDK1
and glycolytic state of 435R cells
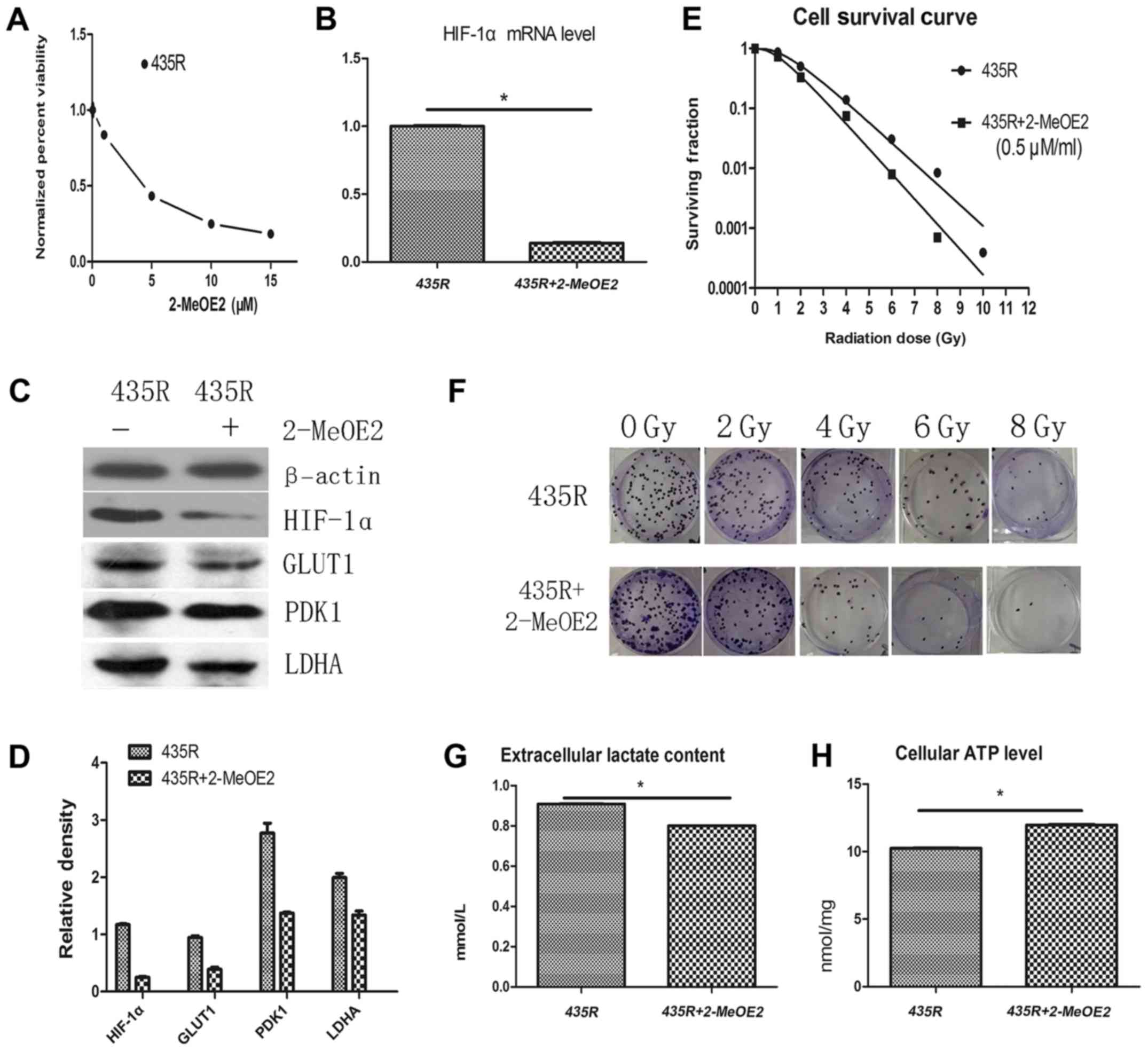
The median IC50 of 2-MeOE2 was 4.3 μM (48 h)
for 435R cells (Fig. 2A). Our data
indicated that 2-MeOE2 at the concentration ≤0.5 μM had no
significant cytotoxic effect on 435R cells. Thus, we chose the
2-MeOE2 with 0.5 μM (incubated with cells 6 h before
irradiation) for our experiments. The expression of HIF-1α at the
mRNA and protein levels in the radioresistant cells were decreased
after using the 2-MeOE2 (Fig. 2B and
C). The results of colony formation assay show that survival
fractions of 435R cells with 2-MeOE2 decreased significantly at
different irradiation doses compared with the cells without 2-MeOE2
(Fig. 2E and F).
Decrease of PDK1 expression of 435R cells was
detected after inhibiting HIF-1α with 2-MeOE2. Extracellular
lactate content and the expression of glycolysis-related proteins
GLUT1 and LDHA of the 435R cells were decreased (Fig. 2C, D and G), and the cellular ATP
level of 435R cells was increased after the 2-MeOE2 treatment
(Fig. 2H). The results indicated
that inhibition of HIF-1α decreased the glycolytic states of 435R
cells.
2-MeOE2 increases DNA damage and
apoptosis of 435R cells after irradiation
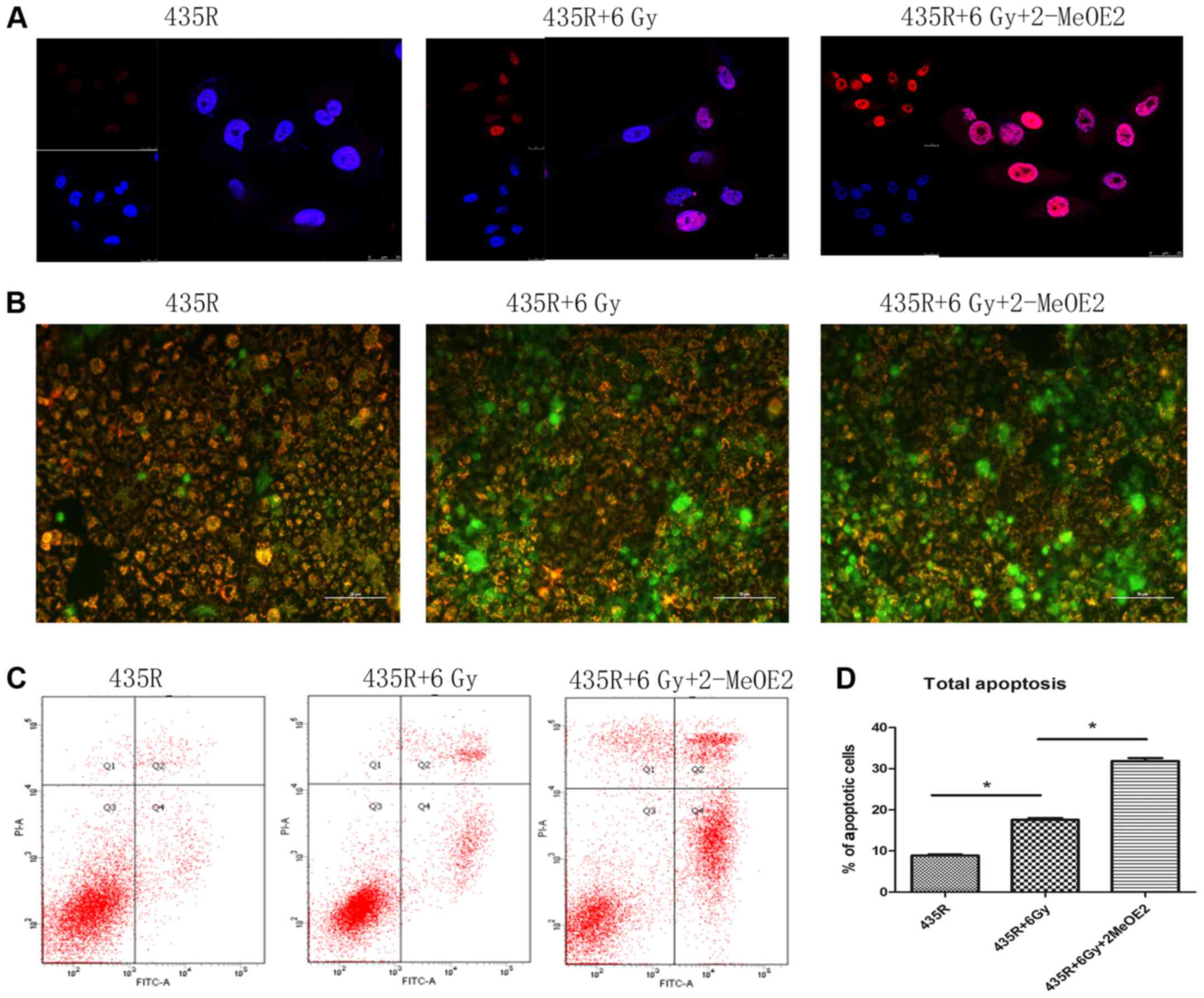
H2AX plays an essential role in the cellular
response to DSBs of DNA induced by irradiation, the number of
γ-H2AX foci is proportional to the amount of DSBs. As shown in
Fig. 3A, The number of γ-H2AX foci
in the 435R cells treated with 2-MeOE2 significantly increased
compared with 435R without this after the same dose of
irradiation.
It is well known that irradiation could cause DNA
damage. If DNA damage can not been adequately repaired after
irradiation, cells may progress towards apoptosis and/or necrosis.
The decrease of mitochondrial membrane potential is an important
factor in the early stage of apoptosis (27). The decrease of red fluorescence
combined with the increase of green fluorescence suggested the loss
of the mitochondrial membrane potential as shown in Fig. 3B. The mitochondrial membrane
potential of 435R cells treated with 2-MeOE2 decreased to a larger
extent than the parental cells after irradiation (Fig. 3B). The results of flow cytometry
showed that inhibition of HIF-1α increased the apoptotic
percentages of radioresistant cells after irradiation (Fig. 3C and D). The results of western
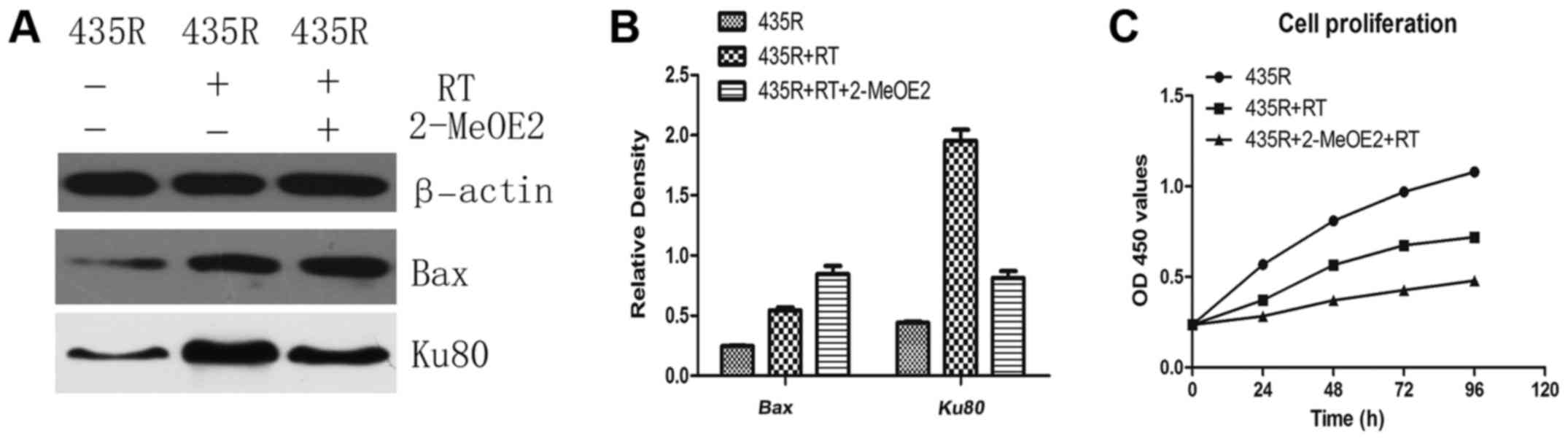
blot analysis showed the expression of Bax was increased after
irradiation treated with 2-MeOE2 (Fig.
4A and B).
2-MeOE2 decreases DNA damage repair and
cell proliferation of 435R cells after irradiation
The results of western blot analysis showed
expression of DNA damage repair-related protein Ku80 in the 435R
cells with 2-MeOE2 was decreased compared the 435R cells without
treatment after irradiation (Fig. 4A
and B). Cell proliferation was detected using CCK-8 assays. The
result showed that inhibition of HIF-1α with 2-MeOE2 can enhance
suppression of cell proliferation of 435R cells induced by
irradiation (Fig. 4C).
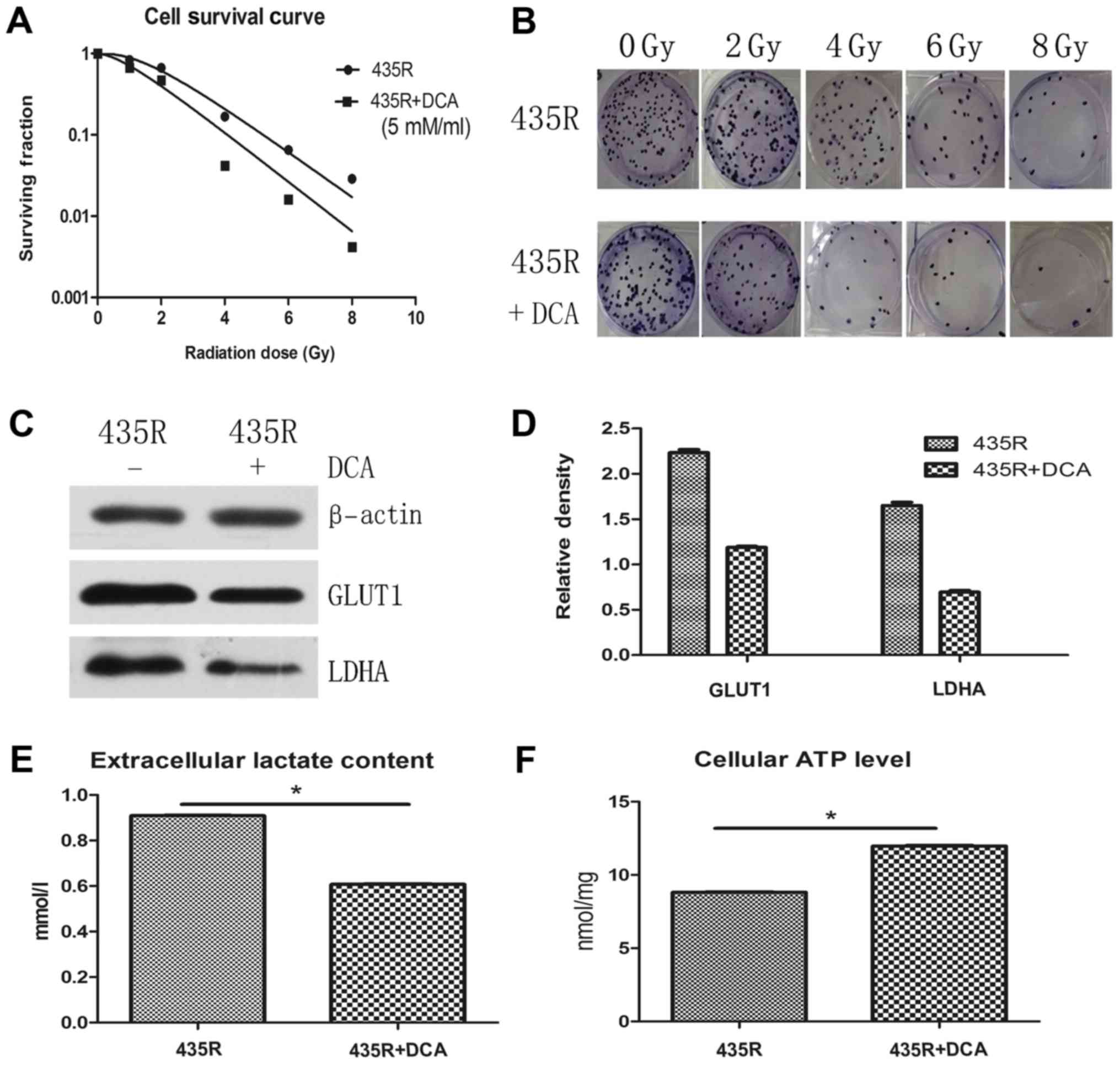
DCA decreases radioresistance and
glycolytic state of 435R cells
In order to verify inhibition of HIF-1α decrease the
radioresistance of 435R cells via HIF-1α/PDK1-mediated glycolysis,
radioresistance of 435R cells were detected after inhibiting
glycolytic state with DCA. The result of colony formation assay
show that survival fractions of 435R cells with DCA decreased
significantly at different irradiation doses compared with those
without DCA (Fig. 5A and B). The
expression of glycolysis related proteins GLUT1, LDHA and the
extracellular lactate content of the 435R cells were decreased
(Fig. 5C–E), cellular ATP level of
the 435R cells was increased after the DCA treatment (Fig. 5F).
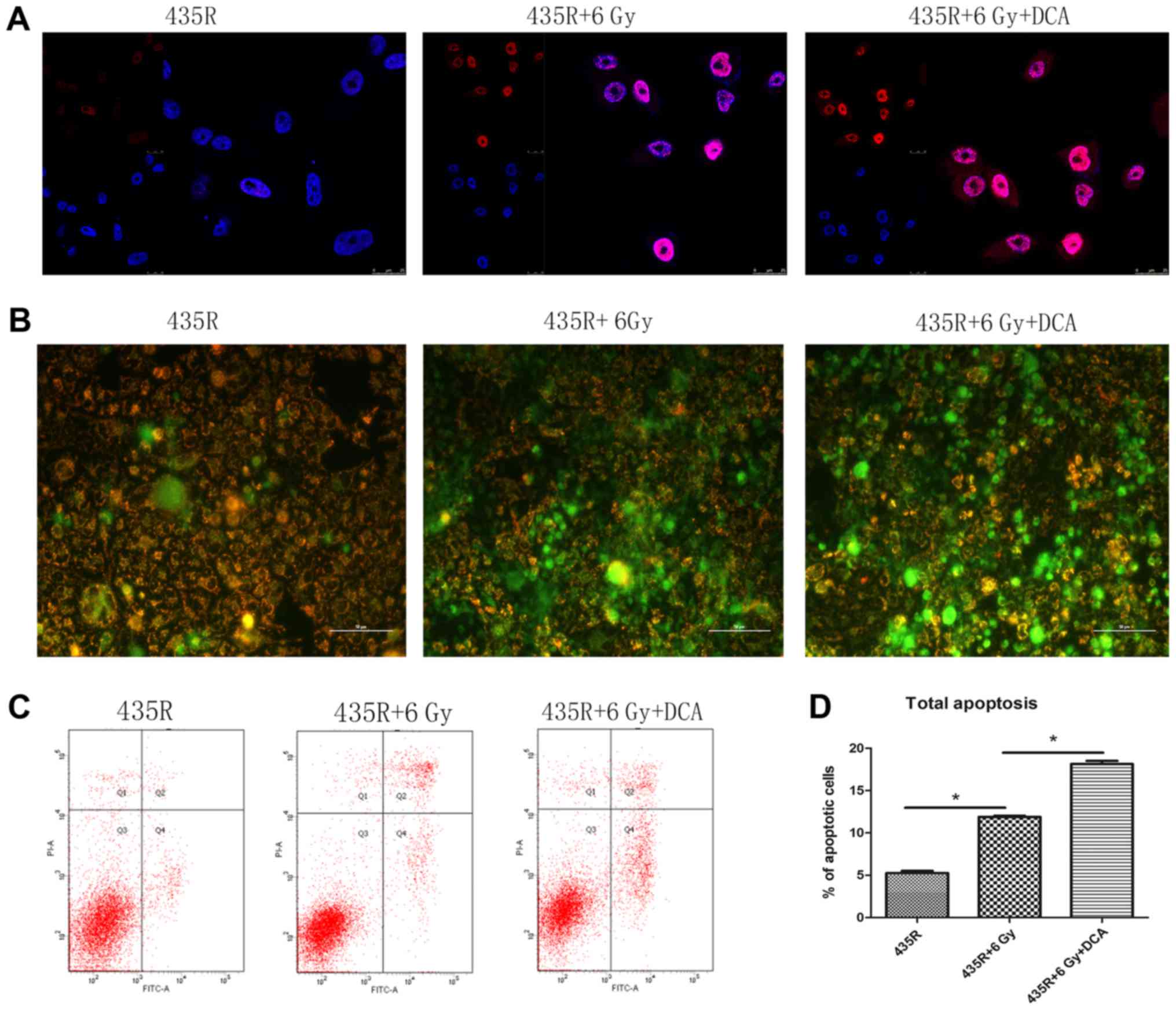
DCA increases DNA damage and apoptosis of
435R cells after irradiation
The number of γ-H2AX foci in the 435R cells treated
with DCA significantly increased compared with 435R without it
after the same dose of irradiation (Fig. 6A). The mitochondrial membrane
potential of 435R cells treated with DCA decreased to a larger
extent than the 435S cells after irradiation (Fig. 6B). The results of flow cytometry
showed that DCA could increase the apoptotic percentage of
radioresistant cells after irradiation (Fig. 6C and D). Furthermore, the
expression of Bax was increased after irradiation when treated with
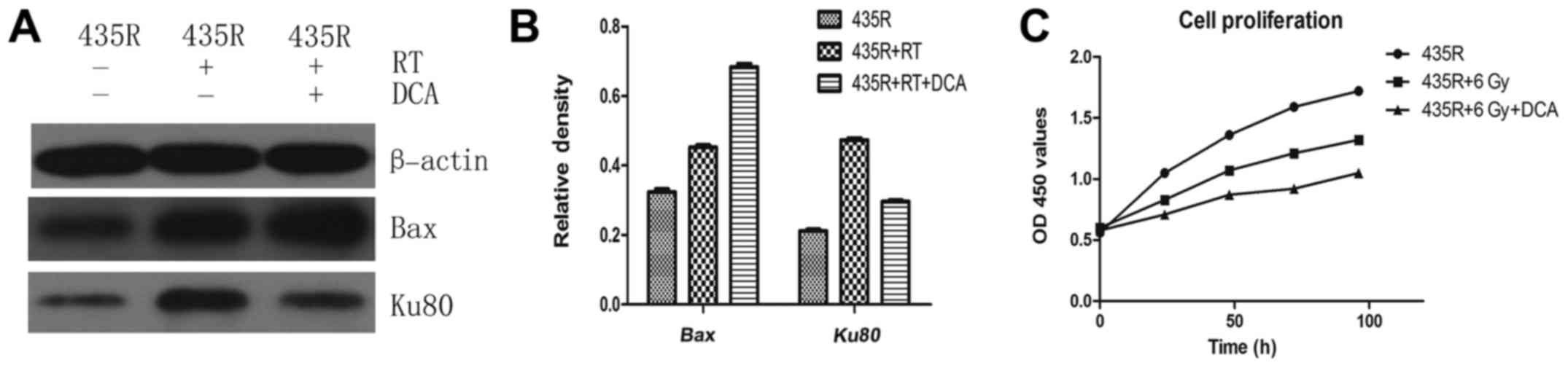
DCA (Fig. 7A and B).
DCA decreases DNA damage repair and cell
proliferation of 435R cells after irradiation
The expression of Ku80 in the 435R with DCA was
decreased compared to those without DCA after irradiation (Fig. 7A and B). The result of cell
proliferation assays showed that inhibition of PDK1 with DCA can
enhance suppression of cell proliferation of 435R cells induced by
irradiation (Fig. 7C).
Discussion
The existence of radioresistance limits the efficacy
of radiotherapy for melanoma. However, recent studies suggest that
under certain clinical circumstances, radiotherapy may play a
significant role in the treatment of melanoma (28–31).
Improving the radiosensitivity of melanoma cells may allow the
expanded use of radiotherapy for melanoma. The results of our study
indicated that the level of HIF-1α and glycolysis in the
radioresistant cells were higher than its parental cells.
Inhibition of HIF-1α sensitizes radioresistant melanoma cells 435R
to X-ray irradiation by targeting the energy metabolism regulated
by glycolysis.
HIF-1α is an important regulator in the cellular and
systemic homeostatic responses to hypoxia by activation of gene
transcription (32). HIF-1α
overexpression is associated with radioresistance of several
tumors. Therefore, selective HIF-1α inhibitors are promising
targeted compounds for adjuvant radiosensitizing therapy. In our
study, we inhibited the expression of HIF-1α in the radioresistant
melanoma cells 435R with the special chemical molecule 2-MeOE2.
2-MeOE2 as a special inhibitor of HIF-1α was shown to inhibit the
expression of HIF-1α in a dose-dependent manner in cancer cells and
blocking HIF-1α nuclear accumulation by depolymerizing microtubules
(4). When detected the expression
of proteins in our study, use of β-tubulin was avoided as the
internal reference to prevent the influence of change of level of
microtubules. We also avoided use of GAPDH as the internal
reference to prevent influence of the change of level of GAPDH
caused by glycolytic activity. 2MeOE-2 is a naturally occurring
derivative of estradiol, which has been shown to be orally active,
well-tolerated small molecule that possesses antitumor and
anti-angiogenic activity (33).
There are several completed Phase I/II clinical trials for the
effectiveness of 2MeOE-2 in various cancers (34–36).
Activation of glycolytic genes by HIF-1α is
considered to be a very important factor for metabolic adaptation
to hypoxia, with increased conversion of glucose to pyruvate and
subsequently to lactate. HIF-1 also suppressed glucose metabolism
through the tricarboxylic acid cycle by directly transactivating
PDK1. PDK1 is an important glucose metabolism enzyme for the
Warburg effect, promotes the conversion of pyruvate to lactic acid
and ATP in the presence of oxygen (aerobic glycolysis), generating
the necessary amount of energy needed for rapid cellular
proliferation (10). Lactate
dehydrogenase A (LDHA) is an enzyme which plays a critical role in
the glucose metabolism. Expression and post-transcriptional
modification of LDHA are regulated by several known oncogenes and
deacetylases, such as MYC and HIF-1α (37–39).
GlUT1 is a facilitative glucose transporter which belongs to the
solute-linked carrier gene family SLC2, and the elevated expression
of GLUT1 has been documented in most cancers (40). We have detected the indicators of
glycolysis in the radioresistant cell model and the parental cells,
and the results show that the expression of PDK1, LDHA and GLUT1 in
the radioresistant cells were higher than its parental cells. In
addition, extracellular lactate production in the radioresistant
cells were also higher than the parental cells. These results show
evidence that highly level of HIF-1α and glycolysis has an
important relationship with the radioresistance of melanoma
cells.
The elevated glycolytic state of cancer cells not
only provides a growth advantage but also correlates strongly with
radioresistance (15,16). Therefore, inhibition of glycolytic
metabolism during or before radiotherapy may be potentially
exploited for identifying new methods to overcome radioresistance.
In our study, inhibition of HIF-1α caused a decrease of PDK1
expression and radioresistance in the radioresistant cells. In
order to verify whether inhibition of HIF-1α decreased the
radioresistance of 435R cells via glycolysis, radioresistance of
435R cells were detected after inhibiting glycolytic state with
DCA. These results demonstrated that inhibition of HIF-1α
sensitizes 435R cells to X-ray irradiation by regulating
PDK1-mediated glycolysis. Some special glycolysis-related enzymes
which can regulate the activity of glycolysis can be the target of
further investigation into combination with radiotherapy.
Radiation can induce single-strand or double-strand
DNA breaks (SSBs and DSBs) by inducing the oxidation of DNA bases
causing lesions in the DNA (41).
As strong activators of apoptosis, DSBs have the most harmful
effect on cell survival (42);
cell death was induced by the persistence of DSBs if not repaired
(43). Zou et al found that
2-MeOE2 could enhance the incidence of radiation-induced genomic
damage (44). Ku80/XRCC5 is one of
the XRCC (X-ray Repair cross-complementing) families which play a
role in protecting mammalian cells from DNA damage caused by
ionizing radiation and antitumor chemotherapy agents (45). Melanoma has been considered to be a
highly radioresistant tumor due to its efficient DNA repair
mechanism (46). Our results
showed that the number of the foci of γ-H2AX in the radioresistant
cells with inhibition of HIF-1α after irradiation was higher than
the cells without this. The expression of Ku80 in the
radioresistant cells with 2-MeOE2 was lower than the cells without
after the same dose of irradiation. It demonstrated that inhibition
of HIF-1α-mediated glycolysis could increase the DSBs and suppress
the DNA damage repair of radioresistant melanoma cells after
irradiation.
Inhibition of HIF-1α can increase the expression of
apoptosis promoting protein Bax and the apoptosis rate of 435R
cells significantly after radiation than the cells after radiation
without the inhibitor. The final results showed that inhibition of
HIF-1α could inhibit the cell proliferation of 435R after
irradiation. Mueck et al observed that under a normoxic
condition, 2-MeOE2 stimulated apoptosis, an effect probably due to
Bcl-2 and Bcl-xL phosphorylation and the subsequent inhibition of
the anti-apoptotic effects (47).
The experiment of Long et al demonstrated that 2-MeOE2
effectively inhibited the protein expression of HIF-1α, which
significantly increased the late stage of radiation-induced
apoptosis of keloid fibroblasts (48). The results of Aquino-Gálvez et
al showed a dose-dependent inhibition of cell growth for
2-ME-treated normoxic cells (49).
In summary, our study detected high expression level
of HIF-1α and more glycolysis activity in the radioresistant
melanoma 435R cell model than its parental cells. HIF-1α, as a key
regulator of PDK1, its inhibition can cause decrease of
radioresistance and PDK1 of 435R cells. The possible mechanism of
this effect might that inhibition of HIF-1α supresses the
glycolysis by PDK1, then the balance of energy metabolism through
glycolysis is disrupted. Since Warburg effect is the major source
of energy in the highly glycolytic cancers (13), imbalanced metabolism may be the
critical factor that influenced biological processes, including
repair of DNA breaks, cell proliferation and other processes which
can change the radiosensitivity of cells. These observations have
raised the possibility that targeting metabolic pathways that the
cancer cell depends on may be a useful therapeutic strategy. Some
special glycolysis-related enzymes could be new targets for further
investigation in combination with radiotherapy, and more cell lines
are needed in the future experiments.
References
|
1
|
Slominski A, Kim TK, Brożyna AA,
Janjetovic Z, Brooks DL, Schwab LP, Skobowiat C, Jóźwicki W and
Seagroves TN: The role of melanogenesis in regulation of melanoma
behavior: Melanogenesis leads to stimulation of HIF-1α expression
and HIF-dependent attendant pathways. Arch Biochem Biophys.
563:79–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aebersold DM, Burri P, Beer KT, Laissue J,
Djonov V, Greiner RH and Semenza GL: Expression of
hypoxia-inducible factor-1alpha: A novel predictive and prognostic
parameter in the radiotherapy of oropharyngeal cancer. Cancer Res.
61:2911–2916. 2001.PubMed/NCBI
|
|
3
|
Koukourakis MI, Giatromanolaki A, Sivridis
E, Simopoulos C, Turley H, Talks K, Gatter KC and Harris AL:
Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and
chemoradiotherapy outcome of squamous cell head-and-neck cancer.
Int J Radiat Oncol Biol Phys. 53:1192–1202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mabjeesh NJ, Escuin D, LaVallee TM,
Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW
and Giannakakou P: 2ME2 inhibits tumor growth and angiogenesis by
disrupting microtubules and dysregulating HIF. Cancer Cell.
3:363–375. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maxwell PH, Pugh CW and Ratcliffe PJ: The
pVHL-hIF-1 system. A key mediator of oxygen homeostasis. Adv Exp
Med Biol. 502:365–376. 2001. View Article : Google Scholar
|
|
6
|
Zeng L, Zhou HY, Tang NN, Zhang WF, He GJ,
Hao B, Feng YD and Zhu H: Wortmannin influences hypoxia-inducible
factor-1 alpha expression and glycolysis in esophageal carcinoma
cells. World J Gastroenterol. 22:4868–4880. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Cao KE, Wang S, Chen J, He B, He
GU, Chen Y, Peng B and Zhou J: MicroRNA-138 suppresses
proliferation, invasion and glycolysis in malignant melanoma cells
by targeting HIF-1α. Exp Ther Med. 11:2513–2518. 2016.PubMed/NCBI
|
|
8
|
Kim JW, Tchernyshyov I, Semenza GL and
Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase
kinase: A metabolic switch required for cellular adaptation to
hypoxia. Cell Metab. 3:177–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujiwara S, Kawano Y, Yuki H, Okuno Y,
Nosaka K, Mitsuya H and Hata H: PDK1 inhibition is a novel
therapeutic target in multiple myeloma. Br J Cancer. 108:170–178.
2013.PubMed/NCBI
|
|
10
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koppenol WH, Bounds PL and Dang CV: Otto
Warburg's contributions to current concepts of cancer metabolism.
Nat Rev Cancer. 11:325–337. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Warburg O: On respiratory impairment in
cancer cells. Science. 124:269–270. 1956.PubMed/NCBI
|
|
13
|
Scott DA, Richardson AD, Filipp FV,
Knutzen CA, Chiang GG, Ronai ZA, Osterman AL and Smith JW:
Comparative metabolic flux profiling of melanoma cell lines: Beyond
the Warburg effect. J Biol Chem. 286:42626–42634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bettum IJ, Gorad SS, Barkovskaya A,
Pettersen S, Moestue SA, Vasiliauskaite K, Tenstad E, Øyjord T,
Risa Ø, Nygaard V, et al: Metabolic reprogramming supports the
invasive phenotype in malignant melanoma. Cancer Lett. 366:71–83.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhatt AN, Chauhan A, Khanna S, Rai Y,
Singh S, Soni R, Kalra N and Dwarakanath BS: Transient elevation of
glycolysis confers radio-resistance by facilitating DNA repair in
cells. BMC Cancer. 15:3352015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhong J, Rajaram N, Brizel DM, Frees AE,
Ramanujam N, Batinic-Haberle I and Dewhirst MW: Radiation induces
aerobic glycolysis through reactive oxygen species. Radiother
Oncol. 106:390–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shimura T, Noma N, Sano Y, Ochiai Y,
Oikawa T, Fukumoto M and Kunugita N: AKT-mediated enhanced aerobic
glycolysis causes acquired radioresistance by human tumor cells.
Radiother Oncol. 112:302–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang C, Qin Y, Zhang H, Ma J and Yang K:
Upregulation of SLC19A2 in stem cell-like cancer cells of
nasopharyngeal carcinoma contributes to increased radioresistance
through enhanced glycolysis. Int J Radiat Oncol Biol Phys.
96:E5822016. View Article : Google Scholar
|
|
19
|
Jiang S, Wang R, Yan H, Jin L, Dou X and
Chen D: MicroRNA-21 modulates radiation resistance through
upregulation of hypoxia-inducible factor-1α-promoted glycolysis in
non-small cell lung cancer cells. Mol Med Rep. 13:4101–4107.
2016.PubMed/NCBI
|
|
20
|
Liu G, Li YI and Gao X: Overexpression of
microRNA-133b sensitizes non-small cell lung cancer cells to
irradiation through the inhibition of glycolysis. Oncol Lett.
11:2903–2908. 2016.PubMed/NCBI
|
|
21
|
Shen H, Hau E, Joshi S, Dilda PJ and
McDonald KL: Sensitization of glioblastoma cells to irradiation by
modulating the glucose metabolism. Mol Cancer Ther. 14:1794–1804.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo YM, Xia NX, Yang L, Li Z, Yang H, Yu
HJ, Liu Y, Lei H, Zhou FX, Xie CH, et al: CTC1 increases the
radioresistance of human melanoma cells by inhibiting telomere
shortening and apoptosis. Int J Mol Med. 33:1484–1490.
2014.PubMed/NCBI
|
|
23
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar
|
|
24
|
Rafehi H, Orlowski C, Georgiadis GT,
Ververis K, El-Osta A and Karagiannis TC: Clonogenic assay:
Adherent cells. J Vis Exp. 49:25732011.
|
|
25
|
Ning S, Shui C, Khan WB, Benson W, Lacey
DL and Knox SJ: Effects of keratinocyte growth factor on the
proliferation and radiation survival of human squamous cell
carcinoma cell lines in vitro and in vivo. Int J Radiat Oncol Biol
Phys. 40:177–187. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rao PN, Cessac JW, Tinley TL and Mooberry
SL: Synthesis and antimitotic activity of novel 2-methoxyestradiol
analogs. Steroids. 67:1079–1089. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu D and Kipps TJ: Reduction in
mitochondrial membrane potential is an early event in
Fas-independent CTL-mediated apoptosis. Cell Immunol. 195:43–52.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jenrette JM: Malignant melanoma: The role
of radiation therapy revisited. Semin Oncol. 23:759–762.
1996.PubMed/NCBI
|
|
29
|
Stevens G and McKay MJ: Dispelling the
myths surrounding radiotherapy for treatment of cutaneous melanoma.
Lancet Oncol. 7:575–583. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rofstad EK: Radiation biology of malignant
melanoma. Acta Radiol Oncol. 25:1–10. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Overgaard J, Overgaard M, Hansen PV and
von der Maase H: Some factors of importance in the radiation
treatment of malignant melanoma. Radiother Oncol. 5:183–192. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pribluda VS, Gubish ER Jr, Lavallee TM,
Treston A, Swartz GM and Green SJ: 2-Methoxyestradiol: An
endogenous antiangiogenic and antiproliferative drug candidate.
Cancer Metastasis Rev. 19:173–179. 2000. View Article : Google Scholar
|
|
34
|
James J, Murry DJ, Treston AM, Storniolo
AM, Sledge GW, Sidor C and Miller KD: Phase I safety,
pharmacokinetic and pharmacodynamic studies of 2-methoxyestradiol
alone or in combination with docetaxel in patients with locally
recurrent or metastatic breast cancer. Invest New Drugs. 25:41–48.
2007. View Article : Google Scholar
|
|
35
|
Bruce JY, Eickhoff J, Pili R, Logan T,
Carducci M, Arnott J, Treston A, Wilding G and Liu G: A phase II
study of 2-methoxyestradiol nanocrystal colloidal dispersion alone
and in combination with sunitinib malate in patients with
metastatic renal cell carcinoma progressing on sunitinib malate.
Invest New Drugs. 30:794–802. 2012. View Article : Google Scholar
|
|
36
|
Sweeney C, Liu G, Yiannoutsos C, Kolesar
J, Horvath D, Staab MJ, Fife K, Armstrong V, Treston A, Sidor C, et
al: A phase II multicenter, randomized, double-blind, safety trial
assessing the pharmacokinetics, pharmacodynamics, and efficacy of
oral 2-methoxyestradiol capsules in hormone-refractory prostate
cancer. Clin Cancer Res. 11:6625–6633. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xiao X, Huang X, Ye F, Chen B, Song C, Wen
J, Zhang Z, Zheng G, Tang H and Xie X: The miR-34a-LDHA axis
regulates glucose metabolism and tumor growth in breast cancer. Sci
Rep. 6:217352016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao D, Zou SW, Liu Y, Zhou X, Mo Y, Wang
P, Xu YH, Dong B, Xiong Y, Lei QY, et al: Lysine-5 acetylation
negatively regulates lactate dehydrogenase A and is decreased in
pancreatic cancer. Cancer Cell. 23:464–476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi M, Cui J, Du J, Wei D, Jia Z, Zhang J,
Zhu Z, Gao Y and Xie K: A novel KLF4/LDHA signaling pathway
regulates aerobic glycolysis in and progression of pancreatic
cancer. Clin Cancer Res. 20:4370–4380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shibuya K, Okada M, Suzuki S, Seino M,
Seino S, Takeda H and Kitanaka C: Targeting the facilitative
glucose transporter GLUT1 inhibits the self-renewal and
tumor-initiating capacity of cancer stem cells. Oncotarget.
6:651–661. 2015. View Article : Google Scholar :
|
|
41
|
Hoeijmakers JH: Genome maintenance
mechanisms for preventing cancer. Nature. 411:366–374. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lips J and Kaina B: DNA double-strand
breaks trigger apoptosis in p53-deficient fibroblasts.
Carcinogenesis. 22:579–585. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fortini P, Ferretti C and Dogliotti E: The
response to DNA damage during differentiation: Pathways and
consequences. Mutat Res. 743–744:160–168. 2013. View Article : Google Scholar
|
|
44
|
Zou H, Zhao S, Zhang J, Lv G, Zhang X, Yu
H, Wang H and Wang L: Enhanced radiation-induced cytotoxic effect
by 2-ME in glioma cells is mediated by induction of cell cycle
arrest and DNA damage via activation of ATM pathways. Brain Res.
1185:231–238. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pierce AJ, Hu P, Han M, Ellis N and Jasin
M: Ku DNA end-binding protein modulates homologous repair of
double-strand breaks in mammalian cells. Genes Dev. 15:3237–3242.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Little JB, Hahn GM, Frindel E and Tubiana
M: Repair of potentially lethal radiation damage in vitro and in
vivo. Radiology. 106:689–694. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mueck AO and Seeger H: 2-Methoxyestradiol
- biology and mechanism of action. Steroids. 75:625–631. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Long F, Si L, Long X, Yang B, Wang X and
Zhang F: 2ME2 increase radiation-induced apoptosis of keloid
fibroblasts by targeting HIF-1α in vitro. Australas J Dermatol.
57:e32–e38. 2016. View Article : Google Scholar
|
|
49
|
Aquino-Gálvez A, González-Ávila G,
Delgado-Tello J, Castillejos-López M, Mendoza-Milla C, Zúñiga J,
Checa M, Maldonado-Martínez HA, Trinidad-López A, Cisneros J, et
al: Effects of 2-methoxyestradiol on apoptosis and HIF-1α and
HIF-2α expression in lung cancer cells under normoxia and hypoxia.
Oncol Rep. 35:577–583. 2016.
|