Introduction
Prostate cancer is the second most frequently
diagnosed cancer in men world-wide (1,2). In
China, the incidence rates of prostate cancer have been increasing
dramatically in the last 10 years, partly due to the gradual
increase of the aging population and wide use of screening tests.
The current screening methods are the prostate-specific antigen
(PSA) test, digital rectal examination (DRE) and transrectal
ultrasonography (TRUS)-guided random biopsy (3,4).
Most prostate cancers (93%) are found when the disease is confined
to the prostate and nearby organs. Patients who are diagnosed with
localized prostate cancer and treated with radical prostatectomy
often respond well to therapy. However, approximately 30% of
patients will endure a relapse, and the 5-year survival rate
declines from 90% for localized prostate cancer to 28% for
metastatic prostate cancer (5).
Although the diagnosis and treatment modality for primary prostate
cancer has improved, there are still no curative treatments for
metastatic prostate cancer at the current time (6). Therefore, identifying new biomarkers
and therapeutic strategies may prove beneficial to prevent
progression and metastasis of this disease.
The sustained growth of solid tumors is dependent on
angiogenesis; receptor tyrosin kinases (RTKs), which participate in
the proliferation, invasion and survival, signal from tumor cells
and stromal cells to endothelial cells that comprise the tumor
vasculature (7). Evidence for the
direct role of vascular endothelial growth factor (VEGF), basic
fibroblast growth factor (bFGF) and platelet-derived growth factor
(PDGF) in angiogenesis has been well documented (8). SU6668 is a small molecular weight
synthetic kinase inhibitor of fetal liver kinase-1/kinase insert
domain-containing receptor (Flk-1/KDR), PDGF receptor β (PDGFRβ)
and FGF receptor 1 (FGFR1), for VEGF, PDGF and bFGF, respectively
(8,9). SU6668 binds to these three tyrosine
kinase receptors and competitively inhibits their phosphorylation,
thereby producing inhibitory effects on vascular endothelial cell
growth. The ability of SU6668 to inactivate signaling makes SU6668
well adapted to inhibit multiple tumor signal transduction pathways
critical to tumor cell growth and survival (10). Until now, the function and
molecular mechanism of SU6668 in angiogenesis has been well
established. However, studies evaluating the role of SU6668 in
tumor cells are very rare and some studies have reported
conflicting results (9,11). Laird et al (9) demonstrated that SU6668 is not a
potent inhibitor of human cancer cells grown in culture. In
contrast, Wang et al (11)
in 2013 found that SU6668 directly suppresses the proliferation of
triple-negative breast cancer cells. These conflicting findings
suggest that the role of SU6668 in human cancer cells needs to be
further studied. Moreover, the effect and potential molecular
mechanism of SU6668 in prostate cancer have not been analyzed in
detail and thus still require clarification (12–15).
Metadherin (MTDH), also known as astrocyte elevated
gene-1 (AEG-1), was first identified in primary human fetal
astrocytes exposed to HIV-1 in 2002 (16–18).
MTDH is overexpressed in many tumor tissues and is considered a
novel oncogene (19–21). Aberrant expression of MTDH is
highly correlated with cell proliferation, migration, invasion,
apoptosis and angiogenesis in a wide range of solid cancers,
including breast cancer, glioblastoma, gastric and prostate cancer
(22–26).
In the present study, we found that SU6668 inhibited
proliferation, invasion and epithelial-mesenchymal transition (EMT)
of prostate cancer cells. After SU6668 treatment, MTDH protein,
which has been reported to be significantly overexpressed in many
human tumor tissues, was downregulated in DU145 and LNCap cells.
Mechanistic investigations identified that the AKT signaling
pathway was inhibited after SU6668 treatment in prostate cancer
cells. Taken together, our findings revealed that SU6668 suppressed
prostate cancer progression by downregulating the MTDH/AKT
signaling pathway.
Materials and methods
Cell cultures
The human prostate cancer cell lines DU145, LNCap
and PC3 were maintained in RPMI-1640 (Gibco/Invitrogen, Sao Paulo,
Brazil) supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin. All cell lines used in the present study
were cultured in a humidified environment containing 5%
CO2 and held at a constant temperature of 37°C.
Real-time PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen) and reverse transcribed using the transcriptase cDNA
synthesis kit (Promega, Fitchburg, WI, USA) according to the
manufacturer's instructions. Real-time PCR analysis was performed
using SYBR Premix Ex Taq™ (cat. no. RR420A; Takara, Dalian, China)
in an Applied Biosystems 7500 Real-Time PCR system according to the
manufacturer's instructions. Primers (F, AAGCAGTGCAAAACAGTTCACG and
R, GCACCTTATCACGTTTACGCT) for MTDH mRNA expression detecting was
synthetized by Sangon Biotech, Co., Ltd. (Shanghai, China).
Cell Counting kit-8
Cells were seeded in 96-well plates and the
proliferation of the cells was assayed at 0, 24, 36 and 48 h using
Cell Counting kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan)
according to the manufacturer's instructions. Cell viability was
assessed by the measurement of absorbance at 450 nm using a
microplate reader.
Western blot analysis
Cells were treated in 6-well plates, washed three
times by phosphate-buffered saline (PBS) and lysed for 10 min on
ice in radioimmunoprecipitation assay (RIPA) buffer containing an
anti-protease mixture. Protein concentration was measured by
bicinchoninic acid assay (BCA). The protein fractions were
resuspended in loading buffer and denatured at 100°C for 10 min.
Total proteins (20 µg/lane) were separated on 10% SDS
polyacrylamide gels and transferred to polyvinylidene fluoride
(PVDF) membranes. The membranes were then blocked in 5% fat-free
milk Tris-buffered saline, 0.1% Tween-20 (TBST) buffer for 2 h at
room temperature. Primary antibodies were used according to the
manufacturer's instructions.
Cell migration and invasion assays
Wound healing assay was used to assess cell
migration. The migration was assessed by measuring the movement of
cells into a scraped area created by a 200 µl pipette tip.
Afterwards, cells were cultured in media supplemented with 0.1% FBS
to eliminate the effect of cell proliferation.
Cell invasion was examined using a reconstituted
extracellular matrix membrane. Cells suspended at 4×104
cells/0.5 ml in serum-free media were placed in the top chambers
and complete medium was added to the bottom chambers. The chambers
were then incubated for 24 h. After incubation, the medium was
removed from the top and bottom wells; the chambers were fixed with
methanol for 30 min and then stained with crystal violet for
another 30 min.
Cell cycle
Cells were harvested, washed once in PBS, and fixed
in 70% ethanol for 48 h. The nuclei were stained with 50
µg/ml propidium iodide (PI) in 1% Triton X-100/PBS
containing 100 µg/ml DNase-free RNase, and the DNA content
was analyzed by flow cytometry. The percentage of cells in each
phase of the cell cycle was determined using the ModFit LT
program.
Cell apoptosis
Cell apoptosis was evaluated using a FITC-Annexin V
apoptosis detection kit. Briefly, cells were harvested and washed
with cold PBS, then were centrifuged at 1000 rpm for 5 min, and the
pellet was resuspended in binding buffer in a 1.5-ml culture tube
and later incubated with 5 µl of a FITC-conjugated Annexin V
and 5 µl PI for 10 min at room temperature in the dark. The
samples were analyzed by flow cytometry.
Statistical analysis
Data in this study were analyzed using Predictive
Analystics Software (PASW) Statistics for Windows (version 18.0;
SPSS, Inc., Chicago, IL, USA). All experiments were conducted at
least three times. Values were presented as the mean ± standard
deviation (SD). The statistical significance of differences between
independent groups was determined by one-way analysis of variance
(ANOVA). P-values <0.05 were considered statistically
significant.
Results
SU6668 downregulates the expression of
MTDH in prostate cancer cells
It has been reported that MTDH is associated with
cancer cell proliferation and invasion (9,11).
To evaluate whether MTDH was affected during SU6668 inhibition of
cancer cell progression, we first examined the protein expression
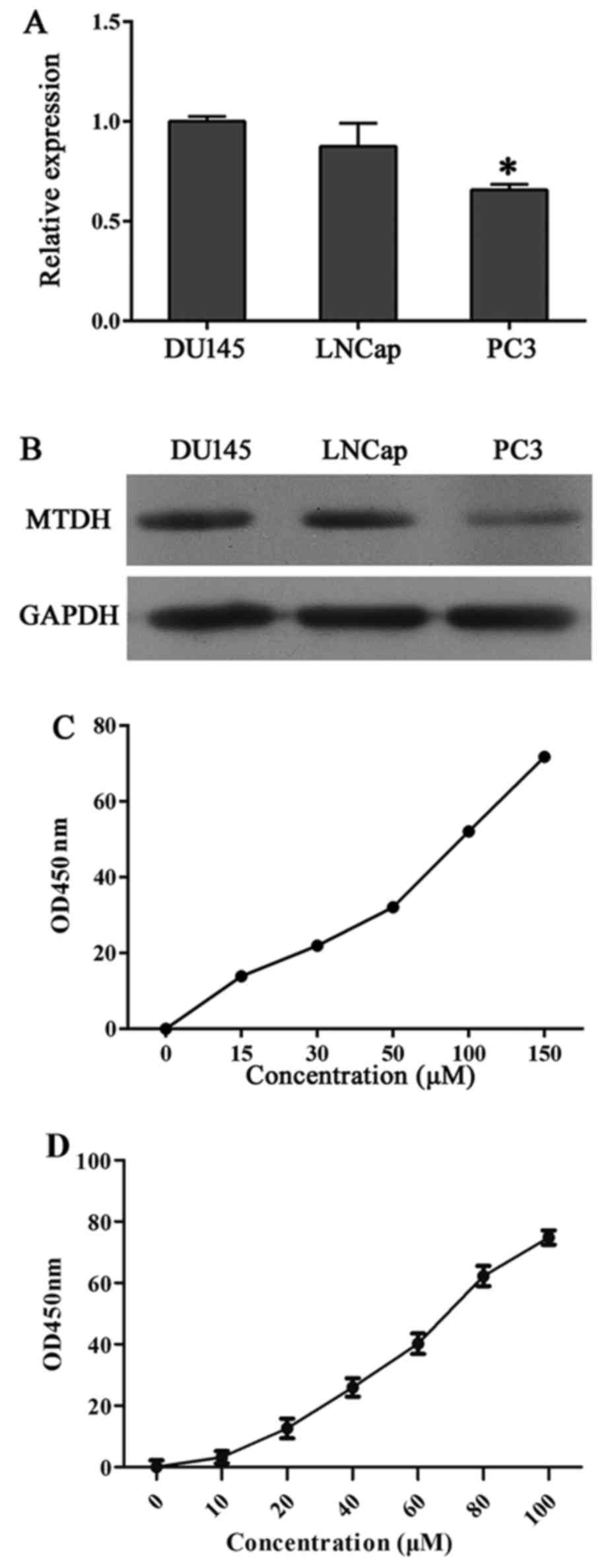
level of MTDH in DU145, LNCap and PC3 cells (Fig. 1A and B). The results showed that
DU145 and LNCap cells expressed higher levels of MTDH compared with
the other cell lines than the PC3 cells, so DU145 and LNCap cells
were chosen for our experiments. Furthermore, IC50 of
DU145 and LNCap to SU6668 was measured through CCK-8 at
concentration of 0, 15, 30, 50, 100 and 150 µM and 0, 10,
20, 40, 60, 80 and 100 µM, respectively. Cell proliferation
is presented in Fig. 1C and D.
IC50 of DU145 and LNCap to SU6668 was 98.6 and 63.976
µM, respectively, and calculated using SPSS.
SU6668 reverses epithelial-mesenchymal
transition (EMT)
EMT is a process by which epithelial cells lose
their cell polarity and adhesion, and gain migratory and invasive
properties to become mesenchymal cells (27–29).
Initiation of metastasis requires invasion, which is enabled by EMT
(29,30). We evaluated whether EMT was
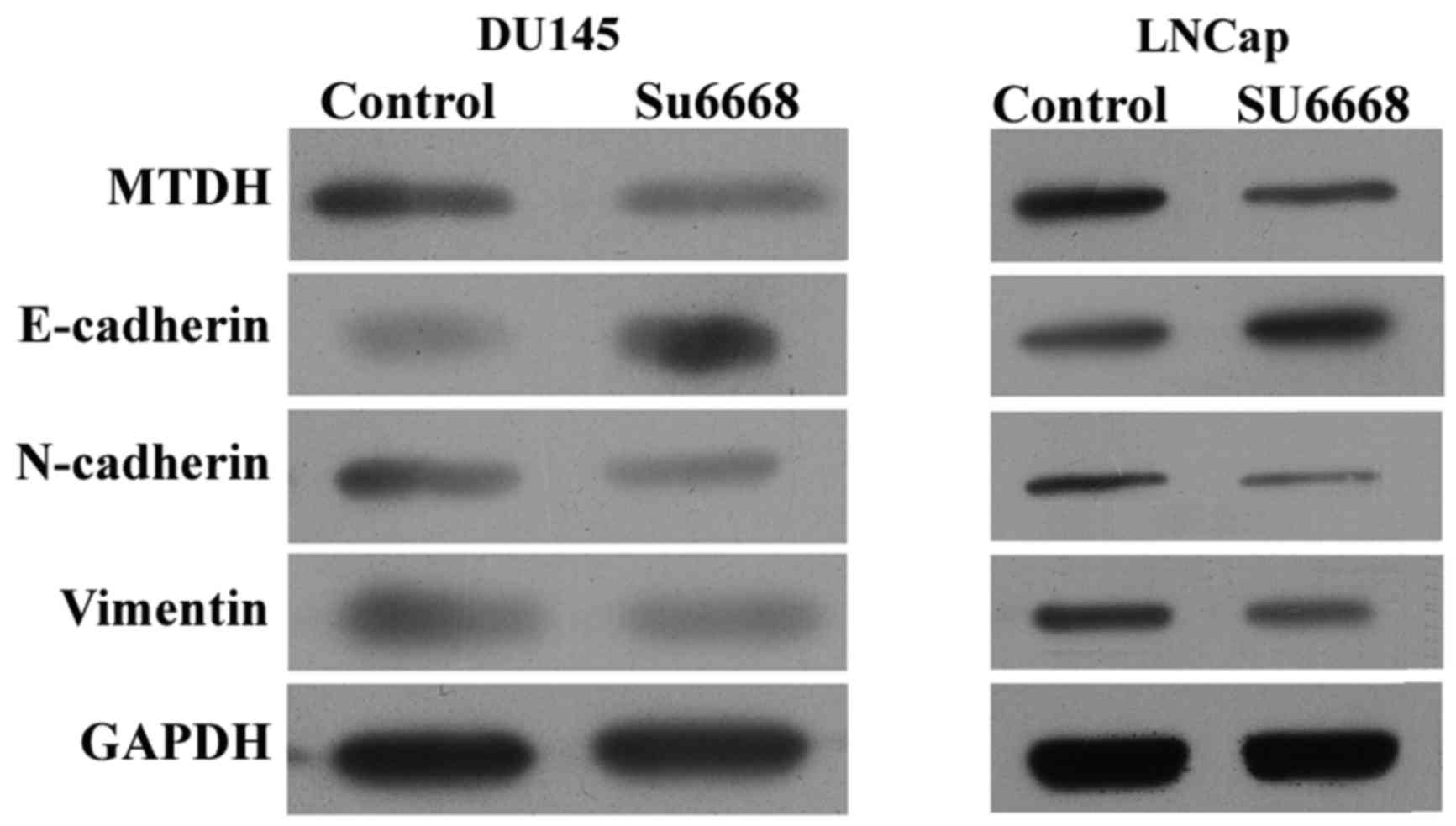
affected in DU145 and LNCap cells after SU6668 treatment. Western
blot assay showed that the protein level of E-cadherin was
increased, while that of N-cadherin and vimentin were decreased
after SU6668 treatment in DU145 and LNCap cells (Fig. 2), suggesting that SU6668 suppressed
transition of epithelial cells to mesenchymal cells, namely the
invasion of epithelial cells.
SU6668 suppresses cell proliferation,
migration and invasion in DU145 and LNCap cells
To determine the role of SU6668 in prostate cancer,
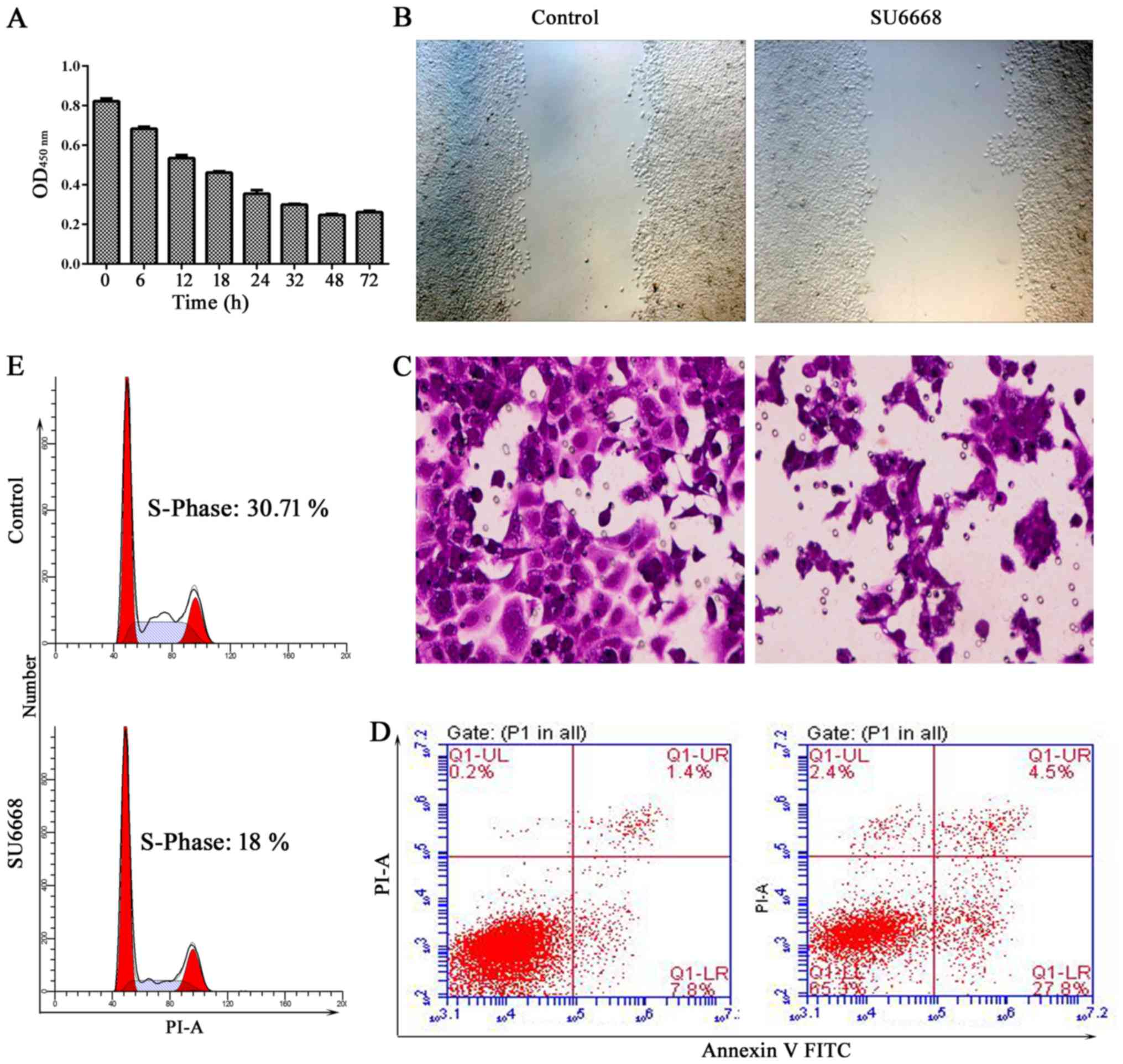
we first measured cell proliferation of DU145 cells after treatment
with (98.6 µM) SU6668 for 6, 12, 18, 24, 32, 48 and 72 h.
CCK-8 assays indicated that cell proliferation was significantly
inhibited at 48 and 72 h (Fig.
3A). The role of SU6668 in the regulation of cell migration and
invasion was assessed by the scratch healing assay and by
reconstituted extracellular matrices in porous culture chambers.
The results showed that cell migration and invasion was suppressed
in DU145 and LNCap cells after treatment with SU6668 (Figs. 3B and C and 4A and B). Cell apoptosis was determined
by staining the cells for Annexin V and PI. Figs. 3D and 4C show that cell apoptosis increased
significantly after SU6668 treatment in DU145 and LNCap cells,
compared with the control group (27.8 vs. 7.8 and 26.6 vs. 6.7%).
SU6668 increased the proportion of cells in the G1 phase and
decreased the proportion of cells in the S phases compared with the
control of DU145 and LNCap cells (30.7 vs. 18 and 29.86 vs. 19.58%;
Fig. 3E and Fig. 4D). Together, these results
demonstrate that SU6668 functions as antitumor agent in prostate
cancer.
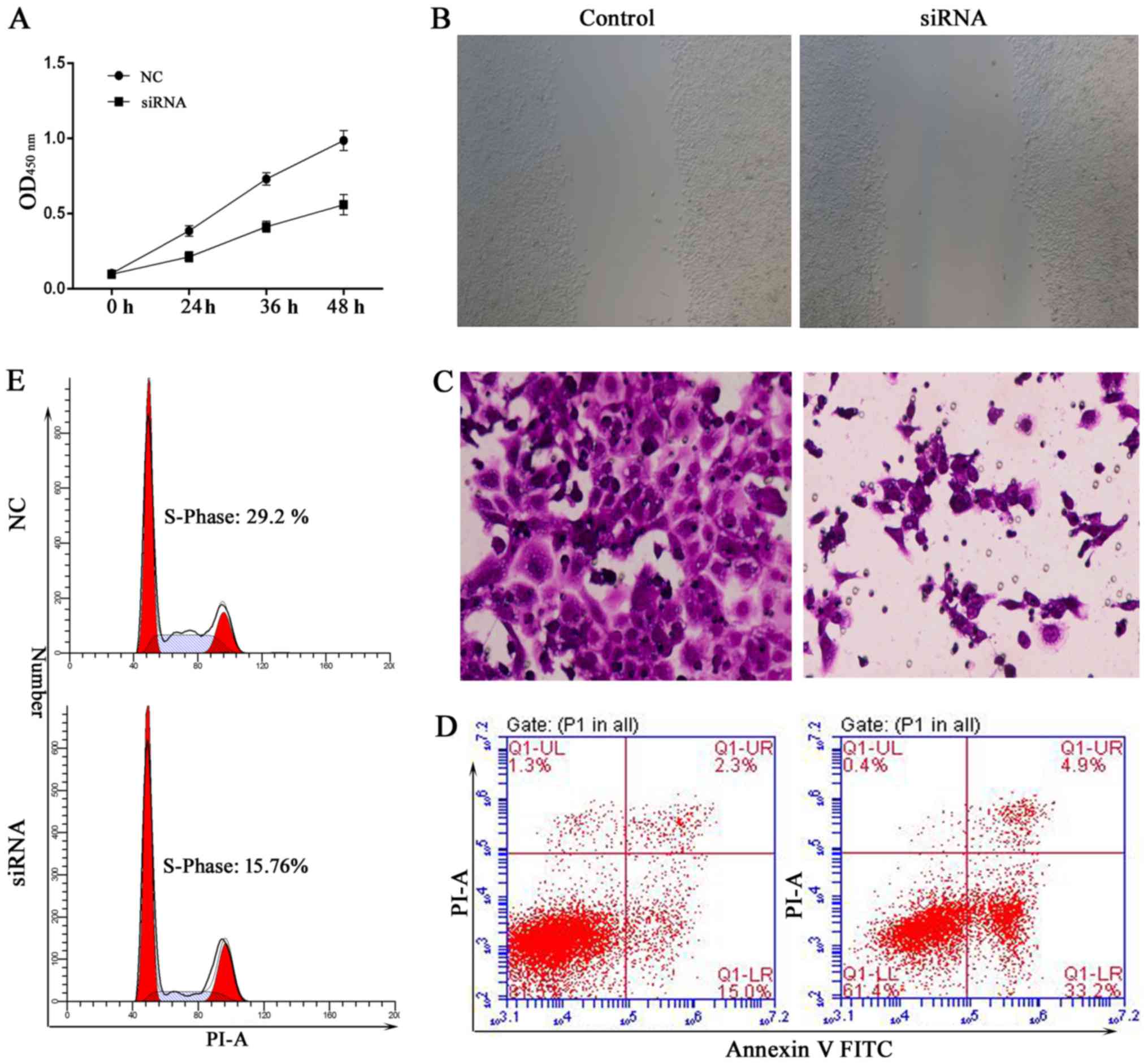
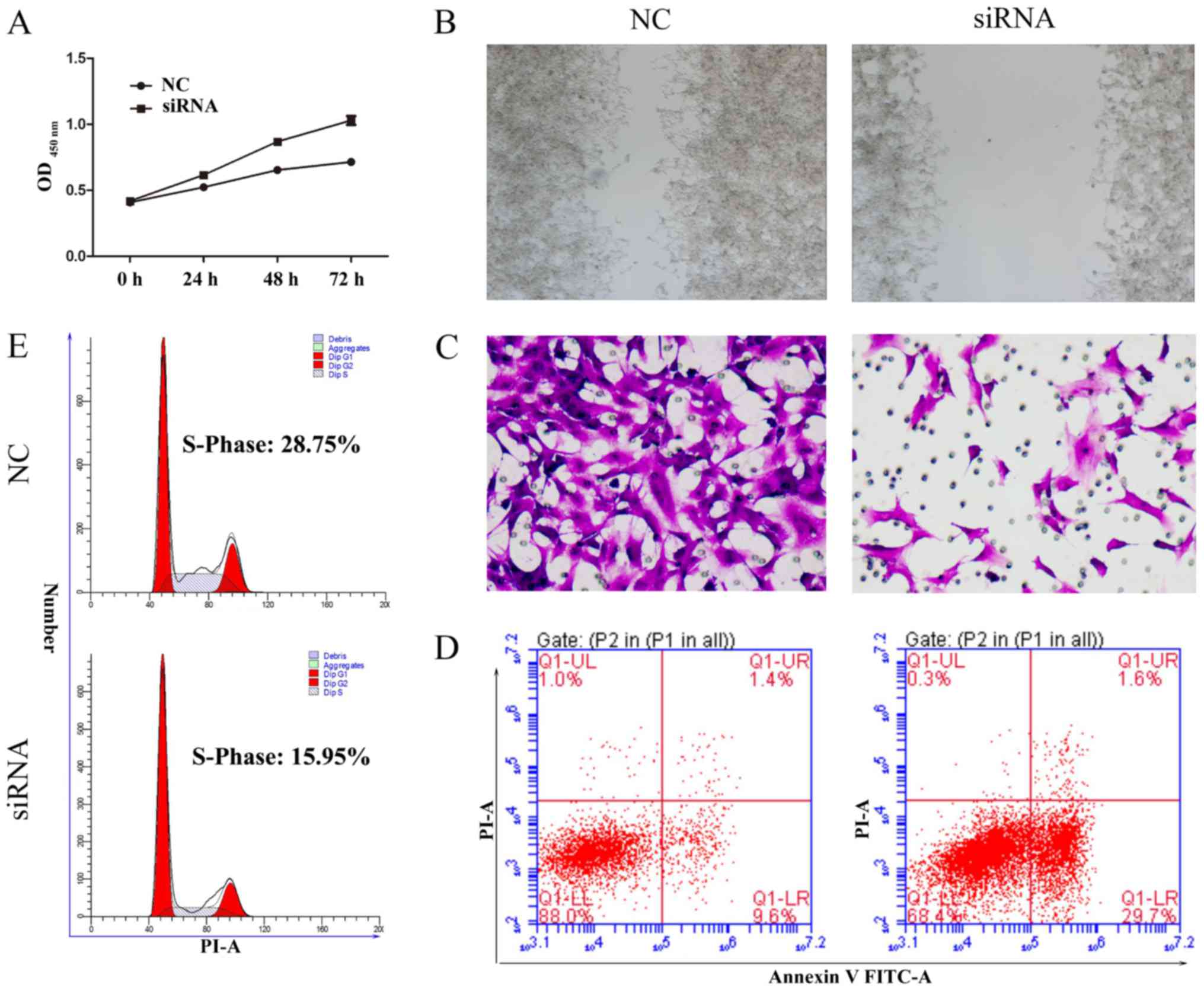
Silencing of MTDH inhibits cell
proliferation and invasion
MTDH acts as an oncogene in melanoma, malignant
glioma, breast cancer and hepatocellular carcinoma (31,32).
To further demonstrate that MTDH is a critical gene involved in the
SU6668-induced phenotypes of prostate cancer cells, we first
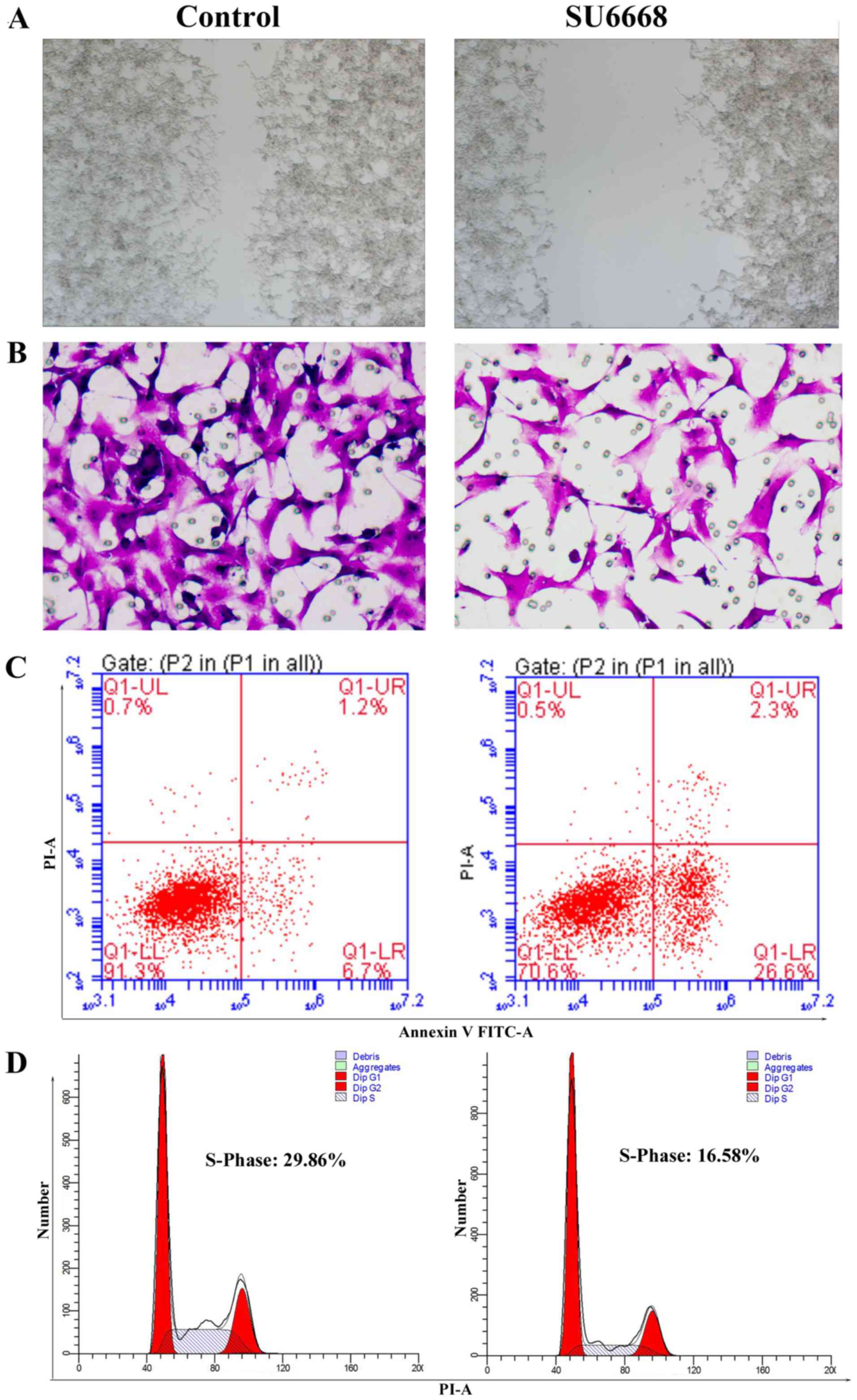
silenced the expression of MTDH by siRNAs in DU145 cells. The role
of MTDH in regulating cell proliferation was assessed by CCK-8
assay. The results showed that knockdown of MTDH expression
arrested cell proliferation compared with the control in DU145 and
LNCap cells (Figs. 5A and 6A). In the wound healing assay, migration
ability of tumor cells was significantly reduced after knockdown of
MTDH expression in DU145 and LNCap cells (Figs. 5B and 6B). Cell invasion ability was assessed by
reconstituted extracellular matrices in porous culture chambers. As
shown in Figs. 5C and 6C, DU145 and LNCap-siRNA cells showed
reduced invasive capacity compared with the control cells. However,
cell apoptosis increased significantly after silencing the
expression of MTDH in DU145 and LNCap cells (Figs. 5D and 6D). Next, we examined the cell cycle
phase distribution by flow cytometry. Only 15.76 of DU145-siRNA
cells and 15.94% of LNCap-siRNA cells were distributed to S phase,
whereas 29.2 of DU145-control cells and 28.75% LNCap-control cells
were in the S phase of the cell cycle (Figs. 5E and 6E). Taken together, these results suggest
that silencing MTDH expression has a significant effect on reducing
cell proliferation and invasion of prostate cancer cells.
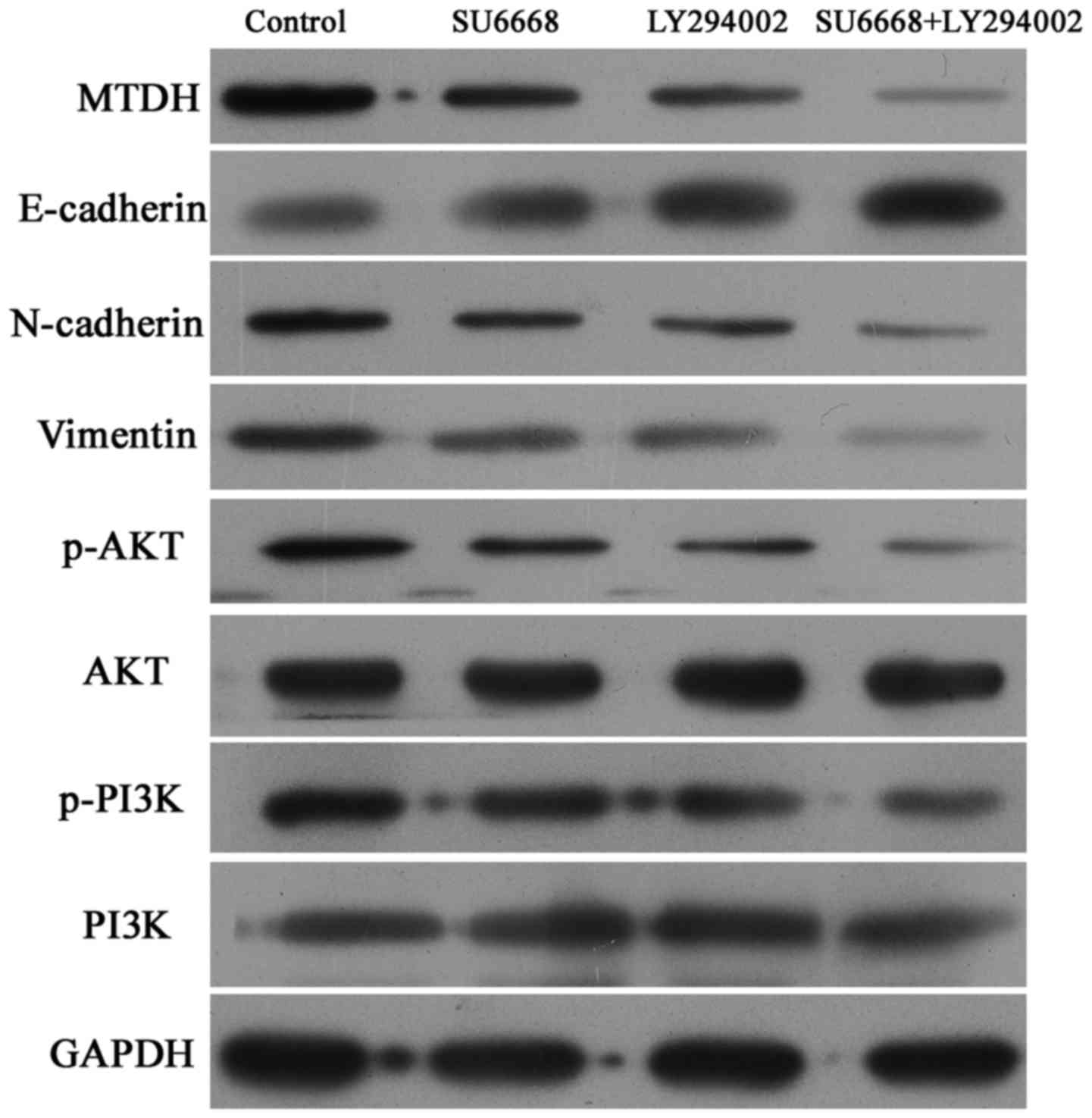
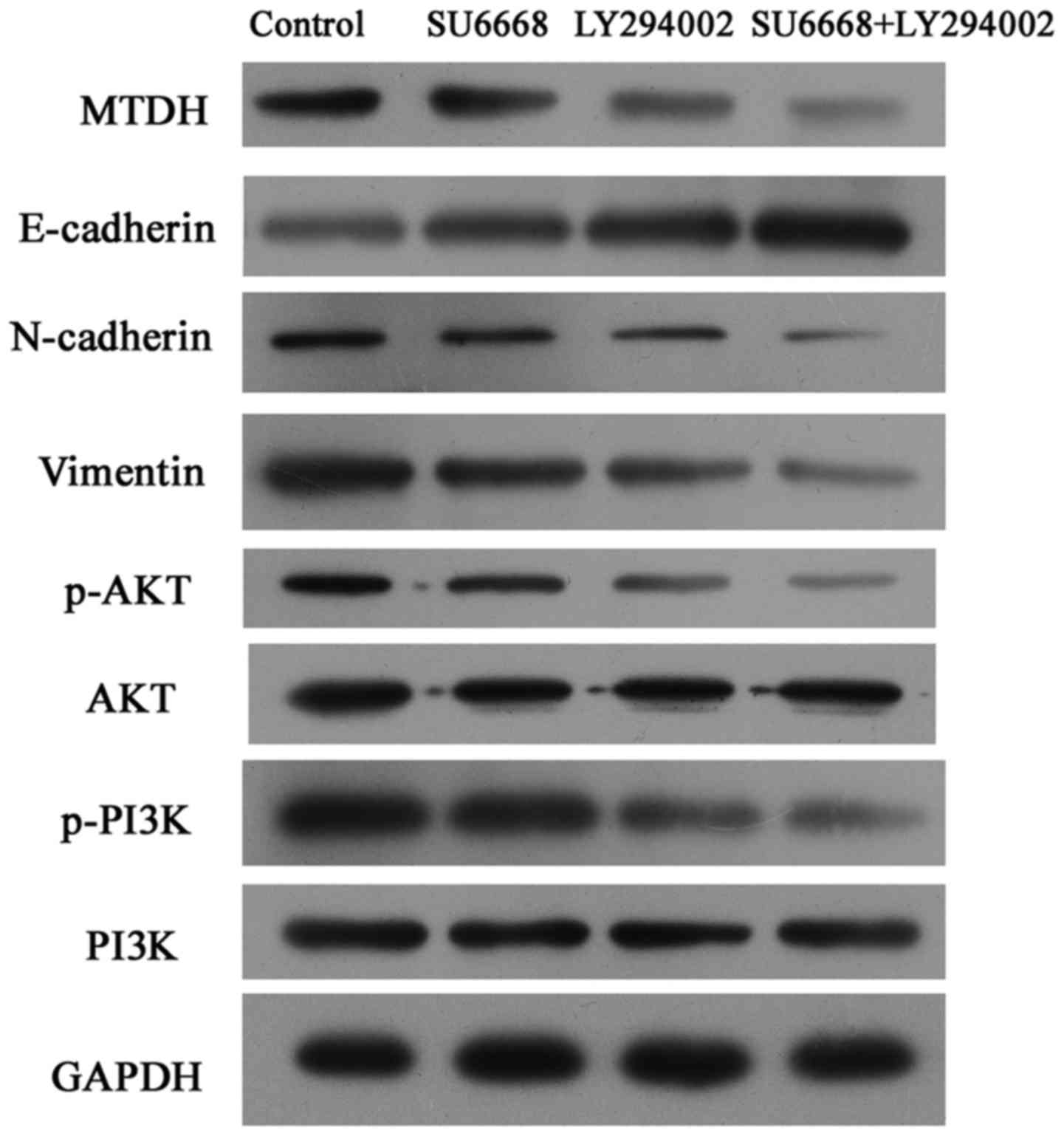
SU6668 suppresses the activity of
PI3K-AKT pathway in prostate cancer cells
Considering that the PI3K-AKT is one of the most
important downstream pathways of MTDH (33), the expression levels of p-AKT and
p-PI3K were measured by western blot analysis in DU145 and LNCap
cells. The results showed that SU6668 downregulated the expression
of MTDH and suppressed activation of the AKT signaling pathway, and
that PI3K-AKT pathway inhibitor LY29004 induced inactivation of the
AKT pathway (Figs. 7 and 8). Moreover, the combination of SU6668
and LY29004 resulted in greater inactivation of AKT pathway
compared with treatment with either of these agents alone (Figs. 7 and 8).
The AKT signaling pathway is involved in
SU6668-induced phenotypes
To determine whether the AKT signaling pathway is
involved in SU6668-induced cell apoptosis and proliferation,
LY29004, a small molecule inhibitor of the PI3K-AKT pathway, was
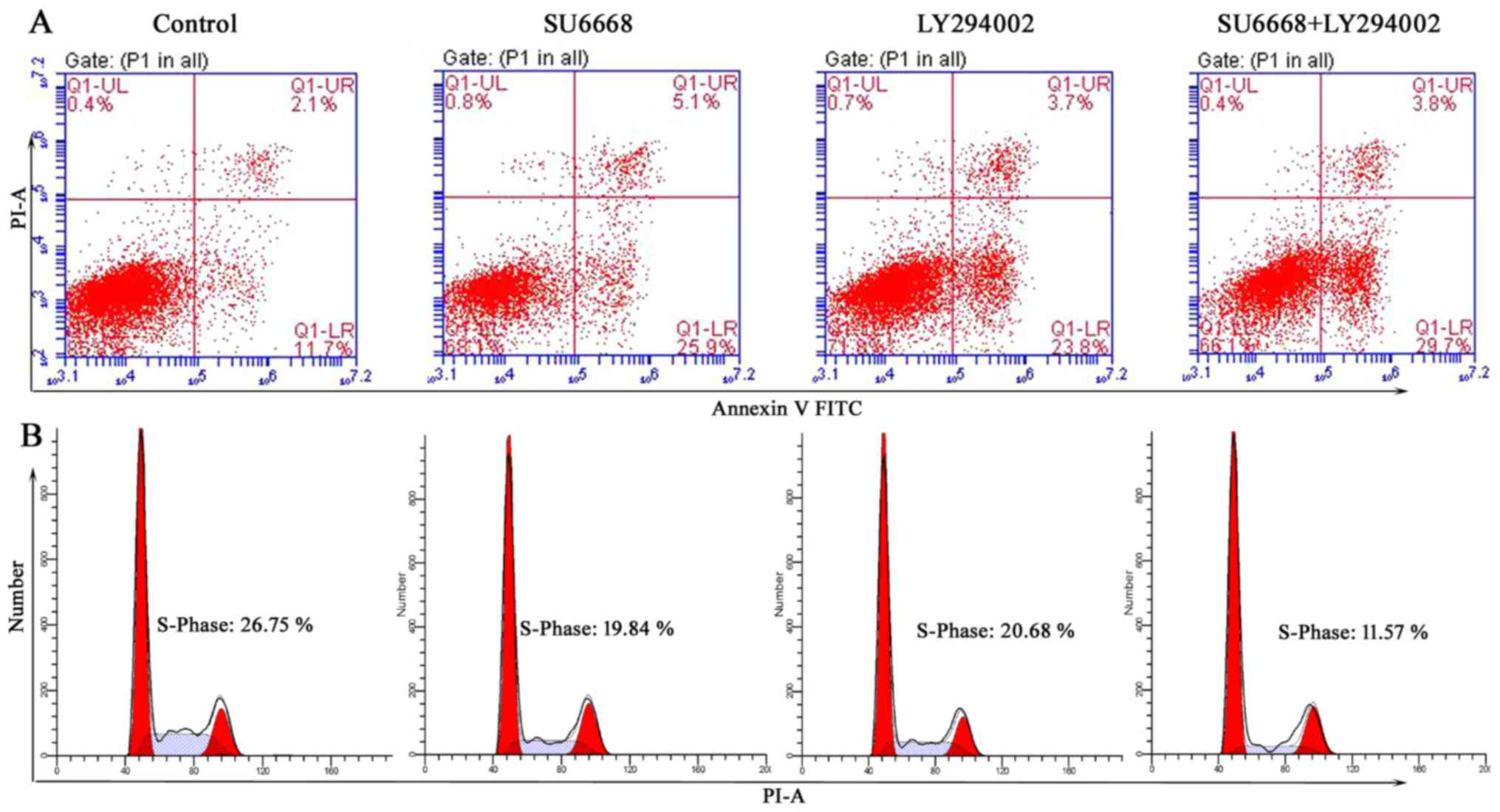
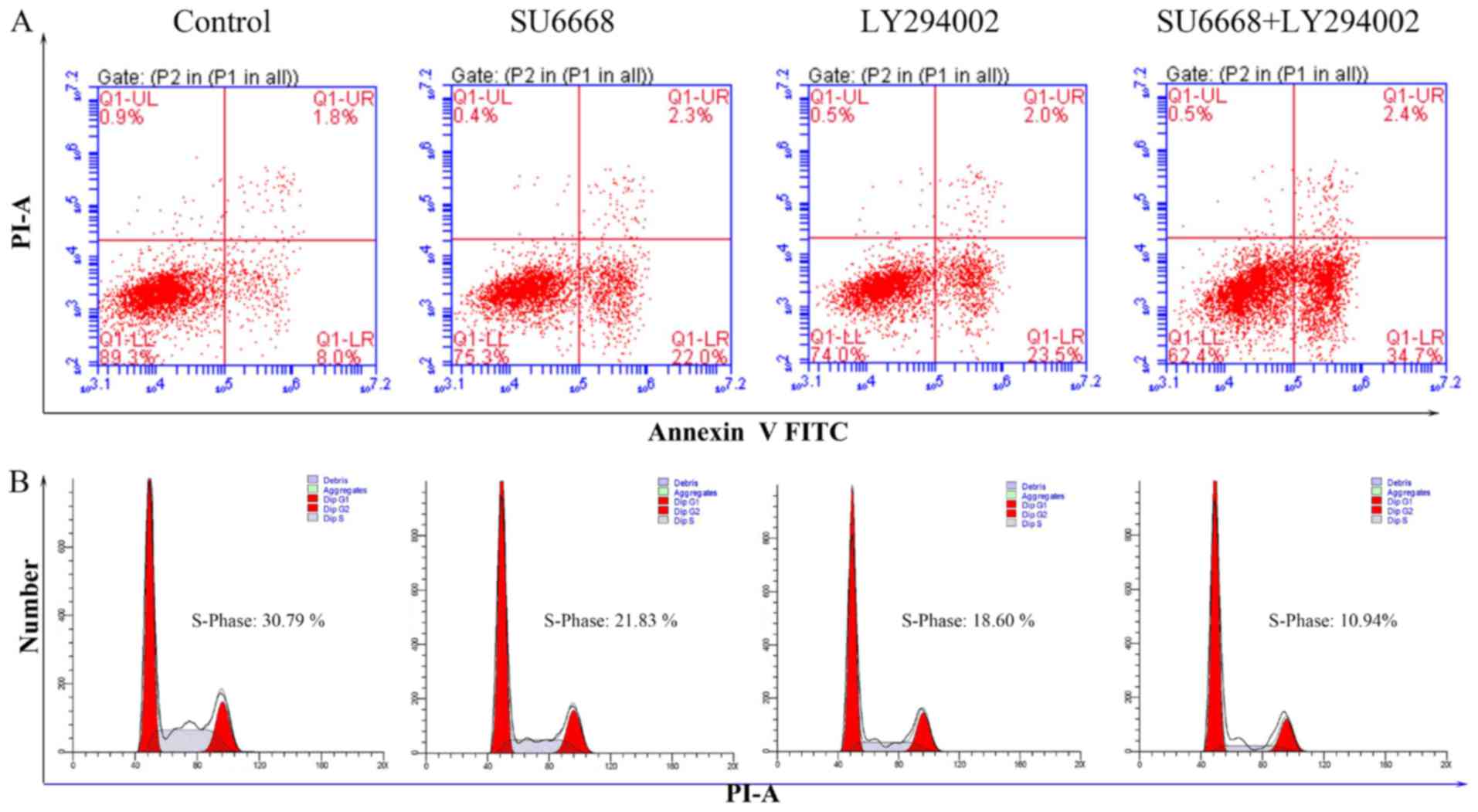
used as a positive control. As shown in Figs. 9 and 10, LY29004 induced cell apoptosis and
cell cycle arrest in DU145 and LNCap cells. Moreover, after SU6668
treatment, the percentage of apoptotic cells increased noticeably
compared with the control (25.9 vs. 11.7 and 22 vs. 8%; Figs. 9A and 10A), consistent with our above results
(Fig. 3). Next, we examined
prostate cancer cell apoptosis after treatment with a combination
of SU6668 and LY29004 in DU145 and LNCap cells and found that the
percentage of apoptotic cells reached 29.7 and 34.7%, respectively,
which is higher than when either agent was used alone (Figs. 9A and 10A). By contrast, flow cytometric
results showed that the percentage of prostate cancer cells in S
phase was markedly reduced after treatment with SU6668 and LY29004
in DU145 cells (11.57 of combination-treated cells vs. 26.75 of
control cells vs. 19.84% of SU6668 treated cells; Fig. 9B) and LNCap cells (10.94 of
combination-treated cells vs. 30.79 of control cells vs. 21.83% of
SU6668 treated cells; Fig. 10B).
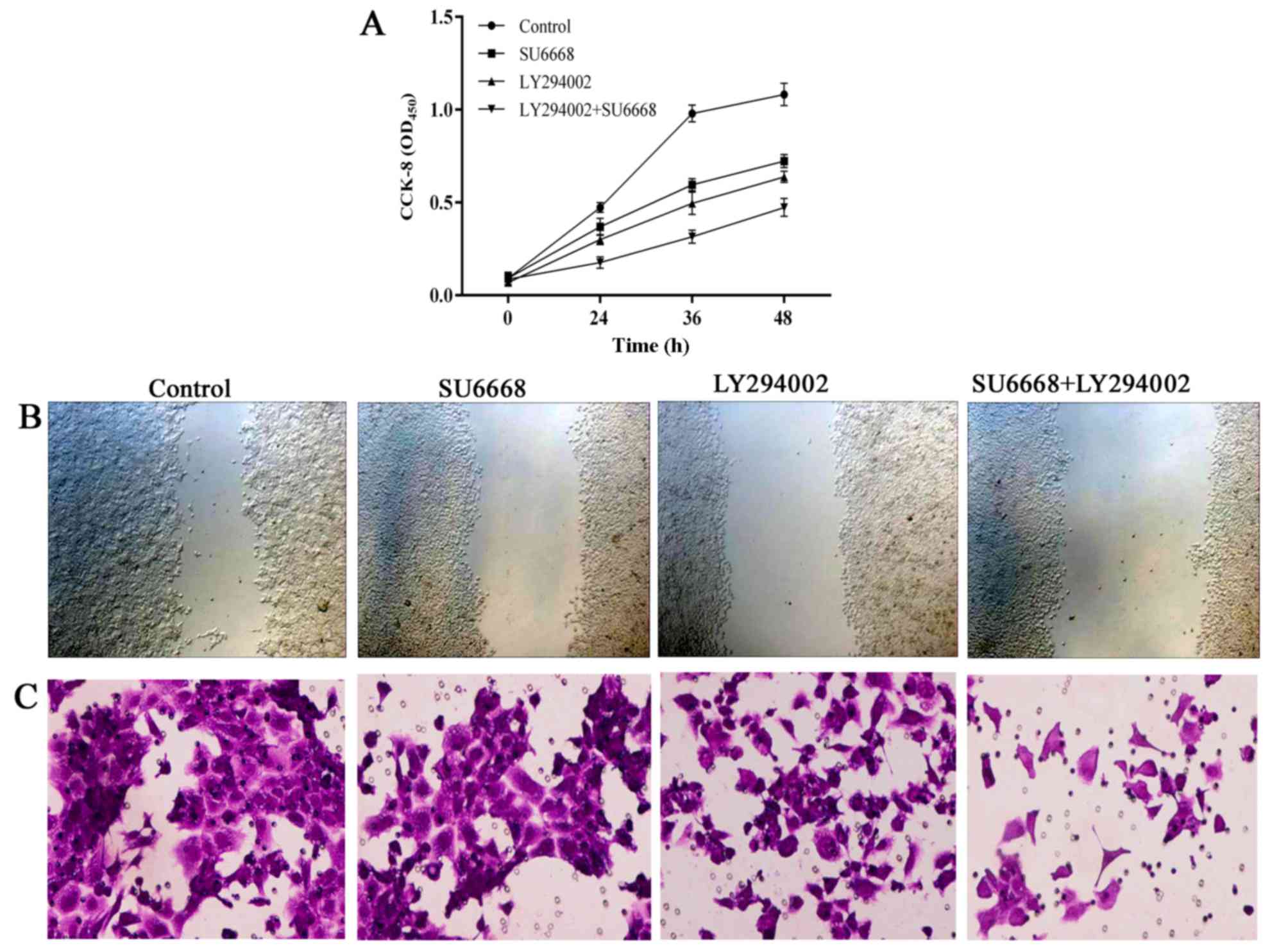
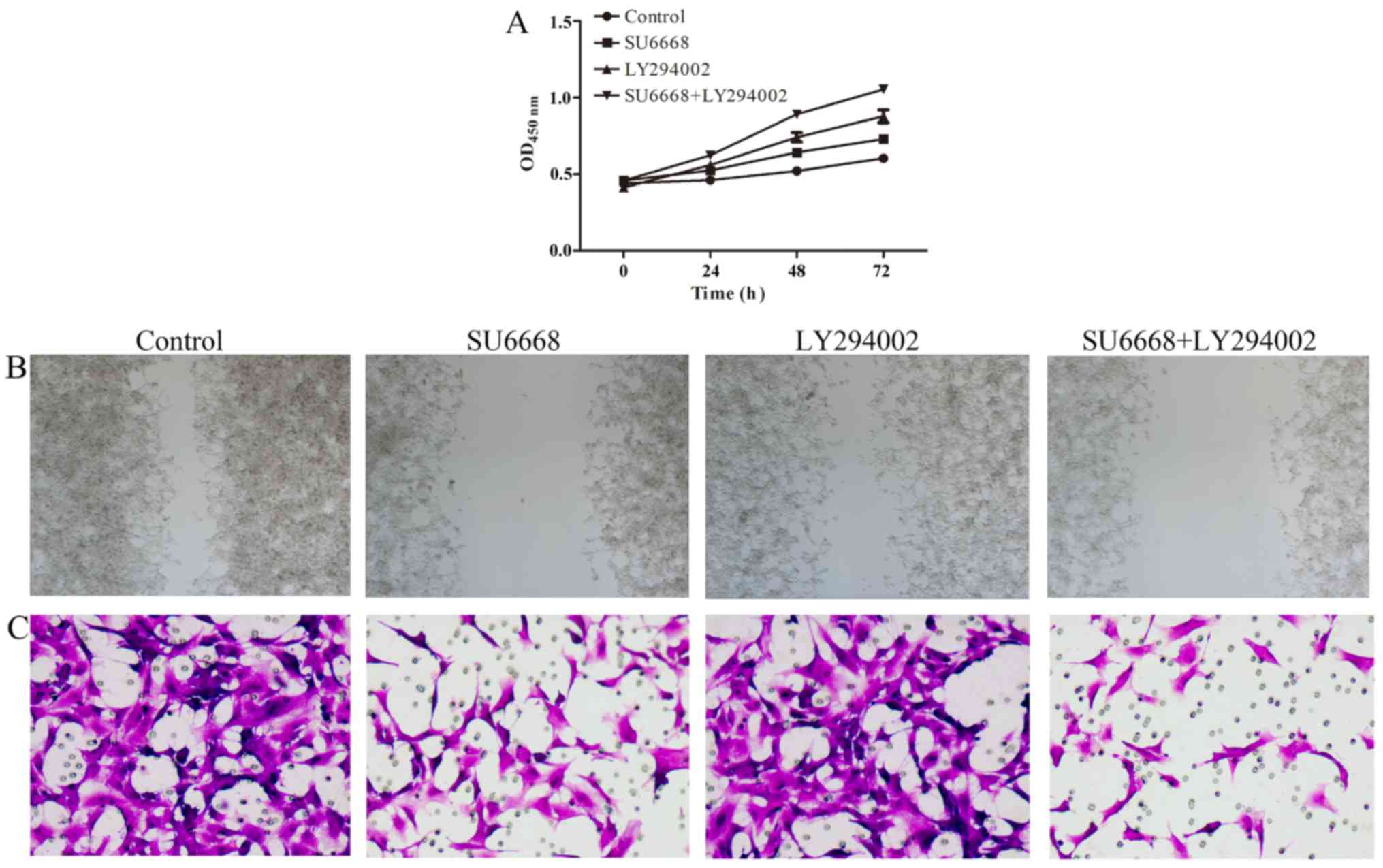
In line with this finding, the migration and invasion ability of
DU145 or LNCap cells was also affected after treatment with SU6668
and LY29004. The ability of cell migration and invasion was
assessed by scratch healing assay and invasion chambers. As shown
in Figs. 11 and 12, compared with the control group, cell
proliferation (Figs. 11A and
12A), migration (Figs. 11B and 12B) and invasion (Figs. 11C and 12C) of the prostate cancer cells was
significantly decreased after treatment with either agent alone or
both in combination in DU145 and LNCap cells, respectively.
Together, these data suggest that PI3K-AKT signaling pathway is
involved in SU6668-induced suppression of cell proliferation,
migration and invasion.
Discussion
SU6668, a novel tyrosine kinase inhibitor, can act
on VEGFR2, PDGFRβ and FGFR1, competing with ATP binding sites so as
to inhibit the activity of several tyrosine and serine/threonine
protein kinases (9,34). SU6668 can target not only
endothelial cells but also tumor cells and surrounding stromal
cells (35). Numerous studies have
demonstrated that SU6668 inhibits tumor vascularization and growth
of tumor xenografts in several types of human cancers (9,13),
including breast, lung cancer, colon cancer and glioma. Prostate
cancer is one of the most frequently diagnosed malignancies in men
all over the world. Unfortunately, there are no curative treatments
for metastatic prostate cancer at the current time. Actually, the
function and molecular mechanism of SU6668 in prostate cancer cells
have not been analyzed in detail. In the present study, we found
that SU6668 inhibited the proliferation and invasion of prostate
cancer cells and induced cell apoptosis. Furthermore, functional
studies also demonstrated that SU6668 inhibited EMT in DU145 and
LNCap cells. These findings suggested that SU6668 acted as a novel
antitumor agent that induced regression of prostate cancer.
It has been reported that the expression level of
MTDH is elevated in many types of human cancer, including breast,
prostate and lung cancer (11,31).
Moreover, overexpression of MTDH is critically involved in many
components of cancer progression, including initiation,
proliferation, migration and invasion (20,36),
which prompted us to investigate whether MTDH was involved in
SU6668-induced suppression of prostate cancer. In the present
study, our data indicated that MTDH protein was downregulated after
treatment with SU6668 in DU145 and LNCap cells (Fig. 1). Knockdown of MTDH expression
decreased the proliferation and invasive capabilities of prostate
cancer cells in vitro, suggesting that SU6668 inhibited cell
proliferation and invasion partly through downstream targeting of
MTDH (Fig. 4). EMT plays an
important role in the tumor invasion process. Our results revealed
that E-cadherin was increased and that N-cadherin and vimentin were
decreased after the expression of MTDH was downregulated in DU145
and LNCap cells (Figs. 7 and
8), suggesting that SU6668 could
inhibit EMT-mediated cell migration and invasion. However, further
experiments are needed to elucidate the mechanism of SU6668 in
regulating MTDH expression and the role of MTDH during EMT-mediated
processes.
The PI3K-AKT-mTOR pathway regulates cell growth and
proliferation and is often dysregulated in cancer due to
post-translational modifications (37). It is an intracellular signaling
pathway that is important for cell apoptosis, malignant
transformation and tumor progression. The activation of the
PI3K-AKT-mTOR pathway has been strongly implicated in prostate
cancer progression (38,39). Taylor et al (38) reported that alterations in the
PI3K-AKT-mTOR pathway were found in 42% of primary prostate tumors
and 100% of metastatic tumors. The PI3K-AKT pathway is a major
signaling pathway regulated by MTDH and produces MTDH-induced
alterations in cancer cell proliferation and invasion (40). In investigating the molecular
mechanisms of MTDH-mediated proliferation and invasion of prostate
cancer cells, we first observed that downregulated expression of
MTDH led to a decrease in p-AKT level (Figs. 7 and 8). Moreover, a combination of SU6668 and
the AKT pathway inhibitor LY29004 resulted in increased inhibition
of cell proliferation and invasion in DU145 and LNCap cells
(Figs. 9Figure 10Figure 11–12). However, our results indicated that
AKT pathway inhibitor LY29004 also downregulated the expression of
MTDH, suggesting the existence of a reciprocal regulatory loop
between MTDH and the PI3K-AKT pathway. Additional work is being
performed to investigate whether there is a reciprocal regulatory
loop.
In summary, the present study revealed that SU6668
suppressed prostate cancer progression via the MTDH/AKT signaling
pathway, providing a potential therapeutic strategy for prostate
cancer.
Acknowledgments
The present study was funded by The Nature Science
Foundation of Fujian, China (nos. 2015J01571 and 2015J01576), and
The Science and Technology Plan Projects Foundation of Ningde,
China (no. 20140176).
References
|
1
|
Castillejos-Molina RA and
Gabilondo-Navarro FB: Prostate cancer. Salud Publica Mex.
58:279–284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Shami K, Oeffinger KC, Erb L, Willis A,
Bretsch JK, Pratt-Chapman ML, Cannady RS, Wong SL, Rose J, Barbour
AL, et al: American Cancer Society Colorectal Cancer Survivorship
Care Guidelines. CA Cancer J Clin. 65:428–455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roehl KA, Antenor JA and Catalona WJ:
Serial biopsy results in prostate cancer screening study. J Urol.
167:2435–2439. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walsh PC: Re: The natural history of
metastatic progression in men with prostate-specific antigen
recurrence after radical prostatectomy: long-term follow-up. J
Urol. 188:8092012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Antonarakis ES, Feng Z, Trock BJ,
Humphreys EB, Carducci MA, Partin AW, Walsh PC and Eisenberger MA:
The natural history of metastatic progression in men with
prostate-specific antigen recurrence after radical prostatectomy:
Long-term follow-up. BJU Int. 109:32–39. 2012. View Article : Google Scholar
|
|
6
|
Sweeney CJ, Chen YH, Carducci M, Liu G,
Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, et
al: Chemohormonal therapy in metastatic hormone-sensitive prostate
cancer. N Engl J Med. 373:737–746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gale NW and Yancopoulos GD: Growth factors
acting via endothelial cell-specific receptor tyrosine kinases:
VEGFs, angiopoietins, and ephrins in vascular development. Genes
Dev. 13:1055–1066. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoekman K: SU6668, a multitargeted
angiogenesis inhibitor. Cancer J. 7(Suppl 3): S134–S138. 2001.
|
|
9
|
Laird AD, Vajkoczy P, Shawver LK, Thurnher
A, Liang C, Mohammadi M, Schlessinger J, Ullrich A, Hubbard SR,
Blake RA, et al: SU6668 is a potent antiangiogenic and antitumor
agent that induces regression of established tumors. Cancer Res.
60:4152–4160. 2000.PubMed/NCBI
|
|
10
|
Laird AD, Christensen JG, Li G, Carver J,
Smith K, Xin X, Moss KG, Louie SG, Mendel DB and Cherrington JM:
SU6668 inhibits Flk-1/KDR and PDGFRbeta in vivo, resulting in rapid
apoptosis of tumor vasculature and tumor regression in mice. FASEB
J. 16:681–690. 2002.PubMed/NCBI
|
|
11
|
Wang L, Liu Z, Ma D, Piao Y, Guo F, Han Y
and Xie X: SU6668 suppresses proliferation of triple negative
breast cancer cells through down-regulating MTDH expression. Cancer
Cell Int. 13:882013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang X, Raskovalova T, Lokshin A,
Krasinskas A, Devlin J, Watkins S, Wolf SF and Gorelik E: Combined
antiangiogenic and immune therapy of prostate cancer. Angiogenesis.
8:13–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Figg WD, Kruger EA, Price DK, Kim S and
Dahut WD: Inhibition of angiogenesis: Treatment options for
patients with metastatic prostate cancer. Invest New Drugs.
20:183–194. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Timke C, Zieher H, Roth A, Hauser K,
Lipson KE, Weber KJ, Debus J, Abdollahi A and Huber PE: Combination
of vascular endothelial growth factor receptor/platelet-derived
growth factor receptor inhibition markedly improves radiation tumor
therapy. Clin Cancer Res. 14:2210–2219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abdollahi A, Lipson KE, Han X, Krempien R,
Trinh T, Weber KJ, Hahnfeldt P, Hlatky L, Debus J, Howlett AR, et
al: SU5416 and SU6668 attenuate the angiogenic effects of
radiation-induced tumor cell growth factor production and amplify
the direct anti-endothelial action of radiation in vitro. Cancer
Res. 63:3755–3763. 2003.PubMed/NCBI
|
|
16
|
Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao
W, Volsky DJ and Fisher PB: Identification and cloning of human
astrocyte genes displaying elevated expression after infection with
HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid
subtraction hybridization, RaSH. Oncogene. 21:3592–3602. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Wei YB, Gao YL, Yan B, Yang JR and
Guo Q: Metadherin in prostate, bladder, and kidney cancer: A
systematic review. Mol Clin Oncol. 2:1139–1144. 2014.PubMed/NCBI
|
|
18
|
Liu X, Wang D, Liu H, Feng Y, Zhu T, Zhang
L, Zhu B and Zhang Y: Knockdown of astrocyte elevated gene-1
(AEG-1) in cervical cancer cells decreases their invasiveness,
epithelial to mesenchymal transition, and chemoresistance. Cell
Cycle. 13:1702–1707. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong L, Qin S, Li Y, Zhao L, Dong S, Wang
Y, Zhang C and Han S: High expression of astrocyte elevated gene-1
is associated with clinical staging, metastasis, and unfavorable
prognosis in gastric carcinoma. Tumour Biol. 36:2169–2178. 2015.
View Article : Google Scholar
|
|
20
|
Hu G, Wei Y and Kang Y: The multifaceted
role of MTDH/AEG-1 in cancer progression. Clin Cancer Res.
15:5615–5620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Tang ZY, Yin Z, Wei YB, Liu LF,
Yan B, Zhou KQ, Nian YQ, Gao YL and Yang JR: Metadherin regulates
epithelial-mesenchymal transition in carcinoma. Onco Targets Ther.
9:2429–2436. 2016.PubMed/NCBI
|
|
22
|
Li J, Zhang N, Song LB, Liao WT, Jiang LL,
Gong LY, Wu J, Yuan J, Zhang HZ, Zeng MS, et al: Astrocyte elevated
gene-1 is a novel prognostic marker for breast cancer progression
and overall patient survival. Clin Cancer Res. 14:3319–3326. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Noch E, Bookland M and Khalili K:
Astrocyte-elevated gene-1 (AEG-1) induction by hypoxia and glucose
deprivation in glioblastoma. Cancer Biol Ther. 11:32–39. 2011.
View Article : Google Scholar :
|
|
24
|
Sun W, Fan YZ, Xi H, Lu XS, Ye C and Zhang
JT: Astrocyte elevated gene-1 overexpression in human primary
gallbladder carcinomas: An unfavorable and independent prognostic
factor. Oncol Rep. 26:1133–1142. 2011.PubMed/NCBI
|
|
25
|
Xu JB, Wu H, He YL, Zhang CH, Zang LJ, Cai
SR and Zhang WH: Astrocyte-elevated gene-1 overexpression is
associated with poor prognosis in gastric cancer. Med Oncol.
28:455–462. 2011. View Article : Google Scholar
|
|
26
|
Kikuno N, Shiina H, Urakami S, Kawamoto K,
Hirata H, Tanaka Y, Place RF, Pookot D, Majid S, Igawa M, et al:
Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer
progression through upregulation of FOXO3a activity. Oncogene.
26:7647–7655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: Emt: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Emdad L, Lee SG, Su ZZ, Jeon HY, Boukerche
H, Sarkar D and Fisher PB: Astrocyte elevated gene-1 (AEG-1)
functions as an oncogene and regulates angiogenesis. Proc Natl Acad
Sci USA. 106:21300–21305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoo BK, Emdad L, Su ZZ, Villanueva A,
Chiang DY, Mukhopadhyay ND, Mills AS, Waxman S, Fisher RA, Llovet
JM, et al: Astrocyte elevated gene-1 regulates hepatocellular
carcinoma development and progression. J Clin Invest. 119:465–477.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sarkar D, Emdad L, Lee SG, Yoo BK, Su ZZ
and Fisher PB: Astrocyte elevated gene-1: Far more than just a gene
regulated in astrocytes. Cancer Res. 69:8529–8535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Godl K, Gruss OJ, Eickhoff J, Wissing J,
Blencke S, Weber M, Degen H, Brehmer D, Orfi L, Horváth Z, et al:
Proteomic characterization of the angiogenesis inhibitor SU6668
reveals multiple impacts on cellular kinase signaling. Cancer Res.
65:6919–6926. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo X, Ying W, Wan J, Hu Z, Qian X, Zhang
H and He F: Proteomic characterization of early-stage
differentiation of mouse embryonic stem cells into neural cells
induced by all-trans retinoic acid in vitro. Electrophoresis.
22:3067–3075. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi X and Wang X: The role of MTDH/AEG-1
in the progression of cancer. Int J Clin Exp Med. 8:4795–4807.
2015.PubMed/NCBI
|
|
37
|
Chang L, Graham PH, Ni J, Hao J, Bucci J,
Cozzi PJ and Li Y: Targeting PI3K/Akt/mTOR signaling pathway in the
treatment of prostate cancer radioresistance. Crit Rev Oncol
Hematol. 96:507–517. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Taylor BS, Schultz N, Hieronymus H,
Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva
B, et al: Integrative genomic profiling of human prostate cancer.
Cancer Cell. 18:11–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Edlind MP and Hsieh AC: PI3K-AKT-mTOR
signaling in prostate cancer progression and androgen deprivation
therapy resistance. Asian J Androl. 16:378–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee SG, Su ZZ, Emdad L, Sarkar D and
Fisher PB: Astrocyte elevated gene-1 (AEG-1) is a target gene of
oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc.
Proc Natl Acad Sci USA. 103:17390–17395. 2006. View Article : Google Scholar : PubMed/NCBI
|