Introduction
Hepatocellular carcinoma (HCC) is among the most
common and highly lethal cancers worldwide, with a depressingly low
long-term survival rate (1). So
far, tumor metastasis remains the primary cause of death for most
HCC patients. Tumor metastasis consists of several discrete
biological processes, initiating from escaping primary tumor site,
invading and surviving in the surrounding tissues, entering the
lymphatic vessels or the bloodstream, eventually transporting to a
remote site and forming new colonies (2).
Breast cancer metastasis suppressor 1 (BRMS1)
is an active tumor metastasis suppressor gene, exhibiting tumor
metastasis suppressive activity in breast cancer, melanoma and
non-small cell lung cancer (3–5).
Many studies have reported that the expression of BRMS1 was
silenced due to gene mutation or promoter hypermethylation in
metastatic tumor cells (6–8). Functional studies revealed that
BRMS1 was involved in the regulation of cell-cell
communication, cell migration, cell invasion, cell apoptosis and
tumor angiogenesis, while no obvious effect has been shown on cell
proliferation, cell-matrix adhesion and matrix degradation
(9). Mechanistically, BRMS1 is an
essential part of mSin3a·HDAC complex, which modulates gene
transcription activity by regulating the acetylation levels of both
histone and transcriptional factors (10). Therefore, several cancer-related
genes have been recently characterized as transcriptional targets
of BRMS1, including CXCR4, cLAP-2, Bcl-xL, uPA and
miR146 (9). We have previously
demonstrated that BRMS1 was able to sensitize HCC cells to
apoptosis through suppressing NF-κB signaling pathway and
osteopontin expression (11,12).
In this study, we started from gene expression
microarray and identified a novel BRMS1 target gene,
death-associated protein kinase 1 (DAPK1), in HCC cells.
DAPK1 is a well-defined tumor suppressor gene, with
significant suppressive effect in both tumor growth and metastasis
in vivo (13). DAPK1
participates in multiple cell death-related signaling pathways,
including caspase-dependent cell apoptosis, mitochondrial-dependent
cell apoptosis and autophagic cell death (14). In a variety of tumor tissues and
cell lines, the promoter region of DAPK1 was
hypermethylated, resulting in significant decrease or loss of
DAPK1 expression (15,16).
Additionally, many transcriptional factors including TP53, C/EBP-β,
HSF1 and SMAD can transcriptionally activate DAPK1
expression, while STAT3 and NF-κB play the opposite role (17).
We reported for the first time that BRMS1 could
transcriptionally activate DAPK1 expression in HCC cells.
Immunohistochemical analysis of human HCC tissues revealed that
DAPK1 expression was specifically silenced in tumor cells.
The association relationship between BRMS1 and DAPK1
expression in paired HCC tissues was studied through western blot
analysis and the transcriptional mechanism of BRMS1 on DAPK1
promoter was further elucidated. Our findings suggested a
functional relationship between BRMS1 and DAPK1, indicating another
potential molecular mechanism accounting for BRMS1's tumor
suppressive role in HCC cells.
Materials and methods
Ethics statement
This study was accomplished with the approval of the
Medical ethics Committee of School of Life Sciences, Fudan
university, Shanghai, China.
Tumor specimens
Fresh surgical specimens of HCC, including tumor
tissues and the neighboring pathologically non-tumorous liver
tissues, were obtained from liver cancer patients at Zhongshan
Hospital (Fudan University, Shanghai, China). All of the samples
were immediately frozen in liquid nitrogen after surgery and then
stored at −80°C before further analysis.
Tissue microarray analysis
Fifty matched pairs of tumor samples and adjacent
normal tissues from clinical HCC patients were used for the
construction of a tissue microarray (Shanghai Biochip Co., Ltd.
Shanghai, China) as previously described (18). In brief, sections (4 μm
thickness, 2 mm diameter) were taken from individual
paraffin-embedded tissues and precisely arrayed on
3-aminopropyltriethoxysilane-coated slides for subsequent staining
with an anti-DAPK1 antibody (Sigma, USA). The immunohistochemistry
analysis was performed in Shanghai Biochip. All the images were
visualized by Leica DC 500 camera on a microscope equipped with
Leica DMRA2 fluorescent optics (LEICA).
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from tissues or cultured
cells using TRIzol reagent (Life Technologies, USA), and 1–2
μg of RNA was used for reverse transcription using
PrimeScript™ RT reagent kit with gDNA eraser (Takara, japan). PCR
analysis was performed using the SYBR green Supermix kit (Takara)
with the CFX Connection detection system (Bio-Rad, USA). Diluted
cDNA was used in a 10-μl real-time PCR reaction in
triplicate for each gene and each sample. Cycle parameters were
95°C for 5 min hot start and 40 cycles of 95°C for 5 sec, 58°C for
10 sec and 72°C for 20 sec. Blank controls with no cDNA templates
were performed to rule out contamination. The specificity of the
PCR product was confirmed by melting curve analysis. BRMS1
primers were: forward, 5′-ACTGAGTCAGCTGC GGTTGCGG-3′; reverse,
5′-AAGACCTGGAGCTGCCTCTGGCGTGC-3′. DAPK1 primers were:
forward, 5′-TGTCTTCCACCAACTCCAGCAG-3′; reverse,
5′-AAATCGCCAACTCCATTCAAATAAGC-3′. 18S rRNA primers were:
forward, 5′-GTAACCCGTTGAACCCCATT-3′; reverse,
5′-CCATCCAATCGGTAGTAGCG-3′.
The expression levels of all genes were normalized
to those of the house keeping gene 18S rRNA. Relative gene
expression levels were calculated by the formula 2−ΔCt,
where ΔCt (Critical threshold) = Ct of genes of interest − Ct of
18S rRNA.
Plasmid construction
Full length of DAPK1 promoter was amplified from
human genomic DNA, forward, 5′-CACTCACTCCCTAGCTGTGT-3′; reverse,
5′-TAGCCCCCTCATGCA-3′. The amplicons were separated by DNA
electrophoresis and purified before being cloned into the pGEM-T
easy Vector (Promega, USA). Both the full-length promoter and three
3′ deletion mutants were further cloned into pGL3-Basic Vectors
(Promega). The relevant primers were as follows:
pGL3-Basic-DAPK1-P(P): forward,
5′-GGGGTACCCACTCACTCCCTAGCTGTGT-3′; reverse,
5′-CCAAGCTTTAGCCCCCTCATGCA-3′. pGL3-Basic-DAPK1-P1(P1):
forward, 5′-GGGGTACCCACTCACTCCCTAGCTGTGT-3′; reverse,
5′-CCAAGCTTGACCGGGTCTCCGGA-3′. pGL3-Basic-DAPK1-P2(P2):
forward, 5′-GGGGTACCCACTCACTCCCTAGCTGTGT-3′; reverse,
5′-CCAAGCTTCCACCTCCAGGGACG-3′. pGL3-Basic-DAPK1-P3(P3):
forward, 5′-GGGGTACCCACTCACTCCCTAGCTGTGT-3′; reverse,
5′-CCAAGCTTGGCGACTCCCTCTCC-3′. Two site-directed mutations of
pGL3-Basic-DAPK1-P, Mut1 and Mut2, were obtained by
KOD-Plus-Mutagenesis kit (Toyobo, japan) according to the
manufacturer′s instructions. The relevant primers were as follows:
Mut1: forward, 5′-TCTGAGCGCCGGGGAGGTCTACTTCCTTTT-3′; reverse,
5′-AACCGCTCGCTGAAGACCGGGTCTCCGGAG-3′. Mut2: forward,
5′-AGGGATACTTCCTTTTGGGGTTGCCATTTT-3′; reverse,
5′-CAACGGCGCTAAGACCCCGCTCGCTGAAGA-3′. Recombinant pCMV-Myc-BRMS1
were constructed as previously described (11).
Cell culture and transfection
Human embryonic kidney cell line 293T and HCC cell
line SK-Hep1 were all cultured with Dulbecco's modified eagle's
medium (DMEM), supplemented with 10% fetal bovine serum (Gibco,
USA) at 37°C in 5% CO2 humidified atmosphere. Cells at
80% confluency were transfected using Lipofectin 2000 (Invitrogen,
USA) according to the manufacturer's instructions.
Dual-luciferase assay
Cells were seeded into 24-well culture plates at a
density of 1×105/well and transfected with indicated
DAPK1 promoter constructs or pGL3-Basic empty vectors. The
pRL-TK control vector (20 ng/well) was used for normalization.
Cells were harvested 36 h after transfection. Firefly and
Renilla activities were determined using GloMax 96
Microplate Luminometer (Promega) according to the manufacturer's
instructions. Data are presented as the changes in Firefly
luciferase activity relative to Renilla luciferase
activity.
Western blot analysis
Protein samples were separated by 10% SDS-PAGE gel
and then transferred to PVDF membranes. Non-specific binding was
blocked by incubation with 5% fat-free milk for 1 h at room
temperature. After blocking, the membranes were incubated with
specific primary antibodies against different proteins at 4°C
overnight, followed by incubation with HRP-conjugated secondary
antibody for 45 min at room temperature. Immunoreactivity was
visualized by enhanced chemiluminescence (Pierce, USA) on a
molecular imager ChemiDoc XRS+ system (Bio-Rad). Related
antibodies included the mouse monoclonal antibody against BRMS1
(Abcam, USA), β-actin and Myc tag (Sigma), the rabbit monoclonal
antibody against DAPK1 (Sigma), peroxidase-conjugated goat
anti-mouse IgG and goat anti-rabbit IgG (Jackson, USA).
Chromatin immunoprecipitation (ChIP)
The ChIP assay was performed according to Farenham
laboratory protocol (19).
Basically, HEK293T cells were seeded at 1×107/dish in
100-mm dishes and transfected with pCMV-Myc-BRMS1 plasmid or empty
pCMV-Myc vector. At 36 h post-transfection, cells were subject to
crosslinking and fixation using 4% formaldehyde (Amresco, USA).
Cell nuclei were released by SDS lysis buffer (1% SDS, 10 mM EDTA,
and 50 mM Tris-HCl, pH 8.1) before sonication. Fragmented genomic
DNA was immuno-precipitated by anti-Myc antibody at 4°C after
precleared with protein A-agarose beads. Mouse IgG was used as the
negative control to exclude genomic contamination. The
immune-precipitated DNA fragments were then collected, washed,
de-crosslinked and digested with Proteinase K. DNA fragments were
recovered by phenol/chloroform extraction and amplified through
qRT-PCR using the following primers: primers covering the putative
NF-κB binding site (P-A): forward, 5′-CAGCGAGCGGGGTCTTAG-3′;
reverse, 5′-GTAAAATGGCAACCCCAAAA-3′. Primers covering the
downstream region of the putative NF-κB binding site (P-B):
forward, 5′-TCTTCAAAAGGACTGGAGACTGA-3′; reverse,
5′-CCTGCCAAGTTCCTCGCC-3′.
Statistical analysis
Comparisons of quantitative data were analyzed by
Student's t-test. Categorical data were analyzed by Fisher's exact
test. We considered p<0.05 to be different and p<0.01 to be
significant different.
Results
DAPK1 is positively regulated by BRMS1 in
HCC cells
In order to find novel transcriptional targets of
BRMS1, BRMS1 stably expressing clone F6 and control clone E6
established in our previous study were analyzed in a whole genome
expression microarray (11). Under
the selection criteria of |Fold change| >2, 260 potential target
genes were found, of which 194 genes were upregulated and the other
66 genes were suppressed by BRMS1 expression (data not shown). A
well-studied tumor suppressor gene, DAPK1 was found to be
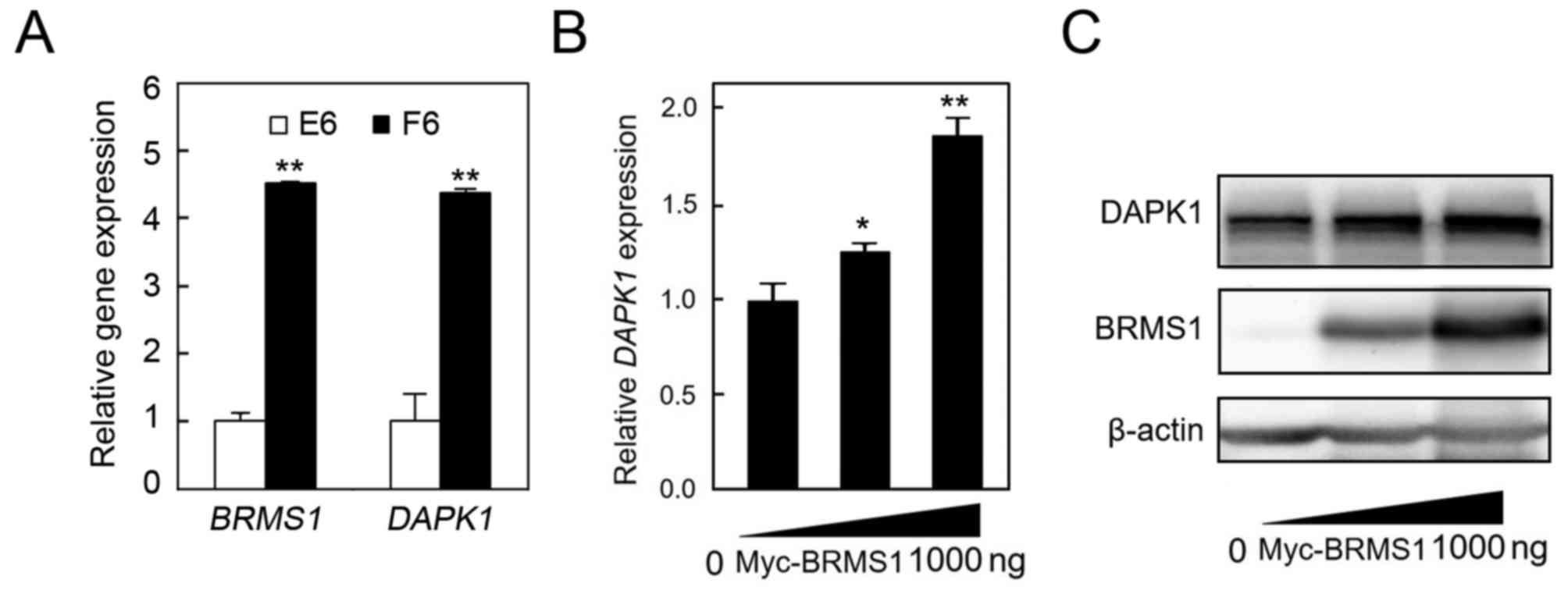
4.46-fold overexpressed in cells stably expressing BRMS1 by
comparison with control cells (Fig.
1A). Next, qRT-PCR and western blot analyses were carried out
to confirm this result. Different dosages of recombinant BRMS1
plasmid were transiently introduced into SK-Hep1 cells. As shown in
Fig. 1B and C, both the mRNA and
the protein expression levels of DAPK1 were, not
surprisingly, upregulated upon BRMS1 overexpression in a
dose-dependent manner. The data strongly suggest that DAPK1 is a
potential transcriptional target of BRMS1 in HCC cells.
DAPK1 is remarkably downregulated in
human HCC tissues
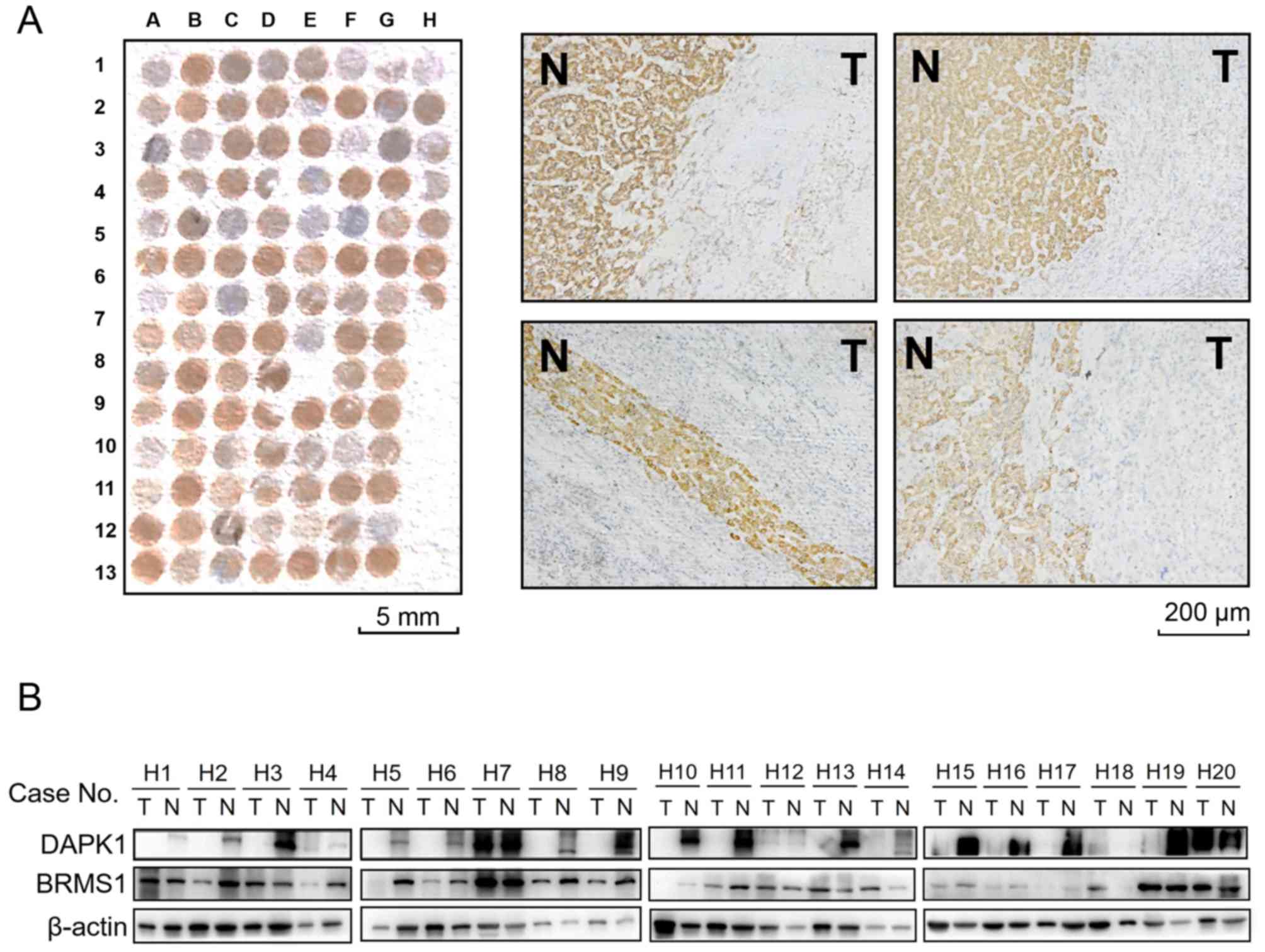
To assess the expressional pattern of DAPK1 in HCC
tissues, immunohistochemical analysis was carried out on a tissue
microarray including 50 paired HCC tissues and adjacent
non-tumorous liver tissues. It was revealed that the immunostaining
signal of DAPK1 is reduced in 37 tumor tissues by comparison with
adjacent non-tumorous tissues, of which, 18 tumor tissues exhibited
remarkably suppressed or even silenced DAPK1 expression. Images
from four representative HCC samples composed of both tumor cells
and normal liver cells are shown in Fig. 2A. In contrast to high DAPK1
expression in normal liver cells, DAPK1 immunostaining signal is
totally lost in the surrounding tumor cells. To further confirm the
expression pattern of DAPK1 in HCC samples, additional 20 paired
HCC tissues and adjacent non-tumorous liver tissues were subjected
to western blot analysis (Fig.
2B). Consistently, DAPK1 protein expression was almost
completely lost in 15 out of 20 tumor tissues. We have previously
investigated the BRMS1 expression in these HCC samples (11), whether DAPK1 expression was
associated with BRMS1 expression in these paired protein samples
needed to be assessed. It was shown that in all 11 samples with
downregulated BRMS1 expression, 10 samples exhibited consistent
DAPK1 downregulation (positive ratio = 90.91%). Moreover, only 5
out of the other 9 samples without suppressed BRMS1 expression
exhibited silenced DAPK1 (positive ratio = 55.56%). This finding
indicates a potential correlation of endogenous BRMS1 and DAPK1
expression in clinical HCC tissues, providing another piece of
evidence that DAPK1 might be transcriptionally regulated by
BRMS1.
DAPK1 promoter is transcriptionally
activated by BRMS1 in a dose-dependent manner
To clarify the transcriptional mechanism between
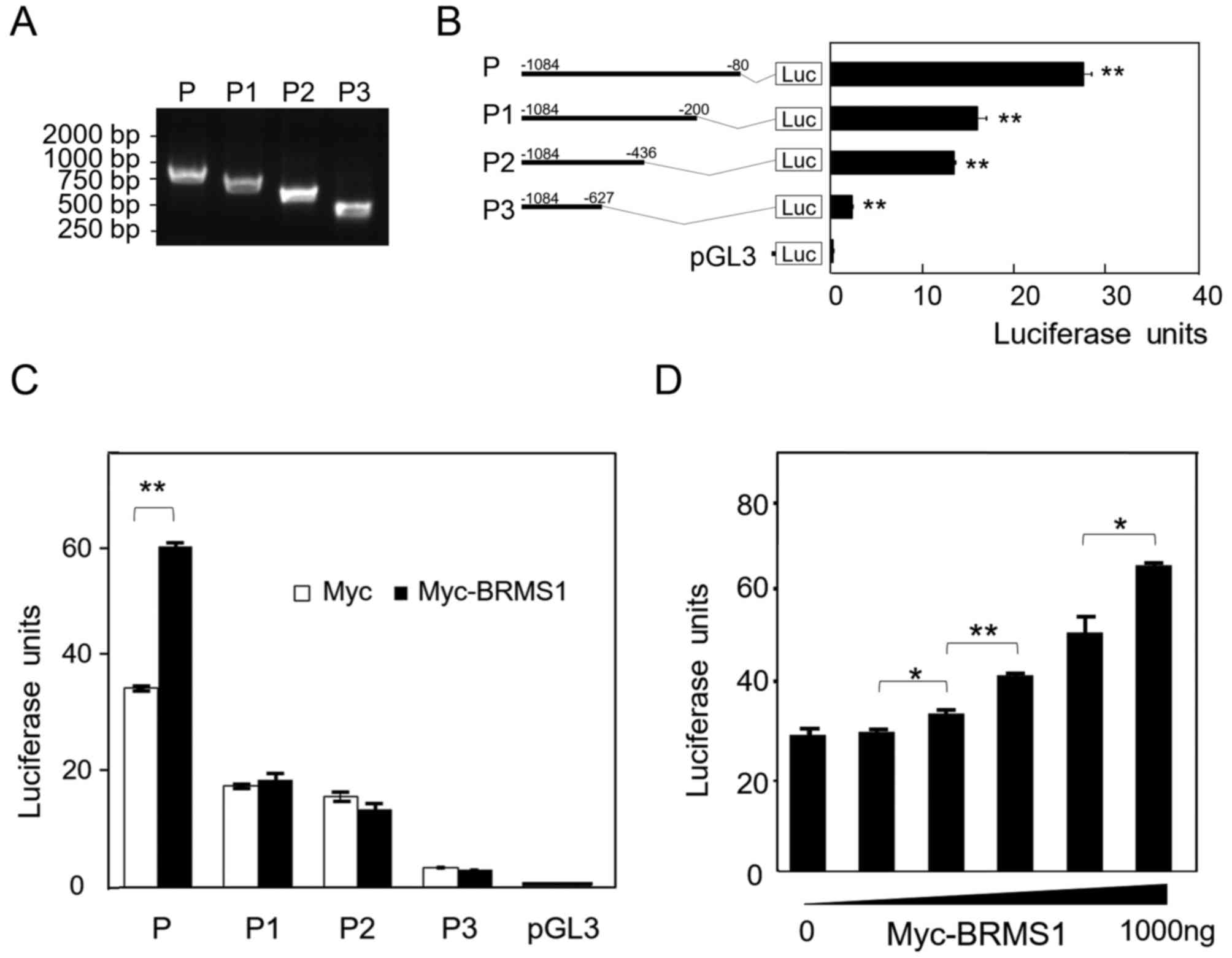
BRMS1 and DAPK1, the promoter region (−1,084 to −80 bp) of
human DAPK1 gene was cloned from human genomic DNA and a
series of 3′ deletion mutants, namely P1 (−1,084 to −200 bp), P2
(−1,084 to −436 bp) and P3 (−1,084 to −627 bp), were constructed
subsequently (Fig. 3A). The four
DNA fragments were then inserted into pgL3-Basic vector to detect
their transcriptional activity in the luciferase assay,
respectively. As shown in Fig. 3B,
by comparison with full length promoter, both pGL3-Basic-P1(P1) and
pGL3-Basic-P2(P2) truncates exhibited a 50% reduction in the report
gene's activity, and pGL3-Basic-P3(P3) almost lost the entire
transcriptional activity. This result suggests that −200 to −80 bp
and −627 to −436 bp are two potential transcriptional active
regions in DAPK1 promoter. Next, Myc-BRMS1 plasmid was
co-introduced into cells to investigate the transcriptional effect
of BRMS1 on DAPK1 promoter. It was found that BRMS1 can
transcriptionally activate the luciferase activity of
pGL3-Basic-P(P), whereas no effect was observed on the other three
truncates (Fig. 3C). Moreover,
when different dosages of Myc-BRMS1 plasmid were utilized, it was
shown that the luciferase activity of pGL3-Basic-P gradually
increased upon the expression of exogenous BRMS1 (Fig. 3D). Taken together, current results
indicate that BRMS1 might be able to activate DAPK1
expression through the −200 to −80 bp region of the promoter.
BRMS1 is able to bind the putative NF-κB
binding sites of DAPK1 promoter
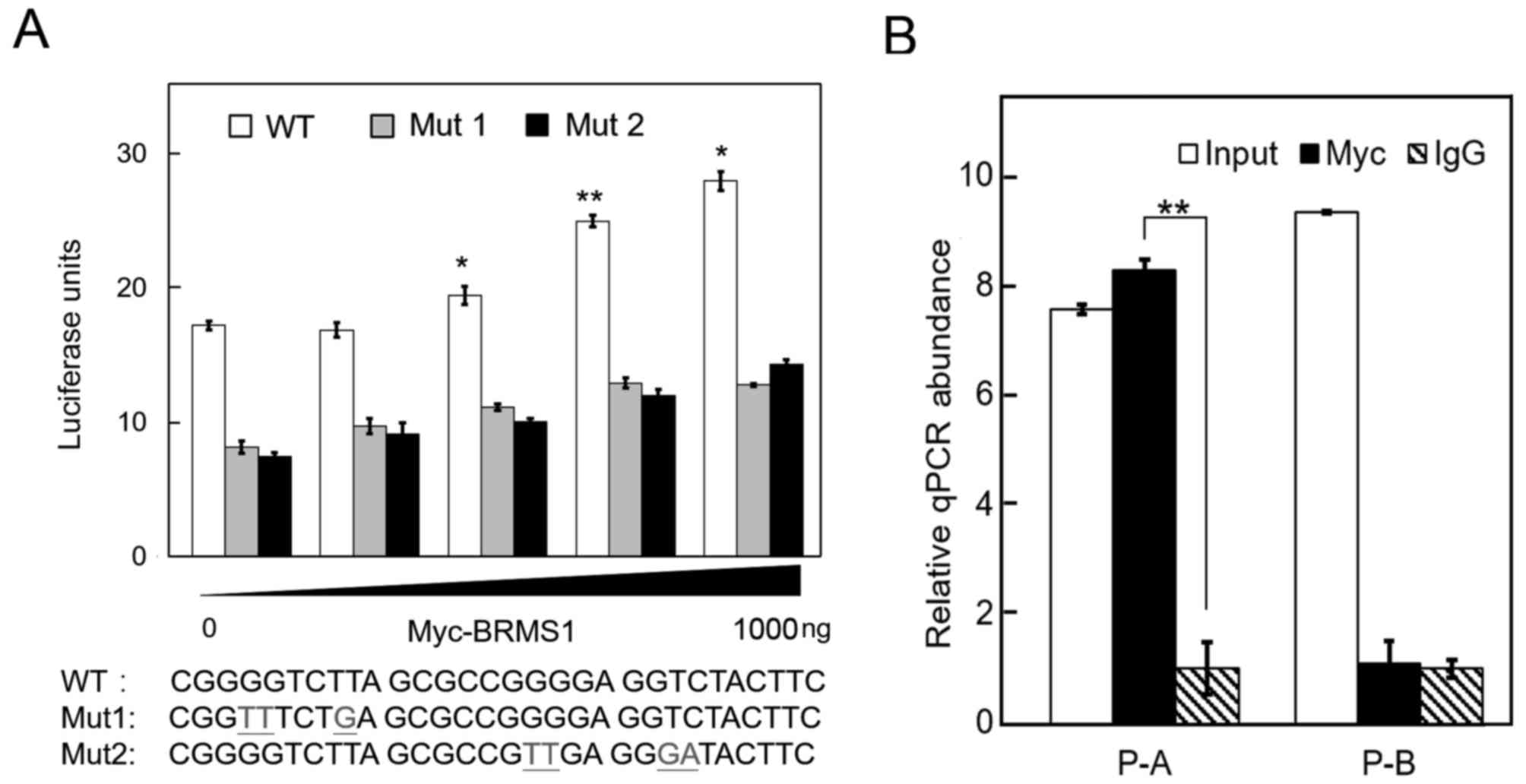
After identifying −200 to −80 bp region of
DAPK1 promoter as the target of interest, we screened this
region using online transcriptional element prediction software to
uncover the potential transcriptional factor binding sites. The
−190 to −181 bp (CGGGGTCTTA) region and the −176 to −167 bp
(CGGGGAGGTC) region of DAPK1 promoter were predicted to be
two tandem putative binding sites for transcription factor NF-κB,
which is demonstrated to be an important deacetylation modification
target of BRMS1 (20–22). To assess whether BRMS1 functions
through these binding sites, two site-directed point-mutations of
pGL3-Basic-P, Mut1 and Mut2, were constructed. As shown in Fig. 4A, both mutations markedly abolished
the transcriptional activation effect of BRMS1 on DAPK1
promoter, strongly suggesting that these putative NF-κB binding
sites might be involved in BRMS1-DAPK1 transcriptional regulation.
Chromatin immunoprecipitation experiment was further carried out in
order to demonstrate whether BRMS1 could bind to this DAPK1
promoter region. Cells overexpressing Myc-BRMS1 were lysed and
immunoprecipitated by anti-Myc antibody or Igg control. Two
different pairs of primers were designed to target the two putative
NF-κB binding sites (P-A) and the downstream region of NF-κB
binding sites (P-B), respectively. As shown in Fig. 4B, only the promoter region
containing NF-κB binding sites was immunoprecipitated by specific
antibody against exogenous BRMS1. These data together demonstrate
that BRMS1 is able to bind DAPK1 promoter through the
putative NF-κB binding sites.
Discussion
After BRMS1 was demonstrated to be a functional
partner of mSin3a·HDAC complex involved in regulating chromatin
status and gene expression, several BRMS1-target genes have been
demonstrated to be involved in BRMS1-mediated tumor metastasis
suppression. Herein, we reported for the first time that
DAPK1 was another transcriptional target of BRMS1 in HCC
cells. DAPK1 was initially identified as a positive mediator of
apoptosis activated by interferon-γ in HeLa cell (23). Soon after that, DAPK1 was well
demonstrated as an important tumor suppressor, which also functions
in suppressing tumor metastasis (24). For example, Inbal et al
found that restoration of DAPK1 to physiological levels in
high-metastatic Lewis carcinoma cells successfully suppressed tumor
metastasis in a mouse model (25).
By utilizing in situ TUNEL staining of tumor sections, they
proposed that loss of DAPK1 expression provides a unique mechanism
that links suppression of apoptosis to metastasis. Kuo et al
reported that DAPK1 can block cell migration and invasion in tumor
cells by blocking the integrin-mediated polarity pathway (26). Additionally, Chen et al
revealed that miR-103/107 promote metastasis of colorectal cancer
probably through targeting DAPK1 (27). It is therefore of great interest to
uncover the relationship between BRMS1 and DAPK1 in HCC tissues and
cell lines.
Consistent with the tumor suppressive function of
DAPK1, clinical studies also revealed that the expression of DAPK1
is significantly decreased in chronic lymphocytic leukemia, breast
cancer and head and neck cancer (16,28,29).
Importantly, loss of DAPK1 expression is associated with advanced
tumor stages and tumor metastasis (24). Studies of DAPK1 expression pattern
and regulation mechanism in HCC are limited. Matsumoto et al
investigated the expression of DAPK1 in 43 Japanese HCC patients
(30). Through association study,
they found DAPK1-negative HCC cases were associated with high serum
AFP, lower tumor differentiation, and less apoptosis. However,
while they revealed that the status of DAPK1 protein expression
correlated with IFN-γ-receptor and Fas expression, but not the
promoter methylation status, how IFN-γ-receptor and Fas control
DAPK1 expression in HCC was not addressed.
In our study, by utilizing 70 pairs of Chinese HCC
specimens, DAPK1 was found to be remarkably reduced or even lost in
HCC tissues by comparison with neighboring non-tumorous tissues,
which is consistent with Matsumoto et al (30). Moreover, the transcriptional
regulation mechanism of DAPK1 has been carefully studied
through luciferase assay and ChIP experiment. Two tandem NF-κB
binding sites locating in −190 to −181 bp and −176 to −167 bp of
DAPK1 promoter could be recognized by BRMS1, and responsible
for BRMS1-mediated transcriptional activation. It has been noted
that NF-κB is an important modification substrate of
BRMS1·mSin3a·HDAC complex (10).
BRMS1 suppresses several metastasis-related genes through
deacetylating NF-κB subunits (21,22,31).
However, Shanmugam et al recently reported that NF-κB was
able to bind to DAPK1 promoter together with HDACs and played a
negative role in regulating DAPK1 expression in acute myeloid
leukemia (32). More
interestingly, Li et al found that the cell cycle regulator
ING4 was specifically induced by BRMS1 through suppressing NF-κB
activities as well, because NF-κB functions as a transcriptional
inhibitor of ING4 (33). Based on
all these pieces of evidence, we speculate that BRMS1 might also be
able to upregulate DAPK1 expression through releasing DAPK1
promoter from the negative regulation of NF-κB. Further experiments
are in progress to demonstrate this hypothesis and elucidate the
underlying molecular mechanism. It would also be important and
interesting to investigate how BRMS1 and DAPK1 collaborate to
regulate tumor metastasis.
Acknowledgments
We thank Dr Hexige Saiyin (Fudan University, China)
for the kind help in analyzing tissue microarray. We also thank Dr
Xuechao Wan (Fudan University, China) for his helpful discussion.
This study was supported by the National Natural Science Foundation
of China (31000558) and Zhuoxue Program of Fudan University.
References
|
1
|
Tang Z-Y, Ye S-L, Liu Y-K, Qin LX, Sun HC,
Ye QH, Wang L, Zhou J, Qiu SJ, Li Y, et al: A decade's studies on
metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol.
130:187–196. 2004. View Article : Google Scholar
|
|
2
|
Fidler IJ: The pathogenesis of cancer
metastasis: The 'seed and soil' hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seraj MJ, Samant RS, Verderame MF and
Welch DR: Functional evidence for a novel human breast carcinoma
metastasis suppressor, BRMS1, encoded at chromosome 11q13. Cancer
Res. 60:2764–2769. 2000.PubMed/NCBI
|
|
4
|
Shevde LA, Samant RS, Goldberg SF,
Sikaneta T, Alessandrini A, Donahue HJ, Mauger DT and Welch DR:
Suppression of human melanoma metastasis by the metastasis
suppressor gene, BRMS1. Exp Cell Res. 273:229–239. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith PW, Liu Y, Siefert SA, Moskaluk CA,
Petroni GR and Jones DR: Breast cancer metastasis suppressor 1
(BRMS1) suppresses metastasis and correlates with improved patient
survival in non-small cell lung cancer. Cancer Lett. 276:196–203.
2009. View Article : Google Scholar
|
|
6
|
Nagji AS, Liu Y, Stelow EB, Stukenborg GJ
and Jones DR: BRMS1 transcriptional repression correlates with CpG
island methylation and advanced pathological stage in non-small
cell lung cancer. J Pathol. 221:229–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Cheng Y, Tai D, Martinka M, Welch DR
and Li G: Prognostic significance of BRMS1 expression in human
melanoma and its role in tumor angiogenesis. Oncogene. 30:896–906.
2011. View Article : Google Scholar :
|
|
8
|
Liu Y, Mayo MW, Nagji AS, Smith PW, Ramsey
CS, Li D and Jones DR: Phosphorylation of RelA/p65 promotes DNMT-1
recruitment to chromatin and represses transcription of the tumor
metastasis suppressor gene BRMS1. Oncogene. 31:1143–1154. 2012.
View Article : Google Scholar
|
|
9
|
Kodura MA and Souchelnytskyi S: Breast
carcinoma metastasis suppressor gene 1 (BRMS1): update on its role
as the suppressor of cancer metastases. Cancer Metastasis Rev.
34:611–618. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meehan WJ, Samant RS, Hopper JE, Carrozza
MJ, Shevde LA, Workman JL, Eckert KA, Verderame MF and Welch DR:
Breast cancer metastasis suppressor 1 (BRMS1) forms complexes with
retinoblastoma-binding protein 1 (RBP1) and the mSin3 histone
deacetylase complex and represses transcription. J Biol Chem.
279:1562–1569. 2004. View Article : Google Scholar
|
|
11
|
Wu Y, Jiang W, Wang Y, Wu J, Saiyin H,
Qiao X, Mei X, Guo B, Fang X, Zhang L, et al: Breast cancer
metastasis suppressor 1 regulates hepatocellular carcinoma cell
apoptosis via suppressing osteopontin expression. PLoS One.
7:e429762012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu J, Wang Y, Qiao X, Saiyin H, Zhao S,
Qiao S and Wu Y: Cloning and characterization of a novel human
BRMS1 transcript variant in hepatocellular carcinoma cells. Cancer
Lett. 337:266–275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bialik S and Kimchi A: The
death-associated protein kinases: Structure, function, and beyond.
Annu Rev Biochem. 75:189–210. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bialik S and Kimchi A: The DAP-kinase
interactome. Apoptosis. 19:316–328. 2014. View Article : Google Scholar
|
|
15
|
Yegnasubramanian S, Kowalski J, Gonzalgo
ML, Zahurak M, Piantadosi S, Walsh PC, Bova GS, De Marzo AM, Isaacs
WB and Nelson WG: Hypermethylation of CpG islands in primary and
metastatic human prostate cancer. Cancer Res. 64:1975–1986. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raval A, Tanner SM, Byrd JC, Angerman EB,
Perko JD, Chen SS, Hackanson B, Grever MR, Lucas DM, Matkovic JJ,
et al: Downregulation of death-associated protein kinase 1 (DAPK1)
in chronic lymphocytic leukemia. Cell. 129:879–890. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Benderska N and Schneider-Stock R:
Transcription control of DAPK. Apoptosis. 19:298–305. 2014.
View Article : Google Scholar
|
|
18
|
Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY,
Xiao YS, Xu Y, Li YW and Tang ZY: Intratumoral balance of
regulatory and cytotoxic T cells is associated with prognosis of
hepatocellular carcinoma after resection. J Clin Oncol.
25:2586–2593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boyd KE, Wells J, Gutman J, Bartley SM and
Farnham PJ: c-Myc target gene specificity is determined by a
post-DNAbinding mechanism. Proc Natl Acad Sci USA. 95:13887–13892.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cicek M, Fukuyama R, Cicek MS, Sizemore S,
Welch DR, Sizemore N and Casey G: BRMS1 contributes to the negative
regulation of uPA gene expression through recruitment of HDAC1 to
the NF-kappaB binding site of the uPA promoter. Clin Exp
Metastasis. 26:229–237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cicek M, Fukuyama R, Welch DR, Sizemore N
and Casey G: Breast cancer metastasis suppressor 1 inhibits gene
expression by targeting nuclear factor-kappaB activity. Cancer Res.
65:3586–3595. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Samant RS, Clark DW, Fillmore RA, Cicek M,
Metge BJ, Chandramouli KH, Chambers AF, Casey G, Welch DR and
Shevde LA: Breast cancer metastasis suppressor 1 (BRMS1) inhibits
osteopontin transcription by abrogating NF-kappaB activation. Mol
Cancer. 6:62007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deiss LP, Feinstein E, Berissi H, Cohen O
and Kimchi A: Identification of a novel serine/threonine kinase and
a novel 15-kD protein as potential mediators of the gamma
interferon-induced cell death. Genes Dev. 9:15–30. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen HY, Lee YR and Chen RH: The functions
and regulations of DAPK in cancer metastasis. Apoptosis.
19:364–370. 2014. View Article : Google Scholar
|
|
25
|
Inbal B, Cohen O, Polak-Charcon S,
Kopolovic J, Vadai E, Eisenbach L and Kimchi A: DAP kinase links
the control of apoptosis to metastasis. Nature. 390:180–184. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuo JJC, Wang WJ, Yao CC, Wu PR and Chen
RH: The tumor suppressor DAPK inhibits cell motility by blocking
the integrin-mediated polarity pathway. J Cell Biol. 172:619–631.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen HY, Lin YM, Chung HC, Lang YD, Lin
CJ, Huang J, Wang WC, Lin FM, Chen Z, Huang HD, et al: miR-103/107
promote metastasis of colorectal cancer by targeting the metastasis
suppressors DAPK and KLF4. Cancer Res. 72:3631–3641. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Levy D, Plu-Bureau G, Decroix Y, Hugol D,
Rostène W, Kimchi A and Gompel A: Death-associated protein kinase
loss of expression is a new marker for breast cancer prognosis.
Clin Cancer Res. 10:3124–3130. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sanchez-Cespedes M, Esteller M, Wu L,
Nawroz-Danish H, Yoo GH, Koch WM, Jen J, Herman JG and Sidransky D:
gene promoter hypermethylation in tumors and serum of head and neck
cancer patients. Cancer Res. 60:892–895. 2000.PubMed/NCBI
|
|
30
|
Matsumoto H, Nagao M, Ogawa S, Kanehiro H,
Hisanaga M, Ko S, Ikeda N, Fujii H, Koyama F, Mukogawa T, et al:
Prognostic significance of death-associated protein-kinase
expression in hepatocellular carcinomas. Anticancer Res.
23B:1333–1341. 2003.
|
|
31
|
Liu Y, Mayo MW, Xiao A, Hall EH, Amin EB,
Kadota K, Adusumilli PS and Jones DR: Loss of BRMS1 promotes a
mesenchymal phenotype through NF-κB-dependent regulation of Twist1.
Mol Cell Biol. 35:303–317. 2015. View Article : Google Scholar
|
|
32
|
Shanmugam R, Gade P, Wilson-Weekes A,
Sayar H, Suvannasankha A, Goswami C, Li L, Gupta S, Cardoso AA, Al
Baghdadi T, et al: A noncanonical Flt3ITD/NF-kappaB signaling
pathway represses DAPK1 in acute myeloid leukemia. Clin Cancer Res.
18:360–369. 2012. View Article : Google Scholar
|
|
33
|
Li J and Li G: Cell cycle regulator ING4
is a suppressor of melanoma angiogenesis that is regulated by the
metastasis suppressor BRMS1. Cancer Res. 70:10445–10453. 2010.
View Article : Google Scholar : PubMed/NCBI
|