Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cancer and the third most important cause of cancer related
death in men (1,2). Each year the growing number of new
HCC cases being diagnosed is nearly equal to the number of deaths
from this disease (1,2). In addition, although for HCC
treatment variable degree of benefit has been observed by use of
chemotherapeutic agents such as interferon (3,4),
adverse effects such as bone marrow suppression after long-term
administration limit their usefulness (5). Therefore, alternative drugs are
needed to improve the prognosis and survival of patients.
Vitamin K2 (VK2), is used clinically as an activator
of homeostasis and an inhibitor of osteoporosis (6,7),
recently has been reported to inhibit the growth of hepatoma cells
(8–10) and in HCC patients decrease serum
levels of des-gammacarboxy prothrombin (DCP), a significant
predisposing factor for the development of portal venous invasion
of HCC (11,12). Furthermore, VK2 is a safe medicine
without prominent adverse effects such as bone marrow suppression
after long-term administration (13).
Although VK2 has potential for the treatment of
liver cancer, it has also been reported that hepatoma is
insensitive to VK2 (14). VK2
administration alone was reported to be insufficient to prevent HCC
recurrence in clinic (15,16). For more effective use of VK2 in HCC
treatment, it is necessary to increase the sensitivity of hepatoma
to VK2.
Cytochrome P450 2E1 (CYP2E1), as an enzyme from CYP2
family, known to regulate cytokine signaling, antigen presentation,
and macromolecular degradation, all of which are crucial to liver
cell function and viability (17).
Furthermore, previous findings indicated that CYP2E1 synergized and
increased the susceptibility of hepatocytes to different chemicals
(18,19). Therefore, we speculated that CYP2E1
may play an important role in the enhanced susceptibility of
hepatomas to VK2. In the present study, we demonstrated that CYP2E1
efficiently enhanced the inhibitory effect of VK2 on the viability
of hepatoma cells. Furthermore, we showed that CYP2E1 was regulated
by VK2 via both post-transcriptional and transcriptional mechanisms
in hepatoma cells.
Materials and methods
Materials
VK2 was purchased from Sigma, St. Louis, MO, USA
(no. V9378). It has also been termed as menatetrenone (MK-4), one
of nine forms of vitamin K2, which is ubiquitously present in
extrahepatic tissues.
Cell culture
Human hepatocellular carcinoma Smmc-7721, QGY-7703
and HepG2 cells were kindly provided by Prof. S. Ge, Shanghai
Jiaotong University, School of Medicine, Shanghai, China. All cells
were checked with the list of known mis-identified cell lines
available from the International Cell Line Authentication Committee
(http://iclac.org/databases/cross-contaminations) and
confirmed that they are not mis-identified or contaminated. All
cells were cultured in RPMI-1640 medium (Thermo Scientific Hyclone,
Logan, UT, USA) supplemented with 12% fetal bovine serum (FBS;
Thermo Scientific Hyclone) in a humidified atmosphere with 5%
CO2 at 37°C.
Viability assays
Cells/well (5×103) were seeded into
96-well plates and treated with VK2 (Sigma) as indicated in the
result section. Cell viability was measured by MTT assay. The
optical density (OD) of each well was measured at 490 nm with the
Thermo Varioskan Flash (Thermo Electron Corp., Vantaa, Finland).
Cell viability was expressed as a percentage of control cells,
which were defined as 100% viable.
CYP2E1 activity assay
Aniline hydroxylase (ANH) activity, a specific
marker of CYP2E1 enzymatic activity, was spectrophotometrically
measured with phenol as a coloring reagent (20). In brief, after treatment cells
(2×107) were treated with RIPA buffer (Beyotime
Institute of Biotechnology, Haimen, China), and protein
concentrations were determined using the BCA protein assay kit
(Beyotime Institute of Biotechnology). Then 10 mM aniline (Sigma)
and 1 mM triphosphopyridine nucleotide (NADPH; Beyotime Institute
of Biotechnology) were added to equal volumes of supernatants.
After incubated for 1 h at 37°C, equal volumes of 20%
trichloroacetic acid (TCA; Sigma) were added to the mixture and
maintained on ice for 5 min. Samples were centrifuged at 1902 × g
for 10 min, and the supernatants were added to equal volumes of 5%
phenol (Sigma) and 1% sodium carbonate (Sigma). The mixture was
incubated for 60 min at RT. ANH activation resulted in producing
4-amino phenol, which was detected spectrophotometrically (630 nm)
with the Thermo Varioskan Flash (Thermo Electron Corp.).
Quantitative RT-PCR
By resuspension in TRIzol reagent (Invitrogen,
Carlsbad, CA, USA) total RNA was extracted from cells. Relative
quantification of the genes of interest was measured by real-time
PCR using the Maxima SYBR Green qPCR Master Mix (Thermo Fisher
Scientific Inc., Waltham, MA, USA). Real-time PCR of the
housekeeping gene β-actin allowed normalization of the expression
of the genes of interest and expression relative to non-treated
control samples was calculated utilizing the ΔΔCT method: Relative
expression level=2−ΔΔCT, where ΔCT=Ct (gene of
interest)-Ct (housekeeping gene), and ΔΔCT=ΔCT (experimental
group)-ΔCT (control group). Reactions were carried out in a
Stratagene Mx3000P qPCR System (Agilent Technologies Inc.,
Waldbronn, Germany). The CYP2E1 primers used for qPCR were:
forward, 5′-GCCGAATCCCTGCCATCAA-3′ and reverse,
5′-GGTGTCTCGGGTTGCTTCAT-3′. The β-actin primers used for qPCR were:
forward, 5′-CGTGCGTGACATTAAGGAGAA-3′ and reverse,
5′-AGGAAGGAAGGCTGGAAGAG-3′.
Western blotting
Cells were lysed by RIPA buffer and the total
proteins were quantified with a BCA protein assay kit (Beyotime
Institute of Biotechnology). After SDS-PAGE electrophoresis
proteins were transferred to a PVDF membrane (Millipore Corp.,
Billerica, MA, USA), which was blocked in blocking buffer and
incubated with primary antibodies. The primary antibodies applied
included rabbit monoclonal anti-human CYP2E1 (1:500 dilution,
catalog no. ab151544, Abcam Inc., Cambridge, MA, USA) and mouse
monoclonal anti-human β-actin (1:5000 dilution, catalog no. 3700,
Cell Signaling Technology, Boston, MA, USA). The second antibody
was the fluorescent secondary antibodies (Alexa Fluor®
790 goat anti-rabbit IgG, 1:10,000 dilution, Catalog no.
111-655-144; Alexa Fluor 680 goat anti-mouse IgG, 1:10,000
dilution, catalog no. 115-625-146; LI-COR Biosciences, Lincoln, NE,
USA). The membranes were detected and analyzed with an
Odyssey® CLx Infrared Imaging System (LI-COR
Biosciences), and the results were analyzed with ImageJ
software.
Immunohistofluorescence assay
Smmc-7721 cells, QGY-7703 cells and HepG2 cells were
fixed with 2% paraformaldehyde (V:V; Sigma) at 37°C for 30 min, and
blocked with PBS+ solution (PBS supplemented with 1%
BSA) for 30 min at RT. Cells were then incubated at 37°C for 1 h
with rabbit monoclonal anti-human CYP2E1 (1:200 dilution, catalog
no. ab151544, Abcam Inc.). Goat anti-rabbit IgG-FITC was used as
secondary antibodies (1:100 dilution, catalog no. sc-2012, Santa
Cruz Biotechnology Inc., Delaware, CA, USA), and nuclei were
stained with Hoechst 33342 (Invitrogen, Eugene, OR, USA).
Photomicrographs were captured with a Leica DMI 4000B microscope
imaging system (Leica Microsystems, Wetzlar, Germany).
Inhibition of CYP2E1 activity
For inhibiting CYP2E1 activity, cells were
pretreated with a CYP2E1 specific inhibitor, diethyldithiocarbamate
(DDC; Sigma; 0.1 mM, final concentration) (21), at 3 h before indicated
treatment.
siRNA transfection
Smmc-7721 cells were transfected with 100 nM of
siRNA specific for human CYP2E1 siRNA (sc-270348, Santa Cruz
Biotechnology Inc.) or control siRNA (sc-37007, Santa Cruz
Biotechnology Inc.) using the Lipofectamine® 2000
Transfection Reagent (Thermo Fisher Scientific Inc., Rockford, IL,
USA) as per the manufacturer's instructions. At 5 h after
transfection, transfection medium was replaced with fresh medium
containing serum and allowed to grow for 24 h. Whole cell extract
was prepared 24 h after transfection for analysis of silencing
efficiency by western blot using antibody against CYP2E1 (rabbit
monoclonal anti-human CYP2E1, 1:500 dilution, catalog no. ab151544,
Abcam Inc.).
Data analysis
All experiments were carried out at least three
times. Data were expressed as the mean ± SD. The results were
analyzed for statistical significance using ANOVA followed by
Duncan's multiple-range test. Values of p<0.05 were considered
to be statistically significant.
Results
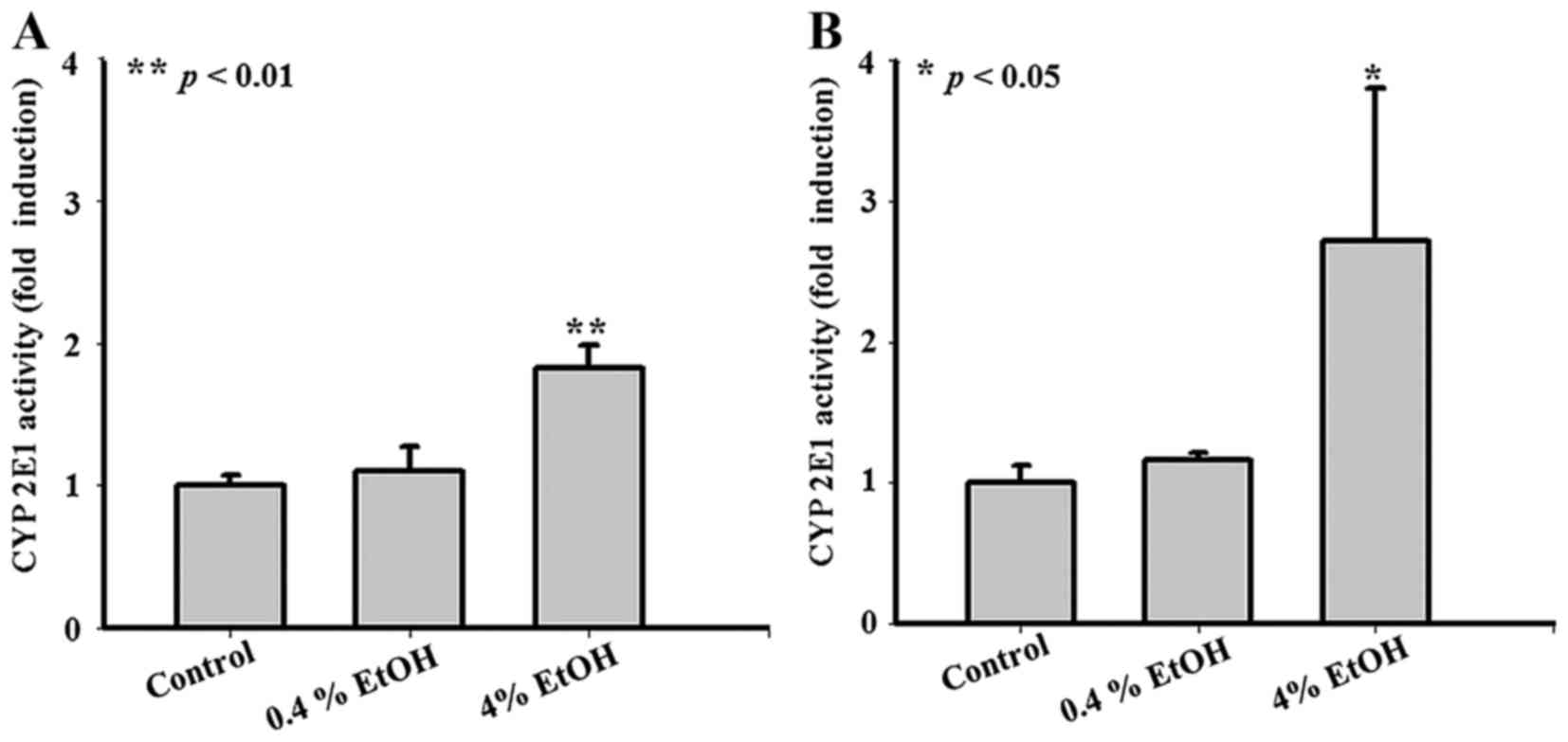
EtOH (V:V) (4%) activates CYP2E1 in
Smmc-7721 cells
Previous findings indicated that CYP2E1 could be
activated by ethanol (EtOH) (22).
As shown in Fig. 1A and B, no
significant changes of CYP2E1 activity were observed in Smmc-7721
cells treated with 0.4% EtOH (V:V) for 24 or 48 h compared with the
untreated group. However, CYP2E1 activity increased to
approximately 1.8-fold in cells treated with 4% EtOH for 24 h
(Fig. 1A) and treated with 4% EtOH
for 48 h CYP2E1 activity increased to approximately 2.7-fold
(Fig. 1B).
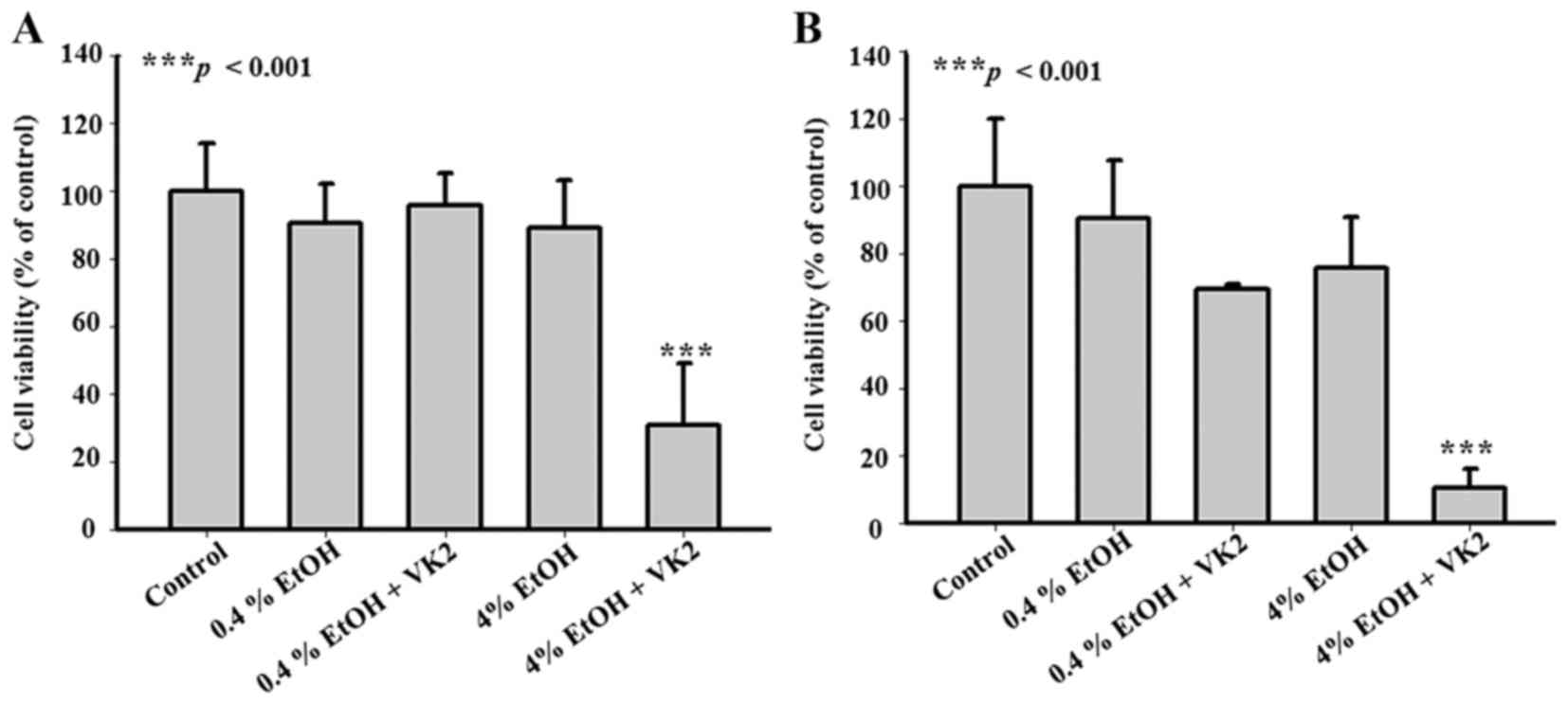
EtOH (4%) enhances the inhibitory effect
of VK2 on cell viability in Smmc-7721 cells
We examined the effects of the combined treatment of
VK2 and EtOH on the viability of Smmc-7721 cells. As shown in
Fig. 2A, compared with the
untreated group, no significant changes of cell viability were
observed in cells treated with 0.4% EtOH, 4% EtOH, or 40 µM
VK2 plus 0.4% EtOH for 24 h. However, cell viability decreased to
around 30% in cells treated with 40 µM VK2 plus 4% EtOH for
24 h (p<0.001) (Fig. 2A). The
same trend was also observed in cells treated for 48 h (Fig. 2B). These results showed that 4%
EtOH could enhance inhibitory effect of VK2 on the cell viability
in Smmc-7721 cells.
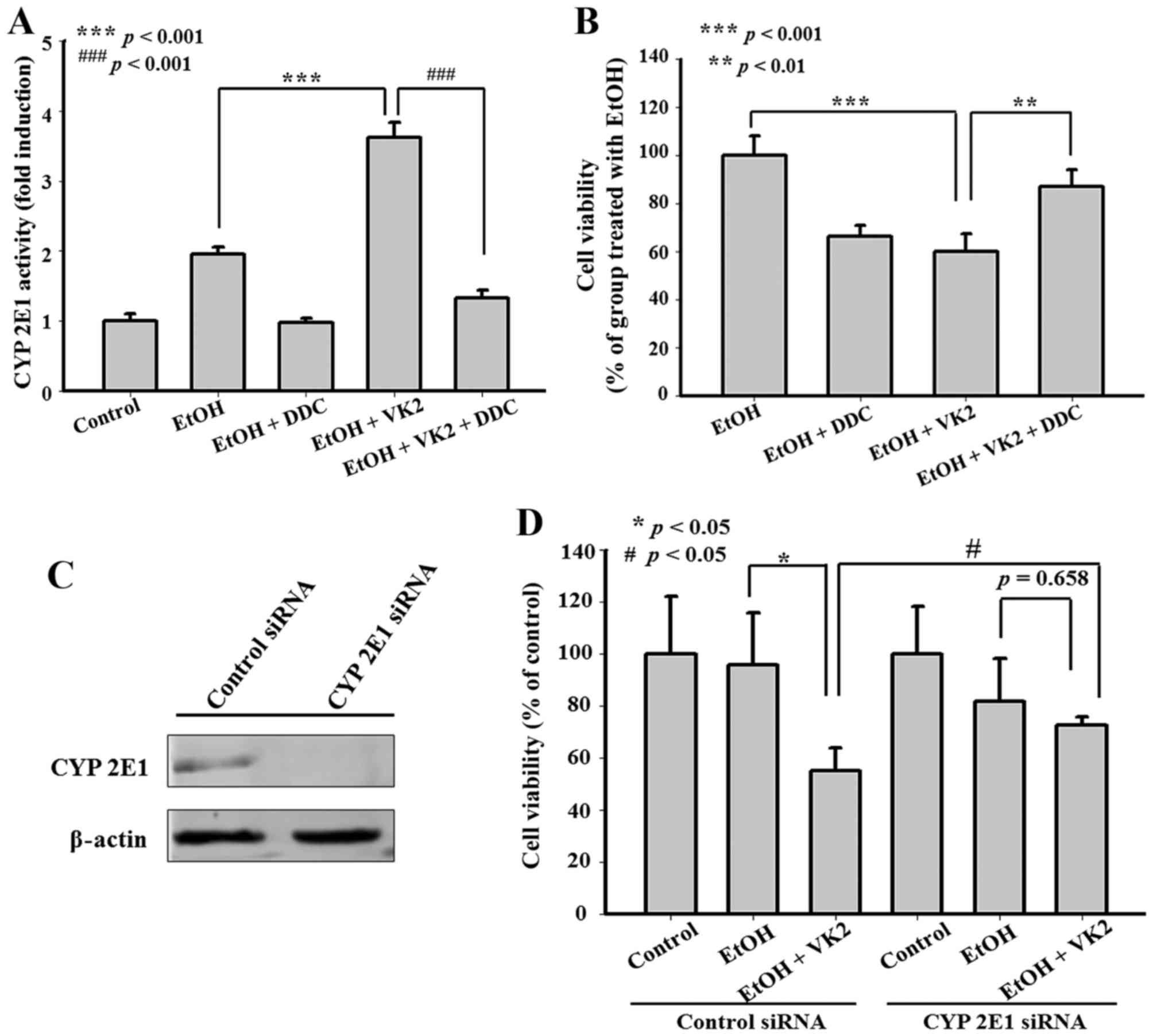
CYP2E1 inhibition attenuates the
synergistic effect of VK2 and EtOH in Smmc-7721 cells
CYP2E1 specific inhibitor and CYP2E1 siRNA were used
to examine the effect of CYP2E1 on the synergistic effect of VK2
and EtOH. As shown in Fig. 3A and
B, 0.1 mM DDC inhibited CYP2E1 activity and attenuated the
synergistic effect of VK2 and EtOH. Moreover, the same attenuated
effect was observed after CYP2E1 silencing by CYP2E1 siRNA
(Fig. 3C and D).
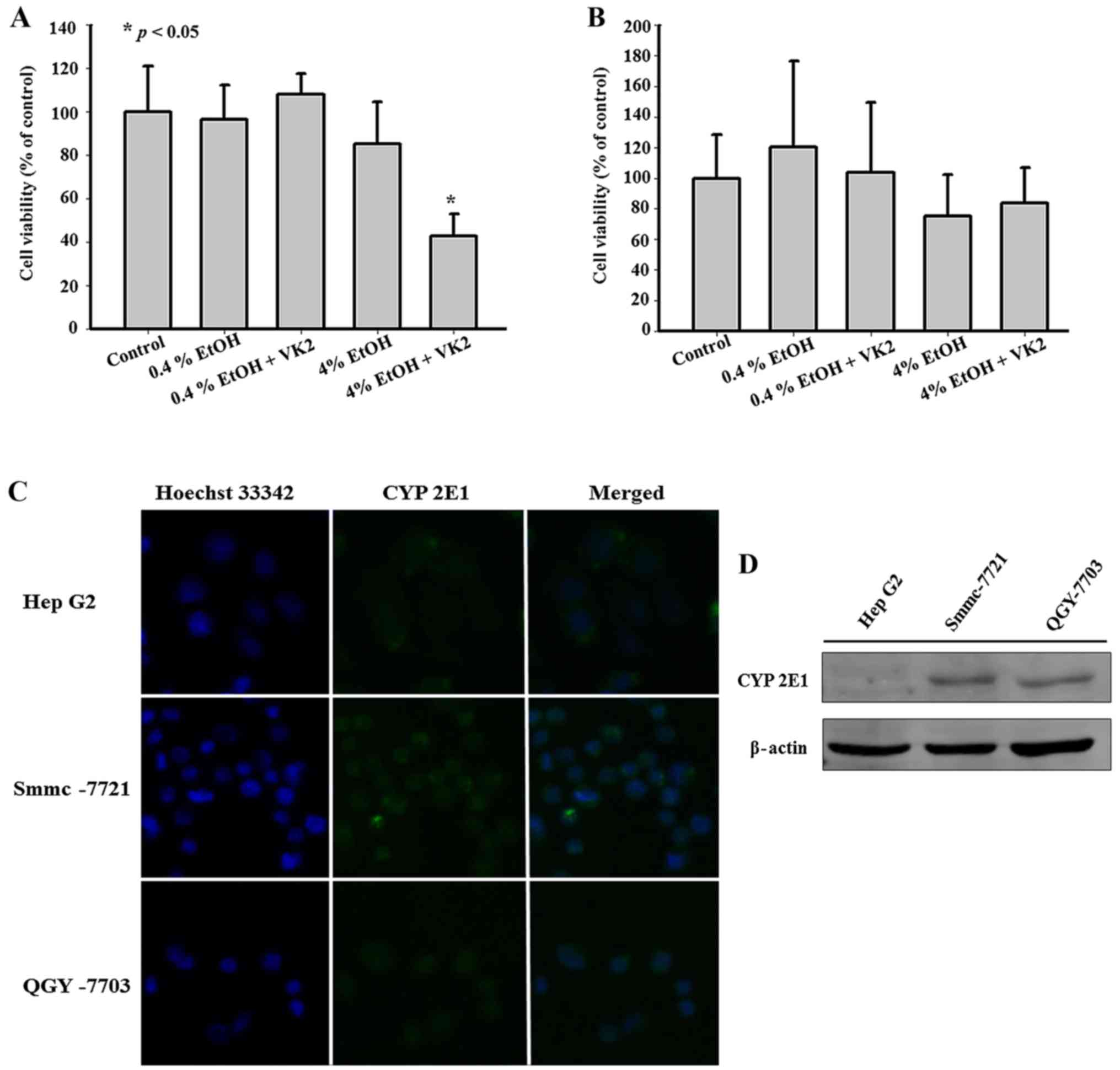
Synergistic effect of VK2 and EtOH was
also observed in QGY-7703 cells, but not in HepG2 cells, which did
not express CYP2E1
In order to identify whether the synergistic effect
of VK2 and EtOH exists in other hepatoma cells, we exposed QGY-7703
cells and HepG2 cells to VK2 plus EtOH. As shown in Fig. 4A, 4% EtOH enhanced the inhibitory effect of
VK2 on the cell viability in QGY-7703 cells treated for 24 h.
However, in HepG2 cells treated for 24 h, 4% EtOH had no
significant effect on the inhibitory effect of VK2 (Fig. 4B). Previous findings reported that
HepG2 cells did not express CYP2E1 (23,24).
Furthermore, exposure of HepG2 cells to EtOH has been shown to have
little effect on the induction of CYP2E1 (24,25).
As shown in Fig. 4C and D, we also
showed that the protein level of CYP2E1 in HepG2 cells was lower
than that in Smmc-7721 cells or QGY-7703 cells.
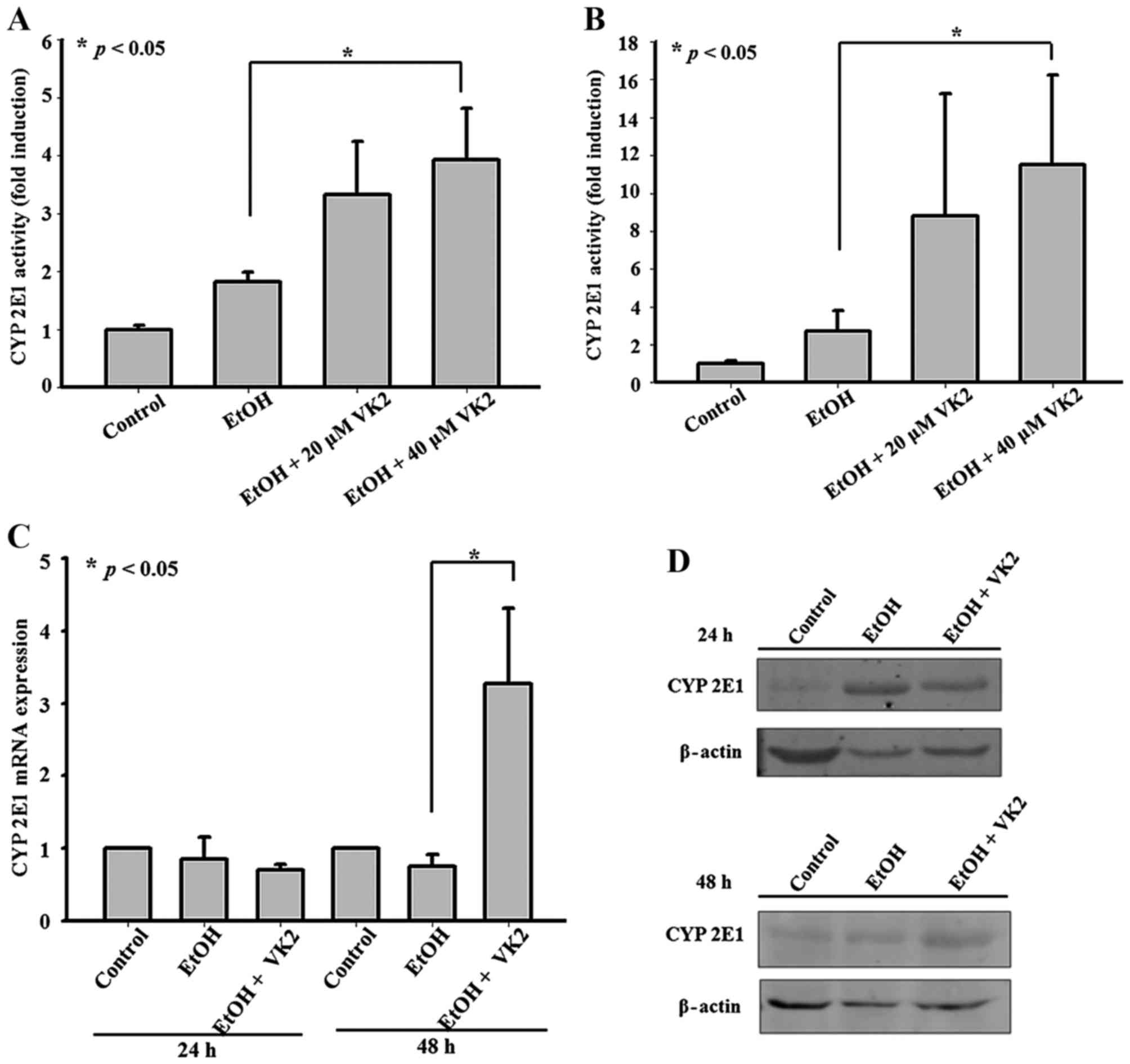
VK2 increases CYP2E1 activity and results
in up regulation of CYP2E1 expression
In order to identify the effect of VK2 on CYP2E1,
CYP2E1 activity and expression were examined in Smmc-7721 cells
treated with different concentrations of VK2. As shown in Fig. 5A and B, 40 µM VK2 increased
CYP2E1 activity in cells treated for 24 h and 48 h. Although no
significant changes of CYP2E1 expression were observed in cells
treated for 24 h, 40 µM VK2 significantly upregulated CYP2E1
expression in cells treated for 48 h (Fig. 5C and D).
Discussion
VK2 is a natural and main form of vitamin K in the
tissue. It has been approved as an anti-osteoporotic medicine by
the Ministry of Health, Labor and Welfare in Japan. Moreover, the
safety of the long-term administration of VK2 has been well
established (26). Although the
exact mechanism has not yet been elucidated in detail, VK2 and
their analogs have been shown to inhibit the survival of various
cancer cell lines (27–29). However, a previous study reported
that hepatoma cells were insensitive to VK2 and, even at higher
concentrations (>100 µM) VK2 could not exhibit
significant inhibitory effect or induce apoptosis in hepatoma cells
(14). As VK2 is a natural, safe
and clinically-utilized agent, we searched for substances that
could enhance the inhibitory effect of VK2 in hepatoma cells. We
found that EtOH, which resulted in an increase of CYP2E1 activity,
could enhance the inhibitory effect of VK2 on the cell viability in
Smmc-7721 cells. CYP2E1 is one of the important hepatic metabolic
enzymes, which is responsible for the catalysis of xenobiotic
(30). Previous studies showed
that CYP2E1 could synergize and increase the susceptibility of
hepatic cells to different chemicals (18,19).
Overexpression of CYP2E1 could enhance sensitivity of hepG2 cells
to fas-mediated cytotoxicity (31). In the present study, we also showed
that CYP2E1 inhibition attenuated the synergetic effect of VK2 and
EtOH in Smmc-7721 cells. These findings indicate that CYP2E1 may be
an attractive target for enhanced sensitivity of hepatoma cells to
VK2.
At present, the effects of CYP2E1 on hepatoma are
limited. Previous studies showed that the expression of CYP2E1 in
tumor cells tended to decrease with the decrease of cell
differentiation degree, and was the lowest in poorly differentiated
HCC (32,33). Ho et al reported that
decreased expression of CYP2E1 was associated with poor prognosis
of hepatocellular carcinoma (34).
In several HCC cell lines the expression of CYP2E1 was absent or
weak (34,35). Furthermore, previous studies showed
that overexpression of CYP2E1 induced or enhanced cytotoxicity to
HepG2 cells, in which the level of CYP2E1 is originally very low
(31,36). In the present study, we also showed
that in those cells with higher levels of CYP2E1, such as Smmc-7721
and QGY-7703 cells, the antitumor effect of VK2 was significantly
enhanced by EtOH. However, in cells with lower levels of CYP2E1,
such as HepG2 cells, EtOH had no influence on antitumor effects of
VK2. These results suggested that induction of CYP2E1 might favor
HCC treatment.
However, previous findings reported that induction
of CYP2E1 caused oxidative stress and resulted in hepatic
cytotoxicity induced by alcohol or other hepatotoxicants (37–39).
Robertson et al reported that in nonalcoholic
steatohepatitis CYP2E1 affected the cell viability of hepatocytes
(40). It is undesirable that
induction of CYP2E1 results in cytotoxicity to hepatocytes. The
effect of CYP2E1 on the hepatic cells is fairly complex.
Schattenberg et al reported that CYP2E1 served both to
protect against and to promote cellular injury in hepatocytes
(18). Overexpression of CYP2E1
sensitized hepatocytes to necrotic death from the polyunsaturated
fatty acid, but surprisingly protected hepatocytes against vitamin
K3 (VK3)-induced apoptotic death (18). Since VK2 and VK3 belong to vitamin
K, this protection of hepatocytes from VK3-induced cell death
inspires that induction of CYP2E1 may protect hepatocytes from
VK2-induced cell death. In fact, our further study showed that VK2
plus 4% EtOH could induce apoptosis in hepatoma cells, but had no
significant effect on hepatocytes (data not shown).
CYP2E1 can be regulated at different levels via
various mechanisms. Many studies reported that CYP2E1 protein
activity were often induced by its own substrates through
post-transcriptional mechanisms (41). Post-transcriptional regulation also
involved CYP2E1 mRNA stabilization (42). Some studies showed that CYP2E1
transcription was influenced by a variety of compounds such as
IL-6, T3 and insulin (42–44). In the present study, our results
showed that VK2-induced initial increase of CYP2E1 activity is not
due to de novo synthesis, suggesting that CYP2E1 could be
induced by VK2 via post-transcriptional regulation. Herein, we also
showed that CYP2E1 could be transcriptional regulated by VK2, but
the molecular mechanism is still poorly understood.
In conclusion, our results suggest that CYP2E1
induction can enhance the inhibitory effect of VK2 on hepatoma
cells. CYP2E1 may be an attractive target for enhanced antitumor
effects of VK2 in HCC. It enlightens us that percutaneous injection
of VK2 plus CYP2E1-inducers by ultrasonic guidance may be an
economic effective treatment for HCC patients without prominent
adverse effects.
Acknowledgments
This study was supported by Anhui Province Natural
Science Foundation Youth Project of China (grant no. 1408085QH171),
Provincial Natural Science Research Project of Anhui Colleges
(grant no. KJ2012Z216), Natural Science Foundation of Anhui
Traditional Chinese Medical University (grant no. 2012qn06 and
2014zr009), and the National Natural Science Foundation of China
(grant no. 81573670).
References
|
1
|
Buonaguro L, Petrizzo A, Tagliamonte M,
Tornesello ML and Buonaguro FM: Challenges in cancer vaccine
development for hepatocellular carcinoma. J Hepatol. 59:897–903.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ikeda K, Arase Y, Saitoh S, Kobayashi M,
Suzuki Y, Suzuki F, Tsubota A, Chayama K, Murashima N and Kumada H:
Interferon beta prevents recurrence of hepatocellular carcinoma
after complete resection or ablation of the primary tumor-A
prospective randomized study of hepatitis C virus-related liver
cancer. Hepatology. 32:228–232. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kubo S, Nishiguchi S, Hirohashi K, Tanaka
H, Shuto T and Kinoshita H: Randomized clinical trial of long-term
outcome after resection of hepatitis C virus-related hepatocellular
carcinoma by postoperative interferon therapy. Br J Surg.
89:418–422. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shiratori Y, Shiina S, Teratani T, Imamura
M, Obi S, Sato S, Koike Y, Yoshida H and Omata M: Interferon
therapy after tumor ablation improves prognosis in patients with
hepato-cellular carcinoma associated with hepatitis C virus. Ann
Intern Med. 138:299–306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabb MM, Sun A, Zhou C, Grün F, Errandi J,
Romero K, Pham H, Inoue S, Mallick S, Lin M, et al: Vitamin K2
regulation of bone homeostasis is mediated by the steroid and
xenobiotic receptor SXR. J Biol Chem. 278:43919–43927. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shiraki M, Shiraki Y, Aoki C and Miura M:
Vitamin K2 (mena-tetrenone) effectively prevents fractures and
sustains lumbar bone mineral density in osteoporosis. J Bone Miner
Res. 15:515–521. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Otsuka M, Kato N, Shao RX, Hoshida Y,
Ijichi H, Koike Y, Taniguchi H, Moriyama M, Shiratori Y, Kawabe T,
et al: Vitamin K2 inhibits the growth and invasiveness of
hepatocellular carcinoma cells via protein kinase A activation.
Hepatology. 40:243–251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Azuma K, Urano T, Ouchi Y and Inoue S:
Vitamin K2 suppresses proliferation and motility of hepatocellular
carcinoma cells by activating steroid and xenobiotic receptor.
Endocr J. 56:843–849. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamamoto T, Nakamura H, Liu W, Cao K,
Yoshikawa S, Enomoto H, Iwata Y, Koh N, Saito M, Imanishi H, et al:
Involvement of hepatoma-derived growth factor in the growth
inhibition of hepatocellular carcinoma cells by vitamin K(2). J
Gastroenterol. 44:228–235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakon M, Monden M, Gotoh M, Kobayashi K,
Kanai T, Umeshita K, Endoh W and Mori T: The effects of vitamin K
on the generation of des-gamma-carboxy prothrombin (PIVKA-II) in
patients with hepatocellular carcinoma. Am J Gastroenterol.
86:339–345. 1991.PubMed/NCBI
|
|
12
|
Koike Y, Shiratori Y, Sato S, Obi S,
Teratani T, Imamura M, Yoshida H, Shiina S and Omata M:
Des-gamma-carboxy prothrombin as a useful predisposing factor for
the development of portal venous invasion in patients with
hepatocellular carcinoma: A prospective analysis of 227 patients.
Cancer. 91:561–569. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshida T, Miyazawa K, Kasuga I, Yokoyama
T, Minemura K, Ustumi K, Aoshima M and Ohyashiki K: Apoptosis
induction of vitamin K2 in lung carcinoma cell lines: The
possibility of vitamin K2 therapy for lung cancer. Int J Oncol.
23:627–632. 2003.PubMed/NCBI
|
|
14
|
Enomoto M, Tsuchida A, Miyazawa K,
Yokoyama T, Kawakita H, Tokita H, Naito M, Itoh M, Ohyashiki K and
Aoki T: Vitamin K2-induced cell growth inhibition via autophagy
formation in cholangiocellular carcinoma cell lines. Int J Mol Med.
20:801–808. 2007.PubMed/NCBI
|
|
15
|
Riaz IB, Riaz H, Riaz T, Rahman S, Amir M,
Badshah MB and Kazi AN: Role of vitamin K2 in preventing the
recurrence of hepatocellular carcinoma after curative treatment: A
meta-analysis of randomized controlled trials. BMC Gastroenterol.
12:170–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida H, Shiratori Y, Kudo M, Shiina S,
Mizuta T, Kojiro M, Yamamoto K, Koike Y, Saito K, Koyanagi N, et
al: Effect of vitamin K2 on the recurrence of hepatocellular
carcinoma. Hepatology. 54:532–540. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Osna NA and Donohue TM Jr:
CYP2E1-catalyzed alcohol metabolism: Role of oxidant generation in
interferon signaling, antigen presentation and autophagy. Subcell
Biochem. 67:177–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schattenberg JM, Wang Y, Rigoli RM, Koop
DR and Czaja MJ: CYP2E1 overexpression alters hepatocyte death from
menadione and fatty acids by activation of ERK1/2 signaling.
Hepatology. 39:444–455. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Lu Y and Cederbaum AI: Induction
of cytochrome P450 2E1 increases hepatotoxicity caused by Fas
agonistic Jo2 antibody in mice. Hepatology. 42:400–410. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martini R, Ingelman-Sundberg M and Murray
M: Pretranslational and post-translational regulation of rat
hepatic CYPs 3A2 and 2E1 by disulfiram. Biochem Pharmacol.
54:1323–1329. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wall KL and Crivello J: Chlorzoxazone
metabolism by winter flounder liver microsomes: Evidence for
existence of a CYP2E1-like isoform in teleosts. Toxicol Appl
Pharmacol. 151:98–104. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bansal S, Anandatheerthavarada HK, Prabu
GK, Milne GL, Martin MV, Guengerich FP and Avadhani NG: Human
cytochrome P450 2E1 mutations that alter mitochondrial targeting
efficiency and susceptibility to ethanol-induced toxicity in
cellular models. J Biol Chem. 288:12627–12644. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Osna NA, Clemens DL and Donohue TM Jr:
Interferon gamma enhances proteasome activity in recombinant Hep G2
cells that express cytochrome P4502E1: Modulation by ethanol.
Biochem Pharmacol. 66:697–710. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu H, Cai P, Clemens DL, Jerrells TR,
Ansari GA and Kaphalia BS: Metabolic basis of ethanol-induced
cytotoxicity in recombinant HepG2 cells: Role of nonoxidative
metabolism. Toxicol Appl Pharmacol. 216:238–247. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilfred de Alwis NM and Day CP: Genetics
of alcoholic liver disease and nonalcoholic fatty liver disease.
Semin Liver Dis. 27:44–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sasaki N, Kusano E, Takahashi H, Ando Y,
Yano K, Tsuda E and Asano Y: Vitamin K2 inhibits
glucocorticoid-induced bone loss partly by preventing the reduction
of osteoprotegerin (OPG). J Bone Miner Metab. 23:41–47. 2005.
View Article : Google Scholar
|
|
27
|
Matsumoto K, Okano J, Nagahara T and
Murawaki Y: Apoptosis of liver cancer cells by vitamin K2 and
enhancement by MEK inhibition. Int J Oncol. 29:1501–1508.
2006.PubMed/NCBI
|
|
28
|
Yaguchi M, Miyazawa K, Katagiri T,
Nishimaki J, Kizaki M, Tohyama K and Toyama K: Vitamin K2 and its
derivatives induce apoptosis in leukemia cells and enhance the
effect of all-trans retinoic acid. Leukemia. 11:779–787. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yokoyama T, Miyazawa K, Naito M, Toyotake
J, Tauchi T, Itoh M, Yuo A, Hayashi Y, Georgescu MM, Kondo Y, et
al: Vitamin K2 induces autophagy and apoptosis simultaneously in
leukemia cells. Autophagy. 4:629–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang CS, Yoo JS, Ishizaki H and Hong JY:
Cytochrome P450IIE1: Roles in nitrosamine metabolism and mechanisms
of regulation. Drug Metab Rev. 22:147–159. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan QG, Shi JG, Zhang F, Zhao QT, Pang XW,
Chen R, Hu PZ, Li QL, Wang Z and Huang GS: Overexpression of CYP2E1
enhances sensitivity of hepG2 cells to fas-mediated cytotoxicity.
Cancer Biol Ther. 7:1280–1287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hirose Y, Naito Z, Kato S, Onda M and
Sugisaki Y: Immuno-histochemical study of CYP2E1 in hepatocellular
carcinoma carcinogenesis: Examination with newly prepared
anti-human CYP2E1 antibody. J Nippon Med Sch. 69:243–251. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Man XB, Tang L, Qiu XH, Yang LQ, Cao HF,
Wu MC and Wang HY: Expression of cytochrome P4502E1 gene in
hepato-cellular carcinoma. World J Gastroenterol. 10:1565–1568.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ho JC, Cheung ST, Leung KL, Ng IO and Fan
ST: Decreased expression of cytochrome P450 2E1 is associated with
poor prognosis of hepatocellular carcinoma. Int J Cancer.
111:494–500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Caro AA and Cederbaum AI: Oxidative
stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol
Toxicol. 44:27–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Q and Cederbaum AI: Cytotoxicity and
apoptosis produced by cytochrome P450 2E1 in Hep G2 cells. Mol
Pharmacol. 53:638–648. 1998.PubMed/NCBI
|
|
37
|
Castillo T, Koop DR, Kamimura S,
Triadafilopoulos G and Tsukamoto H: Role of cytochrome P-450 2E1 in
ethanol-, carbon tetrachloride- and iron-dependent microsomal lipid
peroxidation. Hepatology. 16:992–996. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morimoto M, Zern MA, Hagbjörk AL,
Ingelman-Sundberg M and French SW: Fish oil, alcohol, and liver
pathology: Role of cytochrome P450 2E1. Proc Soc Exp Biol Med.
207:197–205. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nanji AA, Zhao S, Sadrzadeh SM, Dannenberg
AJ, Tahan SR and Waxman DJ: Markedly enhanced cytochrome P450 2E1
induction and lipid peroxidation is associated with severe liver
injury in fish oil-ethanol-fed rats. Alcohol Clin Exp Res.
18:1280–1285. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Robertson G, Leclercq I and Farrell GC:
Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450
enzymes and oxidative stress. Am J Physiol Gastrointest Liver
Physiol. 281:G1135–G1139. 2001.PubMed/NCBI
|
|
41
|
Gonzalez FJ: The 2006 Bernard B. Brodie
Award Lecture. Cyp2e1. Drug Metab Dispos. 35:1–8. 2007. View Article : Google Scholar
|
|
42
|
Woodcroft KJ, Hafner MS and Novak RF:
Insulin signaling in the transcriptional and post-transcriptional
regulation of CYP2E1 expression. Hepatology. 35:263–273. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Johansson I, Lindros KO, Eriksson H and
Ingelman-Sundberg M: Transcriptional control of CYP2E1 in the
perivenous liver region and during starvation. Biochem Biophys Res
Commun. 173:331–338. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lagadic-Gossmann D, Lerche C, Rissel M,
Joannard F, Galisteo M, Guillouzo A and Corcos L: The induction of
the human hepatic CYP2E1 gene by interleukin 4 is transcriptional
and regulated by protein kinase C. Cell Biol Toxicol. 16:221–233.
2000. View Article : Google Scholar : PubMed/NCBI
|