Introduction
The Escherichia coli purine nucleoside
phosphorylase/fludarabine (ePNP/Fludara) suicide system, originally
described by Sorscher et al (1), has been demonstrated to have powerful
killing and bystander effects (2).
Differing from human or mammalian PNP, this bacterial PNP enzyme
converts the low-toxic prodrug Fludara into a very toxic
metabolite, 2-fluoroadenine (F-Ade). F-Ade impairs DNA, RNA and
protein synthesis (3), killing
both dividing and non-dividing cells. However, several drawbacks to
PNP/Fludara suicide system remain to be resolved, including the
side-effects and low efficiency of ePNP gene expression.
Hyperthermia, an inducible antitumor treatment, has
gained acceptance for cancer therapy in breast and colorectal
carcinomas as well as malignant melanomas (4–6).
Recent studies have shown that hyperthermia not only sensitizes
tumor cells to radiation and chemotherapy, but also activates HSP70
expression in some cells. This makes the combination of
hyperthermia and gene therapy possible. Furthermore, hyperthermia
can augment the effects of therapeutic genes in a controlled range
(7). Hyperthermia-induced HSP70
activation is regulated at the transcriptional level (8) and depends on heat shock elements
(HSEs), which are short sequences in the HSP70 promoter that are
essential for heat inducibility. The introduction of HSEs into a
gene transfer vector makes it possible to provide special control
over exogenous gene expression in a locally heated tumor (8).
The human telomerase reverse transcriptase (hTERT)
promoter has been widely used to drive the specific expression of
therapeutic genes for cancer treatment. The hTERT promoter is
significantly weaker than many commonly used viral promoters, such
as the cytomegalovirus (CMV) early promoter and the simian virus 40
(SV40) early promoter (9). This
limitation caused us to hypothesize that the combination of heat
shock elements (HSEs) with the hTERT promoter in a recombinant
lentiviral vector may significantly increase the transcriptional
activity of the hTERT promoter, improving the efficiency of ePNP
gene expression in a locally heated tumor.
Therefore, we designed and constructed a recombinant
lentiviral vector carrying the ePNP gene under the control of the
8HSEs-hTERT promoter, which ensured targeted and powerful gene
expression in tumor cells. We expect that administration of this
recombinant lentiviral vector together with the prodrug Fludara
could provide a new strategy for clinical therapy of solid tumors
together with hyperthermia.
Materials and methods
Reagents
Fludarabine phosphate (Fludara) was obtained from
Sigma-Aldrich (St. Louis, MO, USA) and dissolved in
phosphate-buffered saline (PBS). Rabbit monoclonal antibodies
specific for Bax, Bcl-2, caspase-3, p53, Fas, cyclin D1 and β-actin
were obtained from Epitomics (Burlingame, CA, USA); rabbit
monoclonal antibodies specific for 3FALG were obtained from
Sigma-Aldrich. TurboFect™ transfection reagent was acquired from
Thermo Fisher Scientific (Waltham, MA, USA), and Dual-Glo
Luciferase assay system from Promega (Madison, WI, USA).
Cell culture and in vitro
hyperthermia
Human colorectal cancer SW480 and gastric cancer
MKN74 cells were obtained from Shanghai Institute of Cell Biology,
Chinese Academy of Sciences (Shanghai, China). SW480 and MKN74
cells were cultured in RPMI-1640 supplemented with 10% fetal bovine
serum (FBS), 100 IU/ml penicillin, 100 mg/ml streptomycin and 2 mM
L-glutamine. Cells were grown at 37°C in a humidified atmosphere
containing 5% CO2. Cells were seeded into cell culture
dishes and incubated at 37°C for 24 h. Afterwards, cells were
transferred to a cell culture incubator that was pre-adjusted to
43°C for 1 h every 48 h (data not shown) (10). After incubation for the desired
time, cells were transferred back to the 37°C incubator and
incubated for several hours to recover from the heat treatment.
Construction of plasmid vectors
Preparation of plasmids was accomplished using the
Omega plasmid midi kit (Omega, Norcross, GA, USA). The 295-bp
promoter region (11) of human
TERT [GenBank accession no. AN097365] was amplified by PCR from
human genomic DNA, using the following primer pairs: TERT promoter
(forward, 5′-CTAGCTAGCCACAGACGCCCAGGACCGCGCTTC-3′;
reverse, 5′-CCC AAGCTTCCACGTGCGCCCACGTGCGCCCAC-3′.
The 295-bp fragment was inserted into pGL4.2 (Promega) upstream of
the luciferase reporter gene and verified by DNA sequencing to
generate pGL4.2-hTERTp. Eight heat shock elements (8HSEs) with
optimized AGAACGTTCTAGAAC sequences (10,12)
alternately separated by 5 bp were generated by oligonucleotide
ligation. The 8HSEs fragments were inserted upstream of the
luciferase reporter in pGL4.2-hTERTp to generate
pGL4.2-8HSEs-hTERTp.
The 588-bp CMV early promoter was amplified by PCR
from pLVX-EGFP-3FLAG (GeneChem, Shanghai, China), using the
following primer pairs: forward, 5′-CGCCTCGAGCCGCCTGGCTGACCGCCCA-3′;
reverse, 5′-GCCAGATCTGCCATGGTTCGAATTCAAAT-3′.
The amplified 588-bp fragment was cloned into pGL-4.2 to generate
pGL4.2-CMVp.
Dual luciferase assay
SW480 and MKN74 cells were seeded 5,000 cells per
well into each well of a 96-well plate. The next day, cells were
transfected with 150 ng luciferase reporter plasmid
(pGL4.2-8HSEs-hTERTp or pGL4.2-CMVp) and 30 ng of pGL-4.74
(containing the TK promoter; Promega) as an internal control using
the TurboFect™ Transfection reagent (Thermo Fisher Scientific)
according to the manufacturer's instructions. After 10-h
transfection, the mixture was replaced with fresh medium. After 48
h, cells were subjected to heat treatments. Luciferase assays were
performed 6 h later using the Dual-Glo Luciferase assay system
(Promega). Briefly, 100 μl of Dual-Glo Luciferase assay
reagent was added to each well, followed by the addition of 100
μl Dual-Glo Stop & Glo reagent. Renilla
luminescence was normalized to the internal control vector pGL-4.74
luminescence. Promoter activities were measured as relative
luminescence units (RLU) where the value of the firefly luciferase
luminescence of pGL-4.2 was divided by the Renilla
luciferase pGL-4.74 from the same well.
Establishment of stable
lentivirus-transfected cell lines
Two recombinant lentiviruses
pLVX-8HSEs-hTERTp-ePNP-3FLAG (8HhP) and a negative control
pLVX-Ubi-3FLAG (CON) containing the ubiquitin promoter were
purchased from GeneChem. The recombinant lentiviral vector
(pLVX-8HSEs-hTERTp-ePNP-3FLAG) including the hTERT promoter
modified by the artificial 8HSEs, the ePNP gene and three
consecutive FLAG sequences was constructed using conventional
recombinant techniques. SW480 and MKN74 cells were transduced with
the 8HhP and CON lentiviruses following standard techniques. After
72 h, lentivirus-carrying clones were selected for 15 days in
medium containing 1 mg/ml puromycin (Life Technologies, Grand
Island, NY, USA).
Quantitative reverse transcription
polymerase chain reaction (qRT-PCR)
SW480, MKN74 and the stably transfected SW480-8HhP,
SW480-CON, MKN74-8HhP and MKN74-CON cells were subjected to an RNA
Fast 200 kit (Pioneer Biotech, Xi'an, China) to isolate total RNA
according to the manufacturer's instructions. cDNA was synthesized
from 1 μg of DNase I-treated total RNAs with PrimeScript™ RT
Master Mix (Takara, Dalian, China) according to the manufacturer's
instructions. An aliquot (1 μl) of the cDNA was then
subjected to qRT-PCR analysis of ePNP mRNA using SYBR®
Premix Ex Taq™ II (Takara). The primers were as follows: ePNP
(product: 64 bp) forward, 5′-TGGGTCACGGTATGGGTATC-3′; reverse,
5′-CCGAAATCGGTGATCAGTTC-3′. β-actin (product: 151 bp) forward,
5′-CTTAGCACCCCTGGCCAAG-3′; reverse, 5′-GATGTTCTGGAGAGCCCCG-3′. The
ePNP and β-actin qPCR reaction mixture was denatured at 94°C for 3
min followed by 40 cycles of 94°C for 30 sec, 57°C for 30 sec, and
72°C for 30 sec. All reactions were performed in triplicate and the
relative ePNP mRNA level in each cell line was calculated by the
2−ΔΔCt method, where ACt = Ct (ePNP) - Ct (β-actin).
Protein extraction and western blots
Cells treated with or without Fludara were lysed on
ice with NP-40 buffer containing 40 mM Tris-HCl (pH 6.9), 2 mM
ethylenediaminetetraacetic acid (EDTA, pH 8.0), 100 mM sodium
fluoride, 150 mM NaCl, 10 mM sodium pyrophosphate, 1% Tergitol type
NP-40, 2 mM sodium orthovanadate, 1% Triton X-100, 1.0 mM
phenylmethanesulfonyl fluoride (PMSF), and 1X protease inhibitor
mini-tablet (Roche, Basel, Switzerland). Lysates were then
clarified by centrifugation at 12,000 × g for 10 min at 4°C.
Protein concentration was estimated using the Pierce Protein
Estimation system (Thermo Fisher Scientific) according to the
manufacturer's protocol. Next, equal amounts (30 μg) of
protein were heated at 95°C for 5 min in 5X Laemmli sample buffer,
separated on a 10% SDS-PAGE gel and transferred onto polyvinylidene
difluoride (PVDF) membranes by the wet transfer method.
Non-specific binding was blocked for 1 h at 37°C using 10% fat-free
milk in TBS containing 0.1% Tween-20. The membranes were blotted
with anti-Bax, Bcl-2, caspase-3, p53, Fas, cyclin D1, β-actin or
anti-3FALG primary antibodies at a dilution of 1:2,000. After
washing three times with TBST, horseradish peroxidase-conjugated
secondary antibodies (1:5,000 dilution; Proteintech, Chicago, IL,
USA) were added at 27°C for 1 h. Immunoreactive bands were
visualized using SuperSignal West Femto Maximum Sensitivity
Substrate (Thermo Fisher Scientific) and exposed using the ChemiDoc
XRS+ (Bio-Rad, Hercules, CA, USA).
Cell viability assay
Cell viability was detected using the cell counting
kit-8 (CCK-8, Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). Briefly, 2,000 parental and stably transduced cells were
inoculated into 96-well plates in a final volume of 200 μl
growth medium. The hyperthermia group was treated at 43°C after 24
h. After treatment and incubation, each plate was subjected to the
CCK-8 assay by adding 10 μl of CCK-8 solution to each well,
and the plate was further incubated for 2 h at 37°C. The absorbance
at 450 nm was measured with an EnSpire™ Multilabel Reader 2300
(Perkin-Elmer Inc., Waltham, MA, USA). The relative cellular
survival rate was calculated using the following formula:
(Asample − Ablank)/(Acontrol −
Ablank) × 100% (13).
Flow cytometric apoptosis and cell cycle
distribution assays
SW480, SW480-8HhP, SW480-CON, MKN74, MKN74-8HhP and
MKN74-CON cells were seeded into 6-well plates and the hyperthermia
group was treated at 43°C for 1 h every 48 h. For apoptotic
analysis, apoptosis detection kit (Becton-Dickinson, Franklin
Lakes, NJ, USA) was used to analyze apoptosis rates according to
the manufacturer's instructions. For cell cycle analysis, cells
growing in logarithmic phase were fixed with 75% ethanol for 24 h
at −20°C, incubated with RNase A and Triton X-100 at 37°C for 30
min, and then incubated with propidium iodide at room temperature
for 30 min. Cells were examined by flow cytometry, and CellQuest
software (Becton-Dickinson) was used to conduct data acquisition
and analysis.
Colony formation assay
SW480, SW480-8HhP, SW480-CON, MKN74, MKN74-8HhP and
MKN74-CON cells (200 cells/well) were suspended in 2 ml media and
seeded into 6-well plates. The hyperthermia group was also treated
at 43°C for 1 h every 48 h, after treatment with or without
Fludara, cells were fixed and stained with 1% crystal violet and
cell colonies were counted under an inverted microscope.
Statistical comparison was conducted using Student's t-test.
In vivo gene expression and analysis of
antitumor effects
BALB/c nude mice were used according to the
guidelines for administration to lab animals, issued by the
Ministry of Science and Technology (Beijing, China). Tumors were
established by subcutaneous inoculation with SW480 and MKN74 cells
(100 μl containing 1×107 cells). Tumor volume was
calculated using the empirical formula V = 0.52 × [(shortest
diameter)2 × (longest diameter)]. When the tumor volume
reached 100 mm3, tumor-bearing mice were randomly
divided into six groups of 6 mice each: pLVX-Ubi-3FALG+PBS
(SW480-CON+PBS), pLVX-Ubi-3FALG+Fludara+37°C
(SW480-CoN+Fludara+37°C), pLVX-Ubi-3FALG+Fludara +43°C
(SW480-CON+Fludara+43°C), pLVX-8HSEs-hTERTp-ePNP-3FALG+PBS
(SW480-8HhP+PBS), pLVX-8HS Es-hTERTp-ePNP-3FALG+Fludara+37°C
(SW480-8HhP+Fludara+37°C) and pLVX-8HSEs-hTERTp-ePNP-3FALG+
Fludara+43°C (SW480-8HhP+Fludara+43°C). The mice in each group were
intratumorally administered 100 μl serum-free medium
containing 1×108 pfu pLVX-Ubi-3FALG or
pLVX-8HSEs-hTERTp-ePNP-3FALG. Three days later, lentivirus
administration was repeated. Fludara (10 mg/kg) dissolved in 0.5 ml
PBS was injected intraperitoneally three times daily for three
consecutive days, beginning 48 h after the administration of
recombinant lentivirus. This schedule was counted as a single
course and three consecutive courses were administered (14). On the 11th and 13th day
post-inoculation, mice were placed in a 43°C water bath for 1 h.
The feet of the mice were tied to four nails on a board to ensure
the transplanted tumors were immersed in the water bath (10). Tumor size and growth were monitored
and measured using a caliper at regular intervals. Mice were
sacrificed two weeks after the second virus injection and xenograft
tumors were harvested, measured and photographed. Six mice in each
group were sacrificed and the tumor specimens were subjected to
histopathological analysis and TUNEL staining.
TUNEL assay
Paraffin-embedded tissue slides were prepared from
the xenograft tumors. TUNEL staining was detected by the DeadEnd™
Fluorometric TUNEL system (Promega) according to the manufacturer's
instructions. Cells were fixed and permeabilized with PBS
containing 4% paraformaldehyde and 0.25% Triton X-100. Fluorescence
was measured after incubation with FITC-labeled TUNEL. After TUNEL
staining, the specimens were immersed with a DAPI solution
(Sigma-Aldrich) to stain nuclei. Fluorescence staining was viewed
by laser scanning confocal microscopy (FV300, Olympus, Tokyo,
Japan) (15).
Statistical analysis
Results are shown as means ± standard error.
Differences were evaluated with unpaired two-tailed Student's
t-tests with unequal variance for multiple comparisons using the
SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA). P<0.05
was considered statistically significant. All experiments were
independently repeated at least three times.
Results
Hyperthermia inducibility of the
synthetic 8HSEs-hTERT promoter
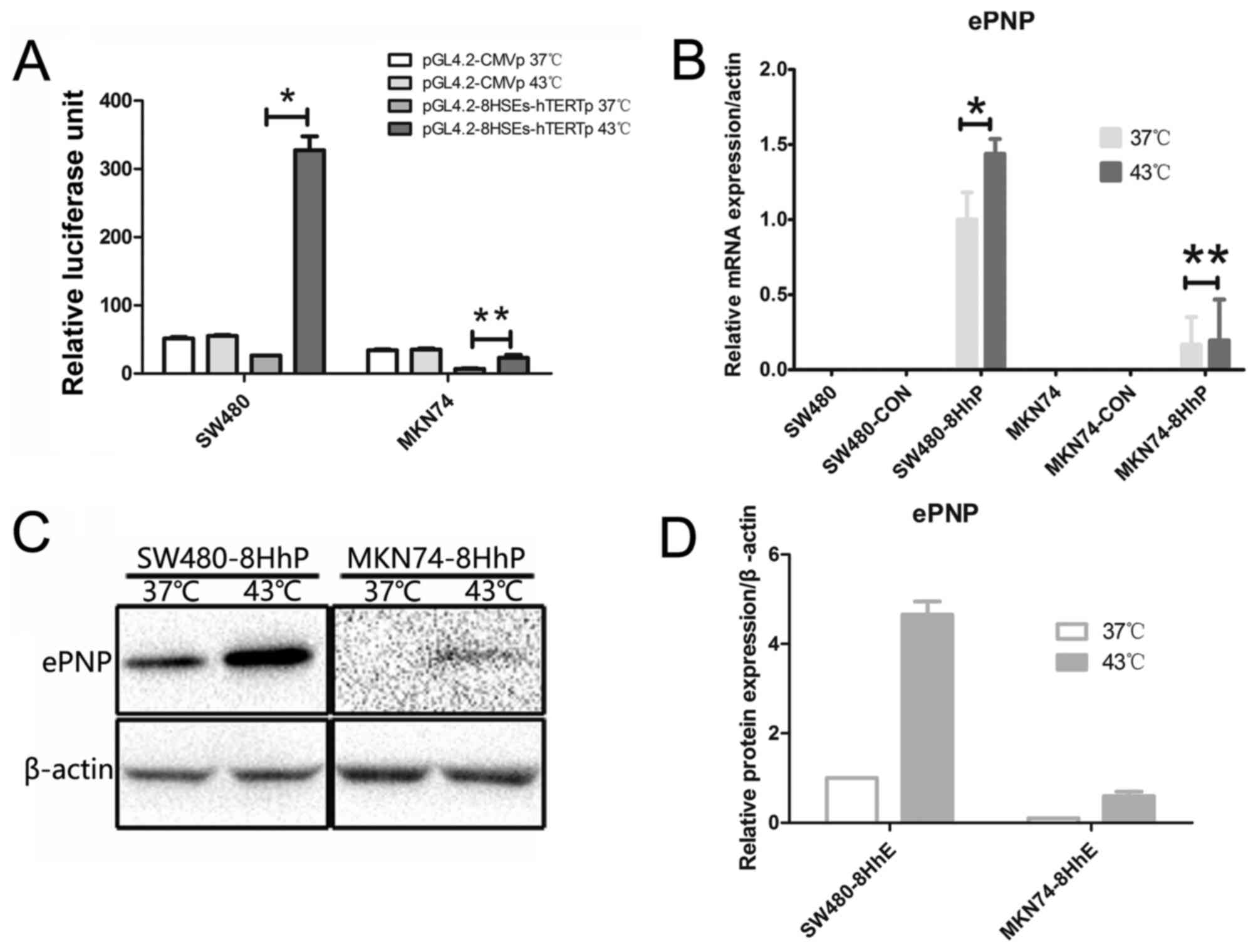
To determine the hyperthermia inducibility of the
synthetic 8HSEs-hTERT promoter after heat treatment, we transfected
SW480 and MKN74 with pGL4.2-8HSEs-hTERTp or pGL4.2-CMVp.
Transfected cells were incubated at either 43°C or 37°C for 1 h and
the resultant luciferase activity was measured. Treatment at 43°C
for 1 h significantly increased the luciferase activity of SW480
but not MKN74 cells (P<0.05 and P=0.219, respectively, Fig. 1A). Contrastingly, 43°C treatment
resulted in no apparent differences in pGL4.2-CMVp luciferase
activity among these cell lines. Moreover, after the 43°C
treatment, the 8HSEs-hTERT promoter luciferase activity was
significantly higher than the CMV promoter. These results
demonstrated that hyperthermia could significantly enhance the
transcriptional activity of the 8HSEs-hTERT promoter in SW480
cells, which endogenously express high hTERT levels. These data
suggested that the 8HSEs-hTERTp promoter might be more efficient
and specific than the CMV promoter for tumor targeting with
hyperthermia.
Overexpression of ePNP in tumor cells
using an 8HSEs-hTERTp-driven expression vector
We tested ePNP expression in hTERT-high expressing
SW480 cells and hTERT-negative MKN74 cells (10) using an 8HSEs-hTERTp-driven
expression vector at 37°C or 43°C. ePNP expression was confirmed by
qRT-PCR and western blotting (Fig. 1B
and C) in SW480-8HhP and MKN74-8HhP cells. SW480-8HhP cells
showed very high levels of ePNP mRNA at 43°C or 37°C, but
MKN74-8HhP only showed low levels of ePNP mRNA at 43°C or 37°C.
Similarly, ePNP protein levels were significantly increased in
SW480-8HhP cells under heat (Fig.
1D). However, ePNP protein levels were very low at 43°C in
MKN74-8HhP cells, and almost absent at 37°C (Fig. 1C and D). As a heterologous gene,
ePNP protein and mRNA were negative in SW480, MKN74, SW480-CON and
MKN74-CON cells at 43°C or 37°C. These data indicated that both
ePNP mRNA and protein were expressed in hTERT-high expressing
SW480-8HhP cells, especially following heat application.
Sensitivity of infected cells to
Fludara
To test whether ePNP could sensitize cancer cells to
Fludara, we tested the proliferation of the different cell lines
using the CCK-8 method to calculate IC50 values for each
line, which was calculated as the drug concentration that inhibited
growth by 50%. When incubated with different concentrations of
Fludara (ranging from 0 to 2 μg/ml), parental, CON- and
8HhP-infected cells were resistant at 37°C. In contrast, SW480-8HhP
cells were susceptible to Fludara at 43°C
(IC50=0.02924), lower than SW480-8HhP cells at 37°C
(IC50=0.07618), SW480 and SW480-CON at 37°C or 43°C
(Fig. 2A). Conversely,
hTERT-negative MKN74 cells showed a higher IC50 at 37°C
or 43°C (Fig. 2A). At a
concentration of 0.05 μg/ml Fludara, the sensitivity of the
infected SW480 cells was time-dependent (Fig. 2B). The data showed that
hyperthermia significantly reduced SW480-8HhP cell viability
compared with either SW480 or SW480-CON cells, under both normal
and heated conditions. However, this result was not replicated in
MKN74-8HhP cells (Fig. 2B),
because MKN74 cells do not express hTERT, and thus, the lentiviral
vector did not induce ePNP expression in these cells (Fig. 2B). These results indicated that
ePNP sensitized cancer cells to Fludara only in hTERT-high
expressing SW480-8HhP cells, especially under heated
conditions.
High level of bystander effect to
Fludara
In the absence of ePNP-positive cells, the growth of
parental cells was not affected even when the concentration of
Fludara was 0.1 μg/ml. At a concentration of 0.05
μg/ml Fludara, a significant bystander effect could be
detected, even at a very low proportion (5%, Fig. 2C) of ePNP-positive SW480-8HhP cells
at 43°C. We detected a weaker bystander effect at the same
proportion of ePNP-positive SW480-8HhP cells at 37°C. Furthermore,
no obvious bystander effect was detected in MKN74-CON and
MKN74-8HhP cells at 43°C or 37°C. These results indicated that a
significant bystander effect was found in SW480-8HhP cells under
heated conditions (Fig. 2C).
Effects of Fludara on SW480
apoptosis
To further elaborate the causes of cell growth
inhibition, we detected apoptosis rates by flow cytometry. The
results showed that 0.05 μg/ml Fludara treatment increased
the rate of spontaneous apoptosis in the infected cells compared
with control cells (Fig. 2D) in
hTERT-high expressing SW480 cells, but not in MKN74 cells. The
greatest increase in the number of apoptotic cells was in
SW480-8HhP, and the ratio was 48.9% at 43°C and 42.1% at 37°C.
These results indicated that the suicide system ePNP/Fludara
inhibited cell proliferation by causing apoptosis, and the
8HSEs-hTERT promoter could enhance the effect only in hTERT-high
expressing cells under heated conditions.
Effects of Fludara on SW480 cell cycle
profiles
To explore the mechanism whereby the suicide system
ePNP/Fludara inhibited cell proliferation, we studied the effect on
the cell cycle. The targeted cells were treated with 0.05
μg/ml Fludara at 43°C or 37°C, and cellular DNA content was
measured by flow cytometry. The data obtained from these studies
demonstrated that ePNP/Fludara induces a G2 cell cycle
arrest only in SW480-8HhP cells (Fig.
3A). At 72 h, flow cytometric analysis of SW480-8HhP cells
showed an ~2-fold increase in the percentage of cells in
G2-M phase (22.89%) compared with cells infected with
CON vector (12.32%; Fig. 3A) under
normal conditions. The addition of hyperthermia produced an
additional increase in G2-M cells to 24.5% in SW480-8HhP
cells. Conversely, few differences were observed between MKN74-CON
and MKN74-8HhP cells at 43°C or 37°C. These results indicated that
the suicide system ePNP/Fludara inhibited cell proliferation by
causing a G2 cell cycle arrest, and the 8HSEs-hTERT
promoter could enhance the effect only in hTERT-high expressing
cells under heated conditions.
Effects of Fludara on SW480 colony
formation
We next evaluated the colony formation ability of
SW480 cells expressing ePNP. After exposure to 37°C or 43°C
treatments, SW480 cells were treated with Fludara. The Fludara plus
43°C treatment significantly reduced SW480-8HhP colony formation
(P<0.05, Fig. 3B). However,
43°C treatment exhibited no effect in all other cells when combined
with either PBS or control vector. The 43°C treatment caused a
significant colony formation decrease only in SW480-8HhP cells
after exposure to Fludara when compared with PBS, control vector,
37°C or MKN74 cells (data not shown). These results indicated that
the suicide system ePNP/Fludara inhibited colony formation, and the
8HSEs-hTERT promoter could enhance the effect only in hTERT-high
expressing cells under heated conditions, demonstrating cancer- and
heat-specificity.
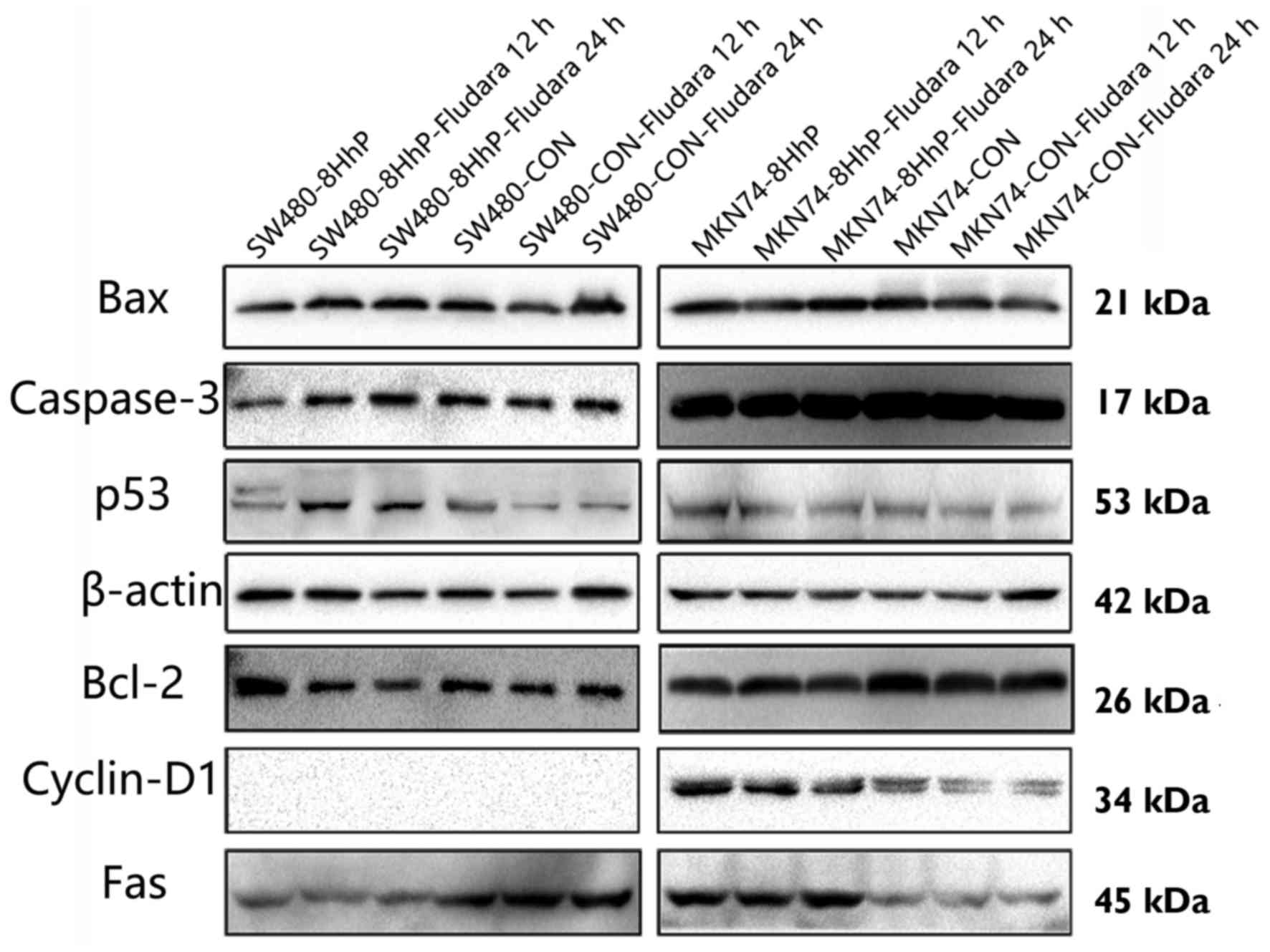
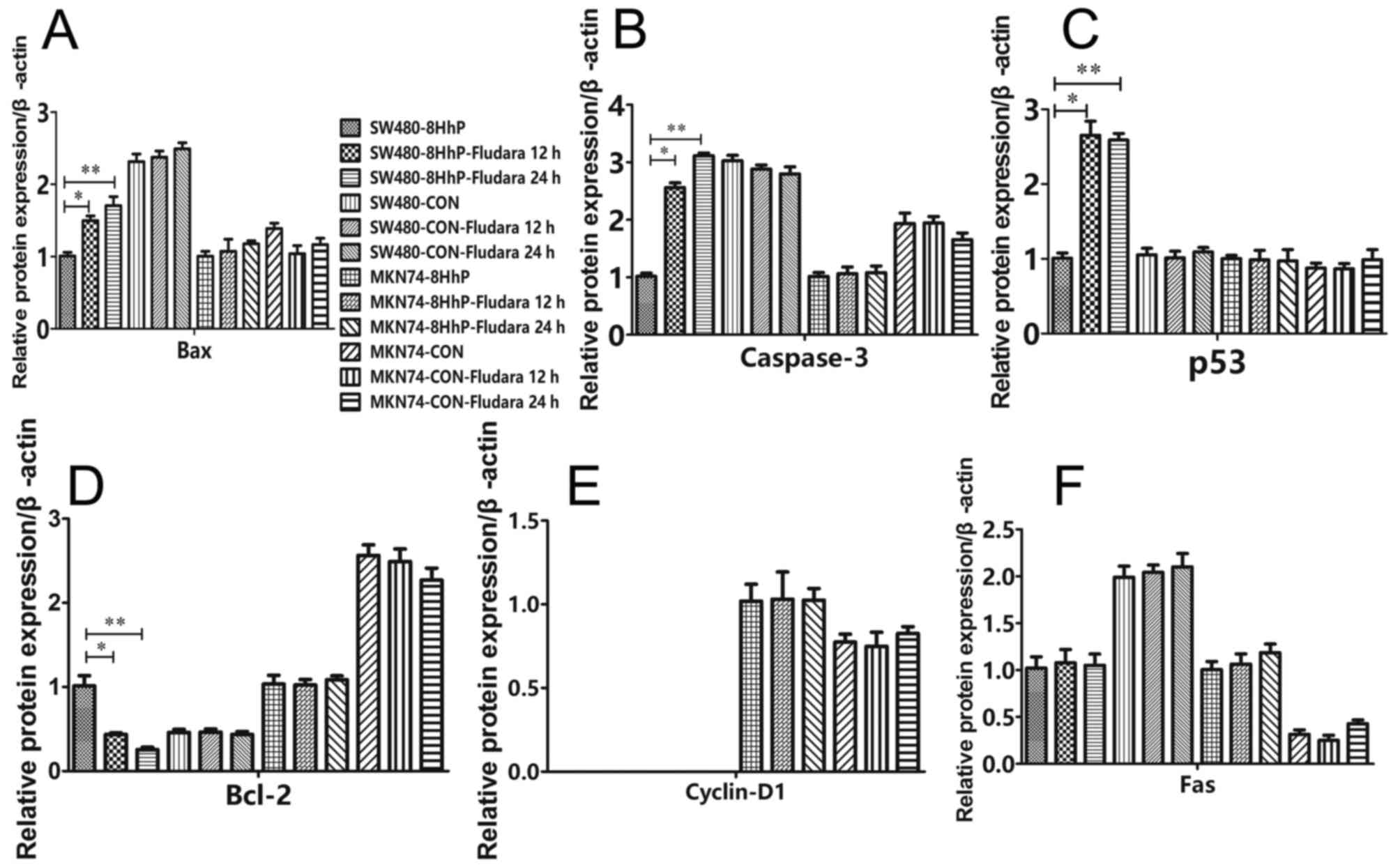
Effects of in vitro ePNP/Fludara on Bax,
caspase-3, p53 and cyclin D1 expression
To investigate the molecular events associated with
ePNP/Fludara-mediated cell cycle arrest and apoptosis, we assessed
whether recombinant 8HhP-regulated ePNP/Fludara could affect the
expression of Bax, Bcl-2, caspase-3, p53, Fas and cyclin D1 under
heated conditions in vitro. A significantly variable
upregulation of the apoptotic proteins Bax, caspase-3 and p53, and
downregulation of the anti-apoptotic protein Bcl-2 were achieved
when ePNP/Fludara was included in SW480 cells (Figs. 4 and 5, *P<0.05, P<0.05). Conversely,
significant changes in expression by ePNP/Fludara did not occur in
the hTERT-negative MKN74 cells. Only Cyclin D1 protein was not
detected in SW480, SW480-CoN and SW480-8HhP cells. These results
indicated that changes in the expression of apoptosis-regulating
proteins was occurring in the ePNP/Fludara treated cells.
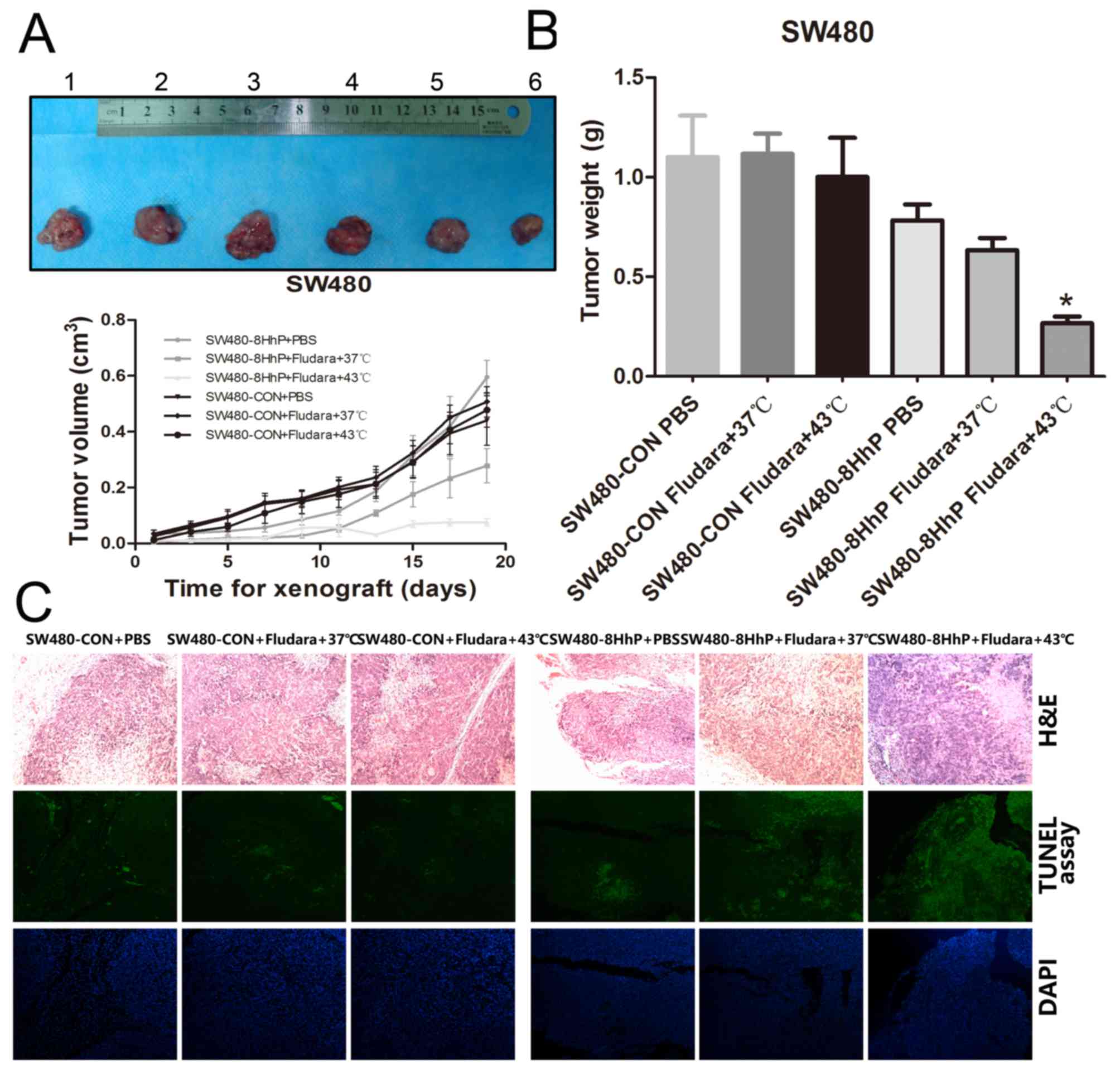
Effects of lentiviral-mediated gene
therapy on a mouse xenograft model
SW480 and MKN74 cells were subcutaneously implanted
in BALB/c nude mice. Mice were monitored every two days for tumor
growth and after 8 days, xenograft tumors formed and grew to 50–100
mm3. Then, we intratumorally injected lentiviruses
(1×108 pfu) in 100 μl of serum-free medium at
three sites per xenograft tumor and repeated the injections on day
4. Two weeks after the second virus injection, mice were sacrificed
and xenograft tumors were collected, measured and photographed.
Compared with PBS at 37°C and the control lentivirus groups, the
size and weight of the tumors were significantly lower in the
SW480-8HhP+Fludara+43°C group (P<0.05; Fig. 6B). Conversely, this it did not
occur in MKN74 cells; the size and weight of tumors showed no
significant differences in any of the MKN74 groups (Fig. 7B). TUNEL assay data also showed
that the suicide system ePNP/Fludara regulated by the recombinant
8HhP lentivirus induced more apoptosis in SW480 xenograft tumors
(Fig. 6C). No obvious differences
in apoptosis were found in the MKN74 xenograft tumors (Fig. 7C). These results demonstrated that
ePNP/Fludara induced apoptosis and the recombinant lentivirus 8HhP
had tumor- and heat-specificity in vivo.
Discussion
In this study, we explored rational strategies for
combining an ePNP/Fludara system controlled by a novel chimeric
promoter with hyperthermia. We cloned the suicide gene ePNP into a
recombinant lentiviral vector to express the ePNP gene with high
efficiency and enhanced target specificity in tumor cells. When the
prodrug Fludara was added, massive cytotoxicity was induced. Both
in vitro and in vivo experiments confirmed the
efficiency and specificity of this system.
We found several advantages to the 8HSE-hTERT
promoter system for cancer therapy. First, the combination of
ePNP/Fludara gene therapy with hyperthermia presents possible
therapeutic advantages in the treatment of solid malignancies.
Second, combining gene therapy and hyperthermia has the potential
to overcome many of the limitations of hyperthermia alone. Third,
8HSEs elements can enhance the activity of hTERT promoter under
heated conditions but do not dampen its specificity to cancer
cells. HSEs have been demonstrated to be necessary for the heat
inducibility of the HSP70B promoter and have been widely used in
gene therapy as an inducible tumor-specific promoter (16). The precise mechanism of this
response and the transcription factors that interact with HSE
elements have yet to be elucidated. However, it is known that
complexes of transcription factors and accessory proteins,
including heat-shock inducible transcription factor (heat shock
factor 1, HSF1), promote gene expression by binding to HSE motifs
(17). In this study, we
demonstrated that eight consecutive HSEs improve the induction
response to hyperthermia, which may be explained simply because
eight target sequences provide efficient binding sites for
HSF1.
The PNP/Fludara system also has several advantages
for treating colorectal cancer. First, PNP converts Fludara into a
highly cytotoxic metabolite F-dAe, which is an inhibitor of both
ribonucleoside reductase and DNA polymerase-A and effectively kills
both dividing and non-dividing cells (18). By contrast, the common suicide
system HSV-TK/GCV can kill dividing cells but not senescent or
slowly-dividing cells (19).
Second, overexpression of ePNP can activate the prodrug Fludara and
kill almost entire populations of tumor cells, even when as few as
5% of the cells express the ePNP gene (20). Hong et al showed that human
glioma tumors in mice were inhibited by adenoviral delivery of ePNP
followed by systemic treatment with the clinically approved
compound Fludara in different proportions of ePNP expressing cells
(21). In this study, we observed
activity of the PNP/Fludara system in SW480 cells when only 5% of
the cells expressed the ePNP gene. Furthermore, the bystander
effect of the PNP/Fludara system was mostly absent in
hTERT-negative MKN74 cells. This indicated that the bystander
effects exhibited dependence on both the level of prodrug
administered and ePNP expression in vitro (Fig. 2). Third, fludarabine has been
studied extensively, and pharmacokinetics of the agent in animal
models are well defined. Conversely, MeP-dR, another prodrug of
ePNP, has to be chemically synthesized in laboratories because
there is no readily available source. Finally, analysis of the
mechanisms of cell death of hepatocellular carcinoma expressing the
suicide gene showed that PNP/Fludara induced apoptosis through p53
accumulation in p53-positive cells compared to p53-negative cells
(22). Our results indicated that
PNP/Fludara-induced apoptosis, might depend not only on p53
accumulation, but also on other factors, such as Bax and caspase-3.
The precise apoptotic mechanisms induced by PNP/Fludara remain to
be determined. Furthermore, we detected a G2/M arrest in
PNP/Fludara-treated cells, as shown previously, suggesting the
PNP/Fludara suicide system might induce irreversible DNA damage
(23). Together, this study
revealed that the mechanism by which PNP/Fludara inhibits the
proliferation of SW480-8HhP cells might be related to inducing
apoptosis and G2/M arrest under heated conditions.
The synergistic effect of ePNP and Fludara, bringing
greater efficacy than the two alone, has important clinical
significance; in particular, the ability to use low doses of
Fludara should translate to reduced side effects and clinical
improvements in quality of life. This is particularly relevant for
patients with ePNP who had previously been or who were undergoing
chemotherapy with Fludara. The reduction in the therapeutic dose of
ePNP also decreases the amount of Fludara required for each
patient. The data obtained in this study warrant further
confirmation in vivo and in vitro. It has been
reported that synergy between ePNP and docetaxel against prostate
cancer cells led to a decrease in tumor load, both in the prostate
and at distant sites in immunocompetent mice (24). However, interactions between PNP
and docetaxel are not yet fully understood (25). A better understanding of these
interactions will help design new studies in patients who have
experienced pre-existing treatment.
To understand the interaction between PNP and
Fludara, molecular studies were performed. Apoptosis has been shown
to play a significant role in the cell death triggered by some
suicide systems (26). The most
effective apoptotic stimulus occurred when PNP interacted with
Fludara, which was reflected in the cell killing observed in our
studies. Pro- or anti-apoptotic proteins and caspases that regulate
apoptosis have been observed in several suicide system treatments
(27). overall, the pro-apoptotic
proteins Bax, cleaved caspase-3 or -9 were upregulated the and
anti-apoptotic protein, Bcl-2 was downregulated when suicide
systems were implemented (28).
Strong expression of both caspase-3 and Bcl-2 in responses to the
suicide system suggests that pathways involving caspases and the
Bcl-2 family may be active in these systems (29). This is the first study to identify
changes in apoptosis-related proteins in response to PNP/Fludara in
SW480 cells. However, our results do not rule out that PNP/Fludara
may be acting through numerous processes ultimately leading to an
upregulation of pro-apoptotic or a downregulation of anti-apoptotic
proteins (30).
To investigate in vivo our in vitro
observation that the 8HSEs-hTERTp-ePNP/Fludara system was capable
of inducing heat- and tumor-specific killing effects, we tested
tumor growth following heat treatment in a mouse xenograft model,
injected with a heat-inducible lentiviral vector. In addition to
tumor-specific killing effects, 8HSEs-hTERTp-ePNP/Fludara system
exhibited optimal tumor xenograft growth inhibition under heated
conditions. Supporting these observations, TUNEL assays showed that
the 8HSEs-hTERTp-ePNP/Fludara system induced obvious apoptosis
following hyperthermia in SW480 xenografts. These results suggest
that the 8HSEs-hTERTp-ePNP/Fludara system might be a promising
method of modulating gene expression during treatment by clinical
hyperthermia.
In conclusion, we have demonstrated that the
8HSEs-hTERTp-ePNP/Fludara suicide system efficiently kills
hTERT-expressing tumor cells in vivo and in vitro.
Inclusion of 8HSEs-hTERTp in the recombinant lentivirus vector
significantly improved the antitumor effects and specificity to
heat treatment of the ePNP/Fludara system. Furthermore, we have
demonstrated that the 8HSEs-hTERTp-ePNP/Fludara suicide system
induced antitumor effects by promoting apoptosis and a
G2 arrest. Our study suggests that by combining
hyperthermia with gene therapy, the 8HSEs-hTERTp-ePNP/Fludara
system, may serve as a powerful strategy for tumor gene therapy
under hyperthermia.
Acknowledgments
This study was supported by a grant from the
National Natural Science Foundation of China (grant serial nos.
81101874 and 81172362), the Science and Technology Project of
Shaanxi Province (grant serial no. 2016SF-015), the Coordinative
and Innovative Plan Projects of the Science and Technology Program
in Shaanxi Province (grant serial nos. 2013KTCQ03-08).
References
|
1
|
Sorscher EJ, Peng S, Bebok Z, Allan PW,
Bennett LL Jr and Parker WB: Tumor cell bystander killing in
colonic carcinoma utilizing the Escherichia coli DeoD gene to
generate toxic purines. Gene Ther. 1:233–238. 1994.PubMed/NCBI
|
|
2
|
Afshar S, Olafsen T, Wu AM and Morrison
SL: Characterization of an engineered human purine nucleoside
phosphorylase fused to an anti-her2/neu single chain Fv for use in
ADEPT. J Exp Clin Cancer Res. 28:1472009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parker WB, Allan PW, Shaddix SC, Rose LM,
Speegle HF, Gillespie GY and Bennett LL Jr: Metabolism and
metabolic actions of 6-methylpurine and 2-fluoroadenine in human
cells. Biochem Pharmacol. 55:1673–1681. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van der Zee J and González DG: Regional
hyperthermia for rectal cancer. Lancet. 356:7722000. View Article : Google Scholar
|
|
5
|
Sherar M, Liu FF, Pintilie M, Levin W,
Hunt J, Hill R, Hand J, Vernon C, van Rhoon G, van der Zee J, et
al: Relationship between thermal dose and outcome in
thermoradiotherapy treatments for superficial recurrences of breast
cancer: Data from a phase III trial. Int J Radiat Oncol Biol Phys.
39:371–380. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wust P, Hildebrandt B, Sreenivasa G, Rau
B, Gellermann J, Riess H, Felix R and Schlag PM: Hyperthermia in
combined treatment of cancer. Lancet Oncol. 3:487–497. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morimoto RI, Sarge KD and Abravaya K:
Transcriptional regulation of heat shock genes. A paradigm for
inducible genomic responses. J Biol Chem. 267:21987–21990.
1992.PubMed/NCBI
|
|
8
|
Brade AM, Ngo D, Szmitko P, Li PX, Liu FF
and Klamut HJ: Heat-directed gene targeting of adenoviral vectors
to tumor cells. Cancer Gene Ther. 7:1566–1574. 2000. View Article : Google Scholar
|
|
9
|
Hildebrandt B, Wust P, Rau B, Schlag P and
Riess H: Regional hyperthermia for rectal cancer. Lancet.
356:771–772. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Zhou P, Sun X, Wei G, Zhang L,
Wang H, Yao J, Jia P and Zheng J: Modification of the hTERT
promoter by heat shock elements enhances the efficiency and
specificity of cancer targeted gene therapy. Int J Hyperthermia.
32:244–253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horikawa I, Chiang YJ, Patterson T,
Feigenbaum L, Leem SH, Michishita E, Larionov V, Hodes RJ and
Barrett JC: Differential cis-regulation of human versus mouse TERT
gene expression in vivo: Identification of a human-specific
repressive element. Proc Natl Acad Sci USA. 102:18437–18442. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cunniff NF and Morgan WD: Analysis of heat
shock element recognition by saturation mutagenesis of the human
HSP70.1 gene promoter. J Biol Chem. 268:8317–8324. 1993.PubMed/NCBI
|
|
13
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Parker WB, Allan PW, Hassan AEA, Secrist
JA III, Sorscher EJ and Waud WR: Antitumor activity of
2-fluoro-2′-deoxyadenosine against tumors that express Escherichia
coli purine nucleoside phosphorylase. Cancer Gene Ther. 10:23–29.
2003. View Article : Google Scholar
|
|
15
|
Pan Z, Sun X, Shan H, Wang N, Wang J, Ren
J, Feng S, Xie L, Lu C and Yuan Y: MicroRNA-101 inhibited
postinfarct cardiac fibrosis and improved left ventricular
compliance via the FBJ osteosarcoma oncogene/transforming growth
factor-β1 pathway. Circulation. 126:840–850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mocna M, Granja C, Leroy C and Stekl I:
Hyperthermia in oncology. AIP Conf Proc. 958:256–257. 2007.
View Article : Google Scholar
|
|
17
|
Akerfelt M, Morimoto RI and Sistonen L:
Heat shock factors: Integrators of cell stress, development and
lifespan. Nat Rev Mol Cell Biol. 11:545–555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Parker WB, Sorscher EJ and Ealick
SE: PNP anticancer gene therapy. Curr Top Med Chem. 5:1259–1274.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Dillen IJ, Mulder NH, Vaalburg W, de
Vries EF and Hospers GA: Influence of the bystander effect on
HSV-tk/GCV gene therapy. A review Curr Gene Ther. 2:307–322. 2002.
View Article : Google Scholar
|
|
20
|
Chaudhary K, Ting LM, Kim K and Roos DS:
Toxoplasma gondii purine nucleoside phosphorylase biochemical
characterization, inhibitor profiles, and comparison with the
Plasmodium falciparum ortholog. J Biol Chem. 281:25652–25658. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong JS, Waud WR, Levasseur DN, Townes TM,
Wen H, McPherson SA, Moore BA, Bebok Z, Allan PW, Secrist JA III,
et al: Excellent in vivo bystander activity of fludarabine
phosphate against human glioma xenografts that express the
Escherichia coli purine nucleoside phosphorylase gene. Cancer Res.
64:6610–6615. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krohne TU, Shankara S, Geissler M, Roberts
BL, Wands JR, Blum HE and Mohr L: Mechanisms of cell death induced
by suicide genes encoding purine nucleoside phosphorylase and
thymidine kinase in human hepatocellular carcinoma cells in vitro.
Hepatology. 34:511–518. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh PP, Joshi S, Russell PJ, Nair S and
Khatri A: Purine Nucleoside Phosphorylase mediated molecular
chemotherapy and conventional chemotherapy: A tangible union
against chemoresistant cancer. BMC Cancer. 11:3682011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang XY, Martiniello-Wilks R, Shaw JM, Ho
T, Coulston N, Cooke-Yarborough C, Molloy PL, Cameron F, Moghaddam
M, Lockett TJ, et al: Preclinical evaluation of a prostate-targeted
gene- directed enzyme prodrug therapy delivered by ovine
atadenovirus. Gene Ther. 11:1559–1567. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan D, Jin L and Zhang X: The latest
advances of experimental research on targeted gene therapy for
prostate cancer. Chinese-German J Clin Oncol. 12:546–550. 2013.
View Article : Google Scholar
|
|
26
|
Barese CN, Felizardo TC, Sellers SE,
Keyvanfar K, Di Stasi A, Metzger ME, Krouse AE, Donahue RE, Spencer
DM and Dunbar CE: Regulated apoptosis of genetically modified
hematopoietic stem and progenitor cells via an inducible caspase-9
suicide gene in rhesus macaques. Stem Cells. 33:91–100. 2015.
View Article : Google Scholar
|
|
27
|
Carlotti F, Zaldumbide A, Martin P,
Boulukos KE, Hoeben RC and Pognonec P: Development of an inducible
suicide gene system based on human caspase 8. Cancer Gene Ther.
12:627–639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Y, Shi S, Wang H, Li N, Su J, Chou G
and Wang S: A Homogeneous polysaccharide from fructus Schisandra
chinensis (Turz.) baill induces mitochondrial apoptosis through the
Hsp90/AKT signalling pathway in HepG2 cells. Int J Mol Sci.
17:10152016. View Article : Google Scholar :
|
|
29
|
Cali U, Cavkaytar S, Sirvan L and Danisman
N: Placental apoptosis in preeclampsia, intrauterine growth
retardation, and HELLP syndrome: An immunohistochemical study with
caspase-3 and bcl-2. Clin Exp Obstet Gynecol. 40:45–48.
2013.PubMed/NCBI
|
|
30
|
Karjoo Z, Chen X and Hatefi A: Progress
and problems with the use of suicide genes for targeted cancer
therapy. Adv Drug Deliv Rev. 99A:113–128. 2016. View Article : Google Scholar
|