Introduction
Psoriasin, also known as S100A7, is a member of the
S100 protein family of calcium binding proteins and was first
identified as being overexpressed in psoriatic skin lesions
(1). It is a 11.4-kDa secretory
protein encoded on chromosome 1q21, clustered alongside 16 other
members of the S100 family within a region known as the epidermal
differentiation complex (EDC) (2,3). The
S100 family encodes both cytoplasmic and secreted proteins that
share EF-hand helix-loop-helix domains, important for their role as
calcium binding proteins (4).
Psoriasin S100 proteins are widely expressed in numerous cell types
localised to the cytoplasm and/or nucleus, or in some cases are
secreted. Due to their role in calcium binding and signalling, they
are involved in numerous cell functions including proliferation,
differentiation, migration and apoptosis.
In addition to its overexpression in psoriatic skin
lesions, aberrant Psoriasin expression has been implicated in a
range of human diseases including cancer. Interestingly, Psoriasin
is a chemotactic factor for keratinocytes and leukocytes (5–7), and
is also upregulated and excreted from cells of the epidermis during
inflammation. Additionally, Psoriasin has been implicated as an
antimicrobial peptide, selectively killing E. coli on the
surface of the skin (8).
Therefore, Psoriasin may be utilised, via the host immune response,
as a selective chemotactic factor both in psoriasis and cancer
(9).
The expression of Psoriasin during carcinogenesis
has been studied in detail, and its overexpression has been linked
to a number of cancers including breast (10), prostate (11), skin (12), head and neck (13), bladder (14) and lung cancer (15). It is also often associated with
poor prognosis. For example in breast cancer, Psoriasin expression
correlates with features of poor prognosis, including oestrogen
receptor (ER) and progesterone receptor (PR) negativity, HER2
positivity, and the presence of lymphocytic infiltration (16–18).
The precise role of Psoriasin in cancer remains unclear. One
hypothesis links Psoriasin expression with stimulation of
angiogenesis regulated by VEGF (19). In the development of breast cancer,
Psoriasin regulates epidermal growth factor (EGF)-induced
phosphorylation of the EGF receptor (EGFR), actin remodelling and
NF-κβ mediated matrix metalloproteinase-9 (MMP-9) secretion,
promoting tumour development and metastasis (20). These results suggest Psoriasin
plays an important role in carcinoma development.
Pancreatic cancer is the fourth leading cause of
cancer deaths in Western countries and carries a very poor
prognosis due to delayed diagnosis and lack of effective
treatments. The majority of patients present with metastasis at
diagnosis; 25% with local metastasis and 55% with regional
metastasis (21). The most common
sites of metastasis are the liver, followed by the peritoneum, lung
and pleura, bones and adrenal glands. Outcome for patients is poor
with a 5-year survival rate of <5% (22). In comparison to the studies of
other malignancies, pancreatic cancer requires more intensive study
for understanding genetic and molecular machinery which is utilised
by cancerous cells during disease progression and metastasis. This
study aimed to examine the role of Psoriasin in pancreatic cancer
with a focus for its involvement in local invasion and distant
metastasis.
Materials and nethods
Materials and cell lines
Pancreatic cell lines (MIA-PaCa-2 and PANC-1) were
purchased from the American Type Culture Collection (ATCC,
Rockville, MD, USA). Both cell lines are derived from primary
tumours of pancreatic cancer. MIA-PaCa-2 was isolated from a
pancreatic carcinoma while PANC-1 was derived from an epithelioid
carcinoma of pancreas. The pancreatic cancer cells were maintained
in Dulbecco's modified Eagle's medium (DMEM)-F12 medium,
supplemented with 10% fetal calf serum (FCS) and antibiotics. LP-9
mesothelial cells were purchased from the Coriell Institute for
Medical Research (Camden, NJ, USA) and maintained in M199 medium
supplemented with 15% FCS and antibiotics. Matrigel (BD Matrigel
Basement Membrane Matrix) was obtained from BD Biosciences (Oxford,
UK).
Construction of ribozyme transgenes
targeting human Psoriasin and Psoriasin overexpression vectors
Anti-human Psoriasin hammerhead ribozymes were
designed based on the secondary structure of the mRNA generated
using Zuker's RNA Mfold program (23). The ribozymes were synthesized and
cloned into pEF6/V5-His-TOPO plasmid vector (Invitrogen, Paisley,
UK). The primers used for ribozyme synthesis are shown in Table I. Similarly, full-length human
Psoriasin coding sequence amplified from normal human prostate
tissues was cloned into the same vector. Constructed plasmids were
extracted using the Genelute Plasmid Mini-Prep kit (Sigma-Aldrich,
Poole, UK). Ribozyme transgenes, overexpression constructs, and
control plasmids were transfected into MIA-PaCa-2 and PANC-1 cells
by electroporation (Gene Pulser Xcell; Bio-Rad, Hertfordshire, UK).
Stable transfectants were obtained and verified following two weeks
selection using blasticidin (5 μg/ml, Melford Laboratories
Ltd., Suffolk, UK). The cells were then cultured in DMEM with
blasticidin at a lower concentration (0.5 μg/ml) to maintain
plasmid expression.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Forward | Reverse |
|---|
|
Psoriasin |
5′-GAGGTCCATAATAGGCATGA |
5′-AGCAAGGACAGAAACTCAGA |
| RAGE |
5′-GATCCAGGATGAGGGGATTT |
5′-GCTACTGCTCCACCTTCTGG |
| Psoriasin
(expression) |
5′-ATGAGCAACACTCAAGCTG |
5′-ACTGGCTGCCCCCGGAACA |
| Psoriasin
(QPCR) |
5′-TGTGACAAAAAGGGCACAAA |
5′-ACTGAACCTGACCGTACACCCAGCAAGGACAGAAACTC |
| CK19
(QPCR) |
5′-CAGGTCCGAGGTTACTGAC |
5′-ACTGAACCTGACCGTACACCGTTTCTGCCAGTGTGTCTTC |
| Cyclin
D1 |
5′-CGGTGTCCTACTTCAAATGT |
5′-ACCTCCTCCTCCTCCTCT |
|
p27Kip1 |
5′-GGAATAAGGAAGCGACCTG |
5′-CCGTCTGAAACATTTTCTTC |
| Caspase
3 |
5′-GGCGTGTCATAAAATACCAG |
5′-ACAAAGCGACTGGATGAA |
| MMP9 |
5′-AACTACGACCGGGACAAG |
5′-ATTCACGTCGTCCTTATGC |
| GAPDH |
5′-GGCTGCTTTTAACTCTGGTA |
5′-AGCAAGGACAGAAACTCAGA |
| Psoriasin
(ribozyme) |
5′-CTGCAGTCACAGGCACTAAGGAAGTTGGGCTGATGAGTCCGTGAGGA |
5′-ACTAGTGGCTGGTGTTTGACATTTCGTCCTCACGGACT |
RNA isolation, reverse transcription-PCR
(RT-PCR) and quantitative PCR (qPCR)
Total RNA was isolated using TRI reagent
(Sigma-Aldrich). First strand cDNA synthesis was undertaken using
the Precision nanoScript Reverse Transcription kit (Primer Design,
Southampton, UK). PCR was performed using GoTaq Green MasterMix
(Promega, Dorset, UK). Cycling conditions were as follows: 94°C for
5 min followed by 36 cycles of 94°C for 30 sec, 55°C for 30 sec,
and 72°C for 40 sec. This was followed by a final extension of 72°C
for 10 min. Products were visualised using 2% agarose gels stained
with SYBR Safe (Invitrogen).
QPCR was performed using the Icycler IQ5 system
(Bio-Rad, Hammel Hempsted, UK). Pancreatic cancer cell cDNA samples
were examined for Psoriasin transcript expression, alongside a set
of standards and negative controls. The Amplifluor system (Intergen
Inc., Oxford, UK) and qPCR mastermix (Bio-Rad) were used. Psoriasin
primer pairs were designed using Beacon design software (Premier
Biosoft, Palo Alto, CA, USA), whereby the reverse primer contained
an additional Z sequence (5′-ACTGAACCTGACCGTACA) complementary to
the universal Z probe (Intergen Inc.). Reaction conditions were as
follows: 94°C for 12 min, followed by 90 cycles of 94°C for 15 sec,
55°C for 40 sec and 72°C for 20 sec.
Quantification of Psoriasin transcripts
in human pancreatic cancer
Human pancreatic tissue was collected from patients
undergoing surgical resection of pancreatic tumours at the Beijing
Cancer Hospital. The specimens retrieved comprised 126 pancreatic
tumours and 114 along with matched adjacent normal pancreatic
tissues over a period from 2002 to 2011 with follow-up information
up to 2013 (median, 12 months, IQR, 6–20.5 months). The tissues
were stored immediately after surgery at −80°C until use.
Clinicopathological factors, including age, sex, histological type,
TNM stage, lymph node metastasis, distant metastases and embolism,
were recorded and stored in the patient database. The protocol and
procedure were reviewed and approved by the Beijing Cancer Hospital
Research Ethics Committee and written consent was obtained from all
patients involved. Total RNA extraction was then performed using
TRI reagent (Sigma-Aldrich). Psoriasin transcripts were determined
using QPCR together with an internal control keratin 19 (CK19).
Normalised Psoriasin transcript levels are shown in Table II. Primer sequences are provided
in Table I.
 | Table IIPsoriasin transcript levels in
pancreatic cancer. |
Table II
Psoriasin transcript levels in
pancreatic cancer.
| N | Mean ± SEM
(copies/25 ngRNA) | p-value |
|---|
| Clinical
samples |
| Tumour | 126 | 162.9±75.8 | 0.32 |
| Normal | 114 | 1,7793±17,660 | |
| Histology |
| Adeno | 108 | 134.7±79 | |
| Ductal | 4 | 981±981 | 0.45 vs adeno |
| Others | 14 | 147±147 | 0.94 vs adeno |
| T staging |
| 1 | 2 | 1.77±1.77 | |
| 2 | 19 | 0.847±0.799 | |
| 3 | 73 | 184.5±97.6 | 0.064 vs T2 |
| 4 | 10 | 644±640 | |
| T1-2 | 21 | | |
| T3-4 | 83 | 240±114 | 0.04 |
| Node status |
| Node-negative | 46 | 272±166 | |
| Node-positive | 4 | 1.77±1.77 | 0.11 |
| Metastasis |
| Yes | 9 |
0.00014±0.00010 | |
| No | 117 | 175.5±81.5 | 0.033 |
| Clinical
outcome |
| Died | 89 | 90.1±64.3 | |
| Alive | 26 | 402±283 | 0.29 |
| Embolism |
| Yes | 35 | 212±162 | |
| No | 73 | 179±105 | 0.87 |
In vitro cell growth assay
A standard growth assay was undertaken as previously
described (15,24). Cells were plated into a 96-well
plate (3,000 cells/well) and growth was assessed after 1, 3 and 5
days. Crystal violet was used to stain the cells, and absorbance
was determined at a wavelength of 540 nm using a spectrophotometer
(Elx800; Bio-Tek, Bedfordshire, UK).
Electric cell-substrate impedance sensing
(ECIS) based cellular migration assays
An ECIS-Ztheta instrument (Applied Biophysics Ltd.;
Troy, NJ, USA) was used with a 96-well 96W1E microarrays and
wounding module. Pancreatic cancer cells were seeded at 40,000
cells per well and cell adhesion was tracked immediately over a
range of frequencies from 1,000 to 64,000 Hz. Once the cells
reached confluence, a current of 2,600 μA was passed across
each well for 20 sec to produce a reproducible wound, after which
cell migration was automatically tracked.
Cell-matrix adhesion assay
A total of 20,000 cells were added to each well of a
96-well plate previously prepared by coating with Matrigel (5
μg/well). The cells were incubated at 37°C in 5%
CO2 for 45 min and the medium was then discarded.
Non-adherent cells were washed off using balanced salt solution
(BSS) buffer. Adherent cells were then fixed in 4% formalin,
stained with crystal violet and absorbance determined at 540
nm.
Cell-cell adhesion assay
LP-9 mesothelial cells were trypsinised and
transferred to a 96-well plate at 30,000 cells per well and grown
to confluence over a 24-h period. Pancreatic cancer cells were
stained with 10 μg/ml DiI membrane stain
(1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine
perchlorate, Life Technologies) before being added at 20,000 cells
per well to the mesothelial cell mono-layer. Pancreatic cancer
cells were left to adhere for 40 min before non-adherent cells were
washed off using BSS buffer. Adherent cells were then fixed (4%
formalin) and images were taken at 549 excitation/565 emission and
phase contrast using an EVOS FL auto (Life Technologies), before
the total number of adherent pancreatic cancer cells was
counted.
In vitro invasion assay
Transwell inserts with 8 μm pore size
(Greiner Bio-One, Gloucester, UK) were coated with 50 μg
Matrigel and air-dried before being rehydrated. A total of 20,000
cells were added to each insert. Following 72-h incubation, cells
that had migrated through the matrix and pores were fixed (4%
formalin), stained in crystal violet and absorbance determined at
540 nm.
Gelatin zymography assay
Pancreatic cancer cells (3×106) were
seeded into a 75-cm2 flask. Following overnight culture,
cells were washed twice with serum-free DMEM and cultured in
serum-free DMEM for 6 h. The conditioned medium was then collected
by centrifugation to remove any cells. Samples were prepared in
non-reducing sample buffer (containing 0.625 mM Tris-HCl, 10%
glycerol, 2% SDS, and 2% bromophenol blue). Each sample (30
μl) was loaded into each lane and separated by 10% sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on
gels containing 1 mg/ml gelatine (Sigma-Aldrich). The gel was then
renatured at room temperature in washing buffer (containing 2.5%
Triton X-100 and 0.02% NaN3), before being incubated in
a buffer containing 50 mM Tris-HCl (pH 7.6), 5 mM CaCl2,
and 0.02% NaN3 for 72 h before staining with Coomassie
blue. The clear bands of gelatin digested by MMPs was documented
and analysed using densitometry.
Cell aggregation assay
Cell aggregation was measured over a time course of
3 h, at 1 h intervals. MiaPa-Ca-1 cells with varying Psoriasin
expression were harvested using trypsin. A small amount was
aliquoted for initial measurements. The remaining solution was left
at 37°C, on a tilt table, to prevent adherence. The collected
samples were kept on ice to prevent additional aggregation. Cells
were aliquoted out at 1, 2 and 3 h. Cell aggregation was measured
using a Neubauer haemocytometer (Celeromics, Cambridge, UK) under a
light microscope, with total individual cells counted followed by
the number of cell groups.
Anoikis assay
Cells (250,000–500,000) per experiment were
transferred to a fresh UC and spun down in a centrifuge at 1700 rpm
for 5 min and washed with ice-cold PBS and re-centrifuged. The
cells were suspended in 250 μl of Annexin V binding
(Invitrogen) buffer per 250,000 cells. This solution (100
μl) was mixed with 5 μl of pre-mixed staining
solution (0.2 μl propidium iodide, 0.8 μl Annexin
V-FITC, 4 μl Annexin V binding buffer) (Invitrogen) and left
in the dark, at room temperature, for 30 min. It was then diluted
with 400 μl of filtered PBS and kept on ice. The remaining
cell solution was incubated at 37°C at 5% CO2 and the
process repeated at 1 and 2 h. The samples were then run through a
flow cytometer (Cyflow, Sysmex UK Ltd.).
Western blot analysis
Equal amount of total proteins of each cell line was
separated on an SDS-PAGE gel. The proteins were then electrically
transferred on to a nitrocellulose membrane and probed with
anti-caspase 3 and GAPDH antibodies (Santa Cruz Biotechnology, CA,
USA), respectively, and also corresponding peroxidase conjugated
secondary antibodies (Sigma-Aldrich). Images of the protein bands
were developed using a chemiluminescence detection kit (Luminata,
Millipore) and a UVITech imager (UVITech, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS18
(SPSS Inc., Chicago, IL, USA). The t-test and Mann-Whitney U test
were used for normally distributed and non-normally distributed
data respectively. Kaplan-Meier analysis was used to analyse the
correlation with patient survival. Differences were considered
statistically significant at p<0.05.
Results
Expression of Psoriasin in pancreatic
cancer, and evaluation of Psoriasin levels in cell lines
In the search of gene expression array data base at
PubMed, a reduction was seen in the expression of Psoriasin in
pancreatic cancers in comparison with the paired adjacent normal
pancreatic tissues (GDS4336) (Fig.
1A). We further determined the transcript levels of Psoriasin
in a cohort of 126 pancreatic tumours and 114 adjacent normal
pancreatic tissues. Psoriasin expression appeared to be reduced in
the tumours but did not reach a significant level in comparison
with the adjacent normal tissues (Table II). An even lower expression of
Psoriasin was seen in tumours with distant metastases.
Interestingly, higher Psoriasin transcript levels were seen in
locally invasive tumours which extended beyond the pancreas (T3 and
T4) in comparison with tumours confined to the pancreas (T1 and T2)
(Table II). It suggests that
Psoriasin plays a significant role in regulation of invasion of
pancreatic cancer cells. To clarify the involvement of Psoriasin in
metastasis, we analysed the expression of Psoriasin in another gene
expression array of pancreatic tumours which includes 145 primary
tumours and 61 metastatic tumours (GSE71729) (25). An increased expression of Psoriasin
was seen in the secondary tumours in comparison with its expression
in primary tumours (Fig. 1B).
The mRNA expression of Psoriasin in three different
pancreatic cancer cell lines was evaluated using RT-PCR (Fig. 1C). RAGE, the receptor of Psoriasin
was detected in the three pancreatic cancer cell lines. PCR
products of 528 bp were amplicons of both variant 2 and 4, while
480-bp products represented amplicons from variant 1, 3, 5, 6, 7, 8
and 9. However, exact variants expressed in these cell lines are
yet to be further determined (Fig.
1C). The expression of Psoriasin in MIA-PaCa-2 is higher than
that for PANC-1, and lowest in AsPc-1. Psoriasin knockdown and
overexpression was verified in the MIA-PaCa-2 and PANC-1 cell lines
transfected with ribozyme transgenes using RT-PCR (Fig. 1D) and qPCR (Fig. 1E). The Psoriasin-modified
MIA-PaCa-2 and PANC-1 cell lines were used for subsequent
experiments.
Effect of Psoriasin knockdown and
overexpression on pancreatic cancer cell growth and migration
A significant decrease in cellular growth was
observed in Psoriasin knockdown MIA-PaCa-2 (Fig. 2A, p<0.001) and PANC-1 (Fig. 2B, p<0.05) cell lines in
comparison with controls. Conversely, overexpression of Psoriasin
resulted in increased growth of MIA-PaCa-2 and PANC-1 cell lines
(p<0.05).
Psoriasin knockdown resulted in decreased migration
of MIA-PaCa-2 (Fig. 2C,
p<0.001) and PANC-1 (Fig. 2D,
p<0.05) cell lines compared to controls. Conversely, Psoriasin
overexpression resulted in increased migration of MIA-PaCa-2
(p<0.001) and PANC-1 (p<0.05) cell lines.
Influence of Psoriasin expression on
pancreatic cancer cell adhesion
An in vitro cell-matrix adhesion assay was
adopted to investigate the effect of Psoriasin knockdown and
overexpression on the adhesive ability of pancreatic cancer cells
to extracellular matrix. MIA-PaCa-2 (Fig. 3A, p<0.05) and PANC-1 (Fig. 3B, p<0.05) Psoriasin
overexpressing cells exhibited decreased cell-matrix adhesion
whilst Psoriasin knockdown resulted in increased adhesion to matrix
(p<0.05).
According to the involvement in adhesion of
pancreatic cancer cells, we were curious about a possible
implication in dissemination through peritoneal cavity. Therefore,
we determined the effect of Psoriasin expression on pancreatic
cancer cell adhesion to mesothelial which constitute peritoneum.
Neither Psoriasin overexpression nor knockdown had any significant
effect on the adhesion of PANC-1 cells to peritoneal mesothelia
compared to control (Fig. 3C).
Effect of Psoriasin expression on
pancreatic cancer cell invasion
The influence of Psoriasin expression on MIA-PaCa-2
and PANC-1 cell invasion was examined. Interestingly, Psoriasin
overexpression resulted in increased invasion of MIA-PaCa-2
(Fig. 4A, p<0.001) and PANC-1
(Fig. 4B, p<0.05) cell lines
whilst Psoriasin knockdown resulted in decreased invasion of
MIA-PaCa-2 (p<0.001) and PANC-1 (p<0.05) cells.
At the mRNA level, MMP-9 expression was
significantly increased in Psoriasin overexpression MIA-PaCa-2
cells compared to controls (Fig.
4C). Conversely, MMP-9 expression was decreased in Psoriasin
knockdown MIA-PaCa-2 cells. As the results from zymography show,
MMP-2 and MMP-9 activity is increased in Psoriasin overexpression
MIA-PaCa-2 cells compared to control (Fig. 4D), whilst MMP-2 and MMP-9 activity
is decreased in Psoriasin knockdown MIA-PaCa-2 cells. As shown in
Fig. 4C, decreased Psoriasin
expression resulted in the decreased expression of cyclin D2 at the
mRNA level in MIA-PaCa-2 cells in which an increased level of
p27Kip1 was also noted. Moreover, overexpression of
Psoriasin resulted in increased cyclin D2 expression.
Influence of Psoriasin on aggregation and
survival of pancreatic cancer cells
In order to evaluate how MIA-PaCa-2 cells interact
with one another in suspension, a cell aggregation assay was
performed. Cells with increased Psoriasin expression demonstrated
an increased propensity to form cell-cell contacts whilst in
suspension, reaching significance at 3 h (Fig. 5, p<0.05). To determine whether
this observation had an impact on the anoikis resistance in this
cell line, an apoptosis assay was performed. We observed cell
survival after suspension was increased in Psoriasin overexpressing
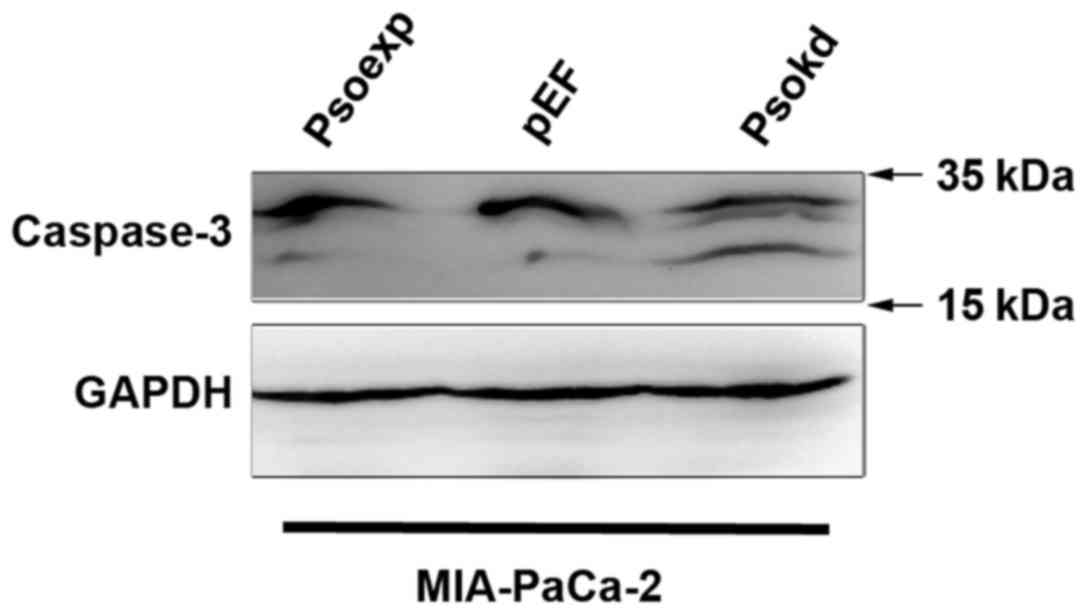
cell lines (Fig. 6). Western blot
analyses of adherent cells showed an enhanced activation of
caspase-3 in MIA-PaCa-2 cells as cleaved proteins in comparison
with the control (Fig. 7). A
reduction in the cleavage of caspase-3 was not observed in the
Psoriasin overexpression cells compared with the control which also
exhibited a lower level of the activation.
Discussion
Psoriasin was first identified as being
overexpressed in the hyperproliferative skin disease psoriasis.
Numerous studies have since demonstrated Psoriasin overexpression
to be associated with tumour progression. Upregulation of Psoriasin
in cancer was initially identified in pre-invasive carcinomas;
whether this upregulation occurs in pancreatic intra-epithelial
neoplasia progression that proceeds to pancreatic adenocarcinoma
remains to be elucidated. Increased Psoriasin expression has also
been identified in cancers at an advanced stage. In breast cancer,
Psoriasin overexpression is associated with metastasis and poor
prognosis. In the present study, Psoriasin expression was
determined in a cohort of pancreatic cancer patients. Psoriasin
expression tended to be reduced in the pancreatic cancers compared
to adjacent normal pancreatic tissues, though did not reach
statistical significance. This observation is similar to findings
from a GEO data set (GDS4336) (Fig.
1A) (26). A reduced
expression was noted in primary tumours (n=9) with distant
metastases. This finding is yet to be verified by examining of a
large cohort pancreatic primary tumours with a reasonable number of
primary tumours which have distant metastasis. In an analysis of a
gene expression array data, an increased expression levels of
Psoriasin was seen in metastases of pancreatic cancer in comparison
with primary tumours (Fig. 1B).
Interestingly, a greater expression of Psoriasin was evident in
locally advanced pancreatic tumours. It suggests that Psoriasin may
play differential roles during the development and progression of
the disease, in particular, the metastatic process.
We determined the expression of Psoriasin in the
cell lines AsPc-1, MIA-PaCa-2 and PANC-1, and expression appeared
highest in MIA-PaCa-2 which is the most tumourigenic of the three
cell lines (27). These results
demonstrate that Psoriasin overexpression is associated with
increased cancer cell growth in vitro, whilst knockdown
inhibits growth. Interestingly, during breast cancer progression,
Psoriasin has been shown to interact with Jab1 (c-jun
activation-domain binding protein), which is involved in
proteosomal degradation and signal transduction. Interaction
between Psoriasin and Jab1 results in increased activator protein-1
(AP-1) activity, increased expression of AP-1 and HIF-1-dependent
genes, and reduced expression of the cell cycle inhibitor
p27Kip1, resulting in enhanced tumourigenesis and
metastasis in vivo (28).
It may be that in pancreatic cancer, the same mechanisms are taking
place promoting tumour growth and metastasis. Jab1 is a negative
regulator of p27Kip1, promoting its degradation
(29). p27Kip1 normally
binds to and prevents the activation of cyclin complexes
controlling cell cycle progression at G1 (30). Thus, it may be that increased
interaction between Psoriasin and Jab1 results in decreased
p27Kip1. In line with this, an increased expression of
p27Kip1 was also observed in the Psoriasin knockdown
cells (Fig. 4C). We also found
that Psoriasin overexpression results in increased expression of
cyclin D2, which may explain the mode of action for
Psoriasin-mediated promotion of cyclin activation and cell cycle
progression. Additionally, differential expression of RAGE, the
receptor of Psoriasin was seen in three pancreatic cancer cell
lines. RAGE has 8 isoforms of protein products encoded by 9
different splicing variants in humans (listed at PubMed Gene).
However, we cannot identify which variants were expressed by these
cell lines. The exact role played by RAGE in mediating functions of
Psoriasin in pancreatic cancer is yet to be investigated.
In this study, Psoriasin expression was inversely
linked to cell-matrix adhesion. This is similar to our results
observed in both prostate and lung cancer (11,15).
It suggests that expression of Psoriasin is a response by cancer
cells to changes of cell-matrix adhesion and reciprocally affects
cell adhesion to matrix. We also found Psoriasin expression has no
effect on the adhesion of pancreatic cancer cells to peritoneal
mesothelial in vitro, and conclude that Psoriasin may not be
required for the adhesion during peritoneal metastasis of
pancreatic cancer.
Psoriasin is regarded as an inflammation-associated
protein and has chemoattractant properties, promoting migration of
granulocytes, lymphocytes, macrophages and monocytes. Our study
showed Psoriasin overexpression results in increased migration of
pancreatic cancer cells. The multi-ligand receptor for advanced
glycation end-products (RAGE) has been identified as a Psoriasin
receptor, and implicated in leukocyte migration and the
inflammatory process. It may be that, similar to leukocyte
migration, Psoriasin attaches to RAGE in order to promote cancer
cell migration (5).
Further to its influences on cell growth, adhesion
and migration, we also examined the effect of Psoriasin on
pancreatic cancer cell invasion. It is well known that activation
of MMP-2 and MMP-9 promotes the invasive and metastatic potential
of pancreatic cancer cells (31,32).
In our recent study of Psoriasin in prostate cancer, Psoriasin has
been shown to have an effect on prostate cancer cell invasion via
MMPs (11). This is subsequently
confirmed herein, whereby Psoriasin overexpression in pancreatic
cancer cells resulted in increased MMP-2 and MMP-9 expression and
activity. Taken together, these data suggest Psoriasin regulates
invasion of pancreatic cancer cells via regulation of MMPs. In a
recent study, Morgan et al observed interaction between
Psoriasin and the cytoplasmic domain of the integrin β6 subunit is
required for αvβ6-dependent breast cancer cell invasion. Similarly,
they showed upregulation of MMPs in Psoriasin overexpressing breast
cancer cells (33). In line with
its higher expression levels in local invasive tumours, Psoriasin
promotes invasion of pancreatic cancer cells which leads to a
greater local invasive expansion of the disease.
A recent report of disruption of cell anchorage
inducing apoptosis led us to investigate whether Psoriasin has a
role of inducing this form of anoikis resistance in pancreatic
cancer cells (34). Anoikis is
referred to as a programmed cell death or apoptosis which happens
to adherent cells lacking anchorage (34). It can occur during the
dissemination of pancreatic cancer cells through circulation and
peritoneal cavity when they lose anchorage. At 3 h we saw a
significant rise of cell-cell aggregates, indicating a role for
Psoriasin in inducing these aggregates. Previous experiments showed
a role for Psoriasin in preventing anoikis (34), a result we confirmed in our cell
lines, hinting that cell-cell aggregation may contribute to
preventing anoikis. Despite this, based on our results, the number
of cell-cell aggregates show a significant increase only at 3 h,
whereas cells display anoikis resistance at 1 h, indicating other
mechanisms must be at play to compensate for the disparity. Western
blot analyses of adherent cells showed an increased activation of
caspase 3 in the Psoriasin knockdown MIA-PaCa-2 cells. The
involvement of intrinsic and extrinsic caspase pathways and
activation of caspase-3 in pancreatic cancer cells undergoing
anoikis remains unclear and is yet to be investigated. Further
investigation is also required to reveal molecules involved in the
enhanced cell-cell adhesion and aggregation.
In conclusion, reduced expression of Psoriasin was
seen in pancreatic cancers with distant metastases, whilst higher
transcript levels were seen in locally advanced tumours. Psoriasin
expression in pancreatic cancer cells is associated with cell
growth, cell-matrix adhesion and migration. Psoriasin regulates
invasion of pancreatic cancer cells via expression of MMPs. The
implication of Psoriasin expression in invasion, disease
progression and potential as a therapeutic target warrants further
investigation.
Acknowledgments
The authors thank the support from Cancer Research
Wales, Life Sciences Research Network Wales (Welsh Government's Ser
Cymru Program) and Albert Huang Foundation. Ying Liu, Yanan Gu and
Gang Chen are recipients of China Medical Scholarship from the
Cardiff University.
References
|
1
|
Madsen P, Rasmussen HH, Leffers H, Honoré
B, Dejgaard K, Olsen E, Kiil J, Walbum E, Andersen AH, Basse B, et
al: Molecular cloning, occurrence, and expression of a novel
partially secreted protein “psoriasin” that is highly up-regulated
in psoriatic skin. J Invest Dermatol. 97:701–712. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoffmann HJ, Olsen E, Etzerodt M, Madsen
P, Thøgersen HC, Kruse T and Celis JE: Psoriasin binds calcium and
is upregulated by calcium to levels that resemble those observed in
normal skin. J Invest Dermatol. 103:370–375. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Børglum AD, Flint T, Madsen P, Celis JE
and Kruse TA: Refined mapping of the psoriasin gene S100A7 to
chromosome 1cen-q21. Hum Genet. 96:592–596. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schäfer BW and Heizmann CW: The S100
family of EF-hand calcium-binding proteins: Functions and
pathology. Trends Biochem Sci. 21:134–140. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wolf R, Howard OM, Dong H-F, Voscopoulos
C, Boeshans K, Winston J, Divi R, Gunsior M, Goldsmith P, Ahvazi B,
et al: Chemotactic activity of S100A7 (Psoriasin) is mediated by
the receptor for advanced glycation end products and potentiates
inflammation with highly homologous but functionally distinct
S100A15. J Immunol. 181:1499–1506. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boniface K, Bernard FX, Garcia M, Gurney
AL, Lecron JC and Morel F: IL-22 inhibits epidermal differentiation
and induces proinflammatory gene expression and migration of human
keratinocytes. J Immunol. 174:3695–3702. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boniface K, Diveu C, Morel F, Pedretti N,
Froger J, Ravon E, Garcia M, Venereau E, Preisser L, Guignouard E,
et al: Oncostatin M secreted by skin infiltrating T lymphocytes is
a potent keratinocyte activator involved in skin inflammation. J
Immunol. 178:4615–4622. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gläser R, Harder J, Lange H, Bartels J,
Christophers E and Schröder JM: Antimicrobial psoriasin (S100A7)
protects human skin from Escherichia coli infection. Nat Immunol.
6:57–64. 2005. View
Article : Google Scholar
|
|
9
|
Jinquan T, Vorum H, Larsen CG, Madsen P,
Rasmussen HH, Gesser B, Etzerodt M, Honoré B, Celis JE and
Thestrup-Pedersen K: Psoriasin: A novel chemotactic protein. J
Invest Dermatol. 107:5–10. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang WG, Watkins G, Douglas-Jones A and
Mansel RE: Psoriasin is aberrantly expressed in human breast cancer
and is related to clinical outcomes. Int J Oncol. 25:81–85.
2004.PubMed/NCBI
|
|
11
|
Ye L, Sun PH, Martin TA, Sanders AJ, Mason
MD and Jiang WG: Psoriasin (S100A7) is a positive regulator of
survival and invasion of prostate cancer cells. Urol Oncol.
31:1576–1583. 2013. View Article : Google Scholar
|
|
12
|
Moubayed N, Weichenthal M, Harder J,
Wandel E, Sticherling M and Gläser R: Psoriasin (S100A7) is
significantly up-regulated in human epithelial skin tumours. J
Cancer Res Clin Oncol. 133:253–261. 2007. View Article : Google Scholar
|
|
13
|
Tripathi SC, Matta A, Kaur J, Grigull J,
Chauhan SS, Thakar A, Shukla NK, Duggal R, DattaGupta S, Ralhan R,
et al: Nuclear S100A7 is associated with poor prognosis in head and
neck cancer. PLoS One. 5:e119392010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Celis JE, Rasmussen HH, Vorum H, Madsen P,
Honoré B, Wolf H and Orntoft TF: Bladder squamous cell carcinomas
express psoriasin and externalize it to the urine. J Urol.
155:2105–2112. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu M, Ye L, Ruge F, Zhi X, Zhang L and
Jiang WG: The clinical significance of Psoriasin for non-small cell
lung cancer patients and its biological impact on lung cancer cell
functions. BMC Cancer. 12:5882012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Enerbäck C, Porter DA, Seth P, Sgroi D,
Gaudet J, Weremowicz S, Morton CC, Schnitt S, Pitts RL, Stampl J,
et al: Psoriasin expression in mammary epithelial cells in vitro
and in vivo. Cancer Res. 62:43–47. 2002.PubMed/NCBI
|
|
17
|
Emberley ED, Alowami S, Snell L, Murphy LC
and Watson PH: S100A7 (psoriasin) expression is associated with
aggressive features and alteration of Jab1 in ductal carcinoma in
situ of the breast. Breast Cancer Res. 6:R308–R315. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watson PH, Leygue ER and Murphy LC:
Psoriasin (S100A7). Int J Biochem Cell Biol. 30:567–571. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shubbar E, Vegfors J, Carlström M,
Petersson S and Enerbäck C: Psoriasin (S100A7) increases the
expression of ROS and VEGF and acts through RAGE to promote
endothelial cell proliferation. Breast Cancer Res Treat. 134:71–80.
2012. View Article : Google Scholar
|
|
20
|
Sneh A, Deol YS, Ganju A, Shilo K, Rosol
TJ, Nasser MW and Ganju RK: Differential role of psoriasin (S100A7)
in estrogen receptor α positive and negative breast cancer cells
occur through actin remodeling. Breast Cancer Res Treat.
138:727–739. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gadiya M, Mori N, Cao MD, Mironchik Y,
Kakkad S, Gribbestad IS, Glunde K, Krishnamachary B and Bhujwalla
ZM: Phospholipase D1 and choline kinase-α are interactive targets
in breast cancer. Cancer Biol Ther. 15:593–601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zuker M: Mfold web server for nucleic acid
folding and hybridization prediction. Nucleic Acids Res.
31:3406–3415. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bonnekoh B, Wevers A, Jugert F, Merk H and
Mahrle G: Colorimetric growth assay for epidermal cell cultures by
their crystal violet binding capacity. Arch Dermatol Res.
281:487–490. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moffitt RA, Marayati R, Flate EL, Volmar
KE, Loeza SG, Hoadley KA, Rashid NU, Williams LA, Eaton SC, Chung
AH, et al: Virtual microdissection identifies distinct tumor- and
stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat
Genet. 47:1168–1178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang G, He P, Tan H, Budhu A, Gaedcke J,
Ghadimi BM, Ried T, Yfantis HG, Lee DH, Maitra A, et al:
Integration of metabolomics and transcriptomics revealed a fatty
acid network exerting growth inhibitory effects in human pancreatic
cancer. Clin Cancer Res. 19:4983–4993. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deer EL, González-Hernández J, Coursen JD,
Shea JE, Ngatia J, Scaife CL, Firpo MA and Mulvihill SJ: Phenotype
and genotype of pancreatic cancer cell lines. Pancreas. 39:425–435.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Emberley ED, Niu Y, Leygue E, Tomes L,
Gietz RD, Murphy LC and Watson PH: Psoriasin interacts with Jab1
and influences breast cancer progression. Cancer Res. 63:1954–1961.
2003.PubMed/NCBI
|
|
29
|
Tomoda K, Kubota Y and Kato J: Degradation
of the cyclin-dependent-kinase inhibitor p27Kip1 is
instigated by Jab1. Nature. 398:160–165. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sgambato A, Cittadini A, Faraglia B and
Weinstein IB: Multiple functions of p27(Kip1) and its alterations
in tumor cells: A review. J Cell Physiol. 183:18–27. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ellenrieder V, Alber B, Lacher U, Hendler
SF, Menke A, Boeck W, Wagner M, Wilda M, Friess H, Büchler M, et
al: Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int
J Cancer. 85:14–20. 2000. View Article : Google Scholar
|
|
32
|
Zhang K, Chen D, Jiao X, Zhang S, Liu X,
Cao J, Wu L and Wang D: Slug enhances invasion ability of
pancreatic cancer cells through upregulation of matrix
metalloproteinase-9 and actin cytoskeleton remodeling. Lab Invest.
91:426–438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morgan MR, Jazayeri M, Ramsay AG, Thomas
GJ, Boulanger MJ, Hart IR and Marshall JF: Psoriasin (S100A7)
associates with integrin β6 subunit and is required for
αvβ6-dependent carcinoma cell invasion. Oncogene. 30:1422–1435.
2011. View Article : Google Scholar
|
|
34
|
Dey KK, Sarkar S, Pal I, Das S, Dey G,
Bharti R, Banik P, Roy J, Maity S, Kulavi I, et al: Mechanistic
attributes of S100A7 (psoriasin) in resistance of anoikis resulting
tumor progression in squamous cell carcinoma of the oral cavity.
Cancer Cell Int. 15:742015. View Article : Google Scholar : PubMed/NCBI
|