Introduction
Gastric cancer is one of the most aggressive
malignancies. It has the second highest mortality rate among
cancers and is the fourth most common cancer in the world (1,2).
Over the past decades, numerous efforts have been made in improving
the treatment of gastric cancer, but unfortunately the outcome has
been disappointing (3). Emerging
evidence suggests that the existence of cancer stem cells (CSCs)
could play pivotal roles in cancer progression and treatment
resistance in gastric cancer (4,5).
CSCs, being the small proportion of cancer cells, have powerful
self-renewal capacity and the potential to differentiate into any
cells in the tumor population, and are responsible for the
resistance to chemotherapy and radiation (6). Additionally, CSCs are considered to
be the most important source of tumor invasion and metastasis.
CSCs have been successfully identified in a variety
of cancers including breast, brain, colon, and gastric cancer
(7–10). Consequently, numerous therapeutic
approaches targeting CSCs have been proposed (11–15).
One of the effective strategies for the identification and
elimination of CSCs is to target several cell surface markers
overexpressed in CSCs (16,17).
Elevated expression of these cell surface markers including CD44,
CD133, CD123, EpCAM (CD326) (18–20)
was correlated with different clinical significance such as
clinical phenotype and prognosis. CD44 is a transmembrane
glycoprotein and used to isolate CSCs in various cancers,
especially in gastric cancer (21,22).
Epithelial-mesenchymal transition (EMT) plays
critical roles not only in tumor metastasis but also in recurrence.
In the EMT process, the expression of E-cadherin is downregulated,
while the expression of the mesenchymal molecules such as vimentin
and N-cadherin are upregulated. In addition, increasing evidence
implicates that EMT-type tumor cells share various biological
characteristics with CSCs. Importantly, microRNAs (miRNAs) can
serve as the key molecule in the EMT and some other biological and
pathologic processes.
miRNAs are endogenous and small non-coding RNA
molecules (~22 nt) that negatively regulate gene expression by
targeting mRNAs posttranscriptionally. miRNAs bind to the partially
complementary target sites in 3′-untranslated region (3′-UTRs) of
mRNA, inducing mRNA degradation or translational inhibition. To
date, abnormal expression of miRNAs has been identified in various
kinds of malignancies, which can function as either oncogenes or
tumor suppressor genes. Moreover, miRNAs play an important role in
modulating CSCs properties such as self-renewal capacity, migration
and invasion (23,24). miR-200c overexpression inhibits
chemoresistance, invasion and colony formation in pancreatic CSCs
(25). The miR-106b family is
overexpressed in several tumors including gastric cancer, which
could regulate CSC properties, particularly EMT characteristics
through the TGF-β signaling pathway (26). miR-19b/20a/92a regulates the
self-renewal ability and proliferation of GCSCs (27).
In this study, we have identified CSCs phenotypes in
CD44(+) stem-like cells in gastric cancer. Then we analyzed the
miRNA expression profiles between CD44(+) and CD44(−) gastric cells
to explore molecular mechanisms related to CSCs characteristics.
Our data demonstrated that upregulated miR-196a-5p markedly
promoted invasion and contributed to stem cell-like phenotypes in
GCSCs by targeting Smad4 and mediating TGF-β-induced EMT. As a
novel miRNA, miR-196a-5p can modulate gastric cancer stem cell-like
characteristics and may be a potential target for gastric cancer
therapy.
Materials and methods
Cell lines and cancer stem cell
culture
The human gastric SNU-5 and BGC-823 cell lines were
purchased from the Chinese Academy of Sciences Cell Bank. SNU-5
gastric cancer cells were maintained in RPMI-1640 medium (Thermo
Fisher Scientific, USA) supplemented with 10% FBS (Thermo Fisher
Scientific), 1% L-glutamine (Gibco, Grand Island, NY, USA) and 1%
penicillin-streptomycin sulfate (Thermo Fisher Scientific). BGC-823
gastric cancer cells were maintained in DH medium (Thermo Fisher
Scientific) supplemented with 10% FBS (Thermo Fisher Scientific),
1% L-glutamine (Gibco) and 1% penicillin-streptomycin sulfate
(Thermo Fisher Scientific).
The serum-free medium (SFM) was composed of
DMEM/F12, 20 µl/ml B27 supplement (Life Technologies), 20
ng/ml basic fibroblast growth factor (bFGF, Gibco), 10 ng/ml EGF
(Gibco) and LIF (Gibco). GCSCs were isolated from SNU-5 or BGC-823
cell line by using SFM. These cells can form sphere-like cell
aggregates in less than 7 days. All cultures were maintained in a
37°C incubator supplemented with 5% CO2.
Flow cytometry analysis and
fluorescence-activated cell sorting
GCSCs were isolated from SNU-5 or BGC-823 cell line
by using SFM in less than 7 days. The cells were stained with a
400-fold dilution of anti-CD44-FITC (BD Biosciences) and incubated
for 1 h at 4°C. Then the cells were washed with PBS. Finally, they
were analyzed and sorted immediately with fluorescence-activated
cell sorting (FACS) AriaIII (BD Biosciences).
miRNA microarray
Total RNAs of CD44(+) and CD44(−) SNU-5 cells were
prepared using TRIzol reagent (Invitrogen) according to the
manufacturer's instructions. Microarray was performed using the
Affymetrix GeneChip miRNA 2.0 platform (CapitalBio Technology,
China). The comparative analysis between the CD44(+) sample and
CD44(−) sample was carried out using fold-change, and miRNA with
2-fold change in expression were considered differentially
regulated. Hierarchical cluster analysis was performed using
complete linkage and Euclidean distance as a measure of
similarity.
Quantitative real-time PCR (qRT-PCR)
The first-strand cDNA was synthesized using iscript
cDNA synthesis kit (Bio-Rad, USA) according to the manufacturer's
instructions. Quantitative real-time PCR analyses were carried out
with a PCR mixture containing 1 µmol/l of each primer and
EvaGreen Supermix (Bio-Rad). The amplifications were performed at
95°C for 15 sec and at 60°C for 30 sec using a StepOnePlus
real-time PCR system (Applied Biosystems). Each sample was examined
in triplicate and the mRNA level of GAPDH was used as an internal
control. Primer sequences are listed in Table I.
 | Table IPrimers used for the real-time PCR
analysis. |
Table I
Primers used for the real-time PCR
analysis.
| Gene | Direction | Primer sequences
(5′-3′) |
|---|
| CD44 | F |
GCTATTGAAAGCCTTGCAGAG |
| R |
CGCAGATCGATTTGAATATAACC |
| Sox2 | F |
AACCAAGACGCTCATGAAGAAG |
| R |
CTGCGAGTAGGACATGCTGTAG |
| Oct4 | F |
GACAACAATGAAAATCTTCAGGAGA |
| R |
TTCTGGCGCCGGTTACAGAACCA |
| Nanog | F |
GTCCCAAAGGCAAACAACCC |
| R |
GCTGGGTGGAAGAGAACACA |
| Smad4 | F |
GCTGCTGGAATTGGTGTTGATG |
| R |
AGGTGTTTCTTTGATGCTCTGTCT |
| E-cadherin | F |
TGCTTGGTTCACCAGTGGAT |
| R |
TTTTGTTGAGCAAGGCAACC |
| N-cadherin | F |
AACTCCAGGGGACCTTTTC |
| R |
CAAATGAAACCGGGCTATC |
| Vimentin | F |
GAGAGGAAGCCGAAAACACC |
| R |
GCTTGGAAACATCCACATCG |
| Slug | F |
CTTCCTGGTCAAGAAGCATT |
| R |
TGAGGAGTATCCGGAAAGAG |
| Snail | F |
GGCCTTCAACTGCAAATACT |
| R |
TTGACATCTGAGTGGGTCTG |
| GAPDH | F |
TGCACCACCAACTGCTTAGC |
| R |
GGCATGGACTGTGGTCATGAG |
To confirmed expression of miR-196a-5p, real-time
qPCR was performed. Total RNA was extracted from culture cells
using mirVana miRNA isolation kit (Ambion). miRNAs expression was
measured using Bulge-Loop miRNA qRT-PCR Primer Set (Ribobio Co.,
Guangzhou, China) and qRT-PCR Starter kit. The amplifications were
performed at 95°C for 20 sec, followed by 40 cycles of amplication
at 95°C for 10 sec, 60°C for 20 sec and 70°C for 10 sec using an
ABI7500 real-time PCR system (Applied Biosystems). The relative
miRNA expression level was calculated from three different
experiments. The fold-change for miRNA relative to U6 was
determined by the formula 2−ΔΔCt.
Sphere colony formation assay
Gastric cancer cells were plated in each well of
ultra-low-attachment 24-well plates (Corning Life Sciences,
Corning, NY, USA) at low density (500 cells per each well) with
0.8% methyl cellulose (Sigma, USA) supplemented with 20
µl/ml B27 supplement (Life Technologies), 20 ng/ml basic
fibroblast growth factor (bFGF, Gibco), 10 ng/ml EGF (Gibco), LIF
(Gibco), 1% L-glutamine (Gibco) and 1% penicillin-streptomycin
sulfate (Thermo Fisher Scientific). Every 3 days, each well was
examined using light microscopy.
Invasion assay
Twenty-four-well, 8.0 µm Transwells (BD
Biosciences) were coated with diluted Matrigel (BD Biosciences) in
PBS and dried for 1 h. For the invasion assay, cells were suspended
in medium without serum, and then 2×105 cells were
seeded in the top chamber. Medium supplemented with 10% FBS was
used as a chemoattractant in the bottom chamber. The cells were
incubated for 8 h in a 37°C incubator supplemented with 5%
CO2. The non-invasive cells on the top chambers were
removed with cotton swabs. The invaded cells on the lower membrane
surface were fixed in 100% methanol for 15 min and washed in PBS
three times, then stained with 4,6-diamidino-2-phenylindole (DAPI).
Invaded cells were counted under a microscope. Three independent
experiments were conducted and the data are presented as the mean ±
SEM.
miRNA inhibitor transfection
miR-196a-5p inhibitor and the negative control were
obtained from Ribobio Co. FACS-sorted CD44(+) cells and CD44(−)
cells were respectively seeded into 6-well plates at a density of
1×105 cells/well in antibiotic-free medium and incubated
overnight. They were transfected with miRNA inhibitor or the
negative control using Lipofectamine 2000 (Invitrogen) according to
the manufacturer's instructions. The final concentration of miRNA
inhibitor was 60 nM. The transfected cells were incubated for 4 h,
and nomal media was added. The cells were harvested for the
extraction of the RNA and protein after cultured for 72 h.
Target prediction
The prediction of the target Smad4 3′-UTR as a miRNA
binding target was performed using Targetscan (www.targetscan.org), microRNA (www.microrna.org) and PicTar (pictar.mdc-berlin.de). miRNAs that were positively
predicted by all these programs were selected for this study.
Luciferase reporter assay
The 3′-UTR of Smad4 containing miR-196a-5p seed
binding sites was cloned into the psiCHECK-2 vector to construct
the wild-type (WT) vector (psicheck-SMAD4-WT1 and
psicheck-SMAD4-WT2). A mutant 3′-UTR of Smad4 was synthesized by
PCR and cloned into the psiCHECK-2 vector to construct the mutation
type (MuT) vector (psicheck-SMAD4-MUT1 and psicheck-SMAD4-MUT2).
SNU-5 sphere-like cells were seeded into 24-well plates
(1×105 cells/well) and transfected with
psicheck-SMAD4-WT/MUT and miR-196a-5p mimics/NC. Luciferase
activities were measured by the dual-luciferase reporter assay
system (Promega).
Plasmid construction and
transfection
The coding domain sequence of Smad4 was synthesized
and the DNA fragment was digested with BamHI and
EcoRI. The resulting fragment was subcloned into the
BamHI and EcoRI sites of the pcDNA 3.1(+) vector
(Invitrogen). The cell transfection was carried out with FuGENE HD
reagent (Roche) according to the manufacturer's instructions.
Protein extraction and western blot
analysis
Cultured cells were harvested after washing twice
with ice-cold PBS. Protein was extracted from collected cells by
Subcellular Protein Fractionation kit (Thermo Scientific Pierce).
The protein concentration was detected using a BCA protein assay
kit (Thermo Scientific Pierce). The same amount of protein was
boiled at 95°C after adding isometric bromophenol blue (Amresco).
Samples were loaded in 12% SDS-PAGE gels for Sox2, Oct4, Nanog,
Smad4, Vimentin, Slug, and Snail and in 6% SDS-PAGE gels for
E-cadherin and N-cadherin, and blotted onto PVDF membranes. After
blocking, nitrocellulose blots were incubated for 1 h with primary
antibodies diluted in TBS/Tween-20 (0.075%) containing 3% Marvel.
Mouse monoclonal antibody directed against SMAD4 (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) was diluted at 1:1,000.
Rabbit monoclonal to CD44 (Abcam, USA) was diluted at 1:5,000.
Rabbit polyclonal anti-Sox2 (Cell Signaling Technology, USA) was
used at 1:1,000 as was Oct4, Nanog, E-cadherin, N-cadherin,
Vimentin, Slug, and Snail. Peroxidase-conjugated affinipure goat
anti-mouse and anti-rabbit antibodies were used as secondary
antibodies correspondingly. After five washes in TBS/Tween-20,
membranes with PBS were developed using the enhanced
chemiluminescence system for the detection of antigen. Protein was
visualized with ECL-chemiluminescent kit (Thermo Fisher
Scientific).
Immunohistochemistery
Paraffin-embeded tissue samples were sectioned and
stained for routine histology (Fuzhou Maixin Biotech Co., Ltd.).
The sections were dewaxed in xylene and dehydrated in alcohol.
Antigen retrieval was achieved by microwaving in citric acid buffer
for 15 min. After the peroxidase activity had been blocked with 3%
H2O2-methanol for 15 min. The sections were
incubated with 10% normal goat serum in PBS to block non-specific
protein binding, followed by incubation with primary antibody
against SMAD4 (1:300; Santa Cruz Biotechnology, Inc.) at 4°C
overnight. After rinsing, the sections were incubated with
biotinylated secondary antibody for 60 min at room temperature.
SMAD4 expression was visualized by 3,3′-diaminobenzidine
tetrahydrochloride (DAB) staining, followed by counterstaining with
hematoxylin.
Statistical analysis
All experiments were repeated at least three times.
All data were summarized and presented as means ± SD. Statistical
analyses were performed using SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA). The differences between two groups were analyzed
using a Student's t-test, and more than two groups were compared by
a one-way analysis of variance (ANOVA). Data were considered
significant at P<0.05 or P<0.001.
Results
Isolation and characteristics of human
GCSCs
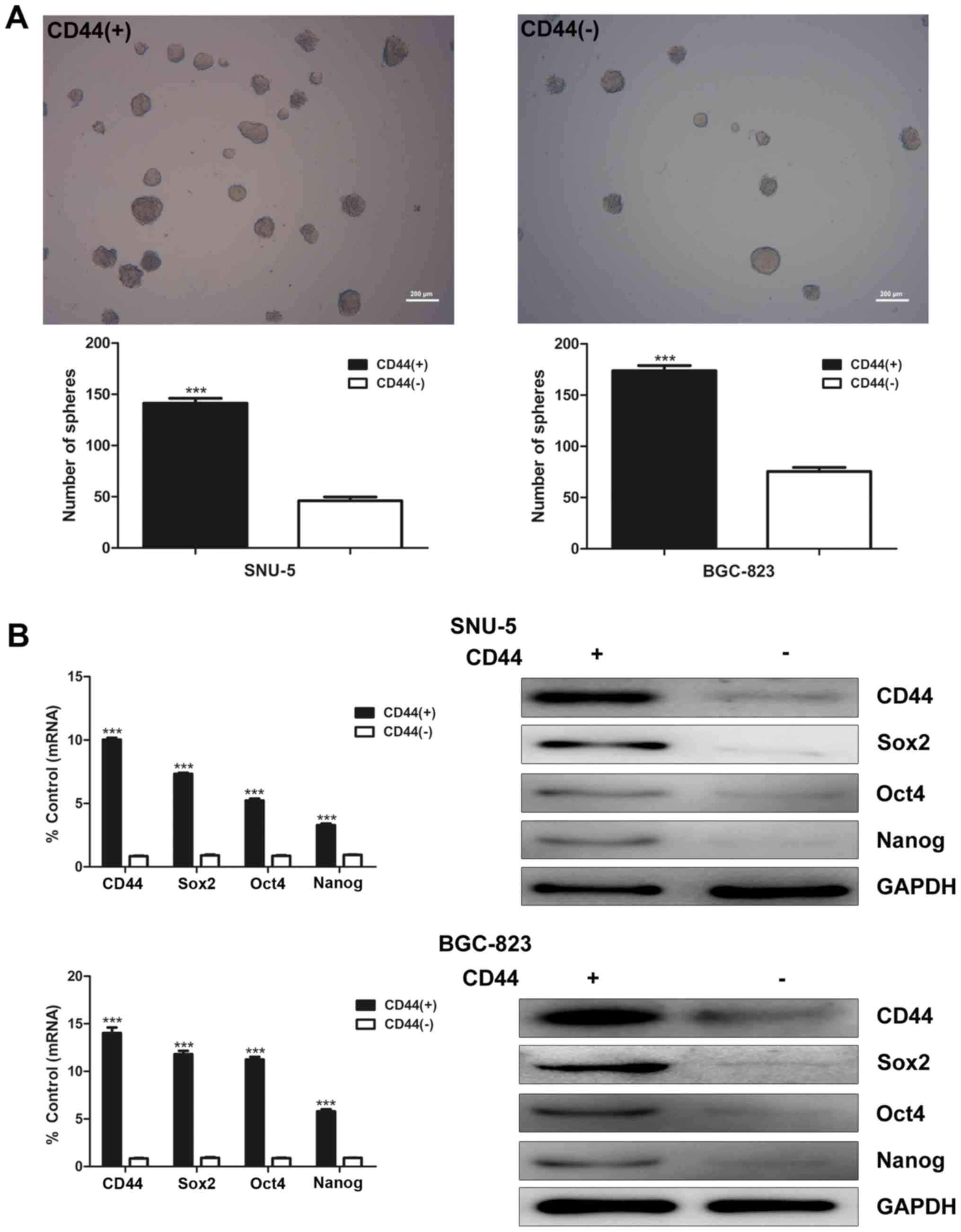
To isolate GCSCs, SNU-5 and BGC-823 cells were
subjected to FACS sorting by anti-CD44 antibody. CD44(+) and
CD44(−) cells were differentially isolated. After growing under
serum-free conditions for 1–2 weeks, CD44(+) cells formed more and
larger spheroid colonies than CD44(−) cells in both SNU-5 and
BGC-823 cell lines (Fig. 1A). To
determine stem cell characteristics of CD44(+) GCSCs, real-time PCR
and western blot analysis were used to evaluate mRNA and protein
expression of stem cell markers Sox2, Oct4, Nanog in CD44(+) and
CD44(−) cells. The results indicated that the mRNA and protein
expression of Sox2, Oct4 and Nanog were both significantly higher
in CD44(+) cells than CD44(−) cells in both SNU-5 and BGC-823 cell
lines (Fig. 1B).
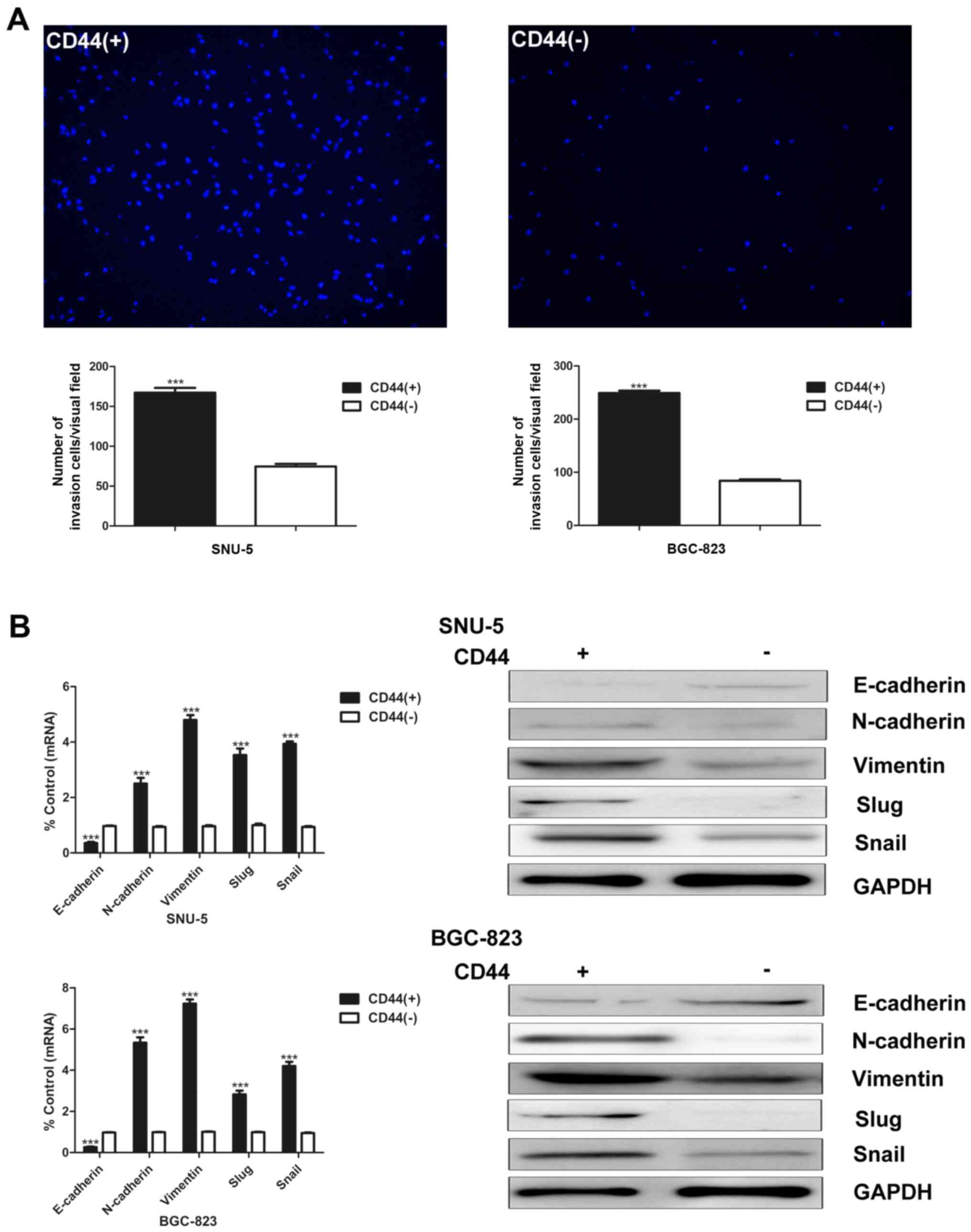
CD44(+) cells show an increased invasion
capacity and EMT characteristics compared with CD44(−) cells
CSCs have higher invasion and migration capacity,
which is considered as the crucial characteristic to facilitate
tumor metastasis and growth. Matrigel invasion assay was used to
compare the invasion abilities of CD44(+) and CD44(−) cells.
CD44(+) cells possess higher invasion capacity than CD44(−) cells
(Fig. 2A).
EMT is a widespread developmental program that
regulates cell migration in many tissues and organs. Growing
evidence reveals that EMT contributes to the invasion and
metastasis of CSCs. We then detected several well-known EMT markers
at both mRNA and protein level. The epithelial marker E-cadherin
was downregulated in CD44(+) cells. On the other hand, the
expression of N-cadherin, vimentin, slug and snail were higher in
CD44(+) SNU-5 and BGC-823 cells (Fig.
2B).
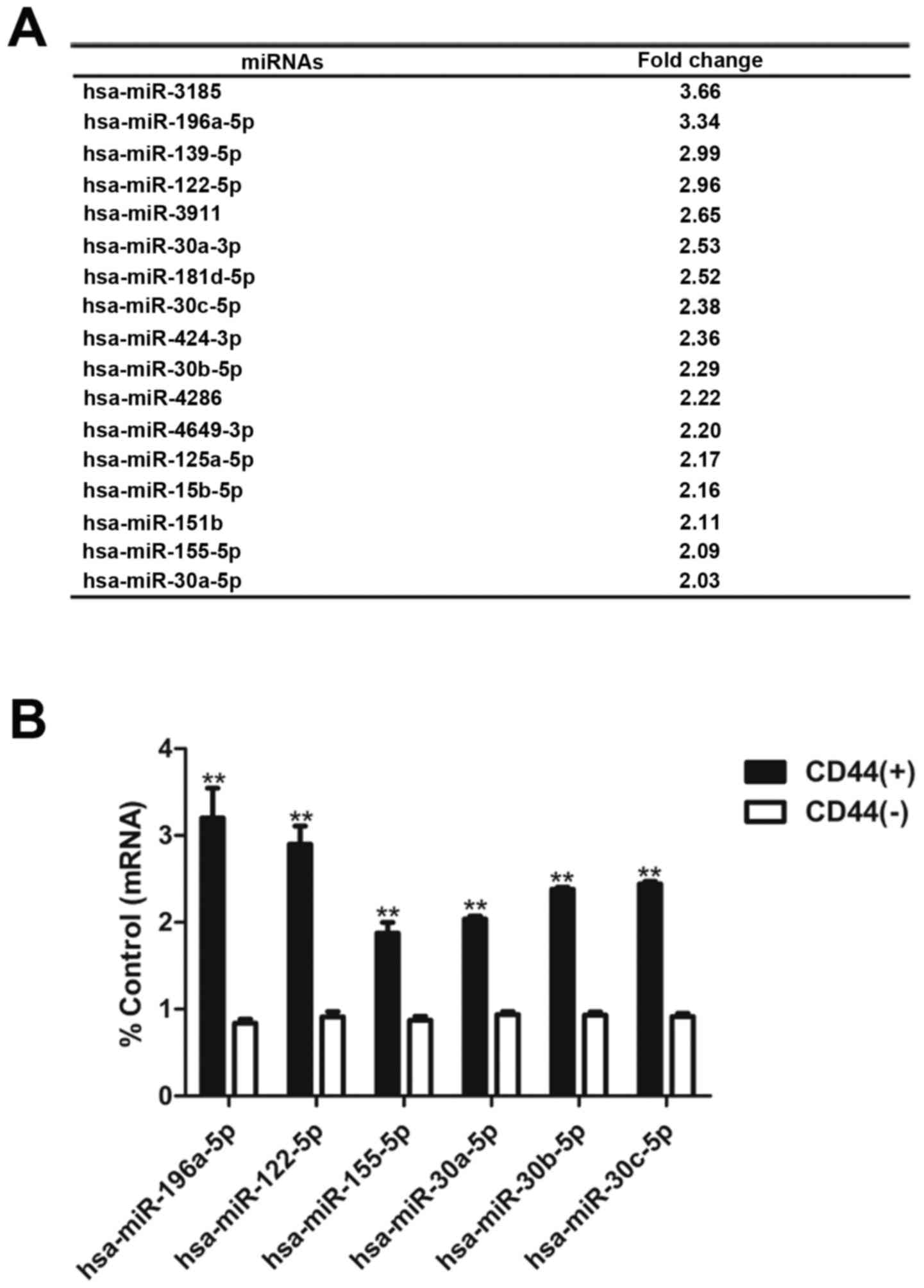
miR-196a-5p is upregulated in CD44(+)
cells in microarray analysis
Microarry analysis revealed a series of miRNAs
upregulated in CD44(+) cells compared with CD44(−) cells (Fig. 3A). Real-time PCR validated that the
expression of miR-196a-5p, miR-122-5p, miR-155-5p, miR-30a-5p,
miR-30b-5p and miR-30c-5p in CD44(−) cells was less than that in
CD44(+) cells (Fig. 3B).
In our pre-screening studies, miR-196a-5p showed the
most significant biological functions on GCSCs among the
upregulated miRNAs identified aforementioned. Additionally, there
is still no relevant research on miR-196a-5p in the field of GCSCs.
This prompted us to carry out further study on the regulatory
mechanism of miR-196a-5p on cancer stem-like cell
characteristics.
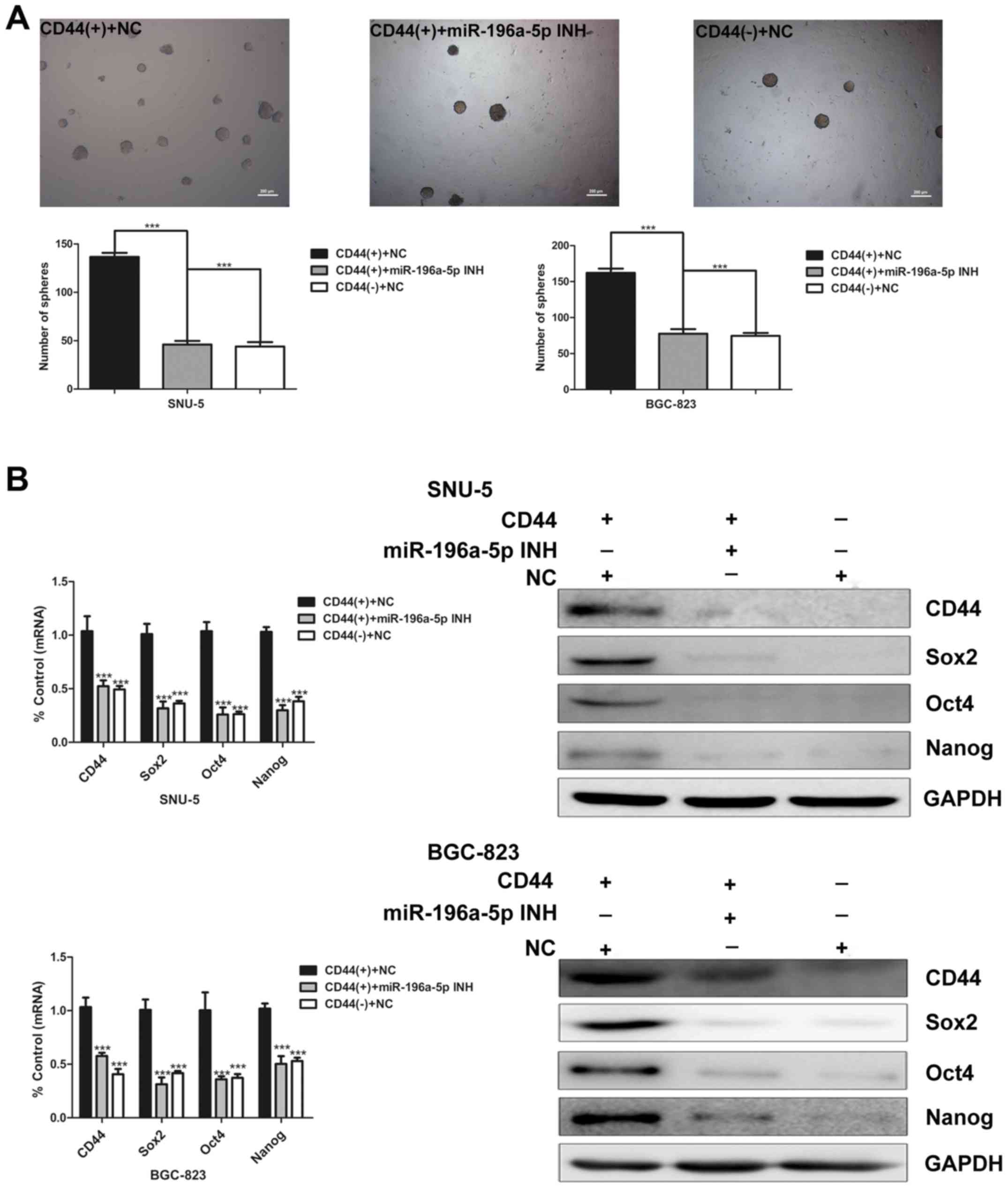
miR-196a-5p regulates the self-renewal
capacity and invasion ability in CD44(+) cells
To evaluate the effect of miR-196a-5p on the
self-renewal capacity, we initially carried out sphere colony
formation assay of CD44(+) and CD44(−) cells transfected with
miR-196a-5p inhibitor. As shown in Fig. 4A, miR-196a-5p knockdown
significantly decreased the sphere colony forming capacity of
CD44(+) cells (Fig. 4A). The
expression of CD44 and cancer stem cell markers at mRNA and protein
levels were determined by real-time PCR and western blot analysis
respectively. Compared with CD44(+) cells transfected with the
negative control, the expression of these genes were relatively
downregulated in the CD44(+) cells transfected with miR-196a-5p
inhibitor to similar levels in CD44(−) cells (Fig. 4B). These data indicate that the
miR-196a-5p expression may promote the self-renewal ability of
GCSCs.
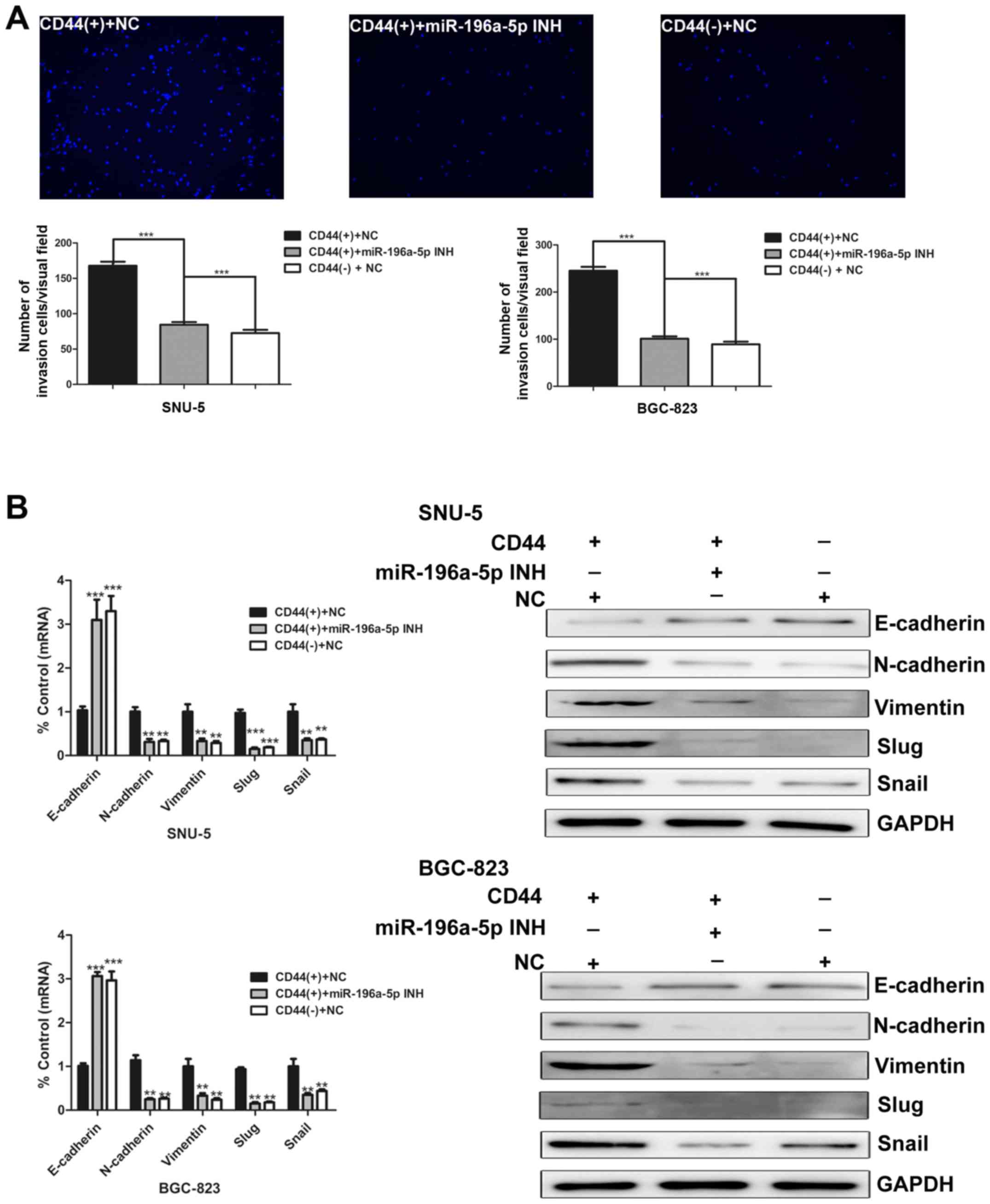
Next, we investigated the role of miR-196a-5p in
regulating invasion ability of GCSCs. After 48 h, the number of
invading cells in each group was counted. The results illustrate
that the number of invasive CD44(+) cells markedly decreased after
miR-196a-5p was inhibited (Fig.
5A). Moreover, the effects of miR-196a-5p on EMT were studied.
As measured by real-time PCR and western blotting, E-cadherin was
upregulated, whereas N-cadherin, vimentin, slug and snail were
downregulated in miR-196a-5p inhibitor-transfected CD44(+) cells
(Fig. 5B). Collectively, these
data demonstrate that miR-196a-5p promotes EMT and invasion of
GCSCs.
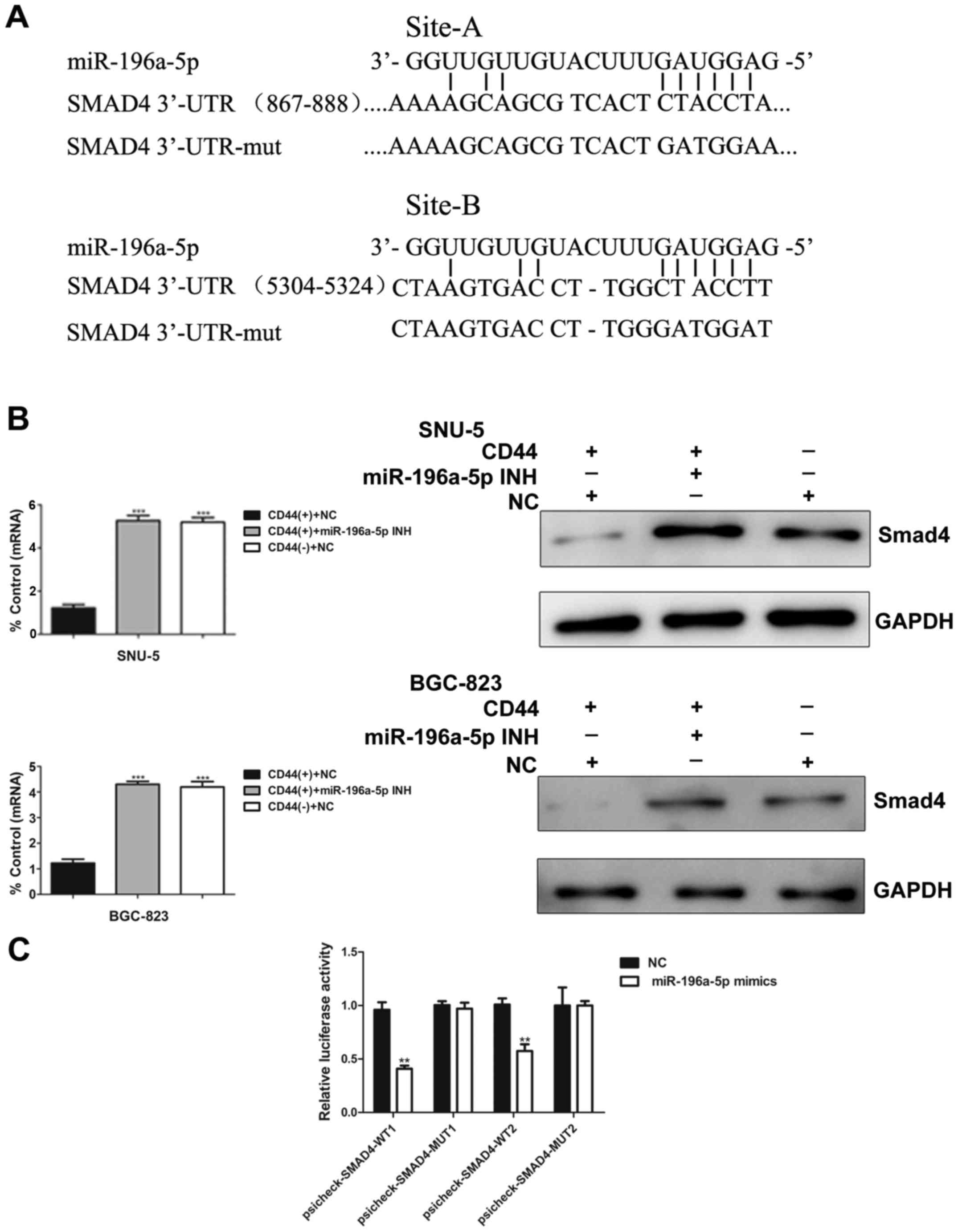
Smad4 is a direct and specific target of
miR-196a-5p in GCSCs
To determine how miR-196a-5p may regulate EMT and
invasion, we applied three bioinformatic algorithms (TargetScan,
PicTar and miRanda) to search for potential targets of miR-196a-5p.
Based on the bioinformatics prediction, there are two conserved
binding sites, 867–888 and 5304–5324, in the 3′-UTR region of the
Smad4 mRNA targeted by miR-196a-5p (Fig. 6A). To validate the results of
computational analysis, we used real-time PCR and western blotting
to compare Smad4 expression in CD44(+) and CD44(−) cells isolated
from two human gastric cancer cell lines which were transfected
with or without miR-196a-5p inhibitor. As shown in Fig. 6B, Smad4 mRNA and protein expression
were significantly increased after inhibition of miR-196a-5p
(Fig. 6B).
To obtain further direct evidence that miR-196a-5p
alters Smad4 expression, we performed luciferasre reporter assays.
Remarkably, luciferase analysis showed that miR-196a-5p mimics
repressed the activities of psicheck-SMAD4-WT1 and
psicheck-SMAD4-WT2. In contrast, psicheck-SMAD4-MUT, in which the
binding sites of miR-196a-5p are mutated, showed higher luciferase
activities than psicheck-SMAD4-WT. Additionally, transfection of
miR-196a-5p mimics had no suppression or activation of
psicheck-SMAD4-MUT1 and psicheck-SMAD4-MUT2 (Fig. 6C). Thus, the expression of
miR-196a-5p is inversely correlated with Smad4, indicating that
Smad4 may be a direct and specific target of miR-196a-5p for
regulatory functions in GCSCs.
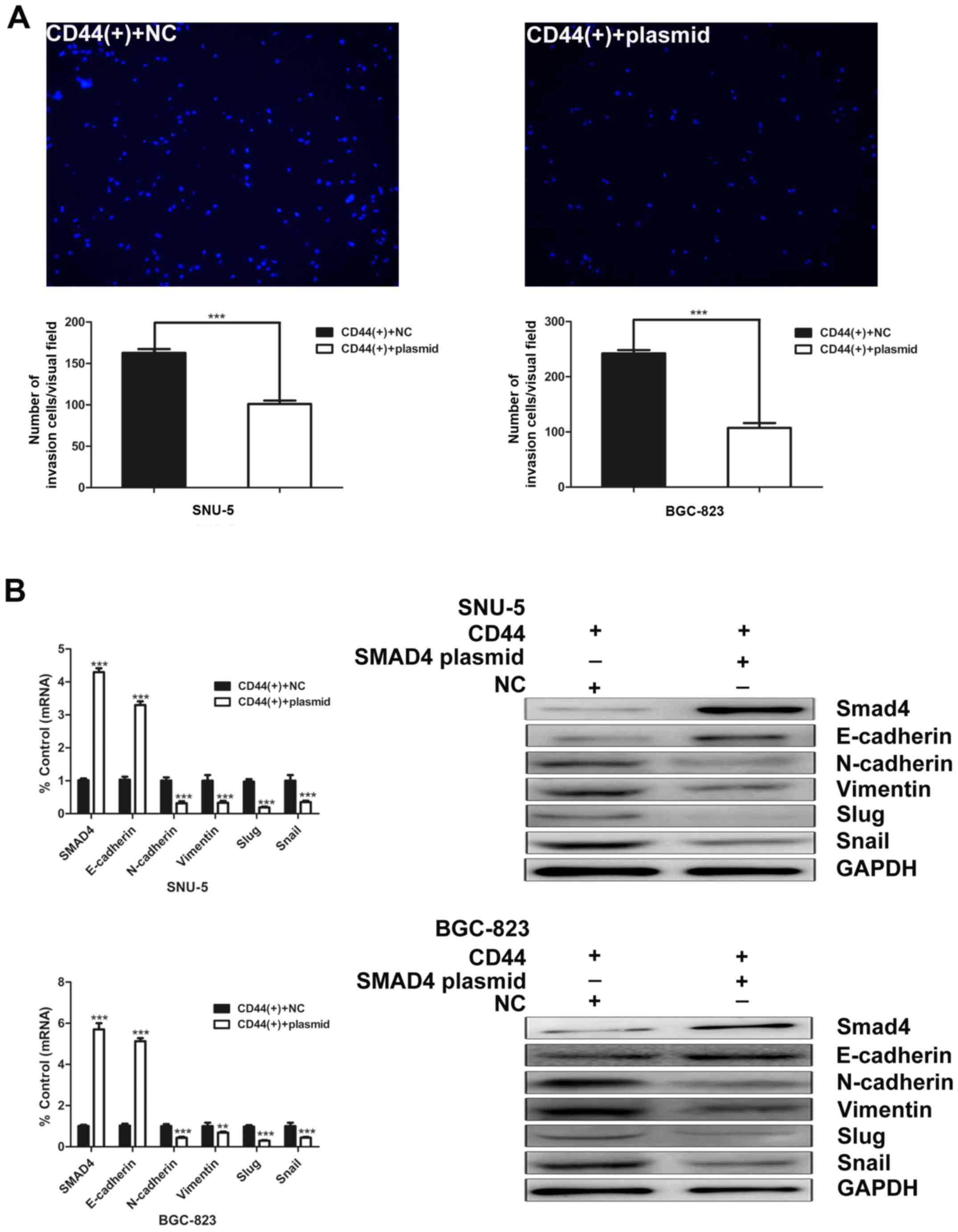
Overexpression of Smad4 decreases GCSCs
invasion ability
To further characterize the function of Smad4 in
GCSCs, we examined the effects of Smad4 overexpression on the
invasion capacity of GCSCs. Overexpression of Smad4 markedly
decreased the invasion ability of CD44(+) GCSCs (Fig. 7A). After Smad4 overexpression in
CD44(+) SNU-5 cells, the expression of N-cadherin, vimentin, slug
and snail were downregulated, whereas E-cadherin was upregulated.
Similar results were observed in BGC-823 cells (Fig. 7B).
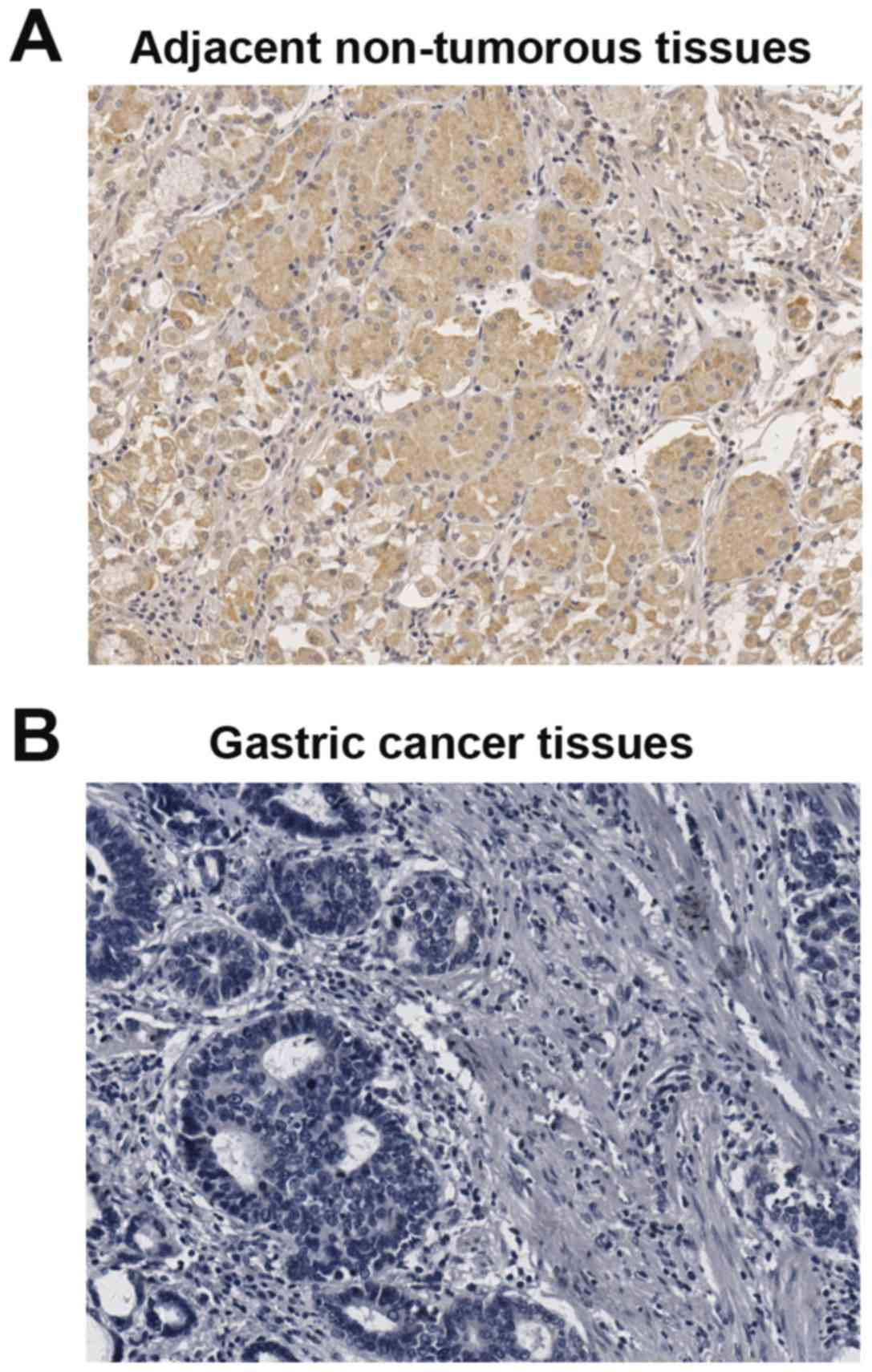
Correlation between the expression of
Smad4 and pathological parameters in gastric cancer patients
To investigate the clinical significance of Smad4 in
gastric cancer development, the expression of Smad4 was analyzed by
immunohistochemistry (IHC) staining in 95 gastric cancer cases and
paired adjacent non-tumorous tissues. IHC staining confirmed that
the downregulation of Smad4 in gastric cancer tissues compared with
adjacent non-tumorous tissues (Fig.
8). Next, we detected the relationship between pathological
characteristics and Smad4 expression levels in gastric cancer
patients. No significant association between Smad4 expression
levels and the patient age, and sex was detected in 95 gastric
cancer cases (Table II). However,
the levels of Smad4 were significantly correlated with
differentiation state, TNM stage and depth of invasion (Table II). Importantly, Smad4 expression
was negatively correlated with differentiation of tumors.
 | Table IIRelationship between Smad4 expression
and pathological parameters in 95 gastric cancer patients. |
Table II
Relationship between Smad4 expression
and pathological parameters in 95 gastric cancer patients.
|
Characteristics | n | Smad4 expression
| P-value |
|---|
| Negative | Positive |
|---|
| Age (years) | | | | |
| <60 | 26 | 23 | 3 | 0.206 |
| ≥60 | 69 | 53 | 16 | |
| Gender | | | | |
| Male | 70 | 54 | 16 | 0.244 |
| Female | 25 | 22 | 2 | |
|
Differentiation | | | | |
| PD | 80 | 69 | 11 | 0.001a |
| MD | 13 | 7 | 6 | |
| WD | 2 | 0 | 2 | |
| TNM stage | | | | |
| I+II | 27 | 16 | 11 | 0.001a |
| III+IV | 68 | 60 | 8 | |
| Depth of
invasion | | | | |
| T1 + T2 | 31 | 21 | 10 | 0.038a |
| T3 + T4 | 64 | 55 | 9 | |
Discussion
RNA is probably the first macromolecule evolved in
life and may play a crucial role in regulating the cell functions.
In addition to the well recognized members of tRNA, mRNA, and rRNA
related to the genetic central dogma, several types of small RNAs,
such as miRNAs, have been recently identified responsible for the
tight regulation of gene expression and cellular function. In the
present study, we have associated the relationship between
miR-196a-5p and gastric cancer, and further narrowed the possible
target Smad4 in affecting the characteristics of gastric cancer
stem cells.
There is increasing evidence that tumors are
composed of phenotypically and functionally diverse populations of
neoplastic cells. Furthermore, the subpopulations with stem cell
properties, the CSCs are closely related to cancer development,
metastasis and resistance to therapy. CSCs are characteristic of
self-renewal and differentiation capacity, high tumorigenesis and
invasiveness, resistance to chemotherapy and radiation, genetic and
epigenetic changes. Numerous therapeutic approaches for eradicating
CSCs have been proposed. Generally, the core idea can be envisaged:
eliminating the CSCs themselves by either killing or
differentiating them, and disruption of niche signaling. According
to ClincalTrials.gov, some anti-CSCs
compounds have been already completed different phases of testing
and successfully entered human clinic trials. Therefore,
therapeutic approaches targeting CSCs bring new hope for future
antitumor treatment. As the crucial regulator of gene expression in
cell cycle, miRNAs act as oncogenes or tumor suppressor genes in
cancer development. Additionally, aberrantly expressed miRNAs play
a major role in the biological properties of CSCs. Therefore,
miRNAs have been identified as the attractive target for anti-CSC
therapies.
In this study, several stem cell surface markers
were tested in order to select the appropriate marker for GCSCs
isolation, such as CD44, EpCAM and CD133. More than 99% of SNU-5
and BGC-823 cell populations expressed the above markers except
CD44 (data not shown). Similar results have been reported
previously (28–30). Based on our previous studies, CD44
was successfully selected as a surrogate marker of GCSCs. Compared
with CD44(−) cells, FACS-sorted CD44(+) cells showed many cancer
stem-like cell charateristics. First, the self-renewal capacity of
cells was examined by sphere formation ability. CD44(+) cells form
more sphere colonies than CD44(−) cells in SNU-5 and BGC-823 cell
lines. The expression of stem cell markers was upregulated at the
mRNA and protein levels. Second, CD44(+) cells possess stronger
invasive ability than CD44(−) cells. In terms of molecular
expression and phenotype of EMT properties, CD44(+) cells
manifested a biological transformation toward mesenchymal cell
types. However, CD44(+) alone cannot be used as an exclusive marker
of CSCs since CD44(−) can also show sphere formation in culture and
develop cancer in animals. Our miRNA microarray data documented
that miR-196a-5p was significantly upregulated in CD44(+) cells
compared with CD44(−) cells. Although there were other miRNAs
overexpressed in CD44(+) cells, miR-196a-5p was firstly selected to
further study according to the following two facets. First,
miR-196a-5p is a newly reported miRNA relevant to multiple cancers,
but there is still no report on its role in CSCs. Second,
miR-196a-5p had the most significant effects on cancer stem-like
cell charateristics in our pre-screening studies.
The present study provided evidence indicating that
the newly found target gene of miR-196a-5p is Smad4, which
functions as Co-Smad in TGF-β/Smad signaling pathway. When TGF-β1
binds to its receptor, Smad2/3 is phosphorylated and binds with
Smad4 to form multimers and translocate to the nucleus to drive the
transcriptional regulation of several target genes (31–34).
Emerging evidence suggests the TGF-β signaling pathway has a major
role in EMT resulting in tumor metastasis and recurrence. Our data
also showed that miR-196a-5p regulated EMT through the TGF-β/Smad
signaling pathway. Interestingly, the stimulation of
epithelial-mesenchymal transition (EMT) by miR-196a-5p in cancer
stem-like cells was abolished by overexpression of Smad4.
Collectively, the expression of Smad4 in gastric cancer tissues was
correlated with differentiation state of tumors, TNM stage and
depth of invasion.
Based on the above-mentioned data, it is strongly
suggested that miR-196a-5p markedly modulates gastric cancer stem
cell characteristics by targeting Smad4. Hence, it is necessary and
valuable to transform this observation to clinical therapeutics.
There are important aspects for our future clinical studies. First,
we will measure the miR-196a-5p expression level in gastric cancer
patients and analyze the relationship between the miR-196a-5p
expression level and clinical characteristics, particularly
metastasis, invasion of gastric cancer. Additionally, the
aforementioned studies can also be observed in other types of
cancer to confirm the general role of miR-196a-5p in affecting the
phenotypes of cancers. Lastly, we will strive to develop
miR-196a-5p-based therapeutic agents by various technologies
including nanocapsules and nanocarriers, liposomes and PEGylated
vesicles.
On the basis of the current state of knowledge, it
is reasonable to suggest that miRNAs-based approaches may represent
one of the novel and promising therapeutics. Currently, several
therapeutic strategies targeting miRNAs have been used in the
clinic. Growing evidence indicates that miRNAs regulate cancer
development in a traditional manner by regulating signaling
pathways and factors. On the other hand, a non-conventional
mechanism of cancer regulation by stem cell reprogramming via a
regulatory network consisting of miRNAs that could modulate stem
cell properties of CSCs.
Acknowledgments
This study was supported by grants from The National
High Technology Research and Development Program of China (863
Program) (2014AA020537), National Natural Science Foundation of
China (31371445).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zhang S, Zhang S, Zhao P,
Li G, Wu L and He J: Report of incidence and mortality in China
cancer registries, 2009. Chin J Cancer Res. 25:10–21.
2013.PubMed/NCBI
|
|
3
|
Kim TH and Shivdasani RA: Stomach
development, stem cells and disease. Development. 143:554–565.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang X, Hua R, Wang X, Huang M, Gan L, Wu
Z, Zhang J, Wang H, Cheng Y and Li J: Identification of stem-like
cells and clinical significance of candidate stem cell markers in
gastric cancer. Oncotarget. 7:9815–9831. 2016.PubMed/NCBI
|
|
6
|
Alison MR, Lin WR, Lim SM and Nicholson
LJ: Cancer stem cells: In the line of fire. Cancer Treat Rev.
38:589–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu TJ, Sun BC, Zhao XL, Zhao XM, Sun T,
Gu Q, Yao Z, Dong XY, Zhao N and Liu N: CD133+ cells
with cancer stem cell characteristics associates with vasculogenic
mimicry in triple-negative breast cancer. Oncogene. 32:544–553.
2013. View Article : Google Scholar
|
|
8
|
Sakakini N, Turchi L, Bergon A, Holota H,
Rekima S, Lopez F, Paquis P, Almairac F, Fontaine D, Baeza-Kallee
N, et al: A positive feed-forward loop associating EGR1 and PDGFA
promotes proliferation and self-renewal in glioblastoma stem cells.
J Biol Chem. 291:10684–10699. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nataraj SM, Prema CL, Vimalambike MG,
Shivalingaiah SC, Sundaram S, Kumar AP, Math AK and Prashant A:
Major protein of carcinoembryonic antigen gene family - CD66c, a
novel marker in colon carcinoma. J Clin Diagn Res. 10:XC01–XC04.
2016.PubMed/NCBI
|
|
10
|
Liu J, Ma L, Xu J, Liu C, Zhang J, Liu J,
Chen R and Zhou Y: Spheroid body-forming cells in the human gastric
cancer cell line MKN-45 possess cancer stem cell properties. Int J
Oncol. 42:453–459. 2013.
|
|
11
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog
pathways. Nat Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar
|
|
12
|
Sounni NE and Noel A: Targeting the tumor
microenvironment for cancer therapy. Clin Chem. 59:85–93. 2013.
View Article : Google Scholar
|
|
13
|
Lim YC, Kang HJ, Kim YS and Choi EC:
All-trans-retinoic acid inhibits growth of head and neck cancer
stem cells by suppression of Wnt/β-catenin pathway. Eur J Cancer.
48:3310–3318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Zhang T, Korkaya H, Liu S, Lee HF,
Newman B, Yu Y, Clouthier SG, Schwartz SJ, Wicha MS and Sun D:
Sulforaphane, a dietary component of broccoli/broccoli sprouts,
inhibits breast cancer stem cells. Clin Cancer Res. 16:2580–2590.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang T and Rycaj K: Targeted therapy
against cancer stem cells. Oncol Lett. 10:27–33. 2015.PubMed/NCBI
|
|
16
|
Leon G, MacDonagh L, Finn SP, Cuffe S and
Barr MP: Cancer stem cells in drug resistant lung cancer: Targeting
cell surface markers and signaling pathways. Pharmacol Ther.
158:71–90. 2016. View Article : Google Scholar
|
|
17
|
Lau WM, Teng E, Chong HS, Lopez KA, Tay
AY, Salto-Tellez M, Shabbir A, So JB and Chan SL: CD44v8-10 is a
cancer-specific marker for gastric cancer stem cells. Cancer Res.
74:2630–2641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mansour SF and Atwa MM:
Clinicopathological significance of CD133 and ALDH1 cancer stem
cell marker expression in invasive ductal breast carcinoma. Asian
Pac J Cancer Prev. 16:7491–7496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishikawa S, Konno M, Hamabe A, Hasegawa
S, Kano Y, Fukusumi T, Satoh T, Takiguchi S, Mori M, Doki Y, et al:
Surgically resected human tumors reveal the biological significance
of the gastric cancer stem cell markers CD44 and CD26. Oncol Lett.
9:2361–2367. 2015.PubMed/NCBI
|
|
20
|
Tseng JY, Yang CY, Yang SH, Lin JK, Lin CH
and Jiang JK: Circulating CD133(+)/ESA(+) cells in colorectal
cancer patients. J Surg Res. 199:362–370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shitara K, Doi T, Nagano O, Imamura CK,
Ozeki T, Ishii Y, Tsuchihashi K, Takahashi S, Nakajima TE, Hironaka
S, et al: Dose-escalation study for the targeting of CD44v cancer
stem cells by sulfasalazine in patients with advanced gastric
cancer (EPOC1205). Gastric Cancer. 20:341–349. 2017. View Article : Google Scholar
|
|
22
|
Wang W, Dong LP, Zhang N and Zhao CH: Role
of cancer stem cell marker CD44 in gastric cancer: A meta-analysis.
Int J Clin Exp Med. 7:5059–5066. 2014.
|
|
23
|
Takahashi RU, Miyazaki H and Ochiya T: The
role of microRNAs in the regulation of cancer stem cells. Front
Genet. 4:2952014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu C and Tang DG: MicroRNA regulation of
cancer stem cells. Cancer Res. 71:5950–5954. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma C, Huang T, Ding YC, Yu W, Wang Q, Meng
B and Luo SX: MicroRNA-200c overexpression inhibits
chemoresistance, invasion and colony formation of human pancreatic
cancer stem cells. Int J Clin Exp Pathol. 8:6533–6539.
2015.PubMed/NCBI
|
|
26
|
Yu D, Shin HS, Lee YS and Lee YC: miR-106b
modulates cancer stem cell characteristics through TGF-β/Smad
signaling in CD44-positive gastric cancer cells. Lab Invest.
94:1370–1381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu Q, Yang Z, Wang F, Hu S, Yang L, Shi Y
and Fan D: MiR-19b/20a/92a regulates the self-renewal and
proliferation of gastric cancer stem cells. J Cell Sci.
126:4220–4229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takaishi S, Okumura T, Tu S, Wang SS,
Shibata W, Vigneshwaran R, Gordon SA, Shimada Y and Wang TC:
Identification of gastric cancer stem cells using the cell surface
marker CD44. Stem Cells. 27:1006–1020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu H, Tian Y, Yuan X, Wu H, Liu Q, Pestell
RG and Wu K: The role of CD44 in epithelial-mesenchymal transition
and cancer development. Onco Targets Ther. 8:3783–3792. 2015.
|
|
30
|
Li LC, Wang DL, Wu YZ, Nian WQ, Wu ZJ, Li
Y, Ma HW and Shao JH: Gastric tumor-initiating CD44 (+) cells and
epithelial-mesenchymal transition are inhibited by γ-secretase
inhibitor DAPT. Oncol Lett. 10:3293–3299. 2015.
|
|
31
|
Zhang W and Li Y: miR-148a downregulates
the expression of transforming growth factor-β2 and SMAD2 in
gastric cancer. Int J Oncol. 48:1877–1885. 2016.PubMed/NCBI
|
|
32
|
Wang XH, Liu MN, Sun X, Xu CH, Liu J, Chen
J, Xu RL and Li BX: TGF-beta 1 pathway affects the protein
expression of many signaling pathways, markers of liver cancer stem
cells, cytokeratins, and TERT in liver cancer HepG2 cells. Tumour
Biol. 37:3675–3681. 2016. View Article : Google Scholar
|
|
33
|
Hoshino Y, Nishida J, Katsuno Y, Koinuma
D, Aoki T, Kokudo N, Miyazono K and Ehata S: Smad4 decreases the
population of pancreatic cancer-initiating cells through
transcriptional repression of ALDH1A1. Am J Pathol. 185:1457–1470.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao S, Ammanamanchi S, Brattain M, Cao L,
Thangasamy A, Wang J and Freeman JW: Smad4-dependent TGF-beta
signaling suppresses RON receptor tyrosine kinase-dependent
motility and invasion of pancreatic cancer cells. J Biol Chem.
283:11293–11301. 2008. View Article : Google Scholar : PubMed/NCBI
|