Introduction
Bladder cancer (BC) is among the most prevalent
cancers worldwide and recently appears to be increasing in
incidence. It is reported that there are ~261,000 new cases and
115,000 deaths from BC each year (1,2). BC
is classified into two major groups with different biological
properties: non-muscle invasive and muscle-invasive bladder cancers
(3). Most cases of BC (75–80%) are
diagnosed as non-muscle invasive tumors, which have high recurrence
rates of 50–70% (4). The remainder
(~15%) are high-grade muscle-invasive tumors that can rapidly
progress to metastasis and death (5). Despite existing multiapproach
treatment regimens, including radical cystectomy, bladder-sparing
therapy with transurethral resection, chemotherapy and
radiotherapy, the median overall survival for metastatic BC is
merely 13–15 months (6). Thus,
there is an urgent need to gain further insight into the molecular
mechanisms of tumorigenesis and metastasis of BC and find novel
therapeutic strategies to improve the outcome of patients with
BC.
DAB2IP acts as a tumor suppressor and is often
downregulated by epigenetic modification in many aggressive types
of cancer including prostate, breast, advanced lung and pancreatic
cancer (7–12). For example, DAB2IP-deficient
prostate cancer cells show great proliferative potential and become
resistant to stress-induced apoptosis and loss of DAB2IP expression
in prostate epithelial cells results in epithelial-mesenchymal
transition, a central step in tumor metastasis (13,14).
In pancreatic cancer, decreased DAB2IP expression is associated
with clinical stage, development and infiltration of pancreatic
cancer (12). Compared with
widespread reports in other cancers, only few studies have
described dysregulated DAB2IP expression and its correlation with
disease outcome in BC (15,16)
and the tumor suppressive role of DAB2IP in BC has not been fully
elucidated.
MicroRNAs (miRNAs), endogenous 19 to 22-nucleotide
non-coding RNAs, negatively regulate gene expression through
complementary binding to the 3′-untranslated region (3′-UTR) of
target mRNAs, which can lead to blockage of translation and
degradation of the mRNA (16–18).
miRNAs can affect various biological behaviors of tumors, such as
proliferation, invasion, apoptosis and metastasis. Increasing
evidence has demonstrated that miRNAs act as oncogenes or tumor
suppressors in many human cancers, including BC (19). Recently, some dysregulated miRNAs
have been identified to regulate tumorigenesis and metastasis of BC
(20–24). For instance, upregulated miRNA-19a
expression in BC tissues can significantly promote growth of BC
cells by targeting PTEN (25) and
miRNA-137, which is identified as a regulator of the tumor
suppressor gene PAQR3, functions as an oncogene by promoting
proliferation, migration and invasion of BC cells (26). Moreover, some miRNAs (miRNA-143,
miRNA-490-5p, miRNA-576-3p, miRNA-204 and miRNA-145) have been
proved to function as tumor suppressors by inhibiting proliferation
of BC (27–31). However, few studies have
investigated which miRNAs directly regulate DAB2IP expression in
BC. Furthermore, the pattern and regulatory mechanism of
interactions between miRNAs and DAB2IP in BC cells remain
unknown.
In the present study, we provide the first
demonstration that DAB2IP is a direct target of a new microRNA
(miRNA-556-3p), and show that endogenous miRNA-556-3p expression
negatively correlates with simultaneous DAB2IP expression in BC
tissues and cells. Functional analysis showed that ectopic
expression of miRNA-556-3p could promote proliferation, invasion,
migration and colony formation by targeting DAB2IP. Further
investigation verified that the tumor promotion effect driven by
miRNA-556-3p in BC cells is partially dependent on activation of
the Ras-ERK pathway.
Materials and methods
Human BC tissue and plasma samples
Human BC tissue samples were obtained from 30
patients with BC who underwent surgery at Hongqi Hospital
(Mudanjiang, China) from 2011 to 2013. Patients who underwent
preoperative chemotherapy and radiotherapy were excluded. In
addition, 30 peritumoral tissues adjacent to the tumor margin were
used as controls. All samples were immediately snap-frozen in
liquid nitrogen and stored at −80°C until RNA and protein
extraction. Blood samples were collected from bladder cancer
patients and from volunteers as controls. All blood samples were
collected in EDTA tubes and processed within 1 h of collection.
Blood samples were centrifuged to obtain plasma, which was stored
at −80°C until RNA extraction. All clinical samples were collected
after obtaining informed consent from participants and with
approval from the ethics committee of Hongqi Hospital.
Cell culture
Five BC cell lines (BIU-87, T24, 5637, J82 and EJ),
a non-tumorigenic bladder cell line (SV-HUC-1) and the viral
packaging cell line (293T) were purchased from the Institutes of
Biochemistry and Cell Biology (Shanghai, China) and originated from
the American Type Culture Collection (ATCC; Manassas, VA, USA).
293T and SV-HUC-1 cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, USA) and other BC
cells were cultured in RPMI-1640 medium. The media were
supplemented with 10% fetal bovine serum (FBS; Invitrogen) and 1%
penicillin-streptomycin. Cells were adherent and passaged by
trypsin digestion, and all cells were maintained at 37°C in a
humidified 5% CO2 incubator.
Quantitative reverse
transcriptase-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from plasma and tissues of
BC patients using TRIzol reagent (Invitrogen). Complementary DNA
was synthesized from total RNA using a M-MLV reverse transcription
kit (Takara Bio, Dalian, China) with the specific primers U6 snRNA
(NM_001101. 3; 5′-TACCTTGCGAAGTGCTTAAAC-3′) and miRNA-556-3p
(5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACAAAGA-3′). cDNA
samples were used as templates for amplification reactions carried
out in a PCR Thermal Cycler Dice Real-Time system with the
SYBR® PrimeScript PCR kit (Takara Bio) following our
previously described procedure (32). The expression of miR-556-3p was
analyzed with the 2−∆∆CT method. For each sample,
triplicate determinations were performed and mean values were
adopted for further calculations. All values were normalized to an
endogenous U6 control. The PCR primers for mature miRNA-556-3p or
U6 were designed as follows: miRNA-556-3p forward,
5′-ATATTACCATTAGCTCATCTTT-3′ and reverse,
5′-GTCGTATCCAGTGCGTGTCGTG-3′; U6 forward,
5′-GTGCTCGCTTCGGCAGCACAT-3′ and reverse,
5′-TACCTTGCGAAGTGCTTAAAC-3′.
RNase protection assay (Rpa)
Mature miRNA-556-3p from bladder cancer cells was
detected by Rpa as previously described (33). Briefly, total mRNA was extracted
from BC cells by the TRIzol method (Invitrogen).
32P-labeled antisense RNA probe
(5′-AAAGAUGAGCUAAUGGUAAUAU-3′) complementary to miR-556-3p was used
in RNase protection assays. The RNA probe was prepared using a
mirVana miRNA Probe Construction kit (AM1550; Invitrogen) following
the manufacturer's protocol. Total RNA (5 µg) was hybridized
with the probe at 52°C for 16 h. RNases A/T1 were added and the
mixture was incubated at 37°C for 30 min. The remaining RNA was
precipitated by ethanol after heat inactivation and separated by
15% denaturing PAGE. The probes were visualized by autoradiography.
Endogenous U6 was used as the internal normalization control.
Western blot analysis
Protein extractions were prepared using a previously
described procedure (32) and
western blot analysis was performed in triplicate experiments.
Protein lysates were subjected to 11% SDS-PAGE and proteins were
electrotransferred to polyvinylidene fluoride membranes (Millipore,
Billerica, MA, USA). Membranes were incubated with 5% non-fat dry
milk in TBS and probed with anti-DAB2IP (1:500), anti-Ras (1:200),
anti-phosphorylated-ERK1/2 (1:400), anti-ERK1/2(1:600) and
anti-GAPDH (1:1,200; Abcam, Cambridge, MA, USA) antibodies in TBST
(0.1% Tween-20 in TBS). Horseradish peroxidase-conjugated
anti-rabbit (or mouse) IgG (Cell Signaling Technology, Danvers, MA,
USA) was used for detection of immunoreactive proteins by
chemiluminescence (Pierce, Rockford, IL, USA) and imaging with
X-ray film. GAPDH was used as an endogenous reference for
normalization.
Cell proliferation assay
Cell proliferation assays were performed using the
Cell Counting kit-8 assay (CCK-8; Dojindo Laboratories, Kumamoto,
Japan) following the manufacturer's protocol. At 72 h after viral
infection, BIU-87, 5637 and T24 cells that were uninfected or
infected with Lv-control, Lv-miRNA-556-3p, or
Lv-miRNA-556-3p-inhibition were seeded into 96-well plates at
1×103 cells/well and grown for 24, 48 and 72 h. At the
end of the incubation, 10 µl of CCK-8 solution was added to
each well and samples were incubated for 4 h at 37°C. Absorbance at
490 nm was read on a microplate reader (Multiskan Spectrum; Thermo
Fisher Scientific, Waltham, MA, USA). All experiments were
performed in triplicate experiments and the average of the results
was calculated.
Colony formation assay
A total of 0.5×103 T24 cells (72 h after
viral infection) were seeded into 6-well plates and cultured for 10
days. Media were replaced with fresh media on days 3 and 6.
Following incubation, colonies were washed with phosphate-buffered
saline (PBS), fixed for 5 min with 4% paraformaldehyde and stained
with 0.1% crystal violet for 30 sec. The colony formation assay was
repeated three times with duplicate wells.
Wound healing assay
A total of 1×105 5637 cells (72 h after
viral infection) were seeded in 6-well plates and cultured until
they reached confluence. Confluent monolayer cells were scratched
with a 200-µl pipette tip (Axygen Scientific, Inc., Union
City, CA, USA) and washed three times with PBS to remove cell
debris and cells in suspension. Fresh serum-free medium was added
and the cells were allowed to close the wound for 48 h under normal
conditions. Images were taken at the same position of the wound
with a computer-assisted microscope (Nikon Corp., Tokyo, Japan) at
24 and 48 h.
Cell invasion assay
Invasion of BIU-87 cells (72 h after viral
infection) was determined using the QCM™ 24-well Fluorimetric Cell
Invasion Assay kit (ECM554; Chemicon, Temecula, CA, USA) according
to the manufacturer's instructions. The kit uses an insert
polycarbonate membrane with 8-µm pore size. The insert was
coated with a thin layer of ECMatrix™ that occluded the membrane
pores and blocked migration of non-invasive cells. Culture medium
(500 µl) supplemented with 10% FBS was used as a
chemoattractant. Cells that migrated and invaded the underside of
the membrane were fixed in 4% paraformaldehyde. The invaded cells
were stained with DAPI.
Construction of recombinant expression
vectors
Human genomic DNA was extracted from BIU-87 cells
and used for amplification of the template for the precursor
sequence of miRNA-556-3p. The primers used were
5′-GGAATTCTTAGAGCTGTAAAACAATTACT-3′ and
5′-CGGGATCCCCTATACTCAAGTCTAACATTC-3′. The PCR product was digested
using EcoRI and BamHI, ligated into a linear
pCDH-EF1-GFP vector (System Biosciences, Palo Alto, CA, USA) and
transformed into Top10 competent cells (Takara Bio). The resultant
vector was called pcDH-miRNA-556-3p.
An inhibition sequence that complementarily binds to
miRNA-556-3p was chosen. The oligonucleotide templates of three
tandem inhibition sequences were chemically synthesized and cloned
into linear pcDH vector (System Biosciences) obtained by digestion
by BamHI and EcoRI and purification by agarose gel
electrophoresis. The recombinant vector was named
pcDH-inhibition-miRNA-556-3p.
The CDS sequence of human DAB2IP (NM_032552.3) was
amplified using the primers 5′-CCCAAGCTTGCCACCATGGAGCCCGACTCCCTT-3′
and 5′-CGGAATTCCTAATGCATACTCTCTTTC-3′, which contain a
HindIII restriction site and Kozak sequence and a
BamHI cutting site, respectively. cDNA was prepared by
reverse transcription of RNA isolated from 293T cells. The PCR
product was digested and cloned into a pcDH-CMV lentiviral
expression vector. The final recombinant vector was named
pcDH-DAB2IP.
The products of the vectors were confirmed by DNA
sequencing. Endotoxin-free DNA was prepared in all cases.
Lentivirus packaging and lentiviral
infection of cells
All recombinant lentivirus vectors
(pcDH-miRNA-556-3p, pcDH-DAB2IP and pcDH-miRNA-556-3p-inhibition)
and lentiviral packaging plasmids (System Biosciences) were
cotransfected into 293T packaging cells using Lipofectamine 2000
(Invitrogen) according to the manufacturer's instruction. At 48 h
after transfection, the supernatant was harvested, cleared by
centrifugation at 5,000 × g at 4°C for 5 min and passed through a
0.45-µm PVDF membrane (Millipore). The titer of virus was
determined by gradient dilution. The packaged lentiviruses were
named Lv-miRNA-556-3p, Lv-DAB2IP and
Lv-miRNA-556-3p-inhibition.
Suspensions of BC cell lines BIU-87, 5637 and T24 in
logarithmic phase were prepared by trypsin digestion and the number
of viable cells was counted with a hemocytometer after trypan blue
staining. Cells were collected by centrifugation at 1,000 × g and
resuspended in complete RPMI-1640 medium to a concentration of
1×106 cells/ml. Cells were seeded on 6-well plates at 2
ml/well and cultured overnight under normal conditions. The medium
was replaced with 2-ml complete medium containing 10 µl of
viral solution. The infection efficiency was observed using the
fluorescent marker 72 h after infection, and the levels of
miRNA-556-3p and DAB2IP in cells were detected by qRT-PCR or
western blotting, respectively. The infected cells were reseeded
and cultured under normal conditions for a further 72 h before use
in assays for proliferation, colony formation, wound healing and
invasion. At 72 h after infection, the cells were collected and
total protein was extracted for detection of Ras levels and ERK1/2
phosphorylation level by western blotting.
3′-TUR luciferase reporter assay
The 3′-untranslated region (253 bp) of human DAB2IP
was amplified from cDNA obtained by reverse transcription of total
RNA of 293T cells using the primers
5′-GCTCTAGAGAGCATCTGCCCCAGGTACACCT-3′ and
5′-GCTCTAGAGAGCATCTGCCCCAGGTACACCT-3′. The amplification parameters
were 32 cycles of denaturation at 95°C for 10 sec, annealing at
58°C for 30 sec and extension at 72°C for 30 sec. The product was
then digested with XbaI and inserted into the pGL3-promotor
vector (Promega, Madison, WI, USA). The seed region was mutated by
point mutagenesis from 5′-GTAATA-3′ to 5′-ATAAGT-3′. The resulting
vectors were named pGL-WT (wild-type)-DAB2IP and pGL-MT (mutated
type)-DAB2IP. The pRL-TK vector (Promega) was used as an internal
control. Luciferase reporter plasmid was cotransfected into 293T
cells with miRNA-556-3p mimics (5′-AUAUUACCAUUAGCUCATCUUUtt-3′),
miRNA-556-3p inhibitor (5′-AAAGAUGAGCUAAUGGUAAUAUtt-3′), or
negative control (NC, 5′-AGUCAUUACAUACUUCUCUAUUtt-3′). After 48 h
of transfection, cells were harvested and assayed with the
Dual-Luciferase assay kit (Promega) according to the manufacturer's
protocol.
Ras GTPase activity assay
To investigate the effect of miRNA-556-3p on Ras
GTPase activity through DAB2IP, we examined Ras GPTase activity in
three cell lines (BIU-87, 5637 and T24) at 72 h after lentiviral
infection strictly following the instructions of Ras GTPase ELISA
kit (chemiluminescent) (ab134640; Abcam).
Statistical analysis
Statistical analysis was performed using the SPSS
statistical software (16.0 for Windows). Experimental data were
expressed as means ± standard deviation (SD). Differences between
groups were analyzed using the Student's t-test and the one-way
analysis of variance (ANOVA). All statistical tests performed were
two-sided. Differences were considered statistically significant at
P<0.05. All experiments were performed at least three times to
insure reproducibility of the results.
Results
DAB2IP expression inversely correlates
with levels of candidate miRNAs targeting DAB2IP in BC tissues and
BC cells
To search for candidate miRNAs that were
differentially expressed in BC, we used TargetScan 6.1 to assess
miRNAs complementary to the DAB2IP 3′-UTR. Two transcript variants
of human DAB2IP mRNA (NM_032552.3 and NM_138709.2) contained 38
miRNAs with a 7-nucleotide seed match at different positions of the
DAB2IP 3′-UTR. Of these, 10 miRNAs exhibited highly conserved
target sites and 28 miRNAs had poorly conserved target sites in the
3′-UTR of DAB2IP (data not shown). The expression of 10 candidate
miRNAs (exhibited highly conserved target sites) targeting DAB2IP
was examined in plasma samples from 30 BC patients and 30 healthy
volunteers by qRT-PCR (Table I).
Of 10 candidate miRNAs, four candidate miRNAs (miRNA-4725-5p,
miRNA-556-3p, miRNA-4691-3p and miRNA-576-5p) were significantly
increased in BC patients compared with healthy volunteers. To
further search for candidate miRNAs, the expression of 4 candidate
miRNAs (miRNA-4725-5p, miRNA-556-3p, miRNA-4691-3p and
miRNA-576-5p) targeting DAB2IP was examined in 30 BC tissues and
adjacent peritumoral tissues. Compared to the expression of
miRNA-4725-5p and miRNA-576-5p, the expression of miRNA-556-3p and
miRNA-4691-3p was remarkable increased in BC tissues than those in
peritumoral tissues, thus, we selected miRNA-556-3p and
miRNA-4691-3p to perform luciferase reporter assays. The luciferase
reporter assays showed that miRNA-556-3p overexpression could
significantly reduce the activity of a luciferase reporter
containing the DAB2IP 3′-UTR (results see below), but the
miRNA-4691-3p could not serve as DAB2IP gene regulator (data not
shown). As a result, we selected miRNA-556-3p for further
experiments.
 | Table ITen candidate miRNAs were detected in
plasma samples from 30 BC patients and 30 healthy volunteers by
qRT-PCR. |
Table I
Ten candidate miRNAs were detected in
plasma samples from 30 BC patients and 30 healthy volunteers by
qRT-PCR.
| Candidate miRNAs
target to DAB2IP | PCR primers for
candidate miRNAs | Result | P-value |
|---|
| hsa-miR-4469 | Forward primer:
GCTCCCTCTAGGGTCGCTCGGA | | |
| Reverse primer:
TCGTATCCAGTGCGTGTCGTG | Yes | P>0.05 |
|
hsa-miR-4725-5p | Forward primer:
AGACCCTGCAGCCTTCCCACC | | |
| Reverse primer:
GTCGTATCCAGTGCGTGTCGTG | Yes | P<0.05a |
| hsa-miR-3119 | Forward primer:
TGGCTTTTAACTTTGATGGC | | |
| Reverse primer:
GTCGTATCCAGTGCGTGTCGTG | Yes | P>0.05 |
| hsa-miR-25 | Forward primer:
CATTGCACTTGTCTCGGTCTGA | | |
| Reverse primer:
GTCGTATCCAGTGCGTGTCGTG | No | – |
| hsa-miR-556-3p | Forward primer:
ATATTACCATTAGCTCATCTTT | | |
| Reverse primer:
GTCGTATCCAGTGCGTGTCGTG | Yes | P<0.05a |
|
hsa-miR-4691-3p | Forward primer:
CCAGCCACGGACTGAGAGTGCAT | | |
| Reverse primer:
GTCGTATCCAGTGCGTGTCGTG | Yes | P<0.05a |
|
hsa-miR-518a-5p | Forward primer:
CTGCAAAGGGAAGCCCTTTC | | |
| Reverse primer:
GTCGTATCCAGTGCGTGTCGTG | Yes | P>0.05 |
| hsa-miR-504 | Forward primer:
AGACCCTGGTCTGCACTCTATC | | |
| Reverse primer:
GTCGTATCCAGTGCGTGTCGTG | No | – |
|
hsa-miR-4735-5p | Forward primer:
CCTAATTTGAACACCTTCGGTA | | |
| Reverse
primer:GTCGTATCCAGTGCGTGTCGTG | Yes | P>0.05 |
| hsa-miR-576-5p | Forward primer:
ATTCTAATTTCTCCACGTCTTT | | |
| Reverse primer:
GTCGTATCCAGTGCGTGTCGTG | Yes | P<0.05a |
The results (Fig.
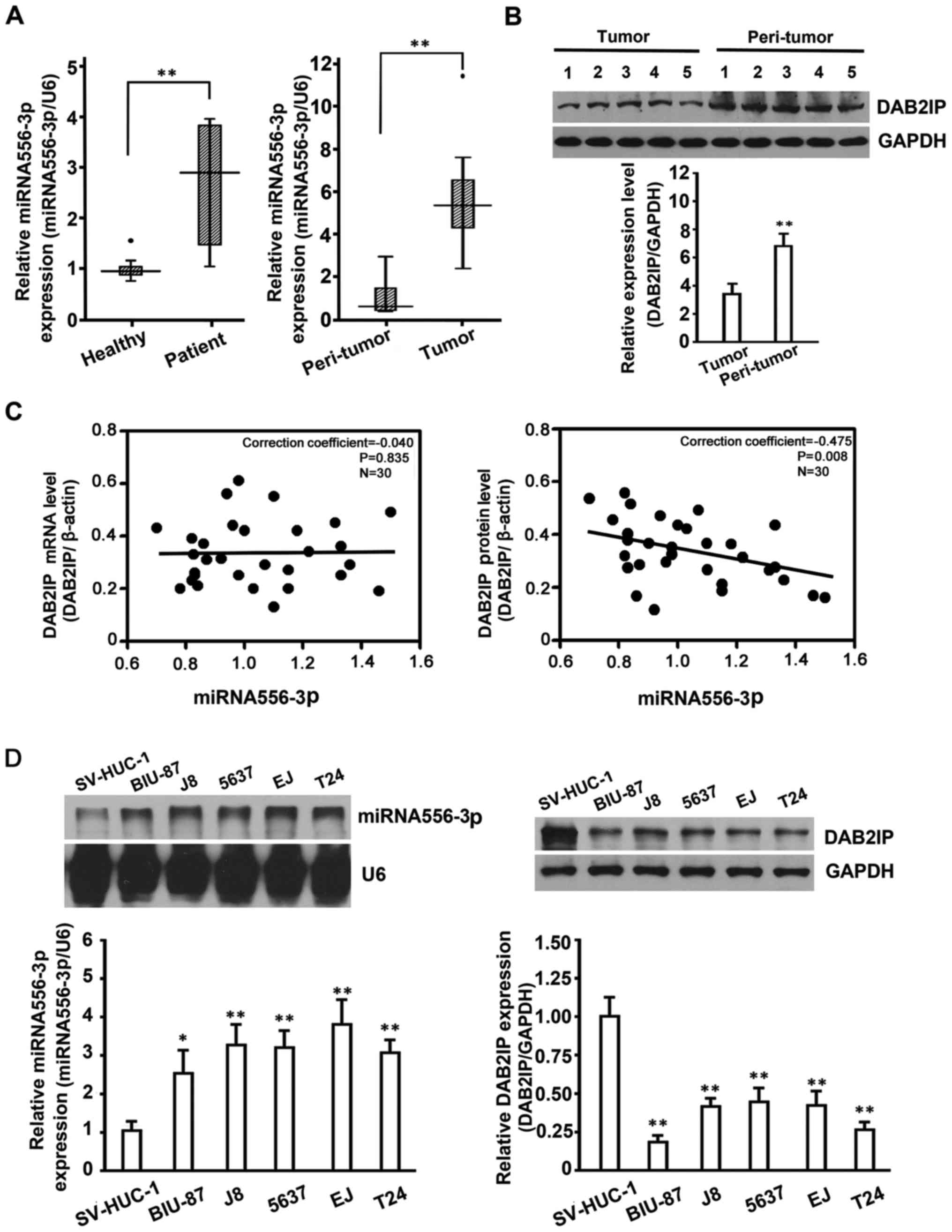
1A, left panel) showed that miRNA-556-3p in plasma samples from
30 BC patients was differentially expressed compared with the
control group (30 healthy volunteers; P<0.05). Subsequent
analysis of the miRNA-556-3p level in 30 paired BC tissues and
adjacent peritumoral tissues by qRT-PCR showed that the
miRNA-556-3p level was significantly increased in BC tissues
compared with controls (Fig. 1A,
right panel). To confirm the association between miRNAs targeting
DAB2IP and DAB2IP expression, we detected DAB2IP protein expression
in BC tissues by western blotting. To show all results in the same
membrane, we included paired samples from BC tissues and adjacent
peritumoral tissues (Fig. 1B),
respectively.
Correlation analysis of DAB2IP protein/mRNA and
miRNA-556-3p in tumor was performed, and spearman analysis showed
that in 30 tumor samples, miRNA-556-3p level was inversely
correlated with DAB2IP protein (correction coefficient, −0.475;
P=0.008) but not DAB2IP mRNA (correction coefficient, −0.040,
P=0.835; Fig. 1C). As confirmed in
other cancers, DAB2IP expression in BC tissues was significantly
decreased compared with controls, indicating that DAB2IP expression
inversely correlated with levels of miRNA-556-3p in BC tissues.
To verify whether endogenous DAB2IP expression in BC
cells also correlated with miRNA-556-3p, the expression of
miRNA-556-3p and DAB2IP in BC cells was detected by Rpa and western
blot analysis, respectively. The results (Fig. 1D) confirmed that BC cell lines
(BIU-87, T24, 5637, J82 and EJ) with high levels of miRNA-556-3p
showed much lower DAB2IP expression than control cells (SV-HUC-1)
with low levels of miRNA-556-3p but higher DAB2IP expression. Taken
together, our results indicated that DAB2IP would be a direct
target of miRNA-556-3p, and that endogenous miRNA-556-3p expression
showed a negative correlation with simultaneous DAB2IP expression
in BC cells.
Prediction of candidate miRNAs targeting
DAB2IP by TargetScan and verification of the relationship between
miRNA-556-3p and DAB2IP
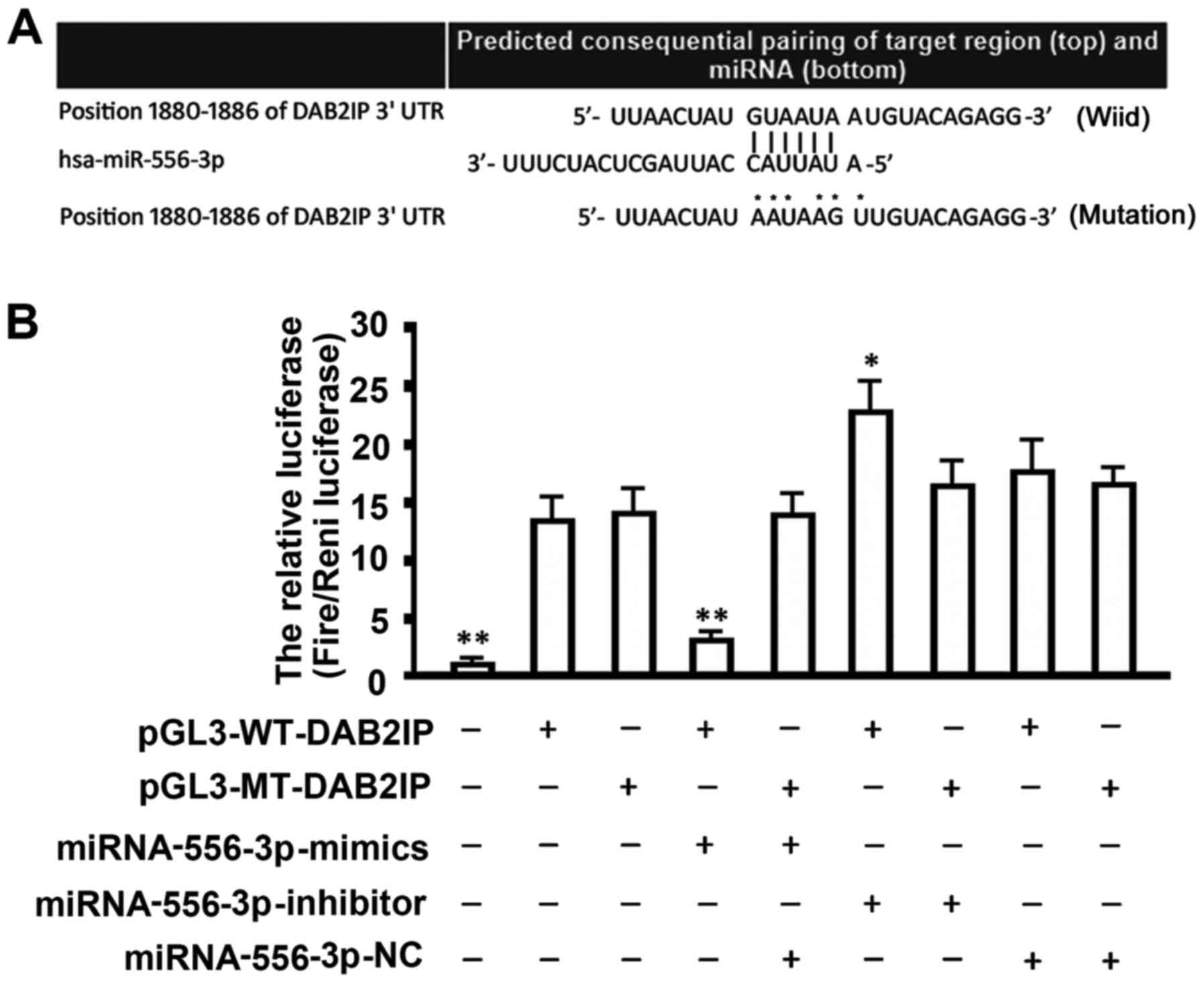
Bioinformatic analysis (Fig. 2A) showed a seeding area of
hsa-miRNA-556-3p in the 3′-UTR of the DAB2IP gene: 5′-GUAAUAA-3′.
To determine whether miRNA-556-3p is a regulator of DAB2IP
expression, we performed luciferase reporter assays in 293T cells.
Luciferase reporter plasmids with wild-type or mutant sequence in
the 3′-UTR of DAB2IP mRNA were cotransfected with miRNA-556-3p
mimics, inhibitor, or negative control (NC) into 293T cells for 48
h, followed by measurement of luciferase activity (Fig. 2B). Our results showed that
cotransfection of miRNA-556-3p mimics significantly inhibited the
activity of firefly luciferase reporter with wild-type 3′-UTR of
DAB2IP, but not of those with the mutant 3′-UTR, whereas inhibition
of miRNA-556-3p by miRNA-556-3p inhibitor exhibited opposite
results (Fig. 2B).
Enhanced miRNA-556-3p expression promoted
proliferation of BC cells
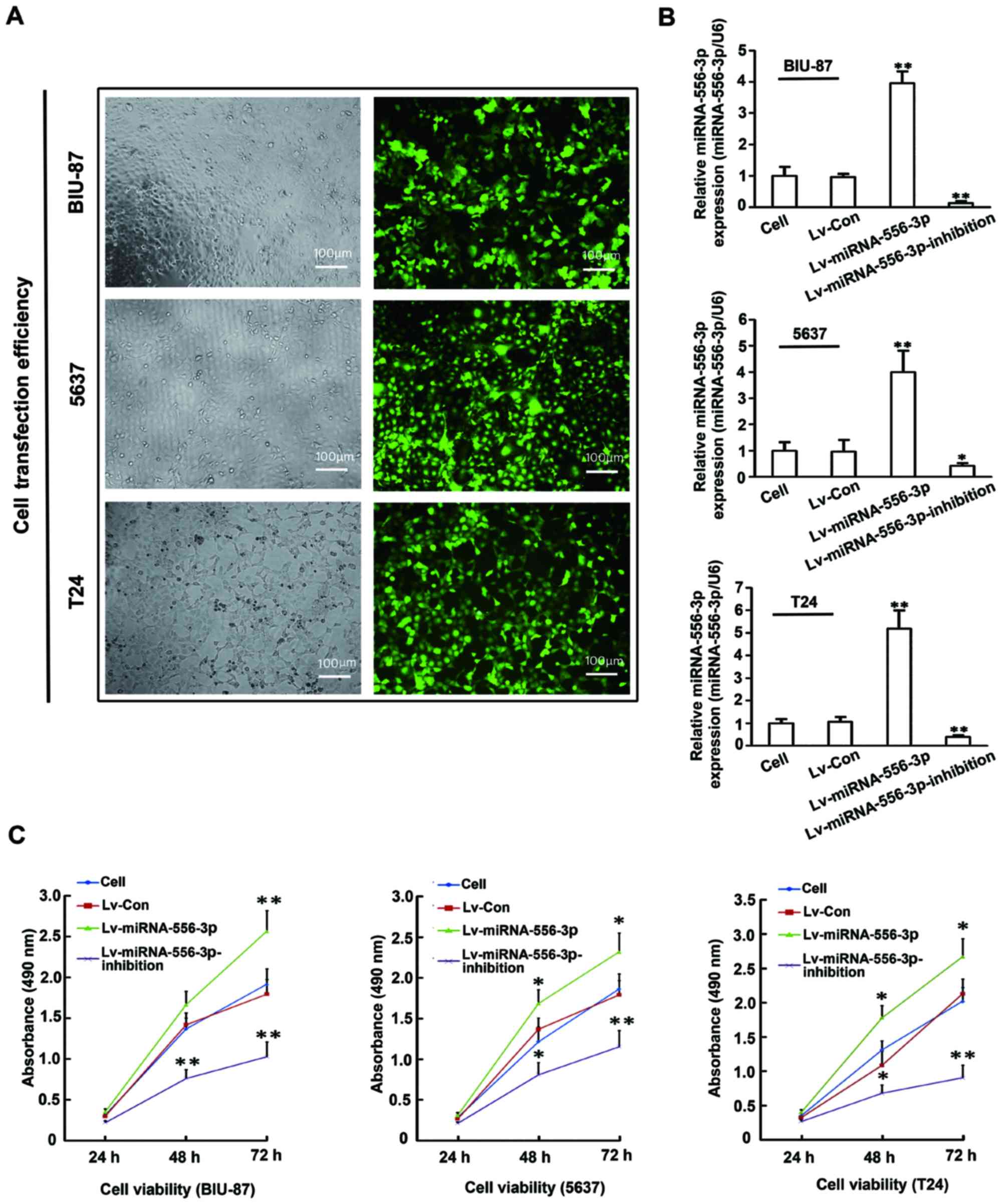
To investigate the effects of miRNA-556-3p in BC
cells, BIU-87, 5637 and T24 cells were genetically engineered with
Lv-miRNA-556-3p, Lv-miRNA-556-3p-inhibition, or Lv-Control. The
pcDH-copGFP lentiviral vector carries a green fluorescent protein
that allows measurement of transfection efficiency. More than 90%
transfection efficiency was confirmed in these cells by fluorescent
microscopy (Fig. 3A). After 72 h
of transfection, multiple cellular RNA preparations were assayed
for miR-556-3p expression by qRT-PCR (Fig. 3B). miRNA-556-3p expression level in
Lv-miRNA-556-3p cells was significantly higher than that in
untreated cells (P<0.05), whereas miRNA-556-3p expression was
markedly inhibited in Lv-miRNA-556-3p-inhibition cells (P<0.05).
There was no difference in miRNA-556-3p expression between Lv-NC
cells and untreated cells (P>0.05). Next, we examined the effect
of miRNA-556-3p on proliferation of BC cells in vitro by
CCK-8 assay. As shown in Fig. 3C,
miRNA-556-3p overexpression significantly increased the growth rate
of BIU-87, 5637 and T24 cells in the presence of Lv-miRNA-556-3p
compared with untreated cells, whereas miRNA-556-3p inhibition
markedly decreased the growth rate of BIU-87, 5637 and T24 cells in
the presence of Lv-miRNA-556-3p-inhibition (P<0.05). No
significant difference was found in cell proliferation between
Lv-control cells and untreated cells (P>0.05). Collectively,
these results indicated that enhanced miRNA-556-3p expression
promoted BC cell proliferation in vitro.
Restored DAB2IP expression attenuates
invasion, migration and colony formation of BC cells
Published data showed that DAB2IP functions as a
suppressor of tumorigenesis and metastasis of BC cells (34). We hypothesized that miRNA-556-3p
affects the biological behavior of BC cells by negatively
regulating DAB2IP. To test this hypothesis, a 'rescue' experiment
with Lv-miRNA-556-3p and Lv-DAB2IP was performed in vitro in
BIU-87, 5637 and T24 cells. After 72 h of transfection, BIU-87, T24
and 5637 cells were harvested for invasion, colony formation and
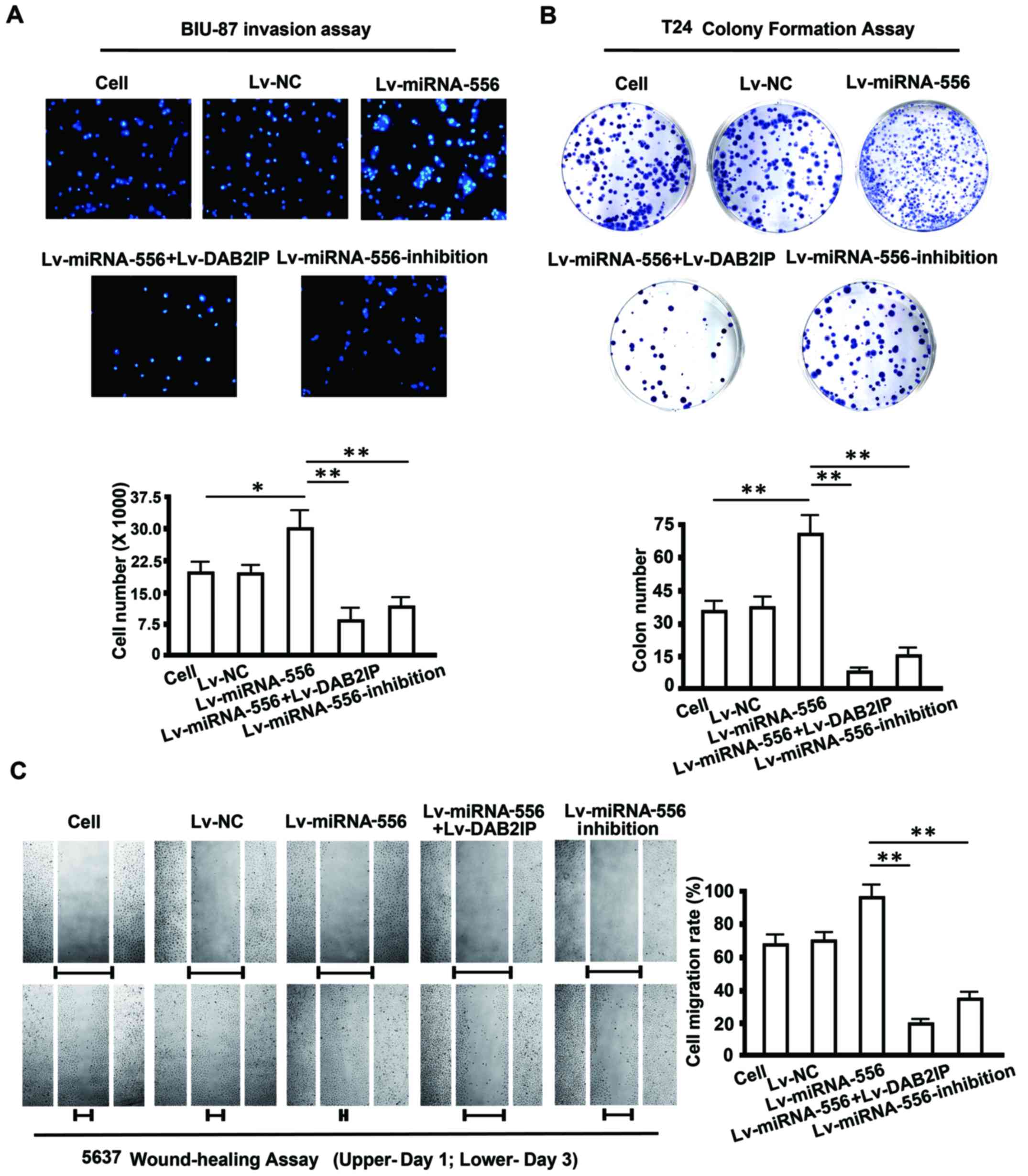
migration assays, respectively. As shown in Fig. 4A, miRNA-556-3p overexpression
significantly promoted invasion of BIU-87 cells invasion compared
with untreated cells, whereas the number of invaded cells was
obviously lower following transfection with Lv-DAB2IP.
Furthermore, cell invasion was significantly
inhibited when Lv-miRNA-556-3p-inhibition was transfected into
BIU-87 cells. A similar result was obtained in the colony formation
assay with T24 cells. Transfection of Lv-miRNA-556-3p alone
enhanced colony formation by 70%. In contrast, cotransfection of
Lv-miRNA-556-3p and Lv-DAB2IP markedly reduced colony formation by
50%, and knockdown of miRNA-556-3p by Lv-miRNA-556-3p-inhibition
also resulted in a 50% decrease of colonies. No difference in
invasion, migration and colony formation assays was observed
between in Lv-NC cells and untreated cells (Fig. 4B).
To measure the influence of restored DAB2IP
expression on cell migration we performed a wound healing assay in
5637 cells. Cells that overexpressed miRNA-556-3p migrated to the
wound more rapidly than untreated cells. Conversely, the motility
of 5637 cells was markedly suppressed after treatment with
Lv-DAB2IP, and introduction of Lv-miRNA-556-3p-inhibition also
exhibited an inhibitory effect on 5637 cell migration (Fig. 4C). Therefore, miRNA-556-3p,
functioned as a tumor promoter during tumorigenesis and metastasis
of BC by directly targeting DAB2IP.
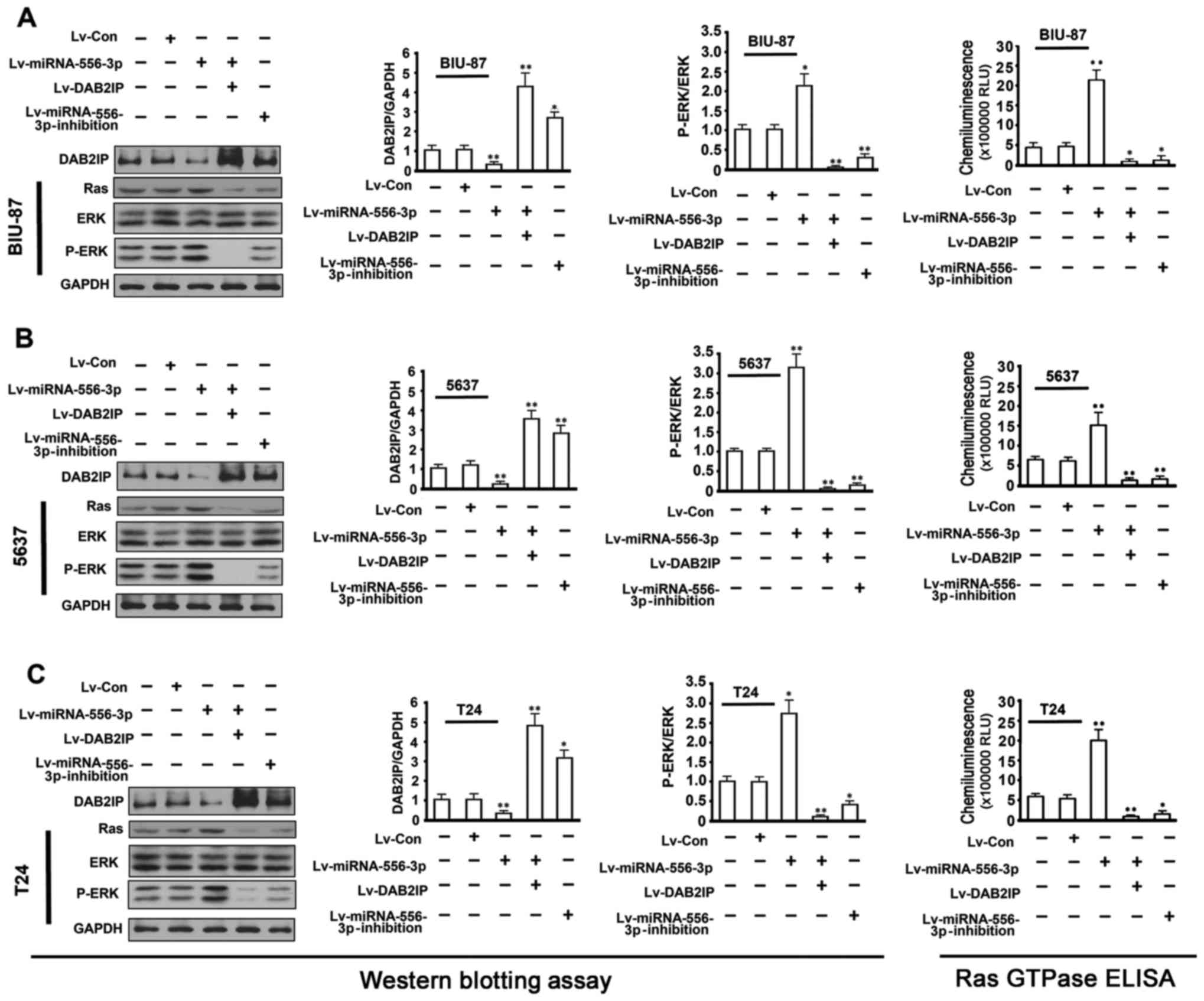
miRNA-556-3p-mediated DAB2IP suppression
plays an oncogenic role by partially activating the Ras-ERK
pathway
We next searched for molecular regulatory mechanisms
that contributed to the above effects. Previous studies revealed
that DAB2IP functions as a tumor suppressor in cancers through
inhibition of the Ras-ERK pathway, a critical pathway for
tumorigenesis (35,36). In the present study, DAB2IP was
identified as a target of miRNA-556-3p. Therefore, we wondered
whether the miRNA-556-3p-mediated changes in biological behavior of
BC cells are dependent on the Ras-ERK pathway. To address this
issue, we examined expression of DAB2IP, Ras and phosphorylated
ERK1/2 (pERK1/2), a central regulator of cell proliferation, in
BIU-87, 5637 and T24 cells. As shown in Fig. 5, western blot analysis showed a
decrease in DAB2IP protein and an increase in Ras and pERK1/2
compared with untreated cells when miRNA-556-3p expression was
enhanced in BIU-87, 5637 and T24 cells by Lv-miRNA-556-3p.
Conversely, inhibition of miRNA-556-3p expression in BIU-87, 5637
and T24 cells by Lv-miRNA-556-3p-inhibition, resulted in
significant upregulation of DAB2IP and downregulation of Ras and
pERK1/2 proteins. Notably, in a rescue assay, cotransfection of
Lv-miRNA-556-3p and Lv-DAB2IP not only markedly restored DAB2IP
expression but also efficiently silenced Ras and pERK1/2 protein to
a barely detectable level in BIU-87, 5637 and T24 cells.
Transfection of Lv-control had no effect on DAB2IP, Ras and pERK1/2
protein expression compared with untreated cells. Ras GTPase ELISA
assay showed that overexpression of miRNA-556-3p in BC cells could
markedly increase Ras GTPase activity. Together, these results
suggested that miRNA-556-3p-mediated DAB2IP suppression at least in
part played an oncogenic role in BC cells by activating the Ras-ERK
pathway.
Discussion
miRNAs are key factors in the regulation of cell
proliferation, apoptosis and other important cellular processes
(37). In past decades, miRNAs
have been the focus of much research in oncology, and there are
great expectations for their utility as cancer biomarkers and
therapeutic targets (38,39). Although many miRNAs are predicted
to regulate target mRNAs and are dysregulated in their respective
cancer types, to date, few studies have reported the expression and
function of miRNAs targeting DAB2IP in bladder cancer. In the
present study, we primarily focus on the identification of miRNAs
targeting DAB2IP and validation of their expression and function in
BC.
It is estimated that ~30% of genes in the human
genome are regulated by miRNAs (39). Therefore, identification of
aberrantly expressed miRNAs is critical in the analysis of human
oncogenesis. To complete the study's main aim of discovering and
validating differentially expressed miRNAs targeting DAB2IP in BC,
we applied TargetScan 6.1 for an initial overview to identify
candidate miRNAs. qRT-PCR and luciferase reporter assays confirmed
that DAB2IP was a direct target of miRNA-556-3p. To the best of our
knowledge, miRNA-556-3p is a newly identified miRNA specific to
DAB2IP in BC and no functional study results on miRNA-556-3p have
previously been published. Identification of miRNA-556-3p offered a
sound basis for further studies to determine its expression and
biological function in BC. Our data revealed that, compared with
controls, endogenous miRNA-556-3p expression was significantly
upregulated in clinical samples of BC patients and BC cell lines,
whereas DAB2IP expression was simultaneously downregulated.
Based on these findings, we speculated that
miRNA-556-3p might function in as a tumor promoter in BC. As
expected, gain or loss of function assays through transfection of
Lv-miRNA-556-3p or Lv-miRNA-556-3p-inhibition indicated that
enhancement of miRNA-556-3p expression could promote proliferation,
invasion and migration, and colony formation of BC cells, whereas
repression of miRNA-556-3p expression yielded opposite results.
More importantly, a 'rescue' experiment with Lv-miRNA-556-3p and
Lv-DAB2IP not only attenuated invasion, migration and colony
formation of BC cells, but also reversed the tumor promotion effect
induced by miRNA-556-3p. In fact, these results underline the great
potential of miRNA-556-3p as an oncogene in the tumorigenesis and
metastasis of BC though inhibition of DAB2IP. In fact, one previous
study also demonstrated that the tumor overexpressive miR-92b
promoted migration and invasion of BC cells, but had no effect on
cell proliferation (15). Herein,
we reported the function of a new important miRNA, miRNA-556-3p not
only promoted BC cells migration and invasion, but also promoted BC
cells proliferation by negatively regulating DAB2IP expression.
Many present studies have clearly indicated that one miRNA could
control multiple oncogenes and antioncogenes, this may be the
reason that miR-92b and miRNA-556-3p have the same targeted gene
but play partly different roles in BC cells, thus further studies
are needed to explore this.
The interaction between miRNA-556-3p and DAB2IP
suggested important changes in molecular pathways in BC cells. In
the present study, we observed a correlation of miRNA-556-3p with
DAB2IP and the Ras-ERK pathway in BC cells. Western blot analysis
showed a decrease in DAB2IP protein and an increase in Ras and
pERK1/2 proteins in BC cells that overexpressed miRNA-556-3p,
whereas introduction of Lv-miRNA-556-3p-inhibition in cells that
underexpressed miRNA-556-3p cells produced opposite results.
Intriguingly, restoration of DAB2IP in a 'rescue' assay efficiently
silenced Ras and pERK1/2 protein to a barely detectable level in BC
cells. Recent investigations have revealed that DAB2IP can modulate
the activities of various pathways including Ras-Raf-ERK, ASK1-JNK
and PI3K-Akt, providing potential mechanisms through which loss of
DAB2IP can deregulate survival and apoptosis pathways, leading to
tumor development (35).
Specifically, DAB2IP inhibits the Ras pathway by directly binding
to and inactivating H-Ras and R-Ras through its Ras GTPase activity
(36). In this study, we detected
the Ras GTPase activity in bladder cancer cell lines, and found
that the overexpression of miRNA-556-3p in BC cells not only
decreased DAB2IP expression, but also markedly increased Ras GTPase
activity and ERK1/2 phosphorylation level. From all the above
results, we propose that miRNA-556-3p-mediated DAB2IP suppression
promoted growth and metastasis of BC cells at least in part by
activation of the Ras-ERK pathway.
There are some important limitations to the present
study that need to be discussed. First, although miRNA-556-3p
expression was significantly upregulated in plasma and tissues from
BC patients compared with controls, the number of clinical samples
examined in this study was limited. Examination of additional
clinical BC samples may provide more persuasive evidence supporting
the relationship between miRNA-556-3p and DAB2IP. Second, a single
target gene is not regulated by a single miRNA (40,41).
Our qRT-PCR analysis of clinical samples of BC patients showed that
four upregulated miRNAs (miRNA-4725-5p, miRNA-556-3p, miRNA-4691-3p
and miRNA-576-5p) might be involved in regulating DAB2IP expression
(data not shown). Expression and function of these other miRNAs in
BC should be investigated in future studies. Third, previous
studies showed that miRNAs can be readily detected in bodily fluids
including serum, plasma, saliva, urine and tears (42–45).
The innate properties of miRNAs make them attractive as potential
biomarkers. In this study, we determined the miRNA-556-3p
expression level in plasma of BC patients and found that
miRNA-556-3p plasma expression was enhanced in accordance with its
high level in BC tissues. However, the relationship of miRNA-556-3p
expression level with clinical features of BC patients, including
diagnosis, therapy response, and prognosis, must be analyzed
further to support the use of plasma miRNA-556-3p as a non-invasive
marker for detection of bladder cancer.
Despite these limitations, we obtained sustainable
evidence that miRNA-556-3p performs an oncogenic function in human
BC cells via targeting DAB2IP. In conclusion, the present study
provides novel insight into the role of miRNA-556-3p in human BC
pathogenesis and suggests its potential application as a promising
molecular target for BC therapy.
Acknowledgments
The present study was supported by the National
Science Foundation of China (no. 81372293 and 81241088 to Y.K.F.;
no. 81273161 to K.J.F.), the Heilongjiang Province Science Funds
for Distinguished Young Scientists (JC2015019 to Y.K.F.), the New
Century Excellent Talents at Heilongjiang Province University
(1254-NECT-023 to Y.K.F.; UNPYSCT-2016113 and 12531736 to Y.M.P.),
the Program for Innovation Research Team in Science and Technology
at Mudanjiang Medical University, Department of Science and
Technology of Heilongjiang Province of China (H201377 to P.S.).
References
|
1
|
Wu M, Dickinson SI, Wang X and Zhang J:
Expression and function of SIRT6 in muscle invasive urothelial
carcinoma of the bladder. Int J Clin Exp Pathol. 7:6504–6513.
2014.PubMed/NCBI
|
|
2
|
Cornu JN, Neuzillet Y, Hervé JM, Yonneau
L, Botto H and Lebret T: Patterns of local recurrence after radical
cystectomy in a contemporary series of patients with
muscle-invasive bladder cancer. World J Urol. 30:821–826. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi SY, Ryu JH, Chang IH, Kim TH, Myung
SC, Moon YT, Kim KD and Kim JW: Predicting recurrence and
progression of non-muscle-invasive bladder cancer in Korean
patients: A comparison of the EORTC and CUETO models. Korean J
Urol. 55:643–649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vinall RL, Ripoll AZ, Wang S, Pan CX and
deVere White RW: MiR-34a chemosensitizes bladder cancer cells to
cisplatin treatment regardless of p53-Rb pathway status. Int J
Cancer. 130:2526–2538. 2012. View Article : Google Scholar
|
|
5
|
Tatarano S, Chiyomaru T, Kawakami K,
Enokida H, Yoshino H, Hidaka H, Nohata N, Yamasaki T, Gotanda T,
Tachiwada T, et al: Novel oncogenic function of mesoderm
development candidate 1 and its regulation by MiR-574-3p in bladder
cancer cell lines. Int J Oncol. 40:951–959. 2012.
|
|
6
|
Teply BA and Kim JJ: Systemic therapy for
bladder cancer - a medical oncologist's perspective. J Solid
Tumors. 4:25–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dai X, North BJ and Inuzuka H: Negative
regulation of DAB2IP by Akt and SCFFbw7 pathways. Oncotarget.
5:3307–3315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsai Y-S, Lai C-L, Lai C-H, Chang KH, Wu
K, Tseng SF, Fazli L, Gleave M, Xiao G, Gandee L, et al: The role
of homeostatic regulation between tumor suppressor DAB2IP and
oncogenic Skp2 in prostate cancer growth. Oncotarget. 5:6425–6436.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kong Z, Xie D, Boike T, Raghavan P, Burma
S, Chen DJ, Habib AA, Chakraborty A, Hsieh JT and Saha D:
Downregulation of human DAB2IP gene expression in prostate cancer
cells results in resistance to ionizing radiation. Cancer Res.
70:2829–2839. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dote H, Toyooka S, Tsukuda K, Yano M,
Ouchida M, Doihara H, Suzuki M, Chen H, Hsieh JT, Gazdar AF, et al:
Aberrant promoter methylation in human DAB2 interactive protein
(hDAB2IP) gene in breast cancer. Clin Cancer Res. 10:2082–2089.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yano M, Toyooka S, Tsukuda K, Dote H,
Ouchida M, Hanabata T, Aoe M, Date H, Gazdar AF and Shimizu N:
Aberrant promoter methylation of human DAB2 interactive protein
(hDAB2IP) gene in lung cancers. Int J Cancer. 113:59–66. 2005.
View Article : Google Scholar
|
|
12
|
Duan YF, Li DF, Liu YH, Mei P, Qin YX, Li
LF, Lin QX and Li ZJ: Decreased expression of DAB2IP in pancreatic
cancer with wild-type KRAS. Hepatobiliary Pancreat Dis Int.
12:204–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie D, Gore C, Liu J, Pong RC, Mason R,
Hao G, Long M, Kabbani W, Yu L, Zhang H, et al: Role of DAB2IP in
modulating epithelial-to-mesenchymal transition and prostate cancer
metastasis. Proc Natl Acad Sci USA. 107:2485–2490. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Min J, Zaslavsky A, Fedele G, McLaughlin
SK, Reczek EE, De Raedt T, Guney I, Strochlic DE, Macconaill LE,
Beroukhim R, et al: An oncogene-tumor suppressor cascade drives
metastatic prostate cancer by coordinately activating Ras and
nuclear factor-kappaB. Nat Med. 16:286–294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang J, Wang B, Hui K, Zeng J, Fan J,
Wang X, Hsieh JT, He D and Wu K: miR-92b targets DAB2IP to promote
EMT in bladder cancer migration and invasion. Oncol Rep.
36:1693–1701. 2016.PubMed/NCBI
|
|
16
|
Kutter C and Svoboda P: miRNA, siRNA,
piRNA: Knowns of the unknown. RNA Biol. 5:181–188. 2008. View Article : Google Scholar
|
|
17
|
Bartels CL and Tsongalis GJ: MicroRNAs:
Novel biomarkers for human cancer. Clin Chem. 55:623–631. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fendler A, Stephan C, Yousef GM and Jung
K: MicroRNAs as regulators of signal transduction in urological
tumors. Clin Chem. 57:954–968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Døssing KBV, Binderup T, Kaczkowski B,
Jacobsen A, Rossing M, Winther O, Federspiel B, Knigge U, Kjær A
and Friis-Hansen L: Down-regulation of miR-129-5p and the let-7
family in neuroendocrine tumors and metastases leads to
up-regulation of their targets Egr1, G3bp1, Hmga2 and Bach1. Genes
(Basel). 6:1–21. 2014.
|
|
21
|
Du C, Lv Z, Cao L, Ding C, Gyabaah OA, Xie
H, Zhou L, Wu J and Zheng S: MiR-126-3p suppresses tumor metastasis
and angiogenesis of hepatocellular carcinoma by targeting LRP6 and
PIK3R2. J Transl Med. 12:2592014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ujihira T, Ikeda K, Suzuki T, Yamaga R,
Sato W, Horie-Inoue K, Shigekawa T, Osaki A, Saeki T, Okamoto K, et
al: MicroRNA-574-3p, identified by microRNA library-based
functional screening, modulates tamoxifen response in breast
cancer. Sci Rep. 5:76412015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu J, Cheng Y, Li Y, Jin Z, Pan Y, Liu G,
Fu S, Zhang Y, Feng K and Feng Y: microRNA-128 plays a critical
role in human non-small cell lung cancer tumourigenesis,
angiogenesis and lymphangiogenesis by directly targeting vascular
endothelial growth factor-C. Eur J Cancer. 50:2336–2350. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Itesako T, Seki N, Yoshino H, Chiyomaru T,
Yamasaki T, Hidaka H, Yonezawa T, Nohata N, Kinoshita T, Nakagawa
M, et al: The microRNA expression signature of bladder cancer by
deep sequencing: The functional significance of the miR-195/497
cluster. PLoS One. 9:e843112014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feng Y, Liu J, Kang Y, He Y, Liang B, Yang
P and Yu Z: miR-19a acts as an oncogenic microRNA and is
up-regulated in bladder cancer. J Exp Clin Cancer Res. 33:672014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiu Y, Liu Z, Xia S, Jin C, Yin H, Zhao W
and Wu Q: MicroRNA-137 upregulation increases bladder cancer cell
proliferation and invasion by targeting PAQR3. PLoS One.
9:e1097342014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin T, Dong W, Huang J, Pan Q, Fan X,
Zhang C and Huang L: MicroRNA-143 as a tumor suppressor for bladder
cancer. J Urol. 181:1372–1380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li S, Xu X, Xu X, Hu Z, Wu J, Zhu Y, Chen
H, Mao Y, Lin Y, Luo J, et al: MicroRNA-490-5p inhibits
proliferation of bladder cancer by targeting c-Fos. Biochem Biophys
Res Commun. 441:976–981. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pignot G, Cizeron-Clairac G, Vacher S,
Susini A, Tozlu S, Vieillefond A, Zerbib M, Lidereau R, Debre B,
Amsellem-Ouazana D, et al: microRNA expression profile in a large
series of bladder tumors: Identification of a 3-miRNA signature
associated with aggressiveness of muscle-invasive bladder cancer.
Int J Cancer. 132:2479–2491. 2013. View Article : Google Scholar
|
|
30
|
Liang Z, Li S, Xu X, Xu X, Wang X, Wu J,
Zhu Y, Hu Z, Lin Y, Mao Y, et al: MicroRNA-576-3p inhibits
proliferation in bladder cancer cells by targeting cyclin D1. Mol
Cells. 38:130–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ichimi T, Enokida H, Okuno Y, Kunimoto R,
Chiyomaru T, Kawamoto K, Kawahara K, Toki K, Kawakami K, Nishiyama
K, et al: Identification of novel microRNA targets based on
microRNA signatures in bladder cancer. Int J Cancer. 125:345–352.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feng Y, Hu J, Ma J, Feng K, Zhang X, Yang
S, Wang W, Zhang J and Zhang Y: RNAi-mediated silencing of VEGF-C
inhibits non-small cell lung cancer progression by simultaneously
down-regulating the CXCR4, CCR7, VEGFR-2 and VEGFR-3-dependent
axes-induced ERK, p38 and AKT signalling pathways. Eur J Cancer.
47:2353–2363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ronchetti S, Nocentini G, Giunchi L,
Bartoli A, Moraca R, Riccardi C and Migliorati G: Short-term
dexamethasone treatment modulates the expression of the murine TCR
zeta gene locus. Cell Immunol. 178:124–131. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen YJ, Kong ZL, Wan FN, Wang HK, Bian
XJ, Gan HL, Wang CF and Ye DW: Downregulation of DAB2IP results in
cell proliferation and invasion and contributes to unfavorable
outcomes in bladder cancer. Cancer Sci. 105:704–712. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang R, He X, Liu W, Lu M, Hsieh JT and
Min W: AIP1 mediates TNF-alpha-induced ASK1 activation by
facilitating dissociation of ASK1 from its inhibitor 14-3-3. J Clin
Invest. 111:1933–1943. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xie D, Gore C, Zhou J, Pong RC, Zhang H,
Yu L, Vessella RL, Min W and Hsieh JT: DAB2IP coordinates both
PI3K-Akt and ASK1 pathways for cell survival and apoptosis. Proc
Natl Acad Sci USA. 106:19878–19883. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chekulaeva M and Filipowicz W: Mechanisms
of miRNA-mediated post-transcriptional regulation in animal cells.
Curr Opin Cell Biol. 21:452–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ratert N, Meyer HA, Jung M, Lioudmer P,
Mollenkopf HJ, Wagner I, Miller K, Kilic E, Erbersdobler A, Weikert
S, et al: miRNA profiling identifies candidate mirnas for bladder
cancer diagnosis and clinical outcome. J Mol Diagn. 15:695–705.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baffa R, Fassan M, Volinia S, O'Hara B,
Liu CG, Palazzo JP, Gardiman M, Rugge M, Gomella LG, Croce CM, et
al: MicroRNA expression profiling of human metastatic cancers
identifies cancer gene targets. J Pathol. 219:214–221. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cho WCS: OncomiRs: The discovery and
progress of microRNAs in cancers. Mol Cancer. 6:602007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Geng Q, Fan T, Zhang B, Wang W, Xu Y and
Hu H: Five microRNAs in plasma as novel biomarkers for screening of
early-stage non-small cell lung cancer. Respir Res. 15:1492014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kosaka N, Iguchi H and Ochiya T:
Circulating microRNA in body fluid: A new potential biomarker for
cancer diagnosis and prognosis. Cancer Sci. 101:2087–2092. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cortez MA, Bueso-Ramos C, Ferdin J,
Lopez-Berestein G, Sood AK and Calin GA: MicroRNAs in body fluids -
the mix of hormones and biomarkers. Nat Rev Clin Oncol. 8:467–477.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Szeto CC: Urine miRNA in nephrotic
syndrome. Clin Chim Acta. 436:308–313. 2014. View Article : Google Scholar : PubMed/NCBI
|