Introduction
Lung cancer is an international health problem and
its incidence and mortality rate show a rising trend among various
common malignant tumors (1). As
early lung cancer is often asymptomatic, more than 20% of non-small
cell lung cancers are classified as stage III disease at the time
of diagnosis (2). Standard
treatment for advanced lung cancer includes surgery and combination
chemotherapy, but there are issues with relapse and chemotherapy
drug resistance, while the toxicity of chemotherapy also limits its
range in clinical applications. Therefore, exploration of new
treatments for lung cancer is a hotspot of current research.
Indoleamine 2,3-dioxygenase-1 (IDO1) is a
rate-limiting enzyme that catalyzes the catabolism of tryptophan by
the kynurenine pathway (3), and
its activity has been detected in various tissues such as lung,
small intestine and placenta (4),
especially in the thymic medulla and secondary lymphoid organs of
the T-cell area immune system (5,6).
IDO1 expression in tumor tissues is significantly higher than that
in normal tissues. However, overexpression of IDO1 gene contributes
to the depletion of local tryptophan, resulting in the suppression
of T-cell and NK-cell functions. These immune cells are sensitive
to tryptophan deficiency. At the same time, the accumulation of
tryptophan metabolites, especially kynurenic acid, can inhibit NK
cell receptor expression, thereby inhibiting the function of NK
cells, thus developing tumor immune tolerance (7). Many studies have shown IDO1
expression in various cancers, including lung (8), colorectal (9), prostate (10), breast (11), and ovarian (12), and the expression of IDO is
associated with poor patient prognosis (13–15).
Angiogenesis is a necessary condition for the growth
and metastasis of solid tumors. It provides the necessary nutrients
for growth, allowing the tumor to enter the rapid growth phase, and
provides the channel for distant metastasis of the tumor (16). Microvessel density (MVD) is one of
the most important parameters in the evaluation of tumor
angiogenesis. As an indirect measure of angiogenesis, MVD can be
used to reflect the biological behavior of malignant tumor. MVD has
been shown to be an independent prognostic marker to predict tumor
growth, metastasis and recurrence in many solid tumors including
lung cancer (17). CD34 is a
highly glycosylated transmembrane protein that allows for
quantitative assessment of microvessel density in the event of
vascular injury (18). CD34 is one
of the markers of vascular endothelium, which is considered to be a
repeatable, stable and mature vascular endothelial marker. CD146 is
a single-chain membrane through glycoprotein, belonging to the
immunoglobulin superfamily members. It has homology with many cell
adhesion molecules. CD146 is involved in tumor angiogenesis, not
only as a cell adhesion molecule, but also as a membrane signal
transduction molecule in tumor-induced angiogenesis (19). We used CD34 and CD146 as markers
for quantitative evaluation of microvessel density in this
study.
Current research of IDO1 is focused on its
immunological function with very few studies on the relationship
between IDO1 expression and angiogenesis. In this study, we aimed
to expound the relationship between IDO1 expression and lung cancer
progression, particularly angiogenesis, and to develop
IDO1-targeted molecular therapy to inhibit angiogenesis of lung
cancer.
Materials and methods
Cell lines and experimental animals
The Lewis lung cancer (LLC) cell line used in this
study was obtained from the American Type Culture Collection (ATCC)
and maintained in DMEM medium (Invitrogen Life Technologies,
Carlsbad, CA, USA) with 10% FBS, L-glutamine, penicillin, and
streptomycin at 37°C in 5% CO2. Four- to six-week-old
female C57BL/6 mice were purchased from The Jackson Laboratory (Bar
Harbour, ME, USA). All experimental protocols and ethics approval
were in accordance with the approved guidelines for safety
requirements of Jiangxi Academy of Medical Sciences, Nanchang
University.
siRNA synthesis and transfection
The siRNA targeting IDO1 mRNA was generated in
accordance with the target sequence selection method described by
Jin et al (20). siRNA was
synthesized by the manufacturer (Sigma, St. Louis, MO, USA). siRNA
targeting luciferase gene GL2 (GL2 siRNA) was used as a
scrambled-silencing control as GL2 is not expressed in treated
cells. IDO1 siRNA and GL2 siRNA were transfected into LLC cells
using Lipofectamine-2000 reagent (Invitrogen Life Technologies).
Briefly, cells were plated into 12-well plates (1×105
cells/well) and allowed to grow overnight to reach approximately
70% confluence. Cell medium was replaced with 300 µl
OptiMEM® serum-reduced medium (Invitrogen Life
Technologies) before transfection. IDO1 siRNA (1 µg) or GL2
siRNA was incubated with 2 µl of Lipofectamine-2000 reagent
in 200 µl of OptiMEM serum-reduced medium at room
temperature for 20 min, and then the mixture was gently added to
each group.
IDO1 mRNA quantification
Cells were lysed using TRIzol Reagent (Invitrogen
Life Technologies) and total RNA was isolated according to the
manufacturer's instructions. Total RNA (1 µg) was
synthesized to cDNA using reverse transcriptase (MMLV-RT,
Invitrogen Life Technologies). The following primers sets were used
for qPCR amplifications: Actin, 5′-AGG GAA ATC GTG CGT GAC AT-3′
(sense) and 5′-AAC CGC TCG TTG CCA ATA GT-3′ (antisense); IDO1,
5′-GTACATCACCATGGCGTATG-3′ (sense) and 5′-CGAGGAAGAAGCCCTTGTC-3′
(antisense); real-time PCR reactions were performed in a Stratagene
Mx3000P QPCR System (Agilent Technologies, Santa Clara, CA, USA)
using SYBR green PCR Master Mix (Invitrogen Life Technologies)
according to manufacturer's protocol. The differences of gene
expression were calculated using the ΔCt method.
Western blot analysis
Cells (1×105) were seeded into a 12-well
plate and grown overnight. The cells were transfected with IDO1
siRNA or GL2 siRNA for 48 h. Cells were harvested, washed twice
with ice-cold PBS, re-suspended in protein lysis buffer with
complete protein inhibitor, and then the container was kept on ice
for 30 min. Lysed cells were centrifuged at 12,000 rpm for 10 min
at 4°C, and the supernatant was collected and stored at −20°C for
future use. Protein concentration was determined by Bio-Rad protein
assay and 40 µg of each group cell lysate was separated on
10% SDS-PAGE, transferred to nitrocellulose membrane, blocked with
5% fat-free milk and 3% BSA in TBST (0.25% Tween-20), probed with a
mouse anti-human IDO1 mAb (Santa Cruz Biotechnology, Santa Cruz,
CA, USA) that binds to both human and mouse IDO1 and monoclonal
anti-β-actin Ab (Santa Cruz Biotechnology) according to the
manufacturer's instructions, and visualized by an ECL assay
(Pierce, Rockford, lL, USA). The gray scale was calculated by
ImageJ software, and the relative expression of IDO1 protein was
respectively calculated by IDO1/β-actin.
Transwell cell migration and invasion
assays
Transwell chambers with an 8 µm pore size
were used to detect migration and invasion abilities of LLC cells
after being transfected with IDO1 siRNA or GL2 siRNA for 24 h. For
migration assays, 8×104 cells in 200 µl DMEM
containing 10% FBS were seeded into the upper chamber and 500
µl DMEM containing 20% FBS was added to the lower chamber.
For invasion assays, the upper chamber (Becton-Dickinson, Bedford,
MA, USA) was precoated with 80 µl 5% Matrigel overnight
before 4×104 cells were seeded. Cells were cultured in
5% CO2 at 37°C for 24 h. Cells on the upper side of
Transwell were then removed, the wells were fixed with
paraformaldehyde for 15 min, and hematoxylin and eosin (H&E)
was used to stain the cells. The migrated and invaded cells were
quantified by light microscopy and the number of cells in five
high-power fields was counted to represent the migrated cell
number.
shRNA expression vector treatment
A lung cancer model was established by inoculating
1×106 LLC cells subcutaneously into the upper hind leg
of C57BL/6 mice. The cancer-bearing mice were treated with 50
µg of IDO1 shRNA or scramble shRNA in 1.0 ml PBS by
hydrodynamic injection through the tail vein at days 3, 7, 14 and
21. Tumor onset day was defined as the time when tumor diameter
reached 5 mm. Tumor volume was measured by caliper every three days
when tumors appeared and the tumor volume was calculated using the
following formula: tumor volume = 0.5 × (tumor width) × (tumor
length). Tumors were weighed using an electronic balance.
Vasculogenic mimicry assay in vitro
LLC cells were transfected with siRNA targeting IDO1
or GL2 (control siRNA), or remained untransfected as a blank
control. At 24 h after gene silencing, the 3×104/well
cells were placed on the Matrigel. After 3 h culture, formations of
vasculogenic mimicry (VM) were observed under microscope. The
numbers of VM tubers formed by the cells transfected with IDO1
siRNA, GL2 siRNA and control cells were counted.
Immunohistochemical staining
Tumor tissues from lung cancer-bearing mice were
collected on day 21 and fixed with PFA. Paraffin sections were
prepared, deparaffinized, and treated with hydrogen peroxide for 20
min to block endogenous peroxidase. The antigen was repaired with
sodium citrate buffer in a pressure cooker. Then, the paraffin
sections were reacted with primary antibody for 16 h at 4°C
overnight. After 3 washes with PBS, sections were incubated with
enzyme-conjugated streptavidin for 30 min. The sections were washed
with PBS 3 times, and color was developed using the DAB method. The
antibodies were as follows: IDO1 (Santa Cruz Biotechnology, 1:50),
CD34 (Abcam, Cambridge, MA, USA; 1:250), CD146 (Abcam, 1:250). The
degree of immunostaining of sections was reviewed and scored
independently by two observers, based on both the proportion of
positively stained tumor cells and the intensity of staining.
Intensity of staining was recorded on a scale of 0, no staining; 1,
weak staining; 2, moderate staining; and 3, strong staining. The
proportion of positively stained tumor cells was graded as follows:
0, no positive tumor cells; 1, <10% positive tumor cells; 2,
10–50% positive tumor cells; 3, 50–80% positive tumor cells; and 4,
>80% positive tumor cells. The staining index = staining
intensity × proportion of positively stained tumor cells (21). We evaluated the expression level of
IDO1 by staining index (scored as 0, 1, 2, 3, 4, 6, 9 or 12) using
this method. We counted the MVD using the classic method reported
by Weidner et al (22). The
distribution of blood vessels in the whole slice was observed under
light microscopy first, and we selected the most active areas of
neovascularization to count microvessels per 200 × field.
Statistical analysis
Numeric data are presented as mean ± SD. Student's
t-test (2-tailed) was used to determine differences between two
means. For comparison of multiple groups, one-way ANOVA test was
applied. The correlation between MVD and IDO1 protein expression
was analyzed by Spearman rank correlation analysis. For all
statistical analyses, differences with p-values <0.05 were
considered significant.
Results
IDO1 siRNA significantly knocks down IDO1
expression in LLC cells
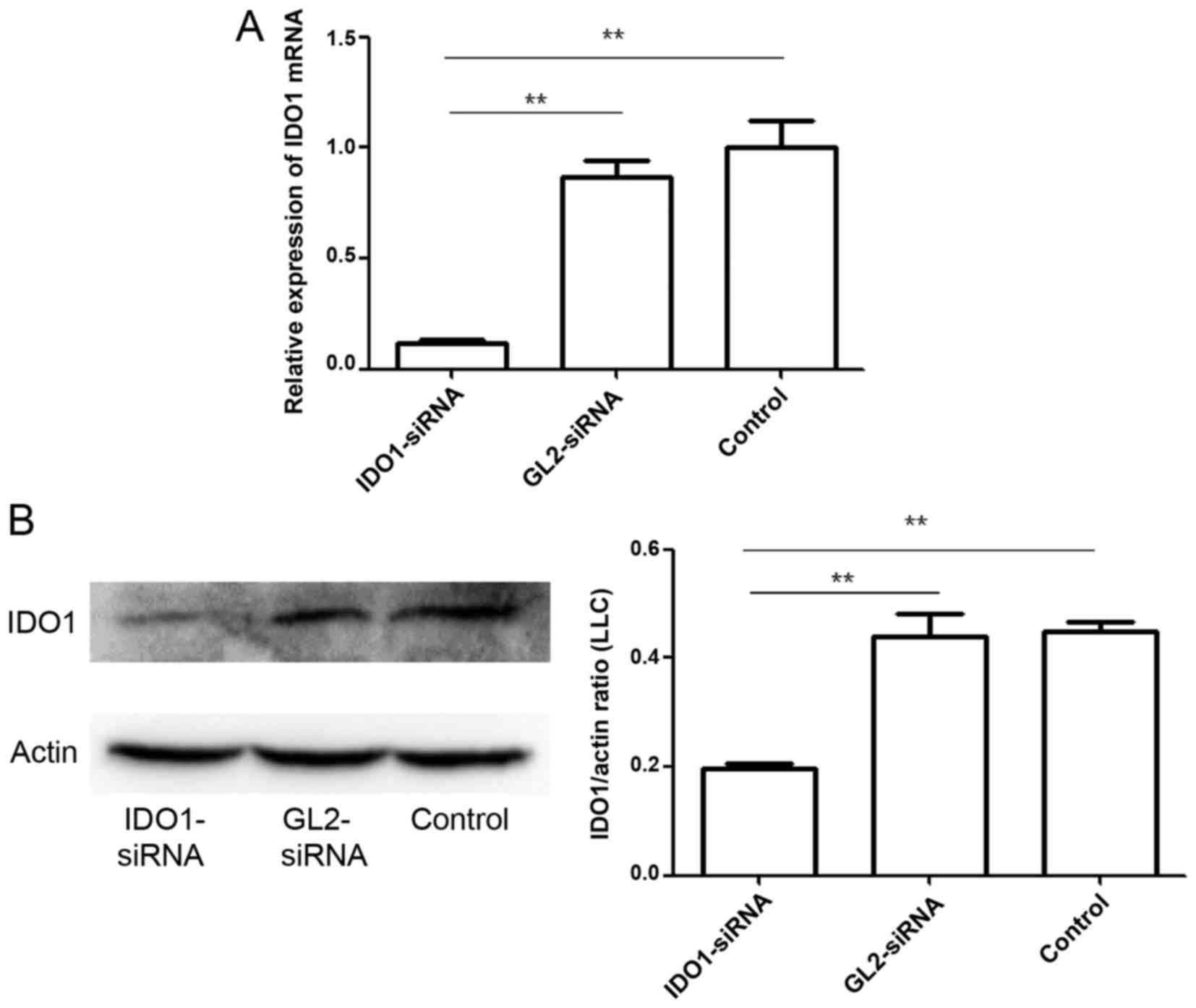
To test the efficacy of gene silencing using IDO1
siRNA, LLC cells were transfected with IDO1 siRNA or GL2 siRNA
(control siRNA). At 24 h after transfection, IDO1 expression was
measured at the transcriptional level by qPCR (Fig. 1A). In the cells with IDO1 siRNA
transfection, IDO1 mRNA expression decreased by >80% (p≤0.01).
IDO1 expression at the protein level was also measured by western
blot analysis (Fig. 1B). Data show
that the designed IDO1 siRNA significantly knocked down IDO1
expression in LLC cells.
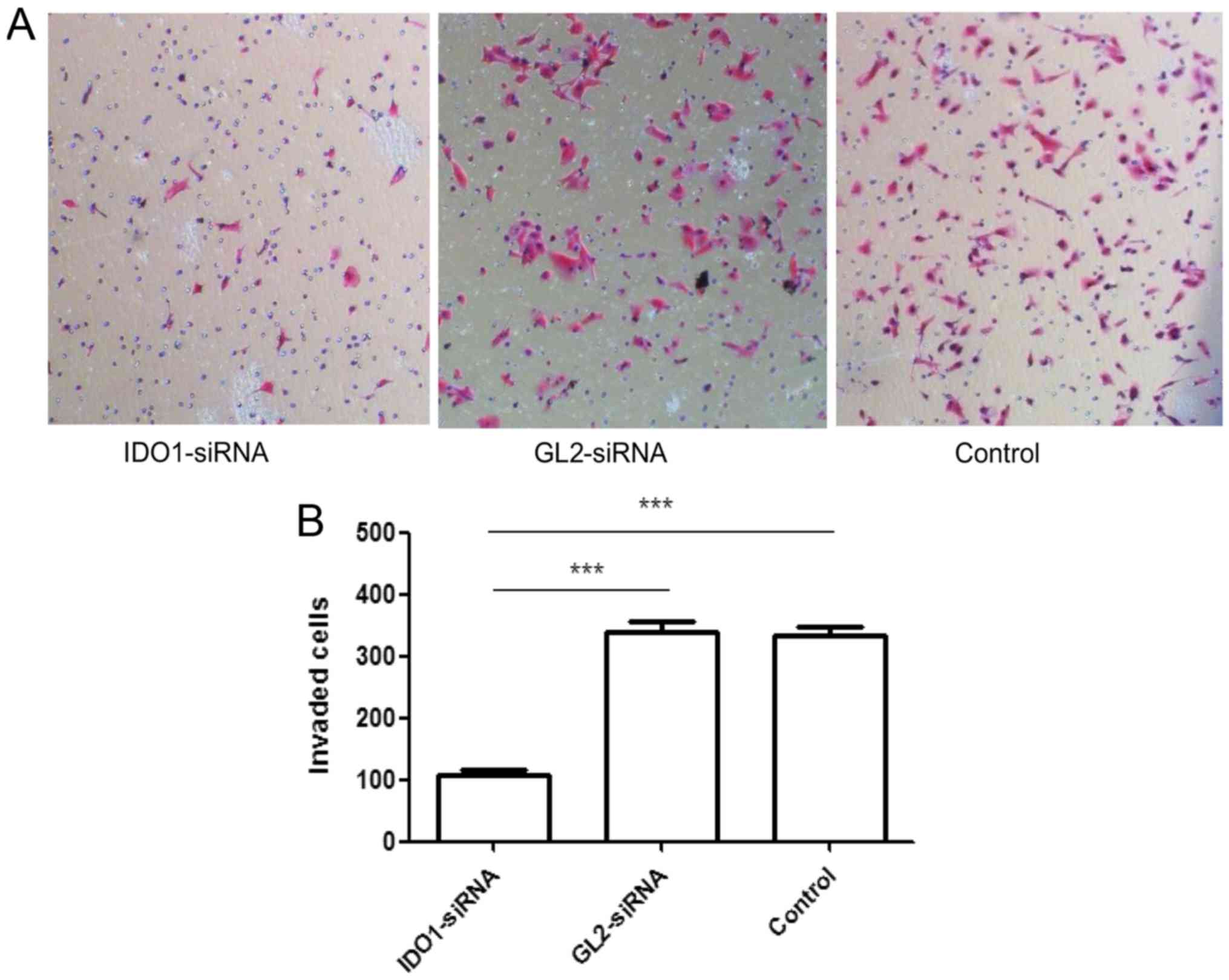
Effect of IDO1 on the invasion abilities
of LLC cells
As shown in Fig. 2,
the numbers of invasion cells tranfected with IDO1 siRNA, GL2
siRNA, and control cells were 108.667±6.658/well,
341.333±16.773/well and 333.333±16.442/well, respectively, showing
significant difference (p≤0.001). In other words, IDO1 expression
can decrease the invasive capacity of LLC cells in
vitro.
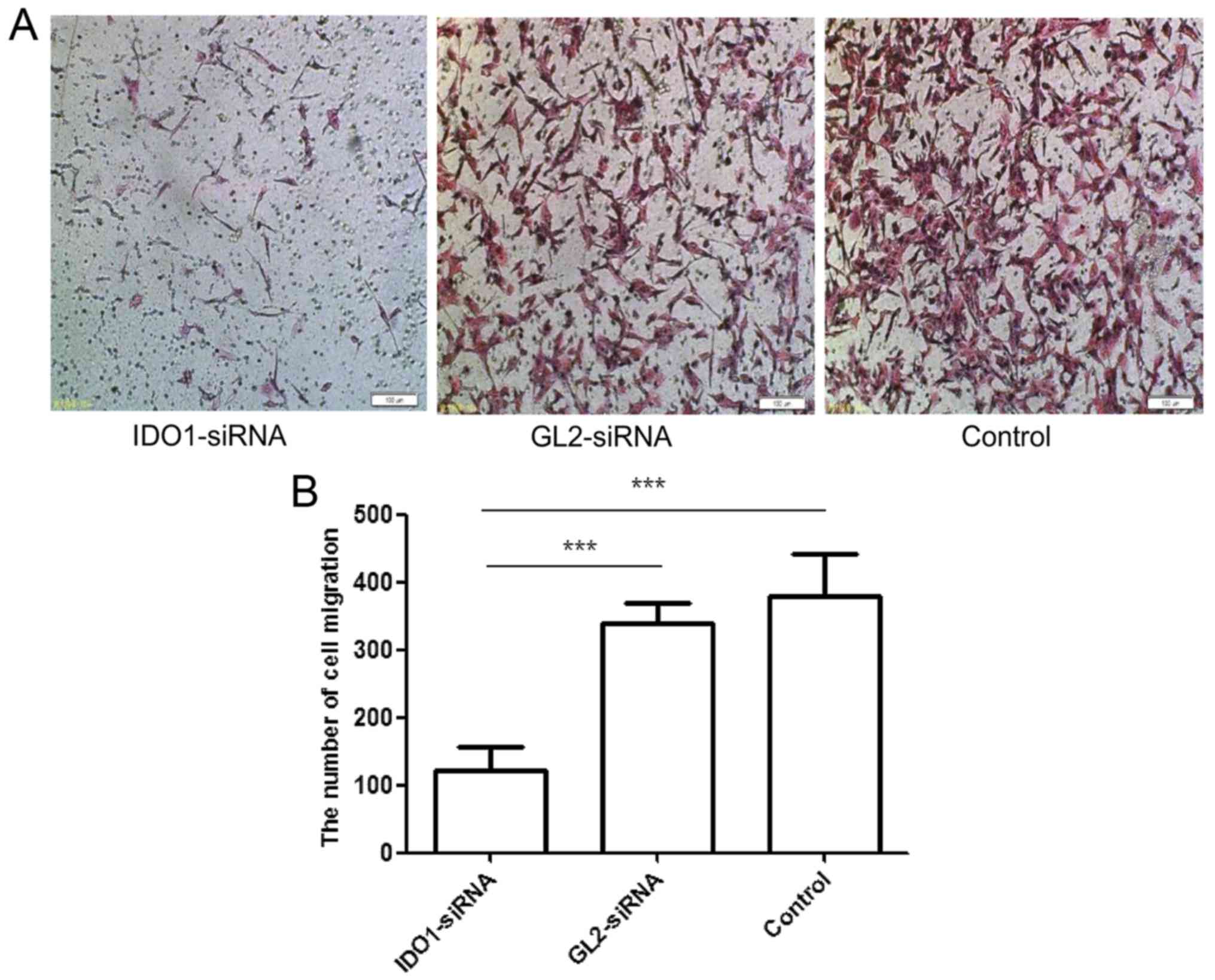
Effect of IDO1 on the migration ability
of LLC cells
Fig. 3 shows the
numbers of migrated cells transfected with IDO1 siRNA, GL2 siRNA,
and control cells: 121.333±35.921/well, 338±31.953/well and
380±61.294/well, respectively, showing a significant difference
(p≤0.001). Thus, IDO1 expression can reduce the migration of LLC
cells in vitro.
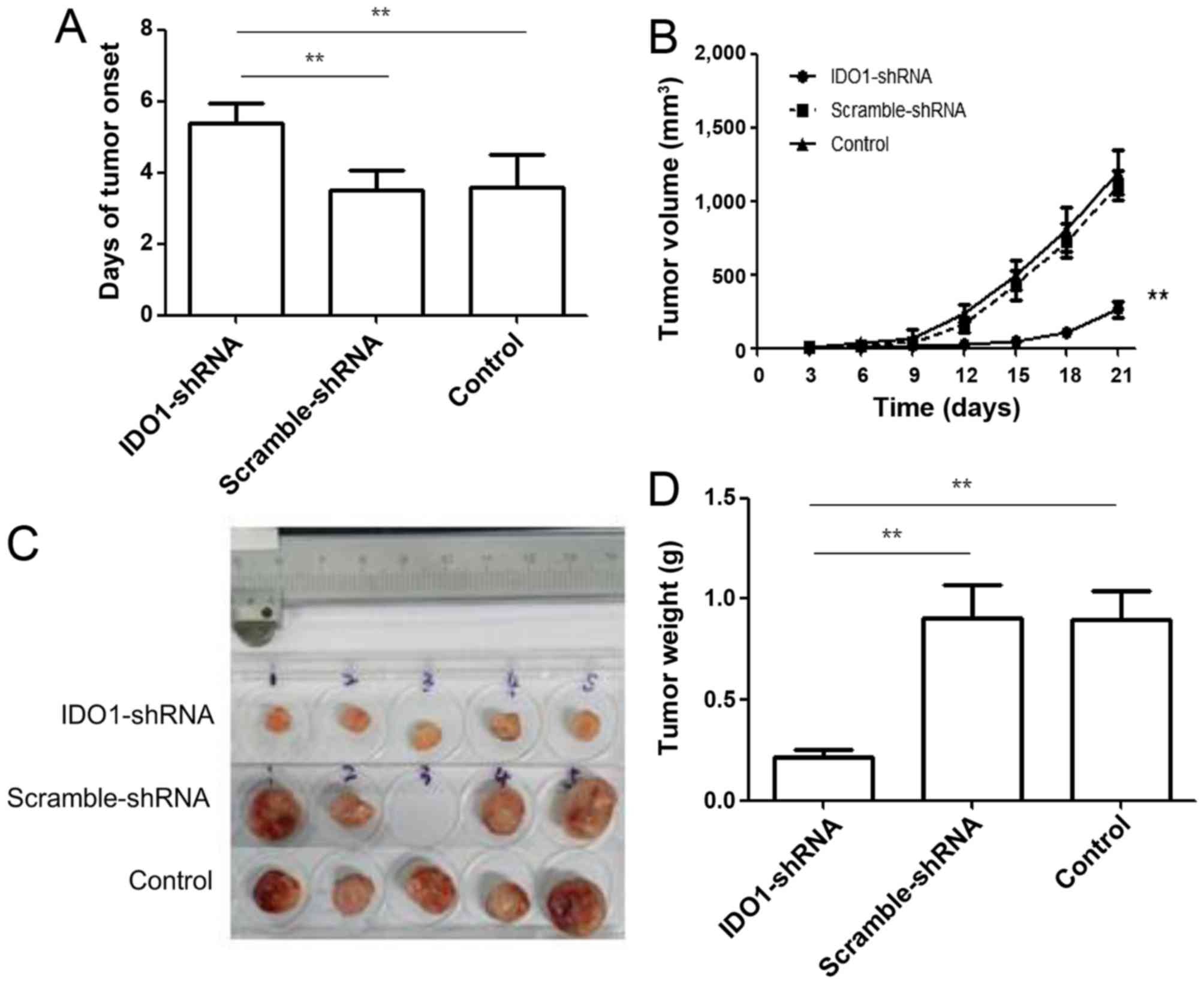
Treatment with IDO1 shRNA in vivo
suppresses tumor growth
To explore the therapeutic effect of silencing IDO1,
we treated tumor-bearing mice with IDO1 shRNA. As shown in Fig. 4A, treatment with IDO1 shRNA delayed
tumor onset (p≤0.01). Tumor growth was significantly slower in IDO1
shRNA-treated mice compared with scrambled shRNA-treated mice and
control group (Fig. 4B). Both
tumor size (Fig. 4C) and weight
(Fig. 4D) were less in IDO1
shRNA-treated mice than in scramble shRNA-treated mice and control
mice. These results indicate that IDO1 shRNA, injected
intravenously, can inhibit tumor growth in a murine lung cancer
model.
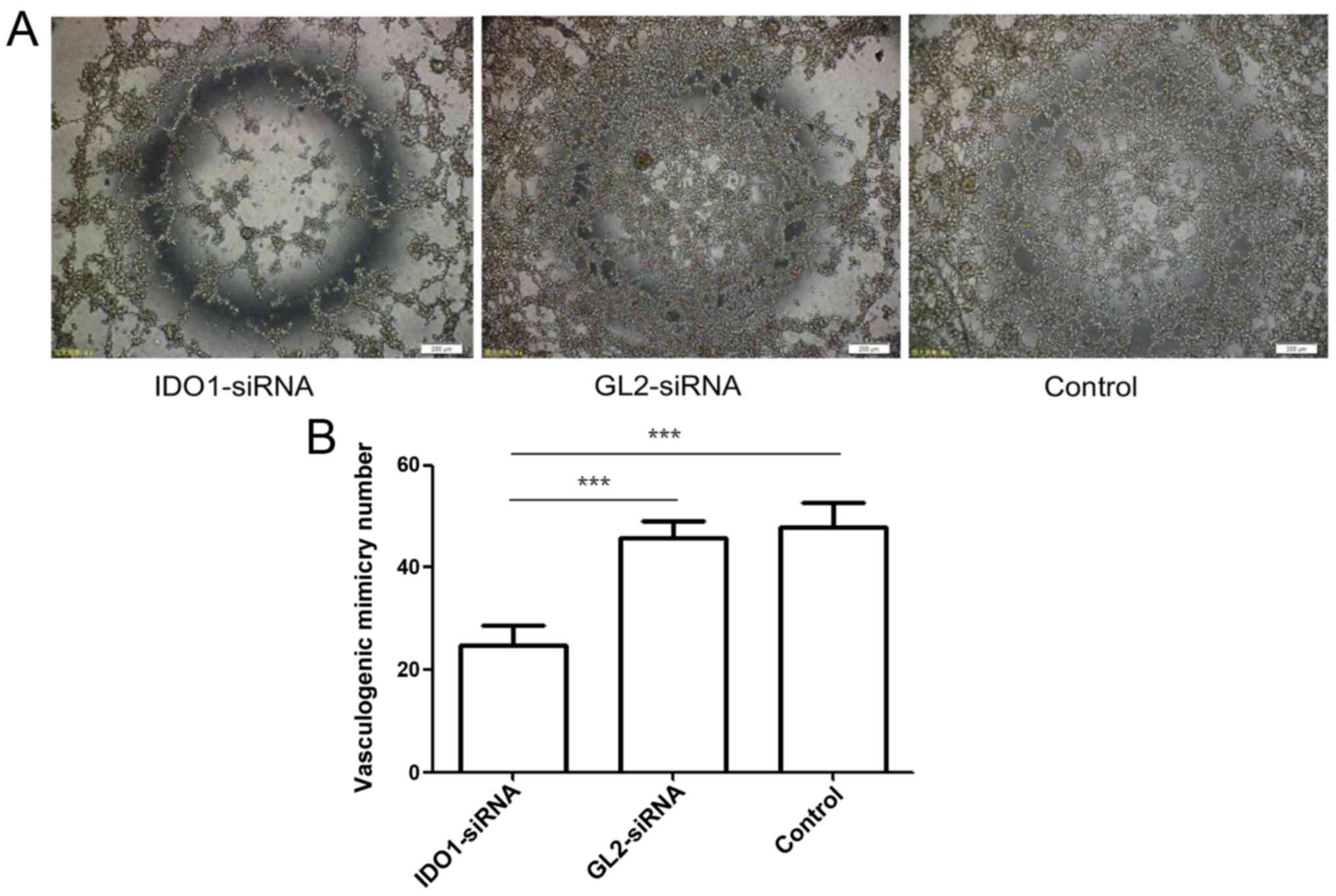
Effect of IDO1 on vasculogenic mimicry
formation of LLC cells
Tumor growth and metastasis depend on angiogenesis.
Tumor cells, through morphological self-deformation and matrix
remodeling, form a unique vascular microcirculation pipeline
structure. This channel without endothelial lining is called
vasculogenic mimicry (VM) (23).
It is an important complement to classical angiogenesis outside the
tumor. Fig. 5 shows the numbers of
VM tubers formed by the cells transfected with IDO1 siRNA, GL2
siRNA and control cells were 24.67±4.041/well, 45.67±3.512/well and
48±4.583/well, respectively, showing significant difference
(p≤0.001). That is, gene silencing of IDO1 can inhibit VM
formation.
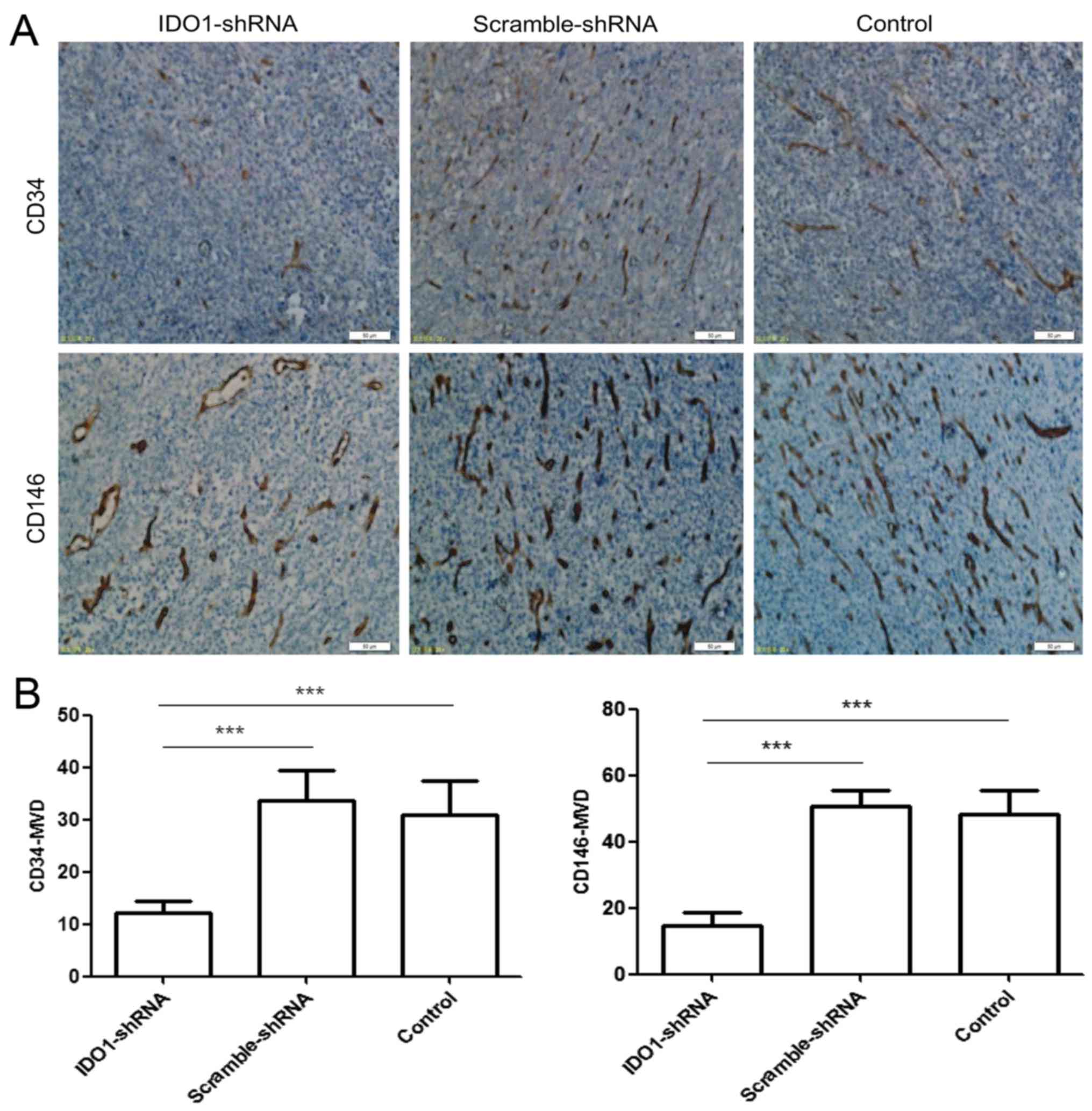
Effect of IDO1 on tumor angiogenesis
Fig. 6A shows that
tumor tissues of mice with scramble shRNA or control group
expressed markedly high levels of CD34 and CD146 protein. In
experimental tumor tissues from IDO1 shRNA-treated mice, there were
significantly decreased levels of CD34 and CD146 protein
expression. Fig. 6B shows that the
number (CD34+MVD, 12.2±1.94; CD146+MVD,
14.8±3.834) of newly formed blood vessels in the IDO1 shRNA tumors
was significantly less than (CD34+MVD, 31±6.442;
CD146+MVD, 48.4±7.197) those in the control tumors.
Thus, IDO1 expression can affect tumor angiogenesis.
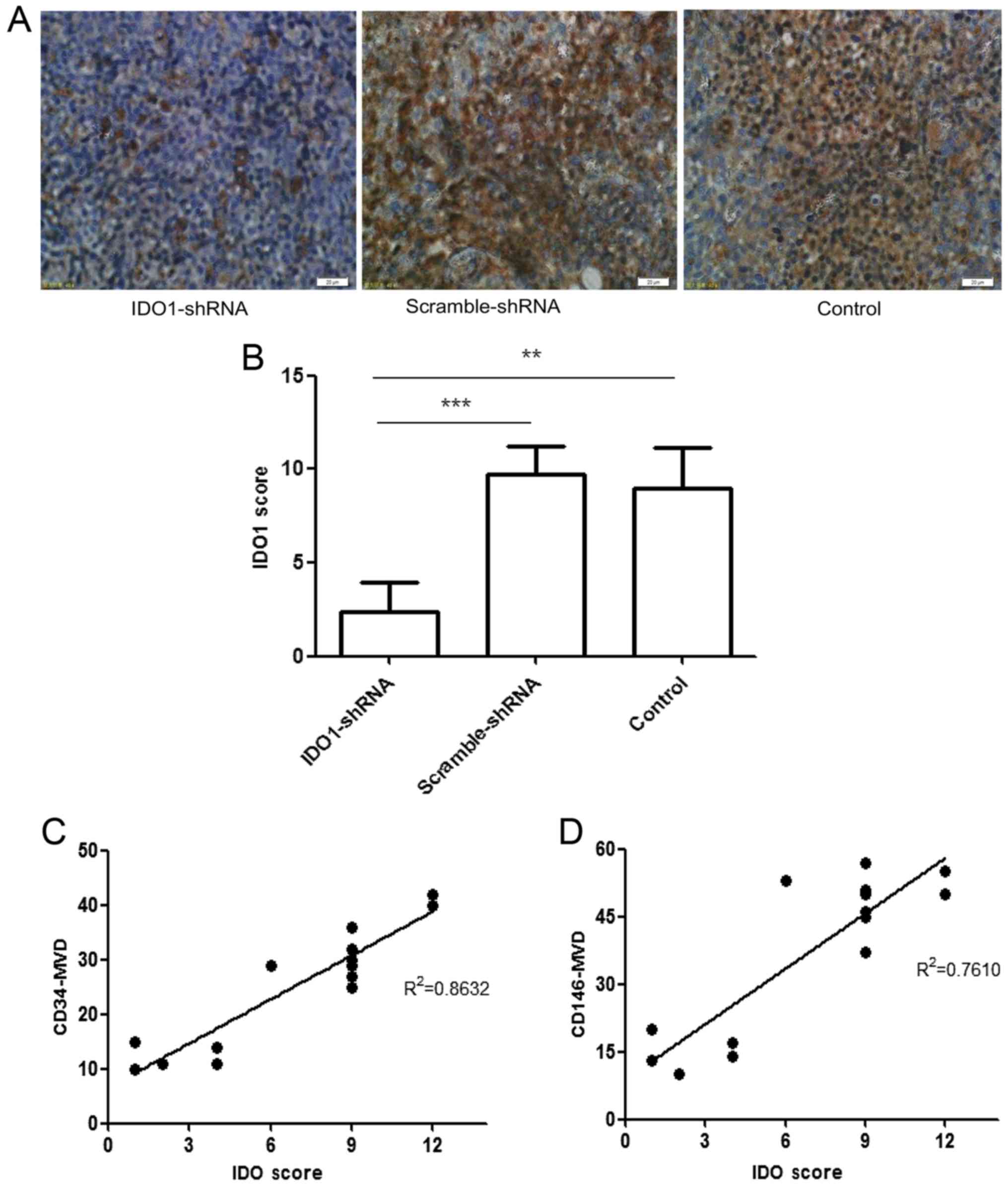
Correlation between MVD and IDO1
expression
Fig. 7 shows that
IDO1 expression was positively correlated to both
CD34+MVD (r2=0.8632) and CD146+MVD
(r2= 0.761). This association between the microvascular
density marked by CD34 or CD146 and the expression of IDO1
indicates that IDO1 has the potential to participate in the
formation of new capillaries.
Discussion
In this study, we aimed to clarify a factor involved
in lung cancer progression, particularly angiogenesis, and to
develop molecular therapy that could target it. We focused on IDO1,
which has been widely reported to be involved in tumor
immunotolerance. First, we attempted to transfect siRNA into the
IDO1-expressing Lewis lung cancer (LLC) cell line to knock down the
IDO1 gene. qPCR and western blot analysis identified that this gene
was silenced successfully. We conducted a basic study using this
gene-silenced cell line, and found that the expression of IDO1 can
affect the invasion and migration of LLC cells in vitro.
Our research indicates that siRNA or its precursor
shRNA can block the expression of the target gene through an RNAi
mechanism (24). RNAi is a
technique of specific gene silencing at the post-transcriptional
level, which can transfect double stranded RNA (dsRNA) of target
sequence into an organism. RNAi has the characteristics of high
efficiency and gene specificity with the potential to replace gene
knockout technology to some extent. Research of RNAi gene silencing
in tumor, virus infection, genetic disease and related diseases has
entered the stage of clinical trials (25,26).
Targeting gene knockout mice using a short hairpin RNA (shRNA)
plasmid through hydrodynamic tail vein injection has been reported
to treat breast cancer, prostate cancer, liver cancer and other
diseases in the mouse model (27–29).
In this study, we designed shRNA to silence IDO1 in treating
C57BL/6-LLC tumor-bearing mice. With hydrodynamic tail vein
injection of IDO1 shRNA plasmids, the speed of tumor growth slowed
significantly. Tumor volume and weight were also reduced markedly.
Immunohistochemistry showed reduced expression of IDO1. The above
experimental results indicate that IDO1 can be knocked out by
injection of IDO1 shRNA plasmids, which then inhibits tumor
angiogenesis and growth.
Maniotis et al (23) reported a new kind of tumor
vascularization pattern, named vasculogenic mimicry (VM). The
authors found that highly invasive melanoma cells can differentiate
into multiple cell phenotypes, which have the characteristics of
endothelial cells. This process can lead to de novo vascular
structure. A growing number of studies have found that many types
of cancer, including breast (30),
ovarian (31), prostate (32), and lung (33) contain VM. The high plasticity of
tumor cells is the formative basis of VM, which could allow certain
malignant cells to obtain an embryonic phenotype. When Maniotis
et al (23) discovered VM,
they also noted that the metastatic capacity of human melanoma
cells can affect the formation of VM. After establishing the LLC
cell line in which IDO1 gene was silenced, we used the Matrigel
matrix three-dimensional cell culture model to observe the ability
of LLC cells to form VM. After knockdown of the IDO1 gene, the
number of VM decreased markedly, and this may be associated with
the silenced IDO1 gene having a reduced metastatic ability.
Therefore, the removal of IDO1 gene leads to a decline in the
ability of LLC cells to form VM.
Angiogenesis is an essential process in growth,
metastasis and invasion of tumor cells. The interactions among
tumor cells, immune regulatory factors, and tumor vessels are
inextricably linked in tumor microenvironment. IDO1 is a classical
negative immunoregulation factor, but its association with tumor
angiogenesis has been rarely studied. As reported by Li et
al (34), IDO was believed to
promote angiogenesis by tryptophan depletion during skin xenograft.
Nonaka et al (35),
reported that IDO promotes the peritoneal dissemination of ovarian
cancer through angiogenesis. Thus, IDO secreted by tumor cells may
facilitate the proliferation, migration, and activiation of
endothelial cells, which leads to increased neovascularization. In
this study, the levels of CD34+MVD and
CD146+MVD both decreased significantly after IDO1 shRNA
treatment. Enhanced IDO expression was positively correlated with
MVD marked by CD34 or CD146. These findings suggest that IDO is
involved in lung cancer progression, and the IDO1 gene can be
successfully silenced using molecular-targeted therapy.
Acknowledgments
This study was partially supported by grants from
the Natural Science Foundation of China (NSFC, no. 81673009),
Jiangxi Science and Technology Innovation Programs (20124ACB00800),
Canadian Institute of Health Research (CIHR).
Abbreviations:
|
IDO1
|
indoleamine 2,3-dioxygenase-1
|
|
siRNA
|
small interfering RNA
|
|
shRNA
|
short hairpin RNA
|
|
LLC
|
Lewis lung cancer
|
|
MVD
|
microvessel density
|
|
VM
|
vasculogenic mimicry
|
|
H&E
|
hematoxylin and eosin
|
References
|
1
|
Zheng R, Zeng H, Zuo T, Zhang S, Qiao Y,
Zhou Q and Chen W: Lung cancer incidence and mortality in China,
2011. Thorac Cancer. 7:94–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rusch VW: Stage III non-small cell lung
cancer. Semin Respir Crit Care Med. 37:727–735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mellor AL and Munn DH: IDO expression by
dendritic cells: Tolerance and tryptophan catabolism. Nat Rev
Immunol. 4:762–774. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamazaki F, Kuroiwa T, Takikawa O and Kido
R: Human indolylamine 2,3-dioxygenase. Its tissue distribution, and
characterization of the placental enzyme. Biochem J. 230:635–638.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grohmann U, Fallarino F and Puccetti P:
Tolerance, DCs and tryptophan: Much ado about IDO. Trends Immunol.
24:242–248. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hirata F, Ohnishi T and Hayaishi O:
Indoleamine 2,3-dioxygenase. Characterization and properties of
enzyme O2- complex. J Biol Chem. 252:4637–4642. 1977.PubMed/NCBI
|
|
7
|
Uyttenhove C, Pilotte L, Théate I,
Stroobant V, Colau D, Parmentier N, Boon T and Van den Eynde BJ:
Evidence for a tumoral immune resistance mechanism based on
tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med.
9:1269–1274. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karanikas V, Zamanakou M, Kerenidi T,
Dahabreh J, Hevas A, Nakou M, Gourgoulianis KI and Germenis AE:
Indoleamine 2,3-dioxygenase (IDO) expression in lung cancer. Cancer
Biol Ther. 6:1258–1262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen IC, Lee KH, Hsu YH, Wang WR, Chen CM
and Cheng YW: Expression pattern and clinicopathological relevance
of the indoleamine 2,3-dioxygenase 1/tryptophan 2,3-dioxygenase
protein in colorectal cancer. Dis Markers. 2016:81697242016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feder-Mengus C, Wyler S, Hudolin T, Ruszat
R, Bubendorf L, Chiarugi A, Pittelli M, Weber WP, Bachmann A,
Gasser TC, et al: High expression of indoleamine 2,3-dioxygenase
gene in prostate cancer. Eur J Cancer. 44:2266–2275. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bi WW, Zhang WH, Yin GH, Luo H, Wang SQ,
Wang H, Li C, Yan WQ and Nie DZ: Analysis of indoleamine 2–3
dioxygenase (IDO) and EGFR co-expression in breast cancer tissue by
immunohistochemistry. Asian Pac J Cancer Prev. 15:5535–5538. 2014.
View Article : Google Scholar
|
|
12
|
Liu J, Zhang H, Jia L and Sun H: Effects
of Treg cells and IDO on human epithelial ovarian cancer cells
under hypoxic conditions. Mol Med Rep. 11:1708–1714. 2015.
|
|
13
|
Creelan BC, Antonia S, Bepler G, Garrett
TJ, Simon GR and Soliman HH: Indoleamine 2,3-dioxygenase activity
and clinical outcome following induction chemotherapy and
concurrent chemoradiation in Stage III non-small cell lung cancer.
Oncoimmunology. 2:e234282013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ino K, Yoshida N, Kajiyama H, Shibata K,
Yamamoto E, Kidokoro K, Takahashi N, Terauchi M, Nawa A, Nomura S,
et al: Indoleamine 2,3-dioxygenase is a novel prognostic indicator
for endometrial cancer. Br J Cancer. 95:1555–1561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takao M, Okamoto A, Nikaido T, Urashima M,
Takakura S, Saito M, Saito M, Okamoto S, Takikawa O, Sasaki H, et
al: Increased synthesis of indoleamine-2,3-dioxygenase protein is
positively associated with impaired survival in patients with
serous-type, but not with other types of, ovarian cancer. Oncol
Rep. 17:1333–1339. 2007.PubMed/NCBI
|
|
16
|
Fus LP and Górnicka B: Role of
angiogenesis in urothelial bladder carcinoma. Cent European J Urol.
69:258–263. 2016.PubMed/NCBI
|
|
17
|
Cox G, Walker RA, Andi A, Steward WP and
O'Byrne KJ: Prognostic significance of platelet and microvessel
counts in operable non-small cell lung cancer. Lung Cancer.
29:169–177. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pereira T, Dodal S, Tamgadge A, Bhalerao S
and Tamgadge S: Quantitative evaluation of microvessel density
using CD34 in clinical variants of ameloblastoma: An
immunohistochemical study. J Oral Maxillofac Pathol. 20:51–58.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng C, Qiu Y, Zeng Q, Zhang Y, Lu D,
Yang D, Feng J and Yan X: Endothelial CD146 is required for in
vitro tumor-induced angiogenesis: The role of a disulfide bond in
signaling and dimerization. Int J Biochem Cell Biol. 41:2163–2172.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin EJ, Choi YA, Sonn JK and Kang SS:
Suppression of ADAM 10-induced Delta-1 shedding inhibits cell
proliferation during the chondro-inhibitory action of TGF-beta3.
Mol Cells. 24:139–147. 2007.PubMed/NCBI
|
|
21
|
Scharl A, Vierbuchen M, Conradt B, Moll W,
Würz H and Bolte A: Immunohistochemical detection of progesterone
receptor in formalin-fixed and paraffin-embedded breast cancer
tissue using a monoclonal antibody. Arch Gynecol Obstet. 247:63–71.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weidner N, Folkman J, Pozza F, Bevilacqua
P, Allred EN, Moore DH, Meli S and Gasparini G: Tumor angiogenesis:
A new significant and independent prognostic indicator in
early-stage breast carcinoma. J Natl Cancer Inst. 84:1875–1887.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jinek M and Doudna JA: A three-dimensional
view of the molecular machinery of RNA interference. Nature.
457:405–412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lares MR, Rossi JJ and Ouellet DL: RNAi
and small interfering RNAs in human disease therapeutic
applications. Trends Biotechnol. 28:570–579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burnett JC, Rossi JJ and Tiemann K:
Current progress of siRNA/shRNA therapeutics in clinical trials.
Biotechnol J. 6:1130–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang X, Liu Y, Zhang G, Shi J, Zhang X,
Zheng X, Jiang AT, Zhang ZX, Johnston N, Siu KS, et al: Synergic
silencing of costimulatory molecules prevents cardiac allograft
rejection. J Transl Med. 12:1422014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vakili S, Ebrahimi SS, Sadeghi A,
Gorgani-Firuzjaee S, Beigy M, Pasalar P and Meshkani R:
Hydrodynamic-based delivery of PTP1B shRNA reduces plasma glucose
levels in diabetic mice. Mol Med Rep. 7:211–216. 2013.
|
|
29
|
Caldas H, Holloway MP, Hall BM, Qualman SJ
and Altura RA: Survivin-directed RNA interference cocktail is a
potent suppressor of tumour growth in vivo. J Med Genet.
43:119–128. 2006. View Article : Google Scholar
|
|
30
|
Shirakawa K, Tsuda H, Heike Y, Kato K,
Asada R, Inomata M, Sasaki H, Kasumi F, Yoshimoto M, Iwanaga T, et
al: Absence of endothelial cells, central necrosis, and fibrosis
are associated with aggressive inflammatory breast cancer. Cancer
Res. 61:445–451. 2001.PubMed/NCBI
|
|
31
|
Sood AK, Fletcher MS, Zahn CM, Gruman LM,
Coffin JE, Seftor EA and Hendrix MJ: The clinical significance of
tumor cell-lined vasculature in ovarian carcinoma: Implications for
anti-vasculogenic therapy. Cancer Biol Ther. 1:661–664. 2002.
View Article : Google Scholar
|
|
32
|
Sharma N, Seftor RE, Seftor EA, Gruman LM,
Heidger PM Jr, Cohen MB, Lubaroff DM and Hendrix MJ: Prostatic
tumor cell plasticity involves cooperative interactions of distinct
phenotypic subpopulations: Role in vasculogenic mimicry. Prostate.
50:189–201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Passalidou E, Trivella M, Singh N,
Ferguson M, Hu J, Cesario A, Granone P, Nicholson AG, Goldstraw P,
Ratcliffe C, et al: Vascular phenotype in angiogenic and
non-angiogenic lung non-small cell carcinomas. Br J Cancer.
86:244–249. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Tredget EE, Ghaffari A, Lin X,
Kilani RT and Ghahary A: Local expression of indoleamine
2,3-dioxygenase protects engraftment of xenogeneic skin substitute.
J Invest Dermatol. 126:128–136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nonaka H, Saga Y, Fujiwara H, Akimoto H,
Yamada A, Kagawa S, Takei Y, Machida S, Takikawa O and Suzuki M:
Indoleamine 2,3-dioxygenase promotes peritoneal dissemination of
ovarian cancer through inhibition of natural killer cell function
and angiogenesis promotion. Int J Oncol. 38:113–120. 2011.
|