Introduction
Cervical cancer is the world's fourth most common
female malignancy and ranks the second in the developing countries
and the third in the developed countries, seriously affecting the
health of women worldwide. Each year there are around 500,000 new
cervical cancer cases and 266,000 deaths, of which more than 80%
are in developing countries. It is estimated that there are
approximately 62,000 new cases and 30,000 dead cases each year in
China, and its annual incidence increases by 2–3%. In addition, the
onset age of cervical cancer patients in recent years has
decreased, and the incidence of cervical cancer increases sharply
in the age group of 25 to 34 years, causing great concern
world-wide (1–3).
According to recent epidemiological investigation,
clinical stage is one of the risk factors affecting prognosis of
patients with cervical cancer: the later the stage, the worse the
prognosis of the patients (4).
These findings indicate that invasion and metastasis are the main
causes for poor prognosis and high mortality of patients with
cervical cancer and these difficult problems need to be solved.
Studies have found that epithelial-mesenchymal transition (EMT) is
the primary step causing cervical cancer invasion and metastasis
and its relationship to tumor progression is gradually drawing more
attention (5–7). EMT is a phenomenon where epithelial
cells lose their polarity and gradually shift to mesenchymal cell
morphology under certain pathological stimulations. The process is
accompanied with cell morphological changes and increased ability
to alter cell motility, thus further affecting cell migration
(8,9).
Recent studies have found that changes in the
mechanical properties of the extracellular matrix, i.e. the
substrate stiffness, could regulate EMT and other biological
functions of tumor cells. In other words, mechanical properties of
extracellular matrix play a major role in tumor progression.
Studies have found that changes in substrate stiffness can affect
EMT of breast cancer cell lines, lung cancer cell lines and
hepatoma cell lines (10,11). In addition, human tissue stiffness
changes significantly with disease progressing. For example, the
stiffness of normal breast tissues is around 4 kPa, while that of
cancerous breast tissues is approximately 12 kPa (7,12–16).
Moreover, studies have shown that tumor-associated fibroblasts and
interstitial fibrosis components which is produced by fibroblasts,
such as α-SMA, laminin and fibronectin increase significantly with
cervical cancer progression, suggesting that the stiffness of
cervical cancer tissues is higher than that of normal cervical
tissues (16). However, whether
the mechanical properties of extracellular matrix can influence EMT
of cervical cancer cells has not been reported.
MicroRNA is a small RNA with post-transcriptional
regulation functions. MicroRNA regulate expression levels of
important oncogenes and tumor suppressor genes, and further
regulate cancer cell invasion and metastasis. Previous studies have
found that the mechanical signals of extra cellular matrix can
alter microRNA expression in cells (17–19).
For example, miR-29 expression was significantly increased by
bladder obstruction-induced increase in bladder tissue stiffness
(19); microRNA expression changes
in alveolar epithelium and mesothelial cells under different
mechanical shear stress. In other words, different substrate
stiffness can affect some microRNA expression in cells, therefore
regulating cell functions by targeting degradation of important
proteins (20). Mouw et al
found that mechanical stiffness of extracellular matrix could
regulate PTEN expression and its downstream PI3K/AKT signaling
pathway and promote breast cancer occurrence through microRNA,
indicating that microRNA is an important key node in transducing
mechanical signals to cells (21).
Our previous study established a related miRNA-mRNA
regulatory network diagram through mRNA and microRNA chips analysis
of normal cervical tissues and cervical cancer tissues and found
that miR-106b is a key node in the network and closely related to
cervical cancer cell migration. In addition, our previous study
also found that miR-106b was significantly upregulated in cervical
cancer tissues and could promote EMT and migration of cancer cells
by degrading its target protein DAB2 (22).
In this study, we explored whether substrate
stiffness can affect EMT of cervical cancer cells, and the role of
miR-106b and its target protein DAB2 in this process.
Materials and methods
Artificial substrate preparation
Artificial substrates with different stiffness were
prepared using acrylamide and bisacrylamide at different ratios and
laid on 24×24 mm slides. After irradiated with UV light for 2 h,
they were used to grow cervical cancer cell lines.
Cell culture
The cervical cancer cell lines SiHa and HeLa were
cultured in DMEM medium supplemented with 10% FBS, 100 U/ml
penicillin and 100 mg/ml streptomycin at 37°C in a humidified
incubator supplemented with 5% CO2. When cells reached
70–80% confluency, they were transferred on artificial
substrates.
Western blotting
Proteins were extracted from cervical cancer cell
lines HeLa and SiHa and semi-quantitated with Coomassie brilliant
blue. A total of 10 µg protein of each sample was separated
on 10% SDS-PAGE and transferred onto PVDF membrane. The membrane
was then blocked with 5% skim milk at 37°C for 1 h. Target proteins
on the membrane were recognized by incubating with appropriate
primary antibodies (rabbit anti-human E-cadherin, mouse anti-human
vimentin, rabbit anti-human Twist1 and rabbit anti-human Eif-5
antibodies, respectively) at room temperature for 4 h. After rinsed
with TBST three times for 5 min each time, the protein-antibody
complexes were recognized by alkaline phosphatase-labeled anti-IgG
(1:1000) antibody by incubating at room temperature for 1 h. After
rinsed with TBST three times for 10 min each, the complexes were
visualized by incubation with NBT/BCIP. The gray scale images were
scanned and protein expression was analyzed using Eif-5 as the
internal control and expressed as relative RGS. Each experiment was
repeated 3 times.
Real-time PCR
Total RNA were extracted using TRIzol from SiHa
cells cultured on substrate with different stiffness. mRNA were
reverse transcribed into cDNA and subjected to fluorescence
quantitative PCR at the condition of denature at 95°C for 2 min
followed by 40 cycles of 95°C for 30 sec, 55°C for 30 sec and 72°C
for 30 sec using primers shown in Table I on Bio-Rad PCR real-time PCR
instrument. Expression levels of DAB2 and vimentin were normalized
to internal control Eif-5. Each experiment was repeated three
times.
 | Table ISequences of primers used in real-time
PCR. |
Table I
Sequences of primers used in real-time
PCR.
| Gene | Primer (5′-3′) |
|---|
| DAB2 | F:
AGCAAGGTTCAAAGGCGATG |
| R:
GTTGTCCCTGAGACCGACC |
| Vimentin | F:
TGCCGTTGAAGCTGCTAACTA |
| R:
CCAGAGGGAGTGAATCCAGATTA |
| N-cadherin | F:
AGCCAACCTTAACTGAGGAGT |
| R:
GGCAAGTTGATTGGAGGGATG |
| GAPDH | F:
CCTCCGGGAAACTGTGGCGTGATGG |
| R:
AGACGGCAGGTCAGGTCCACCACTG |
MicroRNA chip analysis
Total RNA was extracted using TRIzol from SiHa cells
cultured on substrates with stiffness of 1 kPa and 20 kPa as well
as on a glass plate were reverse transcribed with the Qiagen RNeasy
Mini kit and subjected to microRNA chip analysis using Genisphere
FlashTag Labeling kit from Beijing CapitalBio Corp.
Statistical analysis
The results are expressed as mean ± SEM. Differences
among multiple groups were compared using one way ANOVA. Samples
with different variance between two groups were compared using
non-parametric tests. P<0.05 was considered to indicate a
statistically significant difference. Graphs were plotted using
GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA,
USA).
Results
Selection of substrate stiffness
range
It was reported that the average stiffness of human
solid tumors is approximately 20 kPa. The stiffness of most solid
tumor tissues before canci-genesis is higher than that of normal
tissues (7). Therefore, in this
study, the artificial substrates were prepared to have stiffness of
1, 10, 20 and 30 kPa, respectively, and glass plate was used as
control.
Effects of substrate stiffness on the
morphology of cervical cancer cell lines SiHa and HeLa
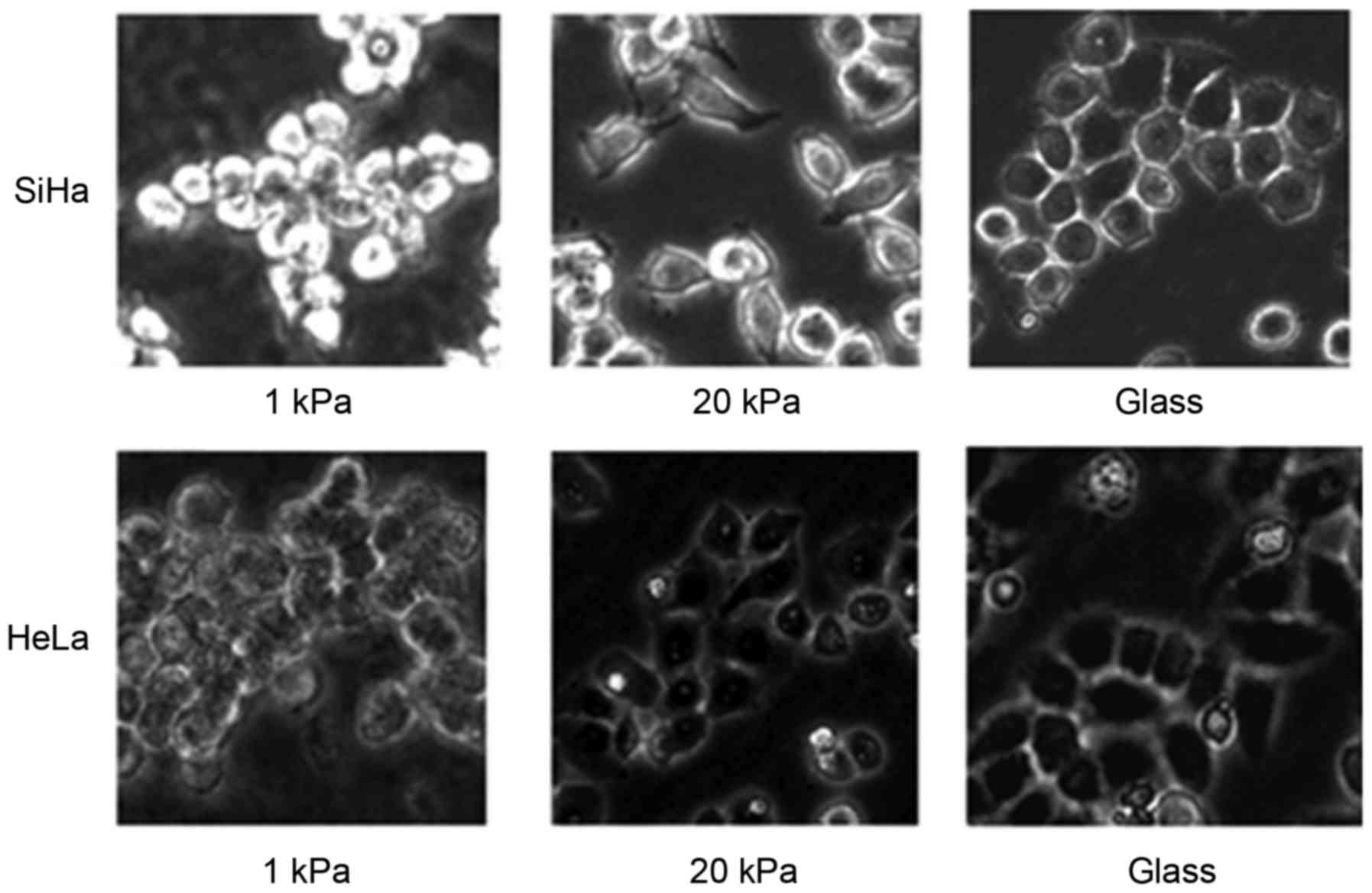
SiHa and HeLa cells were cultured on artificial
substrate with stiffness of 1 kPa to represent their normal
condition and on artificial substrate with stiffness of 20 kPa to
represent cervical tumor tissues. Morphological observation showed
that cells cultured on artificial substrate with stiffness of 1 kPa
were spherical and hardly had pseudopodia, while cells cultured on
artificial substrate with stiffness of 20 kPa were rich in
pseudopodia (Fig. 1), indicating
that SiHa and HeLa cells have stronger migration ability when
cultured on artificial substrate with stiffness of 20 kPa than
cultured on artificial substrate with stiffness of 1 kPa.
Effects of substrate stiffness on EMT of
cervical cancer cell lines SiHa and HeLa
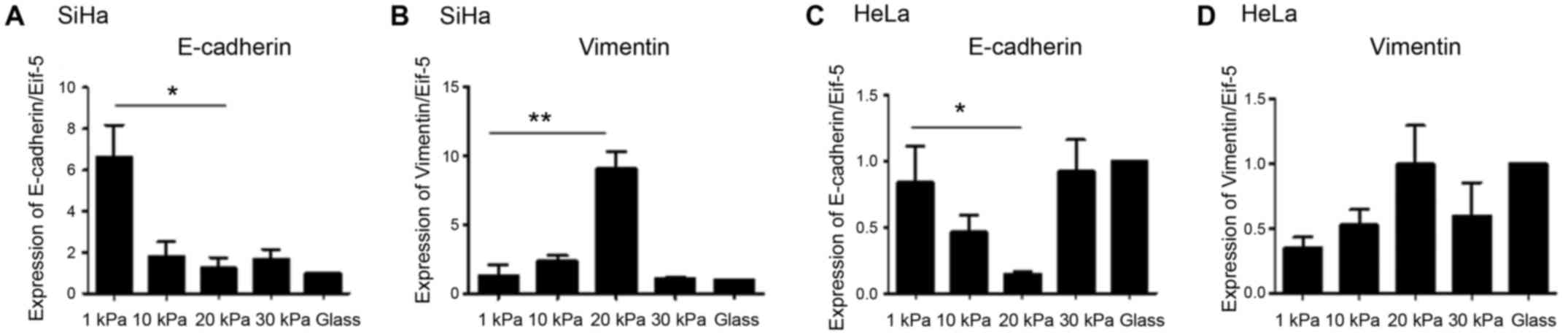
First, we detected the expression level of EMT
markers in SiHa and HeLa cultured at artificial substrate with
different stiffness using real-time PCR. The results showed that
mRNA levels of vimentin were significantly lower in SiHa and HeLa
cells cultured on artificial substrate with stiffness of 20 kPa
than cultured on substrate with stiffness of 1 kPa, and the
expression level of E-cadherin higher in stiffness of 20 kPa than 1
kPa (Fig. 2).
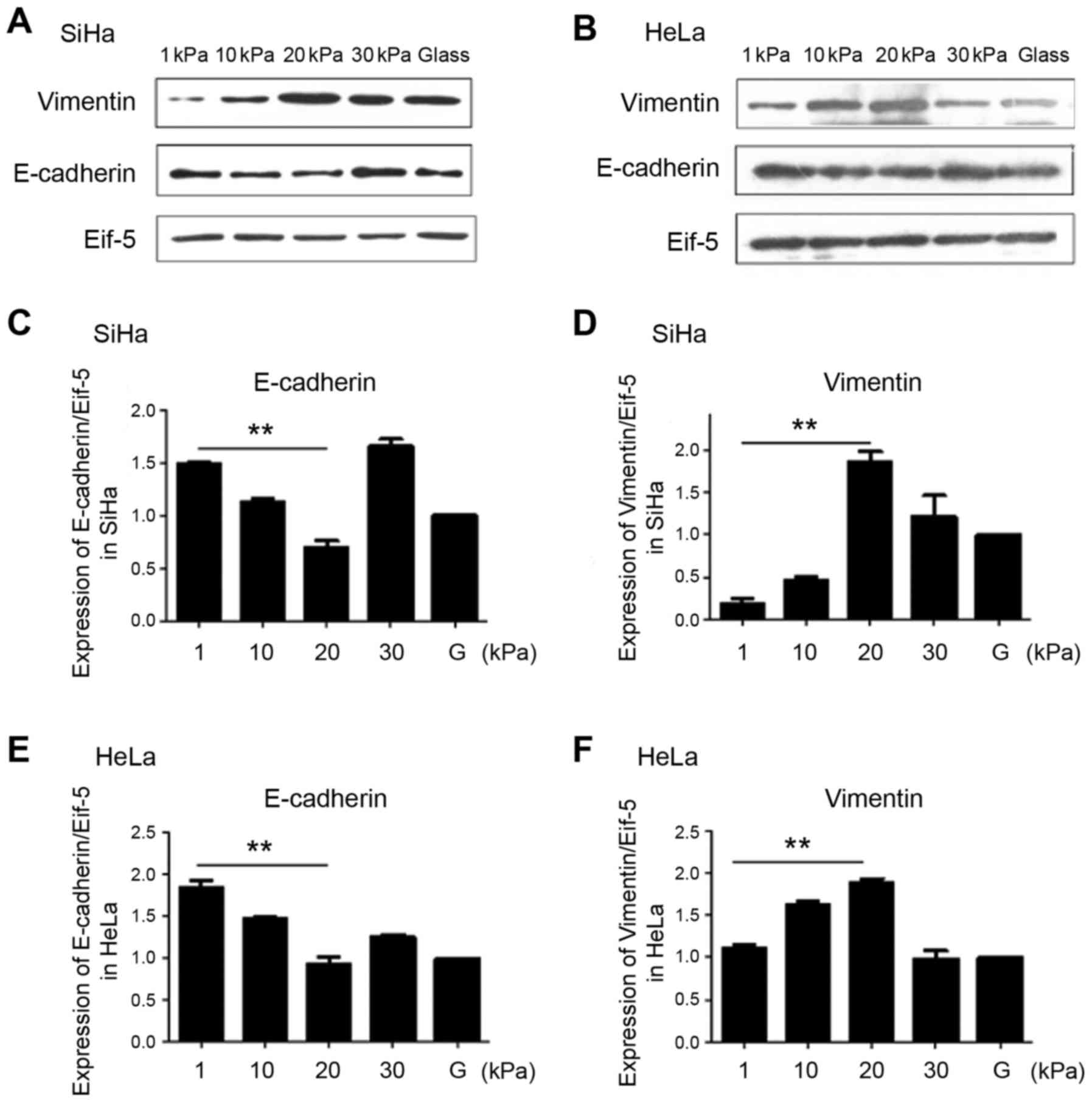
Expression levels of EMT markers E-cadherin and
vimentin in SiHa and HeLa cultured at artificial substrate with
different stiffness was also detected using western blotting. The
results showed that protein levels of E-cadherin is significantly
lower in SiHa and HeLa cells cultured on artificial substrate with
stiffness of 20 kPa than cultured on substrate with stiffness of 1
kPa, and expression levels vimentin is significantly higher in
stiffness of 20 kPa than 1 kPa (Fig.
3).
The above results indicate that cervical cancer cell
line SiHa and HeLa had stronger EMT ability when cultured on
artificial substrate with stiffness of 20 kPa than cultured on
substrate with stiffness of 1 kPa.
Effects of substrate stiffness on Twist1
expression of cervical cancer cell line SiHa
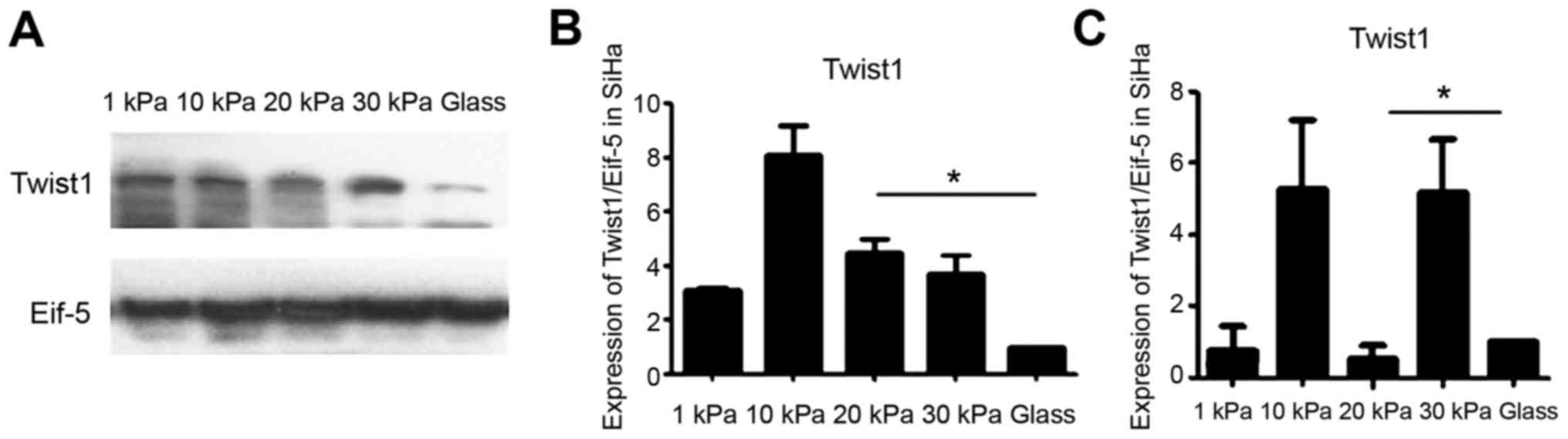
We detected the expression level of Twist1 in SiHa
which were cultured at artificial substrate with different
stiffness using western blotting. The results assessed by western
blotting showed that Twist1 expression was the highest in 30 kPa on
glass, lowest in 10 kPa situation. However, when tested by
Real-time PCR the Twist1 expression was the lowest in 20 kPa
situation excluding on glass. Expression of Twist1 of the cervical
cancer cell line SiHa is not consistent with EMT tendency (Fig. 4).
Effects of substrate stiffness on
microRNA expression in cervical cancer cell lines SiHa and
HeLa
To explore whether microRNA is involved in
regulating EMT of cervical cancer cell lines cultured on substrates
with different stiffness, we tested differential expression of
microRNA using microarray test. SiHa cells cultured on substrates
with stiffness of 1 kPa and 20 kPa as well as on glass plates for
36 h. Their total RNA was extracted and used for microRNA chip
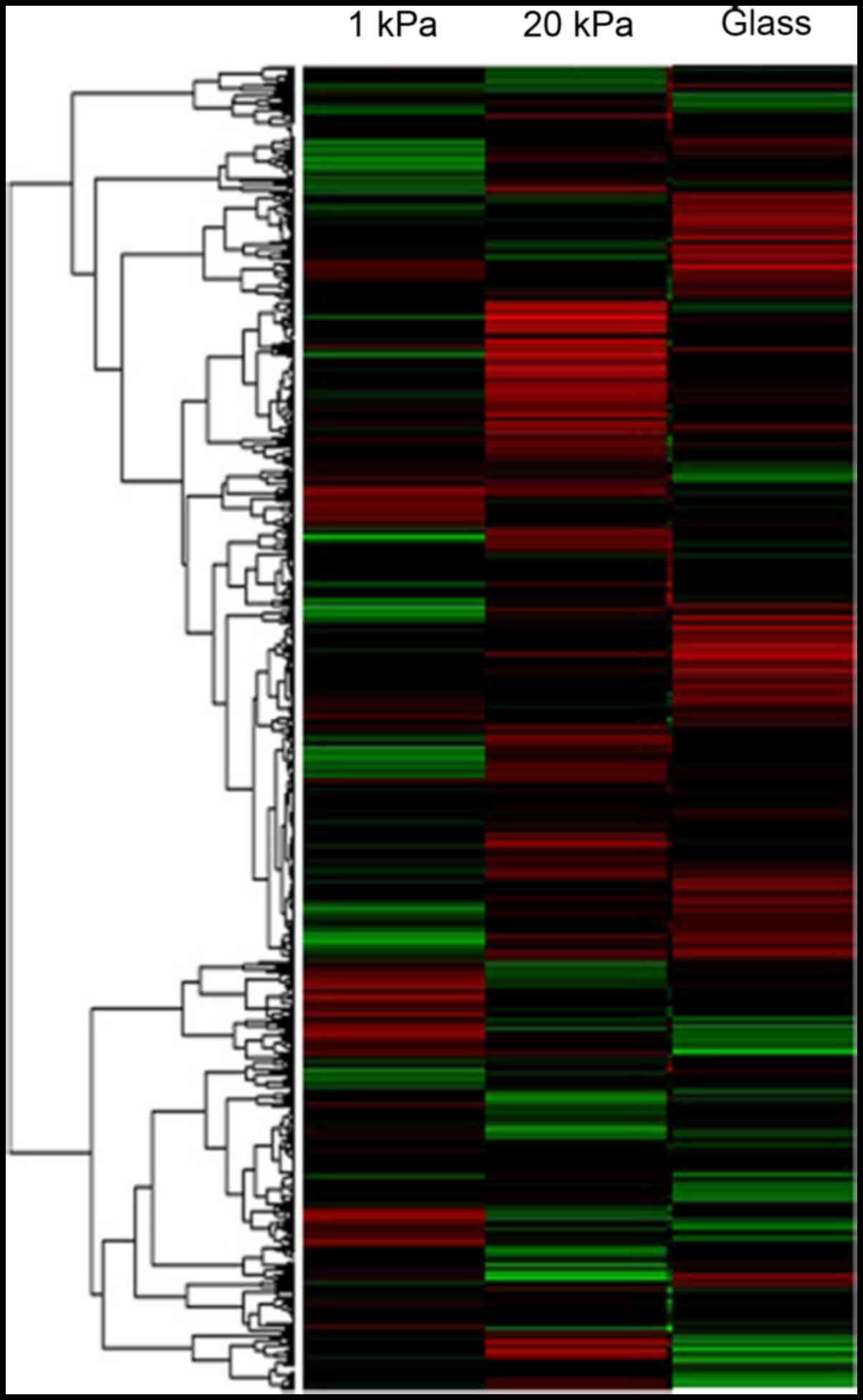
analysis to detect differential expression of microRNA. Cluster
analysis clearly showed that 173 microRNA were deferentially
expressed in cervical cancer cell line SiHa cultured on substrates
with different stiffness (Fig. 5).
Among them, 70 microRNA including miR-106b were upregulated and 103
microRNA were downregulated in SiHa cells cultured on substrates
with stiffness of 20 kPa (p<0.05, FRD<0.05). Tables II and III list the microRNA that were mostly
upregulated and down-regulated in SiHa cells cultured on substrate
with stiffness of 20 kPa compared with cells cultured on substrate
with stiffness of 1 kPa, respectively. Obviously, substrate
stiffness can affect microRNA expression in cervical cancer SiHa
cells, wherein miR-106b may play an important regulatory role.
 | Table IITop differentially upregulated
microRNAs in cervical cancer cells cultured on substrates with
stiffness of 1 and 20 kPa, respectively. |
Table II
Top differentially upregulated
microRNAs in cervical cancer cells cultured on substrates with
stiffness of 1 and 20 kPa, respectively.
| MicroRNA | Fold change | MicroRNA | Fold change |
|---|
| hsa-miR-125a | 17.554 | hsa-miR75b | 3.8857 |
| hsa-miR-21 | 3.8289 | hsa-miR-595 | 1.6495 |
| hsa-miR-150 | 1.067 |
hsa-miR-6785-3p | 8.487 |
|
hsa-miR-6507-5p | 3.5284 | hsa-miR-548u | 2.8511 |
| hsa-miR-211 | 2.6768 | hsa-miR-106b | 2.1564 |
 | Table IIITop differentially downregulated
microRNAs in cervical cancer cells cultured on substrates with
stiffness of 1 and 20 kPa, respectively. |
Table III
Top differentially downregulated
microRNAs in cervical cancer cells cultured on substrates with
stiffness of 1 and 20 kPa, respectively.
| MicroRNA | Fold change | MicroRNA | Fold change |
|---|
| hsa-miR-506b | −30.9045 | hsa-miR-218 | −1.2745 |
| hsa-miR-200b | −0.5418 | hsa-miR-107 | −0.0175 |
| hsa-miR-183 | −0.0517 | hsa-miR-7-1-3p | −0.0197 |
| hsa-miR-1279 | −0.0933 | hsa-miR-522-3p | −0.011 |
| hsa-miR-101-5p | −0.0791 |
hsa-miR-5001-3p | −0.0127 |
Effects of substrate stiffness on
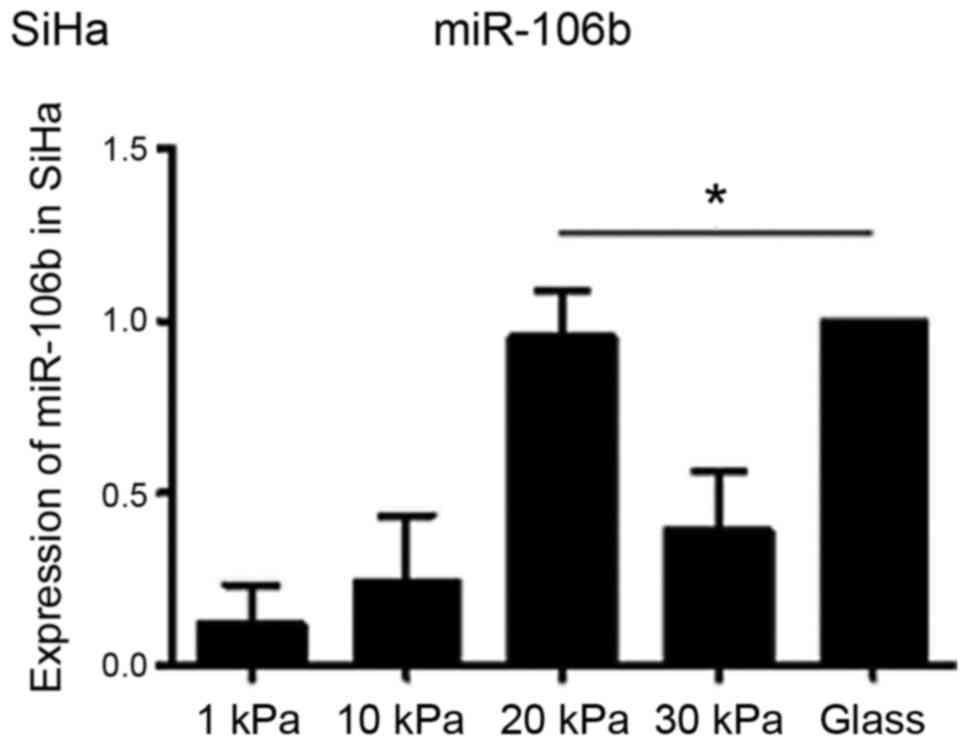
miR-106b expression in cervical cancer cell line SiHa
To understand whether substrate stiffness affects
EMT of cervical cancer cell lines through miR-106b, miR-106b
expression in cervical cancer cells was further measured and
verified using real-time PCR. The results (Fig. 6) confirmed that miR-106b expression
was indeed upregulated in cervical cancer cell line SiHa cultured
on substrate with stiffness of 20 kPa. Considering that SiHa
cultured on substrate with stiffness of 20 kPa had stronger EMT
ability than SiHa cultured on substrate with stiffness of 1 kPa,
the result suggests that substrate stiffness might regulate EMT of
cervical carcinoma cell line SiHa by regulating miR-106
expression.
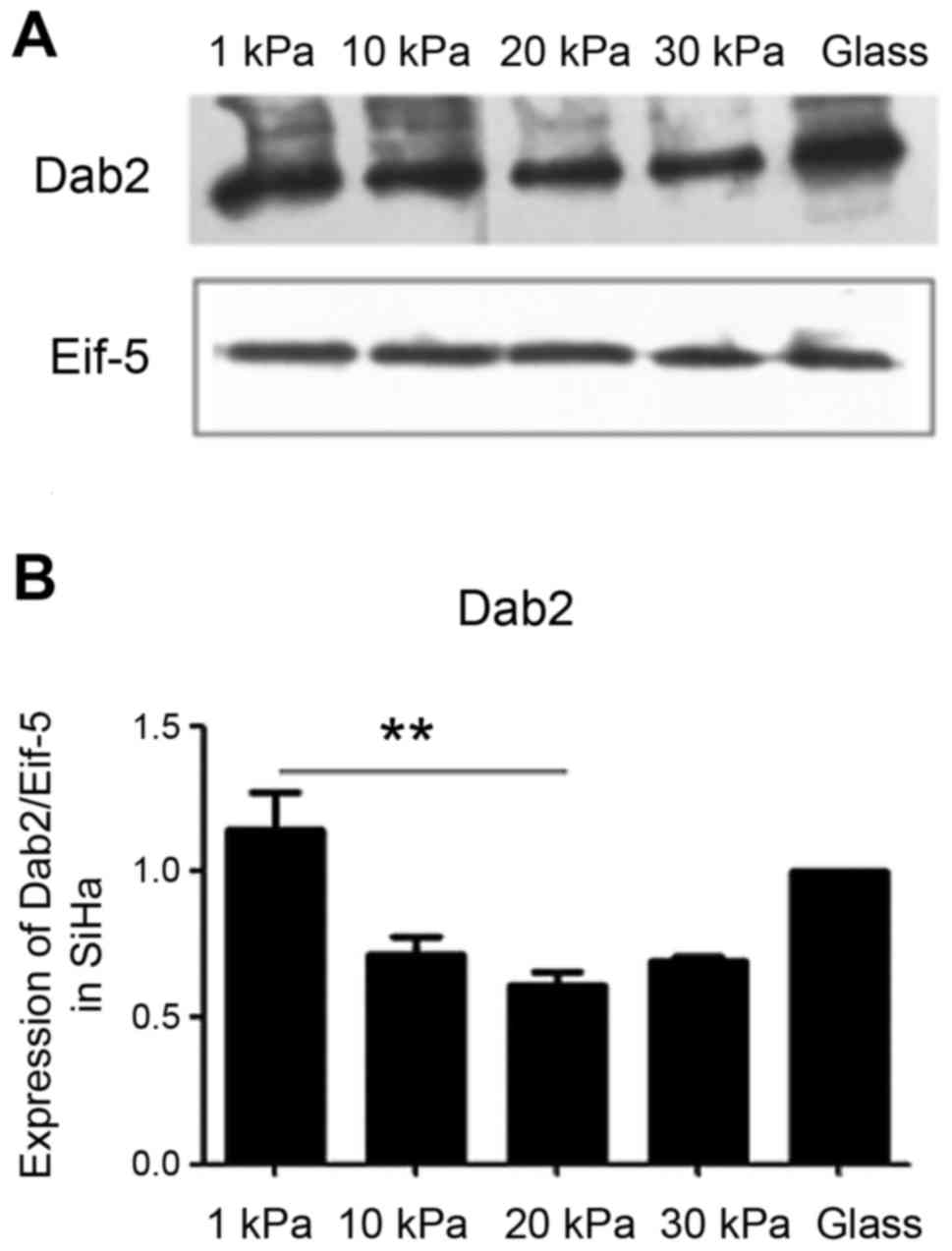
Effects of substrate stiffness on
expression of tumor suppressor protein DAB2 in cervical cancer cell
line SiHa
To further investigate whether substrate stiffness
affects EMT of cervical carcinoma cell line SiHa through miR-106b
and its target protein DAB2, the level of DAB2 in cervical cancer
cell line SiHa was examined by western blotting. The results
(Fig. 7) showed that the
expression level of DAB2 was significantly different in SiHa cells
cultured on substrate with different stiffness and lower in cells
cultured on substrate with stiffness of 20 kPa, suggesting that
substrate stiffness may regulate EMT through miR-106b targeted DAB2
degradation.
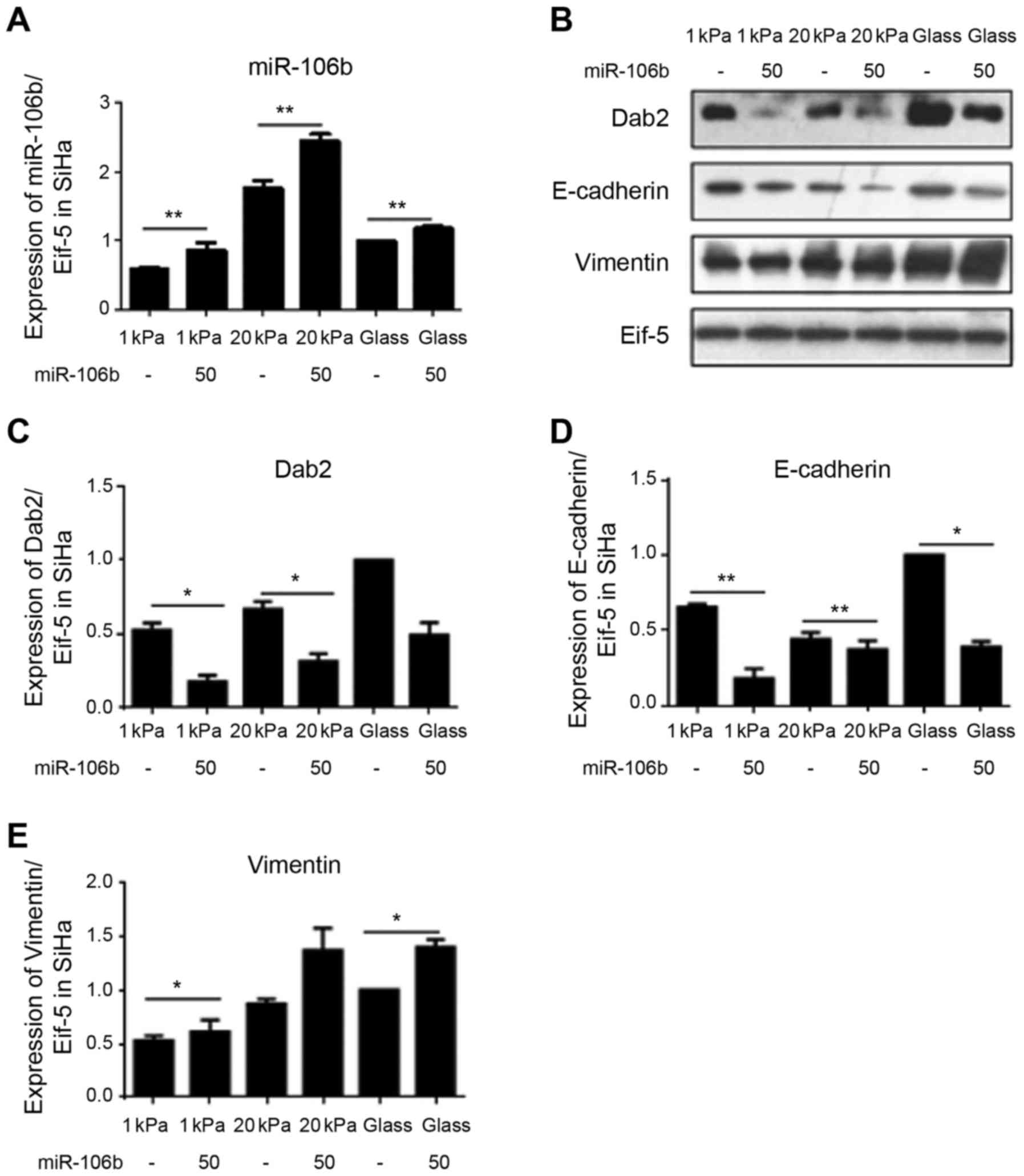
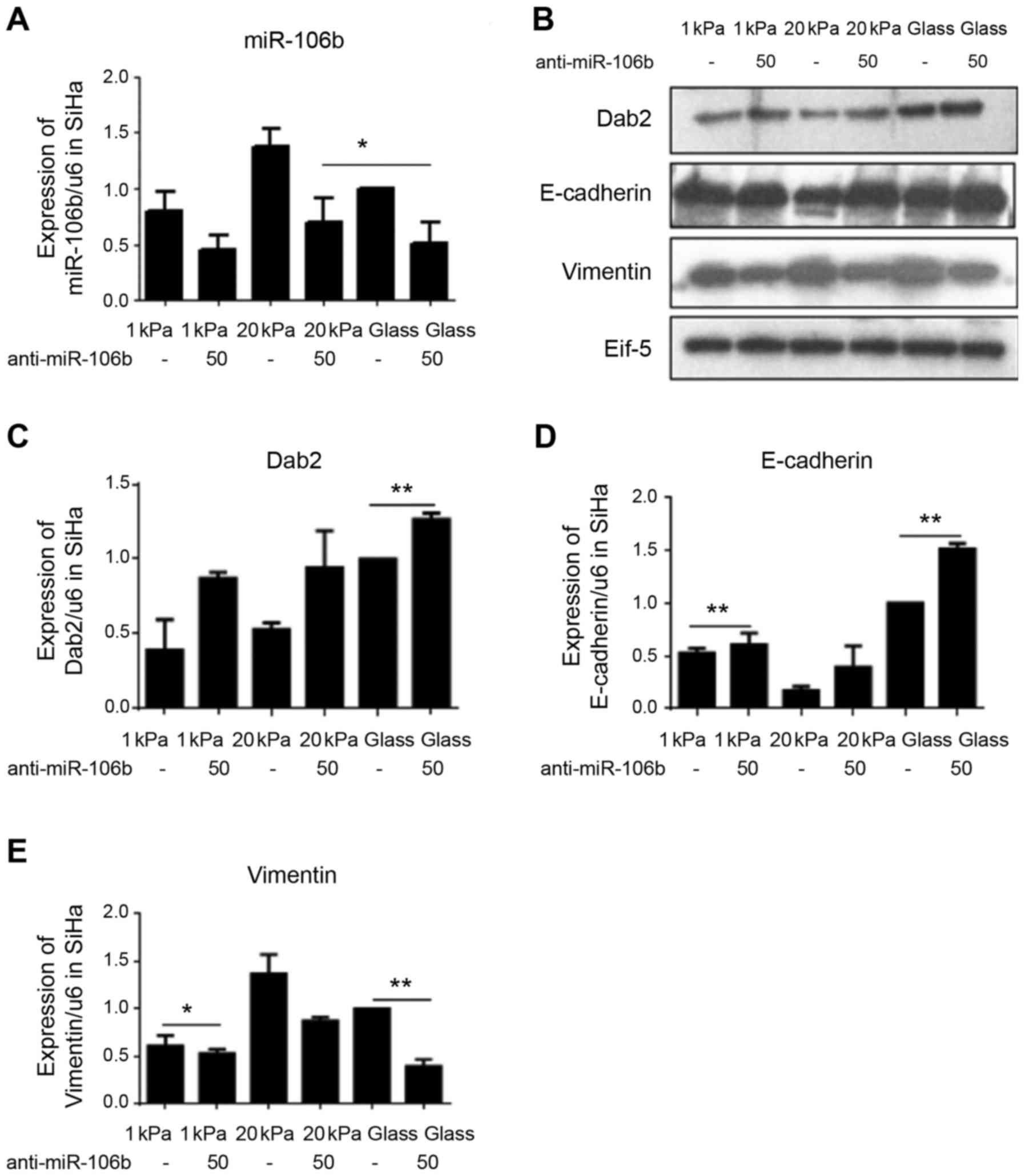
miR-106b is involved in substrate
stiffness regulated EMT of cervical cancer cell line SiHa
To investigate the specific mechanism, substrate
stiffness of 1 and 20 kPa was selected to represent normal cervical
tissue stiffness and cervical cancer tissue stiffness,
respectively, and the changes in expression levels of miR-106b
target protein DAB2 and EMT-related markers vimentin and E-cadherin
after miR-106b over expression and silencing were detected using
western blotting. Fig. 8 shows
that miR-106b overexpression significantly decreased the expression
of DAB2 and vimentin, but increased the expression of E-cadherin in
cervical cancer cell line SiHa cultured on substrate with stiffness
of 20 kPa. Fig. 9 shows that
miR-106b silencing significantly increased the expression level of
DAB2 and vimentin and decreased the expression level of E-cadherin
in cervical cancer cell line SiHa cultured on substrate with
stiffness of 20 kPa. Together, these data suggest that substrate
stiffness regulates EMT of cervical carcinoma cell line SiHa
through miR-106b and its target protein DAB2.
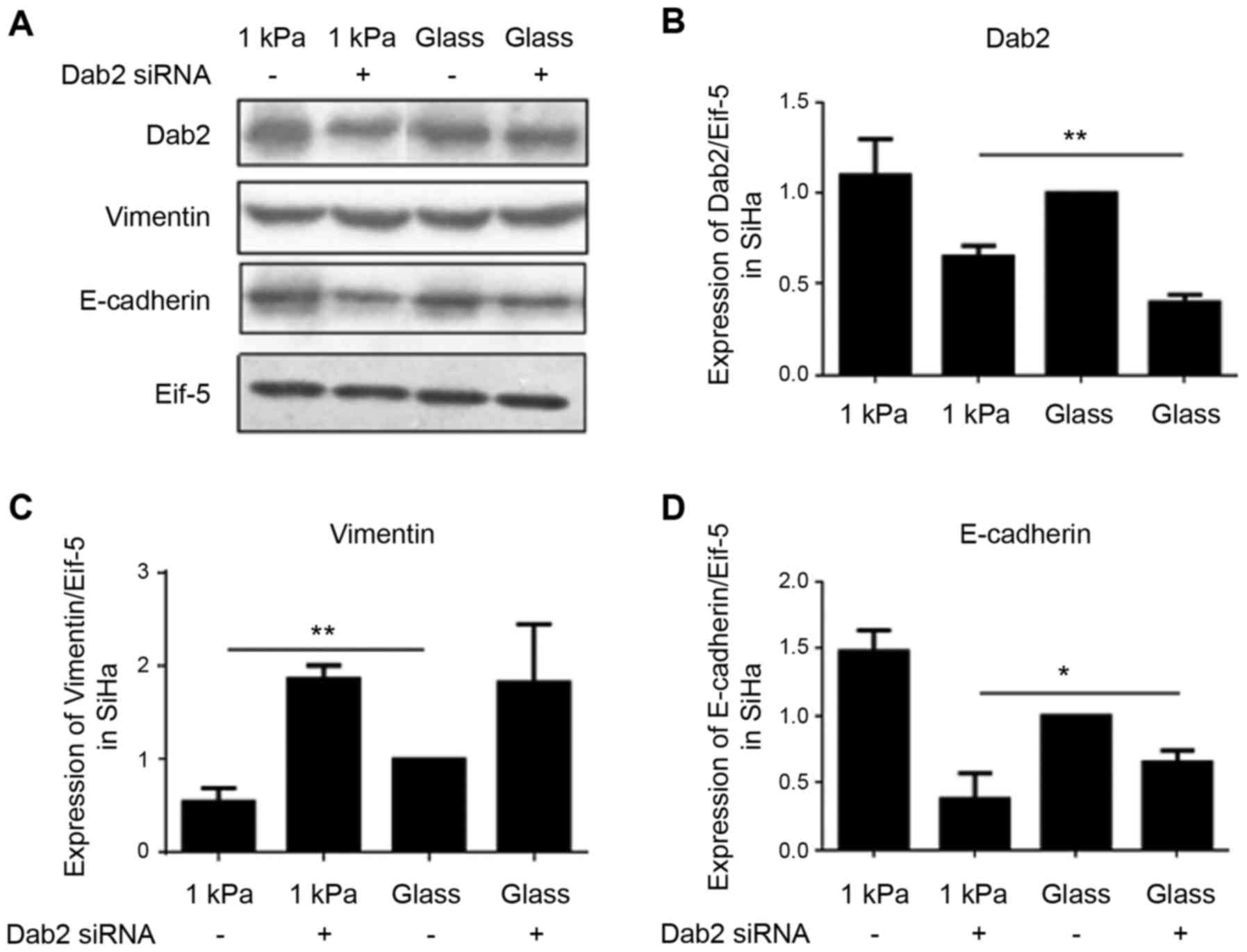
DAB2 is involved in regulating EMT of
cervical cancer cell SiHa
To further investigate whether DAB2 is involved in
regulating EMT of cervical cancer cells in different substrate
stiffness, we explored the effect of DAB2 knockdown on the
expression of E-cadherin and vimentin in cervical cancer cell SiHa
cultured on substrate with the stiffness of 1 kPa using western
blotting. The results showed that DAB2 knockdown significantly
decreased EMT-associated E-cadherin level and increased vimentin
level, i.e. enhanced EMT, indirectly suggesting that DAB2 is
involved in substrate stiffness regulation of EMT of cervical
cancer cell line SiHa (Fig.
10).
Discussion
Substrate stiffness affects EMT of
cervical cancer cells
In this study, we explored the effects of substrate
stiffness on EMT of cervical cancer cells. Changes in substrate
stiffness are important signs of many epithelial tumors and can
regulate EMT of many important cells such as liver epithelial cells
and different types of cells. EMT of hepatocytes is stronger on
substrate with stiffness of 60 kPa (hard substrate) than on
substrate with stiffness of 11 kPa (soft substrate) (23); EMT of mouse mammary epithelial
cells is stronger on substrate with stiffness of 11 kPa (hard
substrate) than on substrate with stiffness of 4 kPa (soft
substrates) (24); EMT of alveolar
epithelial cells is stronger on substrate with stiffness of 17 kPa
than on substrate with stiffness of 4 kPa. All these results
indicated that EMT of different types of tumor cells requires
different extracellular conditions. In our study, we found that
cervical cancer cell lines are more prone to EMT on substrate with
stiffness of 20 kPa than 1 kPa, and less prone to EMT on substrate
with stiffness of 30 kPa than 20 kPa. Substrate stiffness of 20 kPa
is currently considered as average stiffness of human solid tumor
tissues and very close to the stiffness of cervical cancer tissue,
suggesting that cervical cancer cells are prone to EMT at certain
substrate stiffness while are not prone to EMT at other
conditions.
Substrate stiffness affects EMT of
cervical cancer cells through miR-106b and its target DAB2
In recent years, many transcription factors and
signal pathways have been shown closely related with EMT of cancer
cells. Research on transcription factors have not made substantial
progress (5). In our investigation
we also tested the transcription factor Twist1 which has a close
relation with EMT of cervical cancer. We found the expression of
Twist1 1 kPa condition the lowest and the expression of Twist1 the
highest in 30 kPa condition. Thus, the expression of Twist1 does
not match the EMT tendency (Fig.
10). The impact of microRNA on tumorigenesis has gradually
become a hot topic of research. It is well acknowledged that
microRNA is closely related to pathological process of several
kinds of cancers such as cervical cancer (17,18).
Importantly some studies have found that specific
microRNA can regulate EMT of tumor cells (25–32).
In the present study, we found that miR-106b and its target protein
DAB2 play significantly important regulatory roles in EMT process
of cervical cancer cell lines SiHa and HeLa. In our study, we found
that miR-106b and DAB2 play important roles in regulating EMT of
cervical cancer SiHa and HeLa cells which were caused by changes in
substrate stiffness. In other words, with substrate stiffness
changing, miR-106b expression in cervical cancer cell lines changes
significantly. Suppression or overexpression of miR-106b
correspondingly alters the expression of EMT markers, indicating
that substrate stiffness may affect EMT of cervical cancer cells
through microRNA. This view is also supported by other studies.
Mouw et al found that changes in substrate stiffness
increased miR-18a expression and promoted breast cancer development
by regulating expression of its target protein PTEN (21). It is clear that miR-106b is an
important key node in mechanical signaling transduction in cervical
cancer HeLa and SiHa cells and microRNA can be the key component in
mechanical signal transduction process.
It is currently accepted that integrin is the cell
membrane receptor of mechanic signals. It sensors extracellular
mechanical signals and transfers the signals into intracellular
signals to further regulate expression of important genes and
cellular functions such as growth, proliferation, migration and
EMT. It has been reported that some microRNA may further affect
integrin receptor expression or activity through their target
proteins and associated signaling pathways to influence cell EMT,
migration, invasion and other cell functions (30,31).
It is possible that changes in miR-106b expression due to substrate
stiffness may also affect expression or activity of certain
specific cell membrane integrin receptors and further the
transduction of extracellular mechanical signals into intracellular
signals. This hypothesis need to be further explored.
Moreover, microRNA usually regulate the expression
of its target proteins by post-translational degradation. Our
results found that miR-106b target protein DAB2 could regulate EMT
of cervical cancer cell lines cultured on substrates with different
stiffness. Many studies have found that loss of expression of tumor
suppressor protein DAB2 can significantly promote EMT of many tumor
cells, including cervical cancer cells. To our knowledge, whether
different substrate stiffness can regulate DAB2 expression has not
been reported previously. Our results showed that substrate
stiffness could affect DAB2 expression. DAB2 expression is the
lowest in cervical cancer cell lines cultured on substrate with
stiffness of 20 kPa and the highest in cervical cancer cell lines
cultured on substrate with stiffness of 1 kPa.
The characteristics that tumor tissues have
different stiffness in ultrasound and elastography examinations
have been used in clinical medicine for preliminary tumor diagnosis
(32–34). Our results showed that substrate
stiffness can regulate EMT of cancer cells. We have previously
shown that miR-106b expression is higher in cervical tumor tissues
than in normal cervical tissues, and high miR-106b expression
promotes cervical carcinogenesis. In this study, we found that at
cytological level, miR-106b plays an important regulatory role in
EMT of cervical cancer cell lines cultured on substrates with
different stiffness, suggesting that miR-106b and substrate
stiffness play important roles in cervical cancer progression. Our
results also suggest that changes in substrate stiffness can
regulate specific molecular signaling pathways, miR-106b and its
downstream target proteins DAB2, and changes in substrate stiffness
and miR-106b can be targets for treatment of cervical cancer. The
study indicates a new way for treatment of cervical cancer.
Acknowledgments
This work was supported by grants from the National
Natural Science Foundation of China (no. 81472429, 81471893,
81270157) and National Basic Research Program of China (no. 973
Program, 2013CB933702, 2014CBA02003).
References
|
1
|
Poljak M, Kocjan BJ, Oštrbenk A and Seme
K: Commercially available molecular tests for human
papillomaviruses (HPV): 2015 update. J Clin Virol. 76(Suppl 1):
S3–S13. 2016. View Article : Google Scholar
|
|
2
|
Oh J-K and Weiderpass E: Infection and
cancer: Global distribution and burden of diseases. Ann Glob
Health. 80:384–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Xu K, Shi B, Zhang K, Zhou P, Liao
X, Liu G and Zhang Y: Prognosis of 4374 cases of cervical cancer
and its affecting factors. China Cancer. 23:181–188. 2014.
|
|
5
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei SC and Yang J: Forcing through tumor
metastasis: The interplay between tissue rigidity and
epithelial-mesenchymal transition. Trends Cell Biol. 26:111–120.
2016. View Article : Google Scholar :
|
|
7
|
Nagelkerke A, Bussink J, Rowan AE and Span
PN: The mechanical microenvironment in cancer: How physics affects
tumours. Semin Cancer Biol. 35:62–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Huang G, Li X, Zhang Y, Jiang Y,
Shen J, Liu J, Wang Q, Zhu J, Feng X, et al: Hypoxia induces
epithelial-mesenchymal transition via activation of SNAI1 by
hypoxia-inducible factor-1α in hepatocellular carcinoma. BMC
Cancer. 13:1082013. View Article : Google Scholar
|
|
10
|
Brown AC, Fiore VF, Sulchek TA and Barker
TH: Physical and chemical microenvironmental cues orthogonally
control the degree and duration of fibrosis-associated
epithelial-to-mesenchymal transitions. J Pathol. 229:25–35. 2013.
View Article : Google Scholar
|
|
11
|
Young JL, Holle AW and Spatz JP: Nanoscale
and mechanical properties of the physiological cell-ECM
microenvironment. Exp Cell Res. 343:3–6. 2016. View Article : Google Scholar
|
|
12
|
Tilghman RW, Cowan CR, Mih JD, Koryakina
Y, Gioeli D, Slack-Davis JK, Blackman BR, Tschumperlin DJ and
Parsons JT: Matrix rigidity regulates cancer cell growth and
cellular phenotype. PLoS One. 5:e129052010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Das T, Safferling K, Rausch S, Grabe N,
Boehm H and Spatz JP: A molecular mechanotransduction pathway
regulates collective migration of epithelial cells. Nat Cell Biol.
17:276–287. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mierke CT: The fundamental role of
mechanical properties in the progression of cancer disease and
inflammation. Rep Prog Phys. 77:0766022014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parekh A and Weaver AM: Regulation of
invadopodia by mechanical signaling. Exp Cell Res. 343:89–95. 2016.
View Article : Google Scholar :
|
|
16
|
Fullár A, Dudás J, Oláh L, Hollósi P, Papp
Z, Sobel G, Karászi K, Paku S, Baghy K and Kovalszky I: Remodeling
of extracellular matrix by normal and tumor-associated fibroblasts
promotes cervical cancer progression. BMC Cancer. 15:2562015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yehya N, Yerrapureddy A, Tobias J and
Margulies SS: MicroRNA modulate alveolar epithelial response to
cyclic stretch. BMC Genomics. 13:1542012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valastyan S and Weinberg RA: Roles for
microRNAs in the regulation of cell adhesion molecules. J Cell Sci.
124:999–1006. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ekman M, Bhattachariya A, Dahan D, Uvelius
B, Albinsson S and Swärd K: Mir-29 repression in bladder outlet
obstruction contributes to matrix remodeling and altered stiffness.
PLoS One. 8:e823082013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neth P, Nazari-Jahantigh M, Schober A and
Weber C: MicroRNAs in flow-dependent vascular remodelling.
Cardiovasc Res. 99:294–303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mouw JK, Yui Y, Damiano L, Bainer RO,
Lakins JN, Acerbi I, Ou G, Wijekoon AC, Levental KR, Gilbert PM, et
al: Tissue mechanics modulate microRNA-dependent PTEN expression to
regulate malignant progression. Nat Med. 20:360–367. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng Y, Guo Y, Zhang Y, You K, Li Z and
Geng L: MicroRNA-106b is involved in transforming growth factor
β1-induced cell migration by targeting disabled homolog 2 in
cervical carcinoma. J Exp Clin Cancer Res. 35:112016. View Article : Google Scholar
|
|
23
|
Bonnans C, Chou J and Werb Z: Remodelling
the extracellular matrix in development and disease. Nat Rev Mol
Cell Biol. 15:786–801. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee K, Chen QK, Lui C, Cichon MA, Radisky
DC and Nelson CM: Matrix compliance regulates Rac1b localization,
NADPH oxidase assembly, and epithelial-mesenchymal transition. Mol
Biol Cell. 23:4097–4108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng YX, Chen GT, Chen C, Zhang QF, Pan
F, Hu M and Li BS: MicroRNA-200b inhibits epithelial-mesenchymal
transition and migration of cervical cancer cells by directly
targeting RhoE. Mol Med Rep. 13:3139–3146. 2016.PubMed/NCBI
|
|
26
|
Yao J, Deng B, Zheng L, Dou L, Guo Y and
Guo K: miR-27b is upregulated in cervical carcinogenesis and
promotes cell growth and invasion by regulating CDH11 and
epithelial-mesenchymal transition. Oncol Rep. 35:1645–1651.
2016.
|
|
27
|
Peralta-Zaragoza O, Deas J, Meneses-Acosta
A, De la O-Gómez F, Fernández-Tilapa G, Gómez-Cerón C,
Benítez-Boijseauneau O, Burguete-García A, Torres-Poveda K,
Bermúdez-Morales VH, et al: Relevance of miR-21 in regulation of
tumor suppressor gene PTEN in human cervical cancer cells. BMC
Cancer. 16:2152016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qin X, Wan Y, Wang S and Xue M:
MicroRNA-125a-5p modulates human cervical carcinoma proliferation
and migration by targeting ABL2. Drug Des Devel Ther. 10:71–79.
2015.
|
|
29
|
Cheng Y, Ma D, Zhang Y, Li Z and Geng L:
Cervical squamous cancer mRNA profiles reveal the key genes of
metastasis and invasion. Eur J Gynaecol Oncol. 36:309–317.
2015.PubMed/NCBI
|
|
30
|
Zhang J, Na S, Liu C, Pan S, Cai J and Qiu
J: MicroRNA-125b suppresses the epithelial-mesenchymal transition
and cell invasion by targeting ITGA9 in melanoma. Tumour Biol.
37:5941–5949. 2016. View Article : Google Scholar
|
|
31
|
Liu X, Liang Z, Gao K, Li H, Zhao G, Wang
S and Fang J: MicroRNA-128 inhibits EMT of human osteosarcoma cells
by directly targeting integrin α2. Tumour Biol. 37:7951–7957. 2016.
View Article : Google Scholar
|
|
32
|
Fasching PA, Heusinger K, Loehberg CR,
Wenkel E, Lux MP, Schrauder M, Koscheck T, Bautz W,
Schulz-Wendtland R, Beckmann MW, et al: Influence of mammographic
density on the diagnostic accuracy of tumor size assessment and
association with breast cancer tumor characteristics. Eur J Radiol.
60:398–404. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang JM, Park IA, Lee SH, Kim WH, Bae MS,
Koo HR, Yi A, Kim SJ, Cho N and Moon WK: Stiffness of tumours
measured by shear-wave elastography correlated with subtypes of
breast cancer. Eur Radiol. 23:2450–2458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Evans A, Whelehan P, Thomson K, McLean D,
Brauer K, Purdie C, Jordan L, Baker L and Thompson A: Quantitative
shear wave ultrasound elastography: Initial experience in solid
breast masses. Breast Cancer Res. 12:R1042010. View Article : Google Scholar : PubMed/NCBI
|