Introduction
Colorectal cancer (CRC) is one of the commonest
malignancies and also the leading cause of cancer-related deaths
worldwide. The incidence rates of CRC show an increased trend in
the world, including China (1,2). The
majority of the CRC patients developed lymph node metastasis, even
liver, lung, and peritoneum peritoneal metastasis leading to high
rates of death (3). Consequently,
much work remains to be done to fully understand the molecular
mechanisms of the development of CRC, and the importance of markers
that promote the progression of CRC has been emphasized, as they
may function as therapeutic targets (4).
Recently, circadian disruption has become the main
stay in research field of oncology as it affects the genesis,
development and treatment of tumors (5–7).
Growing evidence indicates that disruption of circadian rhythms by
a set of clock genes and proteins significantly increases the
incidence of human CRC. For example, previous studies have reported
higher incidence rates of colorectal, breast, and endometrial
cancers which existed in shift workers with presumed circadian
disruption (8). On the other hand,
many clinical trials have reported strong evidence on the
beneficial effects of chronotherapy, which refer to the delivery of
chemotherapy according to the circadian rhythms (9,10).
These studies showed that chronotherapy significantly increased the
tolerance to high doses of chemotherapeutic drugs, improved
clinical response and prolonged survival in patients with
metastatic CRC (11–13). We aimed to clarify who plays the
key role in circadian disorder and how it affects CRC
progression.
Various living organisms exhibit behavioral and
physiological circadian rhythms, allowing them to adapt to daily
light and dark cycle (14). The
molecular mechanism of circadian oscillations includes eight core
circadian genes (15,16): Period1 (Per1), Period2 (Per2),
Period3 (Per3), Clock, Bmal1, Casein Kinase Iε (CKIε),
Cryptochrome1 (Cry1) and Cryptochrome2 (Cry2). Among them, the gene
Clock is an integral and core component of the circadian pacemaking
system (17,18).
The current study also investigated the role of the
core circadian genes involved in the tumorigenesis of CRC. For
example, in the CRC tumor tissues of patients, expression levels of
Per1, Per2, Per3 were lower compared to their matched healthy
mucosa (19–21), and a low expression of the Per1
gene was correlated with liver metastasis (22). Moreover, Cry1 overexpression in CRC
tissues was correlated with tumor progression and poor prognosis
(23), and genetic variants in the
gene hClock had a significant effect on the risk of death of CRC
patients (24). Lu et al,
on 250 advanced rectal cancer patients, reported that the effect of
neoadjuvant chemotherapy was correlated with expression of the gene
Clock. They have further put forward that circadian genes acted as
potential biomarkers for predicting the beneficial effects of
neoadjuvant chemoradiation therapy in patients with rectal cancer
(25).
Above all, since the hClock gene is the core
component of the circadian gene family, we assume that disruption
of this gene expression may play an important role in the incidence
of CRC, especially in the tumor invasion and metastasis. However,
there are few studies supporting the variations of hClock
expression in the CRC progression, and the inclusion of hClock
working mechanism in the invasion and metastasis of CRC still needs
investigation.
In our previous studies, we found that the circadian
gene human Clock (hClock) was highly expressed in the CRC tissues,
and associated with late TNM stage and positive lymph node
metastasis (26). Our previous
studies also demonstrated the inhibition of tumor cell apoptosis by
hClock in vitro and in vivo, while hClock silencing
reversed this effect (27). Hence,
in this study, we further explored the role and mechanism of hClock
participation in CRC progression in vitro and in
vivo.
Materials and methods
Ethics statement
The study was approved by the institutional review
board of Huashan Hospital Affiliated to Fudan University (HIRB),
Shanghai, China. All patients provided written informed consent.
All in vivo experiments in the study protocol were strictly
in accordance with the National Institutes of Health Guide for Care
and Use of Laboratory Animals and were approved by the Animal Care
and Use Committee of Shanghai Medical College of Fudan University,
Shanghai, China.
CRC specimens and cell lines
CRC specimens including adjacent non-tumor
colorectal tissues were obtained from patients who have undergone
radical surgery for CRC in the Huashan Hospital, Shanghai, China.
None of the patients received chemotherapy or radiotherapy before
operation. The samples were surgically obtained at the following
time points: 23 cases between 10:00 and 12:00, 8 cases between
12:00 and 14:00, 3 cases between 14:00 and 16:00, 3 cases between
16:00 and 18:00, and 1 case at 22:00.
Four human CRC cell lines SW480, SW620, HT29, LoVo,
and human embryonic kidney cell line 293T were cultured in
Dulbecco's modified Eagle's medium (DMEM) with 10% (v/v) newborn
calf serum, 100 U/ml penicillin and 100 ng/ml streptomycin at 37°C
in 5% CO2. All cell lines were obtained from the Cell
Bank of the Chinese Academy of Sciences, Shanghai, China.
Quantitative reverse transcription-PCR,
immunohistochemistry and evaluation of staining
Detailed materials and methods performed were
according to our previous study (28).
Western blotting
Total cellular proteins were extracted and separated
in SDS-PAGE gel, and western blot analysis was performed according
to the standard procedures. GAPDH was used as a loading control on
the same membrane. The antibodies used included anti-hClock,
anti-VEGF (Abcam, Cambridge, UK), anti-GAPDH, anti-HIF-1α,
anti-ARNT, anti-E-cadherin, anti-N-cadherin, anti-vimentin, and
anti-fibronectin (Cell Signaling Technology, Danvers, MA, USA).
Band densitometry was performed using Scion Image software (Scion
Corp., Fredrick, MD, USA).
Plasmid construction
Full-length cDNA of hClock (Genbank accession
number: NM_004898) was amplified and inserted into pcDNA3-Flag
vector (Invitrogen, Carlsbad, CA, USA) to generate
pcDNA3-Flag-hClock expression plasmid. The cDNA fragment of
Flag-hClock was inserted into the pGV186 retroviral vector to
generate pGV186-Flag-hClock. All the constructs were confirmed by
DNA sequencing.
Construction of lentiviral-delivered
hClock shRNA
Detailed materials and methods used in this section
were performed according to our previous work (27).
Infection of target cells
Retroviruses carrying the hClock or hClockRNAi were
generated by cotransfection of recombinant pGV186 or pGV113
plasmids, respectively with pHelper plasmid into 293T cells using
Lipofectamine 2000 (Invitrogen). After 48 h of incubation, the
culture medium containing recombinant virus was harvested and
purified by a 0.45 μm filter. Target cells were seeded
(5×105/well) into 6-well plates and incubated with
recombinant virus was supplemented with 5 μg/ml polybrene
for a spin infection procedure.
Cell migration assay
The migration ability of the cells was measured in
transwell chambers (8 μm pore; BD Biosciences). The bottom
chamber was filled with 600 μl of DMEM containing 10% FBS.
For the migration assay, tumor cells (5×104 cells in a
total volume of 100 μl) were placed in the upper chamber and
incubated at 37°C in 5% CO2 under humidified air
conditions. After 24 h of culture, non-migrating cells on the upper
surface of the membrane were removed, and cells that migrated to
the underside of the polycarbonate membrane were fixed with ethanol
and stained with 1% crystal violet for 10 min. The number of
migrating cells was then determined from 5 independent microscopic
fields. The mean of triplicate assays for each experimental
condition was used for the analysis.
Lung metastatic tumor experiment in nude
mice
Female, 5–6 weeks old, Balb/c nude mice were
obtained from Shanghai Experimental Animal Center and provided with
standard laboratory chow and tap water ad libitum under
special pathogen free (SPF) conditions in the department of
laboratory animal science of Shanghai Medical College of Fudan
University. The mice were then separated into 2 groups randomly and
were injected with SW620-hClock-shRNA 2# or SW620-shRNA-control
infected cells via the caudal veins (10 mice/group,
1×106 cells/mouse). Six weeks after injection, all mice
were euthanatized by cervical dislocation and their lungs were
sectioned and stained with hematoxylin and eosin (H&E), and
then observed by light microscopy for the formation of lung
metastasis. All the animal studies were conducted in accordance
with 'Animal Research: Reporting In vivo Experiments'
(ARRIVE) guidelines and the guidelines of Institutional Animal Care
and Use Committee. All the mice were treated humanely throughout
the experimental period.
Statistical analysis
The data are presented as mean ± SD unless stated
otherwise. A paired t-test was used to test for the differences in
hClock expression between matched tumor and benign mucosa.
χ2-test or Fisher's exact test was performed to analyze
the correlations of hClock protein levels with clinical and
pathologic parameters. The statistical significance of the in
vitro and in vivo studies was analyzed using Student's
t-test. All P-values were two-sided, and P-values of <0.05 were
considered to be statistically significant. All statistical
analyses were conducted using SPSS software package, version 19.0
(SPSS Inc., Chicago, IL, USA).
Results
Analysis of hClock expression in human
CRC specimens and cell lines
In our previous studies, we found that hClock
was highly expressed in CRC tissues when compared with the
peritumoral tissues in CRC patients, and strongly associated with
late TNM stage and positive lymph node metastasis (26). Similar results can be found in the
present study since another additional 8 cases were added to the
current study and were further analyzed.
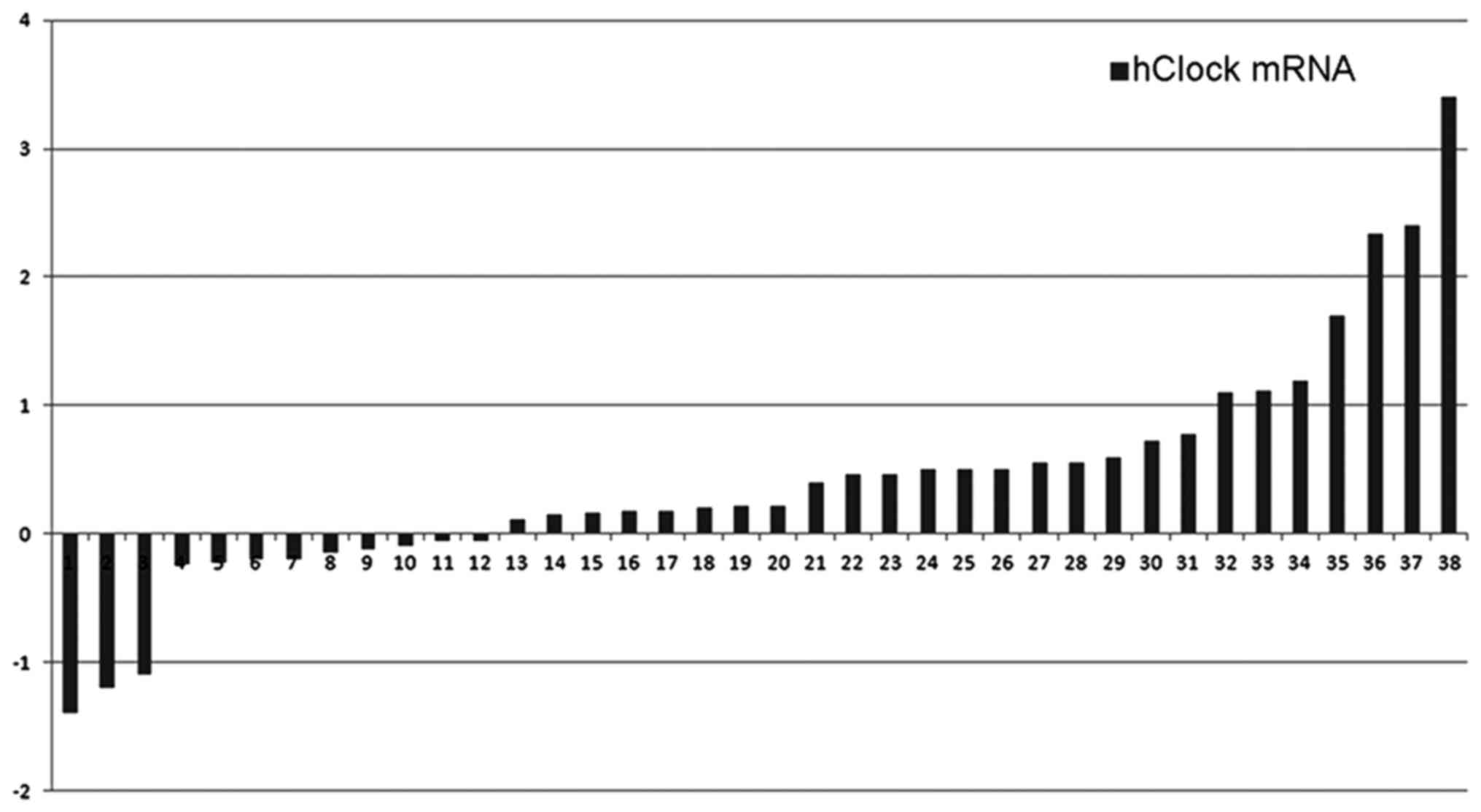
We analyzed hClock mRNA levels in 38 pairs of
human CRC samples, including primary CRC tissues and paired normal
colorectal tissues in this study. Significantly increased
expression of hClock mRNA was observed in 26 of 38 (68.4%)
CRCs compared with the paired normal colorectal tissues
(P<0.01). Relative expression levels of hClock in the CRC
compared with the non-cancerous components were 2.75:1 (Fig. 1).
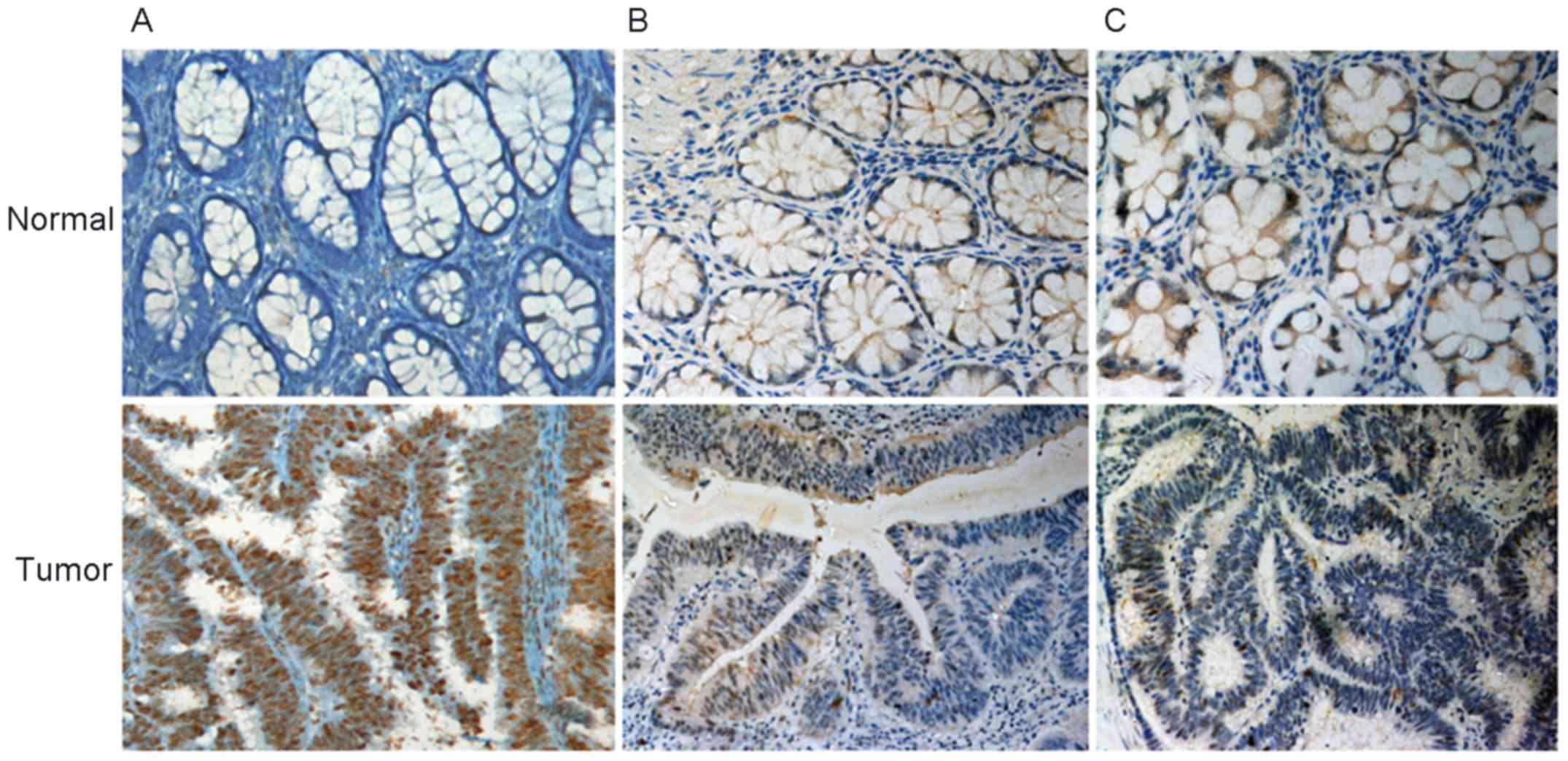
hClock expression at the protein level was further
examined in these 38 pairs of CRC tissues by immunohistochemical
staining. hClock protein was predominantly detected in the
nuclei of tumor cells. In all, there were three different
hClock protein expression patterns: type I (50.0%, 19/38):
staining for hClock was much stronger in the cancerous cells
than in the paired non-cancerous cells (Fig. 2A); type II (44.7%, 17/38): there
were no visually significant differences between the staining of
cancerous and non-cancerous cells (Fig. 2B), although subtle differences in
the hClock protein expression could not be excluded; type
III (5.3%, 2/38): staining of hClock was stronger in
non-cancerous cells than in the paired cancerous cells (Fig. 2C). Furthermore, 26 cases showed
upregulated expression of hClock mRNA in the CRC tissues, 18
specimens demonstrated a higher expression of hClock
protein. Thus, the expression of hClock protein and mRNA
revealed a positive correlation (P<0.01).
We next examined the possible intrinsic relationship
between hClock expression and clinicopathological features.
As shown in Table I, hClock
upregulation did not correlate with sex, age, and tumor sites, but
increased expression of hClock was strongly associated with
positive lymph node metastasis (P=0.003) and advanced TNM staging
(P=0.003). These results suggested that higher hClock
expression was associated with the degree of malignancy for
CRC.
 | Table IRelationship between increased
expression of hClock protein and clinicopathological features. |
Table I
Relationship between increased
expression of hClock protein and clinicopathological features.
| Clinicopathological
features | Total cases N | Increased hClock
expression in tumor n (%) | P-value |
|---|
| Sex | | | NS |
| Male | 18 | 9 (50.0%) | |
| Female | 20 | 10 (50.0%) | |
| Age, yrs. | | | NS |
| ≤50 | 8 | 5 (62.5%) | |
| >50 | 30 | 14 (46.7%) | |
| Tumor site | | | NS |
| Colon | 21 | 11 (52.4%) | |
| Rectum | 17 | 8 (47.1%) | |
| Lymph nodes
spread | | | 0.003 |
| Negative | 21 | 6 (28.6%) | |
| Positive | 17 | 13 (76.5%) | |
| TNM stage | | | 0.003 |
| I+II | 21 | 6 (28.6%) | |
| III+IV | 17 | 13 (76.5%) | |
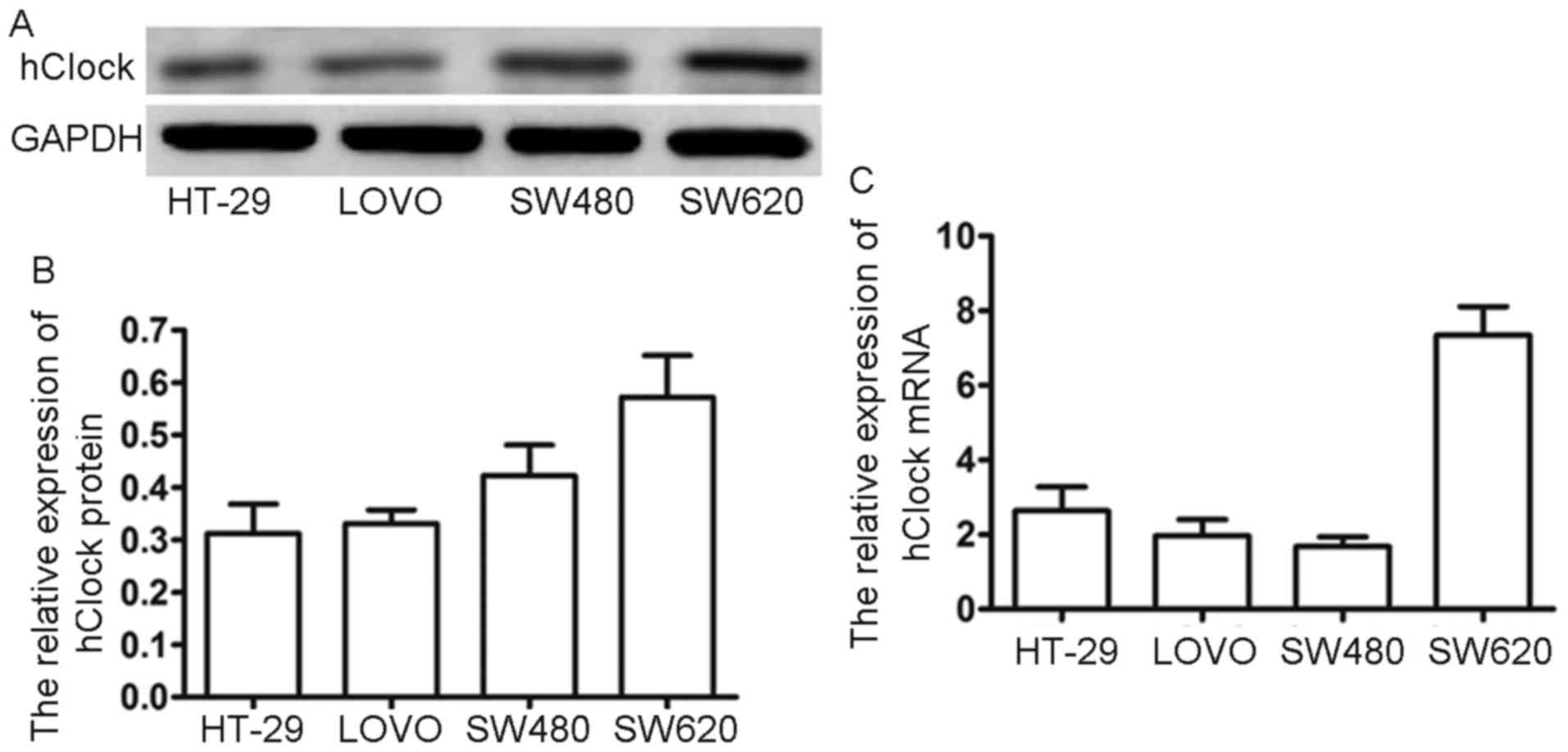
To validate our findings from the clinical CRC
samples, we examined cellular hClock expression in four
different CRC cell lines (HT-29, LoVo, SW480 and SW620). Results of
hClock expression in the four CRC cell lines are shown in
Fig. 1. Noteworthy, hClock
was highly expressed in SW620 both at the mRNA and protein level
(Fig. 3A and B). Since SW620 has
the highest potential of metastasis among these CRC cell lines
(28), our result again suggested
that hClock overexpression significantly correlated with CRC
metastasis.
hClock promotes migration of CRC
cells
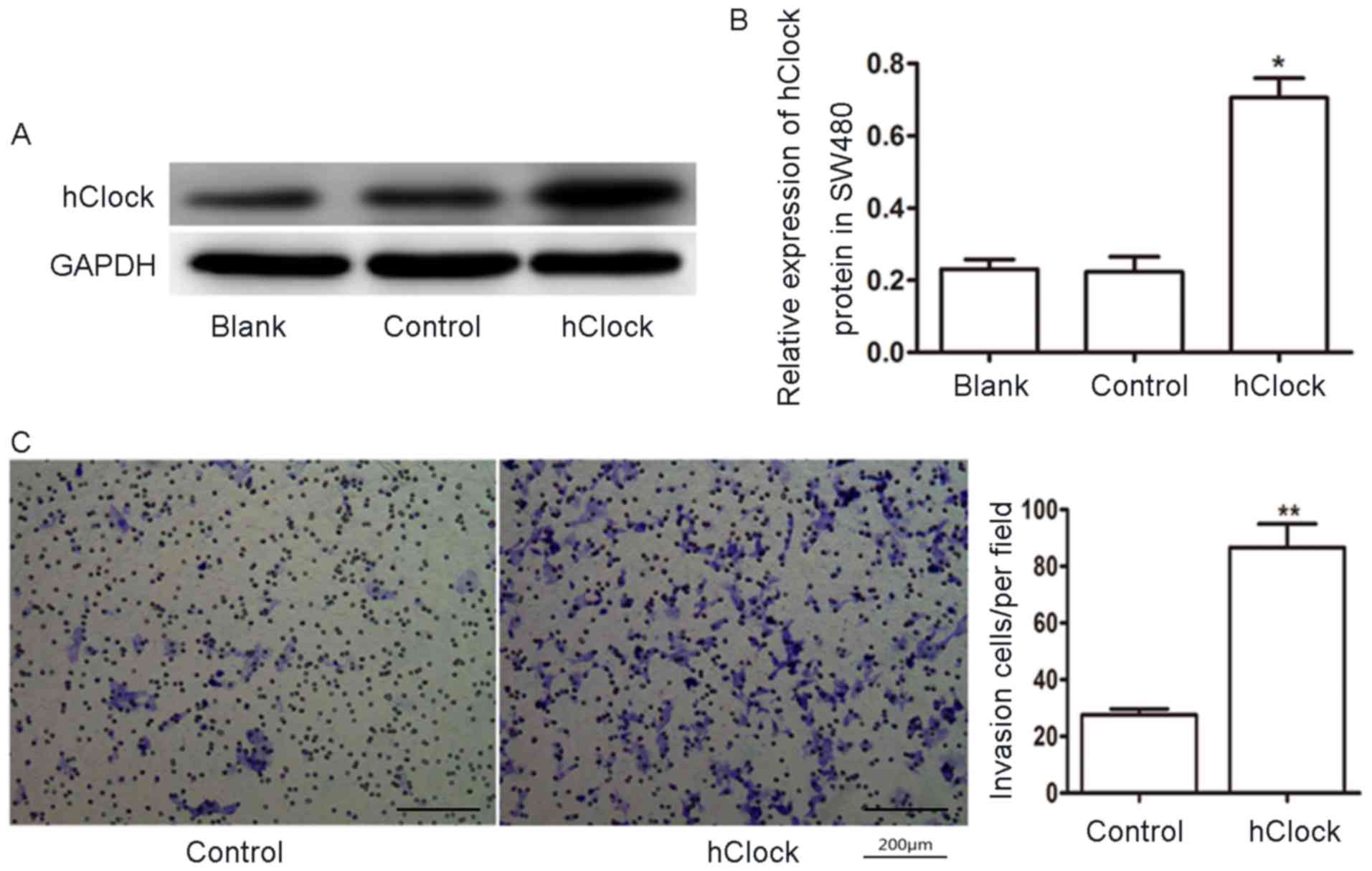
To evaluate the role of hClock in CRC, we
next tested whether overexpression of hClock promoted the
migration of human CRC cells. We used the CRC cell line SW480 which
relatively expressed a lower level of hClock. hClock
was overexpressed in SW480 cells when infected with pGV186-hClock
lentiviruses (Fig. 4A and B).
Transwell migration assay showed that overexpression of
hClock enhanced the migration ability of SW480 CRC cells
(Fig. 4C, P<0.01). These
findings suggested that hClock plays an important role in
the CRC cell migration.
To further investigate the relationship between
hClock upregulation and CRC progression, endogeneous
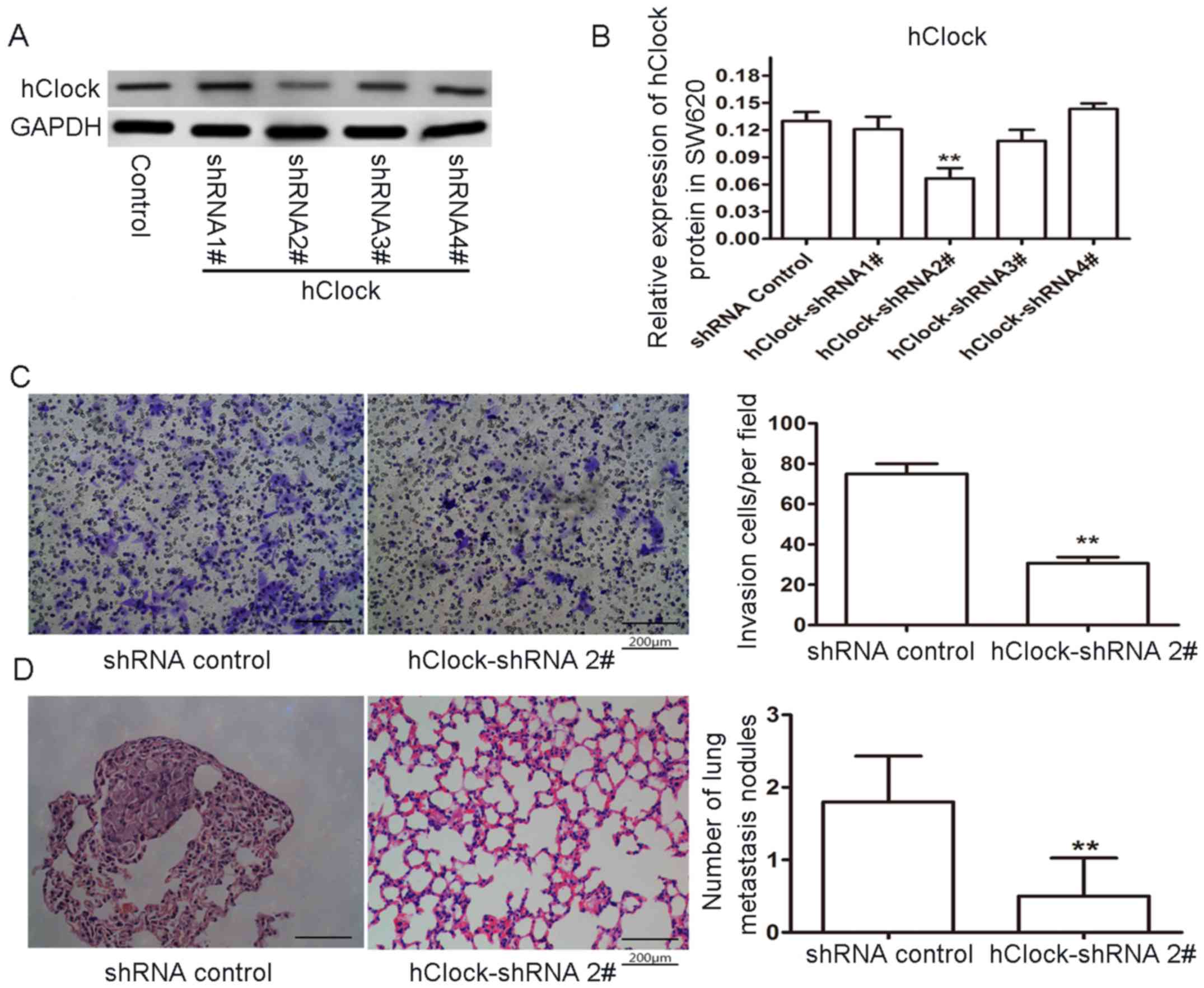
hClock expression in the CRC cell line SW620 was knocked
down and cell migration was examined. Transfection of SW620 cells
with hClock hRNA significantly inhibited the hClock
expression (Fig. 5A and B). To
further examine whether the targeted knockdown of hClock in
SW620 cells affected their migration, we performed an in
vitro Transwell migration assay. The result showed that the
number of hClock-shRNA cells migrated through the filter decreased
markedly by contrast to the control group (Fig. 5C, P<0.01). These results
together indicated that hClock was critical for CRC cell
proliferation and migration in vitro.
To further explore the role of hClock in CRC
metastasis in vivo, SW620 CRC tumor cells transfected with
hClock-shRNA or with control hRNA were injected into the nude mice
via the caudal vein (10 mice/group, 1×106 cells/mouse).
Six weeks after injection, none of the mice had died or behaved
abnormally, and they were euthanatized. Their lungs were sectioned
and stained with H&E, and then observed by light microscopy.
Notably, in contrast to the control group, a significant reduction
in the number of metastatic nodules in the lungs of nude mice
injected with hClock-shRNA-transfected SW620 cells was observed
(Fig. 5D). These results showed
that targeting hClock by shRNA effectively suppressed the
metastatic ability of SW620 CRC cells in nude mice and suggested
that hClock knockdown exerted a strong antitumor effect
in vivo.
Taken together, both our in vitro and in
vivo experiments provided significant evidence that
hClock was strongly associated with CRC metastasis.
Overexpression of hClock promotes
epithelial-mesenchymal (-like) transition (EMT) in CRC cells and
upregulated the expression of tumor angiogenesis-related genes
HIF-1α, ARNT and VEGF
It has been previously reported that EMT was the
driving force of invasion in the epithelial cells (29,30).
In recent years, changes in the level of expression of different
cadherins, so-called cadherin switches have been increasingly used
to monitor EMT. The cadherin switch from E-cadherin to N-cadherin,
which is expressed in mesenchymal cells, fibroblasts, cancer cells,
and neural tissues has often been used to monitor the EMT progress
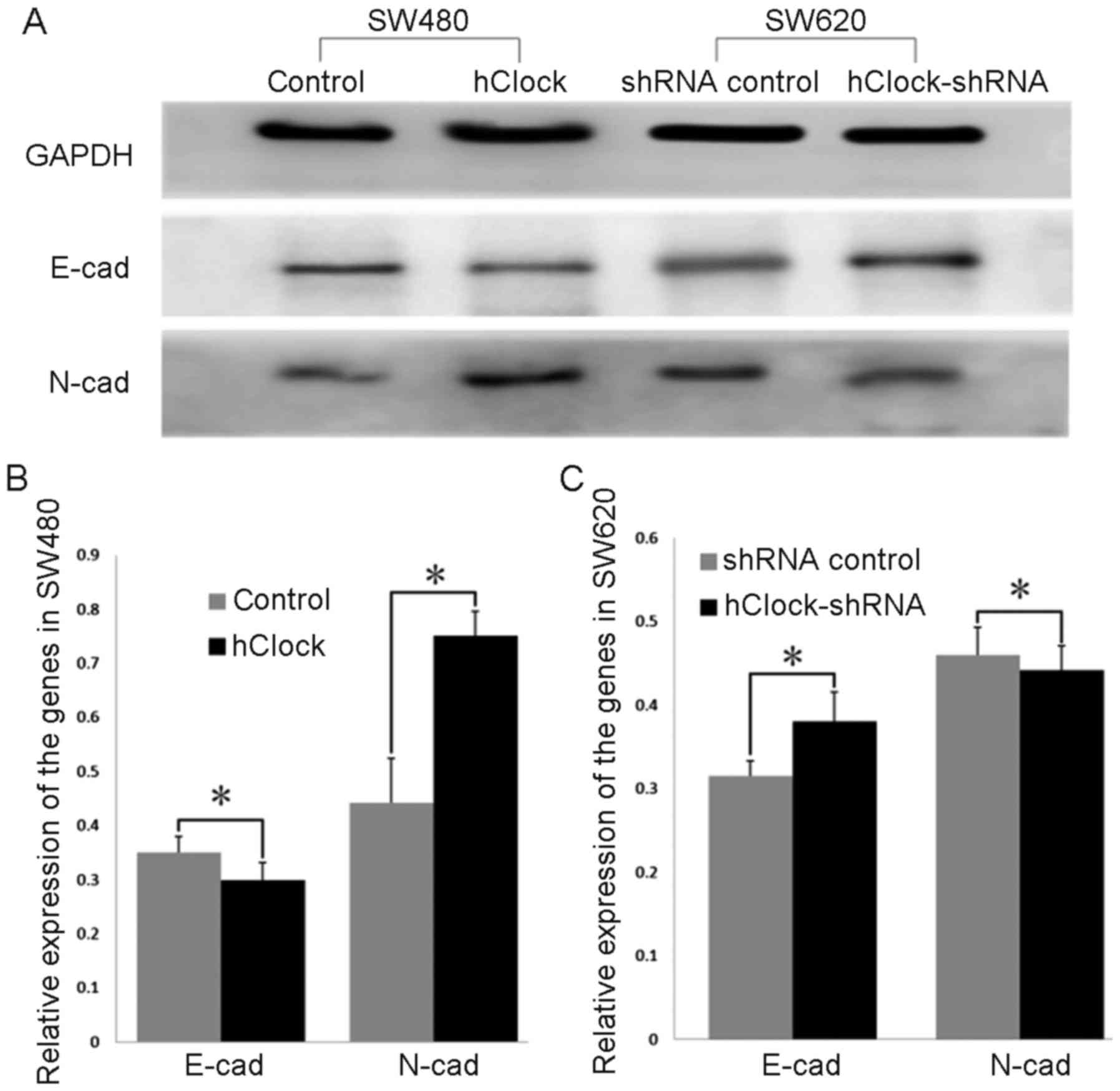
during the embryonic development and cancer progression (31). Thus, to investigate the mechanism
underlying the effects of hClock overexpression on CRC
progression, we examined the expression of EMT markers E-cadherin
and N-cadherin. As shown in Fig. 6A
and B, expression levels of E-cadherin were significantly
decreased in SW480 cells which overexpressed hClock compared
with those of the vector only-treated SW480 cells (P<0.05),
whereas upregulation of hClock resulted in a significant
increase in N-cadherin expression (P<0.05). In contrast,
silencing of endogenous hClock expression resulted in a
marked decrease of N-cadherin expression compared with that of the
shRNA-transfected controls, whereas E-cadherin expression levels
were significantly increased (P<0.05) (Fig. 6A and C).
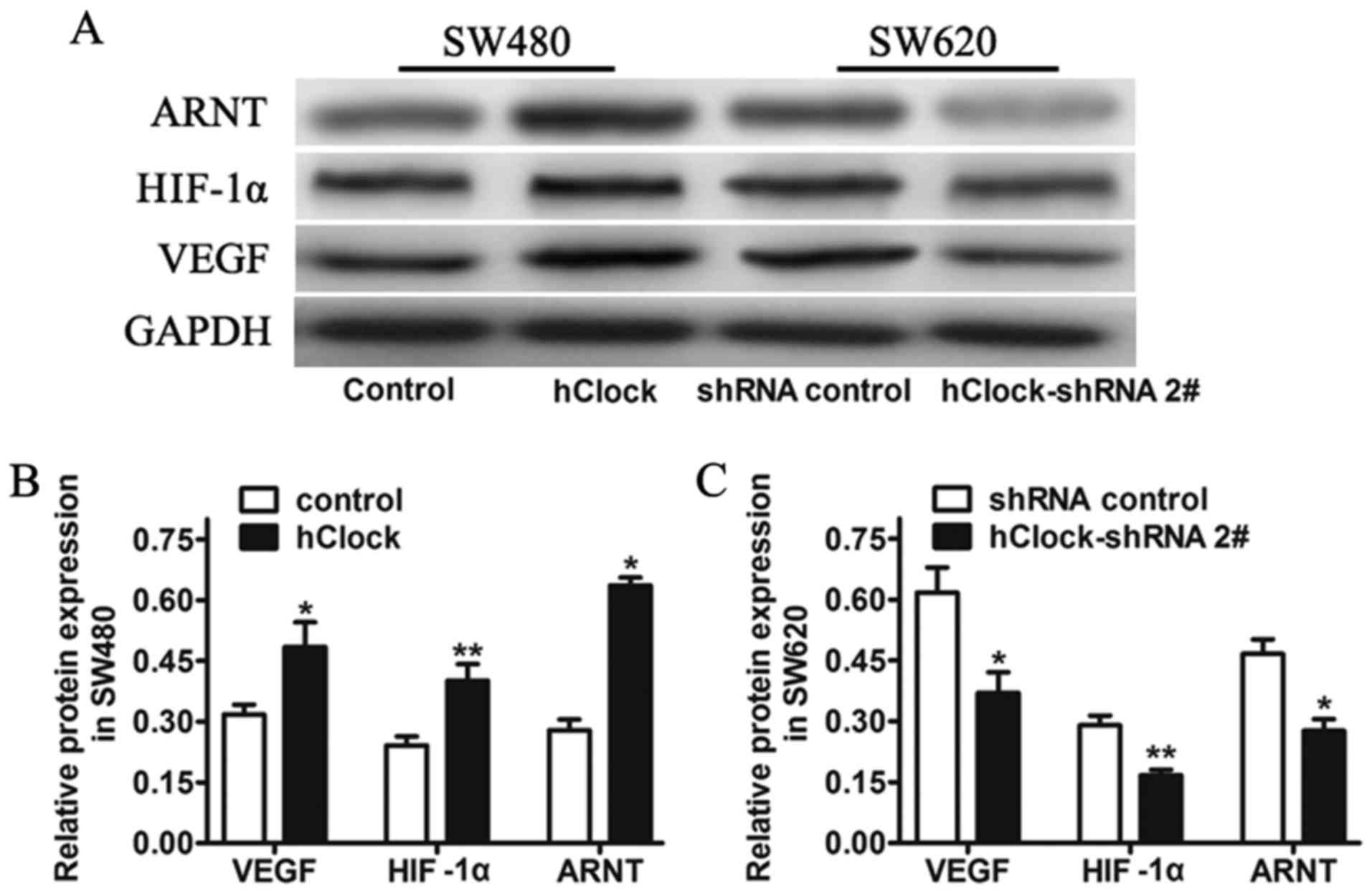
In parallel, increasing evidence demonstrates that
circadian genes participate in the angiogenesis and growth of
tumors (32,33). Thus, we analyzed the expression of
tumor angiogenesis-related genes, such as HIF-1α, ARNT, VEGF in the
CRC cell lines with hClock knockdown or over expression, to
see whether ectopic expression of hClock contributes to
tumor angiogenesis and malignancy. Western blot analysis indicated
that expression of HIF-1α, ARNT and VEGF were decreased in SW620
cells transfected with hClock shRNA, but increased in SW480
cells with hClock overexpression (Fig. 7).
Discussion
CRC patients frequently develop lymph nodes and
hematogenous metastases in the advanced stages. The factors
involved in CRC metastasis are largely unknown, however,
deregulation of molecular processes and signaling pathways are
generally considered to be the main causes which result in the
growth and metastasis of the disease. Consequently, the importance
of molecular markers that promote the development of CRC has been
emphasized, as they might be the therapeutic targets for the
disease (34).
In our present study, we showed that the expression
of hClock increased in the CRC tissues significantly. We also
showed that overexpression of hClock was strongly associated with
lymph node matastasis and late stage of TNM classification in CRC
patients. Since these clinicopathological features indicate
relatively poor prognosis, overexpression of hClock can be regarded
as one of the potential biomarkers for evaluating malignancy of CRC
and requires further investigation in the future. In parallel, we
examined the expression of hClock in CRC cell lines, and observed
statistically higher expression of hClock in SW620 cells compared
to SW480 cells both at mRNA and protein level. SW620 has a higher
metastatic potential than SW480 cells (29), this suggests that hClock
overexpression has a correlation with CRC metastasis. Overall,
these results suggest that disruption of hClock expression is
associated with CRC progression.
The role of hClock in CRC progression is still
poorly understood. Aiming to investigate this biological question,
we first examined in this study the effect of hClock on CRC cell
migration through transwell assay. The results revealed that
repressed hClock expression by shRNA led to the retardation of cell
migration of SW620 CRC cells, while in stable overexpression of
hClock in SW480 CRC cells which enhanced cell migration.
Experiments of tumor lung metastasis were performed in nude mice
which estimated the growth-promoting function of hClock in
vivo. Of note, an antitumor effect of hClock RNAi was observed,
as lung rectal metastasis was suppressed when hClock expression was
knocked down by injecting hClock-shRNA into the nude mice via the
caudal vein. Collectively, these results suggest that hClock can be
used as a marker to evaluate the progression of CRC, and acts as a
strong positive expression of hClock indicating a high risk of
lymph node or hematogenous metastasis. Moreover, our data indicated
that lentivirus vector-mediated knockdown of hClock in CRC cells
significantly inhibited the tumor growth and metastasis both in
vitro and in vivo, which indicated a therapeutic
potential of hClock shRNA on the treatment of CRC.
Regarding the potential mechanisms underlying the
promoting effect of hClock in CRC metastasis, hClock overexpression
promoted the EMT progression in CRC cells. We considered that the
gene hClock may induce the occurrence of EMT in CRC cells, thus
promoting the invasion and metastasis of CRC. Recently, it was
reported that loss of co-repressor circadian gene PER2 under
hypoxic conditions upregulated OCT1-mediated EMT gene expression
and enhanced tumor malignancy (35). It was also reported that circadian
gene Clock contributed to cell proliferation and migration of
glioma by upregulating NF-κB mediated EMT (36). However, to date the role of hClock
in the regulation of EMT to promote CRC progression has not been
well studied. In combination with the findings of this study, we
are interested in research of cytoskeletal protein, transcription
factor, adherence protein, or microRNAs which may mediate this
pathway. Further efforts may be needed to clarify these issues in
our future research.
In 2015, Jensen reviewed recent findings on the role
of circadian rhythms and hypoxia in cancer and metastasis, and
concluded that circadian rhythms and hypoxia are involved in tumor
metastasis at all levels from pathological deregulation of the
cells to the tissues and the whole organism. Pathological tumor
blood vessels caused hypoxia and disruption in circadian
rhythmicity, which in turn commuted to tumor metastasis (37). According to our previous results,
expression of hClock was associated with the overexpression of
HIF-1α, ARNT and VEGF in CRC specimens (26). We hypothesized that these three
genes were clock-controlled genes (CCGs), and their function were
regulated by hClock. Thus, in the present study, we further
analyzed the expression of these angiogenesis- related genes in CRC
cell lines. We found that the expression levels of the genes were
all repressed after the knockdown of hClock expression, and were
increased in cells with hClock overexpression. VEGF is widely known
to play a pivotal role in the tumoral angio-genesis and promote the
invasion and metastasis of cancer cells (38). This progression is induced by
hypoxia along with the major angiogenic transcription factor
hypoxia-inducible factor-1 (HIF-1), which consists of HIF-1α and
HIF-1β (aryl hydrocarbon receptor nuclear translocator, ARNT)
(39). Since angiogenesis is
essential for the development, growth and advancement of solid
tumors (40), our findings
implicated that aberrant overexpression of hClock may accelerate
the angiogenesis pathway in CRC, and may therefore provide a
molecular basis for promoting CRC progression.
In conclusion, our study demonstrated that
overexpression of circadian gene hClock played an important
role in the CRC progression by promoting EMT and angiogenesis of
the CRC cells. Silencing hClock inhibits the metastasis of
CRC cells both in vitro and in vivo. Thus, the gene
hClock may serve as a new diagnostic marker in CRC with high
metastastic potential as well as a potential therapeutic target for
CRC therapy.
Acknowledgments
This work was supported by the National Science
Foundation Fostering Talents in Basic Research of China (no.
J1210041, RZ.Q.); the National Natural Science Foundation of China
(NSFC) (no. 81570771, RZ.Q.).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muzny DM, Bainbridge MN, Chang K, Dinh HH,
Drummond JA, Fowler G, Kovar CL, Lewis LR, Morgan MB, Newsham IF,
et al Cancer Genome Atlas Network: Comprehensive molecular
characterization of human colon and rectal cancer. Nature.
487:330–337. 2012. View Article : Google Scholar
|
|
4
|
Fearon ER: Molecular genetics of
colorectal cancer. Annu Rev Pathol. 6:479–507. 2011. View Article : Google Scholar
|
|
5
|
Fu L and Lee CC: The circadian clock:
Pacemaker and tumour suppressor. Nat Rev Cancer. 3:350–361. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takahashi JS, Hong HK, Ko CH and McDearmon
EL: The genetics of mammalian circadian order and disorder:
Implications for physiology and disease. Nat Rev Genet. 9:764–775.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sahar S and Sassone-Corsi P: Metabolism
and cancer: The circadian clock connection. Nat Rev Cancer.
9:886–896. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Straif K, Baan R, Grosse Y, Secretan B, El
Ghissassi F, Bouvard V, Altieri A, Benbrahim-Tallaa L and Cogliano
V; WHO International Agency For Research on Cancer Monograph
Working Group: Carcinogenicity of shift-work, painting, and
fire-fighting. Lancet Oncol. 8:1065–1066. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Innominato PF, Focan C, Gorlia T, Moreau
T, Garufi C, Waterhouse J, Giacchetti S, Coudert B, Iacobelli S,
Genet D, et al Chronotherapy Group of the European Organization for
Research and Treament of Cancer: Circadian rhythm in rest and
activity: A biological correlate of quality of life and a predictor
of survival in patients with metastatic colorectal cancer. Cancer
Res. 69:4700–4707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Block KI, Block PB, Fox SR, Birris JS,
Feng AY, de la Torre M, Nathan D, Tothy P, Maki AK and Gyllenhaal
C: Making circadian cancer therapy practical. Integr Cancer Ther.
8:371–386. 2009. View Article : Google Scholar
|
|
11
|
Giacchetti S, Perpoint B, Zidani R, Le
Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y,
Coudert B, et al: Phase III multicenter randomized trail of
oxaliplatin added to chronomodulated fluorouracil-leucovorin as
first-line treatment of metastatic colorectal cancer. J Clin Oncol.
18:136–147. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giacchetti S: Chronotherapy of colorectal
cancer. Chronobiol Int. 19:207–219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Focan C, Kreutz F, Graas M-P, Longrée L,
Focan-Henrard D, Demolin G and Moeneclaey N: Phase I - II study to
assess the feasibility and activity of the triple combination of
5-fluorouracil/folinic acid, carboplatin and irinotecan (CPT-11)
administered by chronomodulated infusion for the treatment of
advanced colorectal cancer. Final report of the BE-1603 study.
Pathol Biol (Paris). 61:e27–e31. 2013. View Article : Google Scholar
|
|
14
|
Gery S and Koeffler HP: The role of
circadian regulation in cancer. Cold Spring Harb Symp Quant Biol.
72:459–464. 2007. View Article : Google Scholar
|
|
15
|
Lee C, Etchegaray JP, Cagampang FR, Loudon
AS and Reppert SM: Posttranslational mechanisms regulate the
mammalian circadian clock. Cell. 107:855–867. 2001. View Article : Google Scholar
|
|
16
|
Reppert SM and Weaver DR: Coordination of
circadian timing in mammals. Nature. 418:935–941. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Antoch MP, Song EJ, Chang AM, Vitaterna
MH, Zhao Y, Wilsbacher LD, Sangoram AM, King DP, Pinto LH and
Takahashi JS: Functional identification of the mouse circadian
Clock gene by transgenic BAC rescue. Cell. 89:655–667. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu D, Potluri N, Lu J, Kim Y and
Rastinejad F: Structural integration in hypoxia-inducible factors.
Nature. 524:303–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krugluger W, Brandstaetter A, Kállay E,
Schueller J, Krexner E, Kriwanek S, Bonner E and Cross HS:
Regulation of genes of the circadian clock in human colon cancer:
Reduced period-1 and dihydropyrimidine dehydrogenase transcription
correlates in high-grade tumors. Cancer Res. 67:7917–7922. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mazzoccoli G, Panza A, Valvano MR, Palumbo
O, Carella M, Pazienza V, Biscaglia G, Tavano F, Di Sebastiano P,
Andriulli A, et al: Clock gene expression levels and relationship
with clinical and pathological features in colorectal cancer
patients. Chronobiol Int. 28:841–851. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Hua L, Lu C and Chen Z: Expression
of circadian clock gene human Period2 (hPer2) in human colorectal
carcinoma. World J Surg Oncol. 9:1662011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oshima T, Takenoshita S, Akaike M,
Kunisaki C, Fujii S, Nozaki A, Numata K, Shiozawa M, Rino Y, Tanaka
K, et al: Expression of circadian genes correlates with liver
metastasis and outcomes in colorectal cancer. Oncol Rep.
25:1439–1446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu H, Meng X, Wu J, Pan C, Ying X, Zhou Y,
Liu R and Huang W: Cryptochrome 1 overexpression correlates with
tumor progression and poor prognosis in patients with colorectal
cancer. PLoS One. 8:e616792013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou F, He X, Liu H, Zhu Y, Jin T, Chen C,
Qu F, Li Y, Bao G, Chen Z, et al: Functional polymorphisms of
circadian positive feedback regulation genes and clinical outcome
of Chinese patients with resected colorectal cancer. Cancer.
118:937–946. 2012. View Article : Google Scholar
|
|
25
|
Lu H, Chu Q, Xie G, Han H, Chen Z, Xu B
and Yue Z: Circadian gene expression predicts patient response to
neoadjuvant chemoradiation therapy for rectal cancer. Int J Clin
Exp Pathol. 8:10985–10994. 2015.PubMed/NCBI
|
|
26
|
Wang L, Chen B, Wang Y, Sun N, Lu C, Qian
R and Hua L: hClock gene expression in human colorectal carcinoma.
Mol Med Rep. 8:1017–1022. 2013.PubMed/NCBI
|
|
27
|
Wang Y, Qian R, Sun N, Lu C, Chen Z and
Hua L: Circadian gene hClock enhances proliferation and inhibits
apoptosis of human colorectal carcinoma cells in vitro and in vivo.
Mol Med Rep. 11:4204–4210. 2015.PubMed/NCBI
|
|
28
|
Kubens BS and Zänker KS: Differences in
the migration capacity of primary human colon carcinoma cells
(SW480) and their lymph node metastatic derivatives (SW620). Cancer
Lett. 131:55–64. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY,
Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, et al: Bmi1 is
essential in Twist1-induced epithelial-mesenchymal transition. Nat
Cell Biol. 12:982–992. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koyanagi S, Kuramoto Y, Nakagawa H,
Aramaki H, Ohdo S, Soeda S and Shimeno H: A molecular mechanism
regulating circadian expression of vascular endothelial growth
factor in tumor cells. Cancer Res. 63:7277–7283. 2003.PubMed/NCBI
|
|
33
|
Jensen LD and Cao Y: Clock controls
angiogenesis. Cell Cycle. 12:405–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh R, Lillard JW Jr and Singh S:
Chemokines: Key players in cancer progression and metastasis. Front
Biosci (Schol Ed). 3:1569–1582. 2011.
|
|
35
|
Hwang-Verslues WW, Chang PH, Jeng YM, Kuo
WH, Chiang PH, Chang YC, Hsieh TH, Su FY, Lin LC, Abbondante S, et
al: Loss of corepressor PER2 under hypoxia up-regulates
OCT1-mediated EMT gene expression and enhances tumor malignancy.
Proc Natl Acad Sci USA. 110:12331–12336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li A, Lin X, Tan X, Yin B, Han W, Zhao J,
Yuan J, Qiang B and Peng X: Circadian gene Clock contributes to
cell proliferation and migration of glioma and is directly
regulated by tumor-suppressive miR-124. FEBS Lett. 587:2455–2460.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jensen LD: The circadian clock and hypoxia
in tumor cell de-differentiation and metastasis. Biochim Biophys
Acta. 1850:1633–1641. 2015. View Article : Google Scholar
|
|
38
|
Geva E and Jaffe RB: Role of vascular
endothelial growth factor in ovarian physiology and pathology.
Fertil Steril. 74:429–438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang GL and Semenza GL: Purification and
characterization of hypoxia-inducible factor 1. J Biol Chem.
270:1230–1237. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Folkman J: Tumor angiogenesis. Adv Cancer
Res. 43:175–203. 1985. View Article : Google Scholar : PubMed/NCBI
|