Introduction
Asbestos and silica are the most well-known
causative, environmental and occupational substances that induce
pneumoconiosis (1–3). The initial event following entry of
these substances into the human body involves the action of immune
competent cells such as alveolar macrophages to treat these foreign
materials by activating the NOD-like receptor family, pyrin domain
containing 3 (NLRP3: NALP3) inflammasome to produce interleukin
(IL)-1β and attract fibroblasts (4–6).
Since the immunological effects of asbestos fibers
have not been thoroughly investigated, we decided to study the
various effects of asbestos on human immune competent cells.
Investigation of natural killer (NK) cells using in vitro
chronic exposure in an NK cell line and freshly isolated NK cells
derived from healthy volunteers (HV) showed a reduction in NK cell
activating receptors such as NKG2D and 2B4, as well as
intracellular granzyme A, and decreased phosphorylation of ERK
(7,8). In addition to these findings, studies
of peripheral blood NK cells from asbestos-exposed patients with
pleural plaque (PP) or malignant mesothelioma (MM) showed decreased
expression of NKp46 activating receptor (7–9).
Furthermore, the differentiation and proliferation of
CD8+ cytotoxic T lymphocytes (CTLs) were also impaired
by asbestos exposure. The cytotoxicity for allogeneic targets
decreased in peripheral blood mononuclear cells (PBMCs) exposed to
chrysotile B (CB) asbestos, but not in cells exposed to crocidolite
(CR) asbestos, when compared with PBMCs without exposure during a
mixed lymphocyte reaction (MLR) (10). Exposure to CB during the MLR
resulted in suppression of an increase in granzyme B+
cells and interferon (IFN)γ positive cells. CB exposure also
resulted in suppression of increases in CD45RO+
effector/memory cells and CD25+-activated cells in
CD8+ lymphocytes, and a decrease in CD45RA+
cells. Furthermore, CB exposure suppressed the proliferation of
CD8+ lymphocytes without yielding an increase in Annexin
V+ apoptotic cells in CD8+ lymphocytes.
Moreover, the production of IL-10, IFNγ and tumor necrosis factor
(TNF)-α, but not IL-2, decreased in the presence of CB (10–13).
Our research also established a model involving
chronic and continuous exposure to asbestos for the investigation
of CD4+ T helper (Th) cells. Transient and relatively
high-dose exposure to asbestos caused apoptosis in a human T-cell
leukemia virus type (HTLV)-1 immortalized human polyclonal T-cell
line, MT-2, by the production of reactive oxygen spices (ROS).
Activation of the pro-apoptotic MAK transduction signaling pathway
such as p38 and JUN, and activation of the mitochondrial apoptotic
pathway during continuous and relatively low-dose (in which the
occurrence of apoptosis was <50%) exposure for more than one
year resulted in MT-2 being more resistant to asbestos-induced
apoptosis (14). Several
independent sublines of MT-2 continuously exposed to asbestos
showed upregulation of IL-10 and transforming growth factor (TGF)β,
and activation of signal transducer and activator of transcription
3 (STAT3) and Bcl-2 (15).
Additionally, these sublines showed alterations in cytoskeletal
molecules such as β-actin and vimentin (16). The results obtained from these
sublines continuously exposed to asbestos revealed reduced
expression of C-X-C chemokine receptor type 3 (CXCR3) and a
decreased potential for IFNγ production. These results were also
observed in CD4+ cells derived from patients with PP or
MM (17,18).
The overall findings concerning the immunological
effects of asbestos on human immune competent cells indicated that
chronic exposure to asbestos causes a reduction in antitumor
immunity (19–21). This suggests that asbestos-exposed
individuals possess gradually reduced antitumor immunity and
subsequent increased susceptibility to the onset of cancer. This
may explain the long latency period observed in asbestos-exposed
individuals before they develop malignant tumors following initial
exposure to asbestos, and this sensitivity may lead to the
development of lung cancer and MM, in addition to other
malignancies such as those of the larynx, gastrointestinal tract
and bladder (22,23).
The role of Th17 cells in the development of tumors
has been investigated from various viewpoints, and consideration of
the part played by Th17 cells in carcinogenesis has spawned various
paradigms (24–27). It appears that Th17 cells play a
complex and controversial role in tumor immunity. A study of the
effects of asbestos on Th17 cells in a murine model using erionite,
which has similar chemical and physical properties to asbestos,
demonstrated induced production of IL-17 (28). Similarly, a study utilizing
amphibole asbestos, but not chrysotile asbestos, demonstrated
induced production of IL-17 in a murine model (29). Both of these studies indicated that
the recorded upregulation of IL-17 was related to the production of
autoantibodies and assumed that autoimmune disease might be caused
by asbestos exposure (29–31). However, the precise manner by which
asbestos exposure affects IL-17 production and alters Th cell-type
commensuration is unclear. At the very least, we found that
exposure of T cells to asbestos causes a reduction in CXCR3 and
IFNγ under the experimental conditions employed and in
asbestos-exposed patients with PP or MM (17,18).
These findings were thought to be very important in assessing
antitumor immunity in Th cells since CXCR3− expressing
Th cells recruit IFNγ-producing antitumor Th into the area
surrounding the tumor. However, the activity of both processes
might be reduced in asbestos-exposed patients. Taken together, it
might be of value to determine whether asbestos can induce
inhibition of the cellular features of Th17 cells. The issue
therefore is to determine the manner by which asbestos affects Th17
cells. In an effort to address this matter, freshly isolated human
peripheral CD4+ cells were activated in vitro
with chrysotile asbestos, since the use of chrysotile in industrial
and commercial products is higher than that of amphibole asbestos,
and the immunological effects were found to be similar for both
forms of asbestos as we previously reported (32). We then investigated IL-17
expression and production in relation to CXCR3 expression. Since a
reduction in CXCR3 expression was observed following asbestos
exposure, the cellular roles of IL-17 production and expression in
CXCR3 positive and negative cells were examined.
Materials and methods
Peripheral blood cell preparation and
intracellular staining
Peripheral blood T cells were prepared from three
HV. Peripheral blood was drawn from the vein with the aid of
heparin. PBMCs were isolated using the Ficoll-Hypaque method. Cells
were then stained with anti-CD4 monoclonal antibody (mAb) and
anti-CXCR3 mAb. Both antibodies were purchased from BD Biosciences,
Minneapolis, MN, USA. PBMCs stained by both antibodies were divided
into two fractions comprising CD4+ and surface CXCR3
negative (CD4+sCXCR3−) or positive
(CD4+sCXCR3+) cells (Fig. 1A). Cells in two fractions
(CD4+sCXCR3− or
CD4+sCXCR3+, 1×106 cells) were
stimulated with 1 µg/ml of plate-coated anti-CD3 mAb and 1
µg/ml of soluble anti-CD28 mAb with 10 µg/ml of IL-2
for four weeks (Fig. 1B). These
cultures received 0, 10, 25 or 50 µg/ml of chrysotile
asbestos.
The chrysotile asbestos was removed following
cultivation using the Ficoll-Hypaque method and intracellular
expression of CXCR3, IFNγ and IL-17 was analyzed using mAbs for
these molecules (BD Biosciences). Intracellular staining was
performed using a Cytofix/Cytoperm kit (BD Biosciences) in
accordance with the manufacturer's instructions. Cells were stained
with individual mAbs for 30 min and then subjected to FACSCalibur
flow cytometry.
Additionally, cells cultured with various
concentrations of chrysotile were re-activated using phorbol
12-myristate 13-acetate (PMA) and ionomycin (IM) for 4 h. Human
PBMCs were obtained for this study from H.V. who provided written
informed consent according to guidelines established by the ethics
committee of the Kawasaki Medical School, Kurashiki, Japan.
CBA assay of culture supernatants
As shown in Fig.
1B, activated cells from two fractions
(CD4+sCXCR3− and
CD4+sCXCR3+) co-cultured for four weeks with
various concentrations of chrysotile were re-stimulated by PMA and
ionomycin for 4 h. These supernatants were subjected to CBA assays
(BD Biosciences) to measure the concentration of IL-17 and
IFNγ.
Real-time RT-PCR
Total RNA was extracted from cells re-stimulated by
PMA and ionomycin and derived from two fractions
(CD4+sCXCR3+ and
CD4+sCXCR3−) activated for four weeks and
co-cultured with various concentrations of chrysotile using RNAzol.
Following synthesis of the first strand of cDNA, real-time RT-PCR
was performed using the SYBER Green method (Takara, Shiga, Japan)
with the Mx3000P QPCR System (Agilent Technologies, Inc., Santa
Clara, CA), as previously described (Maeda et al, 16–18,32).
The following primers were used; for CXCR3: forward (F),
5′-ACACCTTCCTGCTCCACCTA-3′, reverse (R),
5′-GTTCAGGTAGCGGTCAAAGC-3′); for IL-17: F,
5′-ACCAATCCCAAAAGGTCCTC-3′, R, 5′-CCCACGGA CACCAGTATCTT-3′; for
T-bet: F, 5′-AGGTGTCGGGG AAACTGAG-3′, R,
5′-ACCACGTCCACAAACATCCT-3′; and for RORγt: F,
5′-AAATCTGTGGGGACAAGTGG-3′, R, 5′-TCCCTCTGCTTCTTGGACAT-3′.
Statistical analysis
Statistical analyses were performed using SPSS
version 21 (IBM Japan, Tokyo, Japan). Group comparisons in this
study were performed as follows. The first comprised a comparison
of intracellular CXCR3, IFNγ or IL-17 expression in
CD4+CXCR3+ or
CD4+CXCR3− fractions derived from cells
cultured in the absence or presence of various concentrations of
chrysotile fibers. The next involved a comparison of IFNγ and IL-17
levels in supernatants of CD4+CXCR3+ or
CD4+CXCR3− fractions from cells cultured in
the absence or presence of various concentrations of chrysotile
fibers. Additionally, real-time RT-PCR was employed to examine mRNA
expression levels of T-bet, IFNγ, RORγT or IL-17 of
CD4+CXCR3+ or
CD4+CXCR3− fractions from cells cultured in
the absence or presence of various concentrations of chrysotile
fibers. All statistical analyses were subjected to the one-way
ANOVA test and subsequent post-hoc comparisons were made using the
Mann-Whitney U test.
Results
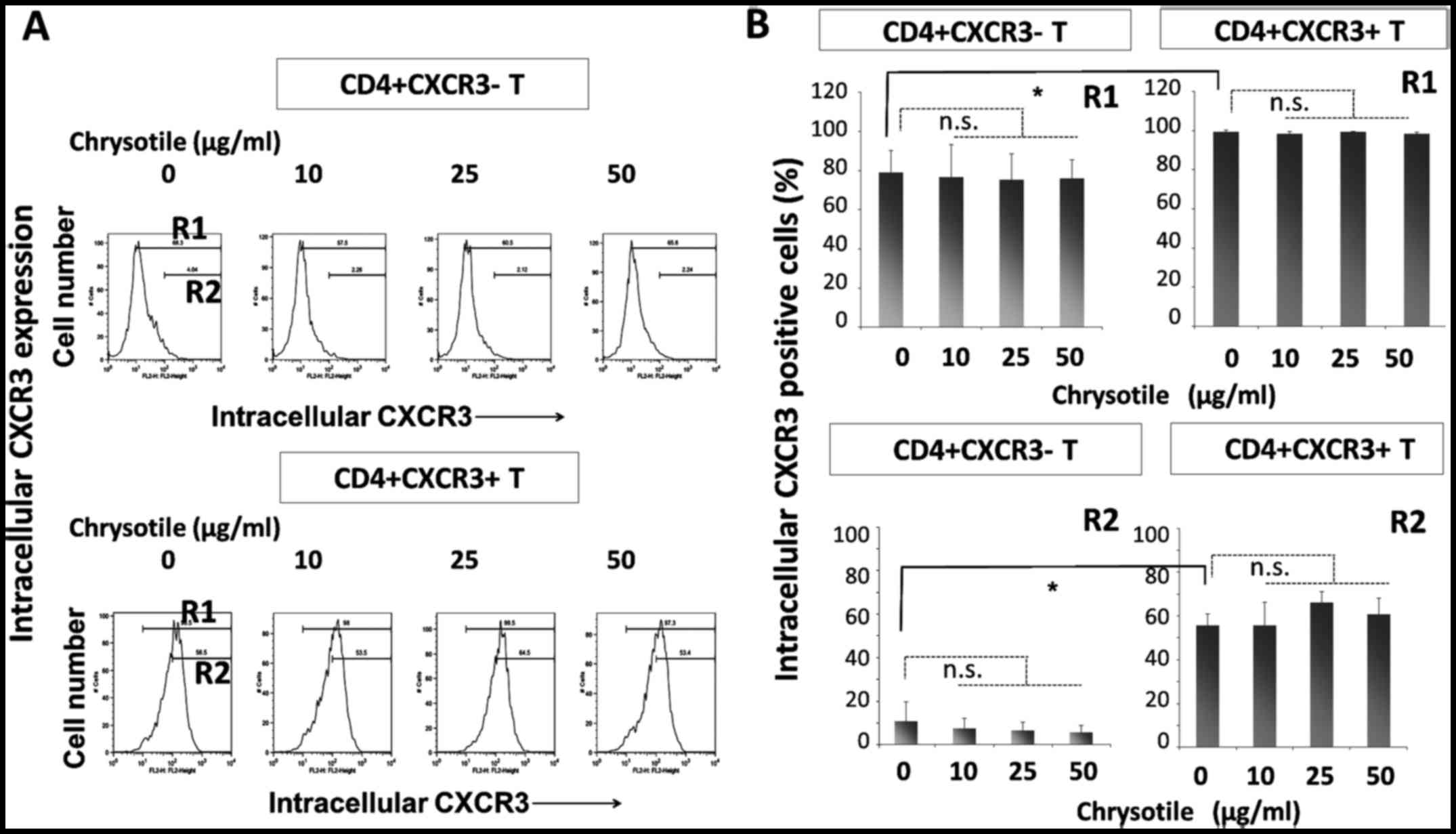
Intracellular CXCR3 expression following
cultivation with chrysotile
CD4+sCXCR3− and
CD4+sCXCR3+ cells were cultured in the
presence of various concentrations of chrysotile (0, 10, 25 or 50
µg/ml) for four weeks with T-cell receptor (TCR) stimulation
and IL-2. Following this activation period, intracellular CXCR3
expression was analyzed by flow cytometry (Fig. 2). The intracellular expression of
CXCR3 was significantly higher in CD4+sCXCR3+
cells compared with CD4+sCXCR3− cells in both
R1 (totally positive cell fraction) and R2 (highly positive
fraction) regions. The results showed that although intracellular
CXCR3 expression remained unaltered in cells derived from
CD4+sCXCR3+ fractions, the presence of
chrysotile tended to reduce the highly positive intracellular
expression of CXCR3 in cells that were initially
CD4+sCXCR3−.
Intracellular IFNγ expression following
cultivation with chrysotile
Levels of IFNγ were examined in
CD4+sCXCR3− and
CD4+sCXCR3+ fractions derived from cells
cultured with various concentrations of chrysotile (Fig. 3). The intracellular expression of
IFNγ was significantly higher in CD4+sCXCR3+
cells compared with CD4+sCXCR3− cells in both
R1 (totally positive cell fraction) and R2 (highly positive
fraction) regions. The results revealed that the initial
CD4+sCXCR3− cells tended to display reduced
IFNγ expression when cells were co-cultured with relatively low
doses of chrysotile, although exposure to chrysotile did not alter
intracellular IFNγ expression in any of the three fractions.
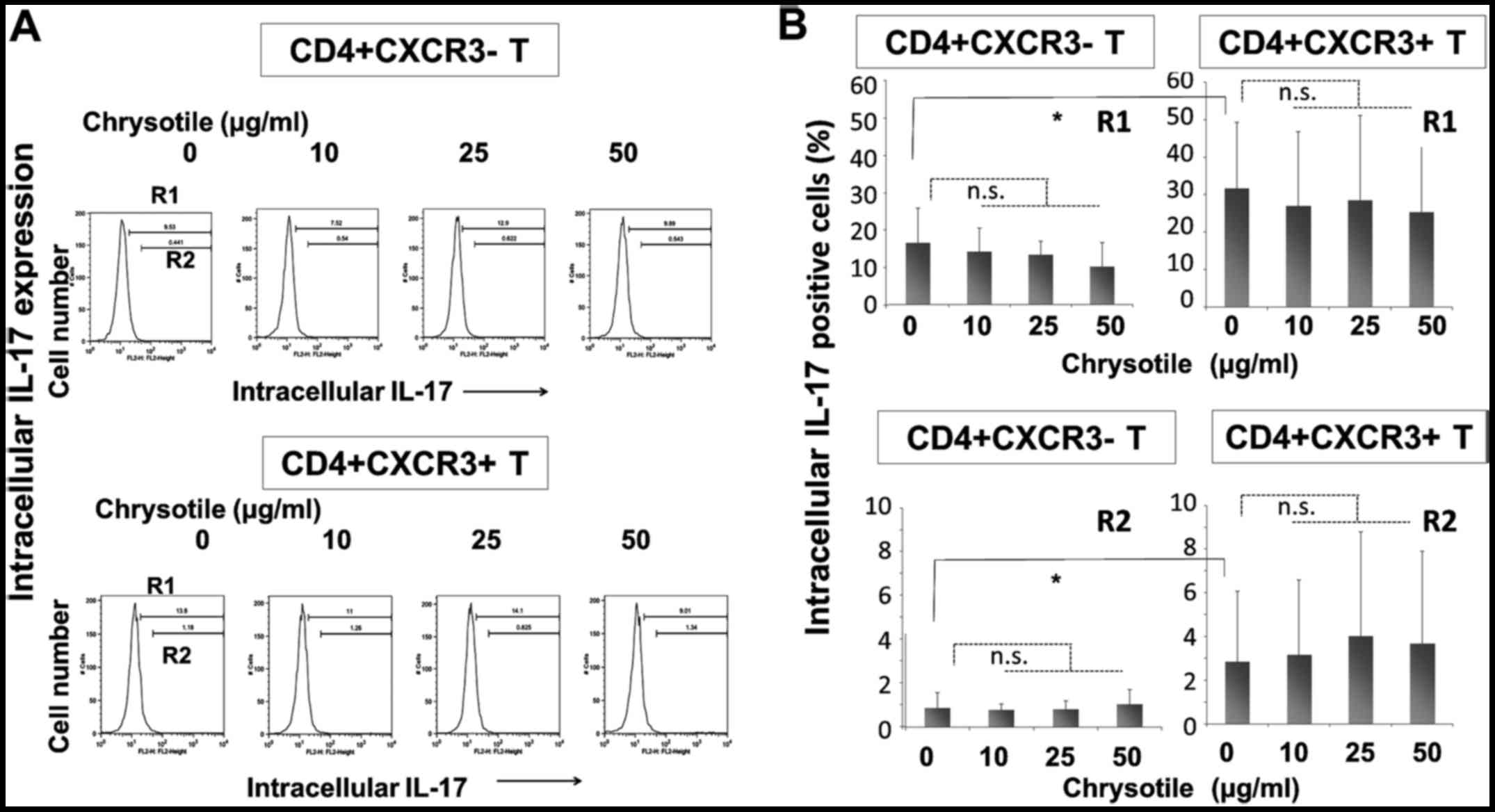
Intracellular IL-17 expression following
cultivation with chrysotile
Intracellular IL-17 expression was analyzed in
CD4+sCXCR3− and
CD4+sCXCR3+ fractions derived from cells
following activation and cultivation with various concentrations of
chrysotile (Fig. 4). The
intracellular expression of IL-17 was significantly higher in
CD4+sCXCR3+ cells compared with
CD4+sCXCR3− cells in both R1 (totally
positive cell fraction) and R2 (highly positive fraction) regions.
The results showed that CD4+ fractions tended to display
increased intracellular IL-17 expression, although there were no
significant differences in intracellular IL-17 expression in any of
the fractions.
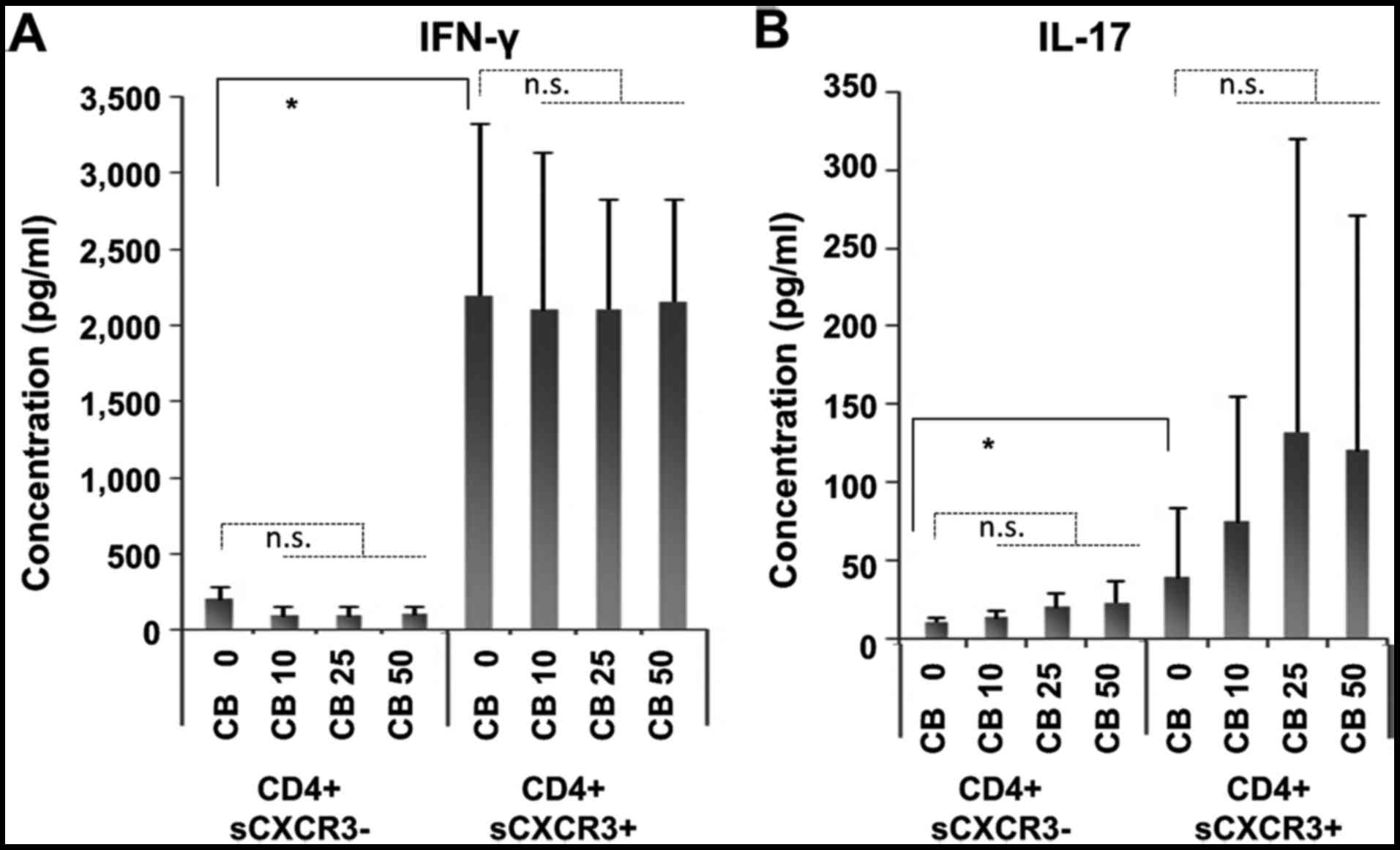
IFNγ and IL-17 production following
stimulation of cells cultured with chrysotile
Since analysis of the intracellular expression of
IFNγ and IL-17 in cell fractions initially divided as
CD4+sCXCR3− and
CD4+sCXCR3+ following four weeks of
cultivation with chrysotile asbestos did not show any marked
alterations, these cells were re-stimulated by PMA and ionomycin
for 4 h as shown in Fig. 1B. The
concentration of IFNγ and IL-17 in culture supernatants was then
measured.
As shown in Fig. 5,
high levels of both IFNγ and IL-17 were found in
CD4+sCXCR3+ fractions. In contrast, IFNγ
production remained unaltered in cells cultured with chrysotile
(Fig. 5A). However, IL-17
production was enhanced in cells cultured with chrysotile in a
dose-dependent manner (Fig. 5B),
although large variations were found.
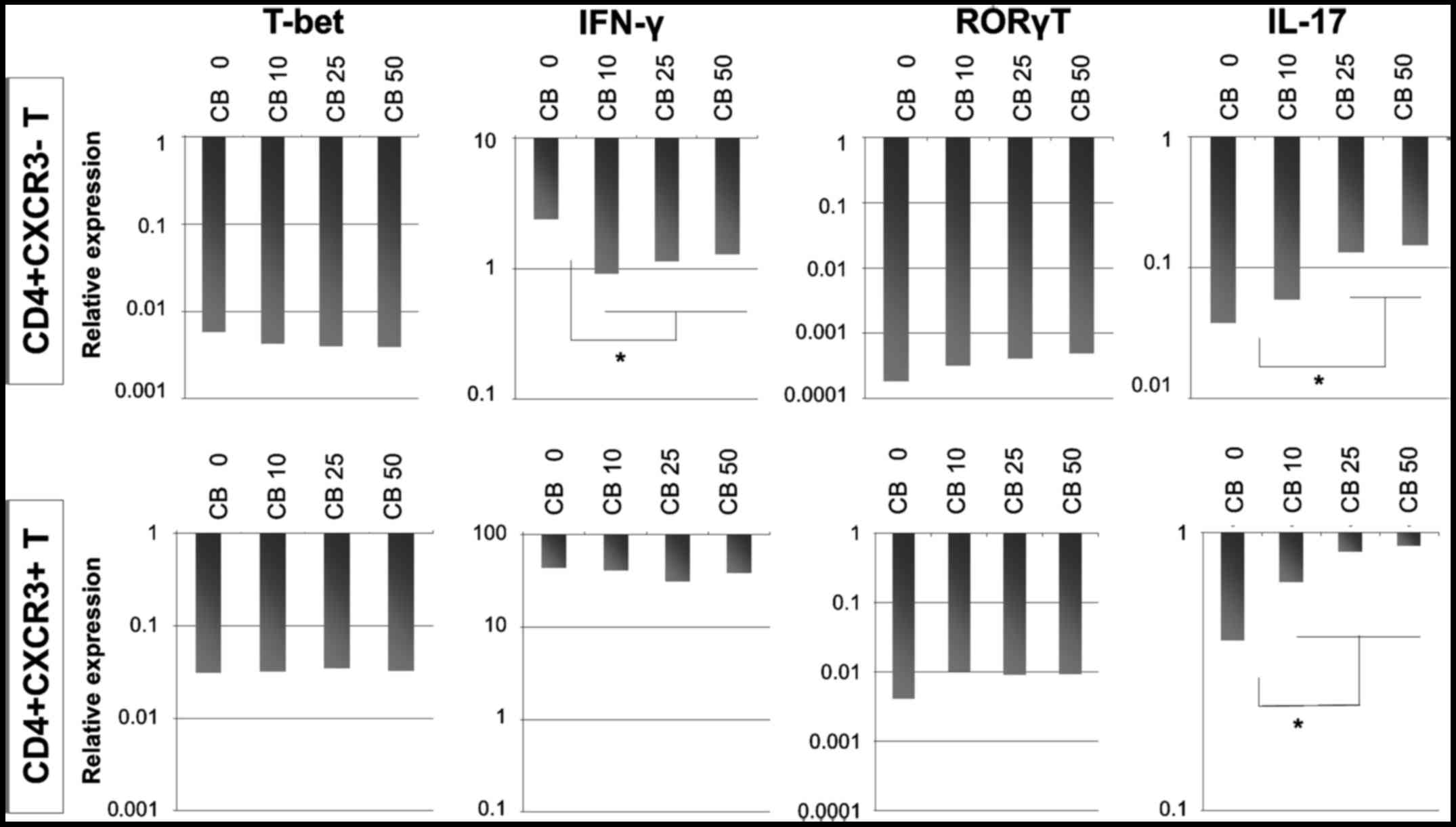
IFNγ, T-bet, RORγT and IL-17 mRNA
expression
Although IFNγ production remained unaltered in
CD4+sCXCR3− and
CD4+sCXCR3+ cells cultured with various
concentrations of chrysotile asbestos, the production of IL-17
increased as shown in Fig. 6.
Consequently, the expression of IFNγ, T-bet, RORγT and IL-17 mRNA
was analyzed.
The results showed that cultivation with chrysotile
did not modify the expression of T-bet, whereas IFNγ expression was
reduced in cells initially categorized as
CD4+sCXCR3−. These results indicated that
although cultivation with chrysotile did not appear to alter the
Th1 population, Th1 function was reduced and depended in particular
on the initial expression of CXCR3.
The Th17 status in these three fractions derived
from cells treated with PMA and ionomycin and followed by T-cell
receptor stimulation with IL-2 and cultivation with various
concentrations of chrysotile asbestos was then examined. IL-17 mRNA
expression was enhanced in both CD4+sCXCR3−
and CD4+sCXCR3+ fractions, although the
expression of RORγT increased slightly in cells co-cultured with
chrysotile.
Discussion
The causative mechanisms of asbestos-induced cancer
are thought to include 1) DNA damage due to ROS production by iron
found in asbestos fibers, 2) direct chromosomal/DNA damage by
physical attack of cells near the inhaled asbestos fibers, and 3)
adsorption of various carcinogenic substances around the fiber
itself (33–36). The effect of these processes
results in the generation of mesothelioma cells containing various
genetic changes such as deletion of p16/p15 cyclin-dependent
kinase-inhibitors (CDK-Is), deletion of NF2/merlin, and alteration
(deletions and mutations) of BRCA1-associated protein 1/ubiquitin
carboxyl-terminal hydrolase (BAP1) (37–39).
Mesothelioma is thought to result after a 30 to 40-year latency
period following initial exposure to asbestos. During these long
latency periods, and under conditions leading to the occurrence of
other malignancies such as cancers of the larynx, gastrointestinal
tract and bladder (22,23), it is thought that individuals
exposed to asbestos and possessing asbestos fibers in their body
might have reduced antitumor immunity due to asbestos exposure and
recurrent and chronic encounters between intra-body fibers and
immune competent cells. It is on this basis that we have been
investigating the immunological effects of asbestos on immune
competent cells, particularly in regard to antitumor immunity
(19–21,40).
Investigation of Th cells showed a reduction in
CXCR3 surface expression and impaired IFNγ production capacity
(17,18). The T-cell line model utilized in
vitro stimulated freshly isolated human peripheral
CD4+ cells and CD4+ cells derived from PP and
MM (17,18). Since CXCR3 is thought to operate
with IFNγ producing cells and functions to attack tumor cells,
these findings also suggest that individuals exposed to asbestos
possess reduced antitumor immunity (40).
We then investigated Th17 cells since they were
considered to be important in the development of dysregulation of
autoimmunity and cancer (24–27).
Some human cancers have been shown to possess increased levels of
Th17 cells in the tumor, such as melanoma, ovarian and prostate
cancers (24–27). Furthermore, the polarization of
Th17 cells was considered to be opposite to that of
CD4+CD25+ and Forkhead box protein P3 (FoxP3)
positive regulatory T cells (Treg) (41,42).
Since enhancement of the volume or function of Treg may cause a
reduction in antitumor immunity that inhibits tumor-recognizing T
cells, the peripheral balance and polarizing conditions defined by
cytokine conditions among IL-6 and TGFβ and between Treg and Th17
(24–27) may be altered, and an increase in
one population may cause a decrease in the other population.
Therefore, an increase in number of cells or enhancement of Th17
function may reflect augmentation of antitumor immunity (24–27).
A consideration of the overall results and our
previous data suggests that asbestos exposure reduces CXCR3
expression and decreases IFNγ production capacity in Th1 cells, and
increases IL-17 production capacity in CD4+ and surface
CXCR3+ fractions. This production capacity appears to be
strongly related to the CXCR3 positive cell fraction (Fig. 5), and reduction in the surface
CXCR3 positive fraction during asbestos exposure was associated
with a reduction in IL-17 production capacity in peripheral blood
derived from asbestos-exposed patients. These findings also
indicated that the immunological effects of asbestos lead to a
reduction in antitumor immunity, and that these mechanisms may play
a role in the subsequent occurrence of mesothelioma and other
cancers in asbestos-exposed individuals.
The status of Treg in asbestos-exposed patients and
the effect of asbestos on Treg require investigation. Recently, we
indicated that asbestos enhances Treg function through a cell-cell
contact pathway, and increases production of typical soluble
factors such as IL-10 and TGFβ. These investigations also showed a
reduction in antitumor immunity in asbestos-exposed patients.
However, it remains unclear whether chronic, recurrent and
continuous exposure of T cells to asbestos influences the
polarization of Th subpopulations such as Th1, Treg and Th17.
Additionally, studies of Treg and Th17 should
determine whether there is an increase in the number and function
of tumors surrounding these subpopulations. However, such
investigations must collect all of the immune competent cells from
rejected tumor specimens. These issues need to be examined in
future studies in an effort to address unresolved questions such as
the alteration of antitumor immunity in mesothelioma patients.
However, our focus is to consider the gradual decrease in antitumor
immunity in asbestos-exposed patients following initial exposure to
asbestos. Therefore, it would be better to analyze a population
that has a significant exposure history to asbestos, but which does
not show any significant physical alterations or the presence of
certain cancers or PP.
The overall results suggest that Treg and Th17
conditions should be analyzed with respect to antitumor immunity
not only in PP and MM patients, but also in individuals who have a
significant history of asbestos exposure without any changes in
health and body-related issues.
An experimental model should be explored in order to
confirm the reduction in antitumor immunity. For example, as
previously reported, continuous exposure of the human NK cell line
YT-A1 to chrysotile asbestos for more than five months resulted in
reduced antitumor killing activity against the human erythroblastic
leukemia cell line K562, a commonly used target cell line to assay
NK cell activity and compared with the original YT-A1 cell line,
which has never been exposed to asbestos fibers (7–9).
Regarding our previous reports showing that asbestos fibers enhance
Treg function (43) and increase
its volume by acceleration of the cell cycle (44), the construction of a cell
co-culture model using human cell lines such as a Treg cell model
or responder T cell model with mesothelioma (or other cancer) model
might be difficult. As we previously reported, subjecting a human
Treg model comprising the MT-2 cell line to continuous exposure to
asbestos resulted in increased production of IL-10 and TGFβ
(43). The effects of soluble
factors such as these cytokines can be assayed using a transwell
culture model. However, these soluble factors act to reduce
antitumor immunity by inhibiting the attack of tumor cells by T
cells. Thus, the model should comprise two cell types such as tumor
cells and tumor-attacking responder T cells. Of course, it would be
possible to match the HLA of some mesothelioma cell line with HV
who could be used to provide PBMCs, including tumor attacking T
cells. Thereafter, PBMCs derived from HLA-matched HV can be
activated in vitro in the absence or presence of asbestos
fibers. The tumor killing activity may then be examined in
vitro with or without cell culture supernatants derived from a
human Treg cell line model cultured in the absence or continuous
presence of asbestos fibers. Animal models transplanting human
mesothelioma cells can also be employed with the induction of
immune cells in the absence or continuous presence of asbestos.
Furthermore, since antibodies are produced against tumor cells,
human myeloma cell lines can be employed and we have successfully
established many of these cell lines (45–47).
These experiments should be performed in an effort to confirm our
hypothesis that asbestos exposure causes a reduction in antitumor
immunity.
In conclusion, we found that in vitro
asbestos exposure of freshly isolated human T-cells induces IL-17
production. Further investigations regarding Th17 status in
asbestos-exposed patients with PP or MM, and in individuals who do
not exhibit any abnormalities, should be conducted to confirm the
status of antitumor immunity in these asbestos-exposed individuals.
Following confirmation of these hypotheses, certain kinds of food
compositions or physiologically active substances derived from
plants or other materials should be tested for their ability to
neutralize the altered antitumor immunity in individuals exposed to
asbestos in an effort to thwart the development of tumors.
Abbreviations:
|
NLRP3/NALP3
|
NOD-like receptor family, pyrin domain
containing 3
|
|
IL
|
interleukin
|
|
NK
|
natural killer
|
|
HV
|
healthy volunteers
|
|
PP
|
pleural plaque
|
|
MM
|
malignant mesothelioma
|
|
CTL
|
cytotoxic T lymphocyte
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
CB
|
chrysotile B
|
|
CR
|
crocidolite
|
|
MLR
|
mixed lymphocyte reaction
|
|
IFN
|
interferon
|
|
TNF
|
tumor necrosis factor
|
|
HTLV
|
human T-cell leukemia virus type
|
|
ROS
|
reactive oxygen species
|
|
TGF
|
transforming growth factor
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
CXCR3
|
C-X-C chemokine receptor type 3
|
|
mAb
|
monoclonal antibody
|
|
PMA
|
phorbol 12-myristate 13-acetate
|
|
IM
|
ionomycin
|
|
F
|
forward
|
|
R
|
reverse
|
|
TCR
|
T-cell receptor
|
|
CDK-I
|
cyclin-dependent kinase-inhibitor
|
|
BAP1
|
BRCA1-associated protein 1/ubiquitin
carboxyl-terminal hydrolase
|
|
Th
|
T helper
|
|
FoxP3
|
Forkhead box protein P3
|
|
Treg
|
regulatory T cells
|
|
R
|
region
|
Acknowledgments
We thank Ms. Naomi Miyahara, Minako Katoh, Misao
Kuroki, Keiko Kimura, Yoshiko Yamashita and Tomoko Sueishi for
their technical assistance. This study was supported by Special
Coordination Funds for Promoting Science and Technology grant
H18-1-3-3-1 (Comprehensive Approach on Asbestos-Related Diseases),
grants from the Ministry of Education, Culture, Sports, Science and
Technology of Japan (18390186, 1965153, 19790411, 20390178 and
22700933), and Kawasaki Medical School Project Grants (18-209T,
19-205Y and 20-210O). This research was also partially supported by
the Translational Research Network Program from the Japan Agency
for Medical Research and Development (AMED).
References
|
1
|
Fujimura N: Pathology and pathophysiology
of pneumoconiosis. Curr Opin Pulm Med. 6:140–144. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mossman BT and Churg A: Mechanisms in the
pathogenesis of asbestosis and silicosis. Am J Respir Crit Care
Med. 157:1666–1680. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seaman DM, Meyer CA and Kanne JP:
Occupational and environmental lung disease. Clin Chest Med.
36:249–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cassel SL, Eisenbarth SC, Iyer SS, Sadler
JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA and
Sutterwala FS: The Nalp3 inflammasome is essential for the
development of silicosis. Proc Natl Acad Sci USA. 105:9035–9040.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dostert C, Pétrilli V, Van Bruggen R,
Steele C, Mossman BT and Tschopp J: Innate immune activation
through Nalp3 inflammasome sensing of asbestos and silica. Science.
320:674–677. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hornung V, Bauernfeind F, Halle A, Samstad
EO, Kono H, Rock KL, Fitzgerald KA and Latz E: Silica crystals and
aluminum salts activate the NALP3 inflammasome through phagosomal
destabilization. Nat Immunol. 9:847–856. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishimura Y, Miura Y, Maeda M, Kumagai N,
Murakami S, Hayashi H, Fukuoka K, Nakano T and Otsuki T: Impairment
in cytotoxicity and expression of NK cell- activating receptors on
human NK cells following exposure to asbestos fibers. Int J
Immunopathol Pharmacol. 22:579–590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishimura Y, Maeda M, Kumagai N, Hayashi
H, Miura Y and Otsuki T: Decrease in phosphorylation of ERK
following decreased expression of NK cell-activating receptors in
human NK cell line exposed to asbestos. Int J Immunopathol
Pharmacol. 22:879–888. 2009. View Article : Google Scholar
|
|
9
|
Nishimura Y, Maeda M, Kumagai-Takei N, Lee
S, Matsuzaki H, Wada Y, Nishiike-Wada T, Iguchi H and Otsuki T:
Altered functions of alveolar macrophages and NK cells involved in
asbestos-related diseases. Environ Health Prev Med. 18:198–204.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumagai-Takei N, Nishimura Y, Maeda M,
Hayashi H, Matsuzaki H, Lee S, Hiratsuka J and Otsuki T: Effect of
asbestos exposure on differentiation of cytotoxic T lymphocytes in
mixed lymphocyte reaction of human peripheral blood mononuclear
cells. Am J Respir Cell Mol Biol. 49:28–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumagai-Takei N, Nishimura Y, Maeda M,
Hayashi H, Matsuzaki H, Lee S, Kishimoto T, Fukuoka K, Nakano T and
Otsuki T: Functional properties of CD8(+) lymphocytes in patients
with pleural plaque and malignant mesothelioma. J Immunol Res.
2014:6701402014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumagai-Takei N, Nishimura Y, Matsuzaki H,
Maeda M, Lee S, Yoshitome K and Otsuki T: Effects of asbestos
fibers on human cytotoxic T cells. Biological Effects of Fibrous
and Particulate Substances (Series Title: Current Topics in
Environmental Health and Preventive Medicine). Otsuki T, Holian A
and Yoshioka Y: Springer-Japan; pp. 211–221. 2015
|
|
13
|
Kumagai-Takei N, Nishimura Y, Matsuzaki H,
Lee S, Yoshitome K, Hayashi H and Otsuki T: The suppressed
induction of human mature cytotoxic T lymphocytes caused by
asbestos is not due to Interleukin-2 insufficiency. J Immunol Res.
2016:74848722016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hyodoh F, Takata-Tomokuni A, Miura Y,
Sakaguchi H, Hatayama T, Hatada S, Katsuyama H, Matsuo Y and Otsuki
T: Inhibitory effects of anti-oxidants on apoptosis of a human
poly-clonal T-cell line, MT-2, induced by an asbestos,
chrysotile-A. Scand J Immunol. 61:442–448. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miura Y, Nishimura Y, Katsuyama H, Maeda
M, Hayashi H, Dong M, Hyodoh F, Tomita M, Matsuo Y, Uesaka A, et
al: Involvement of IL-10 and Bcl-2 in resistance against an
asbestos-induced apoptosis of T cells. Apoptosis. 11:1825–1835.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maeda M, Chen Y, Kumagai-Takei N, Hayashi
H, Matsuzaki H, Lee S, Hiratsuka J, Nishimura Y, Kimura Y and
Otsuki T: Alteration of cytoskeletal molecules in a human T cell
line caused by continuous exposure to chrysotile asbestos.
Immunobiology. 218:1184–1191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maeda M, Nishimura Y, Hayashi H, Kumagai
N, Chen Y, Murakami S, Miura Y, Hiratsuka J, Kishimoto T and Otsuki
T: Reduction of CXC chemokine receptor 3 in an in vitro model of
continuous exposure to asbestos in a human T-cell line, MT-2. Am J
Respir Cell Mol Biol. 45:470–479. 2011. View Article : Google Scholar
|
|
18
|
Maeda M, Nishimura Y, Hayashi H, Kumagai
N, Chen Y, Murakami S, Miura Y, Hiratsuka J, Kishimoto T and Otsuki
T: Decreased CXCR3 expression in CD4+ T cells exposed to
asbestos or derived from asbestos-exposed patients. Am J Respir
Cell Mol Biol. 45:795–803. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumagai-Takei N, Maeda M, Chen Y,
Matsuzaki H, Lee S, Nishimura Y, Hiratsuka J and Otsuki T: Asbestos
induces reduction of tumor immunity. Clin Dev Immunol.
2011:4814392011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuzaki H, Maeda M, Lee S, Nishimura Y,
Kumagai-Takei N, Hayashi H, Yamamoto S, Hatayama T, Kojima Y,
Tabata R, et al: Asbestos-induced cellular and molecular alteration
of immunocompetent cells and their relationship with chronic
inflammation and carcinogenesis. J Biomed Biotechnol.
2012:4926082012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Otsuki T, Matsuzaki H, Lee S,
Kumagai-Takei N, Yamamoto S, Hatayama T, Yoshitome K and Nishimura
Y: Environmental factors and human health: Fibrous and particulate
substance-induced immunological disorders and construction of a
health-promoting living environment. Environ Health Prev Med.
21:71–81. 2016. View Article : Google Scholar :
|
|
22
|
Craighead JE: Nonthoracic cancers possibly
resulting from asbestos exposure. Asbestos and its Diseases.
Craighead JE and Gibbs AR: Oxford University Press; New York: pp.
230–252. 2008, View Article : Google Scholar
|
|
23
|
Freidman GK: Malignant diseases attributed
to asbestos exposure. Asbestos. Risk assessment, epidemiology, and
health effects. Dodson RF and Hammar SP: CRC Press; Boca Raton: pp.
561–592. 2011, View Article : Google Scholar
|
|
24
|
Alizadeh D, Katsanis E and Larmonier N:
The multifaceted role of Th17 lymphocytes and their associated
cytokines in cancer. Clin Dev Immunol. 2013:9578782013. View Article : Google Scholar
|
|
25
|
Bailey SR, Nelson MH, Himes RA, Li Z,
Mehrotra S and Paulos CM: Th17 cells in cancer: The ultimate
identity crisis. Front Immunol. 5:2762014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lakshmi Narendra B, Eshvendar Reddy K,
Shantikumar S and Ramakrishna S: Immune system: A double-edged
sword in cancer. Inflamm Res. 62:823–834. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zou W and Restifo NP: T(H)17 cells in
tumour immunity and immunotherapy. Nat Rev Immunol. 10:248–256.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ferro A, Zebedeo CN, Davis C, Ng KW and
Pfau JC: Amphibole, but not chrysotile, asbestos induces
anti-nuclear autoantibodies and IL-17 in C57BL/6 mice. J
Immunotoxicol. 11:283–290. 2014. View Article : Google Scholar
|
|
29
|
Serve KM, Black B, Szeinuk J and Pfau JC:
Asbestos-associated mesothelial cell autoantibodies promote
collagen deposition in vitro. Inhal Toxicol. 25:774–784. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pfau JC, Serve KM and Noonan CW:
Autoimmunity and asbestos exposure. Autoimmune Dis.
2014:7820452014.PubMed/NCBI
|
|
31
|
Zebedeo CN, Davis C, Peña C, Ng KW and
Pfau JC: Erionite induces production of autoantibodies and IL-17 in
C57BL/6 mice. Toxicol Appl Pharmacol. 275:257–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maeda M, Yamamoto S, Chen Y, Kumagai-Takei
N, Hayashi H, Matsuzaki H, Lee S, Hatayama T, Miyahara N, Katoh M,
et al: Resistance to asbestos-induced apoptosis with continuous
exposure to crocidolite on a human T cell. Sci Total Environ.
429:174–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang SX, Jaurand MC, Kamp DW, Whysner J
and Hei TK: Role of mutagenicity in asbestos fiber-induced
carcinogenicity and other diseases. J Toxicol Environ Health B Crit
Rev. 14:179–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mossman BT and Craighead JE: Mechanisms of
asbestos carcinogenesis. Environ Res. 25:269–280. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moyer VD, Cistulli CA, Vaslet CA and Kane
AB: Oxygen radicals and asbestos carcinogenesis. Environ Health
Perspect. 102(Suppl 10): 131–136. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Toyokuni S: Iron overload as a major
targetable pathogenesis of asbestos-induced mesothelial
carcinogenesis. Redox Rep. 19:1–7. 2014. View Article : Google Scholar
|
|
37
|
LaFave LM, Béguelin W, Koche R, Teater M,
Spitzer B, Chramiec A, Papalexi E, Keller MD, Hricik T,
Konstantinoff K, et al: Loss of BAP1 function leads to
EZH2-dependent transformation. Nat Med. 21:1344–1349. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sekido Y: Molecular pathogenesis of
malignant mesothelioma. Carcinogenesis. 34:1413–1419. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singhi AD, Krasinskas AM, Choudry HA,
Bartlett DL, Pingpank JF, Zeh HJ, Luvison A, Fuhrer K, Bahary N,
Seethala RR, et al: The prognostic significance of BAP1, NF2, and
CDKN2A in malignant peritoneal mesothelioma. Mod Pathol. 29:14–24.
2016. View Article : Google Scholar
|
|
40
|
Maeda M, Yamamoto S, Hatayama T, Matsuzaki
H, Lee S, Kumagai-Takei N, Yoshitome K, Nishimura Y, Kimura Y and
Otsuki T: T cell alteration caused by exposure to asbestos.
Biological Effects of Fibrous and Particulate Substances, (Series
Title: Current Topics in Environmental Health and Preventive
Medicine). Otsuki T, Holian A and Yoshioka Y: Springer; Tokyo: pp.
195–210. 2015
|
|
41
|
O'Connor RA, Taams LS and Anderton SM:
Translational mini-review series on Th17 cells: CD4 T helper cells:
functional plasticity and differential sensitivity to regulatory T
cell-mediated regulation. Clin Exp Immunol. 159:137–147. 2010.
View Article : Google Scholar :
|
|
42
|
Olson NC, Sallam R, Doyle MF, Tracy RP and
Huber SA: T helper cell polarization in healthy people:
Implications for cardiovascular disease. J Cardiovasc Transl Res.
6:772–786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ying C, Maeda M, Nishimura Y,
Kumagai-Takei N, Hayashi H, Matsuzaki H, Lee S, Yoshitome K,
Yamamoto S, Hatayama T, et al: Enhancement of regulatory T
cell-like suppressive function in MT-2 by long-term and low-dose
exposure to asbestos. Toxicology. 338:86–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee S, Matsuzaki H, Maeda M, Yamamoto S,
Kumagai-Takei N, Hatayama T, Ikeda M, Yoshitome K, Nishimura Y and
Otsuki T: Accelerated cell cycle progression of human regulatory T
cell-like cell line caused by continuous exposure to asbestos
fibers. Int J Oncol. 50:66–74. 2017.
|
|
45
|
Tsujioka T, Miura Y, Otsuki T, Nishimura
Y, Hyodoh F, Wada H and Sugihara T: The mechanisms of vitamin
K2-induced apoptosis of myeloma cells. Haematologica. 91:613–619.
2006.PubMed/NCBI
|
|
46
|
Otsuki T, Yata K, Takata-Tomokuni A,
Hyodoh F, Miura Y, Sakaguchi H, Hatayama T, Hatada S, Tsujioka T,
Sato Y, et al: Expression of protein gene product 9.5
(PGP9.5)/ubiquitin-C-terminal hydrolase 1 (UCHL-1) in human myeloma
cells. Br J Haematol. 127:292–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Otsuki T, Yamada O, Kurebayashi J, Moriya
T, Sakaguchi H, Kunisue H, Yata K, Uno M, Yawata Y and Ueki A:
Estrogen receptors in human myeloma cells. Cancer Res.
60:1434–1441. 2000.PubMed/NCBI
|