Introduction
Renal cell carcinoma (RCC) accounting for 80–90%
renal malignancies derives from renal tubule epithelium, which is
also called renal adenocarcinoma. It is estimated that
approximately 62,700 new cases of renal cancer and 14,240 deaths
from renal cancer occurred in the United States in 2016 (1). According to 2004 WHO
histopathological classification criteria, RCC was classified into
a variety of histologic subtypes. Clear cell RCC (ccRCC) represents
the most common subtype of RCC that is characterized by high
frequency of metastasis and mortality, and resistance to
traditional radiotherapy and chemotherapy. Cancer metastasis is the
overwhelming cause of tumor-related mortality in tumor-bearing
patients (2–4). Unfortunately, approximately 30% of
patients with renal cancer have developed local invasion or distant
metastasis when diagnosed, resulting in poor prognosis. Currently,
the underlying molecular mechanisms of metastasis of renal cell
carcinoma are elusive. Therefore, it is greatly needed to make a
thorough inquiry of metastatic mechanisms in RCC, aiming to provide
a novel target for clinical treatment.
Epithelial-mesenchymal transition (EMT), a
fundamental morphogenesis process in which the epithelial cells
transform into motile mesenchymal cells, plays a pivotal role in
embryogenesis (5,6), the formation of organs, the
differentiation of tissues, wound healing, stem cell property, and
malignant tumor progression and metastasis (7,8).
During an EMT process, epithelial cells lose their characteristics,
such as the loss of cell polarity, cell-cell adhesion, and
downregulation of epithelial markers, substituted by mesenchymal
cell traits involving acquisition of migration and invasiveness,
resistance to apoptosis and senescence, stemness and
immunosuppression, and upregulation of mesenchymal markers
(7).
SOX4, belonging to the sex-determining region Y
(SRY)-related high-mobility group (HMG)-box family, is a crucial
transcriptional factor associated with a series of development
procedures, such as retinal differentiation, central nervous
system, heart development, lymphocyte development, via its
transcriptional activation (9–14).
Recently, SOX4, as an oncogenic gene, has aroused great interest of
researchers, and has been implicated in various human malignancies,
including leukemia (15), cancers
of lung (16), prostate (17), liver (18), colon (19), breast (3), and ovarian (20). In breast cancer and prostate
cancer, SOX4 can induce epithelial-mesenchymal transition and
promote metastasis (21,22). In a few human malignancies,
however, SOX4 has been classified as a tumor suppressor gene,
including gallbladder cancer (23), bladder cancer (24), and glioblastoma multiforme
(25). However, few studies have
been carried out to investigate SOX4 expression in renal cell
carcinoma and its biological behavior in renal cancer cells. TGFβ
signaling transduction pathway has been reported to be involved in
EMT and malignancies progression (26). Moreover, TGFβ signaling has been
demonstrated to induce SOX4 expression in breast cancer and
prostate cancer (21,22). Thus, we conjectured that SOX4 might
play a catalytic role in the progression and metastasis of RCC via
induction of EMT.
In this study, we verified SOX4 overexpression in
renal cancer tissues and cell lines. Downregulation of SOX4 greatly
inhibited the migratory and invasive abilities of renal cancer
cells 786-O and SN12-PM6, while overexpression of SOX4 strongly
increased the migratory and invasive properties of the immortalized
non-transformed renal HK-2 cells. Besides, the gain or loss of SOX4
expression could partly induce or reverse the EMT process,
respectively, and upregulate or downregulate the expression of
phospho-AKT (p-AKT) in renal cancer cells. Therefore, we speculated
that SOX4 might play a role in renal cancer cell migration and
invasiveness by inducing EMT through AKT/p-AKT signaling cascade.
These data indicated that SOX4 might be an oncogenic gene through
orchestrating EMT in RCC tumor microenvironment and provided a firm
theoretical principle for consideration of SOX4 as a target for
therapy of RCC.
Materials and methods
Human renal cancer samples
All samples (paired tumor and normal tissues) were
surgically resected from patients with RCC between 2012 and 2014,
at Union Hospital of Huazhong University of Science and Technology
(Wuhan, China). Resected tissues were immediately frozen in liquid
nitrogen for subsequent experiments. None of the patients had
received any antitumor therapy before surgery. Informed written
consent was obtained from each of the RCC patients. This study and
experiment procedures were approved by the Institutional Review
Board of Huazhong University of Science and Technology.
Cell culture
All cell lines were cultured in Dulbecco's Modified
Eagle Medium (DMEM) (Invitrogen, Carlsbad, CA, USA) containing 10%
fetal bovine serum (Gibco-BRL, Grand Island, NY, USA), and 1%
penicillin-streptomycin at 37°C in a humidified 5% CO2
atmosphere. For TGFβ1 treatment, the HK-2 cells were cultured in
DMEM containing 10% fetal bovine serum, and 1%
penicillin-streptomycin and treated with 2.5 ng/ml of TGFβ1 for 0,
3, 7, 10 day(s), respectively.
Transient transfection assay
The si-RNA duplexes targeting SOX4 (si-SOX4) and
si-RNA Negative Control (si-NC) were synthesized and purified by
GenePharma (Shanghai, China). All si-RNA sequences were subjected
to BLAST analysis to guarantee that there is no homology to any
other known coding sequences in the Human Genome Database. The
si-SOX4 and si-NC were transfected at a final concentration of 50
nM with Lipofectamine 2000 reagent (Invitrogen) according to the
manufacturer's recommendations. The plasmid vectors expressing SOX4
(SOX4) or Negative Control (NC) were constructed by Vigene
Biosciences (Shandong, China). A total 2 µg of SOX4 or NC
plasmid were transfected with Lipofectamine 2000 reagent
(Invitrogen) according to the manufacturer's recommendations. At 48
h after transfection, cells were harvested for subsequent
experiments. The si-RNA sequences were as follows: si-SOX4 #1:
5′-UUUGCC CAGCCGCUUGGAGAUCUCG-3′, si-SOX4 #2: 5′-UUGUC
GCUGUCUUUGAGCAGCUUCC-3′, si-NC: 5′-UUCUCCGAACGUGUCACGUTT-3′.
Immunohistochemistry assay
Immunohistochemistry (IHC) assay was performed as
previously described (27).
Briefly, RCC tissues and corresponding normal tissues were fixed in
10% formalin, dehydrated, and embedded in paraffin sequentially.
The sections were incubated with rabbit polyclonal antibody SOX4
(1:100, ab80261, Abcam, Cambridge, MA, USA) overnight at 4°C. After
washing three times with PBS for 10 min each time, the sections
were incubated with anti-rabbit secondary antibodies conjugated to
horseradish peroxidase-labeled polymers. Finally, the sections were
counterstained with hematoxylin.
Immunofluorescence
Appropriate amounts of cells were seeded on glass
coverslips in 12-well plates, washed with PBS three times, fixed in
4% paraformaldehyde and permeabilized with 0.3% TritonX-100 for 10
min. Cells were blocked with 3% BSA for 1 h. Grass coverslips were
incubated with primary antibodies overnight at 4°C, followed by
incubation with CY3-conjugated secondary antibodies for 1 h at room
temperature, and then stained with DAPI. After washing with PBS,
glass coverslips were photographed under a fluorescence
microscope.
Transwell migration and invasion assays
and wound healing assays
In vitro cell migration and invasion assays
were performed using 24-well Transwell chambers with 8.0-µm
pore polycarbonate membrane inserts (Corning). For the migration
assay, 1×104 (786-O, SN12-PM6) or 2.5×104
(HK-2) cells were added to the top chambers. For the invasion
assay, 2×104 (786-O, SN12-PM6) or 5×104
(HK-2) cells were added to the top chambers precoated with Matrigel
(BD Biosciences, Franklin Lakes, NJ, USA). As a chemoattractant,
complete medium supplemented with 10% FBS was added to the bottom
chambers to stimulate migration or invasion. After cells were
incubated for 24 h, cells on the top surface of the membrane were
gently scraped with a cotton swab and cells on the lower surface of
the membrane were fixed with 100% methanol and then stained with
0.1% crystal violet. The cells migrating or invading through the
membrane were counted in 5 random fields. For wound healing assays,
cells transfected with si-RNA were seeded in 6-well plates. When
cells reached 70–80% confluence, a scratch was made using
10-µl pipette tip, washed, then images were at 0 and 24
h.
Quantitative real-time PCR assays
Total RNA of tissues and cells was extracted by
using TRIzol reagent (Invitrogen), and 1 µg RNA samples were
reverse transcribed to cDNA by using reverse transcriptase M-MLV
(Invitrogen). Quantitative real-time PCR (qRT-PCR) was performed
using the SYBR-Green PCR master mix (Invitrogen) on a Roche
LightCycler 480 system (Roche Diagnostics, Mannheim, Germany).
Glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was used as an
endogenous control. Relative expression of genes was calculated
using the power formula: 2−ΔCt (ΔCt = Cttarget -
Ctcontrol), as previously described (28). The gene primer sequences were as
follows: SOX4: forward, 5′-GGCCTCGAGCTGGGAATCGC-3′, reverse,
5′-GCCCACTCGGGGTCTTGCAC-3′; E-cadherin: forward,
5′-GACAACAAGCCCGAATT-3′, reverse, 5′-GGAAACTCTCTCGGTCCA-3′;
N-cadherin: forward, 5′-CGGGTAATCCTCCCAAATCA-3′, reverse,
5′-CTTTATCCCGGCGTTTCATC-3′; Vimentin: forward,
5′-GAGAACTTTGCCGTTGAAGC-3′, reverse, 5′-GCTTCCTGTAGGTGGCAATC-3′;
Twist: forward, 5′-CTGCCCTCGGACAAGCTGAG-3′, reverse,
5′-CTAGTGGGACGCGGACATGG-3′; ZEB1: forward,
5′-TGCTCCCTGTGCAGTTACACCTT-3′, reverse,
5′-CCAGACTGCGTCACATGTCTTTGA-3′; GAPDH: forward,
5′-GAGTCAACGGATTTGGTCGT-3′, reverse,
5′-GACAAGCTTCCCGTTCTCAG-3′.
Western blot assays
Tissues or cells were lysed in RIPA lysis buffer (50
mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.1% SDS1% and sodium
deoxycholate, pH 7.4) containing a protease inhibitor cocktail
tablet (Roche Diagnostics) and 1 mM phenylmethylsulfonyl fluoride
(PMSF). The lysate protein concentration was measured by using BCA
kit (Beyotime Institute of Biotechnology) according to
manufacturer's instructions. Total protein (40 µg) were
subjected to SDS-PAGE electrophoresis and then transferred to a
PVDF membrane (Millipore, Bedford, MA, USA). After being blocked in
PBS containing 5% nonfat milk for 1 h, the membranes were incubated
with antibody against SOX4, N-cadherin, Vimentin, MMP-9, p-AKT, AKT
(all from Abcam), E-cadherin (Cell Signaling Technology, Beverly,
MA, USA), Twist, GAPDH (both from Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) overnight at 4°C. The membranes were then
incubated with secondary antibody conjugated to horseradish
peroxidase for 2 h at room temperature. After washing with
PBS-Tween-20, the proteins were visualized and quantified using
ChemiDoc-XRS+ (Bio-Rad, Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data were analyzed using GraphPad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). Comparisons of
different groups were performed using Student's two-tailed t-test.
Data of each group were expressed as mean ± SEM and P<0.05 was
considered to indicate a statistically significant difference.
Results
The expression of SOX4 is upregulated in
RCC tissues and cell lines
Our previous findings demonstrated low miR-129-3p
levels were associated with short disease-free and overall survival
of RCC patients. miR-129-3p impaired RCC cell migratory and
invasive properties by decreased multiple metastasis-related genes,
including SOX4 (29). Based on
these observations, we first aimed to examine the levels of SOX4
expression in RCC cell lines and tissues by qRT-PCR and western
blot analysis. Of note, the levels of SOX4 mRNA and protein
expression were dramatically upregulated in RCC cell lines (786-O,
SN12-PM6, ACHN and A498) compared with the normal renal epithelial
cell line HK-2 (Fig. 1A and B).
Unexpectedly, no remarkable difference of SOX4 mRNA was observed
between ccRCC and normal tissues. However, RCC tissues exhibited
significantly higher SOX4 protein expression in 17 of 20 pairs RCC
compared with corresponding normal tissues by western blotting and
IHC (Fig. 1C and D). Moreover,
immunofluorescence revealed that SOX4 was overexpressed in RCC
cells compared with the normal renal cells (Fig. 1E). Collectively, these results
indicate SOX4 expression is up regulated in RCC.
The effects of SOX4 on migration and
invasion in 786-O and SN12-PM6 and HK-2 cell lines
To explore the roles of SOX4 upregulation in RCC
cell lines, we then performed a loss-of-function assay by using
small interfering RNA specifically targeting SOX4 (Fig. 2A and D). The effects of SOX4
knockdown on migration and invasion of these two cell lines were
examined using wound healing and Transwell chamber assays. The
results revealed that SOX4 knockdown markedly undermined the
migration and invasion of these two RCC cell lines (Fig. 2B and C, and E and F), however, it
did not influence cell proliferation and apoptosis (data not
shown). To further confirm the involvement of SOX4 in migration and
invasion, we then carried out a gain-of-function assay in HK-2 cell
line by constructing plasmid vector expressing SOX4 (Fig. 2G). The results indicated that SOX4
overexpression prominently promoted the migration and invasion of
HK2 cell line (Fig. 2H), but it
did not affect cell proliferation and apoptosis (data not shown).
In addition, downregulation or upregulation of SOX4 did not cause
significant changes in cell morphology. Therefore, our
loss-of-function and gain-of-function assays demonstrated that SOX4
might promote migration and invasion in RCC cell lines in
vitro.
EMT process exists in RCC tissues and
cell lines
Accumulating evidence demonstrates that EMT has been
implicated in cancer metastasis (2,30,31).
Therefore, in order to investigate the potential metastatic
mechanisms in RCC, we examined the EMT marker expression in RCC
cell lines and tissues by qRT-PCR and western blotting. As shown in
Fig. 3A and B, the RCC cell lines
exhibited a significant downregulation of E-cadherin compared with
the HK-2 cell line; the mesenchymal cell markers N-cadherin, Twist,
and Vimentin were markedly upregulated. Moreover, RCC tissues
exhibited a significant downregulation of E-cadherin compared with
the corresponding normal tissues; whereas, the mesenchymal markers
Vimentin, ZEB1, MMP-9, and Twist were prominently upregulated
(Fig. 3C and D). Data above
indicated that EMT process might have occurred in the formation and
progression of RCC.
The effects of SOX4 on EMT profile in
786-O and SN12-PM6 and HK-2 cell lines
Recent studies have identified SOX4 as a master
regulator of EMT (32). To explore
the potential correlation between SOX4 and EMT in RCC, we
successfully knocked down the expression of SOX4 in 786-O and
SN12-PM6 cell lines that overexpressed endogenous SOX4, by using
small interfering RNA, as confirmed by qRT-PCR and western blotting
(Fig. 4A and B). We then examined
by western blotting the expression of several EMT markers in the
786-O and SN12-PM6 cells that were subjected to SOX4 knockdown. The
results indicated that 786-O and SN12-PM6 cell lines that were
knocked down by SOX4 had a significant higher E-cadherin expression
compared with corresponding control cells; whereas, the mesenchymal
cell markers N-cadherin, Vimentin, and Twist expression were
prominently downregulated (Fig. 4A and
B). To further corroborate the correlation between SOX4 and
EMT, we then overexpressed SOX4 in HK-2 by using plasmid vector
expressing SOX4, as confirmed by qRT-PCR and western blotting
(Fig. 4C). Next, we examined the
EMT markers expression in HK-2 cells that overexpressed the SOX4 by
western blotting. As shown in Fig.
4C, the HK-2 of SOX4 overexpression exhibited a significant
lower E-cadherin expression compared with the corresponding control
cells; whereas, N-cadherin, Vimentin, and Twist expression were
significantly upregulated. These results suggested that SOX4 could
induce EMT in RCC cell lines, and SOX4 knockdown could partially
reverse EMT.
The effects of SOX4 knockdown and rescue
on EMT expression profile in the 786-O and SN12-PM6 and HK-2 cell
lines
To further substantiate the findings, we next
performed SOX4 knockdown and rescue tests in the 786-O and SN12-PM6
and HK-2 cell lines, respectively. As shown, in all the three cell
lines tested, downregulation of SOX4 upregulated epithelial marker
and downregulated mesenchymal markers in 786-O and SN12-PM6 and
HK-2 cell lines; while overexpression of SOX4 yielded opposite
effects (Fig. 5A–C). These results
further solidified our findings that SOX4 could induce the EMT
program.
SOX4 is an important component involving
in TGFβ-induced EMT
Multiple lines of evidence have confirmed the
involvement of TGFβ signaling in EMT program. To further
investigate whether SOX4 was involved in the process of TGFβ
signaling-induced EMT in HK-2 cells, we stimulated HK-2 cells by
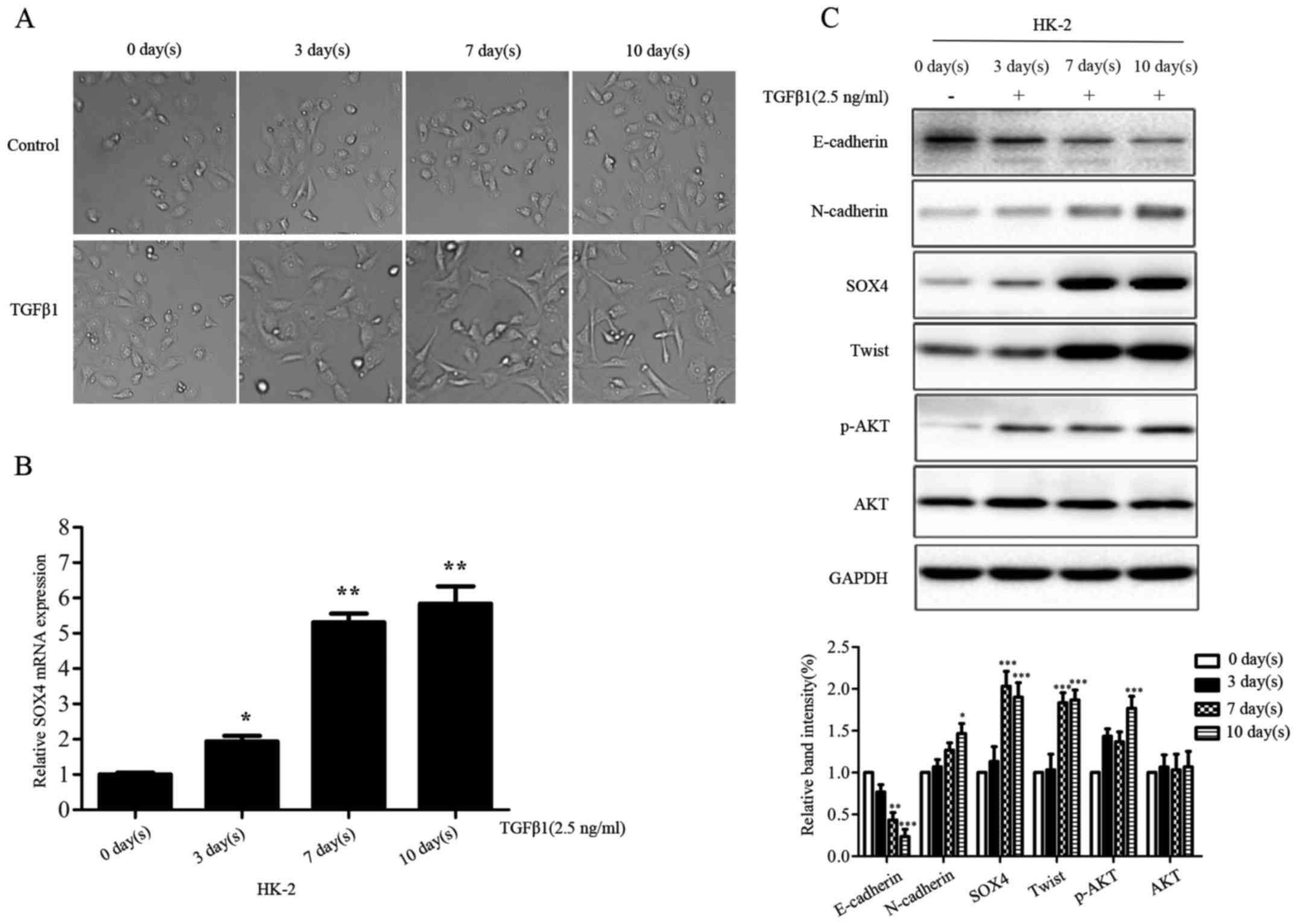
using 2.5 ng/ml concentration TGFβ1 for 0, 3, 7, 10 day(s).
Compared with the parental HK-2 cells, the TGFβ1-treated HK-2 cells
represented morphologic changes from cobblestone-like to
fibroblast-like appearances (Fig.
6A). SOX4 expression was upregulated in HK-2 cells at mRNA and
protein level, in a time-dependent manner following the addition of
TGFβ1 into the cell culture medium (Fig. 6B and C). Moreover, TGFβ1 modulated
the expression of EMT markers and p-AKT at protein level (Fig. 6C). These data indicated SOX4 might
be an important component involving TGFβ-induced EMT.
SOX4 induces EMT possibly via modulating
expression of p-AKT
AKT signaling cascade has been reported involved in
metastasis in a variety of cancers (33–35);
Previous studies also showed that AKT pathway could induce EMT
(36–39). To further explore the molecular
mechanisms how SOX4 induces EMT and metastasis in RCC, we expanded
our study to the metastasis-related protein p-AKT, which may be the
potential downstream target of SOX4. As shown in Fig. 7A, SOX4 knockdown in 786-O and
SN12-PM6 cell lines downregulated the expression of p-AKT, while
overexpression of SOX4 in HK-2 cell line upregulated the expression
of p-AKT (Fig. 7B). Furthermore,
downregulation of p-AKT was accompanied by the upregulation of
E-cadherin, while upregulation of p-AKT reversed this result. Based
on these results, we preliminarily established a pathway diagram
that SOX4 modulated EMT (Fig. 7C).
Collectively, these data indicated that SOX4 might contribute to
RCC metastasis via modulating the AKT/EMT signaling cascade.
Discussion
Accumulating evidence indicates that the SOX4
transcription factor is significantly overexpressed in various
human malignancies, including melanoma (40), leukemia (15), medulloblastoma (41), and caners of colon (19), prostate (17), breast (3), liver (18), and lung (16), indicating that it may play a
crucial role in cancer initiation and progression. Previous study
has reported that miR-335 suppresses breast cancer metastasis by
directly targeting SOX4 (3).
Moreover, SOX4 contributes to breast cancer progression through
inducing EMT (21). In endometrial
cancer, SOX4 knockdown results in attenuation of endometrial cells
growth; however, it has no effects on cell migration or invasion
(42). In oral squamous cell
carcinoma, SOX4 is involved in miR-204-modulated cancer stemness
and EMT and promotes lymph node metastasis (43). In addition, knockdown of SOX4
expression induces apoptosis in adenoid cystic carcinoma (44). Conversely, SOX4 may significantly
impair cell viability and promote cell apoptosis in the bladder
cell line HU609 overexpressing SOX4 (24). Moreover, SOX4 may induce cell cycle
arrest and promote cell apoptosis, and inhibit tumorigenesis in a
p53-dependent manner in HCC116 cells (45). These studies suggest that SOX4 may
play different roles in different malignancies. Different
physiological function of SOX4 in different malignancies may be
attributed to distinct tumor microenvironment and genetic
background. However, the role of SOX4 in RCC is still unclear.
In our studies, we demonstrated that SOX4 expression
was frequently upregulated in RCC tissues and cell lines,
consistent with many of previous reports in other cancer types. Our
results indicate that SOX4 may promote migration and invasion,
which is consistent with the roles of SOX4 in breast cancer and
oral squamous cell carcinoma. However, it has no effects on
proliferation, apoptosis and colony formation in RCC. To understand
the potential molecular mechanisms that SOX4 promoted RCC
metastasis, we examined the EMT marker expression in RCC tissues
and cell lines by qRT-PCR and western blotting. The results
suggested that epithelial cell marker E-cadherin was downregulated
at mRNA level and protein level in RCC tissues and cell lines;
whereas, the mesenchymal cell markers N-cadherin, Vimentin, and
Twist were upregulated, indicating that EMT process happened in RCC
progression.
Based on these results, SOX4 might promote migration
and invasion of RCC through inducing EMT. Previous studies have
confirmed that AKT signaling pathway could induce EMT through
downregulating E-cadherin (36,39).
SOX4 was found to be elevated in prostate cancer and implicated in
the activation of the PI3K/AKT pathway (46). Furthermore, the expression of SOX4
induced by PTEN loss could activate the PI3K-AKT-mTOR signaling in
prostate cancer (17). Therefore,
we investigated the expression of AKT and p-AKT in gain-of-function
and loss-of-function assays of SOX4. The results indicated that the
expression of p-AKT was positively correlated with SOX4 expression.
Thus, we speculated that SOX4 might modulate AKT/p-AKT expression
directly or indirectly. However, there is a limitation in our study
that the roles of SOX4 in vivo are not clear and what
regulates SOX4 expression in RCC remains unknown. Therefore, future
studies that explore the roles of SOX4 in vivo will further
demonstrate the function of SOX4 in RCC.
In conclusion, the current study indicates that SOX4
is overexpressed in RCC tissues and cell lines. Moreover, our
results demonstrate that SOX4 promotes migration and invasion of
RCC by inducing EMT through AKT signaling cascade, which provides
new insight into molecular mechanisms involvement in metastasis of
RCC. Therefore, our study suggests that the function suppression of
SOX4-AKT-EMT axis could be an attractive therapeutic intervention
in RCC metastasis.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (NSFC) (grant no. 81272560), the Open
Research Foundation of the State Key Laboratory of Virology of
Wuhan University (grant no. 2014KF007), Hubei Province Scientific
and Technical Project (grant no. 2011CDB366), and Hubei Provincial
Health Project (grant no. WJ2015MB020) to H.Y. This study was
supported by the National Natural Science Foundation of China
(grant no. 30872924, 81072095 and 81372760), the Program for New
Century Excellent Talents in University from the Department of
Education of China (NCET-08-0223), the National High Technology
Research and Development Program of China (863 Program)
(2012AA021101) to X.Z., and the Natural Science Foundation of
Zhejiang Province (grant no. LY15H160052) to X.C.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tavazoie SF, Alarcón C, Oskarsson T, Padua
D, Wang Q, Bos PD, Gerald WL and Massagué J: Endogenous human
microRNAs that suppress breast cancer metastasis. Nature.
451:147–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pérez-Pomares JM and Muñoz-Chápuli R:
Epithelial-mesenchymal transitions: A mesodermal cell strategy for
evolutive innovation in Metazoans. Anat Rec. 268:343–351. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
She ZY and Yang WX: SOX family
transcription factors involved in diverse cellular events during
development. Eur J Cell Biol. 94:547–563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong CS and Saint-Jeannet JP: Sox proteins
and neural crest development. Semin Cell Dev Biol. 16:694–703.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Potzner MR, Tsarovina K, Binder E,
Penzo-Méndez A, Lefebvre V, Rohrer H, Wegner M and Sock E:
Sequential requirement of Sox4 and Sox11 during development of the
sympathetic nervous system. Development. 137:775–784. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paul MH, Harvey RP, Wegner M and Sock E:
Cardiac outflow tract development relies on the complex function of
Sox4 and Sox11 in multiple cell types. Cell Mol Life Sci.
71:2931–2945. 2014. View Article : Google Scholar
|
|
13
|
Sun B, Mallampati S, Gong Y, Wang D,
Lefebvre V and Sun X: Sox4 is required for the survival of pro-B
cells. J Immunol. 190:2080–2089. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu G and Chen J: A genome-wide regulatory
network identifies key transcription factors for memory
CD8+ T-cell development. Nat Commun. 4:28302013.
|
|
15
|
Zhang H, Alberich-Jorda M, Amabile G, Yang
H, Staber PB, Di Ruscio A, Welner RS, Ebralidze A, Zhang J,
Levantini E, et al: Sox4 is a key oncogenic target in C/EBPα mutant
acute myeloid leukemia. Cancer Cell. 24:575–588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Medina PP, Castillo SD, Blanco S,
Sanz-Garcia M, Largo C, Alvarez S, Yokota J, Gonzalez-Neira A,
Benitez J, Clevers HC, et al: The SRY-HMG box gene, SOX4, is a
target of gene amplification at chromosome 6p in lung cancer. Hum
Mol Genet. 18:1343–1352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bilir B, Osunkoya AO, Wiles WG IV,
Sannigrahi S, Lefebvre V, Metzger D, Spyropoulos DD, Martin WD and
Moreno CS: SOX4 is essential for prostate tumorigenesis initiated
by PTEN ablation. Cancer Res. 76:1112–1121. 2016. View Article : Google Scholar :
|
|
18
|
Liao YL, Sun YM, Chau GY, Chau YP, Lai TC,
Wang JL, Horng JT, Hsiao M and Tsou AP: Identification of SOX4
target genes using phylogenetic footprinting-based prediction from
expression microarrays suggests that overexpression of SOX4
potentiates metastasis in hepatocellular carcinoma. Oncogene.
27:5578–5589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sinner D, Kordich JJ, Spence JR, Opoka R,
Rankin S, Lin SC, Jonatan D, Zorn AM and Wells JM: Sox17 and Sox4
differentially regulate beta-catenin/T-cell factor activity and
proliferation of colon carcinoma cells. Mol Cell Biol.
27:7802–7815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yeh YM, Chuang CM, Chao KC and Wang LH:
MicroRNA-138 suppresses ovarian cancer cell invasion and metastasis
by targeting SOX4 and HIF-1α. Int J Cancer. 133:867–878. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Liang Q, Lei Y, Yao M, Li L, Gao
X, Feng J, Zhang Y, Gao H, Liu DX, et al: SOX4 induces
epithelial-mesenchymal transition and contributes to breast cancer
progression. Cancer Res. 72:4597–4608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, Li Y, Yang X, Yuan H, Li X, Qi M,
Chang YW, Wang C, Fu W, Yang M, et al: ERG-SOX4 interaction
promotes epithelial-mesenchymal transition in prostate cancer
cells. Prostate. 74:647–658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang C, Zhao H, Lu J, Yin J, Zang L, Song
N, Dong R, Wu T and Du X: Clinicopathological significance of SOX4
expression in primary gallbladder carcinoma. Diagn Pathol.
7:412012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aaboe M, Birkenkamp-Demtroder K, Wiuf C,
Sørensen FB, Thykjaer T, Sauter G, Jensen KM, Dyrskjøt L and
Ørntoft T: SOX4 expression in bladder carcinoma: Clinical aspects
and in vitro functional characterization. Cancer Res. 66:3434–3442.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Jiang H, Shao J, Mao R, Liu J, Ma
Y, Fang X, Zhao N, Zheng S and Lin B: SOX4 inhibits GBM cell growth
and induces G0/G1 cell cycle arrest through Akt-p53 axis. BMC
Neurol. 14:2072014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang T, Song W, Chen Y, Chen R, Liu Z, Wu
L, Li M, Yang J, Wang L2, Liu J, et al: Flightless I homolog
represses prostate cancer progression through targeting androgen
receptor signaling. Clin Cancer Res. 22:1531–1544. 2016. View Article : Google Scholar
|
|
28
|
Xu S, Tao Z, Hai B, Liang H, Shi Y, Wang
T, Song W, Chen Y, OuYang J, Chen J, et al: miR-424(322) reverses
chemoresistance via T-cell immune response activation by blocking
the PD-L1 immune checkpoint. Nat Commun. 7:114062016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X, Ruan A, Wang X, Han W, Wang R, Lou
N, Ruan H, Qiu B, Yang H and Zhang X: miR-129–3p, as a diagnostic
and prognostic biomarker for renal cell carcinoma, attenuates cell
migration and invasion via downregulating multiple
metastasis-related genes. J Cancer Res Clin Oncol. 140:1295–1304.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tiwari N, Tiwari VK, Waldmeier L, Balwierz
PJ, Arnold P, Pachkov M, Meyer-Schaller N, Schübeler D, van
Nimwegen E and Christofori G: Sox4 is a master regulator of
epithelial-mesenchymal transition by controlling Ezh2 expression
and epigenetic reprogramming. Cancer Cell. 23:768–783. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho JH, Robinson JP, Arave RA, Burnett WJ,
Kircher DA, Chen G, Davies MA, Grossmann AH, VanBrocklin MW,
McMahon M, et al: AKT1 activation promotes development of melanoma
metastases. Cell Rep. 13:898–905. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuo KT, Chen CL, Chou TY, Yeh CT, Lee WH
and Wang LS: Nm23H1 mediates tumor invasion in esophageal squamous
cell carcinoma by regulation of CLDN1 through the AKT signaling.
Oncogenesis. 5:e2392016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Niessner H, Forschner A, Klumpp B,
Honegger JB, Witte M, Bornemann A, Dummer R, Adam A, Bauer J,
Tabatabai G, et al: Targeting hyperactivation of the AKT survival
pathway to overcome therapy resistance of melanoma brain
metastases. Cancer Med. 2:76–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang PF, Li KS, Shen YH, Gao PT, Dong ZR,
Cai JB, Zhang C, Huang XY, Tian MX, Hu ZQ, et al: Galectin-1
induces hepatocellular carcinoma EMT and sorafenib resistance by
activating FAK/PI3K/AKT signaling. Cell Death Dis. 7:e22012016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Julien S, Puig I, Caretti E, Bonaventure
J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A
and Larue L: Activation of NF-kappaB by Akt upregulates Snail
expression and induces epithelium mesenchyme transition. Oncogene.
26:7445–7456. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grille SJ, Bellacosa A, Upson J,
Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN and
Larue L: The protein kinase Akt induces epithelial mesenchymal
transition and promotes enhanced motility and invasiveness of
squamous cell carcinoma lines. Cancer Res. 63:2172–2178.
2003.PubMed/NCBI
|
|
40
|
Talantov D, Mazumder A, Yu JX, Briggs T,
Jiang Y, Backus J, Atkins D and Wang Y: Novel genes associated with
malignant melanoma but not benign melanocytic lesions. Clin Cancer
Res. 11:7234–7242. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Bont JM, Kros JM, Passier MM,
Reddingius RE, Sillevis Smitt PA, Luider TM, den Boer ML and
Pieters R: Differential expression and prognostic significance of
SOX genes in pediatric medulloblastoma and ependymoma identified by
microarray analysis. Neuro Oncol. 10:648–660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang YW, Liu JC, Deatherage DE, Luo J,
Mutch DG, Goodfellow PJ, Miller DS and Huang TH: Epigenetic
repression of microRNA-129–2 leads to overexpression of SOX4
oncogene in endometrial cancer. Cancer Res. 69:9038–9046. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu CC, Chen PN, Peng CY, Yu CH and Chou
MY: Suppression of miR-204 enables oral squamous cell carcinomas to
promote cancer stemness, EMT traits, and lymph node metastasis.
Oncotarget. 7:20180–20192. 2016.PubMed/NCBI
|
|
44
|
Pramoonjago P, Baras AS and Moskaluk CA:
Knockdown of Sox4 expression by RNAi induces apoptosis in ACC3
cells. Oncogene. 25:5626–5639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pan X, Zhao J, Zhang WN, Li HY, Mu R, Zhou
T, Zhang HY, Gong WL, Yu M, Man JH, et al: Induction of SOX4 by DNA
damage is critical for p53 stabilization and function. Proc Natl
Acad Sci USA. 106:3788–3793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Moreno CS: The Sex-determining region
Y-box 4 and homeobox C6 transcriptional networks in prostate cancer
progression: Crosstalk with the Wnt, Notch, and PI3K pathways. Am J
Pathol. 176:518–527. 2010. View Article : Google Scholar :
|