Introduction
Imaging plays a pivotal role in the staging and
management of breast cancer patients. For instance, breast MRI is
used in stage I disease to rule out additional sites of malignancy
that might be occult at mammography. Other imaging techniques, such
as bone scans, abdominal CT or MR and chest CT are recommended in
stage I-IIB disease in patients with abnormal liver function tests,
alkaline phosphatase, bone pain, abnormal physical examination,
localized bone pain, or with abdominal or pulmonary symptoms and in
stage IIIA disease. PET/CT is considered optional for stage IIIA,
stage IV and recurrent disease (1).
Growing evidence suggests that PET/CT may detect
distant metastases (sensitivity of 78–100%) over conventional
non-metabolic imaging modalities (sensitivity of 37–78.6%)
(2). Specifically, a recent
meta-analysis showed that the detection of distant metastases
increases from 1.2 to 3.3–34.3% if PET/CT is added to conventional
imaging for the staging of patients with stage II breast cancer
(2).
The use of diffusion-weighted imaging (DWI) MRI has
recently increased as a potential alternative to PET/CT for
whole-body staging, prognosis and treatment response assessment of
several malignancies, including malignancies of the breast. Some
studies suggest comparable performance of DWI to PET in disease
staging with the added advantages of lack of radiation, widespread
availability and no additional costs over those related to
operation of a normal MR scanner (3,4).
Other studies have found similar sensitivity of DWI to metabolic
imaging at the expense of reduced accuracy (4–8).
Therefore in this study we also explored, as described below, the
performance of DWI standing alone.
In recent years, PET/MRI has emerged as a new tool
with significant clinical potential for the evaluation and
management of cancer patients (9–12).
PET/MRI allows for improved diagnosis and staging accuracy in a
number of primary and metastatic cancers, including lymphoma, head
and neck, liver and bone tumors (13–19).
Moreover, data suggest that PET/MRI might play a superior role in
affecting oncologic management decisions, compared to PET/CT
(20,21).
Available data supporting the use of PET/MRI in the
evaluation of breast cancer patients remain limited but has been
promising (22–25). While primary lesion detection has
been previously shown to be equivalent between PET/CT and PET/MRI,
PET/MRI might improve detection of metastatic lesions, when
compared to PET/CT and might lead to management changes in up to
one third of the patients, when compared to initial clinical
staging (23,26). Other studies have also shown a role
of combining PET and MRI in characterizing tumor pathology and
predicting response to therapy (27–30).
The aim of the present study was to compare the
staging performance of whole body diffusion-weighted imaging
(WB-DWI), whole body positron emission tomography with computed
tomography (WB-PET/CT), and whole body positron emission tomography
with magnetic resonance imaging (WB-PET/MRI) in staging breast
cancer patients with newly diagnosed invasive ductal carcinoma. To
the best of our knowledge, this is the first study concurrently
assessing the performance of these three modalities in the same
patient population.
Materials and methods
Study design and patient enrollment
A retrospective HIPAA-compliant study was approved
by the institutional review board. Informed consent, that included
the possibility of subsequent usage of imaging and clinical data
for imaging research purposes, was obtained from patients before
undergoing same day PET/MRI and PET/CT. Inclusion criteria
consisted of: i) new, untreated biopsy-proven invasive ductal
carcinoma of the breast; ii) female; iii) 18 years of age or older;
iv) clinical contrast enhanced (CE) PET/CT study; v) same day
CE-PET/MRI study; and vi) availability of pathology or at least
two-years imaging follow-up. Patients were excluded if they met any
of the following criteria: i) pregnancy; ii) blood glucose >140
mg/dl; iii) contraindication to MR imaging; and iv) inclusion in
previous PET/MRI studies.
Imaging protocols
PET/CT imaging
PET/CT images were acquired ~1 h following FDG
administration, mean FDG activity of 4.44 MBq/kg of body weight.
Whole body images were acquired using a 64-detector row PET/CT
scanner (Gemini TF; Philips Medical Systems, Best, The Netherlands)
with time-of-flight capability. Automatic attenuation correction
was performed using attenuation correction maps generated from CT
imaging. Both non-contrast and contrast-enhanced CT images were
collected. Iopamidol (Iopamiro 370; Bracco Imaging, Milan, Italy)
was injected intravenously using a power injector at a rate of 2
ml/sec with a dose of 80 ml in patients weighing <80 kg; and a
dose of 100 ml in patients weighing >80 kg. Bolus care function
was used to acquire diagnostic quality arterial phase images of the
upper abdomen, portal venous phase images of the whole body and
delayed phase of the abdomen and pelvis.
PET/MRI imaging
PET/MRI images were acquired using a Biograph mMR
imager (Siemens Healthcare, Erlangen, Germany) with a 16-channel
head and neck surface coil and three or four 12-channel body coils,
depending on the patient's height. The coils were combined to form
a whole-body coil using total imaging matrix technology. PET/MRI
images were collected ~1.5 h following FDG injection. The following
MRI sequences were obtained concurrently with PET: axial DWI
(b-values 50, 400 and 800 s/mm2), coronal short tau
inversion recovery (STIR), coronal T1-weighted Dixon, axial T2
weighted half Fourier acquired single-shot turbo spin echo (HASTE).
Contrast enhanced-axial and coronal T1-weighted fat saturated
(VIBE, volume interpolated breath-hold examination) images were
acquired after PET completion. PET attenuation correction was
performed using the two-point Dixon sequence. For contrast-enhanced
MR imaging, 0.1 mmol/kg of gadopentetate dimeglumine (Magnevist;
Bayer Schering Pharma, Berlin, Germany) was injected at a rate of 3
ml/sec followed by the same volume of saline at the same rate,
using a power injector. The total time of PET/MRI imaging was ~1
h.
Image post-processing
PET/CT image post-processing was performed using a
dedicated workstation (Extended Brilliance Workstation Philips).
Post-processing of PET/MRI images was done using a Syngovia
workstation (Siemens Healthcare). Image post-processing consisted
of image co-registration and fusion. Images were archived using the
IDS7 image archiving and communication system (Sectra, Linkoping,
Sweden).
Image interpretation
WB-PET/CT, WB-DWI standing alone, WB-PET/MRI that
also included DWI and ADC maps, were randomly presented and
evaluated separately, at least 6 weeks apart, in consensus by a
radiologist (OAC) with 17 years of experience in MR and 5 years in
nuclear medicine and a nuclear medicine physician (AS) with 33
years of experience in nuclear medicine and 20 years in MR.
Specifically, they searched for the occurrence, number and location
of metastatic lesions and recorded for each patient the disease
stage, based on each modality (WB-PET/CT, WB-DWI and WB-PET/MRI)
according to the TNM staging (31). A combination of biopsy, surgical
pathology and 24-month follow-up data were used to define the
ground truth pathologic disease stage for each patient. Readers
were blinded to the final clinical/pathologic stage. Studies for an
individual patient were considered to be concordant if the stage
derived from all three imaging modalities were in agreement,
otherwise it was considered discordant. A modality stage was
defined correct if in agreement with the clinical/pathological
stage, otherwise it was considered incorrect.
Standard of reference
Pathology served as primary standard of reference.
In the case of non-availability of pathology, imaging follow-up,
lasting at least two years, served as secondary standard of
reference.
Statistical analysis
The three methods (WB-PET/CT, WB-DWI and WB-PETMR)
were compared pairwise using the McNemar's test, with Bonferroni
adjustment for multiple comparisons.
Results
Patient demographics
A total of 191 patients with non-treated ductal
invasive breast cancer underwent same-day PET/CT and PET/MRI
imaging between February 2012 and December 2015. One hundred and
forty patients were excluded for the following reasons: 63 for
having been included in previous PET/MRI studies with possibility
of patient recall by the readers and 77 for absence of follow-up or
pathology confirmation. Therefore, the final population consisted
of 51 patients. The average age of study participants was 53 years
with a standard deviation of 14 years (age range, 20–71 years).
Final disease stage was IIA in 8 patients, IIB in 12, IIIA in 4,
IIIC in 7 and stage IV in 20 patients.
Staging by standard of reference
Pathology served as standard of reference for 42
patients: 31 patients with stages II and III, 6 patients with
oligometastatic stage IV, 5 patients with polimetastatic stage
IV.
Follow-up imaging lasting ≥24 months served as
standard of reference for 9 patients with polimetastatic stage
IV.
Final staging was stage IIA 1 patient; stage IIB 12
patients; stage IIC 7 patients; stage IIIA 4 patients; stage IIIC 7
patients; and stage IV 20 patients.
Classification concordance
Thirty-three patients (65%) were correctly and
concordantly staged by WB-PET/CT, WB-DWI and WB-PET/MRI: 7 patients
with stage IIA, 8 patients with stage IIB, 4 patients with stage
IIIC and 14 patients with stage IV disease (Table I and Fig. 1).
 | Table IClassification concordance. |
Table I
Classification concordance.
| Standard of
reference | Concordant staging
(34/51 patients) | Discordant staging
(17/51 patients) |
|---|
| Stage IIA | – | 1 (2%) |
| Stage IIB | 8 (16%) | 4 (8%) |
| Stage IIC | 7 (14%) | – |
| Stage IIIA | – | 4 (8%) |
| Stage IIIC | 4 (8%) | 3 (6%) |
| Stage IV | 14 (27%) | 6 (12%) |
One stage IIIA patient was incorrectly and
concordantly miss-staged as IV by all modalities (Table II). Discordant staging was
reported in 17 patients (33%): 1 patient with stage IIA, 4 patients
with stage IIB, 4 patients with stage IIIA, 3 patients with stage
IIIC and 6 patients with stage IV disease (Tables I–II and Fig.
2).
 | Table IIStaging misclassification by imaging
modality. |
Table II
Staging misclassification by imaging
modality.
| Standard of
reference | WB-DWI | Reason for
discrepancy | WB-PET/CT | Reason for
discrepancy | WB-PET/MR | Reason for
discrepancy | Combined
modalities |
|---|
| Stage IIA | – | | 1 (stage IV) | Sclerosed hepatic
hemangioma miss-interpreted as metastasis (1 pt) | – | | 1 |
| Stage IIB | 1 pt (stage
IV) | Cartilaginous
island miss-interpreted as metastasis (1 pt) | 3 pts (stage
IIA) | Lack of detection
of level I/II lymphadenopathy (3 pts) | – | | 4 |
| Stage IIIA | 2 pts (stage
IV) | Benign red bone
marrow miss-interpreted as metastases (2 pt) | 3 pts (stage IIB,
IV) | Benign red bone
marrow miss-interpreted as metastasis (1 pt) Lack of identification
of internal mammary lymphadenopathy (2 pt) | 1 pt (stage
IV) | Benign red bone
marrow miss-interpreted as metastasis (1pt) | 4 |
| Stage IIIC | – | | 3 pts (stage IIA,
IV) | Lack of detection
of infraclavicular lymphadenopathy (2 pts). Non-regional lymph
nodes miss-interpreted as malignant (1 pt) | – | | 3 |
| Stage IV | 5 pts (stages IIB,
IIIA, IIIC) | Lack of detection
of meta-stases in the liver (2 pts), lung (2 pts) and peritoneum (1
pt) | 3 pts (stage IIB,
IIIA) | Lack of detection
of metastases in the liver (2 pts), bones (1 pt) | | | 6 |
| Total
misclassified | 8 pts WB-DWI | | 13 pts
WB-PET/CT | | 1 pt WB-PET/MR | | 18 pts |
Staging misclassification by imaging
modality
To assess the performance of each imaging modality
in staging newly diagnosed breast cancer, the occurrence of staging
misclassification was determined for each examination: WB-DWI
miss-staged 8 patients, WB-PET/CT miss-staged 13 patients and
WB-PET/MRI miss-staged 1 patient.
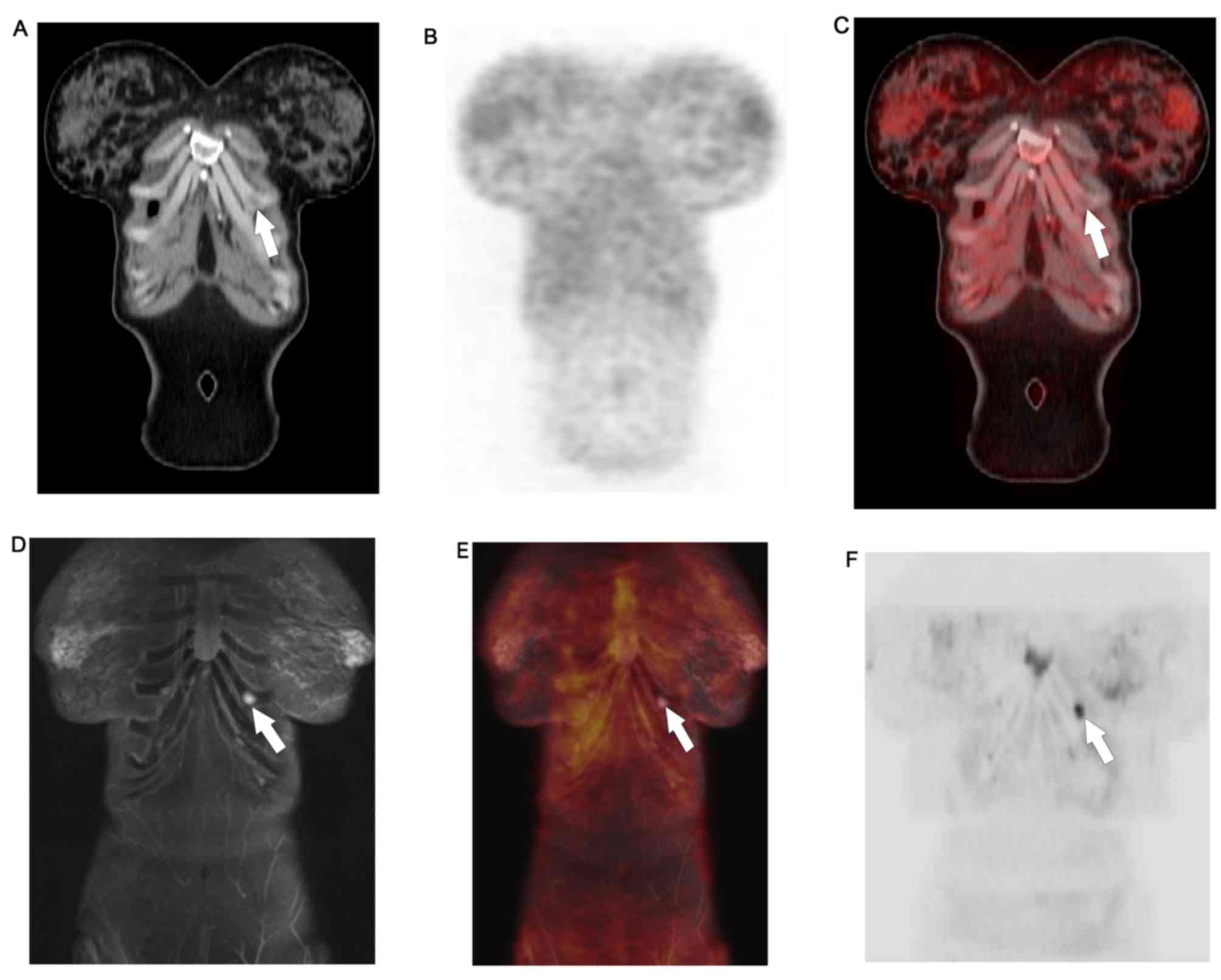
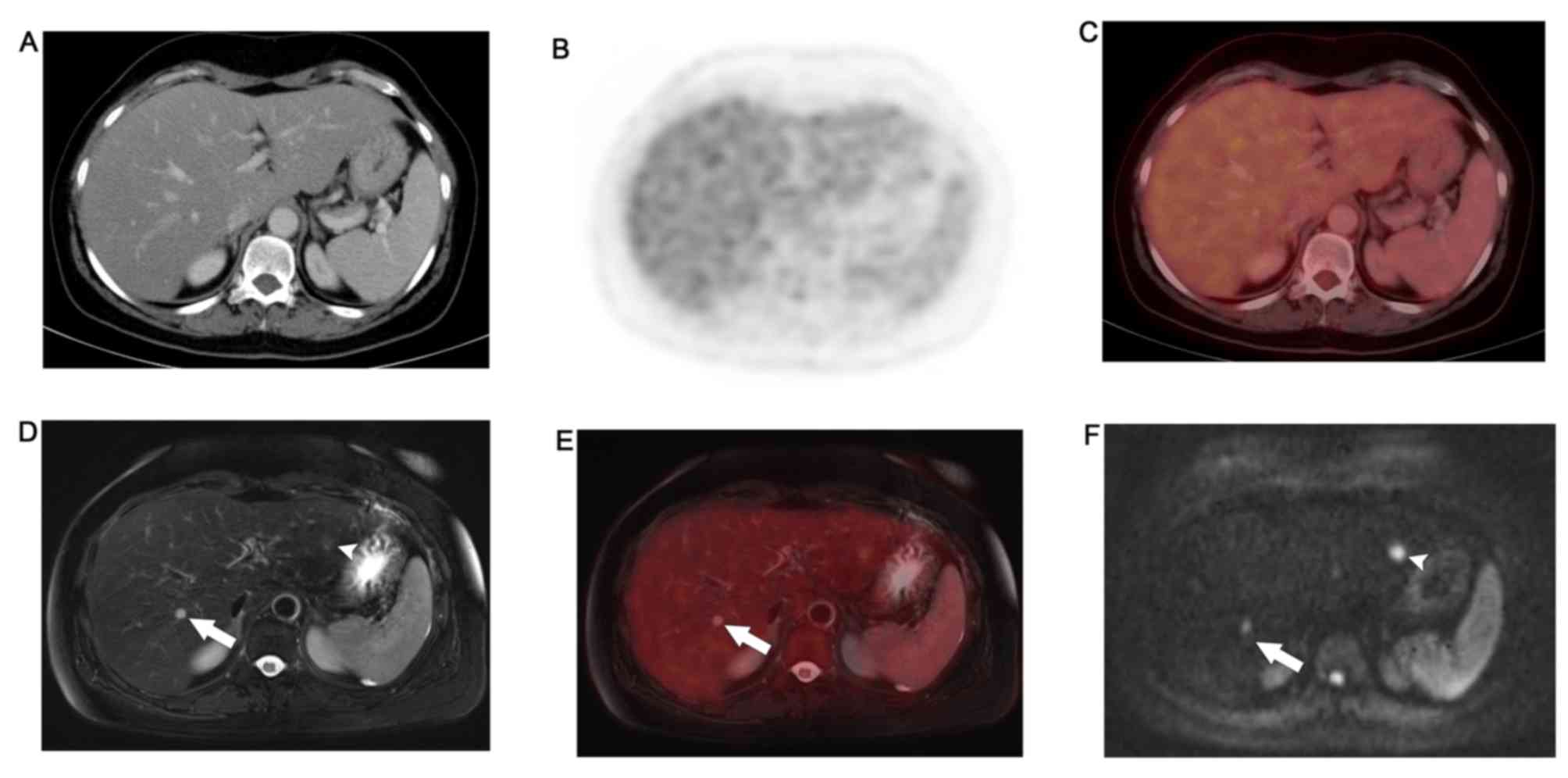
WB-PET/MR detected FDG avid lung metastases and left
liver lobe metastases that were not appreciated on WB-DWI.
Moreover, it ascertained the benign nature of lesions that, due to
T2 shine-trough, retained high signal on high b-values DWI.
WB-PET/MRI identified non-FDG avid permeative bony
metastases and sub-centimeter hepatic metastases that were not
appreciated on WB-PET/CT. Details are provided in Table III.
 | Table IIIStaging performance of the assessed
imaging modalities. |
Table III
Staging performance of the assessed
imaging modalities.
| Patients correctly
staged (n=51) | Accuracy |
|---|
| WB-PET/CT | 38 | 75% |
| WB-DWI | 43 | 84% |
| WB-PET/MRI | 50 | 98% |
Staging performance of WB-PET/CT, WB-DWI
and WB-PET/MRI
The staging accuracy of WB-PET/CT was 75%, of WB-DWI
was 84% and of WB-PET/MRI was 98% (Table III). WB-PET/MRI vs. WB-PET/CT
differ significantly in their agreement with the true stage, with
adjusted P-value of 0.005. On the other hand, the differences
between WB-PET/MR and WB-DWI (P=0.14) and between WB-PET/CT and
WB-DWI (P=0.27) were not statistically significant.
Discussion
In the present study, we assessed the performance of
whole body diffusion-weighted imaging (WB-DWI), whole body positron
emission tomography with computed tomography (WB-PET/CT), and whole
body positron emission tomography with magnetic resonance imaging
(WB-PET/MRI) in patients with untreated invasive ductal breast
cancer. All three modalities correctly staged the cancer in 64% of
patients. In the patients with discordant staging among the imaging
modalities, our results show superior staging accuracy of
WB-PET/MRI in staging metastatic disease, when compared to the
other modalities being assessed.
In particular, when compared to WB-DWI alone, PET/MR
performed better both in detecting FDG avid lung metastases and
left liver lobe metastases, and in ruling out malignancy in the
case of T2 shine-trough retention of signal in some benign lesions.
This was the result of the combined information from FGD uptake and
the entire setting of MR sequences of our protocol. However, these
differences were not statistically significant.
Moreover, since FDG-PET is the same in both
WB-PET/CT and WB-PET/MRI, the improved staging performance of
WB-PET/MR is likely due to the higher sensitivity of MRI in
detecting non-FDG avid lesions, as we found in the case of
permeative bony and sub-centimeter hepatic metastases.
The role of PET/MRI in breast cancer staging is
under investigation. A study examining the performance of PET/MRI
in 36 patients with breast cancer reported correlation of
standardized uptake values as measured on PET/MRI and PET/CT in
primary and metastatic cancerous lesions (22). Another study in 36 patients with
breast cancer showed superior performance of PET/MRI in detecting
metastatic lesions, when compared to PET (23). Furthermore, PET/MRI changed
management decisions in one third of the patients being studied,
when compared to the initial clinical staging (23). Our results add to the existing body
of literature by providing new evidence that PET/MRI outperforms
PET/CT and DWI in staging patients with breast cancer.
PET/MRI has been shown to add complementary
metabolic information to prostate and gynecologic MR imaging,
improving the diagnosis and management of prostate and gynecologic
cancer patients (32–37). Similarly, PET/MRI accurately staged
28 patients with lymphoma, when compared to PET/CT (15). Other studies have also shown
superior performance of PET/MRI in diagnosing primary head and
neck, bone and soft tissue lesions and for detecting metastatic
disease in the brain, liver and bone (15–18,26).
A recent study provided evidence that PET/MRI contributes to the
clinical management of cancer patients more often than PET/CT
(20).
Early and appropriate staging of breast cancer is
especially important in the management of patients with this
disease. Available data suggest longer survival and improved
quality of life with early detection of metastatic disease
(38–40). Our data suggest that PET/MRI is
well-positioned to aid in the staging of breast cancer patients at
the time of their initial diagnosis. PET/MRI performed particularly
well in accurately staging advanced disease, where a higher
proportion of discordant staging was reported by other modalities
in this study (stage IIIA and higher). PET/MRI might have the
potential to play a critical role in affecting treatment decisions
and management in this patient population.
The present study has several limitations, including
the small number of patients and the potential selection bias
introduced by enrolling only untreated ductal invasive breast
cancers. These results might not be applicable to other breast
cancer subtypes or to treated patients. A larger study would be
needed to validate our findings. In this study, the availability of
multiple MR sequences combined with PET helped compensate for the
limitations intrinsic to stand-alone sequences. For example, DWI
helped in detecting liver lesions, and PET was useful in assessing
sub-centimeter metastases in the liver and in lymph nodes.
An additional limitation might have been related to
the delta time with PET/CT, acquired ~60 min after FDG injection
and PET/MRI, acquired ~90 min after FDG injection. Our longer
incubation time for PET/MR is explained by the legal and IRB
requirements that mandated us to acquire PET/MR after a standard of
care PET-CT obtained at 60 min after FDG injection, before being
allowed to acquire any PET-MR study. Although delayed PET
acquisitions might demonstrate lower background activity and
improved lesion visibility, there is no consensus if this
translates into improved accuracy (41–43).
However, it is unlikely that this might have influenced the FDG
uptake obtained by PET/MR. The PET/MR reconstruction software
automatically corrects for incubation time for each bed position.
Moreover, several studies have demonstrated comparable performance
between PET/CT and subsequently acquired PET/MR as quantified by
SUV measurements (22,44,45).
Finally, in the present study, the guidelines of the
European Association of Nuclear Medicine (46) were used to dictate image
acquisition protocols based on local clinical standards.
In conclusion, PET/MRI outperforms PET/CT and is
more accurate in staging untreated patients with invasive ductal
carcinoma. PET/MRI has the potential to affect clinical decision
making and management of breast cancer patients, and should be
considered in the initial staging of this disease.
References
|
1
|
(NCCN) NCCN: Clinical Practice Guidelines
in Oncology. 2016, accessed November 29, 2016.
|
|
2
|
Brennan ME and Houssami N: Evaluation of
the evidence on staging imaging for detection of asymptomatic
distant metastases in newly diagnosed breast cancer. Breast.
21:112–123. 2012. View Article : Google Scholar
|
|
3
|
Albano D, Patti C, La Grutta L, Agnello F,
Grassedonio E, Mulè A, Cannizzaro G, Ficola U, Lagalla R, Midiri M,
et al: Comparison between whole-body MRI with diffusion-weighted
imaging and PET/CT in staging newly diagnosed FDG-avid lymphomas.
Eur J Radiol. 85:313–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jambor I, Kuisma A, Ramadan S, Huovinen R,
Sandell M, Kajander S, Kemppainen J, Kauppila E, Auren J, Merisaari
H, et al: Prospective evaluation of planar bone scintigraphy,
SPECT, SPECT/CT, 18F-NaF PET/CT and whole body 1.5T MRI,
including DWI, for the detection of bone metastases in high risk
breast and prostate cancer patients: SKELETA clinical trial. Acta
Oncol. 55:59–67. 2016. View Article : Google Scholar
|
|
5
|
Albano D, Patti C, Lagalla R, Midiri M and
Galia M: Whole-body MRI, FDG-PET/CT, and bone marrow biopsy, for
the assessment of bone marrow involvement in patients with newly
diagnosed lymphoma. Journal of magnetic resonance imaging. J Magn
Reson Imaging. 45:1082–1089. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Littooij AS, Kwee TC, Barber I, Granata C,
Vermoolen MA, Enríquez G, Zsíros J, Soh SY, de Keizer B, Beek FJ,
et al: Whole-body MRI for initial staging of paediatric lymphoma:
Prospective comparison to an FDG-PET/CT-based reference standard.
Eur Radiol. 24:1153–1165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park SH, Moon WK, Cho N, Chang JM, Im SA,
Park IA, Kang KW, Han W and Noh DY: Comparison of
diffusion-weighted MR imaging and FDG PET/CT to predict
pathological complete response to neoadjuvant chemotherapy in
patients with breast cancer. Eur Radiol. 22:18–25. 2012. View Article : Google Scholar
|
|
8
|
Heusner TA, Kuemmel S, Koeninger A, Hamami
ME, Hahn S, Quinsten A, Bockisch A, Forsting M, Lauenstein T,
Antoch G, et al: Diagnostic value of diffusion-weighted magnetic
resonance imaging (DWI) compared to FDG PET/CT for whole-body
breast cancer staging. Eur J Nucl Med Mol Imaging. 37:1077–1086.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wehner J, Weissler B, Dueppenbecker PM,
Gebhardt P, Goldschmidt B, Schug D, Kiessling F and Schulz V:
MR-compatibility assessment of the first preclinical PET-MRI insert
equipped with digital silicon photomultipliers. Phys Med Biol.
60:2231–2255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Delso G, Fürst S, Jakoby B, Ladebeck R,
Ganter C, Nekolla SG, Schwaiger M and Ziegler SI: Performance
measurements of the Siemens mMR integrated whole-body PET/MR
scanner. J Nucl Med. 52:1914–1922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Delso G and Ziegler S: PET/MRI system
design. Eur J Nucl Med Mol Imaging. 36(Suppl 1): S86–S92. 2009.
View Article : Google Scholar
|
|
12
|
Yoon HS, Ko GB, Kwon SI, Lee CM, Ito M,
Chan Song I, Lee DS, Hong SJ and Lee JS: Initial results of
simultaneous PET/MRI experiments with an MRI-compatible silicon
photomultiplier PET scanner. J Nucl Med. 53:608–614. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boss A, Bisdas S, Kolb A, Hofmann M,
Ernemann U, Claussen CD, Pfannenberg C, Pichler BJ, Reimold M and
Stegger L: Hybrid PET/MRI of intracranial masses: Initial
experiences and comparison to PET/CT. J Nucl Med. 51:1198–1205.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boss A, Stegger L, Bisdas S, Kolb A,
Schwenzer N, Pfister M, Claussen CD, Pichler BJ and Pfannenberg C:
Feasibility of simultaneous PET/MR imaging in the head and upper
neck area. Eur Radiol. 21:1439–1446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heacock L, Weissbrot J, Raad R, Campbell
N, Friedman KP, Ponzo F and Chandarana H: PET/MRI for the
evaluation of patients with lymphoma: Initial observations. AJR Am
J Roentgenol. 204:842–848. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Drzezga A, Souvatzoglou M, Eiber M, Beer
AJ, Fürst S, Martinez-Möller A, Nekolla SG, Ziegler S, Ganter C,
Rummeny EJ, et al: First clinical experience with integrated
whole-body PET/MR: Comparison to PET/CT in patients with oncologic
diagnoses. J Nucl Med. 53:845–855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Buchbender C, Heusner TA, Lauenstein TC,
Bockisch A and Antoch G: Oncologic PET/MRI, part 2: Bone tumors,
soft-tissue tumors, melanoma, and lymphoma. J Nucl Med.
53:1244–1252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buchbender C, Heusner TA, Lauenstein TC,
Bockisch A and Antoch G: Oncologic PET/MRI, part 1: Tumors of the
brain, head and neck, chest, abdomen, and pelvis. J Nucl Med.
53:928–938. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huellner MW, Appenzeller P, Kuhn FP,
Husmann L, Pietsch CM, Burger IA, Porto M, Delso G, von Schulthess
GK and Veit-Haibach P: Whole-body nonenhanced PET/MR versus PET/CT
in the staging and restaging of cancers: Preliminary observations.
Radiology. 273:859–869. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Catalano OA, Rosen BR, Sahani DV, Hahn PF,
Guimaraes AR, Vangel MG, Nicolai E, Soricelli A and Salvatore M:
Clinical impact of PET/MR imaging in patients with cancer
undergoing same-day PET/CT: Initial experience in 134 patients - a
hypothesis-generating exploratory study. Radiology. 269:857–869.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee SI, Catalano OA and Dehdashti F:
Evaluation of gyneco-logic cancer with MR imaging,
18F-FDG PET/CT, and PET/MR imaging. J Nucl Med.
56:436–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pace L, Nicolai E, Luongo A, Aiello M,
Catalano OA, Soricelli A and Salvatore M: Comparison of whole-body
PET/CT and PET/MRI in breast cancer patients: Lesion detection and
quanti-tation of 18F-deoxyglucose uptake in lesions and
in normal organ tissues. Eur J Radiol. 83:289–296. 2014. View Article : Google Scholar
|
|
23
|
Taneja S, Jena A, Goel R, Sarin R and Kaul
S: Simultaneous whole-body 18F-FDG PET-MRI in primary
staging of breast cancer: A pilot study. Eur J Radiol.
83:2231–2239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aklan B, Paulus DH, Wenkel E, Braun H,
Navalpakkam BK, Ziegler S, Geppert C, Sigmund EE, Melsaether A and
Quick HH: Toward simultaneous PET/MR breast imaging: Systematic
evaluation and integration of a radiofrequency breast coil. Med
Phys. 40:0243012013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dregely I, Lanz T, Metz S, Mueller MF,
Kuschan M, Nimbalkar M, Bundschuh RA, Ziegler SI, Haase A, Nekolla
SG, et al: A 16-channel MR coil for simultaneous PET/MR imaging in
breast cancer. Eur Radiol. 25:1154–1161. 2015. View Article : Google Scholar
|
|
26
|
Catalano OA, Nicolai E, Rosen BR, Luongo
A, Catalano M, Iannace C, Guimaraes A, Vangel MG, Mahmood U,
Soricelli A, et al: Comparison of CE-FDG-PET/CT with CE-FDG-PET/MR
in the evaluation of osseous metastases in breast cancer patients.
Br J Cancer. 112:1452–1460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lim I, Noh WC, Park J, Park JA, Kim HA,
Kim EK, Park KW, Lee SS, You EY, Kim KM, et al: The combination of
FDG PET and dynamic contrast-enhanced MRI improves the prediction
of disease-free survival in patients with advanced breast cancer
after the first cycle of neoadjuvant chemotherapy. Eur J Nucl Med
Mol Imaging. 41:1852–1860. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baba S, Isoda T, Maruoka Y, Kitamura Y,
Sasaki M, Yoshida T and Honda H: Diagnostic and prognostic value of
pretreatment SUV in 18F-FDG/PET in breast cancer:
Comparison with apparent diffusion coefficient from
diffusion-weighted MR imaging. J Nucl Med. 55:736–742. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyake KK, Nakamoto Y, Kanao S, Tanaka S,
Sugie T, Mikami Y, Toi M and Togashi K: Journal Club: Diagnostic
value of 18F-FDG PET/CT and MRI in predicting the
clinicopathologic subtypes of invasive breast cancer. AJR Am J
Roentgenol. 203:272–279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Galiza Barbosa F, Delso G, Zeimpekis
KG, Ter Voert E, Hüllner M, Stolzmann P and Veit-Haibach P:
Evaluation and clinical quantification of neoplastic lesions and
physiological structures in TOF-PET/MRI and non-TOF/MRI - a pilot
study. Q J Nucl Med Mol Imaging. May 12–2015.Epub ahead of print.
PubMed/NCBI
|
|
31
|
Sobin LH, Gospodarowicz MK and Wittekind
CH: TNM Classification of Malignant Tumours. 7th edition.
Wiley-Blackwell; Chichester: 2010
|
|
32
|
Wetter A, Lipponer C, Nensa F,
Beiderwellen K, Olbricht T, Rübben H, Bockisch A, Schlosser T,
Heusner TA and Lauenstein TC: Simultaneous 18F choline
positron emission tomography/magnetic resonance imaging of the
prostate: Initial results. Invest Radiol. 48:256–262. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wetter A, Nensa F, Schenck M, Heusch P,
Pöppel T, Bockisch A, Forsting M, Schlosser TW, Lauenstein TC and
Nagarajah J: Combined PET imaging and diffusion-weighted imaging of
intermediate and high-risk primary prostate carcinomas with
simultaneous [18F] choline PET/MRI. PLoS One.
9:e1015712014. View Article : Google Scholar
|
|
34
|
de Perrot T, Rager O, Scheffler M, Lord M,
Pusztaszeri M, Iselin C, Ratib O and Vallee JP: Potential of hybrid
18F-fluorocholine PET/MRI for prostate cancer imaging.
Eur J Nucl Med Mol Imaging. 41:1744–1755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grueneisen J, Beiderwellen K, Heusch P,
Gratz M, Schulze-Hagen A, Heubner M, Kinner S, Forsting M,
Lauenstein T, Ruhlmann V, et al: Simultaneous positron emission
tomography/magnetic resonance imaging for whole-body staging in
patients with recurrent gynecological malignancies of the pelvis: A
comparison to whole-body magnetic resonance imaging alone. Invest
Radiol. 49:808–815. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grueneisen J, Schaarschmidt BM,
Beiderwellen K, Schulze-Hagen A, Heubner M, Kinner S, Forsting M,
Lauenstein T, Ruhlmann V and Umutlu L: Diagnostic value of
diffusion-weighted imaging in simultaneous 18F-FDG
PET/MR imaging for whole-body staging of women with pelvic
malignancies. J Nucl Med. 55:1930–1935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Souvatzoglou M, Eiber M, Takei T, Fürst S,
Maurer T, Gaertner F, Geinitz H, Drzezga A, Ziegler S, Nekolla SG,
et al: Comparison of integrated whole-body [11C]choline
PET/MR with PET/CT in patients with prostate cancer. Eur J Nucl Med
Mol Imaging. 40:1486–1499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Selzner M, Morse MA, Vredenburgh JJ,
Meyers WC and Clavien PA: Liver metastases from breast cancer:
Long-term survival after curative resection. Surgery. 127:383–389.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vogl TJ, Naguib NNN, Nour-Eldin N-EA, Mack
MG, Zangos S, Abskharon JE and Jost A: Repeated chemoembolization
followed by laser-induced thermotherapy for liver metastasis of
breast cancer. AJR Am J Roentgenol. 196:W66–W72. 2011. View Article : Google Scholar
|
|
40
|
Alexander E III, Moriarty TM, Davis RB,
Wen PY, Fine HA, Black PM, Kooy HM and Loeffler JS: Stereotactic
radiosurgery for the definitive, noninvasive treatment of brain
metastases. J Natl Cancer Inst. 87:34–40. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen YM, Huang G, Sun XG, Liu JJ, Chen T,
Shi YP and Wan LR: Optimizing delayed scan time for FDG PET:
Comparison of the early and late delayed scan. Nucl Med Commun.
29:425–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng G, Torigian DA, Zhuang H and Alavi
A: When should we recommend use of dual time-point and delayed
time-point imaging techniques in FDG PET? Eur J Nucl Med Mol
Imaging. 40:779–787. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Laffon E, de Clermont H, Begueret H,
Vernejoux JM, Thumerel M, Marthan R and Ducassou D: Assessment of
dual-time-point 18F-FDG-PET imaging for pulmonary
lesions. Nucl Med Commun. 30:455–461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Atkinson W, Catana C, Abramson JS, Arabasz
G, McDermott S, Catalano O, Muse V, Blake MA, Barnes J, Shelly M,
et al: Hybrid FDG-PET/MR compared to FDG-PET/CT in adult lymphoma
patients. Abdom Radiol (NY). 41:1338–1348. 2016. View Article : Google Scholar
|
|
45
|
Pujara AC, Raad RA, Ponzo F, Wassong C,
Babb JS, Moy L and Melsaether AN: Standardized uptake values from
PET/MRI in metastatic breast cancer: An organ-based comparison with
PET/CT. Breast J. 22:264–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Boellaard R, O'Doherty MJ, Weber WA,
Mottaghy FM, Lonsdale MN, Stroobants SG, Oyen WJ, Kotzerke J,
Hoekstra OS, Pruim J, et al: FDG PET and PET/CT: EANM procedure
guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol
Imaging. 37:181–200. 2010. View Article : Google Scholar
|