Introduction
The phytocannabinoids (CANN) are a group of related
compounds extracted from the cannabis plant (1). The archetypal CANN that is most
widely known is Δ9-tetrahydrocannabinol (THC). It is notorious for
its psychoactive properties; however, it is arguably the only one
to exhibit such an effect out of the >80 members. CANNs interact
with the endocannabinoid system of the human body, and through this
can consequently intrude into a number of physiological aspects
such as appetite (2). Evidence
from the 1970s (3) suggested CANNs
possessed anticancer activity; and since then a large body of in
vitro studies have been developed and performed to confirm this
(4,5).
A number of cells express the cannabinoid receptor
(CBR), of which there are a number of sub-types (e.g. CBR1, CBR2),
and it is believed that signalling through this G-protein coupled
receptor is required for CANN anticancer action. Most of the in
vivo models of anticancer action of CANNs have focussed on
cancers of the brain where there are high levels of CBR1, and a
large proportion of these have indicated that CANN use is
associated with decreased tumour growth and/or increased cell
killing (6). These models have
also shown the use of CANN can successfully support and enhance the
action of other treatment modalities (7,8).
Peripheral cells, mainly immune cells, can also highly express
these receptors (9), in particular
CBR2, and as a consequence the effects of CANNs on cancers
emanating from these cells have also been studied.
Results from numerous in vitro studies have
shown the importance of the CBR in the success of CANNs as
effective anticancer agents. Signalling through the CBR gives CANN
the ability to stimulate pro-apototic elements within the ceramide
pathway (10), as well as being
able to engage autophagy in cells (11). Additionally, CANNs can subsequently
interfere/interact with other intrinsic intracellular signalling
pathways such as PI3-K and ERK via downstream crosstalk, which
offers a way in which they can also fundamentally manipulate key
processes like cell growth and survival (4,12).
The necessity for receptors to be present in order
to elicit these cell killing mechanisms may not, however, be
absolute; anticancer activity has been seen in leukaemia cells that
is independent of the receptors (13), and similarly, minor-occurring
CANNs, which have low binding affinities for these canonical
receptors, are equally as active in these same cell types (14,15).
Furthermore, there have been reports of CANN activity directly on
cancer cells that do not usually express the receptors such as
those of the breast and prostate (16). Together, this suggests the number
of cancers that could respond to CANNs may not be limited to those
expressing the receptors. Nevertheless, the evidence that a number
of CANNs can be used to reduce the growth of leukaemia cells in
vitro is exciting, and warrants further investigation.
As part of our ongoing research efforts
investigating the potential benefits of CANNs in a leukaemia
setting, we have examined further the effects of these drugs
combined with others on cell growth and survival. We paired CANNs
together and specifically examined the activity of these mixes in
leukaemia cells, both alone and in combination with a number of
common anti-leukaemia drugs. We have adopted a number of practical
models to assess drug-drug interactions, and also assessed the
importance of drug sequence in determining the overall efficacy of
the differing treatments.
Materials and methods
Cell culture and drugs
The human cancer cell lines CEM (acute lymphocytic
leukaemia) and HL60 (promyelocytic leukaemia) were purchased from
the European Collection of Authenticated Cell Cultures (Salisbury,
UK), and grown in RPMI-1640 medium (Sigma-Aldrich Co., Ltd.,
Dorset, UK) supplemented with 10% foetal bovine serum (FBS) and 2
mM L-glutamine. All cell lines were incubated in a humidified
atmosphere with 5% CO2 in air at 37°C, and discarded
after ~12 passages. Authentication of the cell lines was performed
by the service provider using the AmpFISTR Identifier Plus PCR
amplification kit looking for the presence of <10 known loci for
each of the cell lines.
Cytarabine (CYT: Sigma) and vincristine (VIN: Sigma)
were reconstituted in PBS at a stock concentration of 10 mM, and
kept at −20°C for no more than four weeks. Cannabidiol (CBD),
cannabigerol (CBG) and THC (all provided by GW Research Ltd.,
Cambridge, UK) were dissolved in ethanol to appropriate
concentrations that ensured a final ethanol concentration in cell
cultures <0.1%. For experiments with treatments, the amount of
FBS in the cell culture medium was reduced to 5%. One aim of the
current study was to investigate the benefit of using two different
CANNs together in a pair. The combinations used here mimic a number
of current and recent clinical trials where a proprietary product
containing CBD and THC was used (www.clinicaltrials.gov - Identifier: NCT01812603 and
NCT01812616). Consequently, our experiments involved using CANNs
paired concomitantly at a 1:1 ratio, where the stated concentration
for them reflected an equal amount of each CANN-component; for
example, 10 µM CBD+THC contained 5 µM CBD and 5
µM THC. A similar approach was adopted and reported in our
earlier studies (8).
Proliferation assays - CANNs alone
To study the effect of the CANNs on cell growth,
leukaemia cells that were growing exponentially were seeded into
96-well plates at a density of 1.5×104/well. CANNs were
then added to the wells at various concentrations, ensuring an
equal volume of 200 µl across the plate. Single-agent
testing: Either CBD, CBG or THC alone was added to the wells at a
concentration range of 1–50 µM. Paired-CANN testing:
CBD+CBG, CBD+THC or CBG+THC was added to the wells at a
concentration range for the paired CANNs of 1–50 µM. The
molarity was based upon the total CANN in each pair. Cell number
was assessed by using a methylthiazoletetrazolium (MTT)-based assay
according to methods previously described (17), and by cell counting using trypan
blue dye as a way of discriminating live and dead cells.
Combination studies - median-effect
analysis
Cells (1.5×104/well) growing
exponentially were reset in fresh culture medium and aliquoted into
96-well plates. A CANN-pair (either CBD+THC or CBD+CBG) was
combined with CYT or VIN at concentrations that were equal ratios
of their respective IC50 according to methodologies
described previously (14,17,18).
Cell number was then assessed after 72 h by the MTT-based assay,
and a combination index (CI) calculated by using the median-effect
equation (19).
Combination studies - modulatory
effect
The ability of CANNs to modify the efficacy CYT and
VIN was studied by assessing and comparing the IC50 of
the anti-leukaemia drugs in the absence and presence of the CANNs.
The CANNs tested were CBD+CBG and CBD+THC (the modulating drug in
this setting), and these were used at a single total sub-optimal
concentration of 1 µM in CEM and 5 µM HL60.
Methodologically, cells (1.5×104/well) growing
exponentially were reset in fresh culture medium and aliquoted into
96-well plates. Drugs were added (CYT and VIN over a range of
concentrations) and cell number determined after 72 h. Parallel
6-well plates containing cells were also prepared and were cultured
with the same treatment combinations described. These allowed for
determination of cell cycle distribution at 72 h by flow cytometry
utilising the nucleic acid stain propidium iodide (17).
Combination studies - drug sequence and
the impact of a recovery phase
CEM and HL60 cells were seeded into 6-well plates at
a density of 1×105/well and then treated according to a
culture schedule that lasted a total of 96 h. The treatment would
involve two separate phases; each lasting 48 h. One set of drugs
would be administered in the first 48 h phase and a second set of
drugs in the following 48 h phase. The culture medium would be
removed by centrifugation after the first treatment to be replaced
with fresh medium in an attempt to remove the drugs used in the
first phase of treatment. The drugs studied were either CBD+CBG (4
µM in CEM and 10 µM in HL60), CBD+THC (4 µM in
CEM and 10 µM in HL60), CYT (10 nM), or VIN (0.1 nM). The
effect of a recovery phase was assessed by keeping the second 48 h
phase of treatment drug-free. Flow cytometry using propidium iodide
staining was performed at the end of the experiment to assess the
extent of cell death/apoptosis.
Immunoblotting analysis
Western blot analyses were performed as previously
described (8). Primary antibody
probing was performed with anti-cyclin B1 and anti-GAPDH (both from
New England Biolabs, Hitchin, UK) and used at a dilution of
1:1,000. Appropriate HRP-conjugated secondary antibodies were then
used (New England Biolabs), and bands were visualised by the
ECL-plus detection system (Amersham Biosciences Ltd., Little
Chalfont, UK).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism or Microsoft Excel, and differences between
treatments and control groups were determined by one-way ANOVA and
subsequently by paired tests. Data values were presented as the
means and SDs of at least three separate experiments.
Results
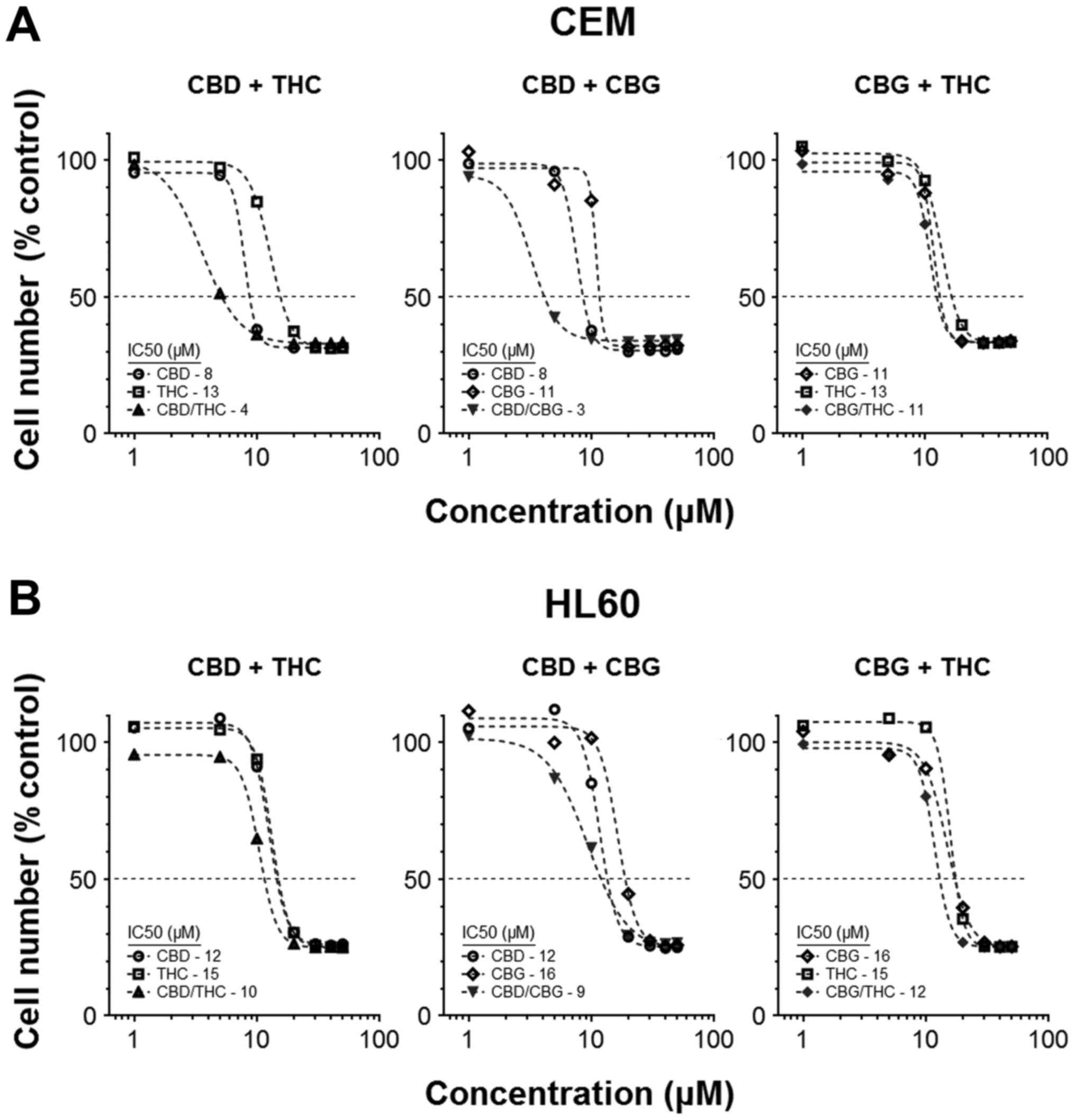
Combining CANNs can improve their overall
activity
Our previous studies showed a small number of CANNs
could be used together to induce a cytotoxic response that was
hyperadditive in nature. We therefore expanded this initial work by
pairing CBD, CBG and THC in different permutations, and assessing
their effects on cell numbers after 72 h treatment. IC50
values for the individual CANNs were determined, and these were
compared with IC50 achieved when the matching CANN-pair
was used. Results showed the virtual IC50 of the
mixtures were generally lower than those for the CANNs when used
individually (Fig. 1A and B). For
example, the IC50 in CEM cells was 7.8±0.21 µM
for CBD alone and 13±0.49 µM for THC alone, compared to
3.6±0.19 µM when CBD and THC were used simultaneously at a
ratio of 1:1 (Fig. 1A).
In this basic paired-model, CEM cells were more
responsive to treatments, as the combination of two CANNs generally
resulted in an improvement in activity. Moreover, combinations
including CBD as one of the partners in a pair usually resulted in
a greater reduction in cell number (IC50 values in CEM
for CBD/THC, CBD/CBG and CBG/THC were 3.6±0.19, 2.8±0.24 and
11±0.55 µM, respectively) (Fig.
1A).
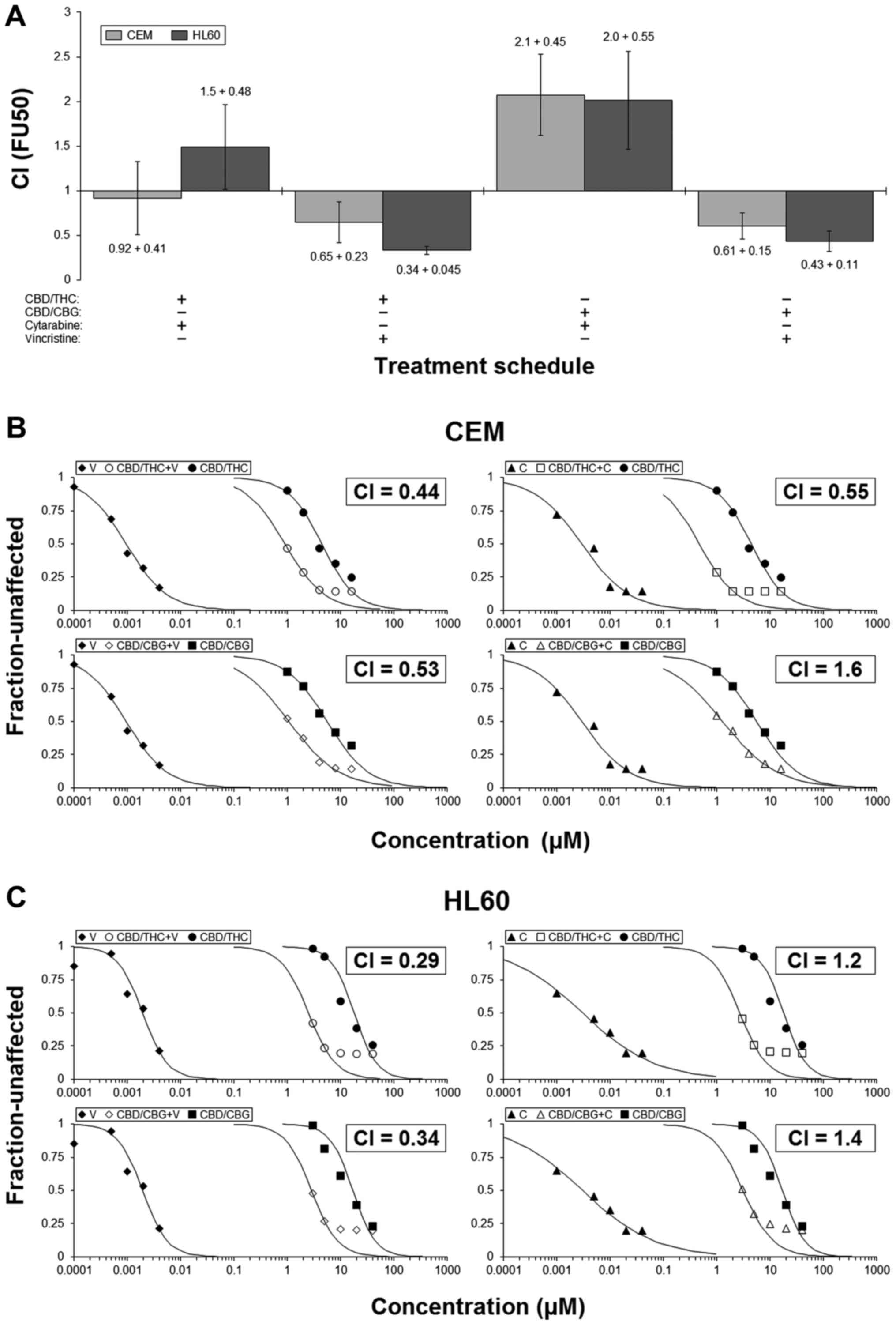
CANN-pairs can cooperate with
anti-leukaemia agents to reduce cell numbers
Median-effect analyses were employed to assess the
interactions between each CANN-pair and common anti-leukaemia
drugs. Guided by our initial results showing CBD-containing pairs
to be most efficacious, we selected the CBD/CBG and CBD/THC pairs
and combined them with either CYT or VIN. CI-values were then
calculated by using these results and used as a way of
understanding the drug-interactions (14,18).
Results showed that outcome of the interactions were
dependent upon both drug and cell line. They also hinted that
combinations involving VIN would more likely result in enhanced
activity, whilst those with CYT may cause additivity/mild
antagonism (Fig. 2A).
Representative examples of the interactions are presented (Fig. 2B and C). Notably, in HL60 cells for
example, the IC50 for CBD/THC was 18 µM and for
VIN was 1.9 nM; however, when these two were used concomitantly,
the IC50 of this combination treatment was 2.5
µM. The calculated CI-value was 0.29, which indicated
synergy (Fig. 2C). This result
shows that in certain circumstances a combination approach can
result in an equivalent level of action even though the
concentrations of the agents used are much lower (in this instance
CBD/THC and VIN were used at ~2.5 µM and ~0.25 nM,
respectively).
CANN-pairs can sensitise cells to the
effects of anti-leukaemia agents
A second model of drug interaction was employed in
our studies. This experiment was designed to test the ability of a
CANN-pair to sensitise cells to the effects of CYT or VIN.
Specifically, the ability of a sub-effective concentration of CANN
to alter the efficacy of CYT or VIN was determined by comparing the
IC50 values of the chemotherapy agents in the absence or
presence of the modulating CANN drug. Results showed adding CANNs
to cells cultured with CYT or VIN only changed the cell number
IC50 values for each chemotherapy drug to a small extent
(Fig. 3A). Changes were most
apparent in treatments where CBD/THC was the modulating drug.
Conversely, however, there were clear changes in the
IC50, when examining the modulatory effect of CANNs on
cell viability (Fig. 3B). For
example, the IC50 for CYT in HL60 was 100 nM; however,
this was reduced to 8 nM if CBD/THC was included to the cultures,
but increased to 150 nM if CBD/CBG was used (Fig. 3B). These results generally agreed
with those from the median-effect combination model, and suggest
combinations of CANNs with VIN would result in hyperadditive
interactions leading to reduced ell numbers.
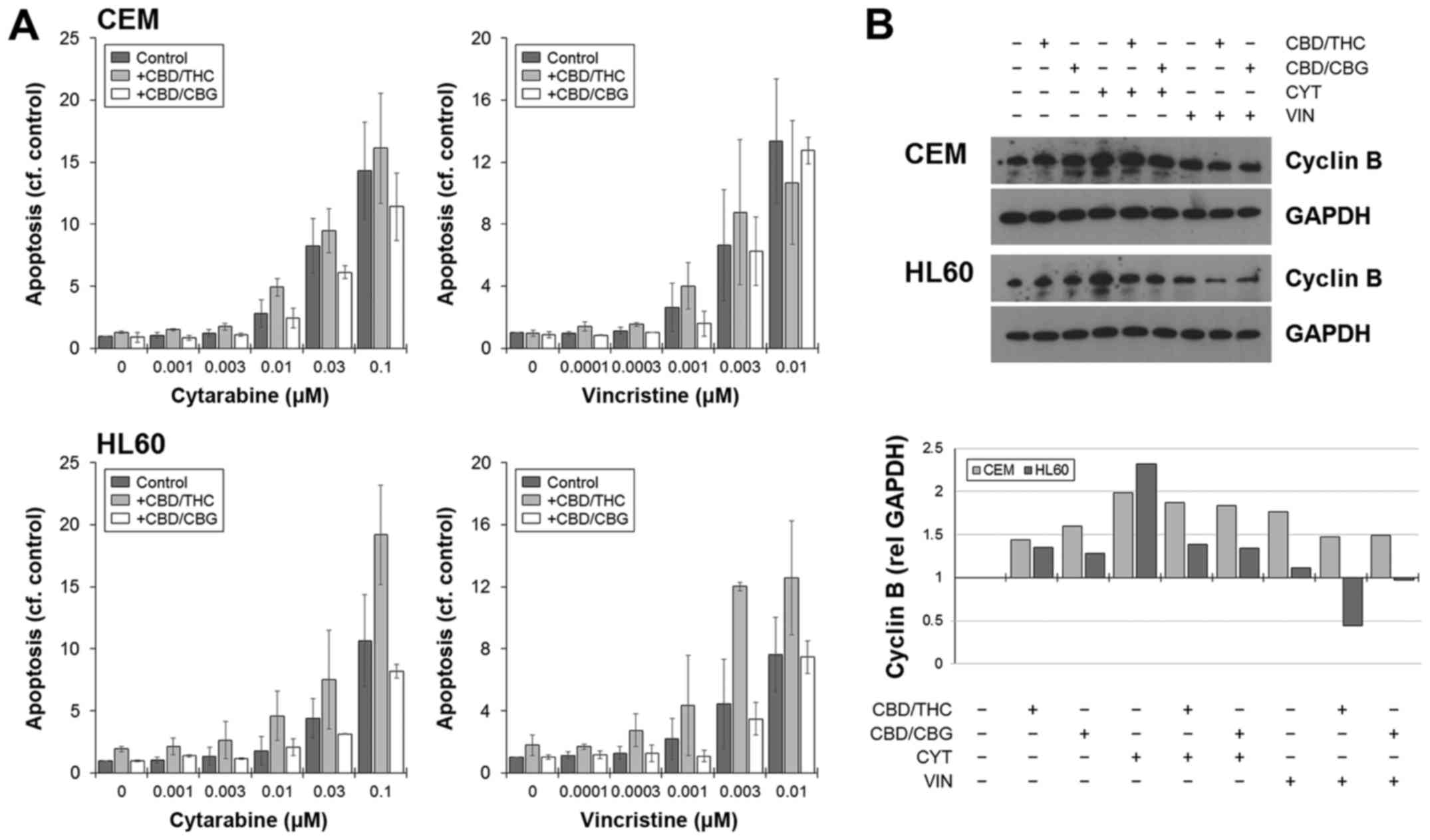
The reduction in viability was associated with an
increase in apoptosis, as shown by flow cytometry, which was
generally higher in combinations involving CBD/THC (Fig. 4A). Cell death was not specific to
any phase of the cell cycle, and the drug-induced arrest in the
S-phase did not significantly impede the ability of cells to
undergo death when CANNs were added (data not shown). The
expression of cyclin B was used as a general marker of cell
cycling, and levels increased when cells were cultured with CYT or
VIN (Fig. 4B). However, this
increase was negated or in the case with HL60, reduced when a CANN
pair was included (Fig. 4B).
The sequence of drugs is important in
determining overall activity
Having seen synergistic interactions between CANNs
and anti-leukaemia drugs when they were used simultaneously, we
next assessed the impact of using the drugs sequentially.
Consequently, cells were cultured according to schedules that
consisted of two rounds of treatment, each lasting 48 h. Each round
of treatment was separated by a washing step to remove drug from
the medium. The order of the drugs were swapped in equivalent
experiments to assess the counter-order of drugs. In some cases, a
treatment schedule could involve the use of a CANN-pair in the
first round of treatment followed by no treatment in the second.
This mimicked a 'recovery' schedule. The duration of each treatment
phase was 48 h to ensure that cells were not overgrown by the end
of the full treatment regimen, which lasted 96 h.
Results showed that, generally, the percentages of
cells within the sub-G1 population of the cell cycle were low in
CEM cells following any treatments (Fig. 5A); however, the order of
administration of the drugs affected the amount of cells in sub-G1.
Typically, using CYT or VIN before a CANN-pair resulted in a
greater amount of cells in sub-G1 compared to schedules in which
the order of drugs was reversed (Fig.
5A). In HL60 cells for example, % sub-G1 was 37% if CBD/THC was
used before CYT, but 72% if CBD/THC was used after CYT.
Furthermore, paired t-test of all the data, irrespective of cell
line and drug used, showed that significantly more apoptosis was
seen if the order of treatment entailed a CANN after chemotherapy
(Fig. 5B).
In accordance with our earlier published data, the
greatest number of cells present in a sub-G1 population was seen
following the schedule where HL60 cells were cultured with CBD/THC
in the first phase of treatment followed by no treatment in the
last phase (92%), in imitation of a recovery phase. This was
considerably higher than the percentage seen when the cells were
cultured with CBD/THC in both rounds of treatment (66%) (Fig. 5A).
Discussion
This work was a continuation study performed to
investigate further the effects that CANNs may have on leukaemia
cells. Our earlier studies showed that a number of CANNs were
capable of eliciting death in cancer cells when used alone or in
combination with each other; however, the benefits of using these
with pre-existing chemotherapy drugs had not been investigated. In
the current study, we showed that combining CANNs with the
anti-leukaemia agents CYT and VIN resulted in enhanced overall
activity. Furthermore, cooperation between CANN and chemotherapy
was sequence-dependent, with a greater level of cell killing seen
when the CANNs were used after the chemotherapy.
There is an increasing body of evidence showing that
CANNs derived from the cannabis plant possess anticancer activity
(12). A number of in vitro
and murine models have shown that the CANNs CBD and THC can alter
the way that tumour cells proliferate, as well as increase the
capacity of these cells to undergo death by apoptosis and/or
autophagy. These effects appear to be both dependent and
independent of signalling via their cognate CBR (13,20).
More recently, in the context of glioma, CANNs have also been shown
to enhance the action of other treatment modalities such as
chemotherapy and irradiation in vivo (7,8).
Although anticancer studies involving CANNs have
rightly concentrated on cancers of the brain (21), a number have focused on their
efficacy in leukaemias (13,22,23).
Earlier investigations studied the action of THC alone in
leukaemia, but limitations to the dosages that could be used in
patients due to the psychoactivity associated with its use, made
THC unattractive. As a result, this hindered its development as a
putative form of therapy. Nonetheless, the concept of using
cannabis-derived substances in leukaemia was revealed. Research
using the non-psychoactive CANNs then rapidly followed, which
recapitulated the results seen with THC. The above also revealed
that combining a number of these minor CANNs could result in
responses that were more active than if the individual CANNs were
used separately (14,23).
These studies fully support the possibility that
mixing CANNs could result in a product that is optimised for
anticancer effect. Crucially, it is important to note that not all
the individual components of a combination need to elicit a direct
cytotoxic effect, but instead can merely support the effect of its
corresponding pair/partner. This cooperative phenomenon has been
described using a number of terms such as an entourage-effect, a
bystander-effect and a compensatory-effect; however, the overall
effect for a combination is simply to induce a measurable response
that is greater than the sum of component's individual ones
(24). The resulting synergy would
be clinically attractive; not only because of an overall increase
in general activity, but also because this improvement would have
arisen concomitantly with a reduction in the dosages of the
individual drugs. Associated with this reduction in dose is the
potential easing of adverse effects that typically accompany the
usage of the individual drugs. A number of recent clinical trials
involving CANNs have tested the efficacy and/or safety of Sativex™,
which is a proprietary product composed of equal amounts of CBD and
THC (www.clinicaltrials.gov: NCT01812603
and NCT01812616). The consideration being that both CANNs possess
anticancer action, and so using them concomitantly would maximise
the chances of a positive effect. These trials are expected to
report soon.
In our current studies, initial experiments were
performed to assess the activity of various CANN pairs and to
identify the most active mix. Our results suggested that pairs
comprising CBD were most active. In agreement with our earlier
results, pairing CBD with CBG was as active as CBD with THC. The
mechanism of this cooperative interaction between CANNs is unknown,
but may simply be a reflection of the sum of the anticancer
properties of the individual agents used (13,25,26).
However, there may also be activation of other unique processes
following the use of two CANNs, as an earlier study of ours showed
a distinct number of genes were activated only when CBD and THC
were used together, and not when they were used separately
(27). These involved a number of
cell cycle and apoptosis genes, suggesting distinct pathways that
may become engaged when the two CANNs were used. Understanding
these interactions may offer ways of developing new treatment
strategies and regimens to best utilise this class of drug.
Generally, HL60 appeared the more sensitive of the cell lines
tested. The reason for this difference could be due to the higher
expression of CBR-2 in HL60 compared to CEM (13), or simply due to differences in the
intrinsic background of intracellular signalling pathways in both,
which we have previously shown to be different (14). This highlights the potential
caveats of selecting the cancers best suited to CANN treatment.
After confirming that these CANNs could be paired
without a loss of anticancer action, we next mimicked the current
clinical path by assessing the effect of combining CBD/THC with
common anti-leukaemia drugs. We first determined the value of using
the CANN pair and chemotherapy at the same time, and results showed
clear improvements in the cytotoxic response. This was indicated by
significant improvements in the IC50 of CYT and VIN if
CBD/THC was included in the treatment. For example, in HL60 cells,
the IC50 for VIN was 20 nM; however, this was reduced to
3.2 nM if a sub-toxic dose of CBD/THC was used with it.
Furthermore, improvements in the IC50 were associated
with increases in apoptosis. Generally, substituting the CBD/THC
pair with CBD/CBG had little effect on the IC50 for CYT
and VIN; however, the IC50 values were reduced and
chemotherapy efficacy improved in some instances.
The sequence in which certain drugs are administered
can influence the overall activity of a treatment course for a
number of cancers (28,29). This should be an important
consideration in any treatment plan as one drug can influence the
action/activity of others. These interactions can be both
beneficial and detrimental to the outcome of the treatment, and as
such, the order of administration should be optimised. When these
interactions are favourable, it is conceivable that one drug alters
the biology of tumour cells to render them more susceptible to
another. For example, it has been suggested that in response to the
inhibition of topoi-somerase I by the drug camptothecin, the
related enzyme topoisomerase II is increased. Thus the use of the
drug etoposide after camptothecin may be fruitful as the specific
target of its action is topoisomerase II (30). In addition to this compensatory
phenomenon, some drugs can work to prime cancer cells to the
cytotoxic action of others by promoting apoptotic pathways. BH3
mimetics serve in such a manner, by removing the 'brakes' in the
form of proteins such as BCL-2 and BCL-xL, that obstruct apoptosis
(31). Therefore, a treatment
regimen could be designed that uses these agents first to lower the
threshold for apoptosis, before using a second drug specifically
chosen to elicit a death signal. Equally, drugs that influence the
cell cycle and modulate the restriction points within it, may
increase the sensitivity of tumour cells to other treatments in a
sequence-dependent manner (32).
Importantly, these drugs can take the form of dedicated cell cycle
inhibitors or those that, through their intrinsic mechanism of
action, disturb the cell cycle (33).
In addition to their cytotoxic features (12), CBD and THC are able to directly
impede the cell cycle through modulation of the cyclin-dependent
kinase inhibitor p21waf1 and p27 (14,34).
We therefore hypothesised that any possible benefit by combining
them with other drugs could be influenced by treatment sequence;
specifically that these could influence any possible benefit of
combining CANNs with other drugs, particularly those that act on
the cell cycle like the anti-leukaemics. As such, we assessed the
level of activity in the cell lines treated with CANNs and
chemotherapy when used sequentially. Results showed sequence of
administration was important, and that significantly greater
amounts of apoptosis was seen when the CANN was used after the
chemotherapy.
In summary, our data showed that a number of CANNs
could be used together in pairs to generate anticancer responses
that are greater than would be expected if the components were used
separately. These CANN pairs can then also be combined
synergistically with common anti-leukaemia agents. Importantly,
results also suggested that the sequence of the drugs may be
crucial in determining the clinical activity of combination
treatment regimens involving CANNs. Specifically, our studies
recommend that if CANNs are to be combined with other
anti-leukaemia drugs, that they should be used either concomitantly
or after them. In conclusion, evidence of CBD activity in patients
with certain forms of cancer linked with a considerable body of
evidence in vitro, support the overall concept that these
plant-derived CANNs are valid therapeutic compounds. However, until
clinical trials that test their value in an oncological setting are
completed and reported, reticence will always remain. Ultimately,
using information from evidence-led in vitro studies is the
best way to predict and determine the treatment combinations and
approaches for CANNs that have the best chance to translate
successfully to the clinic.
Acknowledgments
This work was funded by a research grant awarded to
W.M.L. from GW Research Ltd. (Cambridge, UK).
References
|
1
|
Mechoulam R, Hanuš LO, Pertwee R and
Howlett AC: Early phytocannabinoid chemistry to endocannabinoids
and beyond. Nat Rev Neurosci. 15:757–764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pacher P, Bátkai S and Kunos G: The
endocannabinoid system as an emerging target of pharmacotherapy.
Pharmacol Rev. 58:389–462. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Munson AE, Harris LS, Friedman MA, Dewey
WL and Carchman RA: Antineoplastic activity of cannabinoids. J Natl
Cancer Inst. 55:597–602. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guzmán M: Cannabinoids: Potential
anticancer agents. Nat Rev Cancer. 3:745–755. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fowler CJ: Delta(9)-tetrahydrocannabinol
and cannabidiol as potential curative agents for cancer: A critical
examination of the preclinical literature. Clin Pharmacol Ther.
97:587–596. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ladin DA, Soliman E, Griffin L and Van
Dross R: Preclinical and clinical assessment of cannabinoids as
anti-cancer agents. Front Pharmacol. 7:3612016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Torres S, Lorente M, Rodríguez-Fornés F,
Hernández-Tiedra S, Salazar M, García-Taboada E, Barcia J, Guzmán M
and Velasco G: A combined preclinical therapy of cannabinoids and
temozolomide against glioma. Mol Cancer Ther. 10:90–103. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scott KA, Dalgleish AG and Liu WM: The
combination of cannabidiol and Δ9-tetrahydrocannabinol enhances the
anticancer effects of radiation in an orthotopic murine glioma
model. Mol Cancer Ther. 13:2955–2967. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Basu S and Dittel BN: Unraveling the
complexities of canna-binoid receptor 2 (CB2) immune regulation in
health and disease. Immunol Res. 51:26–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Galve-Roperh I, Sánchez C, Cortés ML,
Gómez del Pulgar T, Izquierdo M and Guzmán M: Anti-tumoral action
of cannabinoids: Involvement of sustained ceramide accumulation and
extracellular signal-regulated kinase activation. Nat Med.
6:313–319. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salazar M, Carracedo A, Salanueva IJ,
Hernández-Tiedra S, Lorente M, Egia A, Vázquez P, Blázquez C,
Torres S, García S, et al: Cannabinoid action induces
autophagy-mediated cell death through stimulation of ER stress in
human glioma cells. J Clin Invest. 119:1359–1372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Velasco G, Sánchez C and Guzmán M:
Anticancer mechanisms of cannabinoids. Curr Oncol. 23:S23–S32.
2016.PubMed/NCBI
|
|
13
|
Powles T, te Poele R, Shamash J, Chaplin
T, Propper D, Joel S, Oliver T and Liu WM: Cannabis-induced
cytotoxicity in leukemic cell lines: The role of the cannabinoid
receptors and the MAPK pathway. Blood. 105:1214–1221. 2005.
View Article : Google Scholar
|
|
14
|
Scott KA, Shah S, Dalgleish AG and Liu WM:
Enhancing the activity of cannabidiol and other cannabinoids in
vitro through modifications to drug combinations and treatment
schedules. Anticancer Res. 33:4373–4380. 2013.PubMed/NCBI
|
|
15
|
Borrelli F, Pagano E, Romano B, Panzera S,
Maiello F, Coppola D, De Petrocellis L, Buono L, Orlando P and Izzo
AA: Colon carcinogenesis is inhibited by the TRPM8 antagonist
cannabigerol, a cannabis-derived non-psychotropic cannabinoid.
Carcinogenesis. 35:2787–2797. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fraguas-Sánchez AI, Fernández-Carballido A
and Torres-Suárez AI: Phyto-, endo- and synthetic cannabinoids:
Promising chemotherapeutic agents in the treatment of breast and
prostate carcinomas. Expert Opin Investig Drugs. 25:1311–1323.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu WM, Gravett AM and Dalgleish AG: The
antimalarial agent artesunate possesses anticancer properties that
can be enhanced by combination strategies. Int J Cancer.
128:1471–1480. 2011. View Article : Google Scholar
|
|
18
|
Liu WM, Scott KA, Shamash J, Joel S and
Powles TB: Enhancing the in vitro cytotoxic activity of
Delta9-tetrahydrocannabinol in leukemic cells through a
combinatorial approach. Leuk Lymphoma. 49:1800–1809. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van Dross R, Soliman E, Jha S, Johnson T
and Mukhopadhyay S: Receptor-dependent and receptor-independent
endocannabinoid signaling: A therapeutic target for regulation of
cancer growth. Life Sci. 92:463–466. 2013. View Article : Google Scholar
|
|
21
|
Rocha FC, Dos Santos Júnior JG, Stefano SC
and da Silveira DX: Systematic review of the literature on clinical
and experimental trials on the antitumor effects of cannabinoids in
gliomas. J Neurooncol. 116:11–24. 2014. View Article : Google Scholar
|
|
22
|
Jia W, Hegde VL, Singh NP, Sisco D, Grant
S, Nagarkatti M and Nagarkatti PS:
Delta9-tetrahydrocannabinol-induced apoptosis in Jurkat leukemia T
cells is regulated by translocation of Bad to mitochondria. Mol
Cancer Res. 4:549–562. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McKallip RJ, Jia W, Schlomer J, Warren JW,
Nagarkatti PS and Nagarkatti M: Cannabidiol-induced apoptosis in
human leukemia cells: A novel role of cannabidiol in the regulation
of p22phox and Nox4 expression. Mol Pharmacol. 70:897–908. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu WM: Enhancing the cytotoxic activity
of novel targeted therapies - is there a role for a combinatorial
approach? Curr Clin Pharmacol. 3:108–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Petrocellis L, Ligresti A, Schiano
Moriello A, Iappelli M, Verde R, Stott CG, Cristino L, Orlando P
and Di Marzo V: Non-THC cannabinoids inhibit prostate carcinoma
growth in vitro and in vivo: Pro-apoptotic effects and underlying
mechanisms. Br J Pharmacol. 168:79–102. 2013. View Article : Google Scholar :
|
|
26
|
Fisher T, Golan H, Schiby G, PriChen S,
Smoum R, Moshe I, Peshes-Yaloz N, Castiel A, Waldman D, Gallily R,
et al: In vitro and in vivo efficacy of non-psychoactive
cannabidiol in neuroblastoma. Curr Oncol. 23:S15–S22.
2016.PubMed/NCBI
|
|
27
|
Scott KA, Dennis JL, Dalgleish AG and Liu
WM: Inhibiting heat shock proteins can potentiate the cytotoxic
effect of cannabidiol in human glioma cells. Anticancer Res.
35:5827–5837. 2015.PubMed/NCBI
|
|
28
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar
|
|
29
|
Dear RF, McGeechan K, Jenkins MC, Barratt
A, Tattersall MH and Wilcken N: Combination versus sequential
single agent chemotherapy for metastatic breast cancer. Cochrane
Database Syst Rev. 12:CD0087922013.
|
|
30
|
Bonner JA and Kozelsky TF: The
significance of the sequence of administration of topotecan and
etoposide. Cancer Chemother Pharmacol. 39:109–112. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Billard C: BH3 mimetics: Status of the
field and new developments. Mol Cancer Ther. 12:1691–1700. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shapiro GI and Harper JW: Anticancer drug
targets: Cell cycle and checkpoint control. J Clin Invest.
104:1645–1653. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shah MA and Schwartz GK: Cell
cycle-mediated drug resistance: An emerging concept in cancer
therapy. Clin Cancer Res. 7:2168–2181. 2001.PubMed/NCBI
|
|
34
|
Caffarel MM, Moreno-Bueno G, Cerutti C,
Palacios J, Guzman M, Mechta-Grigoriou F and Sanchez C: JunD is
involved in the antiproliferative effect of
Delta9-tetrahydrocannabinol on human breast cancer cells. Oncogene.
27:5033–5044. 2008. View Article : Google Scholar : PubMed/NCBI
|