Introduction
Even with the rapid development of surgical
techniques and a broader understanding of oncology over the past
few decades, pancreatic ductal adenocarcinoma (PDAC) remains at a
5-year survival rate of 5–6% (1,2).
Only approximately 15–20% of patients with PDAC are suitable for
surgical resection, which is the best possible treatment for good
oncologic outcome (1,2). For most pancreatic cancers that are
locally advanced or meta-static, the National Comprehensive Cancer
Network (NCCN) recommends chemotherapy (1–4).
However, only a limited subgroup of these patients will really
benefit from chemotherapy; instead, patients often have severe
adverse events, and even a more progressive disease (1–4).
Therefore, it is necessary to select the patients with unresectable
PDAC that could have a better prognosis or benefit from
chemotherapy. Different tumor biological characteristics may
contribute to better outcomes in this subgroup of patients.
Unfortunately, there is still a lack of a simple and effective
marker to identify these patients.
Generally, PDAC, especially advanced cases, is
accompanied by obstruction of the pancreatic duct and varying
degrees of chronic pancreatitis, which contribute to a unique tumor
microenvironment comprising a specific network of multiple immune
and inflammatory factors (5,6). It
was reported that a dynamic imbalance involving CD4+ T
cells and CD8+ T cells contributed to tumor immune
escape and failure of immune surveillance during pancreatic
tumorigenesis (5–7). T regulatory (Treg) cells, one of the
main subsets of CD4+ T helper cells, were reported to be
recruited by the pancreatic tumor microenvironment from a
pre-invasive stage to invasive and metastatic PDAC and played a
crucial role in immunosuppression (6,7).
Previously, we found that peripheral Treg levels were significantly
increased in patients with PDAC compared with that of healthy
donors and patients with benign pancreatic disease; furthermore,
for patients with PDAC who underwent a radical resection, the
percentage of peripheral Tregs was regarded as an independent
prognostic factor (8). Although it
has been widely confirmed that infiltrating immune cells in tumor
tissue have prognostic values (5–7),
whether the systemic quantifiable immune parameters also have
prognostic value is an interesting question. The authors consider
that the data of circulating immune parameters are more widely
available than the data of infiltrating immune cells. Thus, if the
circulating immune parameters have prognostic value, the makers
would be more convenient and accessible to monitor tumor
progression. Therefore, the present study focused on circulatory
immune cells to explore prognostic markers, based on retrospective
analysis. In addition, tissue samples obtained through EUS-FNA in
unresectable pancreatic cancer patiens are rare and limited and
often insufficient for tumor microenvironment analysis. In the
present study, although the results are not direct evidence,
consideration should be given that PDCA caused the abnormal
distribution of peripheral T lymphocyte subsets.
Chemotherapy is a primary treatment for advanced or
metastatic PDAC; however, a new perspective has emerged that the
cytotoxic effects of chemotherapy not only destroys tumor cells,
but also impairs immune cells that subsequently damage the
antitumor response and even promote tumor growth and metastasis
(9–11). In this study, we aimed to observe
the alteration and distribution of peripheral T cell subsets in
patients with unresectable PDAC, evaluate the effect of
chemotherapy on T cell-related immune system, and discover some
effective factors that could predict systemic chemotherapy response
in patients with unresectable PDAC.
Materials and methods
Patients
The present study included all patients with
unresectable pancreatic cancer who received treatment at our center
during October 2010 to December 2015, including locally advanced
and metastatic cases according to the American Joint Committee on
Cancer (AJCC, 7th edition). These patients were diagnosed on the
basis of pathology using biopsy samples obtained by endoscopic
ultrasound-guided fine-needle aspiration (EUS-FNA). Most of the
patients (81.1%) underwent gemcitabine (gemcitabine at 1000
mg/m2 over 30 min, weekly for 3 weeks every 28 days) or
gemcitabine-based combination therapy according to NCCN Guidelines.
This study was approved by the Clinical Research Ethics Committee
of Shanghai Cancer Center of Fudan University, and written informed
consent was provided by each patient. All clinicopathological data
were retrieved from electronic records. Computed tomography (CT)
was used to evaluate the treatment response every 2 months
according to the guidelines of Response Evaluation Criteria in
Solid Tumors (RECIST) 1.0. The primary endpoint of this study was
overall survival (OS), which was measured by comparing the date of
diagnosis to the date of death, and the last follow-up date was
June 2016 with three patients still alive. The median survival of
this cohort of patients is 7 months, with 5.8 months in stage IV
patients and 9.2 months in locally advanced patients.
Blood sample collection and flow
cytometry
Venous blood samples were obtained in heparinized
tubes at admission before or after two-cycle chemotherapy, and the
samples were immediately analyzed by flow cytometry. Monoclonal
antibodies (eBiosciences) used to identify different T lymphocyte
subsets were as follows: anti-CD3 FITC, anti-CD4 PE-Cy™7, and
anti-CD8 APC-Cy7. Anti-CD8 FITC and anti-CD28 PE were used to
detect CD8+CD28+ T cells. Anti-CD4 FITC,
anti-CD25 PE and anti-CD127 APC were applied to identify Tregs.
Statistical analyses involved at least 10,000 events gated on the
population of interest. Detailed steps for the detection of
different T lymphocyte subsets in blood samples are previously
described (8).
Enzyme-linked immunosorbent assay
(ELISA)
Serum samples from patients were prospectively
collected after centrifugation at 3,000 rpm for 10 min at 4°C.
Then, the supernatants were divided and cryopreserved at −80°C. The
following parameters were measured: IL-17A was determined using the
human IL-17A ELISA kit (eBioscience); IL-6 was determined using the
human IL-6 ELISA kit (eBioscience); and TGF-β1 was determined using
the human TGF-β1 ELISA kit (eBioscience). The measurements were
performed according to the manufacturer's protocol. The results are
expressed as pg/ml. In this study, 100 pairs (before and after
two-cycle chemotherapy) of cryopreserved serum samples were
available for analysis.
Statistical analysis
The SPSS version 16.0 statistical software package
and the Graphpad Prism version 6.0 (GraphPad, Inc., San Diego, CA,
USA) were used for statistical analysis. Overall survival curves
were compared using the log-rank test. Univariate and multivariate
analyses were used to examine potentially independent prognostic
factors. Student's t-test was used for continuous variables between
two groups. Differences with a P-value (two-sided) of <0.05 were
considered to be statistically significant.
Results
The patient clinicopathological
characteristics
Two hundred and twelve patients with unresectable
PDAC were included in this study, including 76 locally advanced and
136 metastatic cases, with a median age of 61 years (range, 33–82
years). Among this cohort of patients, 172 (81.1%) patients
underwent gemcitabine-based chemotherapy, and another 40 (18.9%)
patients received only palliative or best supportive care. We
detected peripheral T cell subsets in all 212 patients before any
treatment and monitored the alteration in T cell subsets in 100
patients who underwent two cycles of chemotherapy. The patient
characteristics are summarized in Table I.
 | Table IClinicopathological parameters of
patients with unresectable pancreatic cancer (N=212). |
Table I
Clinicopathological parameters of
patients with unresectable pancreatic cancer (N=212).
| Parameters | N (%) |
|---|
| Age (years)
(median, 61; range, 33–82) | |
| <60 | 90
(42.5) |
| ≥60 | 122 (57.5) |
| Gender | |
| Male | 133 (62.7) |
| Female | 79
(37.3) |
| PS | |
| 90–100 | 78
(36.8) |
| 70–80 | 134 (63.2) |
| Tumor location | |
| Body and tail | 94
(44.3) |
| Head | 118 (55.7) |
| TNM stage | |
| III | 76
(35.8) |
| IV | 136 (64.2) |
| CA19-9 levels
(U/ml) (median, 301.3; range, 0.68–>1000 U/ml) | |
| <301.3 | 106 (50) |
| ≥301.3 | 106 (50) |
| CA125 levels
(U/ml) | |
| <35 | 101 (47.6) |
| ≥35 | 111 (52.4) |
| Chemotherapy | |
| Yes | 172 (81.1) |
| No | 40
(18.9) |
| Survival
status | |
| Alive | 3 (1.4) |
| Dead | 209 (98.6) |
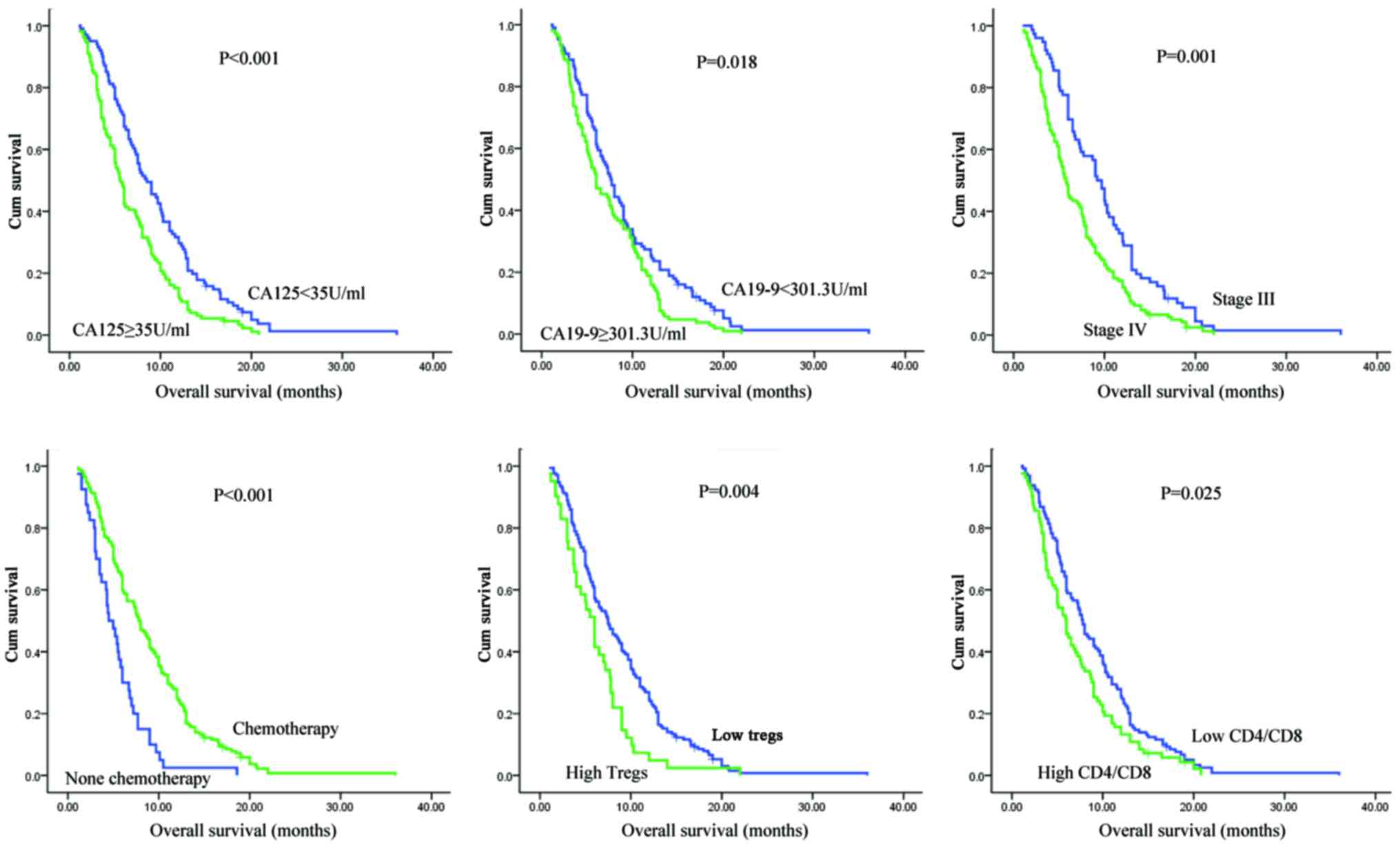
The status of peripheral T cell subsets
predicts overall survival in patients with unresectable pancreatic
cancer before treatment
In univariate and multivariate analyses of
clinicopathological parameters for pancreatic cancer, we found that
the serum level of CA19-9, performance status (PS), TNM stage, and
chemotherapy or no chemotherapy were independent prognostic factors
for unresectable PDAC. For circulating T cell subsets, an initial
CD4/CD8 ratio or Treg level before any treatment was associated
with the prognosis of locally advanced and metastatic disease
(Table II and Fig. 1).
 | Table IIUnivariate and multivariate analyses
of clinicopathological parameters for the prediction of overall
survival in patients with unresectable pancreatic cancer
(N=212). |
Table II
Univariate and multivariate analyses
of clinicopathological parameters for the prediction of overall
survival in patients with unresectable pancreatic cancer
(N=212).
| Univariate analyses
| Multivariate
analyses
|
|---|
| Parameters | P-value | HR (95% CI) | P-value | HR (95% CI) |
|---|
| Age (years) | 0.844 | | 0.899 | |
| <60 vs.
≥60 | | – | | – |
| Gender | 0.405 | | 0.229 | |
| Male vs.
female | | – | | – |
| PS | 0.003 | 0.645 | 0.041 | 0.722 |
| (90–100) vs.
(70–80) | | (0.482–0.862) | | (0.544–0.987) |
| TNM stage | 0.001 | 0.603 | 0.002 | 0.61 |
| III vs. IV | | (0.453–0.804) | | (0.448–0.83) |
| CA19-9 level
(U/ml) | 0.018 | 0.718 | 0.01 | 0.69 |
| <301.3 vs.
≥301.3 | | (0.545–0.945) | | (0.52–0.916) |
| CA125 level
(U/ml) | <0.001 | 0.598 | 0.082 | – |
| <35 vs.
≥35 | | (0.453–0.789) | | |
| Chemotherapy | <0.001 | 0.429 | <0.001 | 0.428 |
| Yes vs. no | | (0.299–0.614) | | (0.292–0.627) |
| CD3+ T
cells | 0.144 | – | | |
| High vs. low
(132/80) | | | | |
| CD4+ T
cells | 0.18 | – | | |
| High vs. low
(124/88) | | | | |
| CD8+ T
cells | 0.527 | – | | |
| High vs. low
(114/98) | | | | |
|
CD8+CD28+ T
cells | 0.126 | | | |
| High vs. low
(181/28) | | | | |
| Tregs
(CD4+CD25+CD127−) | 0.004 | 1.656 | 0.015 | 1.567 |
| High vs. low
(41/171) | | (1.169–2.347) | | (1.09–2.257) |
| CD4/CD8 ratio | 0.025 | 1.379 | 0.032 | 1.374 |
| High vs. low
(83/129) | | (1.043–1.828) | | (1.021–1.835) |
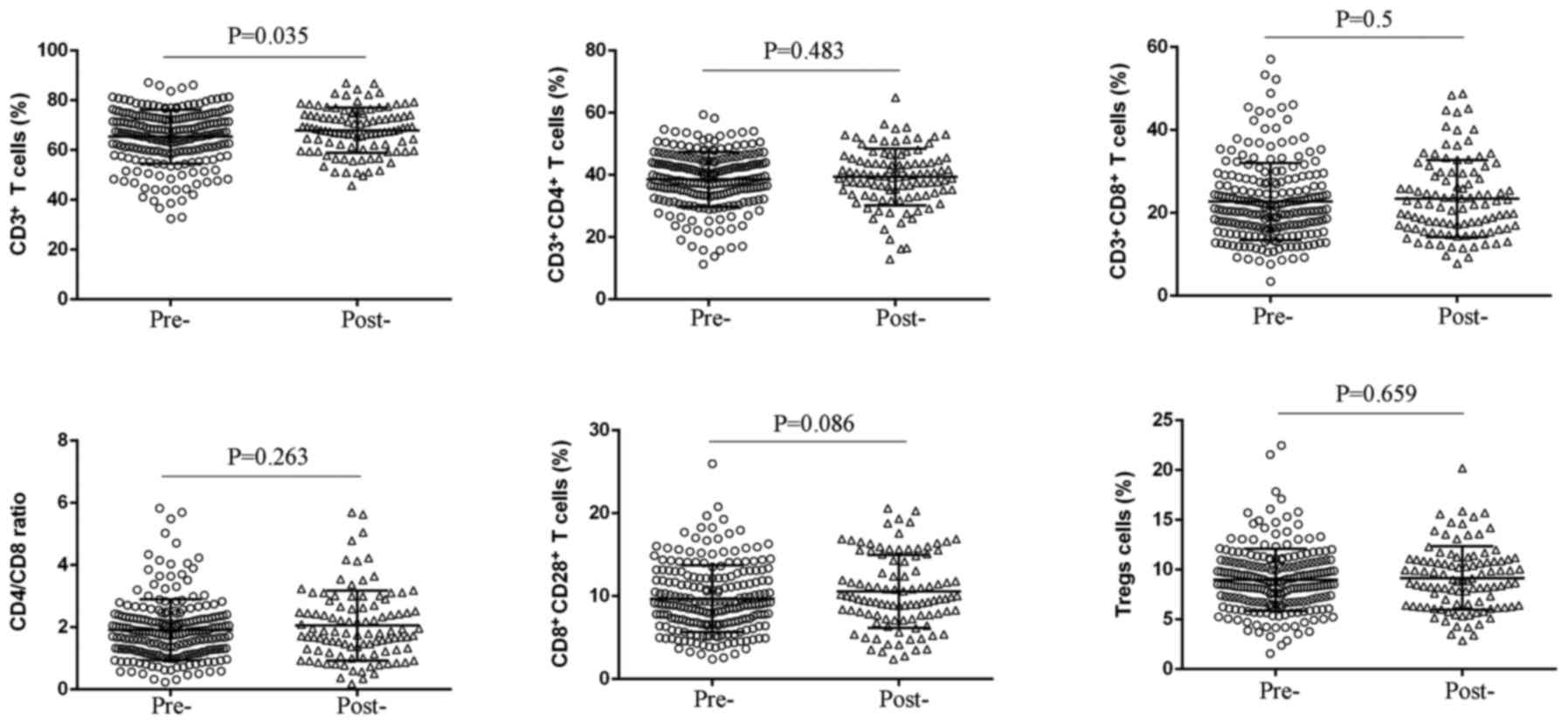
Alteration in peripheral T cell subsets
predicts chemotherapeutic response in patients with unresectable
pancreatic cancer
After two cycles of chemotherapy, we detected the
peripheral T cell subsets to observe the effect of chemotherapy on
T cell immune response, and found that there was no significant
change in percentages of CD3+CD4+ T cells,
CD3+CD8+ T cells, CD4/CD8 ratio,
CD8+CD28+ T cells, and Treg cells, whereas an
elevated level of CD3+ T cells was observed (P=0.035)
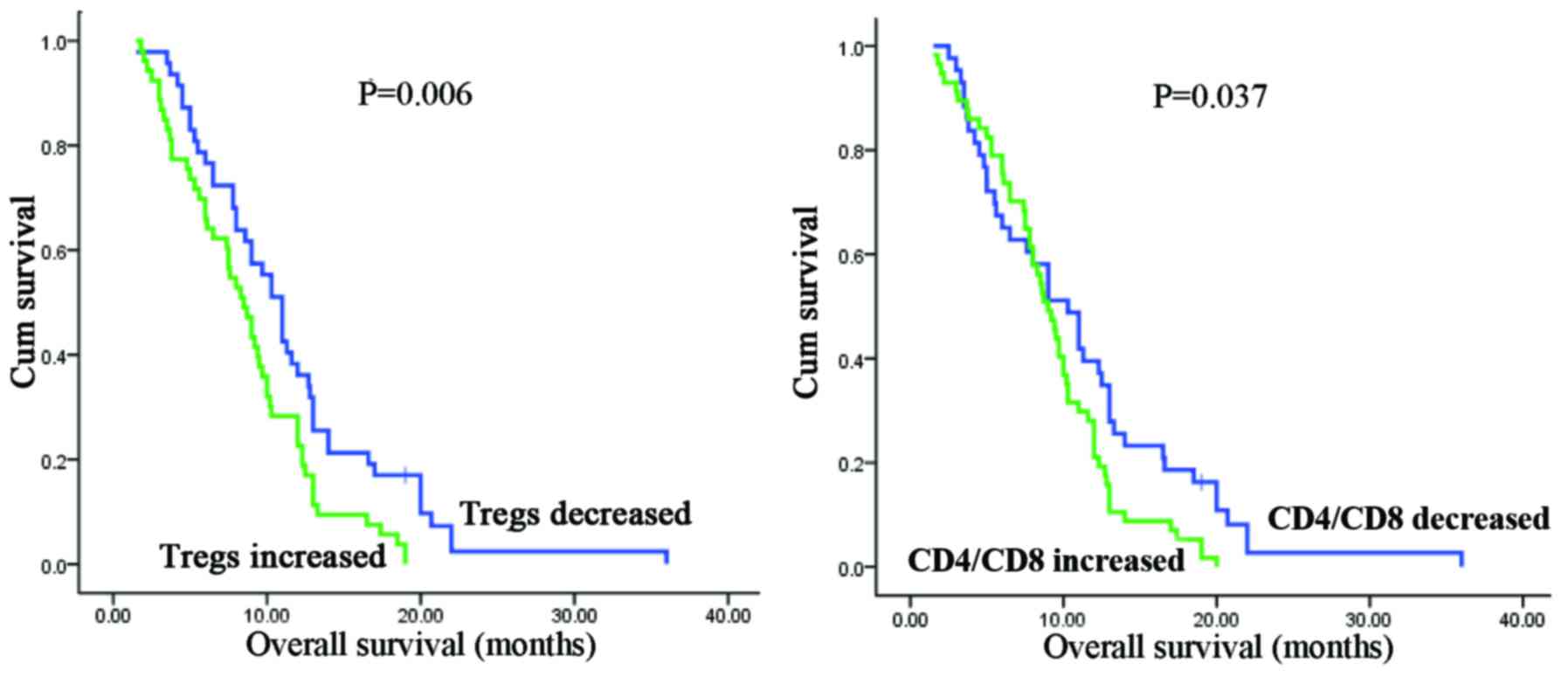
(Fig. 2 and Table III). Remarkably, decreased Tregs
or CD4/CD8 ratio after two cycles of chemotherapy predicts a longer
overall survival in patients with unresectable PDAC (Tregs:
P=0.006; CD4/CD8 ratio: P=0.037; Fig.
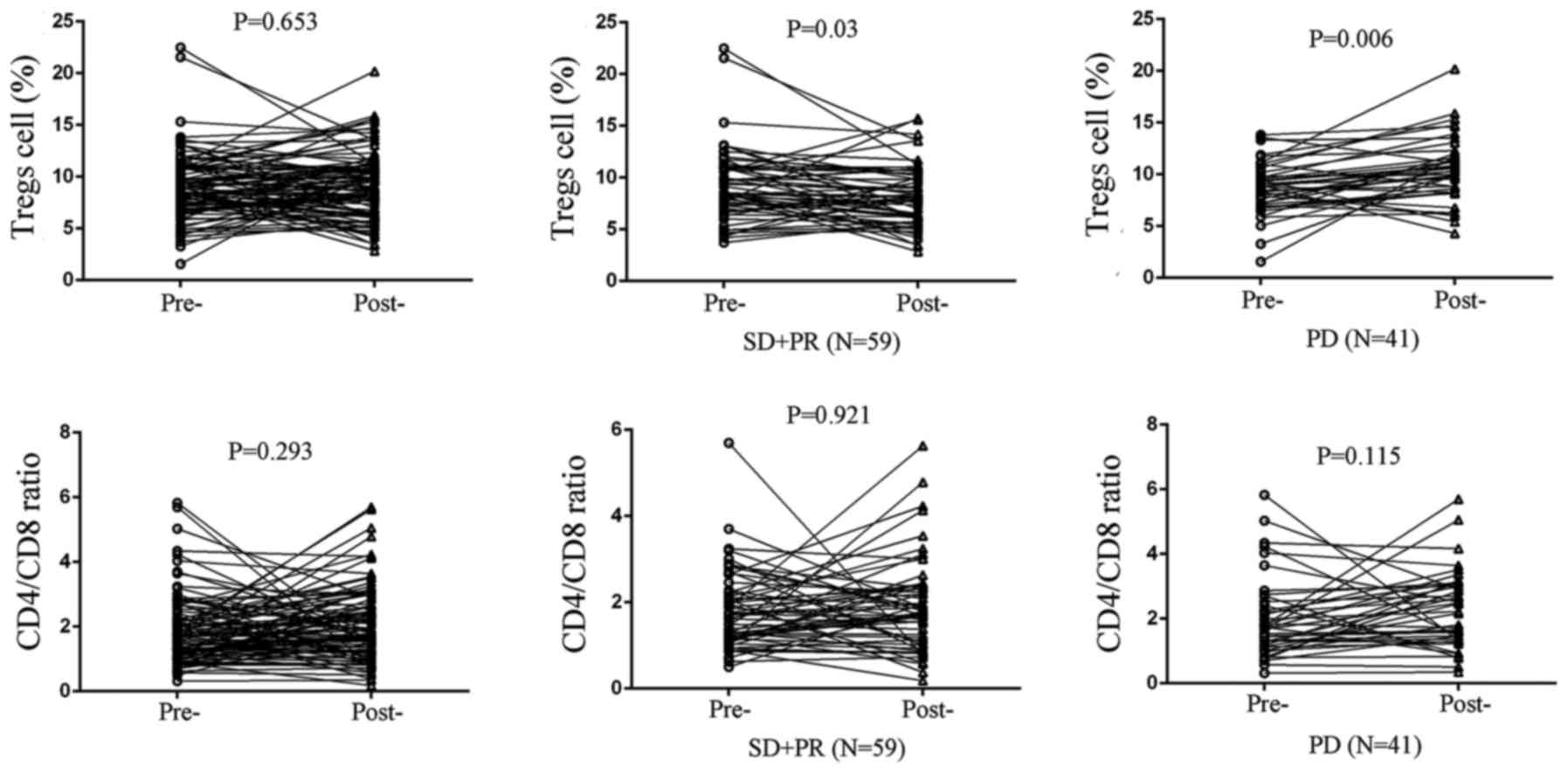
3). Furthermore, circulating Treg levels in stable disease (SD)
and partial remission (PR) cases significantly decreased after
chemotherapy (P=0.030; Fig. 4),
whereas circulating Treg levels increased in progressive disease
(PD) patients (P=0.006; Fig. 4).
However, no statistical significance was found in the cohorts
concerning CD4/CD8 ratio (Fig.
4).
 | Table IIIAnalysis of peripheral T cell subsets
in pancreatic cancer patients before or after chemotherapy. |
Table III
Analysis of peripheral T cell subsets
in pancreatic cancer patients before or after chemotherapy.
| Parameters | Percentages (%)
(means ± SD)
| P-value |
|---|
| Before chemotherapy
(N=212) | After chemotherapy
(N=100) |
|---|
|
CD3+ | 65.34±10.84 | 67.99±9.06 | 0.035 |
|
CD3+CD4+ | 38.58±8.8 | 39.34±9.16 | 0.483 |
|
CD3+CD8+ | 22.73±9.33 | 23.5±9.3 | 0.5 |
| CD4/CD8 ratio | 1.92±0.98 | 2.06±1.13 | 0.263 |
|
CD8+CD28+ | 9.7±4.01 | 10.56±4.41 | 0.086 |
|
CD4+CD25+CD127− | 8.99±3.13 | 9.17±3.31 | 0.659 |
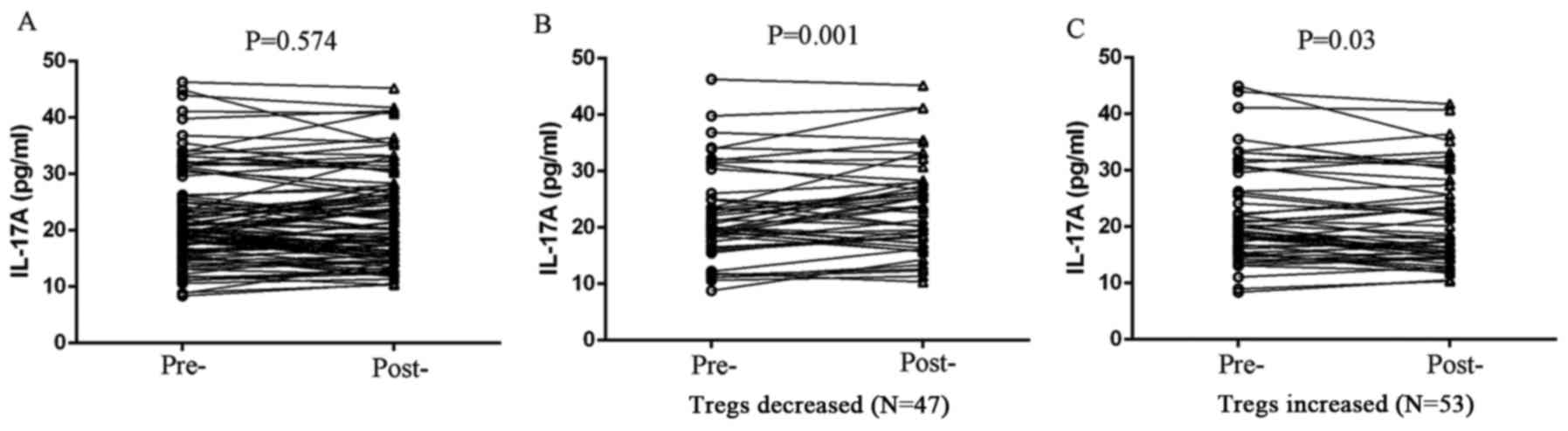
Peripheral IL-17A expression is
negatively associated with Tregs in unresectable pancreatic cancer
patients treated with chemotherapy
We detected peripheral expression of several
cytokines relevant to Treg differentiation, including TGF-β1, IL-6
and IL-17A in patients with unresectable PDAC before and after two
cycles of chemotherapy, and we found that there was no correlation
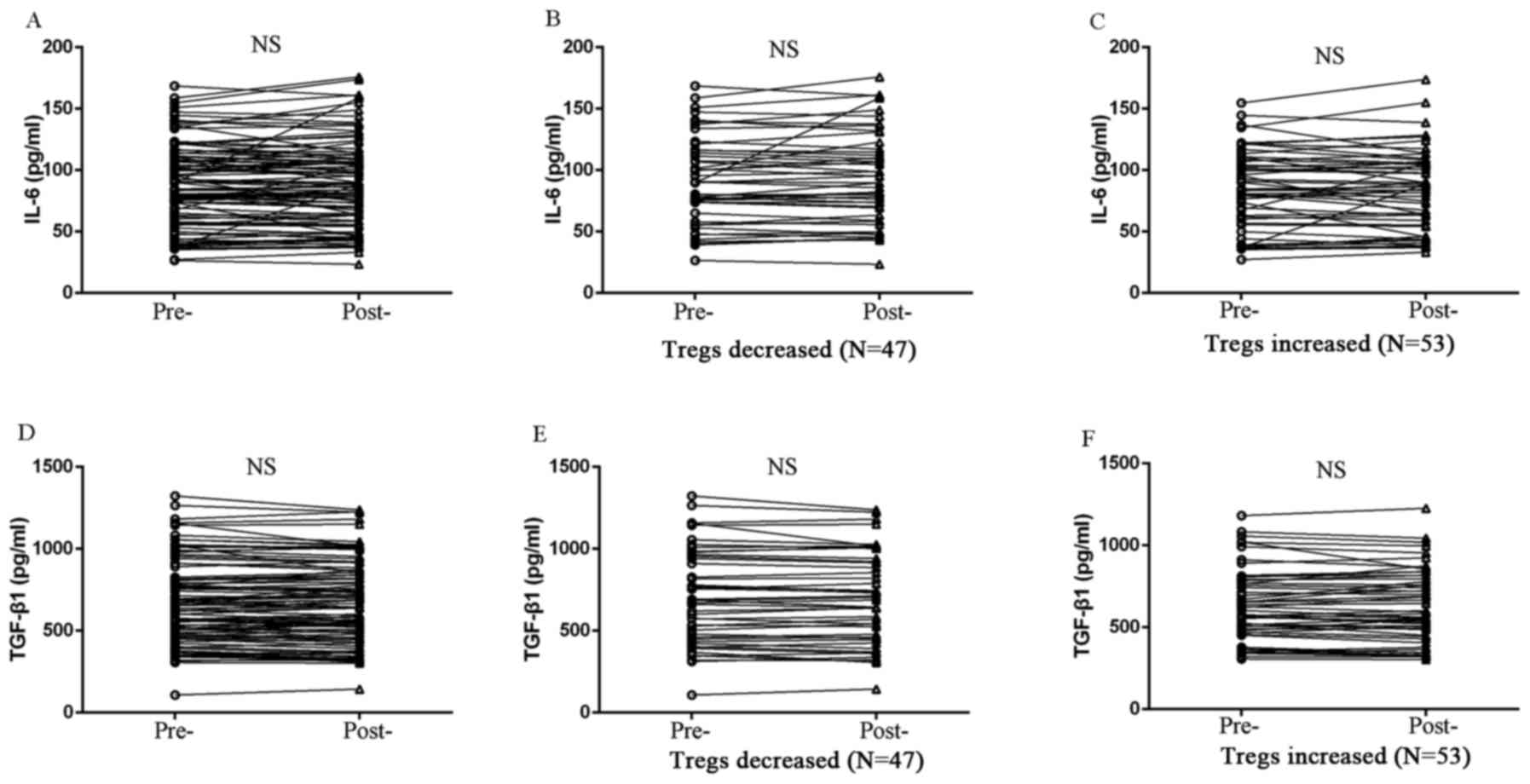
between Tregs and the expression of either TGF-β1 or IL-6 (Fig. 5). However, peripheral IL-17A
expression exhibited a negative correlation with Treg level in
patients treated with chemotherapy. IL-17A expression was
significantly elevated in Treg-decreased patients (P=0.001;
Fig. 6), whereas IL-17A level was
reduced in Treg-increased patients after chemotherapy (P=0.030;
Fig. 6).
Discussion
PDAC contains abundant stroma elements surrounding
cancer cells, and thereby has a unique biologic profile including
chemotherapy resistance and poor prognosis (12). The PDAC specific tumor
microenvironment lacks oxygen and blood supply, which contributes
to metabolic reprogramming and immune cell adaptation (12–14).
Emerging evidence demonstrates that tumor infiltrating T cells such
as Tregs, CD8+ T cells and Th17 cells were associated
with prognosis in various human cancers (15,16).
In a previous study, we investigated the expression of tumor
infiltrating cells within the specimens of resected pancreatic
cancer and found that more infiltration of CD4+ and
CD8+ T lymphocytes indicated a better prognosis after
surgical resection; in contrast, the primary immunosuppressive
factor Tregs and other T helper cell subsets could hardly be
detected in PDAC tissues (16). In
the present study, the percentage of peripheral Tregs and CD4/CD8
ratio before treatment was associated with overall survival of
patients, exhibiting a circulating T lymphocyte signature in
patients with advanced PDAC cases; thus, this may serve as an
effective marker for the prognosis of this subgroup of patients
before any treatment is done.
Because of the unique tumor microenvironment of
PDAC, contributed to by a consistent deprivation of oxygen and
nutrients, there will be a persistence of endoplasmic reticulum
(ER) stress, which is a mechanism of cell self-protection within
the tumor microenvironment of PDAC (17,18).
Given that certain chemotherapeutic agents were also stress
inducers, there existed a sustained stress status and subsequent
tumor metabolism reprogramming in advanced PDAC (19–21).
Prolonged ER stress promotes cell apoptosis, but, conversely, might
induce cancer cells to acquire more aggressive biologic features to
resist chemotherapeutic attack; furthermore, the percentage and
types of immune cells recruited by the tumor microenvironment also
adapted accordingly (13,14,19–21).
In particular, T cell dysfunction owing to nutrient deprivation and
tumor metabolism alteration was regarded as a major issue of
concern in antitumor immune response (22,23).
Immune responses to malignant cells can be categorized as
locoregional or systemic. In situ, the immune contexture
would be essential to accurately define the impact of the local
host-immune reaction in tumor (24). It has been well reported that the
infiltrating immune cells, in tumor site, play important roles in
tumor progression. Unfortunately, in most of the patients with
unresectable PDAC, a sufficient amount of tumor tissues to detect
intratumoral infiltration of immune cells is difficult to obtain,
especially for monitoring chemotherapy-related immune status.
Systemic immunity to tumors, as measured in the peripheral
circulation, is difficult to demonstrate and tumor-specific
responses are particularly elusive. The role of systemic immunity
in tumor progression deserves further study. The available evidence
suggests that both local and systemic antitumor immunity is
compromised in patients with cancer. Many researchers have
investigated the correlation of circulating immune cells and tumor
infiltrating lymphocytes (TIL) in regard to distribution and
function characterization. Circulating T cells obtained from
patients with cancer are either biased in cytokine profile or
otherwise functionally compromised. Furthermore, dysfunction in
circulating lymphocytes was linked to the extent of dysfunction
seen in paired TIL and to the disease stage and/or activity
(25,26). It remains controversial whether
systemic chemotherapy impairs the immune system. A limited number
of patients with pancreatic cancer exhibited a favorable response
to systemic chemotherapy, and only limited available options
including gemcitabine-based or fluoropyrimidine-based regimens were
recommended (9,27). Accordingly, the balance between
benefit and risk of chemotherapy in advanced PDAC should be well
evaluated. Therefore, if the circulating immune parameters have
prognostic value, the markers would be more convenient and
accessible to monitor tumor progression. In the present study, the
changes in circulating Tregs and CD4+/CD8+ T
cell ratio before and after chemotherapy discriminate the
populations that benefit from chemotherapy, to some extent, and
show the divergence in adaption of T cell immune status responsive
to systemic chemotherapy in patients with high PDAC tumor burden.
Furthermore, decreased Tregs was detected more in PR and SD cases.
Moreover, the increased amount of immunosuppressive T cells found
in PD cases also indicated that PDAC response to chemotherapy was
associated with immune adaption of T cell subsets following a local
or systemic stress response or metabolism reprogramming.
The chemotherapeutic agent is a two-edged sword
having both immune suppressing and promoting effects.
CD3+ T cells contain various T cell subsets, such as
CD4+ T cells, CD8+ T cells and Tregs.
Although our results found that the percentage of CD3+ T
cells increased after two cycles of chemotherapy, suggesting
chemotherapy does not lead to total T cell ratio decrease, the
change of other T cell subsets need to be further verified. It was
shown that some chemo-agents could induce T-cell infiltration into
pancreatic cancer. Tsuchikawa et al (28) reported that Foxp3+ T
cell infiltration was significantly lower in the neoadjuvant
chemoradiation therapy cases, while the numbers of CD4+
and CD8+ T cell infiltration had no statistical
difference in the tumor microenvironment. Other studies found that
CD4+ and CD8+ T cells were significantly
increased after neoadjuvant chemotherapy (29). However, for circulating T cells,
although it was reported that circulating CD4+ and
CD8+ T cells were changed after chemotherapy in other
cancers such as breast (30) and
cervical cancer patients (31),
the relationship between circulating T cells and chemotherapy was
still unclear in pancreatic cancer. In the present study,
gemcitabine-based chemotherapy did not exhibit an obviously
impaired effect on human T cell immune system, but underlined some
insights that high tumor burden PDAC and continued chemotherapy
could result in differentiation of T cell subsets.
The change of T cell proportion may be altered by
the chemotherapy or by the shrinkage of the tumor. Many studies
reported that chemotherapy agents could change tumor
microenvironment through various mechanisms, such as increasing
lymphocyte infiltration, depletion of Tregs and inducing
differentiation of MDSC (32).
However, for the circulating lymphocytes, it was still unclear. It
was reported that low Treg percentage in circulation at 1 year
after pancreatic cancer resection was correlated with improved
survival (33). We also previously
reported that high Treg percentage in circulation predicts poor
prognosis in resectable pancreatic cancer patients (8). We consider that circulating
lymphocyte levels are features of immune status in pancreatic
cancer patients. Our data showed that Treg cells decreased after
chemotherapy in SD+PR patients (N=59), while increased in PD
patients (N=41), suggested that patients with good immune status
during the period of chemotherapy, which may be caused by the
shrinkage of tumor, are correlated with prognosis. Metabolic
features of a hypoxic microenvironment, including glycolysis and
Warburg effect, might not only enable cancer cells to develop
different biological properties, but may also influence T cell
function and T cell differentiation (34–36).
Several transcription factors and signaling pathways, such as
HIF-1α, PD-1 and mTOR signaling are involved in T cell immune
adaptation and differentiation (37). Hypoxia and nutrient deprivation
contribute to accumulation of metabolic products and then suppress
CD8+ T effector cells and CD4+ Th1, Th2 and
Th17 subsets, whereas they induce immunosuppressive Treg lineage
(38,39). Generally, the percentage of tumor
infiltrating CD4+ T cells and CD8+ effector T
cells indicates a different prognostic value in cancer (40). For CD4+ T cell subsets,
the plasticity between regulatory T cells and Th17 lineage of T
helper cells has been widely studied recently (41,42).
Although Tregs always accompany poor clinical outcomes, Th17
exhibits some inconsistent prognostic significance in various human
cancers (43,44). Tregs are reported at elevated
frequencies in the peripheral blood and the tumor itself, and
correlates with poor outcome. It was reported that Tregs can
suppress immune cell activity and induce tumor cell escape from
immune surveillance via secreting several cytokines. In addition,
it was shown that Tregs complexed with STAT3, a transcription
factor that is essential for the differentiation of Th17 cells,
then activate IL-17 gene transcription to promote Th17 cell
differentiation (45,46). Tregs and Th17 cells are inversely
associated in the same tumors, and there is a dynamic interaction
between Th17 and Tregs in the tumor microenvironment. The balance
of Tregs/Th17 contributed to the shift between pro-inflammatory and
anti-inflammatory response and somewhat indicates the prognosis of
human cancer (47,48). It was reported that
IL17+FOXP3+ T cells can be detected in
tumors, which also express CD25 and TH17 specific marker RORγt and
have suppressive functions. Moreover, Treg cells can
transdifferentiate towards an IL-17+FOXP3+ T
phenotype and eventually into FOXP3-TH17 cells (49,50).
In the study, we detected several cytokines involved with the
transdifferentiation between Tregs and T helper cell subsets in
advanced PDAC cases. In addition, we found that the expression of
IL-17A, which is characterized by the secretion of Th17, was
significantly elevated in the cases whose Treg level was decreased,
indicating the possibility that a shift from Tregs to Th17 might be
responsible for the sensitivity to chemotherapy in patients with
unresectable PDAC.
The present study included locally advanced and
meta-static cases of PDAC, the basic characteristics in our group
were similar with other studies (3,51).
In addition, we also identified an initial CD4/CD8 ratio or Treg
level before any treatment was associated with the prognosis via
multivariate analyses. Therefore, we concluded the significant
difference is valuable. As limitation in the present study, we
found that the change of proportion in CD3 positive cells had
statistical significance (P=0.035), while the main subsets of
CD3+ cells such as CD4+, CD8+ and
Treg cells remain stable after chemotherapy. However, we did not
detect other T cell subsets (Th1, Th2 or Th17), which might change
after chemotherapy. These results need to be further identified via
expanding sample sizes and detecting more subsets of immune cells.
In addition, we also found that there was no correlation between
Tregs and the expression level of either IL-2 or IL-10 (data not
shown), while other cytokines such as IFN-γ and IL-5, which may be
associated with helper T cell subsets or Tregs, were not detected.
We will continue to collect samples to validate these results and
explore potential mechanisms in future.
Overall, the present study shows a circulating
signature of T cell subsets that could predict the prognosis of
patients with unresectable PDAC before treatment. This finding
could also predict the response to gemcitabine-based chemotherapy,
which could be contributed to by the plasticity of T cell subsets
and their capacity to differentiate from one subset toward another
lineage, depending on a high tumor burden PDAC setting or
chemotherapy-related metabolic changes.
Acknowledgments
The present study is supported by grants from the
National Natural Science Foundation of China (nos. 81370065,
81372653 and SINO-GERMAN GZ857), and the Basic Research Projects of
the Science and Technology Commission of Shanghai Municipality
(15JC1401200).
References
|
1
|
Long J, Luo GP, Xiao ZW, Liu ZQ, Guo M,
Liu L, Liu C, Xu J, Gao YT, Zheng Y, et al: Cancer statistics:
Current diagnosis and treatment of pancreatic cancer in Shanghai,
China. Cancer Lett. 346:273–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luo G, Guo M, Liu Z, Xiao Z, Jin K, Long
J, Liu L, Liu C, Xu J, Ni Q, et al: Blood neutrophil-lymphocyte
ratio predicts survival in patients with advanced pancreatic cancer
treated with chemotherapy. Ann Surg Oncol. 22:670–676. 2015.
View Article : Google Scholar
|
|
4
|
Liu C, Lu Y, Luo G, Cheng H, Guo M, Liu Z,
Xu J, Long J, Liu L, et al: Which patients with para-aortic lymph
node (LN16) metastasis will truly benefit from curative
pancreaticoduodenectomy for pancreatic head cancer? Oncotarget.
7:29177–29186. 2016.PubMed/NCBI
|
|
5
|
Wörmann SM, Diakopoulos KN, Lesina M and
Algül H: The immune network in pancreatic cancer development and
progression. Oncogene. 33:2956–2967. 2014. View Article : Google Scholar
|
|
6
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, et al: Specific recruitment of regulatory T cells in
ovarian carcinoma fosters immune privilege and predicts reduced
survival. Nat Med. 10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clark CE, Beatty GL and Vonderheide RH:
Immunosurveillance of pancreatic adenocarcinoma: Insights from
genetically engineered mouse models of cancer. Cancer Lett.
279:1–7. 2009. View Article : Google Scholar
|
|
8
|
Xu YF, Lu Y, Cheng H, Shi S, Xu J, Long J,
Liu L, Liu C and Yu X: Abnormal distribution of peripheral
lymphocyte subsets induced by PDAC modulates overall survival.
Pancreatology. 14:295–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rébé C and Ghiringhelli F: Cytotoxic
effects of chemotherapy on cancer and immune cells: How can it be
modulated to generate novel therapeutic strategies? Future Oncol.
11:2645–2654. 2015. View Article : Google Scholar
|
|
10
|
Bardeesy N and DePinho RA: Pancreatic
cancer biology and genetics. Nat Rev Cancer. 2:897–909. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neoptolemos JP, Stocken DD, Friess H,
Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C,
Lacaine F, et al European Study Group for Pancreatic Cancer: A
randomized trial of chemoradiotherapy and chemotherapy after
resection of pancreatic cancer. N Engl J Med. 350:1200–1210. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo G, Long J, Zhang B, Liu C, Xu J, Ni Q
and Yu X: Stroma and pancreatic ductal adenocarcinoma: An
interaction loop. Biochim Biophys Acta. 1826:170–178.
2012.PubMed/NCBI
|
|
13
|
Herbel C, Patsoukis N, Bardhan K, Seth P,
Weaver JD and Boussiotis VA: Clinical significance of T cell
metabolic reprogramming in cancer. Clin Transl Med. 5:292016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Molon B, Calì B and Viola A: T cells and
cancer: How metabolism shapes immunity. Front Immunol. 7:202016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshida N, Kinugasa T, Miyoshi H, Sato K,
Yuge K, Ohchi T, Fujino S, Shiraiwa S, Katagiri M, Akagi Y, et al:
A High RORγT/CD3 ratio is a strong prognostic factor for
postoperative survival in advanced colorectal cancer: Analysis of
helper T cell lymphocytes (Th1, Th2, Th17 and regulatory T cells).
Ann Surg Oncol. 23:919–927. 2016. View Article : Google Scholar
|
|
16
|
Wang WQ, Liu L, Xu HX, Wu CT, Xiang JF, Xu
J, Liu C, Long J, Ni QX and Yu XJ: Infiltrating immune cells and
gene mutations in pancreatic ductal adenocarcinoma. Br J Surg.
103:1189–1199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Antonucci L, Fagman JB, Kim JY, Todoric J,
Gukovsky I, Mackey M, Ellisman MH and Karin M: Basal autophagy
maintains pancreatic acinar cell homeostasis and protein synthesis
and prevents ER stress. Proc Natl Acad Sci USA. 112:E6166–E6174.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Zhu F, Jiang J, Sun C, Zhong Q, Shen
M, Wang X, Tian R, Shi C, Xu M, et al: Simultaneous inhibition of
the ubiquitin-proteasome system and autophagy enhances apoptosis
induced by ER stress aggravators in human pancreatic cancer cells.
Autophagy. 12:1521–1537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kong B, Wu W, Valkovska N, Jäger C, Hong
X, Nitsche U, Friess H, Esposito I, Erkan M, Kleeff J, et al: A
common genetic variation of melanoma inhibitory activity-2 labels a
subtype of pancreatic adenocarcinoma with high endoplasmic
reticulum stress levels. Sci Rep. 5:81092015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mahalingam D, Patel S, Nuovo G, Gill G,
Selvaggi G, Coffey M and Nawrocki ST: The combination of
intravenous Reolysin and gemcitabine induces reovirus replication
and endoplasmic reticular stress in a patient with KRAS-activated
pancreatic cancer. BMC Cancer. 15:5132015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng S, Swanson K, Eliaz I, McClintick
JN, Sandusky GE and Sliva D: Pachymic acid inhibits growth and
induces apoptosis of pancreatic cancer in vitro and in vivo by
targeting ER stress. PLoS One. 10:e01222702015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ho PC, Bihuniak JD, Macintyre AN, Staron
M, Liu X, Amezquita R, Tsui YC, Cui G, Micevic G, et al:
Phosphoenolpyruvate is a metaboliccheckpoint of anti-tumor T cell
responses. Cell. 162:1217–1228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Biswas SK: Metabolic reprogramming of
immune cells in cancer progression. Immunity. 43:435–449. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fridman WH, Pagès F, Sautès-Fridman C and
Galon J: The immune contexture in human tumours: Impact on clinical
outcome. Nat Rev Cancer. 12:298–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reichert TE, Strauss L, Wagner EM, Gooding
W and Whiteside TL: Signaling abnormalities, apoptosis, and reduced
proliferation of circulating and tumor-infiltrating lymphocytes in
patients with oral carcinoma. Clin Cancer Res. 8:3137–3145.
2002.PubMed/NCBI
|
|
26
|
Fu J, Zhang Z, Zhou L, Qi Z, Xing S, Lv J,
Shi J, Fu B, Liu Z, Zhang JY, et al: Impairment of CD4+
cytotoxic T cells predicts poor survival and high recurrence rates
in patients with hepatocellular carcinoma. Hepatology. 58:139–149.
2013. View Article : Google Scholar
|
|
27
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuchikawa T, Hirano S, Tanaka E,
Matsumoto J, Kato K, Nakamura T, Ebihara Y and Shichinohe T: Novel
aspects of preoperative chemoradiation therapy improving anti-tumor
immunity in pancreatic cancer. Cancer Sci. 104:531–535. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Homma Y, Taniguchi K, Murakami T, Nakagawa
K, Nakazawa M, Matsuyama R, Mori R, Takeda K, Ueda M, Ichikawa Y,
et al: Immunological impact of neoadjuvant chemoradiotherapy in
patients with borderline resectable pancreatic ductal
adenocarcinoma. Ann Surg Oncol. 21:670–676. 2014. View Article : Google Scholar
|
|
30
|
Bailur JK, Pawelec G, Hatse S, Brouwers B,
Smeets A, Neven P, Laenen A, Wildiers H and Shipp C: Immune
profiles of elderly breast cancer patients are altered by
chemotherapy and relate to clinical frailty. Breast Cancer Res.
19:202017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van Meir H, Nout RA, Welters MJ, Loof NM,
de Kam ML, van Ham JJ, Samuels S, Kenter GG, Cohen AF, Melief CJ,
et al: Impact of (chemo)radiotherapy on immune cell composition and
function in cervical cancer patients. OncoImmunology.
6:e12670952016. View Article : Google Scholar
|
|
32
|
Tsuchikawa T, Takeuchi S, Nakamura T,
Shichinohe T and Hirano S: Clinical impact of chemotherapy to
improve tumor microenvironment of pancreatic cancer. World J
Gastrointest Oncol. 8:786–792. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamamoto T, Yanagimoto H, Satoi S,
Toyokawa H, Hirooka S, Yamaki S, Yui R, Yamao J, Kim S and Kwon AH:
Circulating CD4+CD25+ regulatory T cells in
patients with pancreatic cancer. Pancreas. 41:409–415. 2012.
View Article : Google Scholar
|
|
34
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pavlova NN and Thompson CB: The emerging
hallmarks of cancer metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: Metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Speiser DE, Ho PC and Verdeil G:
Regulatory circuits of T cell function in cancer. Nat Rev Immunol.
16:599–611. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dang EV, Barbi J, Yang HY, Jinasena D, Yu
H, Zheng Y, Bordman Z, Fu J, Kim Y, et al: Control of
TH17/Treg balance by hypoxia-inducible factor
1. Cell. 146:772–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sinclair LV, Rolf J, Emslie E, Shi YB,
Taylor PM and Cantrell DA: Control of amino-acid transport by
antigen receptors coordinates the metabolic reprogramming essential
for T cell differentiation. Nat Immunol. 14:500–508. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang Y, Ma C, Zhang Q, Ye J, Wang F,
Zhang Y, Hunborg P, Varvares MA, Hoft DF, Hsueh EC, et al:
CD4+ and CD8+ T cells have opposing roles in
breast cancer progression and outcome. Oncotarget. 6:17462–17478.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guéry L and Hugues S: Th17 Cell plasticity
and functions in cancer immunity. BioMed Res Int. 2015:3146202015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ivanova EA and Orekhov AN: T Helper
lymphocyte subsets and plasticity in autoimmunity and cancer: An
overview. BioMed Res Int. 2015:3274702015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chaudhary B, Elkord E and Regulatory T:
Regulatory T cells in the tumor microenvironment and cancer
progression: Role and Therapeutic Targeting. Vaccines (Basel).
4:E282016. View Article : Google Scholar
|
|
44
|
Alizadeh D, Katsanis E and Larmonier N:
The multifaceted role of Th17 lymphocytes and their associated
cytokines in cancer. Clin Dev Immunol. 2013:9578782013. View Article : Google Scholar
|
|
45
|
Chaudhry A, Rudra D, Treuting P, Samstein
RM, Liang Y, Kas A and Rudensky AY: CD4+ regulatory T
cells control TH17 responses in a Stat3-dependent manner. Science.
326:986–991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang X, Wang L, Mo Q, Dong Y, Wang G and
Ji A: Changes of Th17/Treg cell and related cytokines in pancreatic
cancer patients. Int J Clin Exp Pathol. 8:5702–5708.
2015.PubMed/NCBI
|
|
47
|
Gagliani N, Amezcua Vesely MC, Iseppon A,
Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limón P, Paiva RS,
Ching T, et al: Th17 cells transdifferentiate into regulatory T
cells during resolution of inflammation. Nature. 523:221–225. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang G, Li H, Yao Y, Xu F, Bao Z and Zhou
J: Treg/Th17 imbalance in malignant pleural effusion partially
predicts poor prognosis. Oncol Rep. 33:478–484. 2015.
|
|
49
|
Chellappa S, Hugenschmidt H, Hagness M,
Line PD, Labori KJ, Wiedswang G, Taskén K and Aandahl EM:
Regulatory T cells that co-express RORγt and FOXP3 are
pro-inflammatory and immunosuppressive and expand in human
pancreatic cancer. OncoImmunology. 5:e11028282015. View Article : Google Scholar
|
|
50
|
Zou W and Restifo NP: TH17
cells in tumour immunity and immunotherapy. Nat Rev Immunol.
10:248–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|