Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common form of cancer and the most aggressive and frequently
diagnosed malignancy of the liver. HCC is responsible for more than
600,000 deaths annually in the world. The main risk factors of HCC
include hepatitis B virus infection, hepatitis C virus infection,
alcohol abuse and tobacco. Recent data indicate that the mortality
of primary liver cancer in China is increasing (1). Surgical resection has been considered
the classical treatment, but only a small proportion of patients
are diagnosed in time to have the chance for surgery. Therefore,
there is a critical need for effective approaches for HCC
treatment, especially in intermediate-stage and end-stage.
The signal transducer and activator of transcription
(STAT) protein family have been shown to play an important role in
tumor cell survival and proliferation (2). Among them, constitutive activation of
STAT3 has been detected in a wide number of human cancer cell lines
and primary tumors, including 50% of HCC (3,4).
STAT3 can be activated by certain cytokines such as interleukin-6
(IL-6) (5), leukemia inhibitory
factor (LIF) (6) and interferon-α
(IFN-α) (7). Among these
inflammation factors inducing STAT3 phosphorylation, IL-6 is
regarded as one of the most vital cytokines in the studies
published in the past few years. Patients with HCC show elevated
levels of IL-6 in their serum compared with those with liver
cirrhosis or healthy individuals (8). The homodimerization of the IL-6
triggers a signaling cascade of phosphorylation of Janus kinases 2
(JAK2) and a downstream effector STAT3, followed by reciprocal
dimerization of the Tyr705 phosphorylated STAT3 (9). Then, the STAT3 downstream target
genes are activated, including Bcl-xl, Bcl-2 and survivin (10,11).
It has been shown that aberrant activation of IL-6/JAK2/STAT3
signaling pathway plays an important role in pathogenesis and
progress of liver cancer (12).
However, there is still no JAK2/STAT3 inhibitors approved to be
used clinically for the treatment or prevention of HCC.
Ursolic acid (UA) is natural triterpenoid compound
found in plants, herbs and other kinds of food. It has been
identified to play noteworthy role in anti-inflammatory,
hepatoprotective and antiallergic activities. It suppresses cell
proliferation of various types of cancer, including multiple
myeloma (13), colon (14), breast (15), pancreatic (16) and prostate cancer (17) by inhibiting the STAT3 signaling
pathway. It has not been reported previously that UA inhibits
phosphorylation of JAK2 and STAT3 in liver cancer cell lines and
suppresses growth of hepatocellular carcinoma. Aberrant activation
of IL-6/JAK2/STAT3 signaling pathway has been shown to play a role
in pathogenesis of liver cancer, which features high malignancy and
relative high resistance to chemotherapy. Due to the fact that
different types of cancer may exhibit diverse resistance or
sensitivity to antitumor agents, it is urgent to detect the levels
of STAT3 phosphorylation and the effect of UA in liver cancer cells
despite previous studies describing the effect of UA in other
cancers.
Materials and methods
Human liver cancer cell lines
Human liver cancer cell lines (Hep3B, HEPG2,
SSMC-7721 and Huh7) were purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA) and cultured in Dulbecco's
modified Eagle's medium (DMEM) high glucose supplemented with 10%
fetal bovine serum (FBS; Hyclone Laboratories, Inc., Logan, UT,
USA) and 1% penicillin/streptomycin. All cancer cell lines were
cultured in a humidified 37°C incubator with 95% air and 5%
CO2.
Compounds
Ursolic acid (UA) was purchased from Sigma-Aldrich
(St. Louis, MO, USA). It was dissolved in sterile dimethyl
sulfoxide (DMSO) to get 20 mM stock solution for cell experiments,
and stored at −20°C until use. IL-6, IFN-α and LIF were from Cell
Signaling Technology (Danvers, MA, USA).
Cell viability assay
Cell viability was measured using the MTT assay. The
MTT cell viability assay kits was from Promoter Biotechnology Ltd.
(Wuhan, China) UA was added in cultured medium to form different
concentration (0, 10, 25, 30, 40 and 50 µM). Four liver
cancer cells types (4,500/well in 96-well plates) were incubated
with the medium at 37°C for 24 h with UA. Then, 10 µl MTT
solution was added to each well and liver cancer cells were
cultured at 37°C. After 4 h, 100 µl MTT formanzan solution
was added to each well and a further 4-h incubation followed. The
optical density (OD) was measured at a wavelength of 570 nm. The
treated cells viability was calculated as follows: (OD of UA
group-OD of blank group/OD of DMSO group-OD of blank group) ×
100%.
Moreover, MTT was performed to determine if the
effect of UA on cell migration was due to its ability to inhibit
cell viability. Huh7 cells were treated with UA for 4 h, then
medium with UA was removed and replaced with fresh medium for
additional 36 h of incubation without UA. Then, 10 µl MTT
solution was added to each well and Huh7 cells were cultured at
37°C. After 4 h, 100 µl MTT formanzan solution was added to
each well and a further 4-h incubation followed. The optical
density (OD) was measured at a wavelength of 570 nm. The treated
cells viability was calculated as follows: (OD of UA group-OD of
blank group/OD of DMSO group-OD of blank group) × 100%.
Colony formation
Liver cancer cells were counted and plated in 6-well
cell culture plates and pretreated with different concentrations of
UA for 4 h at 37°C. The cells were then washed with
phosphate-buffered saline (PBS) twice and seeded on 10-cm plates
for 14 days in culture. After that, cells were fixed with cold
methanol for 15 min and stained with 0.5% crystal violet (25%
methanol) at room temperature for 10 min. Finally, the plates were
washed with distilled water and dried.
Wound healing assay
Liver cell lines were plated in 6-well plate (with
three lines at the external bottom of each well) and cultured in
DMEM)/high glucose supplemented with 10% FBS (HyClone Laboratories)
and 1% penicillin/streptomycin. When the cells grew to a confluence
of 100%, the monolayer cells were scratched using a 10-µl
pipette tip and rinsed with PBS to remove floating cells. Then the
cells were treated with varying concentrations of UA (25 and 50
µM) or DMSO for 4 h. Images of wound closure were taken at
the crossing point of lines and scratches by an inverted microscope
until the wound treated with DMSO was completely closed.
Transwell assay
Cells (5×104) pretreatment of UA for 4 h
were suspended in 200 µl serum-free DMEM medium and seeded
into the upper chamber of each insert. Then, 600 µl of DMEM
containing 10% FBS was added to a 24-well plate. After incubation
at 37°C for 24 h, the cells that migrated were fixed and stained
for 30 min in a dye solution containing 0.1% crystal violet and 20%
methanol.
Western blot analysis
Cancer cells were treated with UA (10, 25 and 50
µM) or DMSO for 12 h. After the treatments, cells were
collected. Cancer cells were serum-starved in media without FBS for
12 h before being treated with UA (10, 25, or 50 µM) or DMSO
for 4 h and incubated with IL-6 (50 ng/ml), LIF (25 ng/ml) or IFN-α
(25 ng/ml) for 30 min. Then the cells were collected and washed
with cold PBS and lysed on ice in a modified RIPA buffer (1% Triton
X-100, 1% deoxycholate, 0.1% SDS) containing protease inhibitors (1
mM PMSF), subjected to SDS-PAGE. Proteins were transferred onto
PVDF membrane and probed with antibodies (Cell Signaling
Technology). Membranes were probed with a 1:1,000 dilution of
primary antibodies (Cell Signaling Technology) against
phospho-specific STAT3 (Tyrosine 705, #9131), phospho-independent
STAT3 (#4904), phospho-specific STAT1 (Tyr 701, #8217),
phospho-specific STAT2 (Tyr 690, #4441), phospho-independent STAT2
(#4597), phospho-specific JAK2 (Tyr 1007/1008, #3776),
phospho-specific Akt (Ser473, #9271), phospho-independent Akt
(#9272), phospho-specific Erk1/2 (Thr 202/Tyr 204, #9101), cleaved
caspase-3 (Asp175, #9661), Bcl-2 (#2876), survivin (#2803), Bcl-xl
(#2762) and GAPDH (#2118). Phospho-independent STAT1 (#D120084) was
purchased from Sangon Biotech, Co., Ltd. (Shanghai, China).
HRP-conjugated secondary antibodies were from Promotor
Biotechnology Ltd. The specific proteins were detected using an
enhanced chemiluminescence (ECL) western blotting kit according to
the manufacturer's instructions.
Immunofluorescence staining
Cells were seeded on sterile glass slides and grew
for 24 h. Hep3B cells were pretreated with UA for 4 h after
serum-free overnight, then IL-6 was added for another 30 min. After
the treatments, the cells were washed with ice-cold PBS buffer, and
were fixed with ice-cold methanol at room temperature for 15 min.
After three washings with ice-cold PBS buffer, the cells were
permeabilized and blocked with PBS buffer containing 0.1% Triton
X-100 and 0.5% normal goat serum at room temperature for at least 1
h. Then the cells were probed with rabbit antibody to
phosphorylated STAT3 (1:50 dilution) at 4°C overnight. After the
overnight incubation, the cells were washed with PBS buffer
containing 0.1% Tween-20. The cells were incubated with
Cy3-conjugated anti-rabbit secondary antibody (1:100; Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) at room
temperature for 1 h. Cells were incubated for 5 min at room
temperature with DAPI (Vector Laboratories, Burlingame, CA, USA) to
stain nuclei and observed using an inverted fluorescence
microscope.
Mouse xenograft tumor model
All animal studies were conducted in accordance with
the standard procedures approved by the Institutional Animal Care
and Use Committee of Tongji Hospital, Huazhong University of
Science and Technology. Known as a classic liver cancer xenograft
model, human liver cancer cell line, HEPG2 (107 cells in
100 µl of sterile PBS and Matrigel), were injected
subcutaneously into the right flank region of mice (4–6 weeks of
age, 18–22 g). After tumor development, the mice were then
randomized into two groups: i) DMSO as vehicle control; and ii) 60
mg/kg UA (~1.2 mg of UA powder dissolved in a 100 µl of
mixture of 10 µl DMSO and 90 µl sterile water, ~2.6
µmol UA in 100 µl). Each mouse was given daily ~100
µl mixture. Vehicle and UA groups were orally treated once
daily for consecutive 15 days. Tumor growth was determined by
measuring the length (L) and width (W) of the tumor every other day
with a caliper. In addition, tumor volume was calculated on the
basis of the following formula: volume = (π/6) × L × W2.
The mice were sacrificed after 15 days of treatment. On the 15th
day, the body weight of mice was measured. The tumors were
harvested after mice were euthanized. We took images of the tumors
and measured the weight of tumors. Then tumors were stored at
−80°C. Tumor tissue homogenates were lysed and separated by
SDS-PAGE. The expression of STAT3, its downstream target genes
Bcl-2 and cleaved caspase-3 in xenograft tumors was examined by
western blot assay. A portion of tumor tissues were fixed by using
formalin and embedded in paraffin. The expression of P-STAT3 (Tyr
705) and Bcl-2 was also examined by immunohistochemistry (IHC)
staining. TUNEL (terminal deoxynucleotidyl transferase dUTP nick
end labeling) assay was used to detect apoptosis in xenograft
tumors.
Statistical analysis
The data are presented as the mean ± SD for at least
three independent experiments. Statistical analysis was performed
with SPSS software (version 13.0). The significant differences
between any of the two groups were evaluated by one-way analysis of
ANOVA. Statistical significance was defined as P<0.05.
Results
Ursolic acid inhibits STAT3
phosphorylation induced by IL-6 in Hep3B liver cancer cells
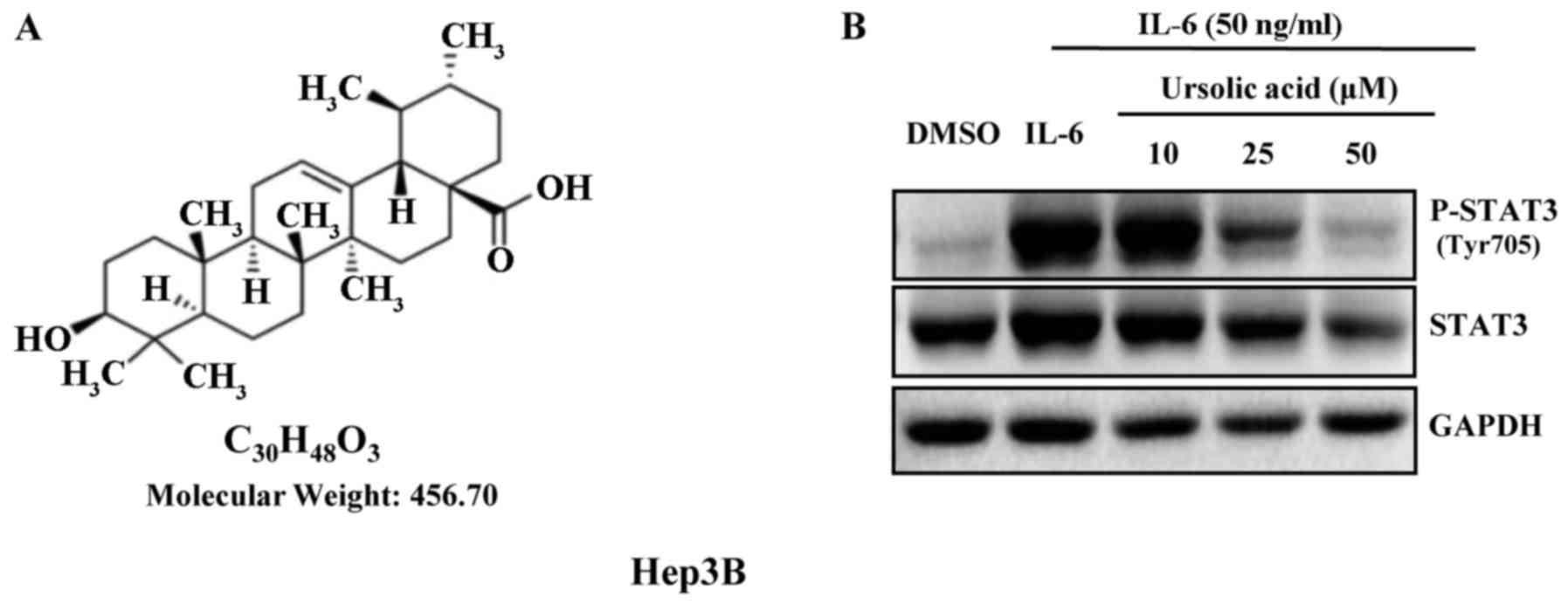
The structure of UA is shown in Fig. 1A. IL-6 is a growth factor and
induces STAT3 phosphorylation (18). UA inhibited STAT3 phosphorylation
induced by IL-6 (50 ng/ml) in a dose-dependent manner in Hep3B
cells. There was marked inhibition of phosphorylation of STAT3 with
UA (50 µM) for 4 h, but less significantly on STAT3
(Fig. 1B).
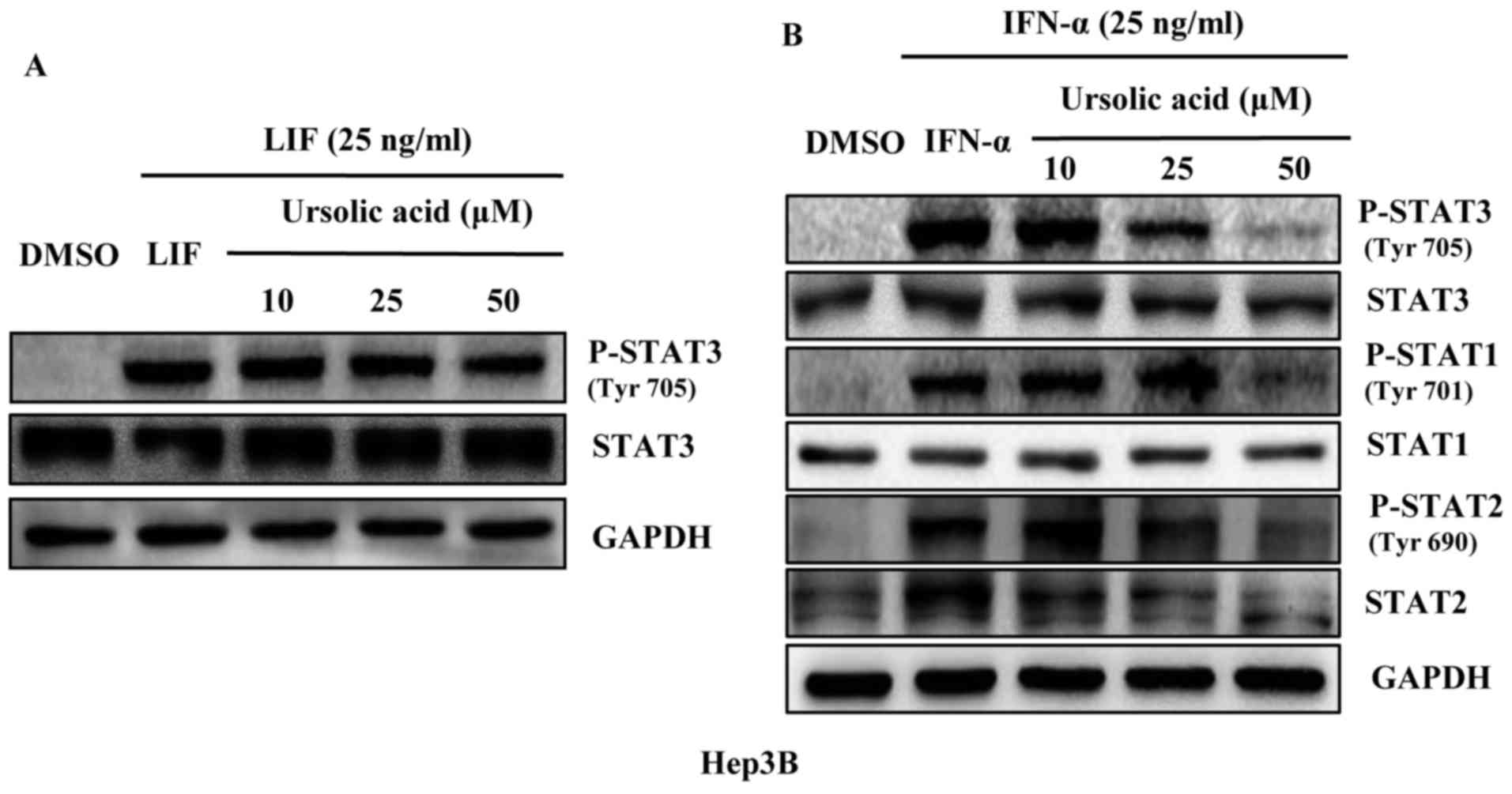
Ursolic acid does not inhibit the
phosphorylation of STAT3 induced by LIF, but inhibits the
phosphorylation of STAT1, STAT2 and STAT3 induced by IFN-α in Hep3B
liver cancer cells
Members of STATs family including STAT1 to STAT6 are
found in many kinds of cells and tissues as important mediators of
cytokine signaling. LIF is expressed in HCC but less than IL-6,
activating STAT3 pathway in liver cancer cells (19). As a member of the type I IFN family
IFN-α affects intracellular signaling through the Jak/Stat pathway.
In HCC, IFN-α can induce activation of STAT1 (20), STAT2 (21) and STAT3 (7). Our results showed that UA did not
inhibit the phosphorylation of STAT3 and total STAT3 induced by LIF
(Fig. 2A). But it could inhibit
the STAT1, STAT2 and STAT3 phosphorylation induced by IFN-α in
Hep3B liver cancer cell line and had a less significant effect on
STAT1, STAT2 and STAT3 (Fig.
2B).
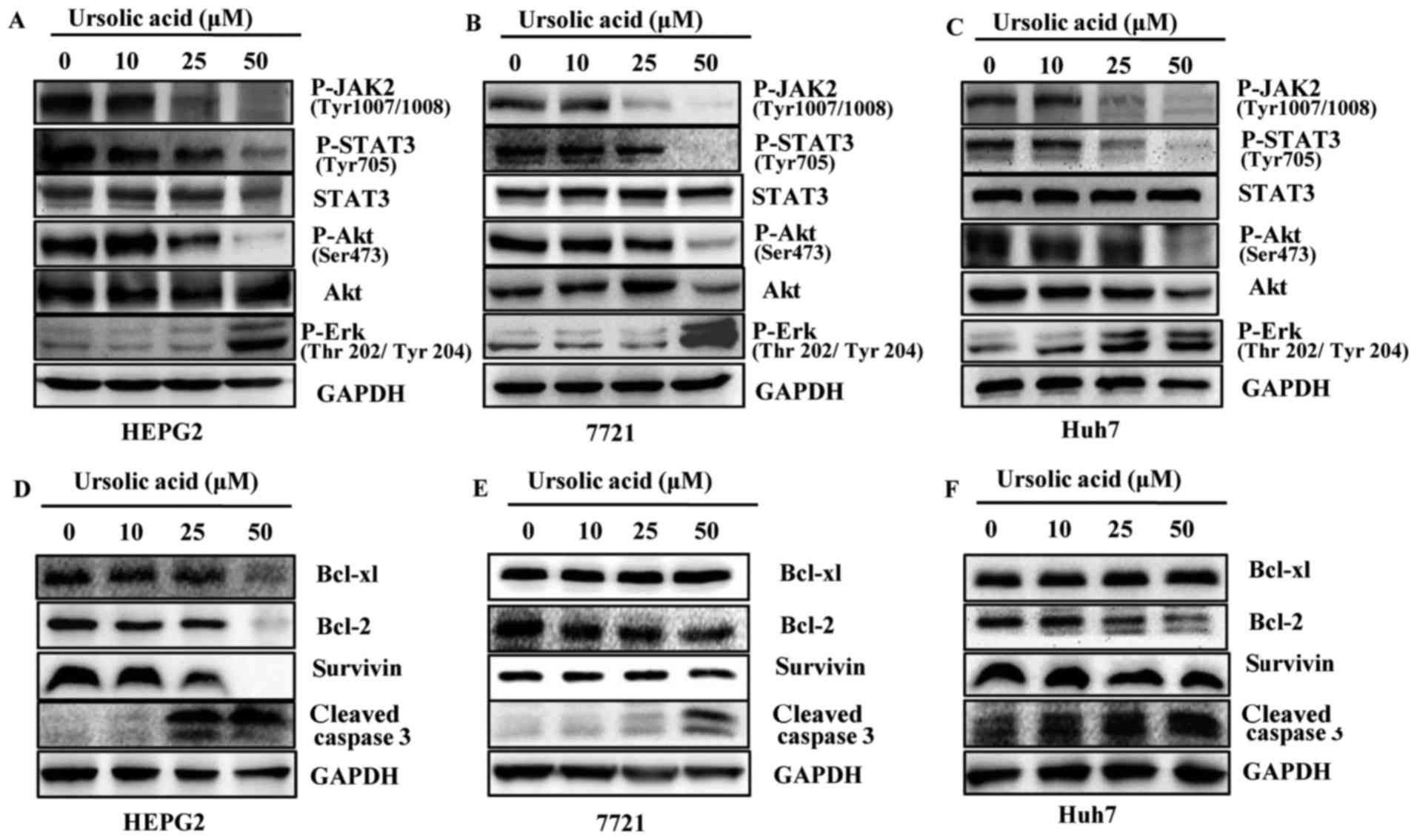
Ursolic acid inhibits JAK2 and STAT3
phosphorylation in HEPG2, 7721 and Huh7 cancer cells
UA inhibited constitutive STAT3 phosphorylation in
HEPG2, 7721 and Huh7 liver cancer cell lines in a dose-dependent
manner but less significantly on the levels of total STAT3
(Fig. 3A–C). STAT3 has been
reported to be activated by JAK2 (9). In our results, UA inhibited
phosphorylation of JAK2 in a dose-dependent manner in HEPG2, 7721
and Huh7 liver cancer cells. The level of survivin, Bcl-2 and
Bcl-xl after treatment of UA was different in HEPG2, 7721 and Huh7
liver cancer cells. Bcl-2 expression was reduced in the Huh7 and
HEPG2 cells while Bcl-xl and survivin expression were only markedly
reduced in the HEPG2 cell line. In 7721 cells, neither Bcl-xl, nor
Bcl-2 or survivin showed reduced expression (Fig. 3D–F). Based on the results, it was
possible that different liver cancer cells responded differently to
UA. We also detected other signaling pathways such as Akt and Erk.
As shown in Fig. 3A–C, we
demonstrated that UA inhibited the phosphorylation of Akt at Ser473
and less significantly on Akt. UA also increased expression levels
of cleaved caspase-3 (Fig. 3D–F).
The levels of P-Erk were increased after UA treatment in a
dose-dependent manner in liver cancer cells as shown in Fig. 3A–C.
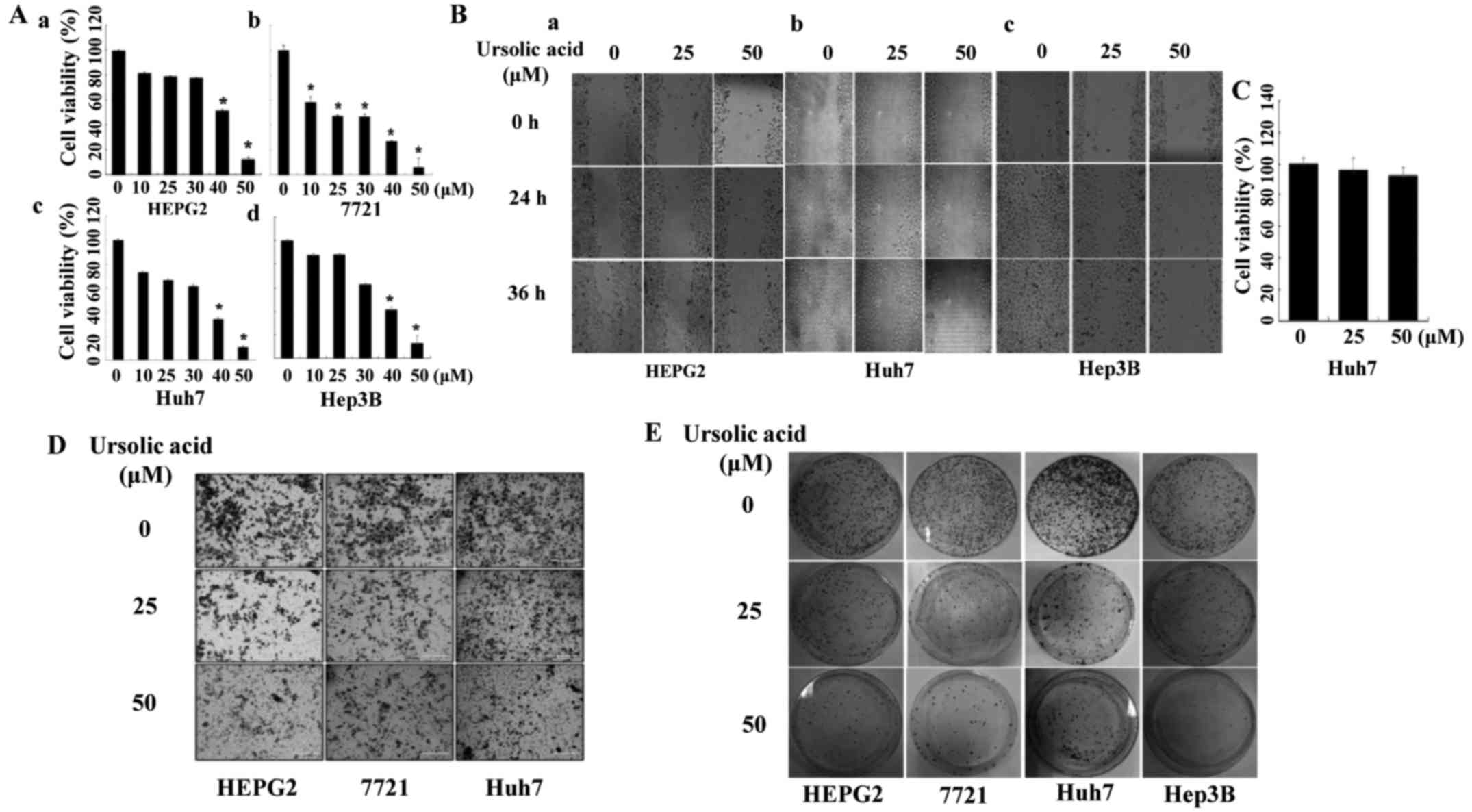
Ursolic acid inhibits cell viability
Our data showed that UA also inhibited the cell
viability of four liver cancer cell lines (Fig. 4A). Cell viability of HEPG2 (A-a),
7721 (A-b) and Huh7 (A-c) liver cancer cells was decreased by 50
µM of UA treatment, with a similar effect on Hep3B (A-d)
cells (P<0.05).
Ursolic acid inhibits cell migration and
invasion in liver cancer cells
We next evaluated the effect of UA on cell migration
by wound healing assay in liver cancer cells. As shown in Fig. 4B, UA suppressed cell migration in a
dose-dependent manner in HEPG2 (B-a), Huh7 (B-b) and Hep3B (B-c).
MTT assay was carried out to determine if the effect of UA on cell
migration was due to its ability to inhibit cell viability. The
time-points (4 h with UA and incubation for additional 36 h without
UA) were also used in the wound healing assay. The ability of UA to
inhibit cell migration did not seem to be due to an inhibition of
cell viability (Fig. 4C).
Transwell assay was performed to determine the effect of UA on cell
invasion in HEPG2, 7721 and Huh7 cancer cells. It showed that UA
(50 µM) could significantly inhibit cell invasion in cell
lines mentioned here (Fig.
4D).
UA inhibits the colony formation in liver
cancer cells
We also investigated the effect of UA on cell colony
formation. The results showed that UA markedly inhibited the colony
formation in HEPG2, 7721, Huh7 and Hep3B liver cancer cell lines
(Fig. 4E).
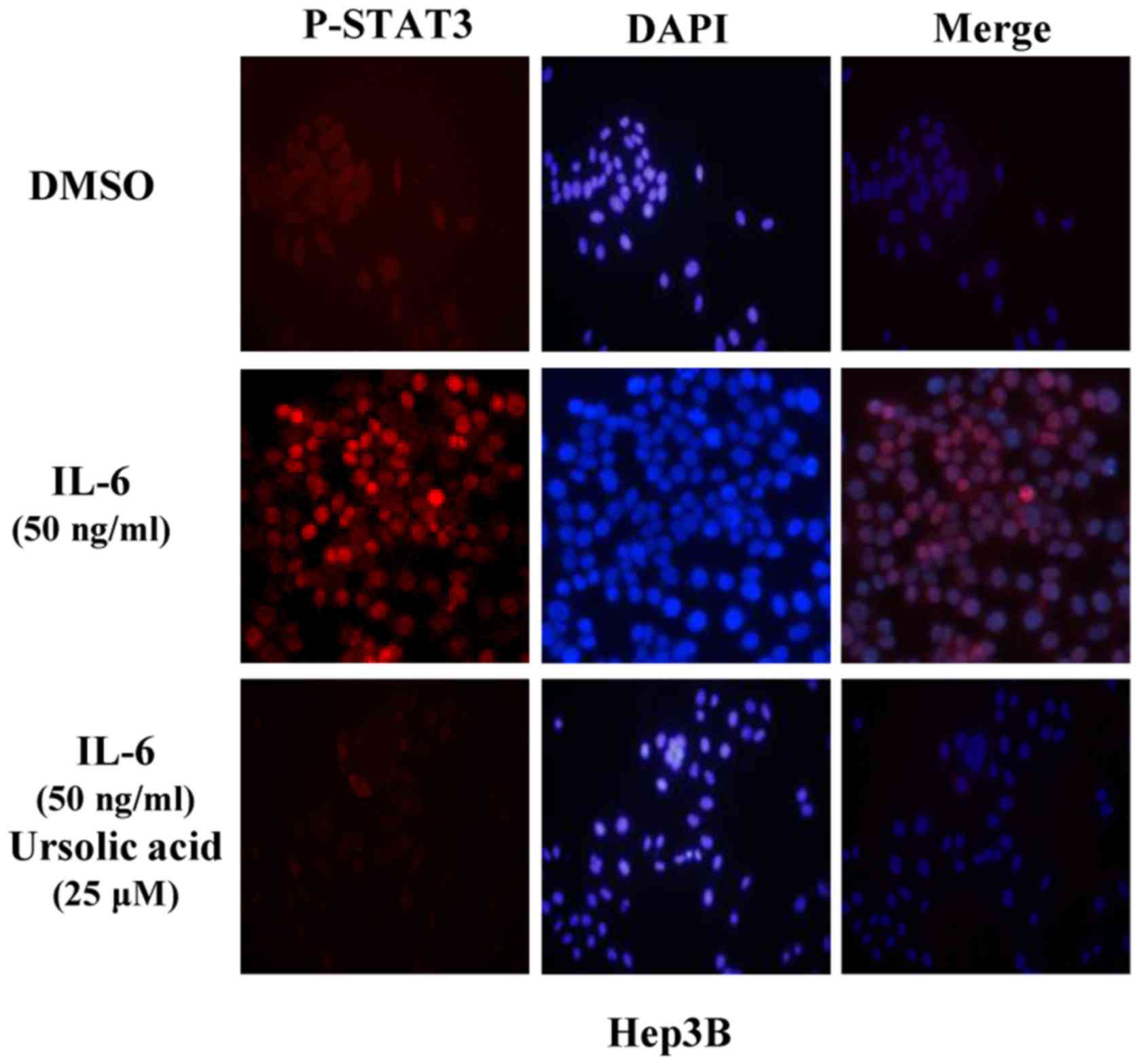
UA inhibits STAT3 activation in the
nucleus in Hep3B liver cancer cells
In Hep3B cells treated with IL-6, STAT3 was
activated in the nucleus. Pre-treated with UA at 25 µM for 4
h, cells were then induced by IL-6 (50 ng/ml) for another 30 min
and P-STAT3 was detected by immunofluorescence staining. These
results indicated that P-STAT3 induced by IL-6 in nucleus could be
inhibited by UA in Hep3B cells (Fig.
5).
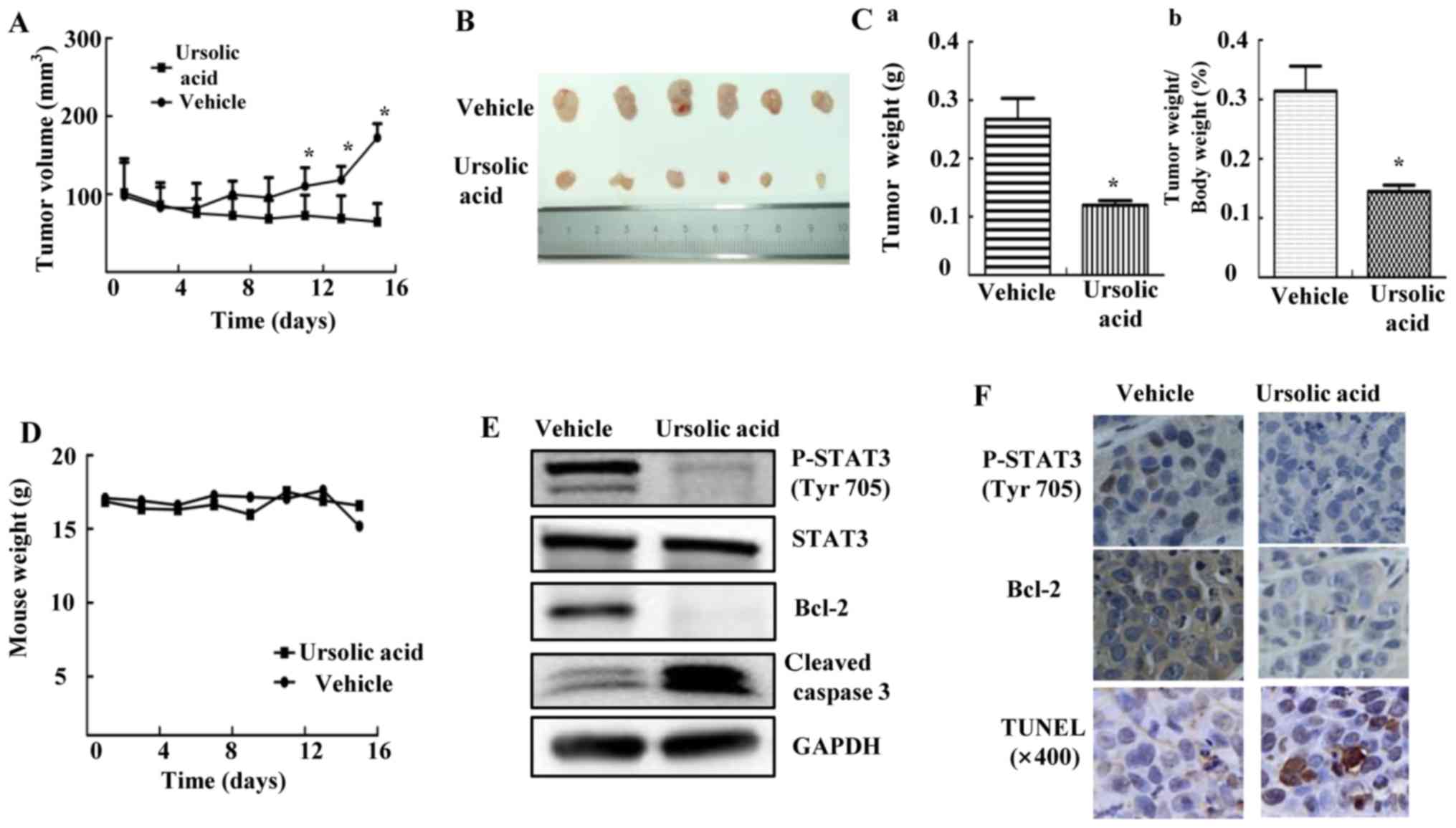
Ursolic acid suppresses tumor growth in
HEPG2 mouse xenograft model in vivo
HEPG2 xenograft experiments were performed to
explore whether UA could inhibit tumor growth in vivo
(22). As shown in Fig. 6A, the tumor volume of mice given
daily 60 mg/kg UA was decreased compared with vehicle group,
measured by caliper every other day. After mice were euthanized, we
isolated tumor tissue as shown in Fig.
6B. The tumor weight of UA group was significantly lower than
that of vehicle group, accompanied by decreased percent of
tumor/body weight (Fig. 6C;
P<0.05). Over the course of treatment, body weight was shown in
Fig. 6D. UA inhibited STAT3
phosphorylation, decreased Bcl-2 and induced cleavage of caspase-3
in xenografts (Fig. 6E). In
addition, UA also inhibited STAT3 phosphorylation, decreased the
expression of Bcl-2 as shown by IHC staining and induced apoptosis
by using TUNEL assay in xenograft tumors (Fig. 6F). These results suggested that UA
inhibited P-STAT3 and suppressed tumor growth in mice, indicating
that UA might be a potent compound in suppressing tumor growth
in vivo.
Discussion
There are studies on the inhibition of STAT3
phosphorylation (both constitutive and IL-6 induced) by UA in
myeloma (13), colon cancer
(14) and prostate cancer cells
(16). As is known, liver cancer
features high malignancy and rapid progress, in addition to its
relatively high resistance to chemotherapy. It has been reported
that elevated level of IL-6 was expressed in serum of patients with
HCC compared with healthy individuals (8), and different types of cancer exhibit
different resistance and sensitivity to antitumor agents. However,
it has not been reported previously that UA inhibits
phosphorylation of JAK2 and STAT3 in liver cancer cell lines and
suppresses growth of hepatocellular carcinoma, in which aberrant
activation of IL-6/JAK2/STAT3 signaling pathway plays an important
role in pathogenesis and progress. In the present study, we
demonstrated that UA decreased JAK2 and STAT3 phosphorylation in
liver cancer cell lines, and inhibited cell viability, cell
migration and colony formation. Moreover, UA inhibited tumor growth
in HEPG2 liver cancer xenograft mice. Thus, UA may have potential
as therapeutic agent for liver cancer. In addition, our results
showed that UA could inhibit STAT3 phosphorylation induced by IL-6,
and also inhibit P-STAT1, 2, 3 induced by IFN-α in Hep3B. However,
UA did not affect P-STAT3 induced by LIF. It suggested that the
inhibitory effects of UA on P-STAT3 induced by diverse stimuli were
different, which were possibly linked to different binding targets
of IL-6, LIF and IFN-α. Leukemia inhibitory factor (LIF) is a
pleiotropic factor belonging to the IL-6 superfamily of cytokines.
It is also expressed in HCC but less than IL-6. LIF can activate
STAT3 pathway in liver cancer cells (19). Interferon-α (IFN-α) is a member of
the type I IFN family known for their antiviral activity (23). In HCC, the activation of STAT1
pathway via IFN-α might have inhibitory effect of hepatocellular
carcinoma (20). IFN-α can also
induce oncogenic signaling of STAT3 (7) and STAT2 (21). Although IL-6, LIF and IFN-α induce
STAT3 activation, their receptor and binding targets are totally
different. It has been reported that IL-6 induces homodimerization
of gp130 by 32F6 (5), LIF leads to
heterodimerization of gp130 with the LIF receptor (LIFR) by B-R3
(6). The IFN-α receptor consists
of two subunits, IFN-αR1 and IFN-αR2, which form a heterodimer upon
IFN-α stimulation (20). The
effect of UA on P-STAT3 induced by LIF or IFN-α has not been
reported before. Our data suggested that UA had different effects
on STAT3 activation induced by different cytokines, indicating that
UA might bind different residues of the different receptors of
IL-6, LIF and IFN-α. These might be helpful to find the exact
mechanism or molecular target by which UA inhibits STAT3
phosphorylation in our future studies. UA might be used as a
leading compound to develop new IL-6/gp130 inhibitors with higher
selectivity and potency. As mentioned above, UA might also inhibit
the activation of other STATs in vitro, which might have
opposite effects on tumor growth. However, our in vivo
experiments revealed that UA suppressed growth in a xenograft model
as shown in Fig. 6.
Furthermore, we also detected other signaling
pathways such as Erk and Akt. The Erk signaling pathway can be
activated in response to a diverse range of extracellular stimuli
including mitogens and cytokines (24). Akt plays a critical role in cell
survival and apoptosis of liver cancer (25). Our results showed that UA inhibited
the phosphorylation of Akt at Ser473. The levels of P-Erk were
increased after treatment of UA in a dose-dependent manner in liver
cancer cells. UA is a natural compound and may play a role in
various signaling pathways including Akt and Erk besides STAT3. In
this study, UA inhibited P-JAK2 and P-STAT3 at a concentration as
low as 25 µM, but inhibited P-Akt at 50 µM. Thus, our
results showed that UA inhibited P-Akt, but inhibited P-STAT3 more
markedly, while increased P-Erk. Our data suggested that inhibition
of STAT3 phosphorylation is one of the mechanism that participated
in inhibitory effect of UA in liver cancer cell lines.
In our previous study, we examined the effect of UA
on HCT116 and SW480 (human colon cancer cells). UA could inhibit
P-STAT3 activation and cell viability significantly at 25 µM
in HCT116 and SW480 cells (14).
In human multiple myeloma, UA inhibited P-STAT3 activation in U266
cells and cell viability in U266, MM1.S and RPMI 8826 at 25
µM (13). In human
pancreatic cancer, UA inhibited cell growth and proliferation at 20
µM in AsPC-1, MIA PaCa-2 and Panc-28 (16). Our results showed that STAT3
phosphorylation could be inhibited at 25 µM which was higher
than other reported cancer cells, suggesting liver cancer cells
might be more malignant and more severely resistant to UA than
other cancer cells. It is of interest to note that the level of
survivin, Bcl-2 and Bcl-xl after treatment of UA was different in
HEPG2, 7721 and Huh7 liver cancer cells. Bcl-2 expression was
reduced in the Huh7 and HEPG2 cell line while Bcl-xl and survivin
expression were only markedly reduced in the HEPG2 cell line. In
7721 cells, neither Bcl-xl, nor Bcl-2 or survivin showed reduced
expression. Based on the results, it was possible that different
liver cancer cell lines responded differently to UA. In this study,
we did not design experiments to explore the different effect of UA
on different liver cancer cell lines, but it does have the
potential to become a novel point in our future studies.
Ursolic acid is a pentacyclic triterpenoid compound
in the form of free acid, which widely exists in apple peels, herb
medicines and many other edible plants. Our data showed that UA
inhibited constitutively activated STAT3 and Akt in HCC cell lines.
We also found that UA suppressed tumor growth in HEPG2 liver cancer
xenograft mice. Thus, UA has the potential to be used clinically as
a candidate for liver cancer therapy with relatively low toxicity
and strong biological stability compared to other artificial
designed compounds. In addition, UA might be used to prevent the
initiation of HCC. Because it is extracted from natural plants and
fruits, such as apples, loquat and olive, people might take UA in
their daily lives to reduce the risk of HCC. Also in our results,
the mice treated with DMSO were not as strong as UA treated group,
which suggested the protecting effect of UA. In conclusion, pure UA
might be developed as a health care product to prevent liver cancer
in future.
In summary, we found that UA, a natural compound
from some plants and fruits, could suppress STAT3 phosphorylation,
cell viability in vitro and xenograft tumor growth in
vivo in HCC. The safety of UA, as natural food ingredient, can
be preserved to be advantage for clinical treatment. UA is a
plant-derived agent and can be modified to design new inhibitors as
antitumor compounds with better cellular permeability or
bioavailability and less side-effects. UA as well as its analogues
might be developed to meet the current FDA standards for future
clinical testing.
Glossary
Abbreviations
Abbreviations:
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
IL-6
|
interleukin-6
|
|
UA
|
ursolic acid
|
|
JAK2
|
Janus kinases 2
|
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China to L.L. (No. 81372402, 81001005
and 81570416) and S.L. (No. 81570337), the Outstanding Young
Investigator Foundation of Tongji Hospital (YXQN009) and the
Fundamental Research Fund for the Central Universities, HUST (No.
0118540019) to L.L.
References
|
1
|
Wang R, Chen XZ, Zhang MG, Tang L and Wu
H: Incidence and mortality of liver cancer in mainland China:
Changes in first decade of 21st century. Hepatogastroenterology.
62:118–121. 2015.PubMed/NCBI
|
|
2
|
Bowman T, Garcia R, Turkson J and Jove R:
STATs in oncogenesis. Oncogene. 19:2474–2488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buettner R, Mora LB and Jove R: Activated
STAT signaling in human tumors provides novel molecular targets for
therapeutic intervention. Clin Cancer Res. 8:945–954.
2002.PubMed/NCBI
|
|
4
|
Subramaniam A, Shanmugam MK, Perumal E, Li
F, Nachiyappan A, Dai X, Swamy SN, Ahn KS, Kumar AP, Tan BK, et al:
Potential role of signal transducer and activator of transcription
(STAT)3 signaling pathway in inflammation, survival, proliferation
and invasion of hepatocellular carcinoma. Biochim Biophys Acta.
1835:46–60. 2013.
|
|
5
|
Chevalier S, Fourcin M, Robledo O,
Wijdenes J, Pouplard-Barthelaix A and Gascan H: Interleukin-6
family of cytokines induced activation of different functional
sites expressed by gp130 transducing protein. J Biol Chem.
271:14764–14772. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hermanns HM, Radtke S, Haan C, Schmitz-Van
de Leur H, Tavernier J, Heinrich PC and Behrmann I: Contributions
of leukemia inhibitory factor receptor and oncostatin M receptor to
signal transduction in heterodimeric complexes with glycoprotein
130. J Immunol. 163:6651–6658. 1999.PubMed/NCBI
|
|
7
|
Wang L, Jia D, Duan F, Sun Z, Liu X, Zhou
L, Sun L, Ren S, Ruan Y and Gu J: Combined anti-tumor effects of
IFN-α and sorafenib on hepatocellular carcinoma in vitro and in
vivo. Biochem Biophys Res Commun. 422:687–692. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ataseven H, Bahcecioglu IH, Kuzu N, Yalniz
M, Celebi S, Erensoy A and Ustundag B: The levels of ghrelin,
leptin, TNF-alpha, and IL-6 in liver cirrhosis and hepatocellular
carcinoma due to HBV and HDV infection. Mediators Inflamm.
2006:783802006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song B, Zhan H, Bian Q and Gu J:
Piperlongumine inhibits gastric cancer cells via suppression of the
JAK1,2/STAT3 signaling pathway. Mol Med Rep. 13:4475–4480.
2016.PubMed/NCBI
|
|
10
|
Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB,
Lee CN and Hsieh CY: Interleukin-6 promotes cervical tumor growth
by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene.
22:1517–1527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin L, Hutzen B, Li PK, Ball S, Zuo M,
DeAngelis S, Foust E, Sobo M, Friedman L, Bhasin D, et al: A novel
small molecule, LLL12, inhibits STAT3 phosphorylation and
activities and exhibits potent growth-suppressive activity in human
cancer cells. Neoplasia. 12:39–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mohan CD, Bharathkumar H, Bulusu KC,
Pandey V, Rangappa S, Fuchs JE, Shanmugam MK, Dai X, Li F,
Deivasigamani A, et al: Development of a novel azaspirane that
targets the Janus kinase-signal transducer and activator of
transcription (STAT) pathway in hepatocellular carcinoma in vitro
and in vivo. J Biol Chem. 289:34296–34307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pathak AK, Bhutani M, Nair AS, Ahn KS,
Chakraborty A, Kadara H, Guha S, Sethi G and Aggarwal BB: Ursolic
acid inhibits STAT3 activation pathway leading to suppression of
proliferation and chemosensitization of human multiple myeloma
cells. Mol Cancer Res. 5:943–955. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang W, Zhao C, Jou D, Lü J, Zhang C, Lin
L and Lin J: Ursolic acid inhibits the growth of colon
cancer-initiating cells by targeting STAT3. Anticancer Res.
33:4279–4284. 2013.PubMed/NCBI
|
|
15
|
Heo TH, Wahler J and Suh N: Potential
therapeutic implications of IL-6/IL-6R/gp130-targeting agents in
breast cancer. Oncotarget. 7:15460–15473. 2016.PubMed/NCBI
|
|
16
|
Prasad S, Yadav VR, Sung B, Gupta SC,
Tyagi AK and Aggarwal BB: Ursolic acid inhibits the growth of human
pancreatic cancer and enhances the antitumor potential of
gemcitabine in an orthotopic mouse model through suppression of the
inflammatory microenvironment. Oncotarget. 7:13182–13196.
2016.PubMed/NCBI
|
|
17
|
Shanmugam MK, Rajendran P, Li F, Nema T,
Vali S, Abbasi T, Kapoor S, Sharma A, Kumar AP, Ho PC, et al:
Ursolic acid inhibits multiple cell survival pathways leading to
suppression of growth of prostate cancer xenograft in nude mice. J
Mol Med (Berl). 89:713–727. 2011. View Article : Google Scholar
|
|
18
|
Kawano M, Hirano T, Matsuda T, Taga T,
Horii Y, Iwato K, Asaoku H, Tang B, Tanabe O, Tanaka H, et al:
Autocrine generation and requirement of BSF-2/IL-6 for human
multiple myelomas. Nature. 332:83–85. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang JF, He ML, Fu WM, Wang H, Chen LZ,
Zhu X, Chen Y, Xie D, Lai P, Chen G, et al: Primate-specific
microRNA-637 inhibits tumorigenesis in hepatocellular carcinoma by
disrupting signal transducer and activator of transcription 3
signaling. Hepatology. 54:2137–2148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li T, Dong ZR, Guo ZY, Wang CH, Tang ZY,
Qu SF, Chen ZT, Li XW and Zhi XT: Aspirin enhances IFN-α-induced
growth inhibition and apoptosis of hepatocellular carcinoma via
JAK1/STAT1 pathway. Cancer Gene Ther. 20:366–374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Testoni B, Schinzari V, Guerrieri F,
Gerbal-Chaloin S, Blandino G and Levrero M: p53-paralog DNp73
oncogene is repressed by IFNα/STAT2 through the recruitment of the
Ezh2 polycomb group transcriptional repressor. Oncogene.
30:2670–2678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wan S, Zhao E, Kryczek I, Vatan L,
Sadovskaya A, Ludema G, Simeone DM, Zou W and Welling TH:
Tumor-associated macrophages produce interleukin 6 and signal via
STAT3 to promote expansion of human hepatocellular carcinoma stem
cells. Gastroenterology. 147:1393–1404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Liu K, Liu Y, Xu Y, Zhang F, Yang H,
Liu J, Pan T, Chen J, Wu M, et al: Exosomes mediate the
cell-to-cell transmission of IFN-α-induced antiviral activity. Nat
Immunol. 14:793–803. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Franke TF, Kaplan DR and Cantley LC: PI3K:
Downstream AKTion blocks apoptosis. Cell. 88:435–437. 1997.
View Article : Google Scholar : PubMed/NCBI
|