Introduction
Prostate cancer (PCa) is one of the most common
malignancies in males in many countries (1). According to statistics in 2016, there
were approximately 3 million men suffering from prostate cancer in
the United States, and additionally, more than 180,000 new cases
were reported (2). In the past
decades, the incidence and mortality of PCa also significantly
increased in China (3). Studies
have confirmed that distant metastasis is the major cause of
PCa-related mortality (4,5).
At present, PSA level is used as an index for
predicting PCa progression include bone metastasis in Western
countries (6,7). However, there were studies considered
it could be different in Asian patients, researchers found bone
metastatic PCa patients in Asian with lower PSA level (7). It is necessary to exploit new
predictive factors and diagnosis patterns for PCa distant
metastasis.
MicroRNAs are a class of non-coding small RNAs,
acting as negative regulators of gene expression through direct
interaction with the 3′-untranslated regions (3′-UTR) of
corresponding mRNA targets (8).
Previous studies had extensively confirmed that MicroRNAs played
vital roles in tumor metastasis (9–11).
Nevertheless, there are very few comprehensive reports on the
prediction and diagnosis values of microRNAs in metastatic PCa
(12).
PrLZ was identified as a novel member of the TPD52
family which is specifically expressed in prostate tissues
(13). Previous studies proved
that PrLZ was associated with the progression of prostate cancer
(14). Increased PrLZ promoted
prostate cancer growth at castration-resistant stage and protected
prostate cancer cells from apoptosis induced by androgen
deprivation (15). However, the
regulation mechanism and function of PrLZ in PCa distant metastasis
is unclear.
In our previous study, we identified that miR-449a
was significantly decreased in primary lesion tissues of bone
metastatic PCa patients. In this study, we further investigated the
function of miR-449a in the process of prostate cancer metastasis.
Furthermore, we demonstrated that miR-449a inhibited prostate
cancer metastasis via targeting PrLZ expression.
Materials and methods
Patients and specimens
Prostate cancer tissues were collected from patients
who underwent curative resection with informed consent between 2011
and 2014 at the Department of Urinary Surgery, the First Affiliated
Hospital of Wenzhou Medical University. These tissues were
immediately transported into liquid nitrogen in operating theatres
and then stored at −80°C. In the present study, specimens were
divided into two groups according to whether the patients relapsed
with distant metastasis in two years after surgical operation. The
demographic information of patients is shown in Table I. The study protocol was approved
by the Ethics Committee of the First Affiliated Hospital of Wenzhou
Medical University, and written informed consents were obtained
from the patients based on the Declaration of Helsinki.
 | Table IDemographic information of
patient. |
Table I
Demographic information of
patient.
| Patient
charectaristics | Distant
metastasis | No distant
metastasis | Total |
|---|
| Number | 17 | 21 | 38 |
| Age (years) | Age range, 42–74
(mean 60.18) | Age range, 41–70
(mean 55.9) | |
| Stage | | | |
| I–II | 4 | 15 | 19 |
| III–IV | 13 | 6 | 19 |
| PSA (ng/ml) | 22.5±9.59 | 41.8±19.81 | |
Cell culture
The human normal prostate epithelial cell line
RWPE-1 and PCa cell lines PC-3, LNcap cells were purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA). PC-3
and LNcap cells were cultured in RPMI-1640 medium with 10% fetal
bovine serum (FBS; Life Technologies, Foster City, CA, USA), 100
units/ml penicillin and 100 units/ml streptomycin (Sigma-Aldrich,
St. Louis, MO, USA). RWPE-1 cells were cultured in Defined
Keratinocyte-SFM (Life Technologies, Carlsbad, CA, USA) with 10%
FBS, 100 units/ml penicillin and 100 units/ml streptomycin. All
these cells were cultured in a humidified environment containing 5%
CO2 and held at a constant temperature of 37°C.
qRT-PCR
Total RNAs for PrLZ and β-actin were extracted from
clinical samples using TRIzol reagent (Life Technologies). cDNA was
synthesized using the PrimeScript RT reagent kit (Takara Bio,
Mountain View, CA, USA). The primers used for the expression
analysis of β-actin and PrLZ were as follow: β-actin forward,
5′-GGCACTCTTCCAGCCTTCC-3′ and β-actin reverse,
5′-GAGCCGCCGATCCACAC-3′; PrLZ forward, 5′-TCTAGCAGAGATCAAGCGGAA-3′
and PrLZ reverse, 5′-ACTGAGCCAACAGACGAAAAA-3′. Total RNAs for
miR-449a and U6 were extracted from clinical samples and cells
using miRNeasy Mini kit (Qiagen, Hilden, Germany). The qRT-PCR
reactions of miR-449a and U6 were performed according to the
manufacturer's instructions of All-in-One™ miRNA qRT-PCR detection
kit (GeneCopoeia, Rockville, MD, USA). iQ-5 (Bio-Rad Laboratories,
Hercules, CA, USA) was used to monitor the qRT-PCR reactions. RNA
expression was quantified using 2−ΔΔCt method.
Western blotting
Total protein was extracted using RIPA protein lysis
buffer (Beyotime Institute of Biotechnology, Shanghai, China) with
added 1% protease inhibitor cocktail and 1 mM phenylmethylsulfonyl
fluoride (PMSF). Cell fractions were prepared using a Nuclear and
Cytoplasmic Protein Extraction kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. Generally,
50 µg of protein was used for western blotting. Samples were
separated by SDS-PAGE and transferred onto PVDF membranes. After
blocking in 5% skim milk, the PVDF membranes were incubated with
primary antibodies in blocking buffer overnight at 4°C and then
with HRP-conjugated secondary antibody for 2 h. The primary
antibodies used were: anti-GAPDH (1:3,000 dilution; Santa Cruz
Biotechnology, Santa Cruz, CA, USA), anti-PrLZ (1:1,000 dilution;
Abcam, Cambridge, UK), anti-E-cadherin (1:1,000 dilution; Abcam),
anti-N-cadherin (1:1,000 dilution; Abcam), anti-CD44 (1:1,000
dilution; Abcam), anti-vimentin (1:1,000 dilution; Abcam),
anti-MMP2 (1:1000 dilution; Abcam), anti-MMP9 (1:500 dilution;
Abcam), anti-RunX2 (1:500 dilution; Abcam). Reactive bands were
visualized with ECL reagent (Pierce, Rockford, IL, USA) and
analyzed. Protein expression was quantified using ImageJ software
(National Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry (IHC)
Formalin-fixed, paraffin-embedded tissues were used
to detect the expression of PrLZ and N-cadherin. The sections were
incubated with anti-PrLZ and anti-N-cadherin rabbit polyclonal
antibodies (Abcam) at 1:200 dilution. A semi-quantitative scoring
system was used to evaluate the intensity of staining: low
(proportion, 0–50%; intensity, no staining to weak) and high
(proportion, >50%; intensity, intermediate to strong).
Oligonucleotide transfection and
luciferase reporter assay
miR-146a mimics and scramble control mimics were
purchased from GeneCopoeia Inc. (Rockville, MD, USA). PC-3 cells
(105 cells) were seeded in 24-well plates before
transfection. Wild-type PrLZ 3′UTR (PrLZ-3′UTR-wt) and miR449a
target site deletion mutation PrLZ 3′UTR (PrLZ-3′UTR-mu) were
constructed into psiCHECK2 plasmid (Promega, Madison, WI, USA).
According to the instructions, miR-449a mimic and PrLZ 3′UTR
Reporter plasmids (psiCHECK-PrLZ-3′UTR-wt or
psiCHECK-PrLZ-3′UTR-mu) were co-transfected using
Lipofectamine® RNAiMAX (Life Technologies) with final
concentration of 50 nM (miR-146a mimic) or 200 ng (PrLZ 3′UTR
Reporters). In addition, 48 h later, the cells were collected and
Luciferase activity was detected using the Dual-Glo luciferase
assay system (Promega) according to the manufacturer's
protocol.
Lentivirus infection
miR-449a overexpression and PrLZ shRNA lentivirus
were purchased from Shanghai R&S Biotechnology, Co., Ltd.
(Shanghai, China). Cells were planted into 10-cm dishes
(3×106 cells/dish) 24 h before the infection. Lentivirus
infection was performed with the MOI (multiplicity of infection) of
30 (LNcap) and 50 (PC-3), respectively. The infection efficiency
was >90%.
Cell proliferation analysis
Cells were seeded in 96-well plates in triplicate at
densities of 5×103/well. Cell viability was evaluated at
desired time-points using CCK-8 kits (Dojindo Molecular
Technologies, Kumamoto, Japan) according to the instructions. Light
absorbance of the solution was measured at 570 nm on a microplate
reader.
Cell invasion assay
Transwell chambers coated with Matrigel (BD
Biosciences, San Diego, CA, USA) were used to analysis cell
invasion. Du145 cells (5×105) in 100 µl
serum-free Dulbecco's modified Eagle's medium (DMEM) were seeded on
upper chambers and DMEM with 10% FBS was added to lower chambers.
After 24-h incubation, invaded cells in the lower side of the
membranes were fixed with methanol and stained with crystal violet.
Images were taken using an inverted microscope. Invaded cells were
counted from three different fields. The experiment was repeated
three times.
Tube formation assay
Forty-eight hours after lentivirus infected, PCa
cells were washed with phosphate-buffered saline (PBS) twice, and
then cultured in medium containing 1% serum for another 24 h.
Conditioned media were then collected, centrifuged at 1,500 rpm/min
and the supernatant was used for tube formation experiments. HUVEC
(2×104 cells/well) were seeded onto the 96-well plates
which were pre-coated with polymerized Matrigel (BD Biosciences)
overnight, and then incubated with 100 µl conditioned media
from PCa cells at 37°C for 4 h in a 5% CO2 incubator.
Six random fields from each well were selected to count the branch
points of tube-like structures (16).
Apoptosis assay
Cell apoptosis was determined by flow cytometry
using the Annexin V-FITC apoptosis detection kit (Nanjing KeyGen
Biotech, Co., Ltd., Nanjing, China). Cells were collected and
incubated with Annexin V and propidium iodide (PI) in a binding
buffer in the dark at room temperature for 10 min. The stained
cells were analyzed using the BD FASAria Cell Sorter.
Tumorigenesis in nude mice
Six-week-old female BALB/c nude mice were injected
with PC-3 cell subcutaneously (107 cells/mouse) into the
left axilla. For lentivirus infection groups, 10 µl
lentivirus were respectively injected into tumors every three days
when the tumor volume up to 50 mm3. The tumor sizes were
measured every three days using micrometer calipers, and tumor
volumes were calculated as following: Tumor volume =
d2xD/2, where d and D represented shortest and the
longest diameters, respectively. All animal experiment protocols
were approved by the Institutional Animal Care and Use
Committee.
Statistical analysis
Results are presented as means ± standard deviations
(SD) of three independent experiments. Significant differences in
the mean values were evaluated by unpaired t-test. One-way ANOVA
was used to compare continuous variables among two or more groups.
Tests of association were conducted using Pearson's χ2
test. P<0.05 was considered statistically significant.
Literature collection was performed by using electronic databases
PubMed. All statistical analyses were executed by using SPSS 20.0
software (IBM, Chicago, IL, USA). Raw and processed data are stored
by the corresponding author and are available upon request.
Results
Downregulation of miR-449a is related to
PCa clinical stage and distant metastasis
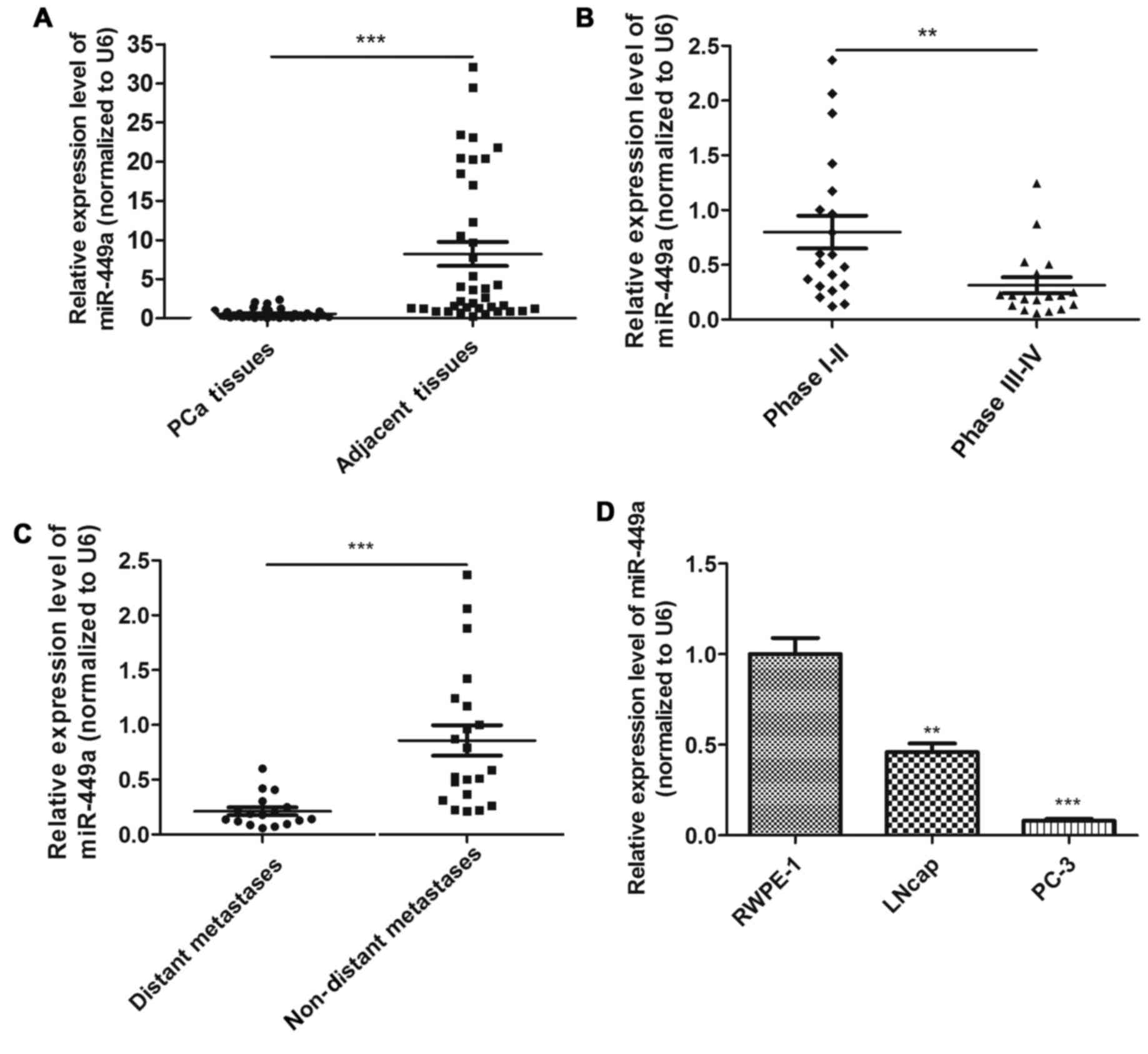
In order to investigate whether miR-449a was
associated with PCa progression, we examined the expression of
miR-449a in 38 PCa tissues using qRT-PCR. We confirmed that
miR-449a was significantly downregulated in PCa tissues compared to
adjacent tissues (Fig. 1A). To
investigate the clinical significance of miR-449a, we assessed the
correlation between miR-449a expression level and pathological
characteristics. The result showed that miR-449a was remarkably
decreased in stage III–IV patients compared with stage I–II
patients (Fig. 1B). As previously
described, PCa tissues were divided into two groups. We found that
miR-449a was obviously lower expressed in distant metastasis
positive specimens (Fig. 1C).
miR-449a expression levels in various prostate derived cells are
presented, miR-449a was remarkably downregulated in prostate cancer
cell lines, especially in androgen-independent prostate cancer cell
PC-3 (Fig. 1D). These results
indicate that miR-449a is related to malignance of prostate
cancer.
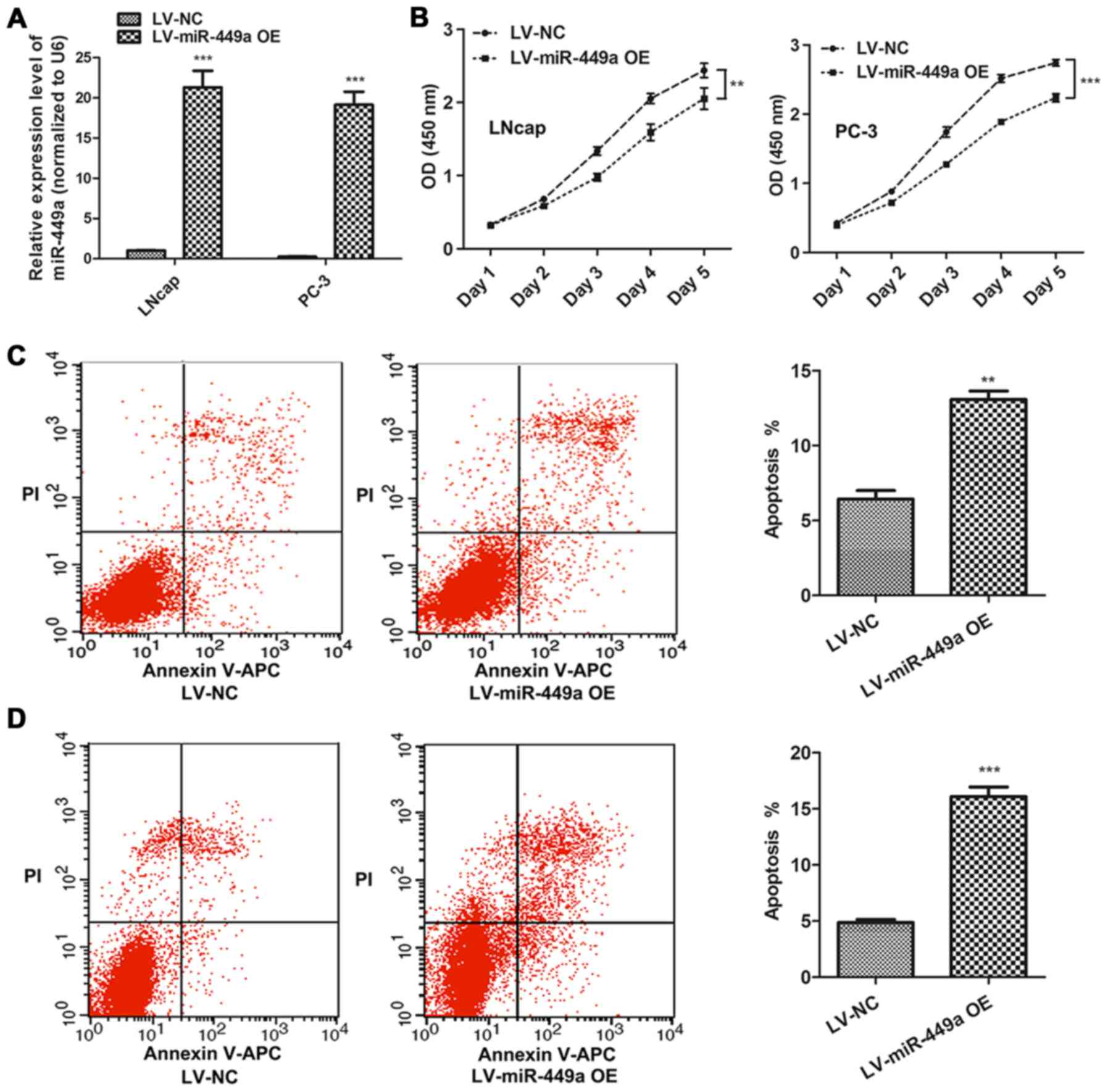
miR-449a inhibits PCa progression
LNcap and PC-3, which represented androgen-dependent
and androgen-independent PCa cells, respectively, were used to
investigate the function of miR-449a in PCa. miR-449a
overexpression lentivirus (LV-miR-449a OE) was performed to
upregulate miR-449a expression in PCa cells. We found that miR-449a
suppressed cell proliferation in LNcap and PC-3 (Fig. 2A). In addition, flow cytometry
assay showed that miR449a remarkably promoted apoptosis in LNcap
and PC-3 cells (Fig. 2B).
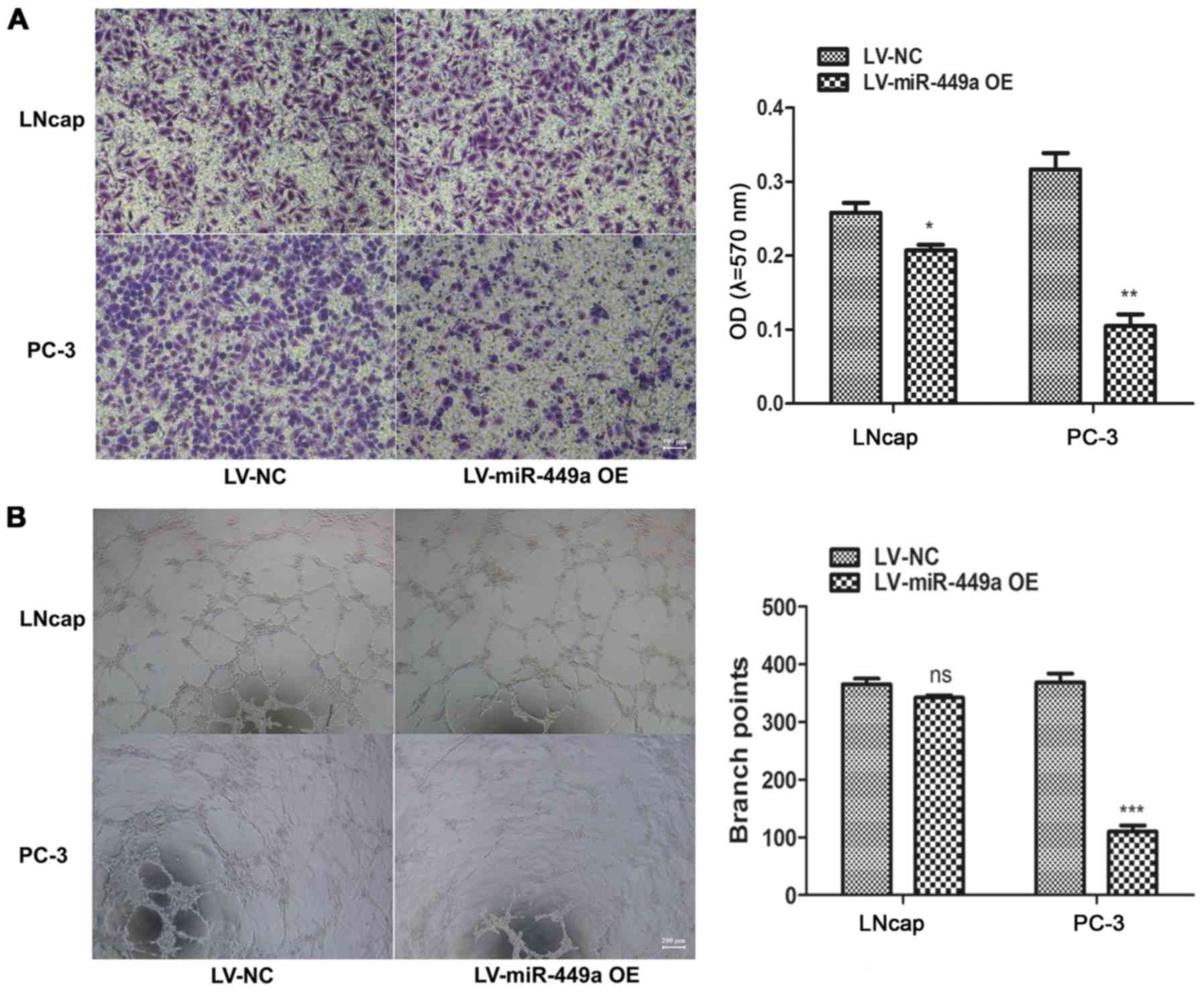
Moreover, we also assessed the impact of miR-449a on metastasis of
PCa, we found that miR-449a obviously restricted invasion and tube
formation ability in LNcap and PC-3 (Fig. 3). These results indicate that
miR-449a is related to malignance of prostate cancer.
miR-449a targets PrLZ expression in PCa
cells
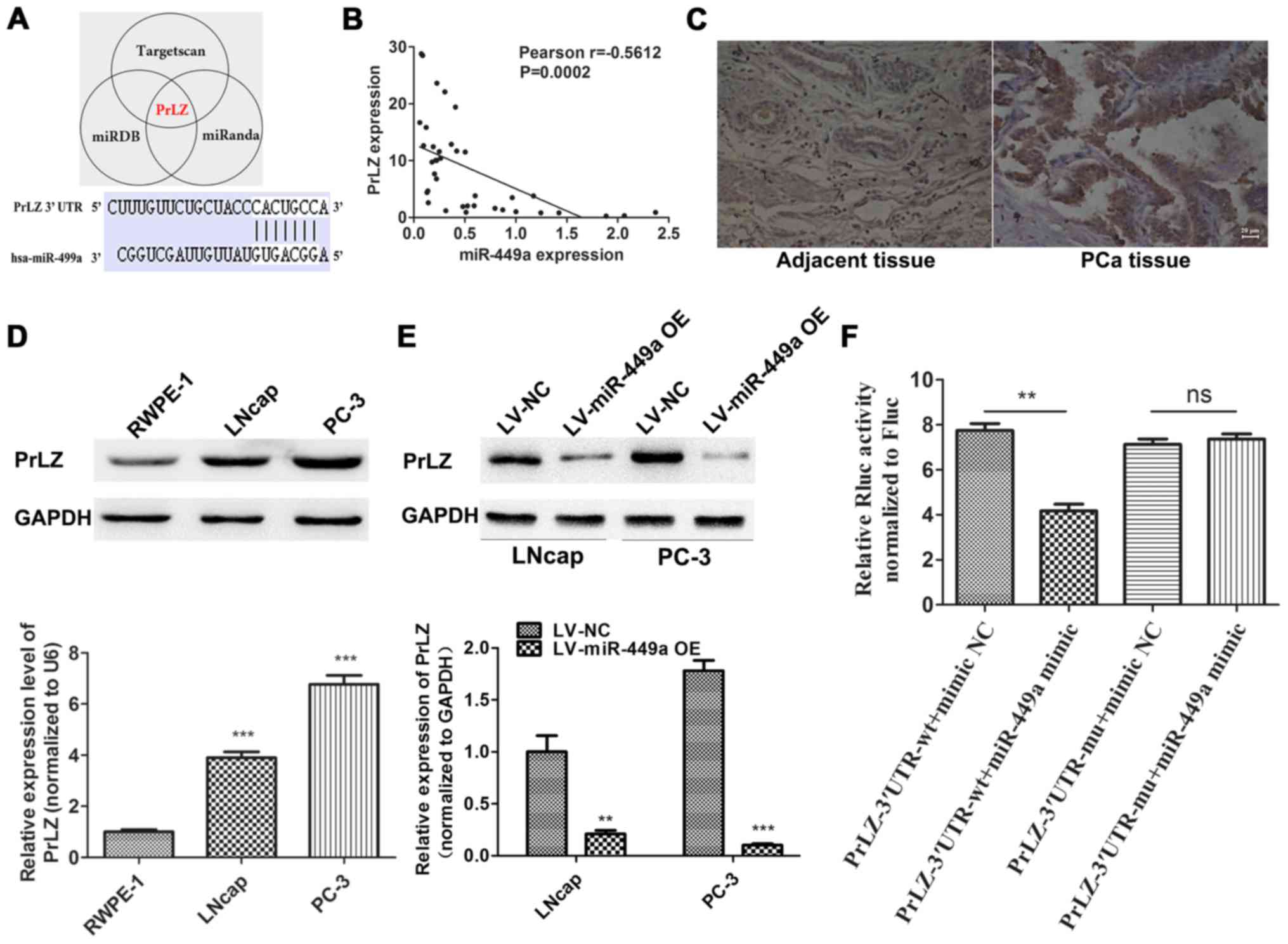
To identify potential target of miR449a, we used a
combination of three algorithms, TargetScan, miRDB and miRanda.
Among these candidates, PrLZ was identified by all the three
programs as potential target of miR-449a with highest predictive
score (Fig. 4A). To validate our
prediction, we detected PrLZ expression in corresponding 38 PCa
specimens by qRT-PCR. Pearson's correlation analysis showed that
the PrLZ expression negative correlated with miR-449a (Fig. 4B). IHC was performed to assess PrLZ
expression in PCa and corresponding adjacent tissues, we found that
PrLZ was upregulated in PCa tissues compared to adjacent specimens
(Fig. 4C). Next, we further
confirmed the target relationship of miR-449a/PrLZ via western
blotting (Fig. 4D and E) and
Luciferase assay (Fig. 4F). Our
results indicate that PrLZ is specifically targeted by miR-449a in
PCa cells.
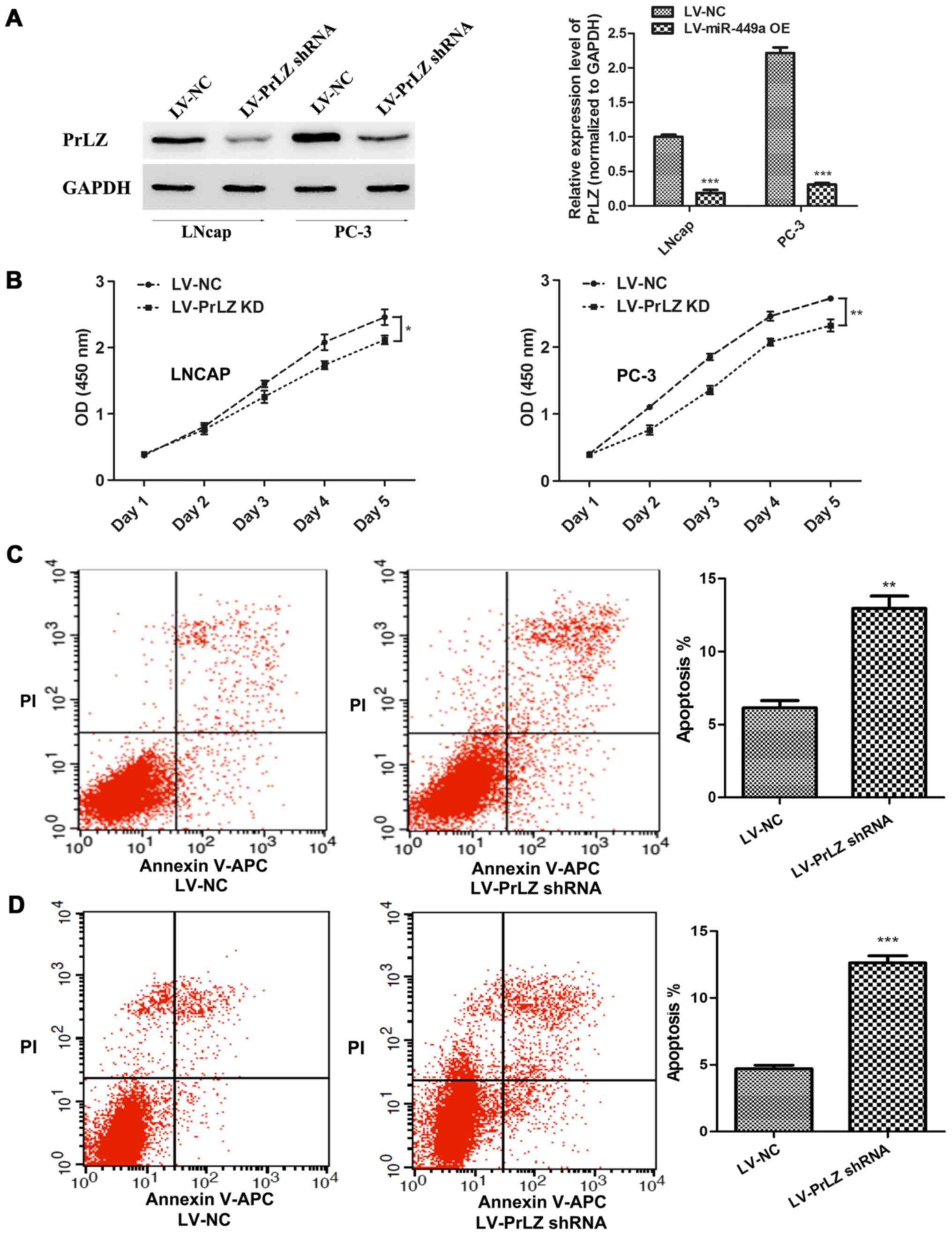
Knockdown of PrLZ inhibits PCa
progression
In order to confirm that miR-449a is a vital cancer
suppressor via regulating PrLZ expression, we specially further
investigated the function of PrLZ in PCa. PrLZ shRNA lentivirus
(LV-PrLZ shRNA) was used to downregulate PrLZ expression, western
blotting showed remarkable decrease of PrLZ in LNcap and PC-3 cells
after PrLZ shRNA lentivirus infection (Fig. 5A). We found that PrLZ knockdown
delayed LNcap and PC-3 cell proliferation (Fig. 5B). In addition, flow cytometry
assay revealed remarkable elevation of apoptosis rate in LNcap and
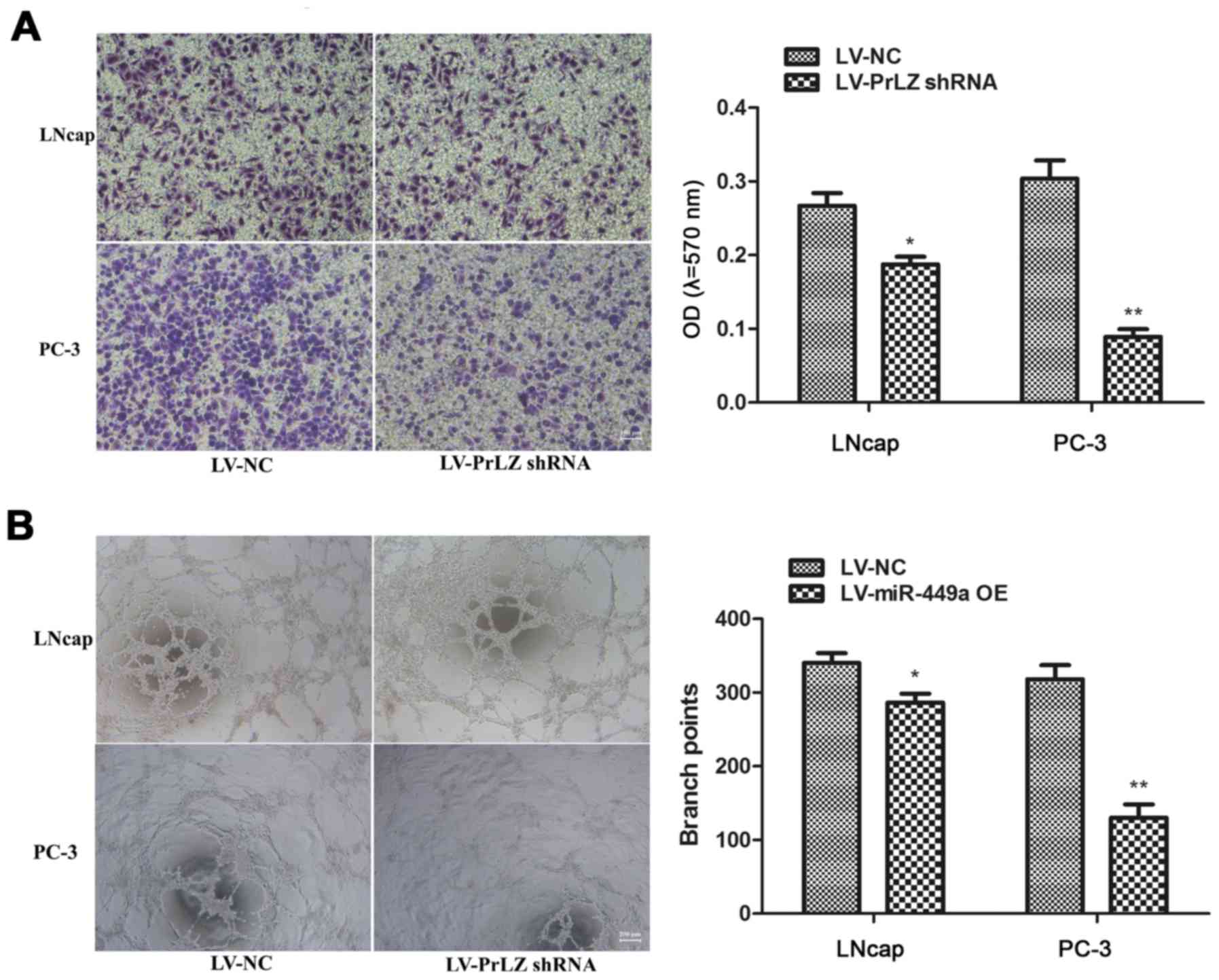
PC-3 cells after LV-PrLZ shRNA infection (Fig. 5C). Similar to miR-449a, PrLZ shRNA
obviously restricted invasion and tube formation ability in LNcap
and PC-3 cells (Fig. 6). Our
results prove that knockdown of PrLZ inhibits PCa progression.
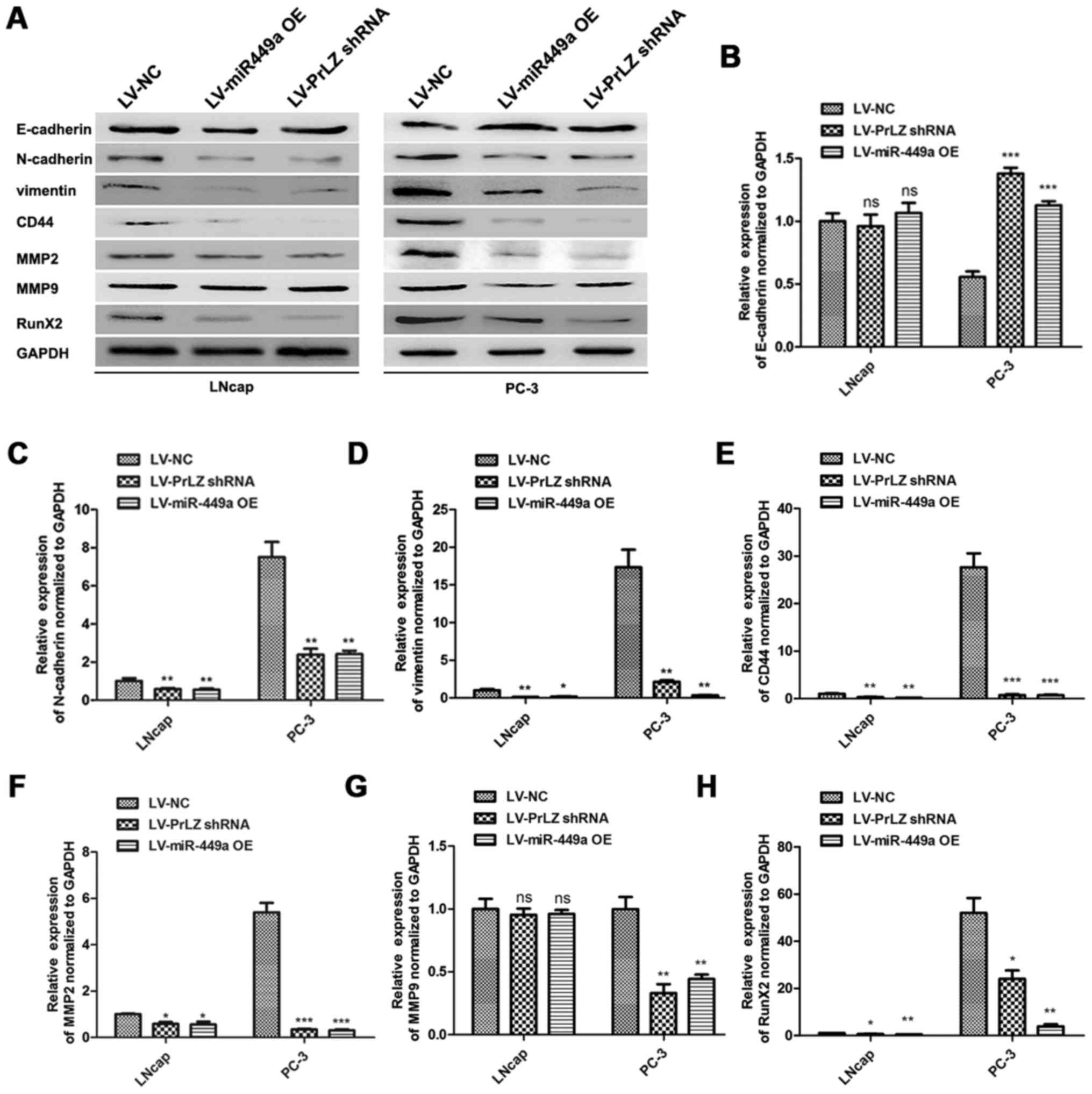
miR-449a/PrLZ axis regulates PCa cell
invasion via adjusting stemness
Cancer stem cell is crucial in cancer progression
and evolution, and the maintenance or resumption of stemness
features are approved to be critical factors for PCa metastasis
(17). We investigated the roles
of miR-449a and PrLZ in regulating stemness features of PCa cells.
E-cadherin, N-cadherin, vimentin, CD44, MMP2, MMP9 and Runx2 were
detected as stemness or metastasis markers by western blotting
(Fig. 7A). We found that
E-cadherin was upregulated in LNcap and PC3 after LV-miR-449a OE or
LV-PrLZ shRNA infection. Oppositely, N-cadherin, vimentin, CD44,
MMP2, MMP9 and Runx2 were remarkably downregulated. Quantitative
analysis results are shown (Fig.
7B–H). These results indicate that miR-449a inhibits stemness
features in LNcap and PC3 cells via targeting PrLZ, and
miR449a/PrLZ axis may regulate PCa cell invasion via adjusting
stemness.
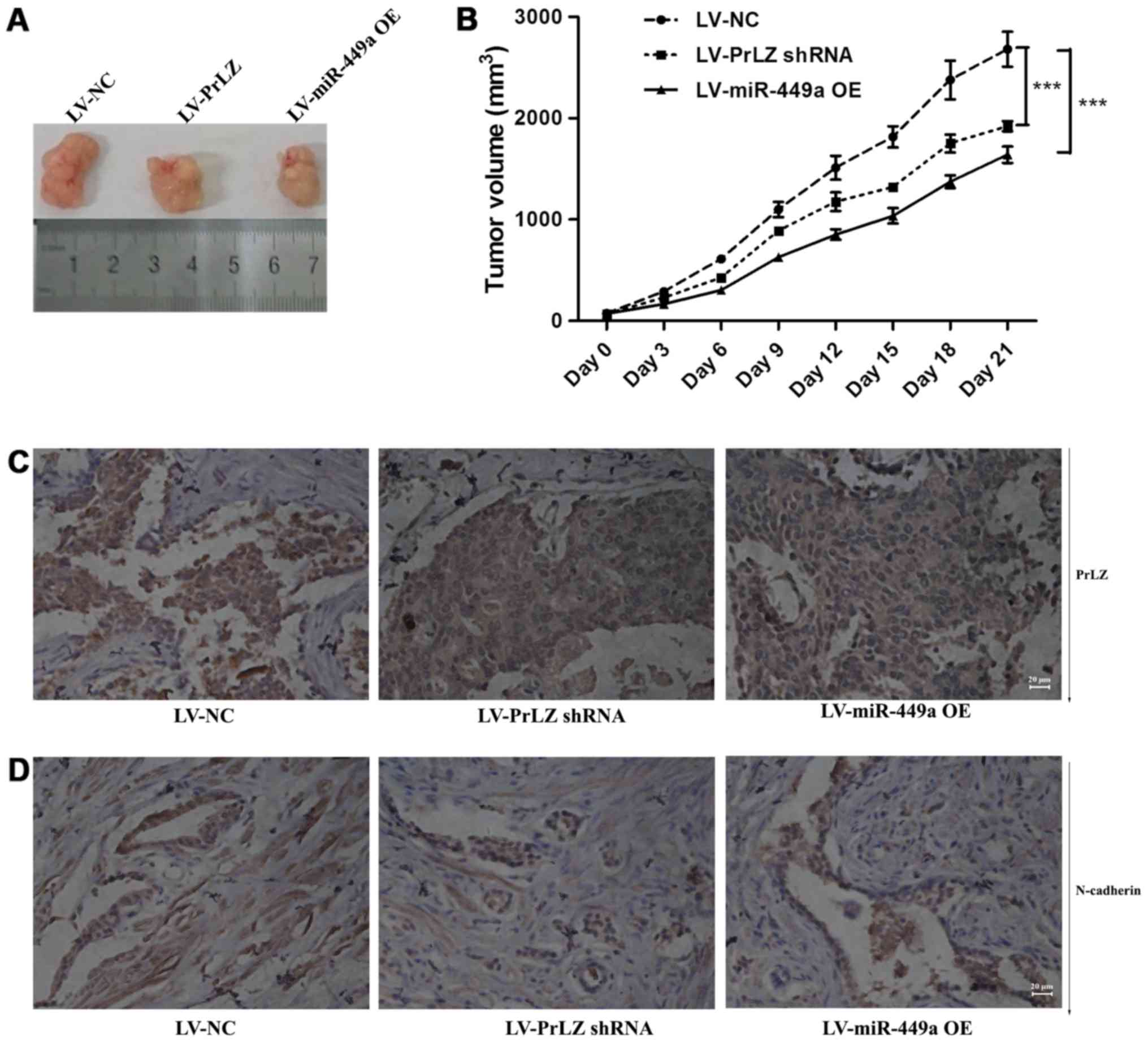
miR-449a/PrLZ axis regulates PC-3 cell
tumorigenesis and metastasis in vivo
PC-3 subcutaneously transplanted nude mice were used
to clarify the function of miR-449a/PrLZ signal axis in
vivo. We found that LV-miR-449a OE virus infection suppressed
tumor formation in vivo. Similarly, PrLZ shRNA inhibited
PC-3 cell tumorigenesis (Fig. 8A and
B). We also observed tumor metastases in vivo, miR-449a
and PrLZ shRNA inhibited the number of animals with tumor
metastases, and data are shown in Table II. We further examined PrLZ and
N-cadherin expression in tumor tissues obtained from nude mice. IHC
showed that PrLZ and N-cadherin were decreased in subcutaneous
tumors (Fig. 8C and D). These
results indicate that miR-449a/PrLZ axis regulates PCa
tumorigenesis and metastasis in vivo.
 | Table IIStatistics of metastasis in
vivo. |
Table II
Statistics of metastasis in
vivo.
| Groups | Total no. of
animals | No. of metastasis
|
|---|
| Liver
metastasis | Lung
metastasis | Total |
|---|
| LV-NC | 13 | 3 | 9 | 9 |
| LV-PrLZ shRNA | 13 | 1 | 3 | 4 |
| LV-miR-449a OE | 15 | 0 | 2 | 2 |
Discussion
As the deadly complication of malignant tumors,
distant metastasis usually occurs in advanced stage of tumors such
as prostate and lung cancer. The most common site of PCa metastasis
is bone. Bone metastasis causes consequences, such as bone pain.
Bone fractures and nerve compression, seriously affect the
patient's quality of life (18,19).
Thus, it is urgent to develop accurate predictors and illuminate
the mechanisms of PCa distant metastasis. Increasing research has
indicated microRNAs play critical roles in tumor progression, and
there is growing evidence that microRNAs are helpful tools in
prostate cancer diagnosis and prognosis, but rarely has microRNA
has been verified to be appropriate predictor of prostate cancer
distant metastasis (20–22).
Previous studies reported that miR-449a was
down-regulated in various malignant tumors, including liver
(23), non-small cell lung
(24), gastric (25) and prostate cancer (26), and Mao et al (27) found that miR-449a enhances
radiosensitivity by downregulation of c-Myc in prostate cancer
cells. Thus, we wondered whether miR-449a was associated with
prostate cancer progression and distant metastasis. In this study,
we demonstrated that miR-449a was downregulated in PCa tissues,
moreover, the expression of miR-449a was significantly lower in
advanced stage of PCa specimens and the primary lesion tissues of
PCa patients with postoperative bone metastasis. These findings
indicated that miR-449a might be related to PCa clinical
progression and distant metastasis. CCK-8 and apoptosis assay
confirmed that the loss of miR-449a promoted PCa cells survival and
proliferation. As the most important characteristics of cancer
cells, invasion and angiogenesis are the necessary abilities for
tumor distal metastasis (28–31).
Our results also showed that miR-449a suppressed invasion and
angiogenesis of LNcap and PC-3 cells, which, respectively,
represented androgen-dependent and androgen-independent PCa. These
above results proved our conjecture that the loss of miR-449a was
an important causality of PCa progression and distant metastasis.
Research has suggested that microRNA exerted physiological function
via negative regulating target genes. Studies reported that
miR-449a targeted several genes in various cancers, including c-MET
and Fos (23), c-Myc (27) and SOX4 (32). In this study, we further identified
PrLZ as a functional target of miR-449a. As a novel
prostate-specific and androgen-responsive gene, PrLZ was proved to
contribute to malignant progression in prostate cancer (13–15,33).
However, there is still no evidence that PrLZ is associated with
prostate cancer metastasis. Here we also further confirmed that
PrLZ promoted PCa cell proliferation. Importantly, we further found
that PrLZ shRNA remarkably supressed invasion and angiogenesis in
LNcap and PC-3 cells. These above results prompted that miR-449a
regulated PCa progression and metastasis via targeting PrLZ. The
decrease of miR-449a caused increase of PrLZ in primary lesions and
is critical in PCa progression and distal metastasis, in
vivo studies further confirmed our conclusions. However, how
did miR-449a/PrLZ axis regulated prostate cancer distant
metastasis? Chen et al (23) revealed that miR-449a inhibited the
epithelial-mesenchymal transition (EMT) of hepatocellular
carcinoma, and as reported in previous studies, stemness and EMT of
cancer cells played critical roles in cancer metastasis (19,34–36),
thus, we wondered whether miR-449a/PrLZ axis was associated with
EMT of prostate cancer cells. We found that miR-449a and PrLZ shRNA
significantly suppressed N-cadherin, vimentin and CD44 expression
in PCa cells. IHC assay also displayed that N-cadherin decreased in
tumors resected from PC-3 xenograft models, in which PrLZ was
downregulated by miR449a or PrLZ shRNA. Our results indicated that
miR-449a/PrLZ axis might regulate prostate cancer invasion through
EMT and stemness maintenance.
In conclusion, in the present study, we confirmed
that the loss of miR-449a caused PrLZ overexpression promoting
prostate cancer progression and distant metastasis, the clinical
diagnotic value of miR-449a and PrLZ need further study.
Abbreviations:
|
PCa
|
prostate cancer
|
|
PI
|
propidium iodide
|
|
UTR
|
untranslated region
|
|
LV-miR-449a OE
|
miR-449a overexpression lentivirus
|
|
LV-PrLZ shRNA
|
PrLZ shRNA lentivirus
|
Acknowledgments
We wish to acknowledge the patients enrolled in the
present study for their participation, and the Department of
Urology, The First Affiliated Hospital of Wenzhou Medical
University, for its collaboration in providing the human specimens
and clinical information used in this study. We also particularly
acknowledge Shanghai R&S Biotechnology Co., Ltd., for its
technical assistance and providing lentivirus used in this study.
The study was also supported by grants from the National Natural
Science Funds of China (no. 81301791) and the Foundation for
Outstanding Young Scientist of Shandong Province (no.
BS2013YY030).
References
|
1
|
Adisa JO, Egbujo EC, Ibrahim B, Musa B and
Madukwe J: Expression of some selected cytokeratins and Ki67
protein in prostatic tumor: Can these be used as tumor markers. Pan
Afr Med J. 20:462015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qi JL, Wang LJ, Zhou MG, Liu YN, Liu JM,
Liu SW, Zeng XY and Yin P: Disease burden of prostate cancer among
men in China from 1990 to 2013. Zhonghua Liu Xing Bing Xue Za Zhi.
37:778–782. 2016.In Chinese. PubMed/NCBI
|
|
4
|
Pound CR, Partin AW, Eisenberger MA, Chan
DW, Pearson JD and Walsh PC: Natural history of progression after
PSA elevation following radical prostatectomy. JAMA. 281:1591–1597.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Logothetis CJ and Lin SH: Osteoblasts in
prostate cancer metastasis to bone. Nat Rev Cancer. 5:21–28. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kosuda S, Yoshimura I, Aizawa T, Koizumi
K, Akakura K, Kuyama J, Ichihara K, Yonese J, Koizumi M, Nakashima
J, et al: Can initial prostate specific antigen determinations
eliminate the need for bone scans in patients with newly diagnosed
prostate carcinoma? A multicenter retrospective study in Japan.
Cancer. 94:964–972. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka N, Fujimoto K, Shinkai T, Nakai Y,
Kuwada M, Anai S, Miyake M, Hirayama A, Hasegawa M and Hirao Y:
Bone scan can be spared in asymptomatic prostate cancer patients
with PSA of <=20 ng/ml and Gleason score of <=6 at the
initial stage of diagnosis. Jpn J Clin Oncol. 41:1209–1213. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lilleby W and Fosså SD: Chemotherapy in
metastatic renal cell cancer. World J Urol. 23:175–179. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Todorova K, Metodiev MV, Metodieva G,
Zasheva D, Mincheff M and Hayrabedyan S: miR-204 is dysregulated in
metastatic prostate cancer in vitro. Mol Carcinog. 55:131–147.
2016. View
Article : Google Scholar
|
|
10
|
Siu MK, Tsai YC, Chang YS, Yin JJ, Suau F,
Chen WY and Liu YN: Transforming growth factor-β promotes prostate
bone metastasis through induction of microRNA-96 and activation of
the mTOR pathway. Oncogene. 34:4767–4776. 2015. View Article : Google Scholar
|
|
11
|
Browne G, Taipaleenmäki H, Stein GS, Stein
JL and Lian JB: MicroRNAs in the control of metastatic bone
disease. Trends Endocrinol Metab. 25:320–327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abba M, Patil N, Leupold JH and Allgayer
H: MicroRNAs - from metastasis prediction to metastasis prevention?
Mol Cell Oncol. 3:e10743362015. View Article : Google Scholar
|
|
13
|
Wang R, Xu J, Mabjeesh N, Zhu G, Zhou J,
Amin M, He D, Marshall FF, Zhau HE and Chung LW: PrLZ is expressed
in normal prostate development and in human prostate cancer
progression. Clin Cancer Res. 13:6040–6048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L, Zhang D, Zhang L, Zhu G, Sun Y, Wu
K, Wang X and He D: PrLZ expression is associated with the
progression of prostate cancer LNCaP cells. Mol Carcinog.
48:432–440. 2009. View
Article : Google Scholar
|
|
15
|
Li L, Xie H, Liang L, Gao Y, Zhang D, Fang
L, Lee SO, Luo J, Chen X, Wang X, et al: Increased PrLZ-mediated
androgen receptor transactivation promotes prostate cancer growth
at castration-resistant stage. Carcinogenesis. 34:257–267. 2013.
View Article : Google Scholar :
|
|
16
|
Upile T, Jerjes W, Radhi H, Al-Khawalde M,
Kafas P, Nouraei S and Sudhoff H: Vascular mimicry in cultured head
and neck tumour cell lines. Head Neck Oncol. 3:552011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deep G, Jain AK, Ramteke A, Ting H,
Vijendra KC, Gangar SC, Agarwal C and Agarwal R: SNAI1 is critical
for the aggressiveness of prostate cancer cells with low
E-cadherin. Mol Cancer. 13:372014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Keller ET, Dai J, Escara-Wilke J, Hall CL,
Ignatoski K, Taichman RS and Keller J: New trends in the treatment
of bone metastasis. J Cell Biochem. 102:1095–1102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weidle UH, Birzele F, Kollmorgen G and
Rüger R: Molecular mechanisms of bone metastasis. Cancer Genomics
Proteomics. 13:1–12. 2016.
|
|
20
|
Singh R, Ramasubramanian B, Kanji S,
Chakraborty AR, Haque SJ and Chakravarti A: Circulating microRNAs
in cancer: Hope or hype? Cancer Lett. 381:113–121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
White NM, Fatoohi E, Metias M, Jung K,
Stephan C and Yousef GM: Metastamirs: A stepping stone towards
improved cancer management. Nat Rev Clin Oncol. 8:75–84. 2011.
View Article : Google Scholar
|
|
22
|
Ell B and Kang Y: MicroRNAs as regulators
of bone homeostasis and bone metastasis. Bonekey Rep. 3:5492014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen SP, Liu BX, Xu J, Pei XF, Liao YJ,
Yuan F and Zheng F: MiR-449a suppresses the epithelial-mesenchymal
transition and metastasis of hepatocellular carcinoma by multiple
targets. BMC Cancer. 15:7062015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo W, Huang B, Li Z, Li H, Sun L, Zhang
Q, Qiu X and Wang E: MicroRNA-449a is downregulated in non-small
cell lung cancer and inhibits migration and invasion by targeting
c-Met. PLoS One. 8:e647592013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Q, Peng J, Li X, Leng A and Liu T:
miR-449a targets Flot2 and inhibits gastric cancer invasion by
inhibiting TGF-β-mediated EMT. Diagn Pathol. 10:2022015. View Article : Google Scholar
|
|
26
|
Noonan EJ, Place RF, Pookot D, Basak S,
Whitson JM, Hirata H, Giardina C and Dahiya R: miR-449a targets
HDAC-1 and induces growth arrest in prostate cancer. Oncogene.
28:1714–1724. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mao A, Zhao Q, Zhou X, Sun C, Si J, Zhou
R, Gan L and Zhang H: MicroRNA-449a enhances radiosensitivity by
downregulation of c-Myc in prostate cancer cells. Sci Rep.
6:273462016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Graff JR and Zimmer SG: Translational
control and metastatic progression: Enhanced activity of the mRNA
cap-binding protein eIF-4E selectively enhances translation of
metastasis-related mRNAs. Clin Exp Metastasis. 20:265–273. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kelly T, Suva LJ, Huang Y, Macleod V, Miao
HQ, Walker RC and Sanderson RD: Expression of heparanase by primary
breast tumors promotes bone resorption in the absence of detectable
bone metastases. Cancer Res. 65:5778–5784. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyamoto N, Yamamoto H, Taniguchi H,
Miyamoto C, Oki M, Adachi Y, Imai K and Shinomura Y: Differential
expression of angiogenesis-related genes in human gastric cancers
with and those without high-frequency microsatellite instability.
Cancer Lett. 254:42–53. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perlikos F, Harrington KJ and Syrigos KN:
Key molecular mechanisms in lung cancer invasion and metastasis: A
comprehensive review. Crit Rev Oncol Hematol. 87:1–11. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sandbothe M, Buurman R, Reich N, Greiwe L,
Vajen B, Gürlevik E, Schäffer V, Eilers M, Kühnel F, Vaquero A, et
al: The microRNA-449 family inhibits TGF-β-mediated liver cancer
cell migration by targeting SOX4. J Hepatol. 66:1012–1021. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang H, Wang J, Pang B, Liang RX, Li S,
Huang PT, Wang R, Chung LW, Zhau HE, Huang C, et al: PC-1/PrLZ
contributes to malignant progression in prostate cancer. Cancer
Res. 67:8906–8913. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakazawa M and Kyprianou N:
Epithelial-mesenchymal-transition regulators in prostate cancer:
Androgens and beyond. J Steroid Biochem Mol Biol. 166:84–90. 2017.
View Article : Google Scholar
|
|
35
|
Diepenbruck M and Christofori G:
Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no,
maybe? Curr Opin Cell Biol. 43:7–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim EK, Choi EJ and Debnath T: Role of
phytochemicals in the inhibition of epithelial-mesenchymal
transition in cancer metastasis. Food Funct. 7:3677–3685. 2016.
View Article : Google Scholar : PubMed/NCBI
|