Introduction
Colorectal cancer is the third most common cancer in
the world, with nearly 1.4 million new cases diagnosed in 2016
(1). Currently, methods of
treatment for colorectal cancer include surgery, radiotherapy,
chemotherapy, and immunotherapy. However, the curative effect of
these methods, particularly for advanced colorectal cancer, is
limited (2–4). Tumor-targeted gene therapy is a new
and promising method for the effective treatment of colorectal
cancer. Gene therapy that uses vectors to bring nucleic acids into
cells to alter gene expression can prevent or reverse malignant
progression of the tumor. The clinical trials on gene therapy are
increasing worldwide. Although tumor-selective delivery systems
have made significant progress in the past 20 years, development of
therapeutic vectors based on promoters that are specifically
expressed in cancer cells is still a challenge. Therefore, many
strategies use specific gene enhancers, promoters, and
5′-untranslated regions that respond to tumor-targeted
transcription factors, in order to overexpress tumor suppressor
genes or to reduce cancer gene expression, have appeared.
In this study, we focused on the intestinal
transcription factor caudal type homeobox 2 (CDX2). CDX2
participates in the development, proliferation, and differentiation
of intestinal epithelial cells (5–7).
Furthermore, CDX2 reduces the mobility and dissemination of colon
cancer cells both in vitro and in vivo (8). CDX2 has tumor-inhibition properties
in colorectal cancer (9–11), reduced CDX2 expression is connected
with poor survival in patients with colorectal cancer (12). In our previous studies, forced
expression of CDX2 by a cytomegalovirus promoter inhibited invasion
of LoVo colon cancer cells (13).
However, targeted expression of CDX2 to cancer cells is
required.
In most cancer cells, but not normal cells, the
human telomerase reverse transcriptase (hTERT) promoter is
activated (14,15). Therefore, the hTERT promoter has
been used in many tumor-targeted gene therapy studies to target
cancer cells, including A549 human lung adenocarcinoma cells and
human gastric cancer MKN45 cells (16,17).
Hypoxia is a major feature of solid tumors and
induces hypoxia-inducible factor-1α (HIF-1α) which binds to the
hypoxia-response elements (HREs) of various target genes and
activates their transcription to regulate glucose transport and
angiogenesis, and potentially enhance the survival of tumor cells
(18–22). Our previous studies have shown that
hypoxia modulates the downregulation of CDX2 in colorectal cancer
(23). In contrast, in this study
we engineered a system in which hypoxia promotes CDX2 expression.
To restore CDX2 expression in colon cancer cells, we constructed an
expression vector carrying CDX2 under the control of the
hypoxia-inducible hTERT promoter (pLVX-5HRE-hTERTp-CDX2-3FLAG).
Targeted genes simultaneously can be dramatically upregulated by 5
copies of a hypoxia response element (HRE) under hypoxic conditions
(24,25).
We evaluated the effects of restored CDX2 expression
on LoVo colon cancer cell viability, cell cycle distribution,
apoptosis, and colony formation and invasion ability in
vitro and on xenograft tumor growth in vivo. This study
further explored potential strategies of targeted gene therapy for
colon cancer.
Materials and methods
Cell lines and cell cultures
All cell lines were purchased from the Shanghai
Institute of Cell Biology, Chinese Academy of Sciences (Shanghai,
China). The LoVo human colon cancer cells were cultured in DMEM
(Gibco BRL, Carlsbad, CA, USA) supplemented with 10% FBS (Hyclone,
Logan, UT, USA) in a humidified atmosphere with 5% CO2
at 37°C. For hypoxic cell culture, cells were incubated with medium
containing a hypoxia-mimicking agent, CoCl2.
Lentivirus infection of LoVo cells
The recombinant plasmid pLVX-5HRE-hTERTp-CDX2-3FLAG
(designated as 5HhC) and the control plasmids
pLVX-5HRE-hTERTp-EGFP-3FLAG (i.e., 5Hh) and pLVX-hTERTp-CDX2-3FLAG
(i.e., hC) have been previously described (26). These vectors and corresponding
viruses (1×108 pfu) were custom constructed and prepared
by GeneChem Co. Lentivirus infection was performed in the presence
of polybrene (GeneChem) in accordance with the manufacturer's
protocol. LoVo cells at 5×105/well were cultured in
6-well plates overnight, infected with 5HhC lentivirus or control
5Hh and hC lentiviruses, and exposed to puromycin (800
µg/ml) for 2 weeks. The 5HhC/LoVo, 5Hh/LoVo, and hC/LoVo
cells were cloned routinely. Drug-resistant clones were identified
and used in the following experiments.
Western blot analysis
The hC/LoVo, 5Hh/LoVo, 5HhC/LoVo, and untransfected
LoVo cells were cultured under normoxic or hypoxic conditions (200
µmol/l CoCl2) for 24 h. The relative ratios of
CDX2, collagen IV, laminin-1, TGF-β, cyclin D1, uPA, MMP-2, MMP-9,
bcl-2, and bax protein to control β-actin were determined by
western blot analysis. Briefly, the hC, 5Hh or 5HhC
lentivirus-infected LoVo cells (1×106 cells) were lysed
with 150 µl of lysis buffer (50 mM Tris, 150 mM NaCl, 5 mM
ethylenediaminetetraacetic acid (EDTA), 5 mM EGTA and 1% SDS, pH
7.5) and gently sonicated. After quantification with Bradford
reagent (Thermo Fisher Scientific, Waltham, MA, USA), the protein
lysates (80 µg/lane) of each sample were subjected to
SDS-PAGE (Shaanxi Pioneer Biotech) on 10% acrylamide gels and
transferred to polyvinylidene fluoride membranes (Millipore,
Billerica, MA, USA). After blocking with 5% fat-free dry milk, the
membranes were incubated with 1:1000 diluted monoclonal rabbit
anti-CDX2 (Epitomics, Burlingame, CA, USA) or 1:3000 diluted
polyclonal rabbit anti-β-actin (Bioworld Technology, St. Louis
Park, MN, USA) overnight at 4°C, and then horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody
(Zhongshan Goldbridge Biotechnology, Beijing, China). This was
visualized with enhanced chemiluminescence (Amresco, Solon, OH,
USA).
Reverse transcriptase polymerase chain
reaction (RT-PCR) and quantitative RT-PCR analysis
The hC/LoVo, 5Hh/LoVo, 5HhC/LoVo and LoVo cells were
cultured under normoxic or hypoxic conditions (200 µmol/l
CoCl2), and the transcription of recombinant CDX2 mRNA
was determined by RT-PCR using the primers listed in Table I. In brief, total RNA was extracted
from the infected cells using TRIzol (Gibco Life Technologies,
Grand Island, NY, USA), in accordance with the manufacturer's
guidelines. The resulting RNAs were treated with RNase-free DNase
(Promega, Madison, WI, USA) and reverse transcribed into cDNA using
a RT-PCR kit (Invitrogen, Carlsbad, USA), in accordance with the
manufacturer's instructions. PCR amplification with the specific
primers (Table I) was performed in
duplicate at 98°C for 2 min; 35 cycles of 98°C for 20 sec, 59°C for
30 sec, and at 72°C for 1 min; and then an extension at 72°C for 10
min. The PCR products were resolved via agarose gel
electrophoresis. RT-PCR was performed in triplicate for each cell
sample using a ChemiDoc System (ChemiDoc MP System 170-8280;
Bio-Rad Laboratories). The cDNA was subjected to quantitative
RT-PCR analysis of CDX2 mRNA using SYBR Premix Ex Taq II (Takara)
and specific primers (Table I) in
an iQ5 multicolor Realtime PCR Detection System (Bio-Rad, Hercules,
CA, USA). Each reaction was performed in triplicate and the mean
CDX2 mRNA level in each group was calculated by the
2−ΔΔCt method, where Ct is the cycle threshold.
 | Table ISequences of PCR primers. |
Table I
Sequences of PCR primers.
| Gene | | Primer | Base sequence
5′-3′ | PCR product
(bp) |
|---|
| CDX2 | RT-PCR | Forward |
CGGAATTCATGTACGTGAGCTACCTCCTGGACAAGGAC | 943 |
| Reverse |
CGGGATCCGTCTGGGTGACGGTGGGGTTTAGCACCCCCCCAGTTG |
| CDX2 | Quantitative
RT-PCR | Forward |
TTCACTACAGTCGCTACATCACC | 100 |
| Reverse |
ACTGCGGTTCTGAAACCAGATT |
| β-actin | RT-PCR and
quantitative RT-PCR | Forward |
AATCTGGCACCACACCTTCTA | 170 |
| Reverse |
ATAGCACAGCCTGGATAGCA |
MTT assay
The growth of LoVo cells of all experimental groups
was measured by methyl thiazol tetrazolium (MTT) assay. The cells
(hC/LoVo, 5Hh/LoVo, 5HhC/LoVo, and LoVo; 5×103/well in
triplicate) were cultured under normoxic or hypoxic conditions (200
µmol/l CoCl2) for 1, 3, 5 or 7 days.
Subsequently, the plates were washed extensively with serum-free
DMEM to remove CoCl2 and dead cells, and were exposed to
20 µl (5 g/l) of MTT (Amersco) for 4 h. The resulting
formazan crystals were dissolved in 200 µl of DMSO
(Sigma-Aldrich), and the absorbance was measured at 490 nm in a
microplate reader (Victor3, Perkin-Elmer, Waltham, MA, USA).
Colony formation assay
Two hundred cells (hC/LoVo, 5Hh/LoVo, 5HhC/LoVo, or
LoVo), plated in 60-mm cell culture dishes, were cultured for 3
weeks. The hypoxic group was also treated with CoCl2
(200 µmol/l). Colony-forming clones were fixed with methanol
at room temperature for 15 min and then stained with Giemsa
solution, and clones containing 50 or more cells were considered to
be true clones. Colonies were counted under an inverted microscope
(Leica Microsystems GmbH, Heidelberg, Germany).
Flow cytometric apoptosis and cell cycle
distribution assays
The hC/LoVo, 5Hh/LoVo, 5HhC/LoVo and LoVo cells were
seeded into 60-mm culture dishes and the hypoxia group was treated
with CoCl2 (200 µmol/l) for 24 h. For analysis of
apoptosis, an FITC Annexin V Apoptosis detection kit I (Becton
Dickinson, Franklin Lakes, NJ, USA) was used, in accordance with
the manufacturer's instructions. For cell cycle analysis, cells
were fixed overnight in 75% ethanol at −20°C, incubated with RNase
A at 37°C for 30 min, and then incubated with propidium iodide at
room temperature for 30 min. Cells were examined by flow cytometry
and the data were analyzed using CellQuest version 3.3 software
(Becton Dickinson).
Wound-healing assay
The cells (hC/LoVo, 5Hh/LoVo, 5HhC/LoVo and LoVo;
2×104) were cultured in a 6-well plate. After 12 h of
culture, pipette tips (200 µl) were used to scratch 3
parallel vertical lines in each well. The wells were washed with
PBS, and then the cells were cultured in serum-free DMEM. Scratch
lines were observed under a microscope and scratch distances were
measured, with images captured at 24 h after scratching.
Migration and invasion assay
For the migration assay, all groups of cells
(hC/LoVo, 5Hh/LoVo, 5HhC/LoVo, and LoVo) were digested with
trypsin-EDTA (Sigma, St. Louis, MO, USA), and 5×103
cells were suspended in serum-free medium supplemented with 0.5%
bovine serum albumin (BSA; Sigma). For the hypoxia group,
CoCl2 (200 µmol/l CoCl2) was also
added to all cells before digestion. Cell suspensions were seeded
into the inserts of Transwells (Corning Inc., New York, NY, USA)
and incubated at 37°C for 48 h. All Transwell inserts were then
washed with fresh PBS and non-migratory cells on the upper surface
of the Transwell inserts were removed. The migratory cells on the
underside of the membrane were fixed with 95% alcohol and stained
with crystal violet (Beyotime, Jiangsu, China). For the invasion
assay, the upper chamber was pre-coated with 50 mg/l Matrigel
(Sigma) prior to the addition of 1×104 cells in
serum-free medium supplemented with BSA. The number of migratory or
invading cells per membrane was counted under an inverted
microscope. Three randomly selected fields of fixed cells were
taken and counted.
Nude mouse xenograft assay
All animal procedures were approved by the
Institutional Animal Care and Use Committee at the First Affiliated
Hospital of Xi'an Jiaotong University. Four-week-old female BALB/c
athymic (nude) mice with body weights of approximately 20 g were
purchased from Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai,
China) and housed in the Laboratory Animal Centre of Xi'an Jiaotong
University. After one week of acclimatization, all nude mice were
randomly divided into four groups (hC/LoVo, 5Hh/LoVo, 5HhC/LoVo,
and LoVo). The mice were subcutaneously inoculated at the right
flanks with cells (1×107/ml) of the four groups in the
logarithmic growth period. At 18 days post-injection, tumor sizes
were monitored every 3 days using calipers, and tumor volumes were
calculated according to the standard formula: width2 ×
length/2 and expressed as mm3 (27). At the end of 18 days, the mice were
sacrificed by cervical dislocation and the tumor tissues of each
group were fixed with 10% formaldehyde (Boster Biological
Engineering) solution for subsequent immunohistochemical
analysis.
Immunohistochemistry
Tumor tissues were embedded in paraffin (Xi'an
Chemical Reagents Instruments, Xi'an, China) and the tissue
sections (4-µm) were immunostained for CDX2 (1:400) or Ki-67
(1:400). In accordance with the manufacturer's instructions, an ABC
Elite kit (Boster, Biological Technology) was used to visualize
antibody binding, and the slides were subsequently counterstained
with hematoxylin (Boster Biological Engineering). Negative controls
were included by replacement of the primary antibody with PBS.
Images were captured using a microscope (Leica Microsystems GmbH).
The intensity of the staining was scored as 1 (negative), 2 (weakly
positive), 3 (moderately positive) or 4 (strongly positive). The
extent of the staining was categorized as 1 (stained cells: 1–25%),
2 (26–50%), 3 (51–75%) or 4 (76–100%). The final staining score was
the product of the intensity and the extent scores. Images of five
random fields were taken from each specimen for quantitative
analysis (28).
Statistical analysis
The data are representative of three independent
experiments and are presented as the mean ± standard deviation
(SD). A two-sample t-test was performed to analyze two independent
samples, whereas analysis of variance was conducted for comparison
among multiple groups. SPSS 13.0 software (SPSS, Chicago, IL, USA)
was used to calculate the P-value and a P<0.05 was considered to
indicate a statistically significant difference.
Results
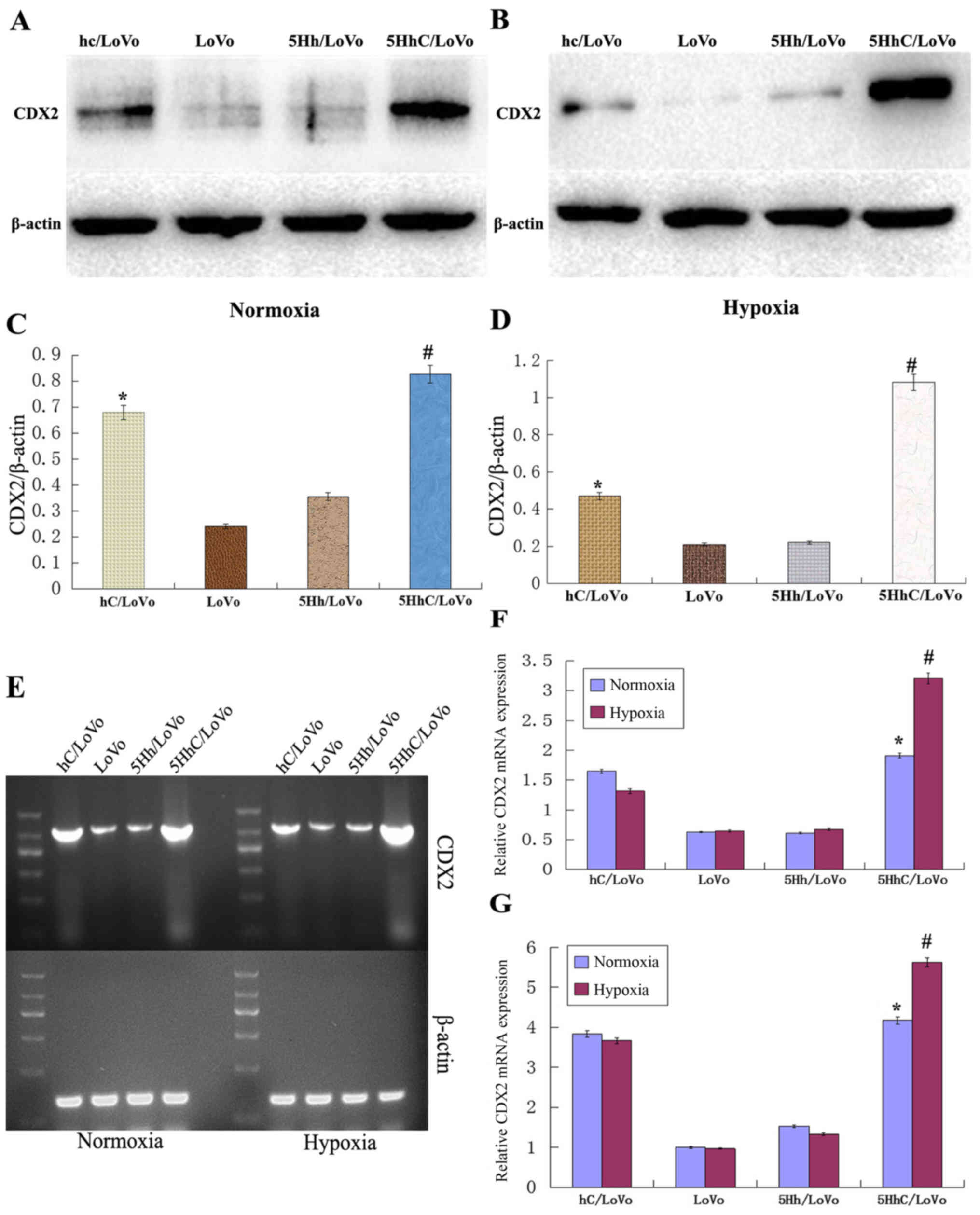
Expression of CDX2 in hC/LoVo, LoVo,
5Hh/LoVo, 5HhC/LoVo under normoxic or hypoxic conditions
To investigate the role of CDX2 expression in LoVo
colon cancer cells, we stably transfected LoVo cells with 5HhC or
its control plasmids hC or 5Hh expression vector. We confirmed the
expression of CDX2 in transfected LoVo cells, especially under
hypoxic conditions, by western blot (Fig. 1A–D), RT-PCR (Fig. 1E and F) and real-time PCR (Fig. 1G). Compared to the control LoVo
cells, mock-transfected cells, or cells transfected with the 5Hh
vector, the expression of CDX2 was higher in hC/LoVo and 5HhC/LoVo.
The highest expression of CDX2 was observed in 5HhC/LoVo cells
under hypoxic conditions (P<0.01).
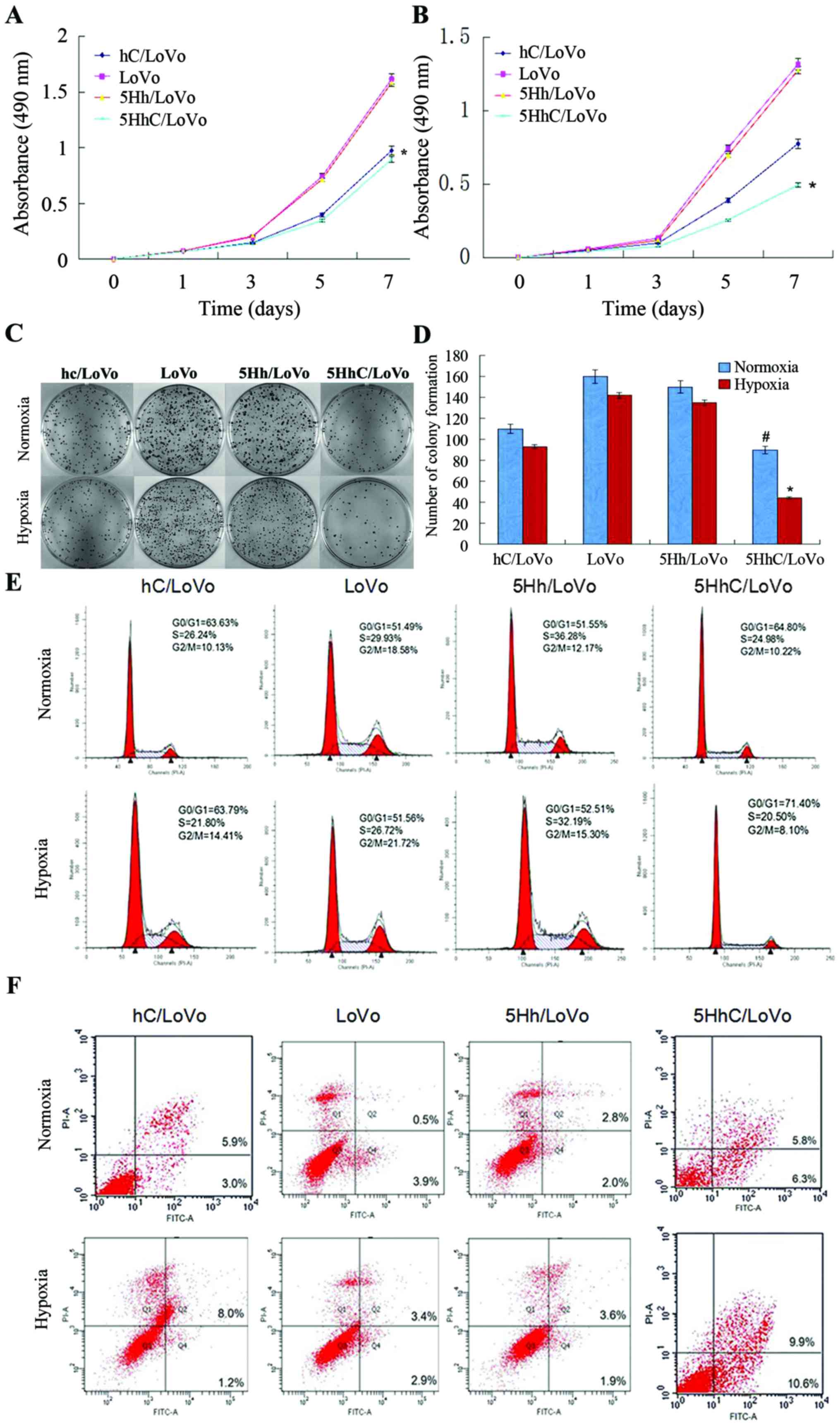
Expression of CDX2 in LoVo cells inhibits
cell proliferation and clonogenicity by restraining the G1 to S
transition
To investigate the effects of exogenous CDX2
expression in 5HhC/LoVo, we performed MTT and colony-formation
assays using hC/LoVo, LoVo, 5Hh/LoVo, and 5HhC/LoVo cells under
normoxic or hypoxic conditions. The MTT assay showed that hC/LoVo
and 5HhC/LoVo cells grew much slower than the control LoVo and
5Hh/LoVo cells under normoxic conditions (Fig. 2A). 5HhC/LoVo cells under hypoxic
conditions showed the least growth among all cells (Fig. 2B), suggesting that CDX2 expression
in 5HhC/LoVo inhibited cell proliferation. As shown in Fig. 2C and D, exogenous CDX2 expression
in 5HhC/LoVo also led to decreased colony numbers and colony size
in the colony-formation assay, especially under hypoxic conditions.
These observations indicated that the CDX2 expression reduced
proliferation and clonogenic growth of LoVo cells in
vitro.
Furthermore, the cell cycle analysis of the hC/LoVo
and 5HhC/LoVo cells showed a higher population of cells in the
G0/G1 phases (63.59 and 64.82%) compared to the control LoVo and
5Hh/LoVo cells (51.38 and 51.59%) under normoxic conditions. The
highest percentage of cells in the G0/G1 phases of 5HhC/LoVo cells
was observed under hypoxic conditions (Fig. 2E). These results suggest that CDX2
inhibits cell proliferation and tumorigenicity by preventing
G1-to-S transition.
Exogenous CDX2 expression in LoVo cells
promotes apoptosis
Annexin V-FITC/propidium iodide flow cytometry was
used to assess the effect of CDX2 expression on apoptosis. In
Fig. 2F, the fourth quadrant (Q4)
represents early apoptotic cells and the second quadrant (Q2)
represents late apoptotic and necrotic cells. The results showed
that hC/LoVo and 5HhC/LoVo underwent increased apoptosis, compared
with LoVo and 5Hh/LoVo cells, with the greatest effect observed
under hypoxic conditions (Table
II).
 | Table IIPercentages of cells in each phase of
the cell cycle and apoptotic cells. |
Table II
Percentages of cells in each phase of
the cell cycle and apoptotic cells.
| Group | Condition | G0/G1 | S | G2/M | Apoptosis |
|---|
| hC/LoVo | Normoxia | 63.59±0.55 | 26.01±2.21 | 10.40±1.32 | 9.03±1.93 |
| Hypoxia | 63.65±0.44 | 21.94±2.04 | 14.41±0.98 | 9.25±1.69 |
| LoVo | Normoxia | 51.38±0.70 | 30.02±0.66 | 18.60±1.13 | 4.25±1.17 |
| Hypoxia | 51.44±0.56 | 26.73±0.61 | 21.83±1.25 | 5.97±0.85 |
| 5Hh/LoVo | Normoxia | 51.59±0.38 | 36.19±1.12 | 12.22±0.30 | 4.92±0.92 |
| Hypoxia | 52.60±0.99 | 32.04±1.23 | 15.36±0.17 | 5.65±0.99 |
| 5HhC/LoVo | Normoxia | 64.82±2.22a | 24.88±1.98 | 10.30±0.32 | 12.58±2.38a |
| Hypoxia | 71.38±3.02b | 20.62±1.59 | 8.00±0.33 | 21.2±2.26b |
Effects of CDX2 overexpression in
5HhC/LoVo on cell invasion and migration potential
To investigate the effect of CDX2 overexpression in
5HhC/LoVo on cell invasion and migration, wound-healing, and
Transwell assays were performed in all groups of LoVo cell lines
under normoxic or hypoxic conditions. The wound-healing assay
clearly showed that 5HhC/LoVo cells had reduced migration,
especially under hypoxic conditions, since the remaining scratches
in the wells containing CDX2-expressing cells were wider than in
the controls (Fig. 3A and B).
Furthermore, random microscopic fields of invading and migrating
cells transfected with hC, 5Hh, 5HhC, or untreated LoVo cells under
normoxic and hypoxic conditions are shown in Fig. 3C–F. The histogram shows that the
number of invading or migrating cells transfected with hC and 5HhC
was significantly lower than in cells transfected with 5Hh or
untreated LoVo cells. The lowest number of invading or migrating
cells was seen in 5HhC/LoVo cells under hypoxic conditions. These
results showed that overexpression of CDX2 substantially decreased
migration and invasion of LoVo cells, especially under hypoxic
conditions.
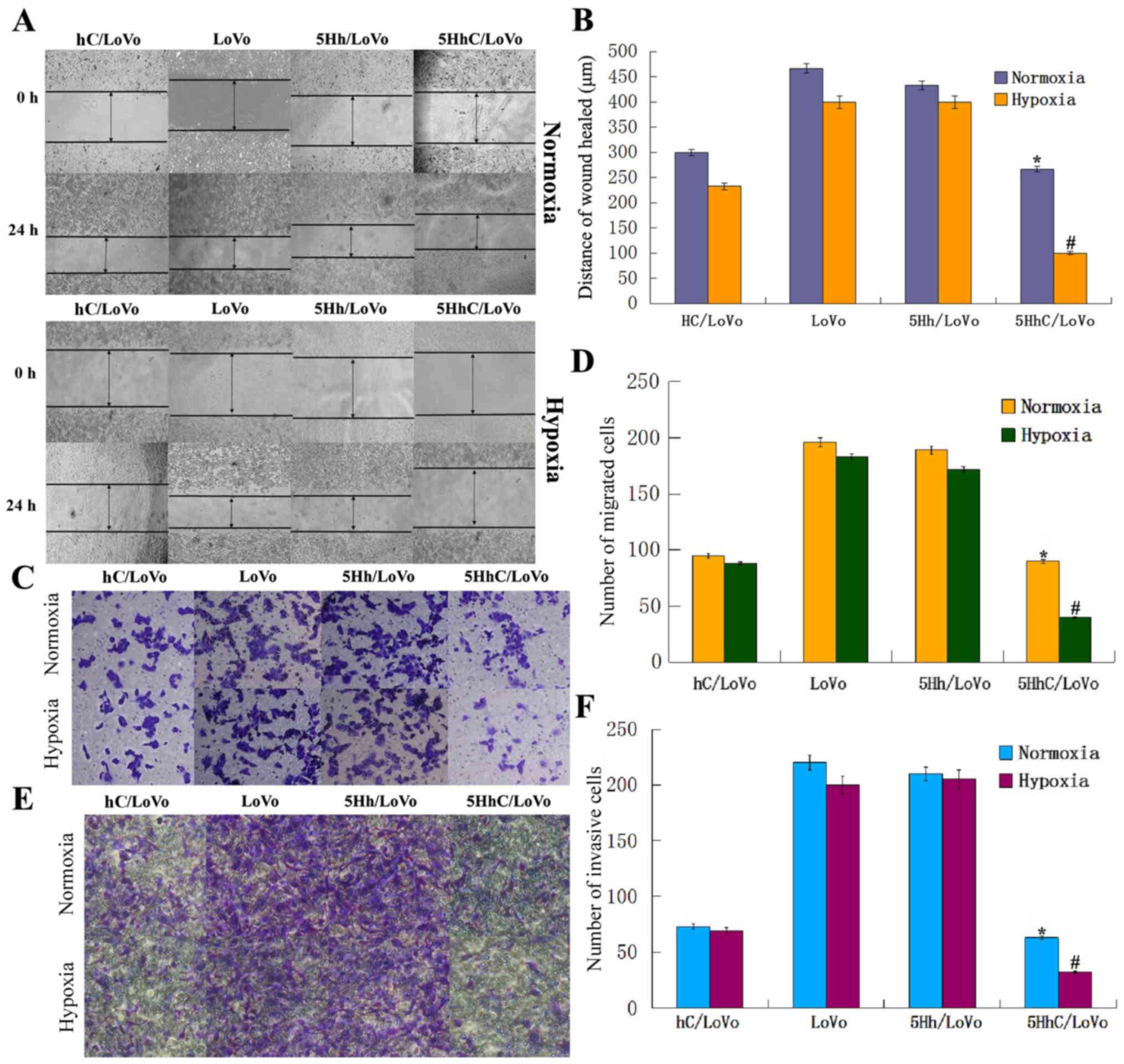 | Figure 3Migration and invasion of LoVo cells
in all groups under normoxic and hypoxic conditions. (A and B)
5HhC/LoVo cells in the wound-healing assay migrated slower compared
with control LoVo and 5Hh/LoVo cells, especially under hypoxic
condition. (*P<0.05 compared to LoVo, 5Hh/LoVo under
normoxia; #P<0.05 compared to hc/LoVo, LoVo, 5Hh/LoVo
under hypoxia). (C and D) 5HhC/LoVo cells displayed decreased
migration ability compared with control LoVo and 5Hh/LoVo cells,
especially under hypoxic condition, (*P<0.05 compared
to LoVo, 5Hh/LoVo under normoxia; #P<0.05 compared to
hc/LoVo, LoVo, 5Hh/LoVo under hypoxia). (E and F) 5HhC/LoVo cells
displayed decreased invasion ability compared with control LoVo and
5Hh/LoVo cells, especially under hypoxic conditions.
(*P<0.05 compared to LoVo, 5Hh/LoVo under normoxia;
#P<0.05 compared to hc/LoVo, LoVo, 5Hh/LoVo under
hypoxia). The data shown are representative images of each group of
cells from 3 separate experiments. The results are shown as means ±
SD. |
The effect of CDX2 on the expression of
collagen IV, laminin-1, TGF-β, cyclin D1, uPA, MMP-2, MMP-9, bcl-2,
and bax
We used western blotting to examine how CDX2
regulates the expression of proteins involved in proliferation,
apoptosis, migration, and invasion under normoxic conditions. The
western blot results showed that overexpression of CDX2 in hC/LoVo
and 5HhC/LoVo cells upregulated expression of collagen IV,
laminin-1 and bax protein. In contrast, the expression of TGF-β,
cyclinD, uPA, MMP-9, MMP-2, and BCL-2 protein was decreased by CDX2
(Figs. 4 and 5).
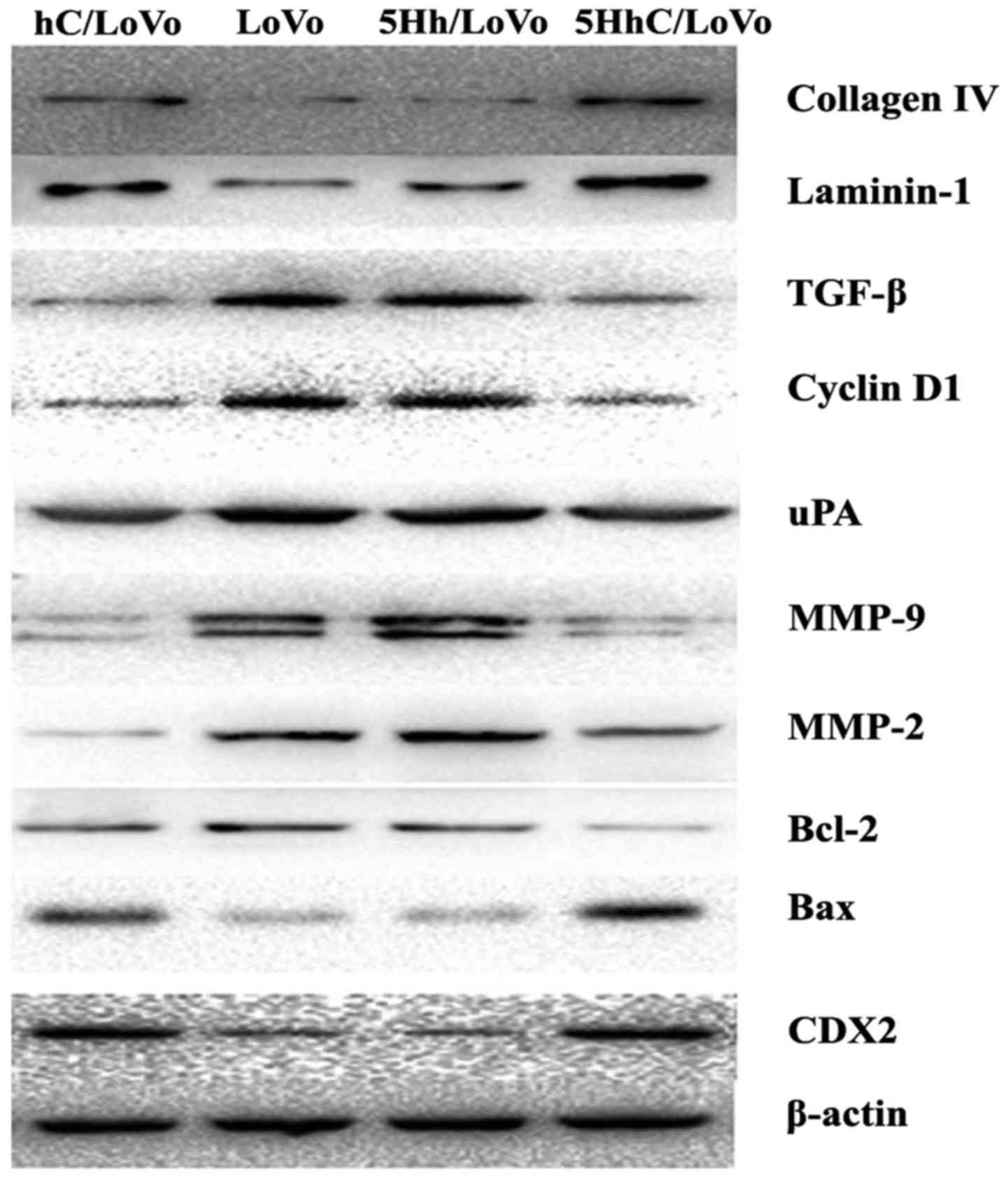 | Figure 4Expression of collagen IV, laminin-1,
TGF-β, Cyclin D, uPA, MMP-2, MMP-9, bcl-2 and bax protein in
hC/LoVo, LoVo, 5Hh/LoVo, 5HhC/LoVo cells. |
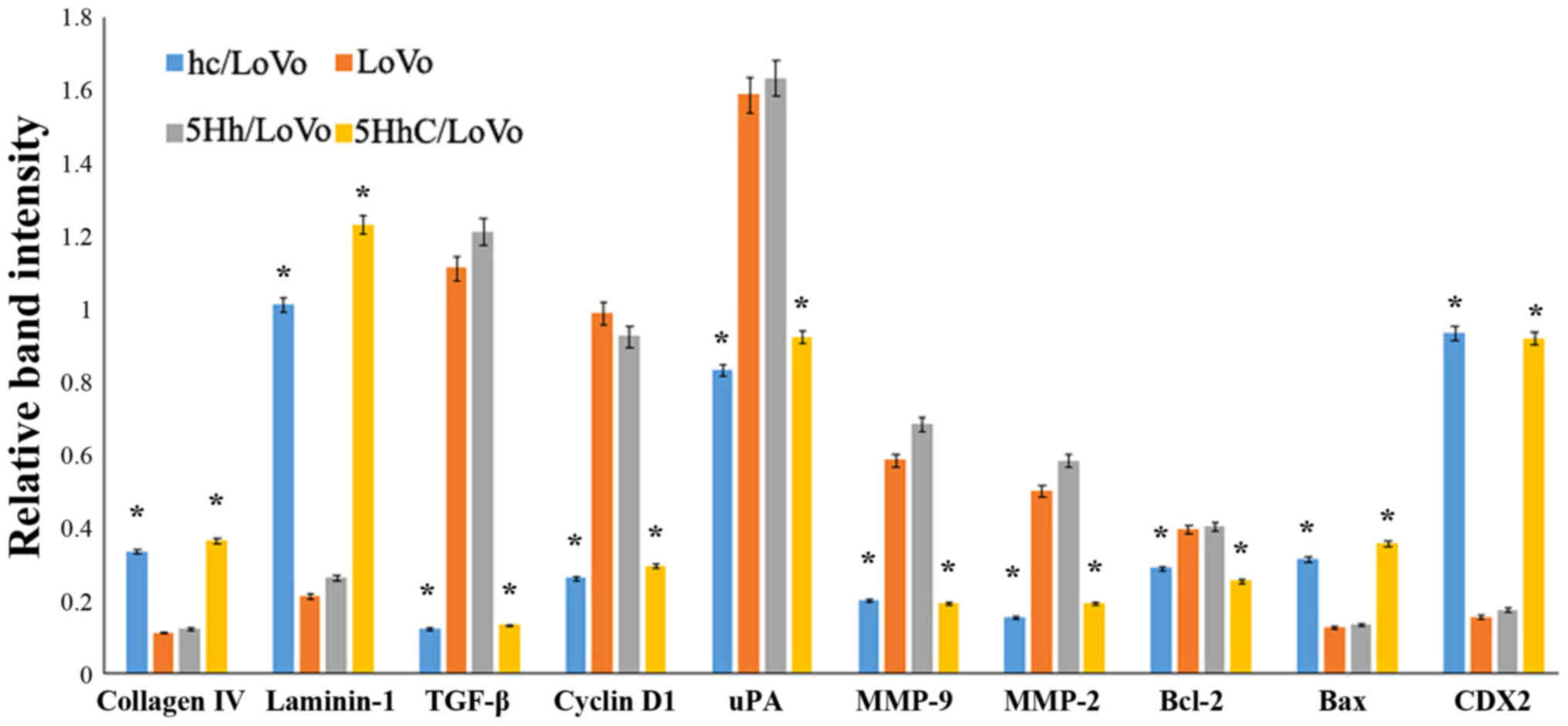 | Figure 5Relative expression of collagen IV,
laminin-1, TGF-β, Cyclin D, uPA, MMP-2, MMP-9, bcl-2 and bax
protein in hC/LoVo, LoVo, 5Hh/LoVo, 5HhC/LoVo cells.
(*P<0.05 compared to LoVo, 5Hh/LoVo under normoxia).
Each experiment was performed in triplicate. |
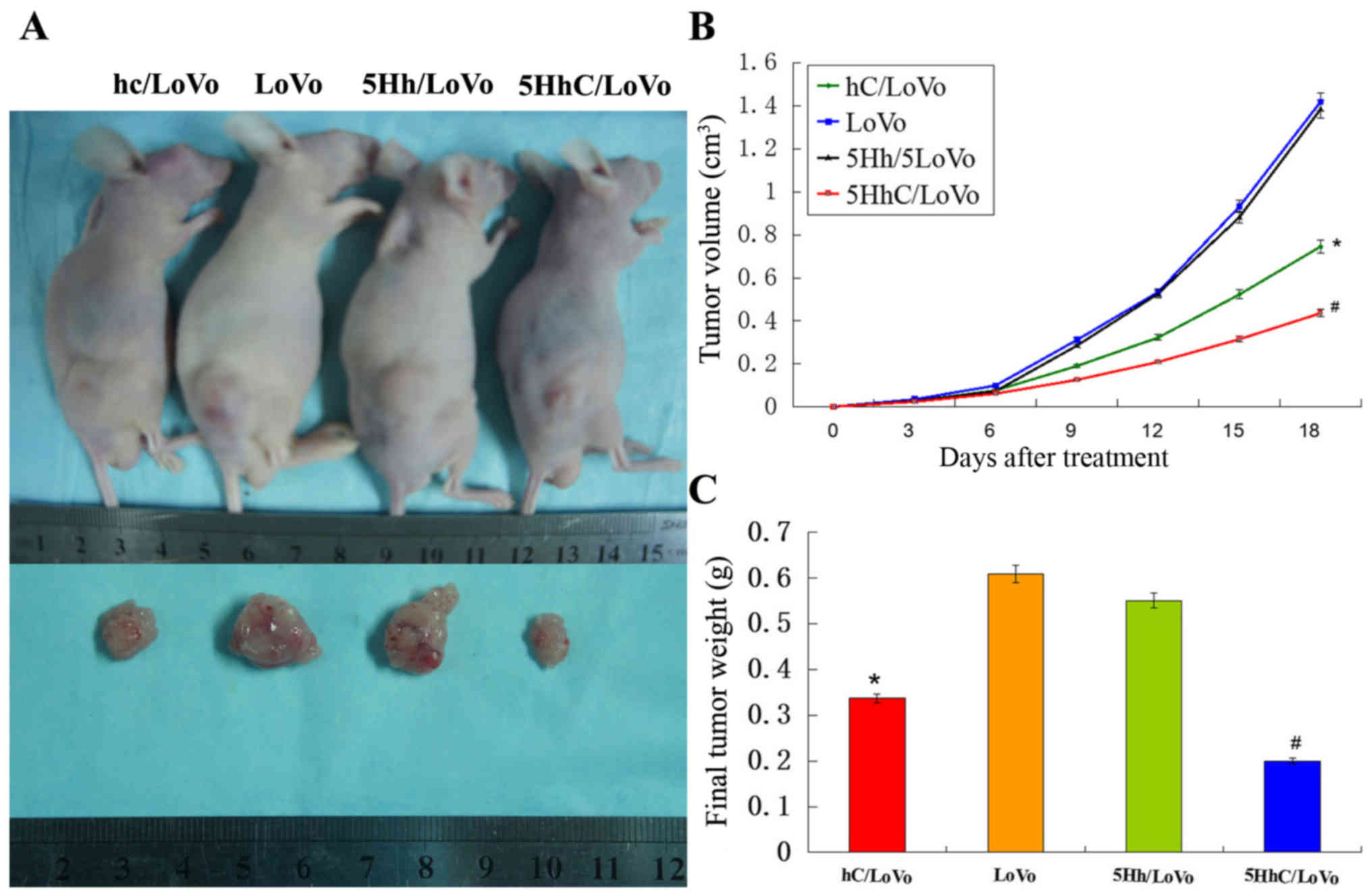
Overexpression of CDX2 in 5HhC/LoVo
inhibited tumorigenicity in vivo
LoVo cells (5×106) were injected into
athymic nude mice and monitored for 18 days. At the end of the
study, the tumors were removed and dissociated, and both the volume
and the weight of the tumors were determined (Fig. 6A). The volume of xenograft tumors
in the hC/LoVo and 5HhC/LoVo groups revealed an obvious difference
compared with the LoVo and 5Hh/LoVo groups (Fig. 6B), especially in the 5HhC/LoVo
group. Similarly, hC/LoVo and 5HhC/LoVo cells formed smaller
subcutaneous tumors than did LoVo and 5Hh/LoVo cells (Fig. 6C). The mean tumor weights in mice
of the hc, LoVo, 5Hh/LoVo, and 5HhC groups were 0.337±0.106 g,
0.609±0.302 g, 0.551±0.158 g and 0.201±0.112 g, respectively. The
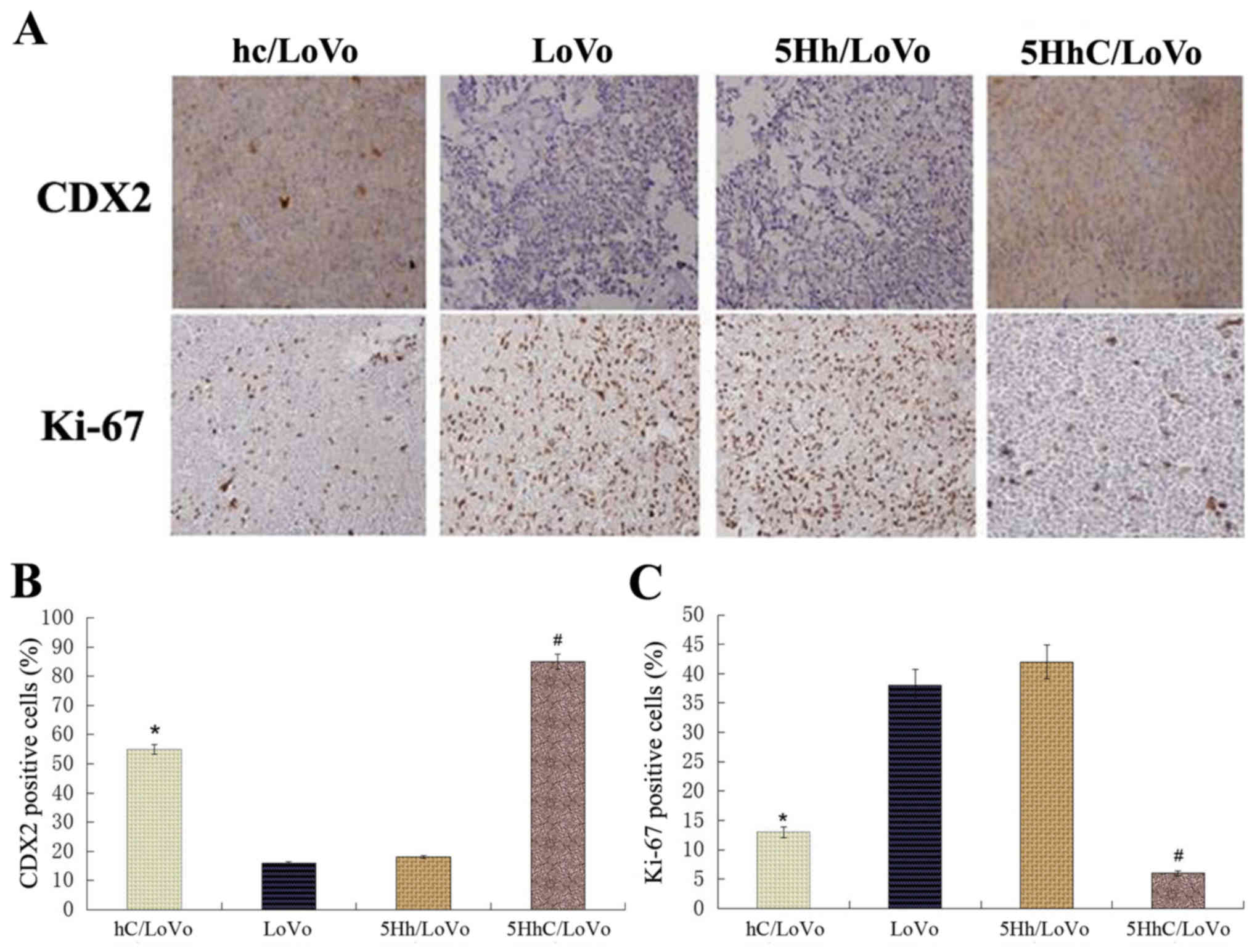
tumors formed by hC/LoVo and 5HhC/LoVo cells showed less Ki-67
expression than tumors formed by LoVo and 5Hh/LoVo cells, with the
lowest level of Ki-67 expression in the 5HhC/LoVo group (Fig. 7). These results confirmed that CDX2
inhibits proliferation of cancer cells in vivo.
Discussion
Gene therapy is a promising option for the treatment
of human cancers. However, two keys to success, the persistent
expression of anticancer gene products, and a tumor-selective
delivery system, remain elusive. CDX2 expression in adults is
restricted to intestinal epithelial cells, where it maintains
differentiated phenotype of mature enterocytes by regulating
expression of intestine-specific genes, including lactase,
sucrase-isomaltase, LI-cadherin, and guanylyl cyclase C (GCC)
(29). In addition, CDX2 also
functions as a tumor suppressor gene in the adult colon. Clinically
and pathologically, CDX2 expression is decreased in human
colorectal cancer, and reduced expression of CDX2 is associated
with poor overall survival rates in colorectal cancer patients
(12,30,31).
Histopathological studies have further established that CDX2
expression is reduced in invasive colorectal cancer cells, but is
restored in metastases (32,33).
Our previous study confirmed that upregulation of CDX2 expression
in human colon cancer cells inhibits invasion and migration in
vitro and tumorigenicity in vivo (13,34).
Therefore, we chose to investigate further CDX2 as a potential
agent for anticancer gene therapy.
A tumor-selective delivery system is the key to
successful tumor gene therapy. The hTERT promoter, active in most
cancer cells, but not in normal tissues, is used as a strategy for
tumor-selective delivery (16,35–37).
Hypoxia plays an important role in tumor development and tumor
progression (38). Herein, we used
5 copies of the hypoxia responsive element (HRE) as an hTERT
promoter enhancer (5HRE). The 5HRE element has previously been used
as an enhancer to utilize the hypoxic microenvironment (39). Harvey et al (40) developed a hypoxia-targeted gene
therapy strategy using the herpes simplex virus thymidine kinase
and bacterial nitroreductase pro-drug-activating genes and showed
that 5HRE linked to the CMV minimal promoter could induce optimum
luciferase reporter gene expression. In our previous study, gene
therapy vectors under the control of 5HRE and a minimal tumor
specific promoter also displayed optimal activation at a low oxygen
tension in hepatoma and gastric cancer cells (25,41).
For gene therapy in colon cancer, we previously
generated a recombinant lentivirus vector for hypoxia-inducible,
hTERT promoter-driven, and tissue-specific expression of CDX2:
pLVX-5HRE-hTERTp-CDX2-3FLAG (5HhC) (26). To verify the specificity and the
activity of pLVX-5HRE-hTERTp-CDX2-3FLAG, the recombinant lentiviral
vector was transfected into hTERT+ cells (LoVo) and
hTERT− cells (HK-2). We confirmed by
immunohistochemistry that the hTERT+ LoVo cells were
infected with the recombinant lentiviral vector 5HhC, while the
hTERT− HK-2 cells were not. The expression of CDX2
protein and mRNA was further increased by hypoxia in 5HhC/LoVo
cells, which was confirmed by western blotting and RT-PCR. Thus, we
concluded that hypoxic microenvironment can increase the expression
of CDX2 using gene therapy vector 5HhC which is regulated by the
hypoxia-induced enhancer (HRE) and the hTERT promoter in
hTERT+ LoVo cells (26). In the current study LoVo cells
infected with pLVX-5HRE-hTERTp-CDX2-3FLAG lentivirus showed reduced
cell viability, lower colony formation and invasive ability, but
displayed increased apoptosis and cell cycle arrest under hypoxic
conditions. Most significantly, pLVX-5HRE-hTERTp-CDX2-3FLAG
suppressed colon cancer xenograft tumor formation and growth in
nude mice. Although hypoxia causes downregulation of CDX2
expression in vivo and promotes progression of colorectal
cancer (23), our current data
indicate that pLVX-5HRE-hTERTp-CDX2-3FLAG can effectively utilize
hypoxia to drive the antitumor activity of CDX2. Our current data
strongly support the potential usefulness of
pLVX-5HRE-hTERTp-CDX2-3FLAG as an effective antitumor treatment
option for colorectal cancer.
However, the mechanism whereby CDX2 exerts antitumor
properties and the downstream signaling pathways in colorectal
cancer have not been elucidated. Some studies reported that CDX2
expression depends on the microenvironment and is regulated by
laminin-1 and collagen-1 (42,43).
In this study we demonstrated that CDX2 regulates the expression of
collagen IV, laminin-1, TGF-β, cyclin D1, uPA, MMP-2, MMP-9, bcl-2
and bax protein in vitro, and of Ki-67 in vivo. The
changes in the expression of these proteins may mediate the tumor
suppressor role of CDX2. Our previous study established that
exogenous expression of CDX2 in LoVo cells results in a significant
decrease in MMP-2 secretion, which, subsequently, restrains cell
invasion and migration in vitro (13). Supporting this result, Gross et
al (8) showed increased
expression of MMP-2 mRNA in SW480 cells upon siRNA-mediated
inhibition of CDX2 and found that CDX2 expression is regulated by
epithelial-mesenchymal transition (EMT)-inducing transcription
factors such as Snail and Slug. Yusra et al (44) demonstrated that transduction of
CDX2-expression vector into CD133+ SW480 cells
effectively suppressed TGFBR1 and TGFBR3 expression but treatment
with TGF-β restored CD133+ SW480 cells and induced MT.
Wei et al (45) indicated
that CDX2 promoted apoptosis in the MGC-803 human gastric cancer
cell line in vitro and in vivo. Overexpression of
CDX2 upregulated expression of Bax and downregulated levels of
survivin, Bcl-2, cyclin D1, Skp2, and c-Myc in tumor tissues. Seno
et al (46) showed that
CDX2-positive gastric cancer tissue samples showed a significantly
lower index for Ki-67 immun ostaining. Even among intestinal-type
gastric cancer cases, the CDX2-positive group showed a lower Ki-67
index and longer postoperative survival than did the CDX2-negative
group. All of the above studies are in agreement with our current
results, which confirmed that CDX2 acts as a tumor suppressor in
colon cancer.
Much more work needs to be done to confirm the
safety and efficiency of the pLVX-5HRE-hTERTp-CDX2-3FLAG lentivirus
vector before our results can be translated into clinical trials.
Our system may help to solve two key problems in gene therapy, that
is, specificity and efficiency. We showed that by using this vector
we can regulate gene expression in hTERT-positive colon cancer
cells under hypoxic conditions, further ensuring the cancer
cell-specific expression of therapeutic genes. In addition, this
study confirms that the expression of CDX2 effectively inhibits the
growth of colon cancer cells in vitro and in vivo.
The system described in this study may provide a potential tool for
treatment and gene therapy of colon cancer.
Acknowledgments
This work was supported by a grant from the National
Natural Science Foundation of China (grant serial nos.: 81101874,
81172362), the Science and Technology Project of Shaanxi Province
(grant serial no.: 2016SF-015), the Coordinative and Innovative
Plan Projects of the Science and Technology Program in Shaanxi
Province (grant serial no.: 2013KTCQ03-08), and a Clinical
Innovation Fund of First Affiliated Hospital of XJTU (grant serial
nos.: 12ZD12, 12ZD21).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shayakhmetov DM, Di Paolo NC and Mossman
KL: Recognition of virus infection and innate host responses to
viral gene therapy vectors. Mol Ther. 18:1422–1429. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen EQ, Song XQ, Wang YL, Zhou TY, Bai L,
Liu L, Liu C, Cheng X and Tang H: Construction of a highly-active,
liver-specific transcriptional regulatory element through
combination of the albumin promoter and α-fetoprotein enhancer.
Plasmid. 65:125–131. 2011. View Article : Google Scholar
|
|
4
|
Dong K, Wang R, Wang X, Lin F, Shen JJ,
Gao P and Zhang HZ: Tumor-specific RNAi targeting eIF4E suppresses
tumor growth, induces apoptosis and enhances cisplatin cytotoxicity
in human breast carcinoma cells. Breast Cancer Res Treat.
113:443–456. 2009. View Article : Google Scholar
|
|
5
|
Akhavan-Niaki H and Samadani AA: Molecular
insight in gastric cancer induction: An overview of cancer stemness
genes. Cell Biochem Biophys. 68:463–473. 2014. View Article : Google Scholar
|
|
6
|
Natoli M, Christensen J, El-Gebali S,
Felsani A and Anderle P: The role of CDX2 in Caco-2 cell
differentiation. Eur J Pharm Biopharm. 85:20–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin ME, Huang D, Deng BH, Lv YS, Rong L
and Yao YS: Expression and functional role of Cdx2 in intestinal
metaplasia of cystitis glandularis. J Urol. 190:1083–1089. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gross I, Duluc I, Benameur T, Calon A,
Martin E, Brabletz T, Kedinger M, Domon-Dell C and Freund JN: The
intestine-specific homeobox gene Cdx2 decreases mobility and
antagonizes dissemination of colon cancer cells. Oncogene.
27:107–115. 2008. View Article : Google Scholar
|
|
9
|
Aoki K, Tamai Y, Horiike S, Oshima M and
Taketo MM: Colonic polyposis caused by mTOR-mediated chromosomal
instability in Apc+/Delta716
Cdx2+/− compound mutant mice. Nat Genet.
35:323–330. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chawengsaksophak K, James R, Hammond VE,
Köntgen F and Beck F: Homeosis and intestinal tumours in Cdx2
mutant mice. Nature. 386:84–87. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olsen AK, Coskun M, Bzorek M, Kristensen
MH, Danielsen ET, Jørgensen S, Olsen J, Engel U, Holck S and
Troelsen JT: Regulation of APC and AXIN2 expression by intestinal
tumor suppressor CDX2 in colon cancer cells. Carcinogenesis.
34:1361–1369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong KD, Lee D, Lee Y, Lee SI and Moon HY:
Reduced CDX2 expression predicts poor overall survival in patients
with colorectal cancer. Am Surg. 79:353–360. 2013.PubMed/NCBI
|
|
13
|
Zheng JB, Sun XJ, Qi J, Li SS, Wang W, Ren
HL, Tian Y, Lu SY and Du JK: Effects of homeodomain protein CDX2
expression on the proliferation and migration of lovo colon cancer
cells. Pathol Oncol Res. 17:743–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang W, Jin B, Li W, Xu CX, Cui FA, Liu B,
Yan YF, Liu XX and Wang XL: Targeted antitumor effect induced by
hTERT promoter mediated ODC antisense adenovirus. Mol Biol Rep.
37:3239–3247. 2010. View Article : Google Scholar
|
|
15
|
Bougel S, Renaud S, Braunschweig R,
Loukinov D, Morse HC III, Bosman FT, Lobanenkov V and Benhattar J:
PAX5 activates the transcription of the human telomerase reverse
transcriptase gene in B cells. J Pathol. 220:87–96. 2010.
View Article : Google Scholar
|
|
16
|
Zhang P, Tan J, Yang DB, Luo ZC, Luo S,
Chen P, Sun P, Zhou Y, Chen XC, Wei YQ, et al: Gene therapy using
the human telomerase catalytic subunit gene promoter enables
targeting of the therapeutic effects of vesicular stomatitis virus
matrix protein against human lung adenocarcinoma. Exp Ther Med.
4:859–864. 2012.PubMed/NCBI
|
|
17
|
Hioki M, Kagawa S and Fujiwara T, Sakai R,
Kojima T, Watanabe Y, Hashimoto Y, Uno F, Tanaka N and Fujiwara T:
Combination of oncolytic adenovirotherapy and Bax gene therapy in
human cancer xenografted models. Potential merits and hurdles for
combination therapy. Int J Cancer. 122:2628–2633. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gout S and Huot J: Role of cancer
microenvironment in metastasis: Focus on colon cancer. Cancer
Microenviron. 1:69–83. 2008. View Article : Google Scholar
|
|
19
|
Jubb AM, Buffa FM and Harris AL:
Assessment of tumour hypoxia for prediction of response to therapy
and cancer prognosis. J Cell Mol Med. 14:18–29. 2010. View Article : Google Scholar
|
|
20
|
Law AY, Ching LY, Lai KP and Wong CK:
Identification and characterization of the hypoxia-responsive
element in human stanniocalcin-1 gene. Mol Cell Endocrinol.
314:118–127. 2010. View Article : Google Scholar
|
|
21
|
Zhang J, Shi Q, Chen X, Yang P, Qi C,
Zhang J, Lu H, Liu J, Jiao Q, Zhao L, et al: Hypoxia-regulated
neurotrophin-3 expression by multicopy hypoxia response elements
reduces apoptosis in PC12 cells. Int J Mol Med. 30:1173–1179.
2012.PubMed/NCBI
|
|
22
|
Hu J, Stiehl DP, Setzer C, Wichmann D,
Shinde DA, Rehrauer H, Hradecky P, Gassmann M and Gorr TA:
Interaction of HIF and USF signaling pathways in human genes
flanked by hypoxia-response elements and E-box palindromes. Mol
Cancer Res. 9:1520–1536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng J, Sun X, Wang W and Lu S:
Hypoxia-inducible factor-1α modulates the down-regulation of the
homeodomain protein CDX2 in colorectal cancer. Oncol Rep.
24:97–104. 2010.PubMed/NCBI
|
|
24
|
Shibata T, Giaccia AJ and Brown JM:
Development of a hypoxia-responsive vector for tumor-specific gene
therapy. Gene Ther. 7:493–498. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou PH, Zheng JB, Wei GB, Wang XL, Wang
W, Chen NZ, Yu JH, Yao JF, Wang H, Lu SY, et al:
Lentivirus-mediated RASSF1A expression suppresses aggressive
phenotypes of gastric cancer cells in vitro and in vivo. Gene Ther.
22:793–801. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He S, Sun XJ, Zheng JB, Qi J, Chen NZ,
Wang W, Wei GB, Liu D, Yu JH, Lu SY, et al: Recombinant lentivirus
with enhanced expression of caudal-related homeobox protein 2
inhibits human colorectal cancer cell proliferation in vitro. Mol
Med Rep. 12:1838–1844. 2015.PubMed/NCBI
|
|
27
|
Ji J and Zheng PS: Expression of Sox2 in
human cervical carcinogenesis. Hum Pathol. 41:1438–1447. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Wang K, Ren Y, Zhang L, Tang XJ,
Zhang HM, Zhao CQ, Liu PJ, Zhang JM and He JJ: MAPK signaling
mediates sino-menine hydrochloride-induced human breast cancer cell
death via both reactive oxygen species-dependent and -independent
pathways: An in vitro and in vivo study. Cell Death Dis.
5:e13562014. View Article : Google Scholar
|
|
29
|
Witek ME, Snook AE, Lin JE, Blomain ES,
Xiang B, Magee MS and Waldman SA: A novel CDX2 isoform regulates
alternative splicing. PLoS One. 9:e1042932014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bae JM, Lee TH, Cho NY, Kim TY and Kang
GH: Loss of CDX2 expression is associated with poor prognosis in
colorectal cancer patients. World J Gastroenterol. 21:1457–1467.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Olsen J, Eiholm S, Kirkeby LT, Espersen
ML, Jess P, Gögenür I, Olsen J and Troelsen JT: CDX2 downregulation
is associated with poor differentiation and MMR deficiency in colon
cancer. Exp Mol Pathol. 100:59–66. 2016. View Article : Google Scholar
|
|
32
|
Dawson H, Koelzer VH, Lukesch AC, Mallaev
M, Inderbitzin D, Lugli A and Zlobec I: Loss of Cdx2 expression in
primary tumors and lymph node metastases is specific for mismatch
repair-deficiency in colorectal cancer. Front Oncol. 3:2652013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Olsen J, Espersen ML, Jess P, Kirkeby LT
and Troelsen JT: The clinical perspectives of CDX2 expression in
colorectal cancer: A qualitative systematic review. Surg Oncol.
23:167–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng JB, Qiao LN, Sun XJ, Qi J, Ren HL,
Wei GB, Zhou PH, Yao JF, Zhang L and Jia PB: Overexpression of
caudal-related homeobox transcription factor 2 inhibits the growth
of transplanted colorectal tumors in nude mice. Mol Med Rep.
12:3409–3415. 2015.PubMed/NCBI
|
|
35
|
Xu Y, Hou J, Liu Z, Yu H, Sun W, Xiong J,
Liao Z, Zhou F, Xie C and Zhou Y: Gene therapy with tumor-specific
promoter mediated suicide gene plus IL-12 gene enhanced tumor
inhibition and prolonged host survival in a murine model of Lewis
lung carcinoma. J Transl Med. 9:392011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Toh L, Lau P and Wang X: Human
telomerase reverse transcriptase (hTERT) is a novel target of the
Wnt/β-catenin pathway in human cancer. J Biol Chem.
287:32494–32511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shepelev MV, Kopantzev EP, Vinogradova TV,
Sverdlov ED and Korobko IV: hTERT and BIRC5 gene promoters for
cancer gene therapy: A comparative study. Oncol Lett. 12:1204–1210.
2016.PubMed/NCBI
|
|
38
|
Paolicchi E, Gemignani F, Krstic-Demonacos
M, Dedhar S, Mutti L and Landi S: Targeting hypoxic response for
cancer therapy. Oncotarget. 7:13464–13478. 2016.PubMed/NCBI
|
|
39
|
Zhang H, Liang C, Hou X, Wang L and Zhang
D: Study of the combined treatment of lung cancer using gene-loaded
immunomagnetic albumin nanospheres in vitro and in vivo. Int J
Nanomed. 11:1039–1050. 2016. View Article : Google Scholar
|
|
40
|
Harvey TJ, Hennig IM, Shnyder SD, Cooper
PA, Ingram N, Hall GD, Selby PJ and Chester JD: Adenovirus-mediated
hypoxia-targeted gene therapy using HSV thymidine kinase and
bacterial nitroreductase prodrug-activating genes in vitro and in
vivo. Cancer Gene Ther. 18:773–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang W, Sun X, Lu L, Zheng JB, Tian Y and
Wang W: Cytotoxicity of lymphocytes activated by superantigen
toxic-shock-syndrome toxin-1 against colorectal cancer LoVo cells.
Mol Cell Biochem. 376:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brabletz T, Spaderna S, Kolb J, Hlubek F,
Faller G, Bruns CJ, Jung A, Nentwich J, Duluc I, Domon-Dell C, et
al: Down-regulation of the homeodomain factor Cdx2 in colorectal
cancer by collagen type I: An active role for the tumor environment
in malignant tumor progression. Cancer Res. 64:6973–6977. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Turck N, Gross I, Gendry P, Stutzmann J,
Freund JN, Kedinger M, Simon-Assmann P and Launay JF: Laminin
isoforms: Biological roles and effects on the intracellular
distribution of nuclear proteins in intestinal epithelial cells.
Exp Cell Res. 303:494–503. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yusra, Semba S and Yokozaki H: Biological
significance of tumor budding at the invasive front of human
colorectal carcinoma cells. Int J Oncol. 41:201–210.
2012.PubMed/NCBI
|
|
45
|
Wei W, Li L, Wang X, Yan L, Cao W, Zhan Z,
Zhang X, Yu H, Xie Y and Xiao Q: Overexpression of caudal type
homeobox transcription factor 2 inhibits the growth of the MGC-803
human gastric cancer cell line in vivo. Mol Med Rep. 12:905–912.
2015.PubMed/NCBI
|
|
46
|
Seno H, Oshima M, Taniguchi MA, Usami K,
Ishikawa TO, Chiba T and Taketo MM: CDX2 expression in the stomach
with intestinal metaplasia and intestinal-type cancer: Prognostic
implications. Int J Oncol. 21:769–774. 2002.PubMed/NCBI
|