Introduction
Breast cancer is the most common cancer among women
worldwide and persists as one of the leading causes of
cancer-related deaths despite advances in early detection,
diagnosis, and targeted treatment options such as Herceptin
(trastuzumab) (1,2). The need for novel therapeutic
strategies remains paramount given the sustained development of
drug resistance, tumor recurrence, and metastasis (1,2). In
this regard, the successful clinical efficacy of arsenic trioxide
(As2O3, a trivalent arsenic derivative) in
the treatment of acute promyelocytic leukemia (APL) has resulted in
further investigations exploring its potential treatment
application for other malignancies, including solid tumors
(3,4). Trivalent arsenic (arsenite,
AsIII) has been demonstrated to exhibit inhibitory
effects against breast cancer cells (5–7),
raising the possibility of utilizing arsenic compounds to treat
patients with breast cancer. The side effects of AsIII,
in particular, AsIII-mediated QT prolongation, remain a
serious concern limiting its clinical application. Solid tumors,
including breast cancer, also demonstrate a lower susceptibility to
arsenic compounds (8–10). Therefore, a growing need exists to
create new approaches aimed at improving its efficiency and
reducing its side effects.
Tetrandrine (Tetra), a bis-benzylisoquinoline
alkaloid isolated from the root of Stephania tetrandra S
Moore, has a long history in Chinese medicine treating diverse
diseases such as silicosis, inflammatory pulmonary diseases, and
hypertension (11). Furthermore,
Tetra has been demonstrated to not only possess the capacity to
inhibit the growth of several different types of cancer cell lines,
but also show a potential for increasing the efficacy of
chemotherapy drugs in combined treatment (12–14).
These previous findings suggest that Tetra may serve as a promising
adjuvant to enhance the efficacy of conventional anticancer drugs.
While Tetra has been shown to enhance the cytotoxicity of
AsIII in HepG2 (human hepatoma cell line) and A549
(human lung carcinoma cell line) (13), the effects of combining
AsIII and Tetra against breast cancer cells have yet to
be evaluated.
Cell cycle arrest, necrosis, as well as autophagic
cell death have been viewed as the major underlying mechanisms for
the cytocidal effects of most chemotherapeutic drugs (7,13,15-18).
The cell cycle is a complex process that is precisely regulated by
vital molecules known as cyclin-dependent kinases (CDKs) and CDK
inhibitors such as p21 Waf1/Cip1 (p21) and p27 Kip1 (p27) (7,19,20).
Of note, forkhead box transcription factor (FOXO3a), which is
considered to be involved in the development of breast cancer and
may also serve as a prognostic marker in breast cancer (21), has been implicated in the control
of genes involving multiple cellular processes, including cell
cycle, migration, invasion, and cell death (2,20–24).
Survivin, another important cancer-associated protein that is
highly expressed in most human tumors, is similarly known to
participate in the above cellular processes (25–28).
However, whether and how these molecules contribute to the
potential cytotoxic effects induced by a combination of
AsIII and Tetra against breast cancer cells remain to be
seen.
In the clinical treatment of cancer, combination
therapy has been widely recognized to decrease cell viability and
clonogenic growth, and reduce toxicity, although sequential
chemotherapy remains the standard of care for a variety of
malignancies including breast cancer (29). In this regard, previous reports
(30,31) have revealed that Tetra enhances
cytotoxicity of conventional anticancer drugs such as cisplatin,
daunorubicin and doxorubicin by inhibition of some ATP-binding
cassette (ABC) transporters including ABCC1/multidrug
resistance-associated protein 1 (MRP1) and ABCB1/multidrug
resistance 1 (MDR1), all of which are known to mediate drug efflux
and play a prominent role in the chemoresistance to several
cytotoxic agents including arsenic compounds (3). These previous findings suggest that
Tetra may be a promising candidate for combination chemotherapy
regimens through the manipulation of drug efflux transporters
thereby enhancing the efficacy of anticancer drugs. Although
treatment with As2S2 dramatically increases
the expression of ABCG2/breast cancer resistance protein (BCRP),
another important multidrug resistance-conferring ABC transporter,
in a myeloid leukemia cell line K562 (32), the relevance of BCRP to arsenic
compound-mediated cytotoxicity as well as arsenic resistance is not
known.
In this study, in order to provide novel insight
into the development of new therapeutic strategies to combat breast
cancer using AsIII-based combination therapy, the cyto
toxicity of a combination of sodium arsenite (another trivalent
arsenic compound) and Tetra was investigated in the human breast
cancer cell line MCF-7 by focusing on cell cycle arrest, necrosis,
and autophagic cell death. Key regulatory molecules associated with
the cell cycle and death were investigated to further elucidate
cytotoxic mechanisms. Intracellular arsenic accumulation (As[i])
was also evaluated in order to clarify the contribution of
multidrug efflux transporters including BCRP to cytotoxicity.
Materials and methods
Materials
Sodium arsenite (NaAsO2,
AsIII) and tetrandrine (Tetra) were purchased from Tri
Chemical Laboratories (Yamanashi, Japan) and National Institutes
for Food and Drug Control (Beijing, China), respectively. Fetal
bovine serum (FBS) was purchased from Nichirei Biosciences (Tokyo,
Japan). RPMI-1640 medium, phenazine methosulfate (PMS), dimethyl
sulfoxide (DMSO) and Giemsa stain solution were obtained from Wako
Pure Chemical Industries (Osaka, Japan). Propidium iodide (PI),
ribonuclease A (RNaseA), and
2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbony]
-2H-tetrazolium hydroxide (XTT) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Ko134, an inhibitor of BCRP,
was obtained from Solvo Biotechnology (Kyoto, Japan). ReverTra Ace
qPCR RT Master Mix with gDNA Remover, Thunderbird SYBR qPCR Mix,
RNase inhibitor, Can Get Signal® Immunoreaction Enhancer
Solution were purchased from Toyobo Co., Ltd. (Osaka, Japan).
Cell culture and treatment
MCF-7 human breast adenocarcinoma cells were
obtained from the RIKEN Cell Bank (Ibaraki, Japan). Cells were
cultured in RPMI-1640 medium supplemented with 10% heat-inactivated
FBS and 100 U/ml of penicillin and 100 μg/ml of streptomycin
in a humidified 5% CO2 atmosphere at 37°C. Cells were
treated with AsIII and Tetra, alone or in combination,
in the presence or absence of BCRP inhibitor Ko134 at the indicated
concentrations. Tetra was dissolved in DMSO, and no cytotoxicity of
the final concentrations of DMSO was observed in the current
experimental system.
Cell viability and clonogenic
survival
After treatment with various concentrations of
AsIII and Tetra, alone or in combination, for 48 h, cell
viability was measured by the XTT assay as previously described
(33). The relative cell viability
was expressed as the ratio of the absorbance of each treatment
group against those of the corresponding untreated control group.
The IC50 values of AsIII were calculated
using GraphPad Prism® 6 software. In order to evaluate
whether the two drugs generated synergistic, antagonistic, or
additive effects, a combination index (CI) was determined as
reported previously, using the computer software ComboSyn (ComboSyn
Inc. Paramus, NJ, USA) for drug combinations and for general
dose-effect analysis, which was developed by Chou (34,35).
The effect of the combination treatment was defined as a
synergistic effect if CI<1, an additive effect if CI=1 or an
antagonistic effect if CI>1 (13,19).
For clonogenic survival assays, MCF-7 cells were seeded at 500
cells/well in 12-well plates, followed by the treatment with
indicated concentrations of AsIII and Terta, alone or in
combination, for 24 h. The medium was then replaced with fresh
media and the cells were allowed to grow for 8–12 days in a
humidified 5% CO2 atmosphere at 37°C before staining
with Giemsa stain solution.
Wound healing assay
Cell migration was evaluated using an in
vitro wound scratching assay as previously described (36). Briefly, MCF-7 cells were seeded at
a density of 1×105 cells/ml in 24-well plates and
allowed to form a confluent monolayer. The layer of cells was
scraped with a 20–200 μl micropipette tip to create a wound
of approximately 0.5 mm in width, and then the cells were gently
rinsed twice by PBS, followed by treatment with the indicated
concentrations of AsIII and Tetra, alone or in
combination, for 48 h. The cells were photographed at 0 and 48 h
using an inverted microscope (CK2, Olympus, Tokyo, Japan) fitted
with a digital camera WRAYCAM-NF300 (Osaka, Japan). The distance
between the edges of the cell-free areas was measured and the cell
migration was calculated using the following equation: %R =
[1−(wound length at T48 h / wound length at T0
h)] ×100% where %R is the percent recovery, T0 h
is the wound length at 0 h, and T48 h is the wound
length at 48 h after injury.
Cell cycle analysis
After treatment with the indicated concentrations of
AsIII and Tetra, alone or in combination, for 48 h, cell
cycle analysis was performed using a FACSCanto flow cytometer
(Becton Dickinson, San Jose, CA, USA) according to a method
previously reported (37).
Briefly, cells were washed twice with phosphate-buffered saline
(PBS), fixed with 1% paraformaldehyde/PBS for 30 min, washed twice
again with PBS, permeabilized in 70% (v/v) cold ethanol and kept at
−20°C for at least 4 h. Cell pellets were then washed twice with
PBS after centrifugation and incubated with 0.25% Triton-X 100 for
5 min on ice. After centrifugation and washing with PBS, cells were
resuspended in 500 μl of PI/RNase A/PBS (5 μg/ml of
PI and 0.1% RNase A in PBS) and incubated for 30 min in the dark at
room temperature. A total of 10,000 events were acquired and Diva
software and ModFit LT™ Ver.3.0 (Verity Software House, Topsham,
ME, USA) were used to calculate the number of cells at each
G0/G1 and S phase fraction.
Lactate dehydrogenase (LDH) assay
After treatment with the indicated concentrations of
AsIII and Tetra, alone or in combination, for 48 and 72
h, LDH leakage from cells was measured using the LDH-Cytotoxic Test
Wako kit (Wako Pure Chemical Industries) according to the method
previously described with slight modifications (15,33).
Briefly, culture supernatants were collected by centrifugation at
2,500 rpm for 5 min at 4°C. Non-treated cells were lysed in culture
medium containing 0.2% Tween-20, and mixed aggressively using a
vortex mixer, followed by the centrifugation at 12,000 × g for 10
min and the cell lysate was used as the positive control. Culture
medium served as the negative control. Culture supernatants were
collected then diluted 16-fold with PBS and 50 μl of the
diluted solution was transferred into wells of a 96-well plate. LDH
activities were determined by adding 50 μl of 'substrate
solution' from the kit, followed by incubation at room temperature
for 30 min. The reaction was stopped by the addition of 100
μl of 'stopping solution' and the absorbance at 560 nm was
measured with a microplate reader (Safire, Tecan, Switzerland).
Cell damage was calculated as a percentage of LDH leakage from
damaged cells using the following formula: LDH leakage (%) =
(Sup-NC)/(P-NCT) ×100 where Sup, NC, P and NCT refer to the
absorption of the culture supernatant, negative control, positive
control and culture medium containing 0.2% Tween-20,
respectively.
RNA extraction, reverse transcription
(RT), and real-time PCR
Total RNA isolation and complementary DNA were
prepared according to a method previously reported with
modifications (33,38). Briefly, total RNA was extracted
from cells using an RNA extraction kit, Isogen II (Nippon Gene,
Tokyo, Japan) and quantified by BioSpec-nano (Shimazu Corp., Kyoto,
Japan). Complementary DNA was synthesized from 500 ng of RNA using
ReverTra Ace® qPCR RT Master Mix with gDNA Remover
according to the manufacturer's protocol. Real time RT-PCR assay
was performed using the CFX Connect (Bio-Rad Laboratories,
Hercules, CA, USA) thermal cycler detection system. DNA primers for
real-time PCR were purchased from Sigma-Aldrich (Tokyo, Japan)
using the forward primer (5′-ccagatgacgaccccatagag-3′) and reverse
primer (5′-ttgttggtttcctttgcaatttt-3′) for survivin (GenBank
accession no.: NM_001168) (39);
and the forward primer (5′-catccctgcctctactggcg-3′) and reverse
primer (5′-agcttcccgttcagctcagg-3′) for glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) (GenBank accession no.: NM_002046.3) served
as a housekeeping gene. Thermal cycling conditions consisted of 10
sec denaturation at 95°C, 15 sec annealing at 60°C, and 20 sec
primer extension at 70°C, and 45 cycles were conducted. A 1-min
activation step preceded cycling (95°C). A fold change in relative
expression of survivin was calculated based on the comparative Ct
(2−ΔΔCt) method. Analysis of melting curves was applied
to confirm whether all PCR products are single.
Western blot analysis
Western blot analysis was carried out according to
the methods previously described (40). Briefly, after separation of
proteins on a sodium dodecyl sulfate (SDS) polyacrylamide gel
electrophoresis, followed by transferring to a polyvinylidene
difluoride (PVDF) membrane (Millipore Corp., Bedford, MA, USA),
protein bands were detected using the following primary antibodies
and dilution ratios: mouse anti-human β-actin (1:5,000 dilution,
Sigma-Aldrich, St. Louis MO, USA); rabbit anti-human FOXO3a
(1:1,000 dilution), rabbit anti-human p27 (1:1,000 dilution), mouse
anti-human p21 (1:1,000 dilution), rabbit anti-human cyclin D1
(1:1,000 dilution), mouse anti-human survivin (1:1000 dilution),
rabbit anti-human phospho-AMPKα1 (Ser485) and AMPKα (1:1,000
dilution), rabbit anti-human phospho-mTOR (Ser2448) and mTOR
(1:1,000 dilution), rabbit anti-human Beclin-1 (1:1,000 dilution),
rabbit anti-human Atg7 (1:1,000 dilution), rabbit anti-human LC3
(1:1,000 dilution) (Cell Signaling Technology, Danvers, MA, USA).
Blotted protein bands were detected with respective horseradish
peroxidase-conjugated secondary antibody and an enhanced
chemiluminescence (ECL) Western blot analysis system (Amersham
Pharmacia Biotech, Buckinghamshire, UK).
Analysis of intracellular arsenic
accumulation (As[i])
After exposure of MCF-7 cells to 3 μM
AsIII alone or in combination with 1 μg/ml Tetra
or 2 μM BCRP inhibitor Ko134 for 0, 1, 2, 4, 8 h, the cells
were gently washed three times with PBS and harvested in 2% SDS
solution. Protein concentrations were determined by Bradford's
method using the protein assay dye reagent (Bio-Rad Laboratories)
according to the manufacturer's instructions, and using BSA as the
standard. The quantitation of As[i] was performed by external
calibration. Concentrations of As[i] were calculated from
calibration curve of standard arsenic compounds. The As[i] was
normalized by the amount of proteins and reported as parts per
billion (ppb) of arsenic per mg of proteins. The analysis of total
arsenic was performed by inductively coupled plasma-mass
spectrometry (ICP-MS) (Perkin-Elmer Sciex, Thornhill, ON, Canada)
according to the methods previously reported (17,33,41).
Statistical analysis
Experiments were independently repeated three times,
and reported as the means ± standard deviation (SD) of the three
assays. Statistical analysis was conducted using one-way ANOVA
followed by Dunnett's post-test. A probability level of P<0.05
was considered to indicate a statistically significant
difference.
Results
Synergistic cytotoxic effect of
AsIII and Tetra in human breast cancer cell line
MCF-7
A significant decrease in cell viability was
observed in a dose-dependent manner in MCF-7 cells after treatment
with various concentrations of AsIII or Tetra alone for
48 h (Fig. 1A), and the
IC50 values were 6.1±0.9 μM and 3.5±0.6
μg/ml for AsIII and Tetra treatment,
respectively. In order to evaluate if the two drugs generated
synergistic, antagonistic, or additive effects, based upon the
above-described IC50 values, the two-drug combination in
constant ratio was determined according to the median-effect method
of Chou (34,35). As shown in Fig. 1A, combination treatment was
significantly more cytotoxic than either drug alone (p<0.05).
Furthermore, the dose-effect curves of single or combined drug
treatment analyzed by the median-effect method demonstrated that
the values of combination index (CI) were <1, except for the
combination of 4 μM AsIII with 2 μg/ml
Tetra (Fig. 1B and C, Table I), indicating that the two drugs
performed in a synergistic manner.
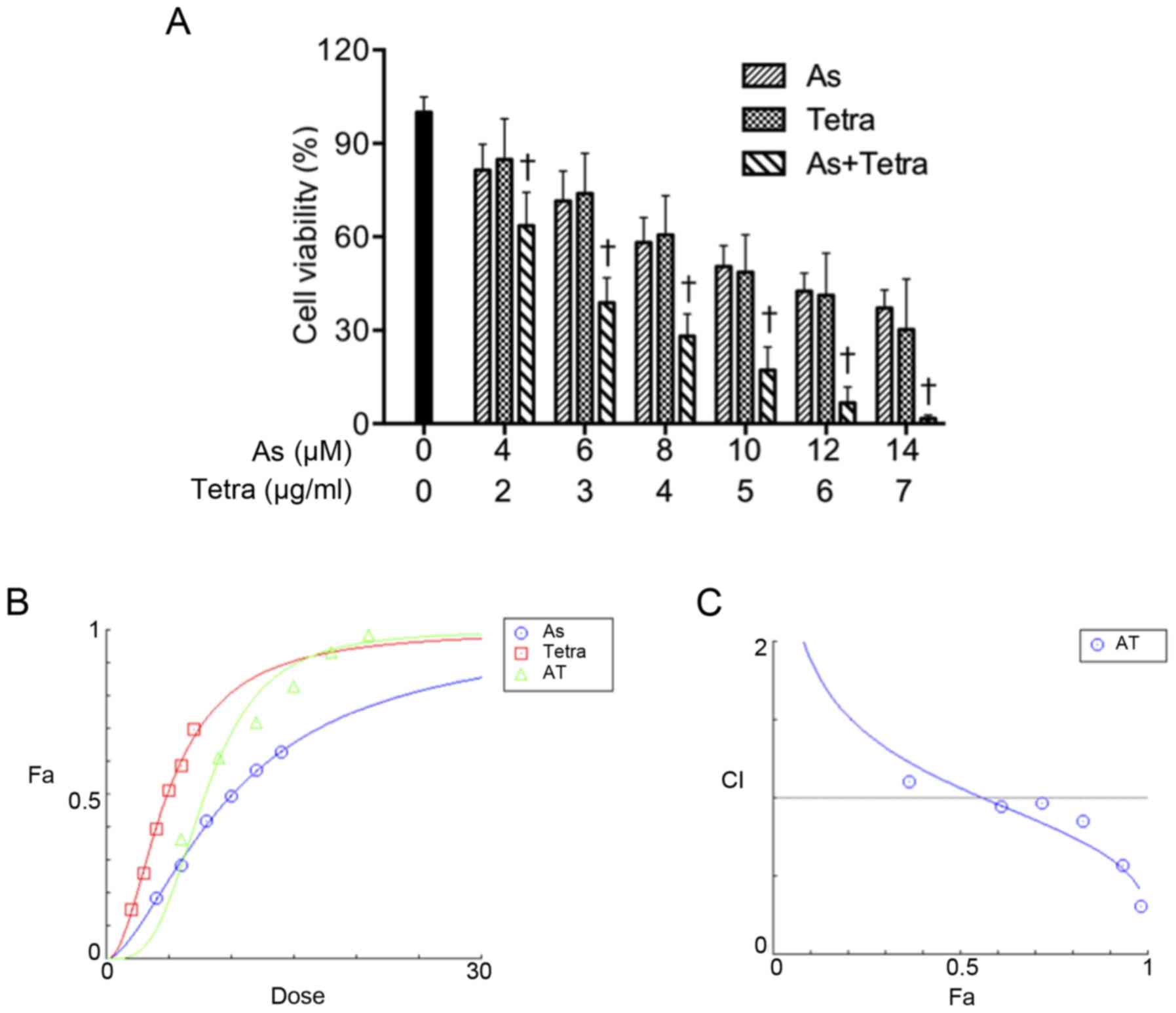 | Figure 1Synergistic cytotoxic effect of
AsIII and Tetra in human breast cancer cell line MCF-7.
(A) Cell viability was determined by XTT assay after the treatment
with various concentrations of AsIII alone (4, 6, 8, 10,
12 and 14 μM), Tetra alone (2, 3, 4, 5, 6 and 7
μg/ml), or their combination for 48 h. Relative cell
viability was calculated as the ratio of the absorbance at 450 nm
of each treatment group against those of the corresponding
untreated control group. Data are shown as the means ± SD (n=3).
†P<0.05 vs. each alone. (B and C) Combination of
AsIII and Tetra exerts synergic effects on MCF-7 cells,
as reflected by the median-effect method of Ting-Chao Chou. The
dose-effect curves of single or combined drug treatment analyzed by
the median-effect method demonstrated that the values of
combination index (CI) were <1, indicating that the two drugs
performed in a synergistic manner. As, AsIII; Tetra,
tetrandrine, AT, AsIII + tetrandrine. |
 | Table ICI values of AsIII at
concentrations in combination with Tetra in MCF-7 cells. |
Table I
CI values of AsIII at
concentrations in combination with Tetra in MCF-7 cells.
| As (μM) | Tetra
(μg/ml) | Fa | CI value |
|---|
| 4 | 2 | 0.36365 | 1.10171 |
| 6 | 3 | 0.61133 | 0.94380 |
| 8 | 4 | 0.71851 | 0.96387 |
| 10 | 5 | 0.82821 | 0.84989 |
| 12 | 6 | 0.93335 | 0.56980 |
| 14 | 7 | 0.98316 | 0.30671 |
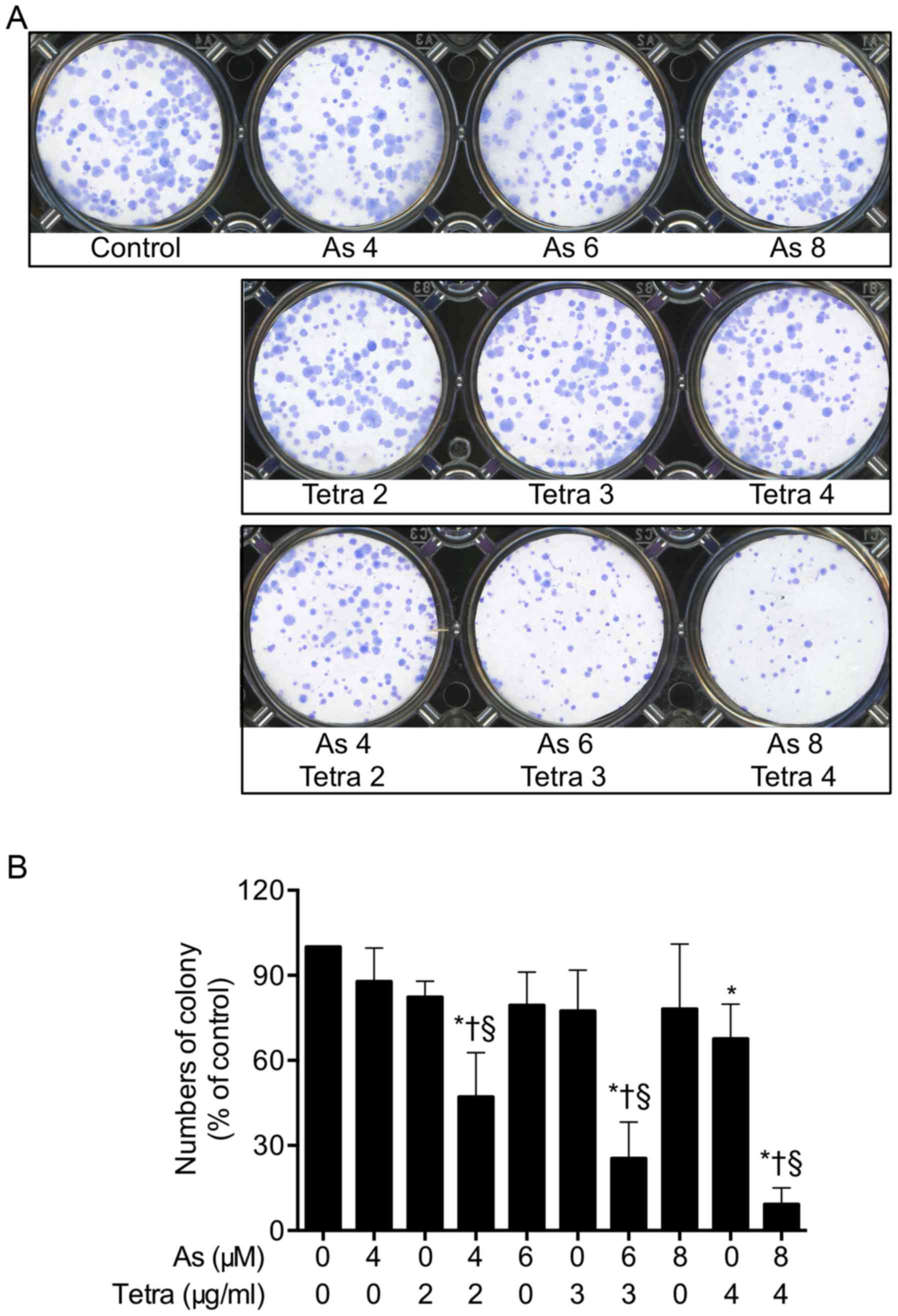
Inhibition of colony formation of MCF-7
cells by AsIII in combination with Tetra
To explore whether exposure to AsIII
alone or in combination with Tetra suppressed the surviving
fraction of MCF-7 cells, a colony formation assay was applied. As
shown in Fig. 2, long-term
treatment with AsIII combined with Tetra significantly
suppressed the colony numbers of MCF-7 cells by 52.8, 74.6 and
90.8% for the combination of 4 μM AsIII + 2
μg/ml Tetra, 6 μM AsIII + 3 μg/ml
Tetra, and 8 μM AsIII + 4 μg/ml Tetra,
respectively, confirming the synergistic cytotoxic effect of
AsIII and Tetra against the cells.
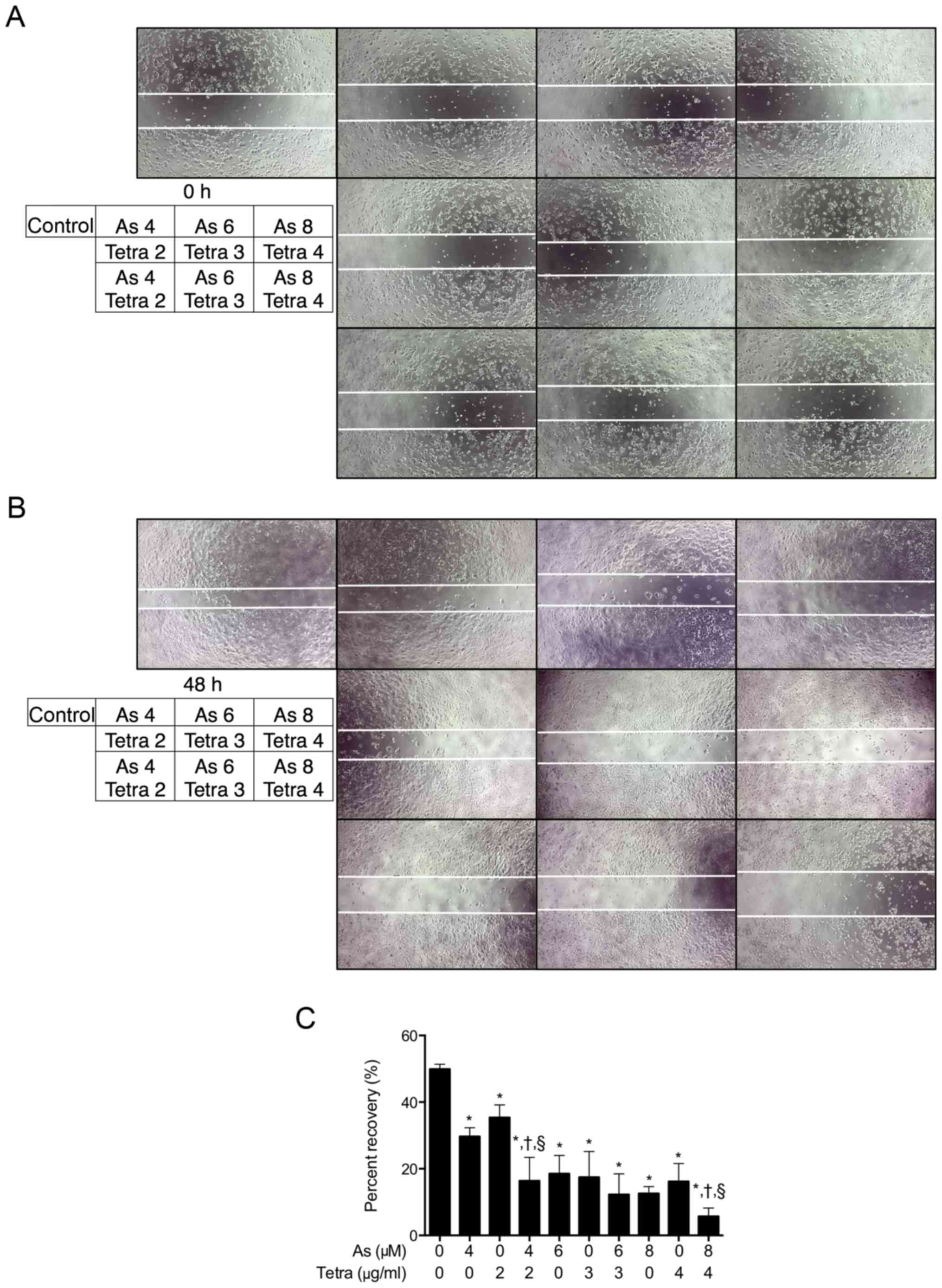
Inhibition of the migration ability of
MCF-7 cells by AsIII in combination with Tetra
To test the effect of AsIII and Tetra,
alone or in combination, on the proliferation and migration of
MCF-7 cells, a scratch wound healing assay was conducted. Confluent
monolayers of MCF-7 cells were scraped with a 20-200 μl
micropipette tip to create a gap as shown in Fig. 3A (0 h), followed by the treatment
with indicated concentrations of AsIII and Tetra, alone
or in combination. As shown in Fig.
3B, after 48 h incubation, the gaps that remained unfilled by
the migrated cells in the treated groups were wider than that in
the untreated group. Furthermore, compared to groups treated with
either AsIII or Tetra alone, combinatorial treatment
effectively reduced the migration of MCF-7 cells into the wounded
area, although there was no significant difference between the
treatment of 6 μM AsIII + 3 μg/ml Tetra or
each alone (Fig. 3C).
Effects of AsIII and Tetra,
alone or in combination, on the cell cycle profiling and the
expression level of cell cycle related-proteins in MCF-7 cells
To explore whether cell cycle arrest is involved in
the cytotoxic effect of AsIII and Tetra, cell cycle
analyses were performed using flow cytometry. As shown in Fig. 4A, after treatment with various
concentrations of AsIII and Tetra, alone or in
combination, at 48 h, a significant increase in the number of cells
in G0/G1 phase was induced by
AsIII alone, but not by Tetra alone. Furthermore, a
slight rise in the number of cells was found in the combination
treatment compared to AsIII treatment alone.
Concomitantly, a similar trend towards a decrease in the number of
cells in S phase was also observed (Fig. 4B). As shown in Fig. 4C, in comparison to control group,
the expression of FOXO3a was upregulated by AsIII and
Tetra, each alone, and further strengthened by their combination.
Noteworthy, the expression level of p21 was upregulated by
AsIII, and slightly enhanced by the addition of Tetra,
although Tetra alone did not affect its expression level. The
expression level of p27 was slightly increased by either
AsIII or Tetra alone compared to the control, and only a
small enhancement in its expression was observed in the combined
treatment group. Furthermore, a substantial decrease in the
expression level of cyclin D1 was observed in cells treated with
the combination of 6 μM AsIII + 3 μg/ml
Tetra, and 8 μM AsIII + 4 μg/ml Tetra,
respectively.
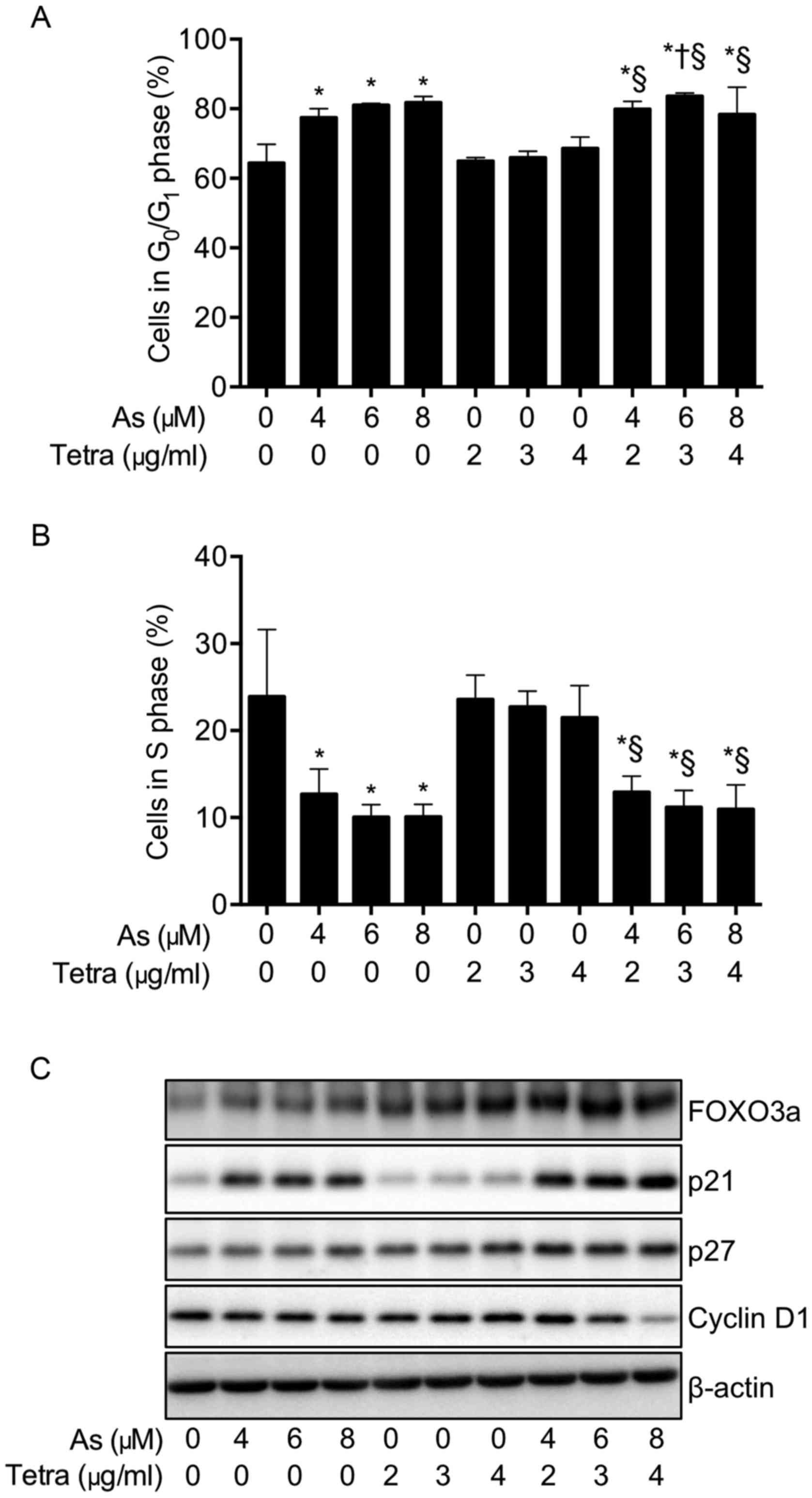 | Figure 4Effects of AsIII and
Tetra, alone or in combination, on the cell cycle profiling and the
expression level of cell cycle related-proteins in MCF-7 cells.
After treatment with various concentrations of AsIII (4,
6 and 8 μM) and Tetra (2, 3 and 4 μg/ml), alone or in
combination, for 48 h, cell cycle analysis was performed using a
FACSCanto flow cytometer (A and B), and the expression profile of
cell cycle-related proteins was analyzed using western blotting (C)
as described in Materials and methods. Results are shown as the
means ± SD from three independent experiments. Significant
difference between control and treatment with AsIII and
Tetra are shown (*P<0.05 vs. control;
†P<0.05 vs. AsIII alone;
§p<0.05 vs. Tetra alone). Representative image of the
expression profile of each protein is shown from three independent
experiments. As, AsIII; Tetra, tetrandrine. |
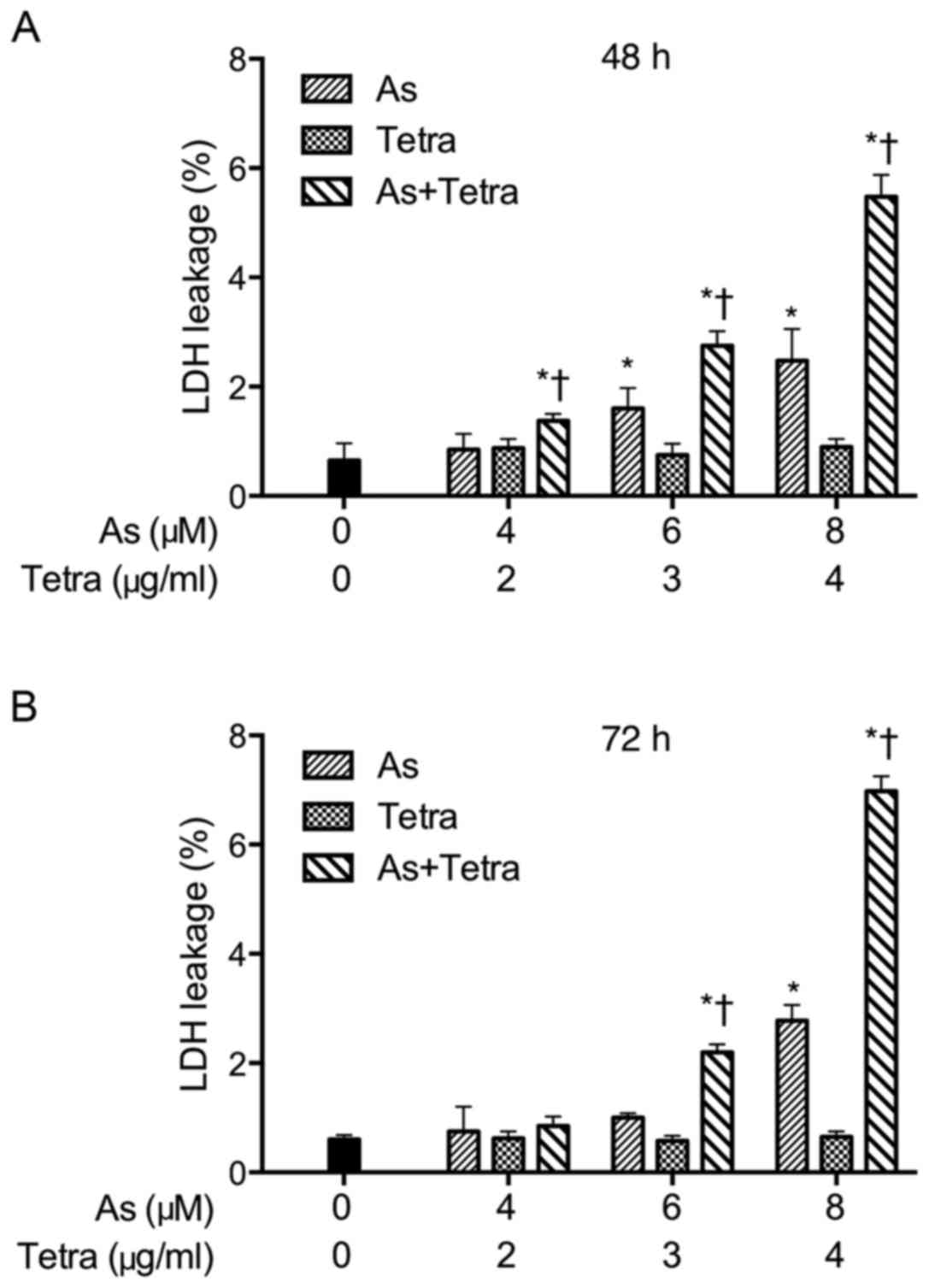
Synergistic effect between
AsIII and Tetra in inducing LDH release in MCF-7
cells
After treatment with various concentrations of
AsIII and Tetra, alone or in combination, for 48 and 72
h, LDH leakage analysis was performed to examine whether the
treatments affected cell membrane integrity. As shown in Fig. 5, a dose- and time-dependent LDH
leakage was observed in MCF-7 cells treated with AsIII
alone. Furthermore, the synergistic effect between AsIII
and Tetra in inducing LDH leakage was observed during combination
treatments, although only slight LDH leakage was detected in the
cells treated with Tetra alone.
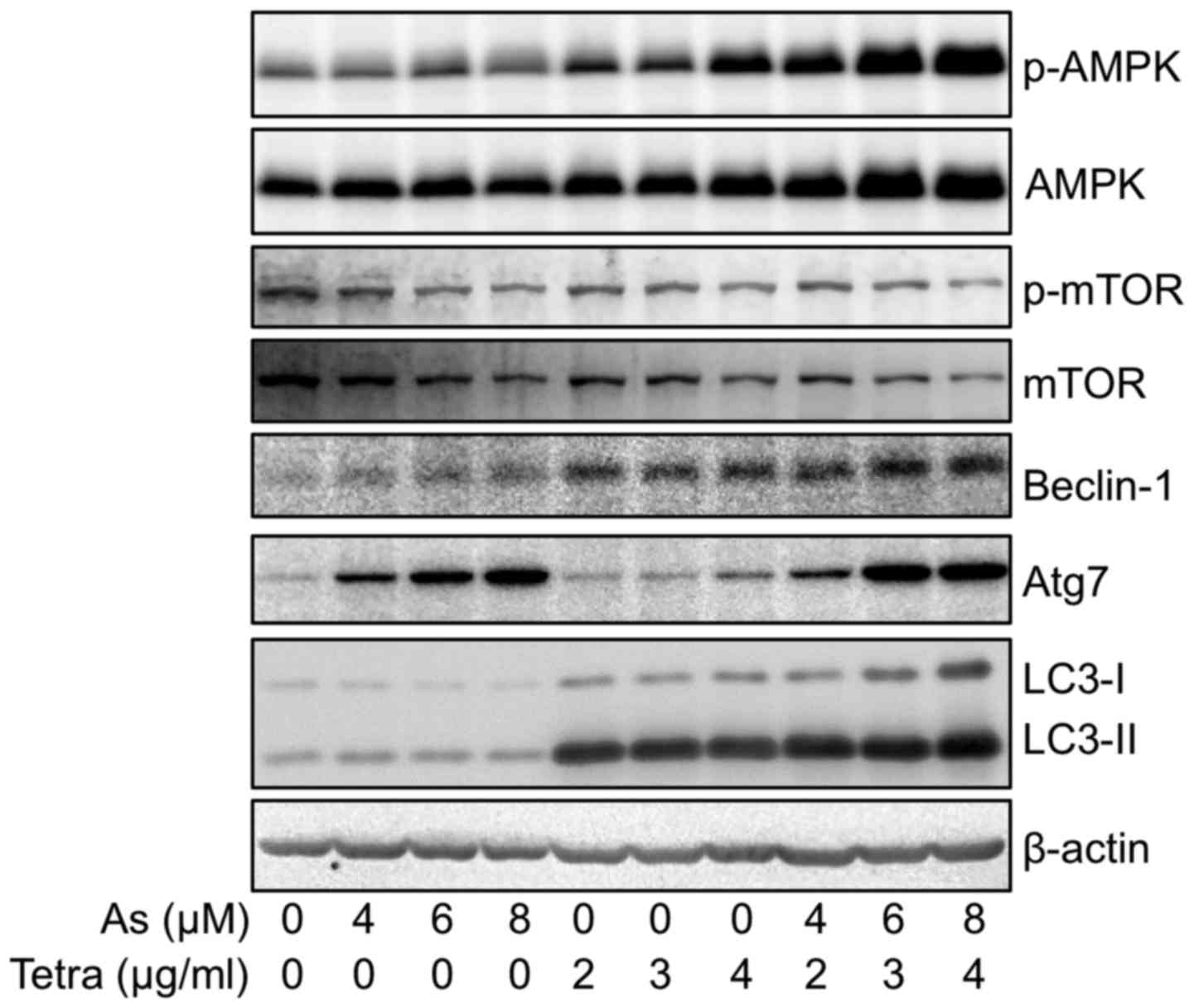
Activation of autophagic pathway in MCF-7
cells treated with AsIII and Tetra, alone or in
combination
Western blotting was performed to explore whether
activation of an autophagic pathway occurred in MCF-7 cells after
treatment with AsIII and Tetra, alone or in combination.
As shown in Fig. 6, the expression
level of LC3, an autophagic marker, was dramatically upregulated by
Tetra. Although only a modest increase in the expression level of
LC3 was observed when treated with AsIII alone, the
upregulation was further enhanced by their combination. In order to
clarify the mechanisms underlying the signaling pathway activating
autophagy, the expression of a several of autophagy-related
proteins was evaluated. The expression levels of phosphorylated
AMP-activated protein kinase (AMPK) (phospho-AMPK) were clearly
upregulated by Tetra in a dose-dependent manner, while only a
modest upregulation was observed when treated with AsIII
alone. In comparison, a remarkable upregulation of phospho-AMPK as
well as total-AMPK was observed in the cells treated with the
combination treatment. Furthermore, the alteration of the
expression levels of phosphorylated mammalian target of rapamycin
(phospho-mTOR) and total-mTOR demonstrated an almost opposite
behavior, showing a trend towards down-regulation of their
expression in the treated cells compared to controls. These results
indicate that AMPK-mediated mTOR deactivation is involved during
the autophagy induction. Similar to the alterations of the
expression levels of phospho-AMPK, the expression levels of
Beclin-1 were modestly and clearly upregulated in the cells treated
with AsIII, and Tetra, respectively, and the increment
was further strengthened by the combinational treatment.
Intriguingly, a dramatic increase in the expression level of Atg-7
was observed in AsIII-treated cells, however, a modest
increase in its expression was observed in Tetra-treated cells.
Again, compared to the treatment with AsIII or Tetra
alone, their combination treatment further enhanced the expression
levels of Atg-7.
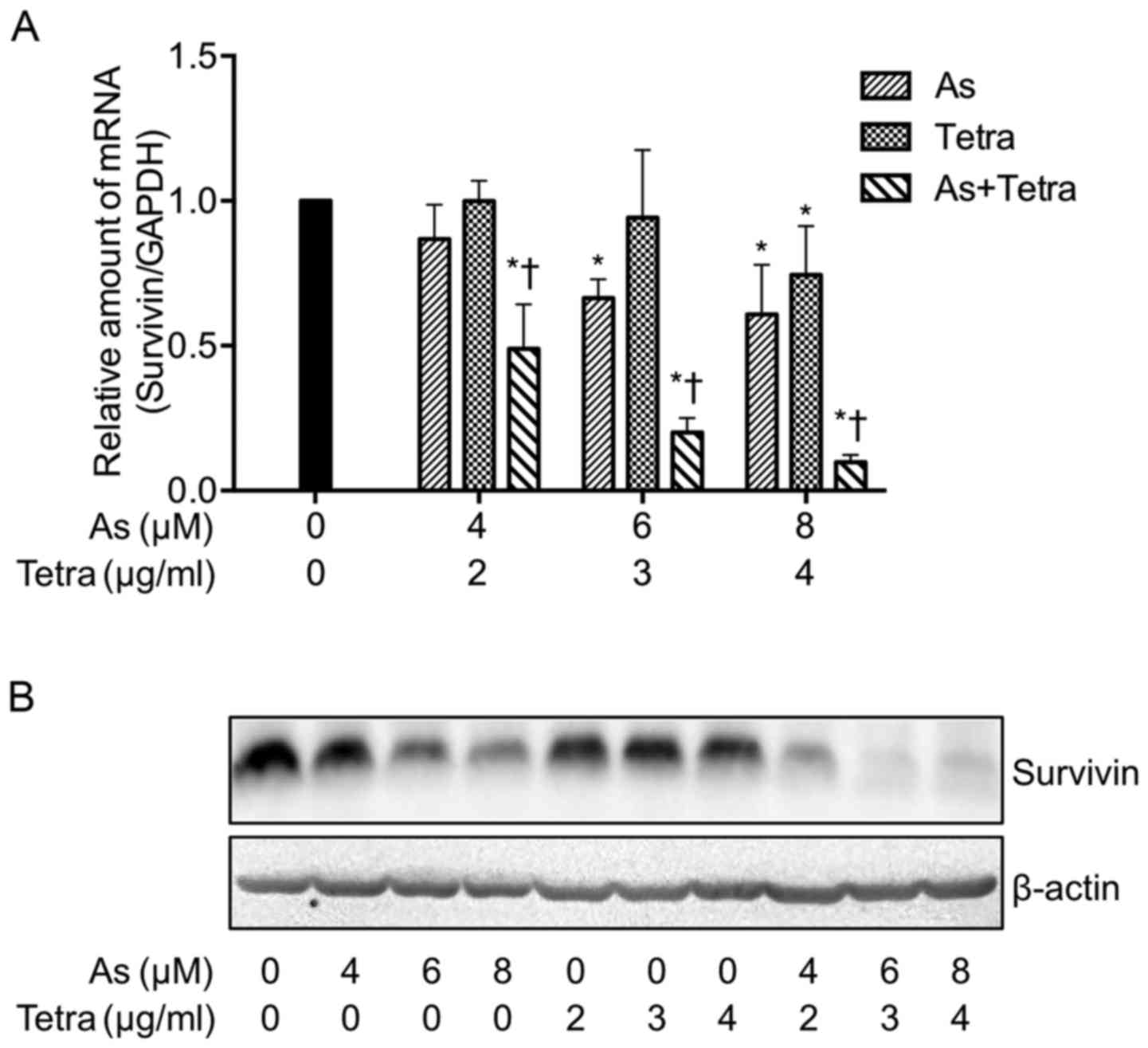
Expression profile of survivin gene in
MCF-7 cells treated with AsIII and Tetra, alone or in
combination
After treatment with various concentrations of
AsIII and Tetra, alone or in combination, for 48 h, the
expression profile of the survivin gene was analyzed using
real-time PCR and western blotting. Treatment with a relatively
high concentration of either AsIII (6 and 8 μM)
or Tetra (4 μg/ml) resulted in a significant decrease in the
expression level of survivin mRNA (Fig. 7A). Similarly to the phenomena
observed in proliferation inhibition and LDH leakage induced by
AsIII and Tetra, a synergistic effect between
AsIII and Tetra in suppressing the expression level of
survivin mRNA was also observed (Fig.
7A). Consistent with the expression profile of survivin mRNA,
the synergistic effects in suppressing its protein expression level
was further confirmed (Fig.
7B).
Enhanced As[i] along with synergistic
cytotoxicity in MCF-7 cells treated with AsIII combined
with Tetra or Ko134
In order to clarify the correlation between
synergistic cytotoxicity and As[i], arsenic uptake was first
measured to examine whether Tetra affected As[i] in MCF-7 cells
when combined with AsIII. After exposure of MCF-7 cells
to 3 μM AsIII alone or in combination with 1
μg/ml Tetra for 0, 1, 2, 4 and 8 h, As[i] was measured by
ICP-MS. The levels of As[i] increased with time in the cells
following treatment with AsIII alone (Fig. 8A). In comparison, Tetra in
combination with AsIII further enhanced the levels of
As[i] in the cells (Fig. 8A).
Analogous augmentation in the levels of As[i] was also observed
when treated with AsIII in combination with 2 μM
BCRP inhibitor Ko134 (Fig. 8B). In
parallel, synergistic cytotoxicity was also observed in these
combinational treatments (Fig. 8C and
D), indicating the synergistic action of AsIII and
Tetra/Ko134 was attributed to the enhanced As[i].
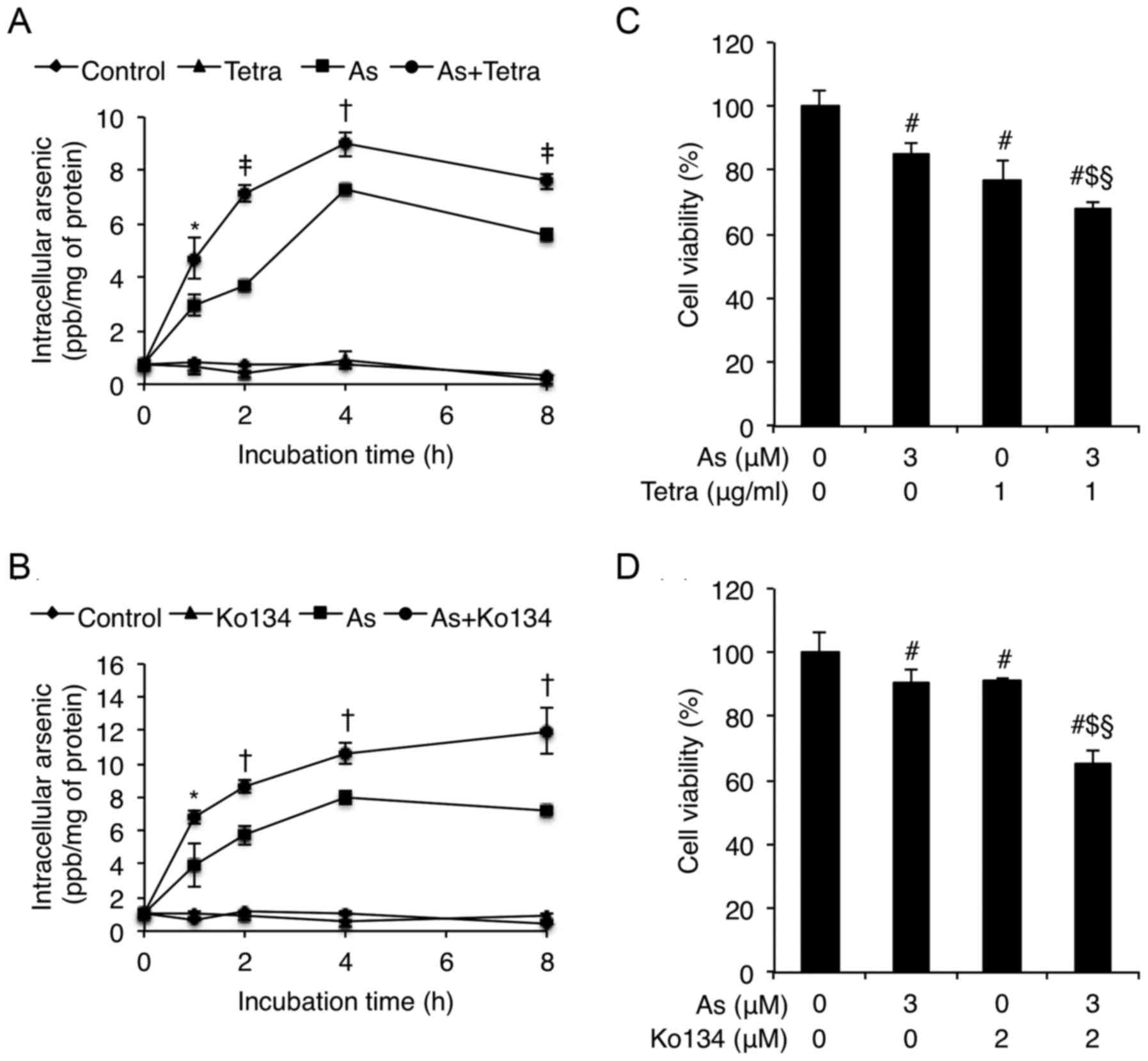 | Figure 8Enhanced As[i] along with synergistic
cytotoxicity in MCF-7 cells treated with AsIII combined
with Tetra or Ko134. After exposure of MCF-7 cells to 3 μM
AsIII alone or in combination with 1 μg/ml Tetra
(A) or 2 μM BCRP inhibitor Ko134 (B), respectively, for 0,
1, 2, 4 and 8 h, As[i] was measured by ICP-MS, as described in
Materials and methods. Furthermore, cell viability was determined
by XTT assay after treatment with 3 μM AsIII
alone or in combination with 1 μg/ml Tetra (C) or 2
μM BCRP inhibitor Ko134 (D), respectively, for 48 h. Results
are shown as the means ± SD from three independent experiments.
P<0.05 was considered as statistically significant
(*P<0.05; †P<0.01 As vs. As+Tetra or
As+Ko134; ‡P<0.001 As vs. As+Tetra;
#P<0.05 vs. control; $P<0.05 vs. As alone;
§P<0.05 vs. Tetra or Ko134 alone). As, 3 μM
AsIII; Tetra, 1 μg/ml tetrandrine. |
Discussion
It has been demonstrated that AsIII
exhibits inhibitory effects on breast cancer cells (5–7),
raising the possibility of utilizing arsenic compounds to treat
patients with breast cancer. However, due to the lower
susceptibility of solid tumors, including breast cancer, to arsenic
compounds, there is a growing need to develop a novel therapeutic
strategy aimed at improving its efficiency and reducing its side
effects (8–10). Results from this study clearly
demonstrate that Tetra significantly enhances the cytotoxicity of
AsIII in MCF-7 cells in a synergistic manner as
evidenced by the XTT assay, as well as inhibition of colony
formation and migration of the cells (Figs. 1Figure 2–3). Similarly, Tetra has been shown to
augment the cytocidal effects of chemotherapeutic agents, including
AsIII, in different types of solid tumor cells (13,14).
While QT prolongation is a major complication in AsIII
therapy (3), closely related to
the intracellular [Ca2+] overload induced by
AsIII (42), Tetra, on
the other hand, has been demonstrated to serve as a calcium channel
antagonist significantly decreasing intracellular [Ca2+]
within ventricular cells (43).
Collectively, the combination regimen of Tetra and AsIII
may be expected not only to achieve improved efficacy of
AsIII, but also overcome its adverse cardiac effects
secondary to Tetra functioning as calcium channel blocker (44).
Cell cycle arrest, necrosis, as well as autophagic
cell death have all been viewed as the major underlying mechanisms
for the cytocidal effects of most drugs used against tumors
(7,13,15–18).
To examine the probable mechanisms underlying the cytocidal effect
of the combination of AsIII and Tetra, cell cycle arrest
was first examined in MCF-7 cells treated with AsIII and
Tetra, alone or in combination. A clear G0/G1
cell cycle arrest was observed when treated with AsIII
alone, and was slightly strengthened by the addition of Tetra
(Fig. 4A). These findings are
supported by previous studies showing that AsIII
inhibits the cellular proliferation of MCF-7 cells via a
G1 and/or G2/M phase arrest (7,45).
We further demonstrated that the expression level of FOXO3a, was
upregulated by AsIII and Tetra, both alone and then
further enhanced by their combination (Fig. 4C).
FOXO3a has been implicated in cell cycle arrest
leading to growth inhibition via upregulation of p21, p27 and
downregulation of cyclin D1 in various cancers including breast
cancer (2,20–22).
In line with these previous findings, a concomitant increase and
decrease in the expression levels of p21, p27, and cycline D1,
respectively, was observed in MCF-7 cells treated with
AsIII and/or Tetra, although a synergistic effect on the
alteration of each gene was not clear in the combined treatment
group (Fig. 4C). We have also
noted that the magnitude of cell cycle arrest does not completely
correlate to the degree of alterations in the expression level of
the above-mentioned cell cycle arrest-related genes. In this
regard, besides contributing to cell cycle arrest, upregulation of
FOXO3a expression is known to inhibit migration and invasion of
different types of solid tumors such as gastric and renal cancer
through inactivating epithelial-mesenchymal transition (EMT) of
cancer cells as a result of downregulation of SNAIL1, a key
regulator of EMT (23,24). Therefore, our results suggest that
the alterations of FOXO3a along with p21, p27, cyclin D1 are
responsible for not only G0/G1 cell cycle
arrest but also migration inhibition of MCF-7 cells induced by
AsIII and/or Tetra, although a more thorough analysis
must be performed to confirm this correlation.
We further demonstrated that combining Tetra with
AsIII synergistically induced LDH release in MCF-7 cells
(Fig. 5). Since the release of LDH
provides an accurate measure of the cell membrane integrity and
cell viability (15,33), our results suggest the involvement
of necrotic cell death in the synergistic action of the
combination. AsIII has been demonstrated to induce
necrotic cell death through a regulated, Bcl-xL-sensitive
mitochondrial pathway that is largely caspase-independent,
providing a plausible explanation for its capability of triggering
cell death in a variety of drug-resistant cell types including
tumor cells even with defects in caspase activation (46).
Induction of autophagy by various anticancer drugs
has also been suggested as a potential therapeutic strategy for
cancer (16,25,47,48).
In this respect, Tetra is known to function as a potent agonist for
cell autophagy in numerous cancer cells including breast cancer
cells (47,49). Moreover, autophagy has been
demonstrated to partially contribute to AsIII-mediated
cytotoxicity in human glioma and fibrosarcoma cells in vitro
and in vivo (25,50). In line with previous reports,
autophagy induction was clearly observed in MCF-7 cells treated
with AsIII and Tetra, alone or in combination, as
evidenced by the marked increase in expression levels of LC3, an
autophagic marker (16,47,48),
along with the activation of the autophagic pathway involving a
number of important molecules including phospho-AMPK, Beclin-1, and
Atg-7 (Fig. 6). It is well known
that AMPK is key energy sensor and an upstream promoter of
autophagy induction (22), and
that Beclin-1 is an autophagic mediator which is deleted in 50% of
breast tumors (18,51). Atg-7 is also known as a key player
in autophagosomes formation and to be involved in plant-derived
polyphenol-mediated autophagic cell death in breast cancer cells
(48). Our results suggest that
the synergistic cytotoxic effect of AsIII and Tetra
against MCF-7 cells should be attributed to
G0/G1 cell cycle arrest, LDH leakage, and
autophagy induction.
The cancer-associated protein survivin is highly
expressed in most human tumor cell lines, in particular breast and
lung cancer cell lines (52).
Therefore, the inhibition of survivin has been pursued as a
compelling strategy for cancer therapy (53). Besides its critical role in
apoptosis inhibition, survivin is also known to regulate cell cycle
arrest and autophagy (25–28). Lee et al have demonstrated
that suberoylanilide hydroxamic acid (SAHA), a novel histone
deacetylase inhibitor, induces autophagy and cell viability
reduction in human breast cancer cells by downregulating the
expression of survivin, as a result of inducing survivin protein
acetylation and impairing its stability (26). Chiu et al also demonstrated
that AsIII-induced autophagy was paralleled by the
downregulation of survivin in human glioma cells in vitro
and in vivo (25). In this
study, the synergistic inhibitory effect of AsIII and
Tetra on the expression levels of survivin was observed (Fig. 7). Taking the previous results and
our observations into account, we suggest that survivin plays a
vital role in the cytocidal effects of the combined regimen of
AsIII plus Tetra, leading to potentially new options for
the combination therapy in patients with breast cancer.
Since drug action usually requires uptake of the
drug, it was considered that As[i] might determine the sensitivity
of cancer cells to arsenic compounds (3). Enhanced As[i] along with synergistic
cytotoxicity was observed in our experiments, suggesting a positive
correlation between synergistic cytotoxicity of combination
treatment and enhanced As[i] in MCF-7 cells. Wang et al
(31) have demonstrated that Tetra
enhances cytotoxicity of cisplatin in human drug-resistant
esophageal squamous carcinoma cells by inhibition of MRP1, which is
also known to be involved in the efflux of AsIII
(3). We previously demonstrated
that Tetra can serve as a potent inhibitor of MDR1 (also known as
P-glycoprotein, P-gp) to reverse multidrug resistance to anticancer
drugs such as daunorubicin, vinblastine and doxorubicin in a human
T lymphoblastoid leukemia MOLT-4 MDR cell line (30), although the roles of P-gp in
arsenic efflux remain controversial (3). Despite the fact that BCRP is known to
mediate concurrent resistance to chemotherapeutic agents including
mitoxantrone, doxorubicin, and daunorubicin in MCF-7/AdrVp, a
multidrug-resistant human breast cancer subline (54), its relevance for resistance to
arsenic is unknown. By demonstrating the enhanced As[i] along with
the synergistic cytotoxicity in MCF-7 cells when treated with
AsIII combined with Ko134, this suggests for the first
time the possibility of manipulating BCRP to overcome the
resistance to the arsenic-based regimens, although a previous study
reported that BCRP-overexpressing MDA-MB-231-BCRP cells were not
more resistant to AsIII than their drug-sensitive
counterparts (55). Therefore,
further studies need to be launched in order to draw a solid
conclusion about the involvement of BCRP in arsenic resistance.
Collectively, our results imply that Tetra and/or BCRP inhibitor
probably intervene in the occurrence of resistance to arsenic
therapy by enhancing the As[i] via modulation of multidrug efflux
transporters such as MRP1, P-gp, and BCRP.
Our results suggest that Tetra can be a useful
combination anticancer agent to enhance the therapeutic effect of
AsIII for patients with breast cancer by enhancing the
As[i] and consequently strengthening AsIII-mediated
growth inhibition associated with cell cycle arrest, necrosis, and
autophagic cell death, all of which seemed to be related to the
downregulation of survivin. Therefore, these results may provide a
rational molecular basis for the combination regimen of
AsIII plus Tetra, facilitating the development of
AsIII-based anticancer strategies or combination
therapies for patients with solid tumors, especially breast
cancer.
Glossary
Abbreviations
Abbreviations:
|
AsIII
|
trivalent arsenic (arsenite)
|
|
Tetra
|
tetrandrine
|
|
p21
|
p21 Waf1/Cip1
|
|
p27
|
p27 Kip1
|
|
FOXO3a
|
forkhead box transcription factor
3a
|
|
ABC transporters
|
ATP-binding cassette transporters
|
|
MRP1
|
ABCC1/multidrug resistance-associated
protein 1
|
|
MDR1
|
ABCB1/multidrug resistance 1
|
|
BCRP
|
ABCG2/breast cancer resistance
protein
|
|
CI
|
combination index
|
|
LDH
|
lactate dehydrogenase
|
|
As[i]
|
intracellular arsenic
accumulation
|
|
ICP-MS
|
inductively coupled plasma-mass
spectrometry
|
|
phospho-AMPK
|
phosphorylated AMP-activated protein
kinase
|
|
phospho-mTOR
|
phosphorylated mammalian target of
rapamycin
|
Acknowledgments
We deeply thank Associate Professor Marcus Ferrone
of University of California, San Francisco (UCSF) School of
Pharmacy for the critical reading of this manuscript. This work was
supported by The Japan Society for the Promotion of Science (JSPS)
KAKENHI Grant to B.Y. (grant no. 26460233).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taylor S, Lam M, Pararasa C, Brown JE,
Carmichael AR and Griffiths HR: Evaluating the evidence for
targeting FOXO3a in breast cancer: A systematic review. Cancer Cell
Int. 15:12015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuan B, Yoshino Y, Kaise T and Toyoda H:
Application of arsenic trioxide therapy for patients with leukemia.
Biological Chemistry of Arsenic, Antimony and Bismuth. Sun H: John
Wiley & Sons, Ltd; Chichester: pp. 263–292. 2011
|
|
4
|
Dilda PJ and Hogg PJ: Arsenical-based
cancer drugs. Cancer Treat Rev. 33:542–564. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chow SK, Chan JY and Fung KP: Suppression
of cell proliferation and regulation of estrogen receptor alpha
signaling pathway by arsenic trioxide on human breast cancer MCF-7
cells. J Endocrinol. 182:325–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu W, Gong Y, Li H, Jiang G, Zhan S, Liu
H and Wu Y: Arsenic trioxide-induced growth arrest of breast cancer
MCF-7 cells involving FOXO3a and IκB kinase β expression and
localization. Cancer Biother Radiopharm. 27:504–512. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Gao P, Long M, Lin F, Wei JX, Ren
JH, Yan L, He T, Han Y and Zhang HZ: Essential role of cell cycle
regulatory genes p21 and p27 expression in inhibition of breast
cancer cells by arsenic trioxide. Med Oncol. 28:1225–1254. 2011.
View Article : Google Scholar
|
|
8
|
Kasukabe T, Okabe-Kado J, Kato N, Honma Y
and Kumakura S: Cotylenin A and arsenic trioxide cooperatively
suppress cell proliferation and cell invasion activity in human
breast cancer cells. Int J Oncol. 46:841–848. 2015.
|
|
9
|
Lin CC, Hsu C, Hsu CH, Hsu WL, Cheng AL
and Yang CH: Arsenic trioxide in patients with hepatocellular
carcinoma: A phase II trial. Invest New Drugs. 25:77–84. 2007.
View Article : Google Scholar
|
|
10
|
Vuky J, Yu R, Schwartz L and Motzer RJ:
Phase II trial of arsenic trioxide in patients with metastatic
renal cell carcinoma. Invest New Drugs. 20:327–330. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu T, Liu X and Li W: Tetrandrine, a
Chinese plant-derived alkaloid, is a potential candidate for cancer
chemotherapy. Oncotarget. 7:40800–40815. 2016.PubMed/NCBI
|
|
12
|
Fu LW, Zhang YM, Liang YJ, Yang XP and Pan
QC: The multidrug resistance of tumour cells was reversed by
tetrandrine in vitro and in xenografts derived from human breast
adenocarcinoma MCF-7/adr cells. Eur J Cancer. 38:418–426. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Li P, Yang S, Tong N, Zhang J and
Zhao X: Tetrandrine enhances the anticancer effects of arsenic
trioxide in vitro. Int J Clin Pharmacol Ther. 52:416–424. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei J, Liu B, Wang L, Qian X, Ding Y and
Yu L: Synergistic interaction between tetrandrine and
chemotherapeutic agents and influence of tetrandrine on
chemotherapeutic agent-associated genes in human gastric cancer
cell lines. Cancer Chemother Pharmacol. 60:703–711. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kon A, Yuan B, Hanazawa T, Kikuchi H, Sato
M, Furutani R, Takagi N and Toyoda H: Contribution of membrane
progesterone receptor α to the induction of progesterone-mediated
apoptosis associated with mitochondrial membrane disruption and
caspase cascade activation in Jurkat cell lines. Oncol Rep.
30:1965–1970. 2013.PubMed/NCBI
|
|
16
|
Wang X, Qi W, Li Y, Zhang N, Dong L, Sun
M, Cun J, Zhang Y, Lv S and Yang Q: Huaier extract induces
autophagic cell death by inhibiting the mTOR/S6K pathway in breast
cancer cells. PLoS One. 10:e01317712015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan B, Okusumi S, Yoshino Y, Moriyama C,
Tanaka S, Hirano T, Takagi N and Toyoda H: Delphinidin induces
cytotoxicity and potentiates cytocidal effect in combination with
arsenite in an acute promyelocytic leukemia NB4 cell line. Oncol
Rep. 34:431–438. 2015.PubMed/NCBI
|
|
18
|
Chen N and Karantza-Wadsworth V: Role and
regulation of autophagy in cancer. Biochim Biophys Acta.
1793:1516–1523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mei L, Chen Y, Wang Z, Wang J, Wan J, Yu
C, Liu X and Li W: Synergistic anti-tumour effects of tetrandrine
and chloroquine combination therapy in human cancer: A potential
antagonistic role for p21. Br J Pharmacol. 172:2232–2245. 2015.
View Article : Google Scholar :
|
|
20
|
Lin CH, Chang CY, Lee KR, Lin HJ, Chen TH
and Wan L: Flavones inhibit breast cancer proliferation through the
Akt/FOXO3a signaling pathway. BMC Cancer. 15:9582015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang Y, Zou L, Lu WQ, Zhang Y and Shen
AG: Foxo3a expression is a prognostic marker in breast cancer. PLoS
One. 8:e707462013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chiacchiera F and Simone C: The
AMPK-FoxO3A axis as a target for cancer treatment. Cell Cycle.
9:1091–1096. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Y, Fu LX, Zhu WL, Shi H, Chen LJ and Ye
B: Blockade of CXCR6 reduces invasive potential of gastric cancer
cells through inhibition of AKT signaling. Int J Immunopathol
Pharmacol. 28:194–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ni D, Ma X, Li HZ, Gao Y, Li XT, Zhang Y,
Ai Q, Zhang P, Song EL, Huang QB, et al: Downregulation of FOXO3a
promotes tumor metastasis and is associated with metastasis-free
survival of patients with clear cell renal cell carcinoma. Clin
Cancer Res. 20:1779–1790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiu HW, Ho YS and Wang YJ: Arsenic
trioxide induces autophagy and apoptosis in human glioma cells in
vitro and in vivo through downregulation of survivin. J Mol Med
(Berl). 89:927–941. 2011. View Article : Google Scholar
|
|
26
|
Lee JY, Kuo CW, Tsai SL, Cheng SM, Chen
SH, Chan HH, Lin CH, Lin KY, Li CF, Kanwar JR, et al: Inhibition of
HDAC3-and HDAC6-promoted survivin expression plays an important
role in SAHA-induced autophagy and viability reduction in breast
cancer cells. Front Pharmacol. 7:812016. View Article : Google Scholar
|
|
27
|
Li Y, Liu D, Zhou Y, Li Y, Xie J, Lee RJ,
Cai Y and Teng L: Silencing of survivin expression leads to reduced
proliferation and cell cycle arrest in cancer cells. J Cancer.
6:1187–1194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu W, Zhu F, Jiang Y, Sun D, Yang B and
Yan H: siRNA targeting survivin inhibits the growth and enhances
the chemosensitivity of hepatocellular carcinoma cells. Oncol Rep.
29:1183–1188. 2013.
|
|
29
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu ZL, Hirano T, Tanaka S, Onda K and Oka
K: Persistent reversal of P-glycoprotein-mediated daunorubicin
resistance by tetrandrine in multidrug-resistant human T
lymphoblastoid leukemia MOLT-4 cells. J Pharm Pharmacol.
55:1531–1537. 2003. View Article : Google Scholar
|
|
31
|
Wang TH, Wan JY, Gong X, Li HZ and Cheng
Y: Tetrandrine enhances cytotoxicity of cisplatin in human
drug-resistant esophageal squamous carcinoma cells by inhibition of
multidrug resistance-associated protein 1. Oncol Rep. 28:1681–1686.
2012.PubMed/NCBI
|
|
32
|
Wang YS, Zhou ST and Wei HL: Apoptosis
effects of drug sensitivity leukemia cells induced by nanorealgar.
Zhongguo Zhong Yao Za Zhi. 38:2202–2205. 2013.In Chinese.
PubMed/NCBI
|
|
33
|
Yoshino Y, Yuan B, Kaise T, Takeichi M,
Tanaka S, Hirano T, Kroetz DL and Toyoda H: Contribution of
aquaporin 9 and multidrug resistance-associated protein 2 to
differential sensitivity to arsenite between primary cultured
chorion and amnion cells prepared from human fetal membranes.
Toxicol Appl Pharmacol. 257:198–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
El-Aarag BY, Kasai T, Zahran MA, Zakhary
NI, Shigehiro T, Sekhar SC, Agwa HS, Mizutani A, Murakami H, Kakuta
H, et al: In vitro anti-proliferative and anti-angiogenic
activities of thalidomide dithiocarbamate analogs. Int
Immunopharmacol. 21:283–292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kikuchi H, Yuan B, Yuhara E, Takagi N and
Toyoda H: Involvement of histone H3 phosphorylation through p38
MAPK pathway activation in casticin-induced cytocidal effects
against the human promyelocytic cell line HL-60. Int J Oncol.
43:2046–2056. 2013.PubMed/NCBI
|
|
38
|
Kikuchi H, Yuan B, Yuhara E, Imai M,
Furutani R, Fukushima S, Hazama S, Hirobe C, Ohyama K, Takagi N, et
al: Involvement of histone H3 phosphorylation via the activation of
p38 MAPK pathway and intracellular redox status in cytotoxicity of
HL-60 cells induced by Vitex agnuscastus fruit extract. Int J
Oncol. 45:843–852. 2014.PubMed/NCBI
|
|
39
|
Zekri A, Ghaffari SH, Yousefi M,
Ghanizadeh-Vesali S, Mojarrad M, Alimoghaddam K and Ghavamzadeh A:
Autocrine human growth hormone increases sensitivity of mammary
carcinoma cell to arsenic trioxide-induced apoptosis. Mol Cell
Endocrinol. 377:84–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yuan B, Ohyama K, Takeichi M and Toyoda H:
Direct contribution of inducible nitric oxide synthase expression
to apoptosis induction in primary smooth chorion trophoblast cells
of human fetal membrane tissues. Int J Biochem Cell Biol.
41:1062–1069. 2009. View Article : Google Scholar
|
|
41
|
Yoshino Y, Yuan B, Miyashita SI, Iriyama
N, Horikoshi A, Shikino O, Toyoda H and Kaise T: Speciation of
arsenic trioxide metabolites in blood cells and plasma of a patient
with acute promyelocytic leukemia. Anal Bioanal Chem. 393:689–697.
2009. View Article : Google Scholar
|
|
42
|
Zhang Y, Dong Z, Jin L, Zhang K, Zhao X,
Fu J, Gong Y, Sun M, Yang B and Li B: Arsenic trioxide-induced hERG
K(+) channel deficiency can be rescued by matrine and oxymatrine
through up-regulating transcription factor Sp1 expression. Biochem
Pharmacol. 85:59–68. 2013. View Article : Google Scholar
|
|
43
|
Liu QY, Karpinski E and Pang PK:
Tetrandrine inhibits both T and L calcium channel currents in
ventricular cells. J Cardiovasc Pharmacol. 20:513–519. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ficker E, Kuryshev YA, Dennis AT,
Obejero-Paz C, Wang L, Hawryluk P, Wible BA and Brown AM:
Mechanisms of arsenic-induced prolongation of cardiac
repolarization. Mol Pharmacol. 66:33–44. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chow SK, Chan JY and Fung KP: Inhibition
of cell proliferation and the action mechanisms of arsenic trioxide
(As2O3) on human breast cancer cells. J Cell
Biochem. 93:173–187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Scholz C, Wieder T, Stärck L, Essmann F,
Schulze-Osthoff K, Dörken B and Daniel PT: Arsenic trioxide
triggers a regulated form of caspase-independent necrotic cell
death via the mitochondrial death pathway. Oncogene. 24:1904–1913.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu T, Men Q, Wu G, Yu C, Huang Z, Liu X
and Li W: Tetrandrine induces autophagy and differentiation by
activating ROS and Notch1 signaling in leukemia cells. Oncotarget.
6:7992–8006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang L, Shamaladevi N, Jayaprakasha GK,
Patil BS and Lokeshwar BL: Polyphenol-rich extract of Pimenta
dioica berries (Allspice) kills breast cancer cells by autophagy
and delays growth of triple negative breast cancer in athymic mice.
Oncotarget. 6:16379–16395. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang H, Liu T, Li L, Wang Q, Yu C, Liu X
and Li W: Tetrandrine is a potent cell autophagy agonist via
activated intracellular reactive oxygen species. Cell Biosci.
5:42015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chiu HW, Lin JH, Chen YA, Ho SY and Wang
YJ: Combination treatment with arsenic trioxide and irradiation
enhances cell-killing effects in human fibrosarcoma cells in vitro
and in vivo through induction of both autophagy and apoptosis.
Autophagy. 6:353–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tsai JH, Hsu LS, Huang HC, Lin CL, Pan MH,
Hong HM and Chen WJ:
1-(2-Hydroxy-5-methylphenyl)-3-phenyl-1,3-propanedione induces G1
cell cycle arrest and autophagy in HeLa cervical cancer cells. Int
J Mol Sci. 17:172016. View Article : Google Scholar
|
|
52
|
Tamm I, Wang Y, Sausville E, Scudiero DA,
Vigna N, Oltersdorf T and Reed JC: IAP-family protein survivin
inhibits caspase activity and apoptosis induced by Fas (CD95), Bax,
caspases, and anticancer drugs. Cancer Res. 58:5315–5320.
1998.PubMed/NCBI
|
|
53
|
Chen X, Duan N, Zhang C and Zhang W:
Survivin and tumorigenesis: Molecular mechanisms and therapeutic
strategies. J Cancer. 7:314–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Doyle LA, Yang W, Abruzzo LV, Krogmann T,
Gao Y, Rishi AK and Ross DD: A multidrug resistance transporter
from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA.
95:15665–15670. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sertel S, Tome M, Briehl MM, Bauer J, Hock
K, Plinkert PK and Efferth T: Factors determining sensitivity and
resistance of tumor cells to arsenic trioxide. PLoS One.
7:e355842012. View Article : Google Scholar : PubMed/NCBI
|