Introduction
Breast cancer is a prevalent type of cancer and in
2012, it was found to be the leading cause of cancer-related
mortality in women worldwide (1).
Clinically, breast cancer can be divided into distinct subtypes
based on the expression of estrogen receptor (ER), progesterone
receptor (PR), and amplification of HER-2/Neu, that have prognostic
and therapeutic implications (2).
Triple-negative breast cancer (TNBC) which is defined by the lack
of ER, PR and HER-2 expression, accounts for ~15% of all breast
carcinomas (3). In particular,
patients with TNBC have a poor outcome compared to the other
subtypes of breast cancer, with the 5-year survival rate being
lower than 30% (4).
There have been significant advances in detection
and chemotherapy, which provide the best prognosis for long-term
survival and improve quality of life. However, ~70% of patients
with breast cancer are inoperable due to tumor outgrowth or bone
metastasis (5), possibly as a
result of induced hypoxia (6). The
hypoxic regions are easily found in most solid tumors due to the
severe structural abnormality of tumor microvessels (7). Hypoxia plays a role as a negative
prognostic and predictive factor owing to its multiple
contributions to chemoresistance, radioresistance, angiogenesis,
vasculogenesis, invasiveness, metastasis, resistance to cell death,
altered metabolism and genomic instability (8). Thus, it is not surprising that
hypoxia is associated with reduced survival in patient with several
cancers (9). It has been reported
that the hypoxic condition can govern TNBC progression (10), promoting adaptation through genes
within the major hallmarks of cancer (11). TNBC is the breast cancer subtype
most frequently associated with hypoxia and displays overexpression
of hypoxia-inducible factor (HIF) target genes (12). Given the role in hypoxia and the
activation of HIF-dependent gene networks is particularly robust in
TNBC, targeting HIF directly might provide a new therapeutic option
for patients with TNBC (13).
There is no effective therapeutic agent readily available for TNBC
at present. Therefore, more successful therapeutic strategies are
required for breast cancer, such as TNBC, via the targeting of
hypoxic conditions (14).
HIF is a crucial transcription factor that responds
to hypoxic conditions. It transactivates a large number of genes
involved in promoting angiogenesis, anaerobic metabolism and
resistance to apoptosis. HIFs are heterodimers composed of one of
three major oxygen-labile HIF-α subunits (HIF-1α, HIF-2α, or
HIF-3α), and a constitutive HIF-1β subunit, which together form the
HIF-1, HIF-2 and HIF-3 transcriptional complexes, respectively
(15). Under aerobic conditions,
HIF-1/2α is hydroxylated by prolyl hydroxylases (PHDs) at two
conserved proline residues in the oxygen-dependent degradation
domain (ODD). The hydroxylation of HIF-1/2α facilitates binding of
the von Hippel-Lindau protein (pVHL) to HIF-1/2α ODD, which causes
poly-ubiquitination and proteasomal degradation of HIF-1/2α
(16). However, under hypoxic
conditions, hydroxylation does not occur and HIF-1/2α are
stabilized and accumulate. HIFs then bind to a conserved DNA
sequence known as the hypoxia response elements, and activate the
transcription of a variety of hypoxia-responsive genes. The most
potent proangiogenic growth factor, vascular endothelial growth
factor (VEGF), is one of the HIF-1α-regulated genes and mediates
hypoxia-driven angiogenesis. As a result of dysregulated and rapid
cell proliferation, which is a characteristic of cancer cells and
functionally abnormal blood vessels that form in solid tumors, the
environment around cancer cells changes from normoxia (~21%
O2) to hypoxia (~1% O2) (17,18).
HIFs mediate the adaptation of cancer cells to an explicit hypoxic
microenvironment. This mediation leads to VEGF expression, followed
by the stimulation of angiogenesis, and thereby, increased
O2 delivery. By repeating this process, hypoxic cancer
cells acquire invasive and metastatic properties, as well as
resistance to cancer therapy, which together constitute the lethal
cancer phenotype. Given these factors, compounds that can inhibit
HIF-1 may have the potential for use as anticancer agents.
Resveratrol (3,4,5-trihydroxy-trans-stilbene;
Fig. 1A), a polyphenol derived
from grapes and peanuts, has been shown to possess a wide range of
health benefits, including cardio-protective, antioxidant,
anti-inflammatory and anti-aging effects (19). Intense efforts over the past
decades have indicated that resveratrol exhibits chemopreventive
and therapeutic effects against a wide range of cancers (20). In spite of resveratrol's great
anticancer potential, its utility as a therapeutic anticancer agent
is limited by its relatively low bioavailability, photosensitivity
and metabolic instability. Thus, numerous approaches are being
undertaken to overcome these limitations and to obtain synthetic
analogues superior to resveratrol.
HS-1793 [4-(6-hydroxy-2-naphthyl)-1,3-benzenediol;
Fig. 1B], a novel synthetic
resveratrol analogue, has been shown to exert stronger antitumor
effects than those of resveratrol in a variety of cancer cell lines
(21–25). Furthermore, it induced the
modulation of tumor-derived T lymphocytes, especially in its
suppressive role on the Treg cell population (26). It exhibits apoptogenic activity in
a wide range of cancer cells, including breast (21,22,27),
prostate (24), colon (23,28),
leukemia (23) and renal carcinoma
cells (25). The way in which this
resveratrol analogue exerts its antiproliferative effects has not
been fully elucidated. HS-1793 has been shown to induce G2/M cell
cycle arrest (27), downregulate
Bcl-2 and Bcl-xL expression (23–25,27),
activate caspase pathways (27),
induce endoplasmic reticulum stress-mediate apoptosis and
inactivate Akt (28,29). We have previously shown that this
resveratrol analogue can inhibit hypoxia-induced HIF-1α and VEGF
expression in PC-3 prostate cancer cells via inhibition of
phosphorylation of PI3K and Akt (30), and cause cell cycle arrest and
apoptotic cell death in MCF-7 (hormone-dependent, wild-type p53)
and MDA-MB-231 (TNBC, mutated p53) breast cancer cells (27). However, most of the studies
investigating HS-1793's anticancer potential have been carried out
in vitro. Only a limited number of animal studies have been
conducted to reveal its anticancer activities.
In the present study, therefore, we used resveratrol
and its synthetic analogue, HS-1793, to investigate and compare
their effects on the expression of HIF-1 and VEGF in MCF-7 and
MDA-MB-231 breast cancer cells in vitro and further
anticancer effects in vivo with triple-negative MDA-MB-231
breast cancer xenografts in nude mice.
Materials and methods
Chemicals
Resveratrol and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
trypan blue stain and antibody against β-actin were purchased from
Sigma-Aldrich (St. Louis, MO, USA). HS-1793 was synthesized and
supplied by Professor Hongsuk Suh (Department of Chemistry, Pusan
National University, Busan, Korea). A 100 mM solution of
resveratrol or HS-1793 was prepared in ethanol and stored in small
aliquots at −20°C. The stock solution was diluted, when required,
in cell culture medium. The maximum concentration of ethanol did
not exceed 0.1% (v/v) in the treatment range, at which it did not
influence cell growth. Anti HIF-1α was purchased from BD
Transduction Laboratories (San Jose, CA, USA). Antibodies against
VEGF and histone H1 were obtained from Santa Cruz Biotechnology
(Dallas, TX, USA). Matrigel was purchased from BD Biosciences (San
Jose, CA, USA).
Cell culture
The cancer cell lines used in the present study
included human breast carcinoma (MCF-7 and MDA-MB-231) with normal
human breast epithelial cell line (MCF-10A) serving as control.
MCF-7 and MDA-MB-231 were obtained from the American Type Culture
Collection (ATCC; Manassas, VA, USA). MCF-10A cells were generously
provided from Dr Robert J. Pauley (The Barbara Ann Karmanos Cancer
Institute, Detroit, MI, USA). MCF-7 and MDA-MB-231 cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; HyClone
Laboratories, Inc., Logan, UT, USA) containing 10% heat-inactivated
fetal bovine serum (FBS; HyClone Laboratories) and 1%
antibiotic-antimycotic solution (HyClone Laboratories) at 37°C in a
humidified atmosphere with 5% CO2, not exceeding passage
number 20. MCF-10A cells were maintained in DMEM/F-12 (1:1) with 5%
horse serum (Thermo Fisher Scientific, Waltham, MA, USA) in 37°C
incubator supplied with 5% CO2.
Hypoxia experiments
Experiments to investigate the effects of hypoxia
were carried out in a hypoxia chamber in an anaerobic system
(Thermo Fisher Scientific, Marietta, OH, USA) as previously
described (30). Hypoxic
conditions were designated as 1% O2 and 5%
CO2 with the temperature maintained at 37°C. Normoxia
was defined as the conditions in a standard CO2
incubator (21% O2 and 5% CO2). For hypoxia
experiments, MCF-7 and MDA-MB-231 cells were grown to 50%
confluency in a standard CO2 incubator at 37°C.
Twenty-four hours prior to experiments, aliquots of cell culture
media were placed in normoxic and hypoxic chambers to allow
equilibration to the corresponding conditions. Immediately before
each experiment, cell culture media were withdrawn from MCF-7 and
MDA-MB-231 cells and replaced with equilibrated media.
MTT assay and growth inhibition
Cell viability was determined by a colorimetric MTT
assay as previously described (31). Briefly, cells were seeded onto
6-well plates at a density of 2×105 cells/well and
allowed to adhere and grow overnight. Cells were then treated with
increasing concentrations of resveratrol, HS-1793, or ethanol
vehicle for 24 h in normoxic condition or for 4 h in hypoxic
condition. Fresh medium with MTT was added to the wells, and the
plate was incubated at 37°C for 2 h. The medium was discarded, the
formazan crystals were dissolved in dimethyl sulfoxide, and the
absorbance at 540 nm was measured using an ELISA plate reader
(Thermo Fisher Scientific, Vantaa, Finland). The ethanol
vehicle-treated cells served as the indicator of 100% cell
viability. Percentage of cell viability was calculated using the
following calculation formula: Cell viability (%) = (OD sample/OD
control) × 100%. IC50 value (concentration of
resveratrol or HS-1793 that reduce 50% cell viability compared to
ethanol vehicle-treated control cell) was determined from the graph
of viability (%) vs. resveratrol or HS-1793 concentration ranging
between 100 and 12.5 μM by 2-fold serial dilution. All cell
lines were assayed for three biological replicates each with
triplicates.
Western blot analysis
Cells were homogenized in protein lysate buffer, and
the debris was removed by centrifugation at 12,000 rpm for 10 min
at 4°C. The nuclear and cytosolic fractions from tumor tissue were
prepared as previously described (32). The protein concentrations in all
samples were determined by protein assay reagents (Bio-Rad
Laboratories, Hercules, CA, USA). Equal quantities of proteins were
subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis. They were then transferred onto polyvinylidene
fluoride membranes, and blocked with 5% non-fat dried milk for 1 h
at 37°C. The membranes were probed with specific primary antibodies
overnight at 4°C, and then incubated with the corresponding
secondary antibodies for 1 h at 37°C. The specific protein bands
were visualized with an ECL detection system (GE Healthcare,
Piscataway, NJ, USA).
VEGF ELISA
To analyze VEGF expression quantitatively, MCF-7 and
MDA-MB-231 cells were pretreated with resve-ratrol, HS-179, or
vehicle for 30 min. The treatment was then removed and replaced
with fresh media, which were preconditioned in normoxic or hypoxic
conditions. Cells were incubated in the presence or absence of
resveratrol or HS-1793 at corresponding conditions for 24 h. The
supernatants in the wells were collected, cleared by centrifugation
and stored at −20°C. ELISA was performed using the human VEGF
Quantikine kit (R&D Systems, Minneapolis, MN, USA) according to
the manufacturer's suggested protocol.
RNA extraction and RT-PCR
Total RNA isolated from breast cancer cells using a
RNeasy Mini kit reagent (Qiagen, La Jolla, CA, USA), was reverse
transcribed using a Bioneer RT/PCR PreMix in the presence of oligo
dT (Bioneer Corp., Daejeon, Korea). The resulting complementary DNA
was amplified with the following sets of oligonucleotide primers:
VEGF (sense, 5′-AGGAGGGCAGAATCATCACG-3′ and antisense,
5′-CAAGGCCCACAGGGATTTTCT-3′), and glyceraldehyde-3-phosphate
dehydrogenase (GADPH; sense, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and
antisense, 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′). GAPDH served as an
internal control. PCR products were analyzed by electrophoresis on
a 1.5% agarose gel (Bio Basic, Inc., Markham, ON, Canada) in the
presence of ethidium bromide, and were visualized with a UV
transilluminator (MultiImage™ Light Cabinet; Alpha Innotech Corp.,
San Leandro, CA, USA).
Animal studies
The animal protocol used in the present study was
reviewed and approved by the Pusan National
University-Institutional Animal Care and Use Committee (PNU-IACUC,
Busan, Korea) in terms of ethical procedures and scientific care
(approval number, PNU-2015-0318). Five-week-old female BALB/c nude
mice (Japan SLC, Inc., Hamamatsu, Japan) were used for in
vivo experiments. The animals were maintained in constant,
specific pathogen-free laboratory conditions for a 12 h light/dark
cycle. They were given water and fed standard mouse chow ad
libitum. For injections, MDA-MB-231 cells were trypsinized and
counted using trypan blue to identify viable cells. Animals were
injected with 1×106 MDA-MB-231 cells [in 100 μl
of phosphate buffered saline (PBS) and Matrigel, 1:1] in the right
flank and allowed to form xenografts. When the average tumor volume
reached 40 mm3, mice were randomly assigned to one
vehicle and three treatment groups (6 mice per group): i) vehicle;
ii) HS-1793 (5 mg/kg); iii) HS-1793 (10 mg/kg); and iv) resveratrol
(20 mg/kg). HS-1793 and resveratrol were dissolved in PBS
containing 0.1% v/v dimethyl sulfoxide (DMSO) and administered
intraperitoneally twice a week. Tumor diameters were determined
with a caliper, and the tumor volume was calculated using a
standard formula: tumor volume (mm3) = L1 ×
(L2)2 × 0.5236, where L1 is the long
diameter and L2 is the short diameter. Toxicity was assessed
by survival, activity and changes in body weight. At the completion
of 4 weeks of treatment, the mice were euthanized and tumor samples
were dissected out, weighed, fixed in formalin, and processed to
determine the expression of target proteins as described in the
sections on immunohistochemical analysis and western blotting.
Immunohistochemical analysis of
tumors
Tumor tissues were fixed in 10% v/v neutral buffered
formalin, embedded in paraffin, sectioned to 5 μm and
mounted on slides. The sections were blocked with normal goat serum
and incubated with the following antibodies; anti-CD31 (Abcam,
Cambridge, MA, USA) and anti-Ki-67 (Abcam). Stained slides were
visualized with an Axiovert 100 microscope (Zeiss Carl, Göettingen,
Germany) and the images were captured at a ×200 magnification.
Statistical analyses
Results were expressed as the mean ± standard
deviation (SD) of three separate experiments and analyzed using the
Student's t-test. Means were considered significantly different at
P<0.05 or P<0.01.
Results
HS-1793 suppresses proliferation of MCF-7
and MDA-MB-231 cells
We examined the effects of resveratrol or HS-1793 on
the viability of MCF-7, MDA-MB-231 and MCF-10A cells cultured in
normoxic condition for 24 h. Table
I summarized the IC50 value of resveratrol and
HS-1793 on all the tested cells. The IC50 values in
MCF-7, MDA-MB-231 and MCF-10A cells treated with resveratrol were
88.2±4.7, 90.6±2.9 and >100 μM, respectively. The
IC50 values in MCF-7 and MDA-MB-231 cells treated with
HS-1793 were 26.3±3.2, 48.2±4.2 and >100 μM,
respectively. Therefore, HS-1793 treatment exhibited 3.3- and
1.9-fold more anti-proliferative effects against MCF-7 and
MDA-MB-231 cells than resveratrol. However, no significant
differences were observed between resveratrol and HS-1793 against
the non-malignant normal MCF-10A cells.
 | Table IThe values of IC50 of
resveratrol or HS-1793 in MCF-7, MDA-MB-231 and MCF-10A.a |
Table I
The values of IC50 of
resveratrol or HS-1793 in MCF-7, MDA-MB-231 and MCF-10A.a
| Cells | Resveratrol
(μM) | HS-1793
(μM) |
|---|
| MCF-7 | 88.2±4.7 | 26.3±3.2 |
| MDA-MB-231 | 90.6±2.9 | 48.2±4.2 |
| MCF-10A | >100 | >100 |
HS-1793 inhibits hypoxia-induced HIF-1a
protein in MCF-7 and MDA-MB-231 cells
To examine the effects of resveratrol and HS-1793 on
HIF-1α expression, we first exposed breast cancer cells to hypoxic
conditions and measured the HIF-1α protein level to determine the
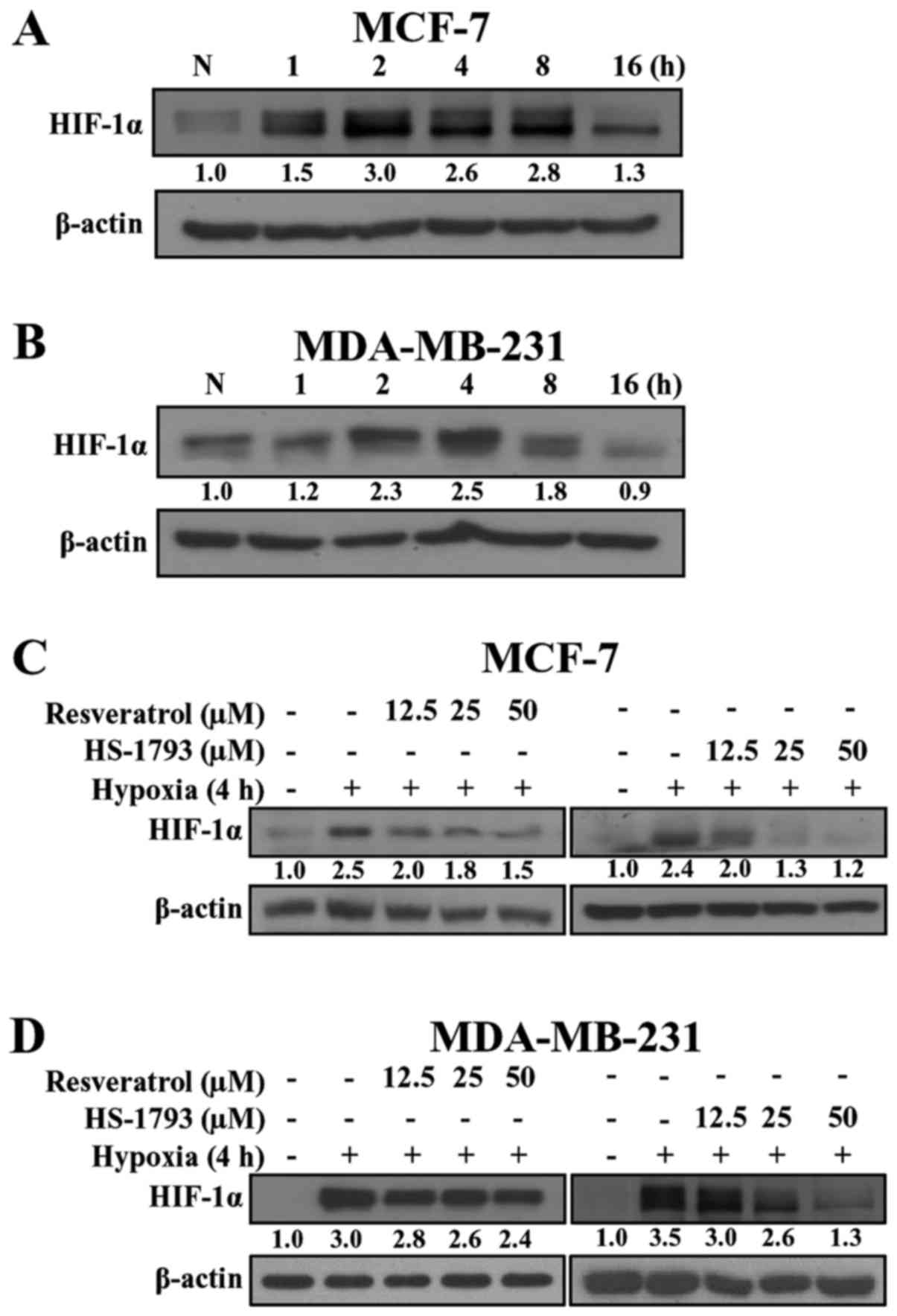
optimum conditions. As shown in Fig.
2, hypoxia induced the expression of HIF-1α in MCF-7 and
MDA-MB-231 cells in a time-dependent manner. In MCF-7 cells
(Fig. 2A), the induction of HIF-1α
protein was observed within 1 h after switching to hypoxic
conditions, and activation continued for 16 h. Unlike MCF-7 cells,
in MDA-MB-231 cells the expression of HIF-1α started at 2 h,
reached a maximum at 4 h, and decreased at 8 h (Fig. 2B). Since both breast cancer cell
lines exhibited the highest expression of HIF-1α at 2-4 h, we used
the 4 h time-point in subsequent experiments.
We next determined whether resveratrol and HS-1793
could modulate the expression of HIF-1α protein under hypoxic
conditions. As shown in Fig. 2C and
D, both resveratrol and HS-1793 downregulated HIF-1α expression
in a concentration-dependent manner. Notably, HS-1793 more
effectively decreased the level of HIF-1α when compared to
resveratrol in both cell lines. In addition, a significant decrease
in the HIF-1α level was demonstrated in response to resveratrol and
HS-1793 in MDA-MB-231 cells (Fig.
2D).
Decrease of HIF-1α protein levels by
HS-1793 is unrelated to cell death
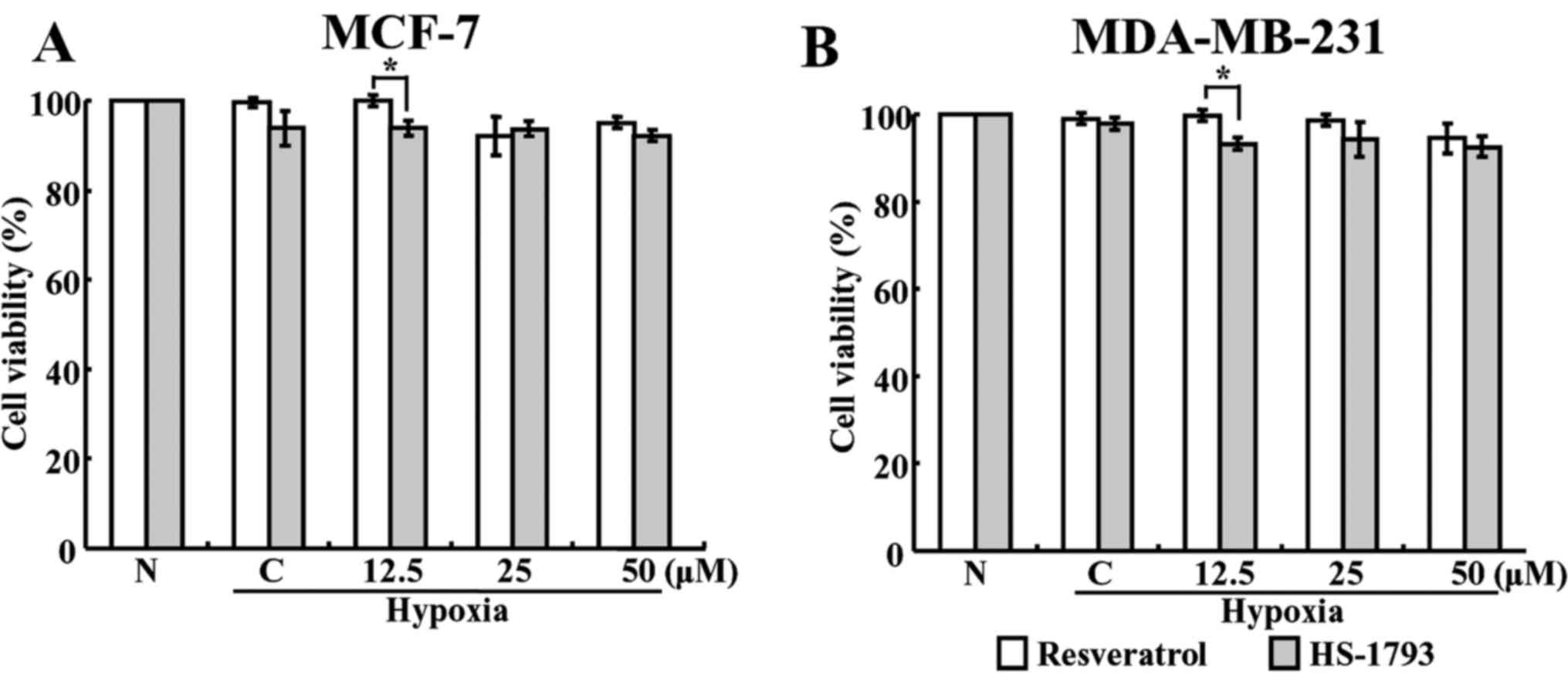
To investigate whether resveratrol and
HS-1793-induced cell death was responsible for the suppression of
HIF-1α accumulation, the cell viabilities in normoxic and hypoxic
conditions were determined using the MTT assay. When both cells
were treated with various concentrations of resveratrol and HS-1793
for 4 h in hypoxic conditions, no significant reductions in the
viability was observed at any concentration (Fig. 3). These results suggest that the
decrease in HIF-1α under hypoxic conditions may not be due to cell
death.
HS-1793 downregulates hypoxia-induced
VEGF expression in breast cancer cells
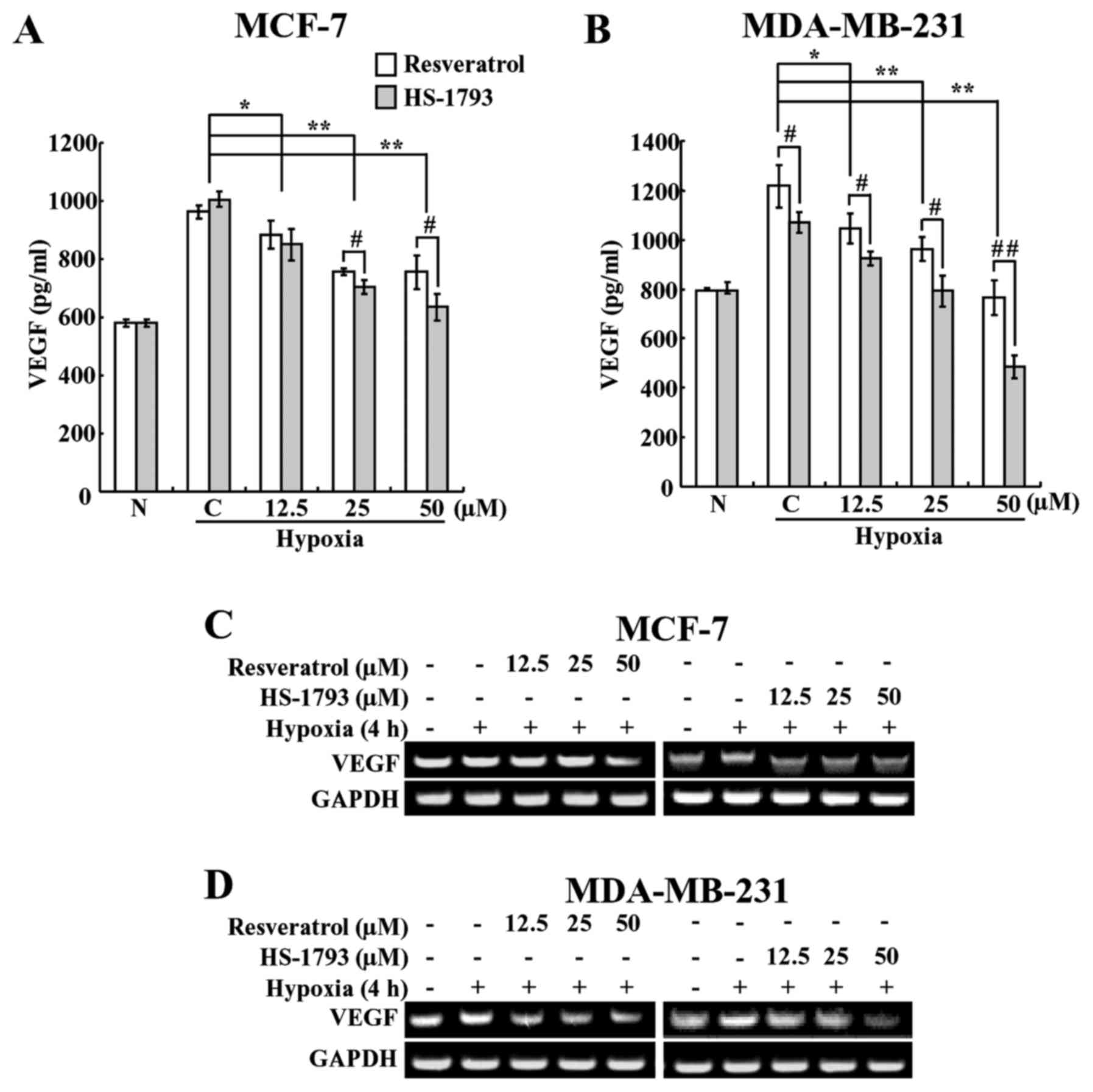
VEGF is one of the downstream target genes of
HIF-1α; therefore, its level increases under hypoxic conditions,
and it plays a crucial role in tumor angiogenesis (33). Therefore, we investigated whether
HS-1793 inhibits VEGF under hypoxic conditions. In order to do
this, we first performed ELISA to determine whether HS-1793 and
resveratrol affect VEGF levels in breast cancer cells. As expected,
hypoxia caused a rise in the VEGF level when compared with normoxia
(Fig. 4A and B). However, the
expression levels of VEGF were decreased in a
concentration-dependent manner following treatment with resveratrol
and HS-1793 treatments (Fig. 4A and
B). Moreover, HS-1793 reduced the VEGF level to a greater
extent than resveratrol in both cell types. These results showed
that HS-1793 is more effective than resveratrol at inhibiting the
production of VEGF in both cancer cell lines. In addition, HS-1793
treatment at 50 μM concentration more efficiently reduced
the expression levels of VEGF in MDA-MB-231 cells than in MCF-7
cells.
HS-1793 suppresses hypoxia-induced mRNA
expression of VEGF at the transcriptional level
In order to determine whether HS-1793 induces a
reduction of VEGF through suppressing the expression of VEGF, we
determined its mRNA levels under hypoxic conditions using reverse
transcription-polymerase chain reaction (RT-PCR). Breast cancer
cells were pretreated with various concentrations of resveratrol or
HS-1793 and incubated for 4 h under hypoxic conditions, and the
mRNA levels were measured. As shown in Fig. 4C and D, HS-1793 downregulated the
expression of VEGF mRNA, with the more marked results
observed in MDA-MB-231 cells. In MCF-7 cells, the effect was
observed at a concentration of 12.5 μM HS-1793, a
concentration at which resveratrol had no significant effects on
the mRNA expression of VEGF (Fig. 4C). In addition, we saw the
suppression of VEGF mRNA by resveratrol only at the highest
concentration (50 μM) used in this study. We also found that
resveratrol slightly inhibited the mRNA expression of VEGF
in MDA-MB-231 cells, whereas HS-1793 highly effective at inhibiting
VEGF mRNA expression in a concentration-dependent manner
(Fig. 4D). Neither resveratrol nor
HS-1793 had any effect on the mRNA expression of HIF-1α
under these experimental conditions (data not shown). Therefore,
these results indicate that the inhibition of VEGF expression by
HS-1793 occurs at the transcriptional level. In summary of in
vitro experiments, HS-1793 treatments showed more efficient
downregulation of HIF-1α and VEGF expression levels in MDA-MB-231
cells than in MCF-7 cells, we decided to use TNBC MDA-MD-231 cells
for further in vivo xenograft experiment.
HS-1793 effectively inhibits the growth
of human breast xenografts
To explore the therapeutic effects of HS-1793 as a
potentially clinically useful agent, we compared the in vivo
efficacy of resveratrol and its synthetic analogue, HS-1793, in
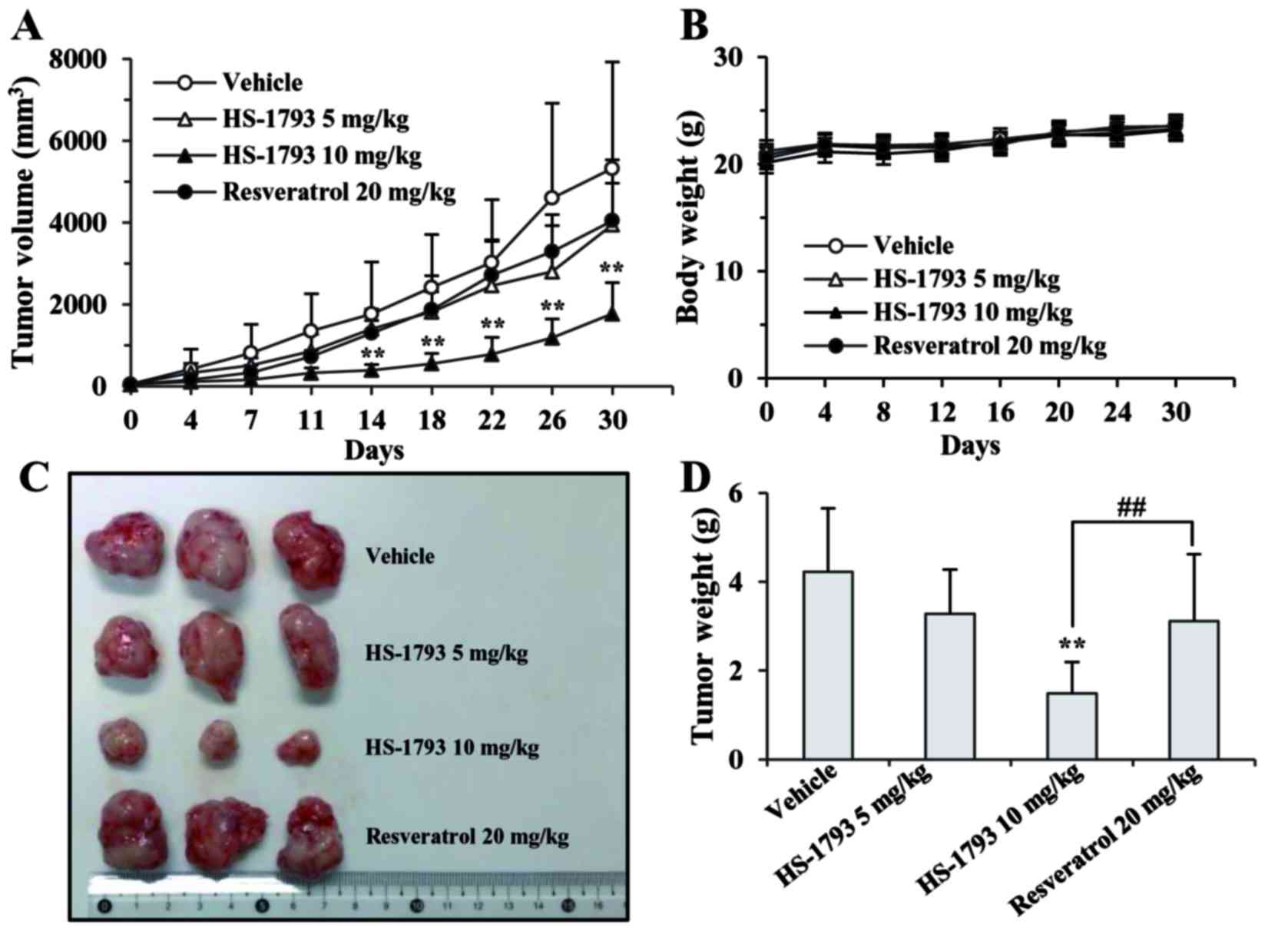
TNBC MDA-MB-231 tumor-bearing mice. As shown in Fig. 5A, tumor growth was rapid in the
vehicle-treated control group, whereas HS-1793 significantly
inhibited MDA-MB-231 xenograft tumor growth in a dose-dependent
manner. Notably, the tumor-inhibitory effects of low-dose HS-1793
(5 mg/kg twice a week) and a 4-fold higher dose (20 mg/kg twice a
week) of the parent agent, resveratrol, were similar, although no
statistical significance was observed (Fig. 5A, C and D). HS-1793 (5 mg/kg twice
a week) showed 2-fold higher maximum growth inhibition when
compared to resveratrol (Fig. 5A and
D), indicating that the in vivo efficacy of HS-1793 was
superior to that of resveratrol. In addition, there were no adverse
side-effects, such as weight loss, ulcerations, or general
decreases in well-being in the drug-treated mice in comparison to
the vehicle-treated control group during the experimental period.
This indicates the non-toxicity of both resveratrol and HS-1793
(Fig. 5B).
To shed light on the mechanism by which HS-1793
inhibited tumor growth in nude mice, we examined the presence of
proliferation markers in tumor tissues from vehicle- and
HS-1793-treated groups. Ki-67 (a proliferation marker) expression
was significantly lower in the HS-1793-treated group than in the
vehicle-control group (Fig. 6A).
As shown in Fig. 6A (right panel),
HS-1793 was more effective than resveratrol (Fig. 6A, bottom panel) in downregulating
Ki-67 levels. The results of proliferation index were summarized in
Fig. 6B. We also examined the
effects of HS-1793 and resveratrol on tumor-associated
angiogenesis, as this process is critical for tumor survival and
proliferation (34). Change in
angiogenesis was validated using immunohistochemistry of CD31
expression in the sections of tumors. Results showed that as
compared to vehicle-treated and resveratrol-treated tumors, the
expression of CD31 was significantly lower in tumors treated with
HS-1793 (Fig. 6C). This result
suggests relatively hampered angiogenesis in HS-1793-treated
tumors, which may be contributing in the slower tumor growth in
this group of mice.
HS-1793 inhibits HIF-1 and VEGF
expression in xenograft tumors from mice
Up to this point, our results indicated that HS-1793
can inhibit the proliferation and vessel formation which linked to
tumor development. We next sought to determine the effects of
resveratrol and HS-1793 treatment on the expression of HIF-1α and
its-regulated gene product VEGF in tumor tissue. As shown in
Fig. 7, the tumor tissues from
vehicle-treated mice groups expressed HIF-1α and VEGF. Treatments
of resveratrol (20 mg/kg) and HS-1793 (5 and 10 mg/kg) successfully
suppressed the expression of HIF-1α and VEGF in tumor tissues. The
results also indicated that the tumor tissues expressed VEGF
(Fig. 7A). However, HS-1793 is
more effective than resveratrol in suppressing the expression of
HIF-1α and VEGF in vivo (Fig.
7B).
Discussion
Numerous studies have suggested that the
transcription factor HIF-1α is a crucial mediator of the hypoxic
response, which plays a role in triggering tumor metastasis and
developing chemoresistance in cancer cells. HIF-1α overexpression
is closely associated with the unfavorable prognosis and increased
mortality in cancer patients (35,36).
Thus, new agents that target this transcription factor have gained
attention. The goal of the present study was to determine whether
the novel resveratrol analogue HS-1793 could inhibit HIF-1α, which
is closely linked with cancer cell proliferation, invasion and
angiogenesis.
Our results showed that HS-1793 reduced the protein
level of HIF-1α without affecting its mRNA level, therefore,
suggesting that its action occurs at the post-transcriptional
level. We found that HS-1793 activates the ubiquitin-proteasome
pathway, which is responsible for HIF-1α protein degradation (data
not shown). This is evidenced by the fact that the 26S
proteasome-specific protease inhibitor, MG132, is able to delay the
degradation of HIF-1α in the presence of HS-1793. This is further
supported by our previous report that showed HS-1793 promoted the
degradation of HIF-1α via the ubiquitin-proteasome pathway in
prostate cancer cells (30).
However, a recent study has documented that the autophagy-lysosome
pathway involves degradation of the HIF-1α protein (37), thus, HS-1793 could induce autophagy
in cancer cells (unpublished data). The precise mechanism by which
HS-1793 regulates HIF-1α needs to be elucidated in future
studies.
This study demonstrated that HS-1793 decreased
hypoxia-induced mRNA expression and secretion of VEGF in breast
cancer cells, which is in agreement with our previous finding in
prostate cancer cells (30). VEGF
is known to be a major signaling molecule involved in tumor
angiogenesis, and is regulated by HIF-1α (38). Therefore, the observed inhibition
of HIF-1α may have accounted for the downregulation of VEGF by
HS-1793. We also found that HS-1793 decreased angiogenesis in
vivo, as indicated by the inhibition of CD31, a marker for
microvessel density and the suppression of VEGF. The downregulation
of the levels of these proteins suggests that this resveratrol
analogue possesses anti-angiogenic potential.
Accumulating evidence indicates that resveratrol
requires relatively high doses and frequent injections to exhibit
its tumor growth inhibitory effect. This is due to its poor
bioavailability, as a result of its low intestinal uptake and short
initial half-life (39–42). Furthermore, there has been
controversy about the antitumor effects of resveratrol on mammary
tumors, with early reports showing that resveratrol (25 mg/kg
bodyweight, intra-peritoneal injection) inhibited the growth of
MDA-MB-231 tumors (43). However,
Castillo-Pichardo et al (44) recently reported that a range of
concentrations (0.5, 5 and 50 mg/kg body weight) of resveratrol
promotes mammary tumor growth in mice. We found that HS-1793 at 5
mg/kg showed a modest inhibitory effect on the growth of breast
cancer in a xenograft implanted nude mouse model, with a further
enhancement of the antitumor effects at 10 mg/kg. A significant 70%
reduction in tumor growth was observed at 10 mg/kg HS-1793 when
compared with growth in the vehicle control. This growth-inhibitory
effect of HS-1793 occurred in a dose-dependent manner. In addition,
all animals tolerated HS-1793 very well, as indicated by the lack
of significant body weight differences between agent-treated and
vehicle-treated mice from the start of the study. HS-1793, as low
as 5 mg/kg of body weight, inhibited tumor growth (volume) by 26%
when compared with the vehicle-control, which was similar to that
of resveratrol at a dose of 20 mg/kg. These results were further
corroborated by earlier studies showing that HS-1793 exerted a
considerable effect on the in vivo growth of an FM3A breast
tumor in CH3/He mice (26).
Furthermore, at a comparable dose of resveratrol, it neither
inhibited the growth, nor suppressed metastasis in 4T1 breast
cancer-bearing mice (45).
Although we showed, for the first time, that HS-1793
had therapeutic effects against human breast cancer xenografts;
there are some reports about the anticancer effects of this
resveratrol analogue in vivo. Previously, HS-1793 has been
shown to have therapeutic effects on established tumors in FM3A
tumor-bearing mice via the suppression of Treg cells (26). The same study also showed the
chemopreventive effect of HS-1793 (26). Jeong et al (46) showed that HS-1793-administration
increased the number of interferon (IFN)-γ-secreting cells in
splenocytes, which lead to the switch-off of M-2 polarized
tumor-associated macrophages with immunosuppressive and tumor
progressive properties, which likely contributes the antitumor
effect of HS-1793.
In conclusion, we provide novel evidence that
HS-1793 exhibits its anticancer activity, at least in part, by
modulating HIF-1α and its regulating gene, VEGF. In our
xenograft mouse study with TNBC MDA-MB-231 cells, which bear an
aggressive phenotype, HS-1793 not only inhibited tumor growth, but
also suppressed microvessel formation, which strongly correlated
with the inhibition of cell proliferation, and the decrease in
angiogenesis. These findings provide a rationale for further
investigation into this novel resveratrol analogue for
chemoprevention and/or treatment in human breast cancer.
Acknowledgments
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF), and was funded by the Ministry of Education, Science, and
Technology (2012R1A1A2006753, 2014R1A1A2055336). This study was
also supported by the National Research Foundation of Korea (NRF)
grant funded by the Korea government (MSIP) (No. 2009-0083538). We
thank Aging Tissue Bank for providing research information. This
study was also financially supported by the 2016 Post-Doc.
Development Program of Pusan National University.
References
|
1
|
Torre LA, Islami F, Siegel RL, Ward EM and
Jemal A: Global cancer in women: Burden and trends. Cancer
Epidemiol Biomarkers Prev. 26:444–457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brenton JD, Carey LA, Ahmed AA and Caldas
C: Molecular classification and molecular forecasting of breast
cancer: Ready for clinical application? J Clin Oncol. 23:7350–7360.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anders CK and Carey LA: Biology,
metastatic patterns, and treatment of patients with triple-negative
breast cancer. Clin Breast Cancer. 9(Suppl 2): S73–S81. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yadav BS, Sharma SC, Chanana P and Jhamb
S: Systemic treatment strategies for triple-negative breast cancer.
World J Clin Oncol. 5:125–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tao M, Ma D, Li Y, Zhou C, Li Y, Zhang Y,
Duan W, Xu X, Wang R, Wu L, et al: Clinical significance of
circulating tumor cells in breast cancer patients. Breast Cancer
Res Treat. 129:247–254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Voss MJ, Möller MF, Powe DG, Niggemann B,
Zänker KS and Entschladen F: Luminal and basal-like breast cancer
cells show increased migration induced by hypoxia, mediated by an
autocrine mechanism. BMC Cancer. 11:1582011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pouysségur J, Dayan F and Mazure NM:
Hypoxia signalling in cancer and approaches to enforce tumour
regression. Nature. 441:437–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilson WR and Hay MP: Targeting hypoxia in
cancer therapy. Nat Rev Cancer. 11:393–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Semenza GL: Oxygen sensing,
hypoxia-inducible factors, and disease pathophysiology. Annu Rev
Pathol. 9:47–71. 2014. View Article : Google Scholar
|
|
10
|
Semenza GL: The hypoxic tumor
microenvironment: A driving force for breast cancer progression.
Biochim Biophys Acta. 1863:382–391. 2016. View Article : Google Scholar
|
|
11
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: Cancer's Achilles' heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bernardi R and Gianni L: Hallmarks of
triple negative breast cancer emerging at last? Cell Res.
24:904–905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cancer Genome Atlas N; Cancer Genome Atlas
Network: Comprehensive molecular portraits of human breast tumours.
Nature. 490:61–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stopeck AT, Brown-Glaberman U, Wong HY,
Park BH, Barnato SE, Gradishar WJ, Hudis CA and Rugo HS: The role
of targeted therapy and biomarkers in breast cancer treatment. Clin
Exp Metastasis. 29:807–819. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar
|
|
16
|
Semenza GL: Hypoxia-inducible factors:
Mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Semenza GL: Hypoxia-inducible factor 1:
Master regulator of O2 homeostasis. Curr Opin Genet Dev.
8:588–594. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ke Q and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smoliga JM, Baur JA and Hausenblas HA:
Resveratrol and health - a comprehensive review of human clinical
trials. Mol Nutr Food Res. 55:1129–1141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aggarwal BB, Bhardwaj A, Aggarwal RS,
Seeram NP, Shishodia S and Takada Y: Role of resveratrol in
prevention and therapy of cancer: Preclinical and clinical studies.
Anticancer Res. 24:2783–2840. 2004.PubMed/NCBI
|
|
21
|
Jeong SH, Song IS, Kim HK, Lee SR, Song S,
Suh H, Yoon YG, Yoo YH, Kim N, Rhee BD, et al: An analogue of
resveratrol HS-1793 exhibits anticancer activity against MCF-7
cells via inhibition of mitochondrial biogenesis gene expression.
Mol Cell. 34:357–365. 2012. View Article : Google Scholar
|
|
22
|
Kim HJ, Yang KM, Park YS, Choi YJ, Yun JH,
Son CH, Suh HS, Jeong MH and Jo WS: The novel resveratrol analogue
HS-1793 induces apoptosis via the mitochondrial pathway in murine
breast cancer cells. Int J Oncol. 41:1628–1634. 2012.PubMed/NCBI
|
|
23
|
Jeong SH, Jo WS, Song S, Suh H, Seol SY,
Leem SH, Kwon TK and Yoo YH: A novel resveratrol derivative,
HS1793, overcomes the resistance conferred by Bcl-2 in human
leukemic U937 cells. Biochem Pharmacol. 77:1337–1347. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeong NY, Yoon YG, Rho JH, Lee JS, Lee SY,
Yoo KS, Song S, Suh H, Choi YH and Yoo YH: The novel resveratrol
analog HS-1793-induced polyploid LNCaP prostate cancer cells are
vulnerable to downregulation of Bcl-xL. Int J Oncol. 38:1597–1604.
2011.PubMed/NCBI
|
|
25
|
Jeong SH, Lee JS, Jeong NY, Kim TH, Yoo
KS, Song S, Suh H, Kwon TK, Park BS and Yoo YH: A novel resveratrol
analogue HS-1793 treatment overcomes the resistance conferred by
Bcl-2 and is associated with the formation of mature PML nuclear
bodies in renal clear cell carcinoma Caki-1 cells. Int J Oncol.
35:1353–1360. 2009.PubMed/NCBI
|
|
26
|
Jeong MH, Yang KM, Choi YJ, Kim SD, Yoo
YH, Seo SY, Lee SH, Ryu SR, Lee CM, Suh H, et al: Resveratrol
analog, HS-1793 enhance anti-tumor immunity by reducing the
CD4+CD25+ regulatory T cells in FM3A tumor
bearing mice. Int Immunopharmacol. 14:328–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim JA, Kim DH, Hossain MA, Kim MY, Sung
B, Yoon JH, Suh H, Jeong TC, Chung HY and Kim ND: HS-1793, a
resveratrol analogue, induces cell cycle arrest and apoptotic cell
death in human breast cancer cells. Int J Oncol. 44:473–480.
2014.
|
|
28
|
Um HJ, Bae JH, Park JW, Suh H, Jeong NY,
Yoo YH and Kwon TK: Differential effects of resveratrol and novel
resveratrol derivative, HS-1793, on endoplasmic reticulum
stress-mediated apoptosis and Akt inactivation. Int J Oncol.
36:1007–1013. 2010.PubMed/NCBI
|
|
29
|
Kim DH, Hossain MA, Kim MY, Kim JA, Yoon
JH, Suh HS, Kim GY, Choi YH, Chung HY and Kim ND: A novel
resveratrol analogue, HS-1793, inhibits hypoxia-induced HIF-1α and
VEGF expression, and migration in human prostate cancer cells. Int
J Oncol. 43:1915–1924. 2013.PubMed/NCBI
|
|
30
|
Kim DH, Kim MJ, Sung B, Suh H, Jung JH,
Chung HY and Kim ND: Resveratrol analogue, HS-1793, induces
apoptotic cell death and cell cycle arrest through downregulation
of AKT in human colon cancer cells. Oncol Rep. 37:281–288.
2017.
|
|
31
|
Tada H, Shiho O, Kuroshima K, Koyama M and
Tsukamoto K: An improved colorimetric assay for interleukin 2. J
Immunol Methods. 93:157–165. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sung B, Park S, Ha YM, Kim DH, Park CH,
Jung KJ, Kim MS, Kim YJ, Kim MK, Moon JO, et al:
Salicylideneamino-2-thiophenol modulates nuclear factor-κB through
redox regulation during the aging process. Geriatr Gerontol Int.
15:211–219. 2015. View Article : Google Scholar
|
|
33
|
Semenza GL: Hypoxia, clonal selection, and
the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol.
35:71–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29(Suppl 16): 15–18. 2002.
View Article : Google Scholar
|
|
35
|
Schindl M, Schoppmann SF, Samonigg H,
Hausmaninger H, Kwasny W, Gnant M, Jakesz R, Kubista E, Birner P
and Oberhuber G; Austrian Breast and Colorectal Cancer Study Group:
Overexpression of hypoxia-inducible factor 1alpha is associated
with an unfavorable prognosis in lymph node-positive breast cancer.
Clin Cancer Res. 8:1831–1837. 2002.PubMed/NCBI
|
|
36
|
Huang M, Chen Q, Xiao J, Yao T, Bian L,
Liu C and Lin Z: Overexpression of hypoxia-inducible factor-1α is a
predictor of poor prognosis in cervical cancer: A clinicopathologic
study and a meta-analysis. Int J Gynecol Cancer. 24:1054–1064.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hubbi ME, Gilkes DM, Hu H, Kshitiz, Ahmed
I and Semenza GL: Cyclin-dependent kinases regulate lysosomal
degradation of hypoxia-inducible factor 1α to promote cell-cycle
progression. Proc Natl Acad Sci USA. 111:E3325–E3334. 2014.
View Article : Google Scholar
|
|
38
|
Forsythe JA, Jiang BH, Iyer NV, Agani F,
Leung SW, Koos RD and Semenza GL: Activation of vascular
endothelial growth factor gene transcription by hypoxia-inducible
factor 1. Mol Cell Biol. 16:4604–4613. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carbó N, Costelli P, Baccino FM,
López-Soriano FJ and Argilés JM: Resveratrol, a natural product
present in wine, decreases tumour growth in a rat tumour model.
Biochem Biophys Res Commun. 254:739–743. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu SL, Sun ZJ, Yu L, Meng KW, Qin XL and
Pan CE: Effect of resveratrol and in combination with 5-FU on
murine liver cancer. World J Gastroenterol. 10:3048–3052. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kimura Y and Okuda H: Resveratrol isolated
from Polygonum cuspidatum root prevents tumor growth and metastasis
to lung and tumor-induced neovascularization in Lewis lung
carcinoma-bearing mice. J Nutr. 131:1844–1849. 2001.PubMed/NCBI
|
|
42
|
Marier JF, Vachon P, Gritsas A, Zhang J,
Moreau JP and Ducharme MP: Metabolism and disposition of
resveratrol in rats: Extent of absorption, glucuronidation, and
enterohepatic recirculation evidenced by a linked-rat model. J
Pharmacol Exp Ther. 302:369–373. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Garvin S, Ollinger K and Dabrosin C:
Resveratrol induces apoptosis and inhibits angiogenesis in human
breast cancer xenografts in vivo. Cancer Lett. 231:113–122. 2006.
View Article : Google Scholar
|
|
44
|
Castillo-Pichardo L, Cubano LA and
Dharmawardhane S: Dietary grape polyphenol resveratrol increases
mammary tumor growth and metastasis in immunocompromised mice. BMC
Complement Altern Med. 13:62013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bove K, Lincoln DW and Tsan MF: Effect of
resveratrol on growth of 4T1 breast cancer cells in vitro and in
vivo. Biochem Biophys Res Commun. 291:1001–1005. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jeong SK, Yang K, Park YS, Choi YJ, Oh SJ,
Lee CW, Lee KY, Jeong MH and Jo WS: Interferon gamma induced by
resveratrol analog, HS-1793, reverses the properties of tumor
associated macrophages. Int Immunopharmacol. 22:303–310. 2014.
View Article : Google Scholar : PubMed/NCBI
|