Introduction
Colorectal cancer is one of the leading causes of
cancer-associated morbidity and mortality worldwide. Despite recent
advances in the management, including surgery, chemotherapy, and
radiotherapy, the overall survival of patients with advanced
colorectal cancer still remains low (1–3).
Therefore, the development of clinically effective anticancer
agents with minimal side effects is needed to enhance treatment and
to improve the survival of patients with advanced colorectal
cancer.
Plant-associated microorganisms are known as an
important sources of diverse natural compounds with novel
structures and biological activities. A substantial body of
evidence demonstrates that various compounds and extracts isolated
from plant-associated microorganisms present significant
pharmacological and biological activities (4–8). For
instance, the bioactive compounds isolated from endophytic fungi
that reside on medicinal plants have been reported (4–8).
Malformins, which are a group of cyclic pentapeptides with a
disulfide bond from two cysteine thiols, were originally discovered
and isolated from the culture broth of the fungus Aspergillus
niger (A. niger). It typically induces malformations in
bean plants and in the curvature of corn roots (9–12).
To date, three sub-groups of Malformins have been identified: A,
produced by A. niger strain 56–39, B, by A. niger
strain 56–30, and C, by A. niger strain AN-1 (9–12).
Malformin A mainly consists of Malformin A1, A2, A3, and A4,
indicated by both amino acid analyses and molecular formula.
Malformin A1 (MA1) is a bicyclic pentapeptide containing five amino
acids: L-isoleucine, L-valine, D-leucine, and two D-cysteines
(12). MA1 is the most
well-studied out of the malformin subtypes, and its various
biological activities have been reported previously, including
causing malformations in plants, having some antibacterial effects
(13–15), enhancing cellular fibrinolytic
activity (16–18), preventing IL-1-induced procoagulant
reaction (19,20), and inhibiting the cell cycle at G2
checkpoint followed by DNA damage (21,22).
Additionally, the anticancer activity of MA1 has been reported in
various human cancer cells including colorectal cancer (9,22–24).
Therefore, understanding these functional and biological properties
of MA1, as well as its mechanism of action is very important for
its application into mainstream of medicine. The aim of this study
is to investigate the impact of MA1 on tumor cell behavior and its
associated oncogenic signaling pathways in human colorectal cancer
cells.
Materials and methods
Cell culture and materials
The SW480 and DKO1 human colorectal carcinoma cell
lines were obtained from the American Type Culture Collection (ATCC
CRL 1589, Manassas, VA, USA). The SW480 and DKO1 cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand
Island, NY, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco) and 1% penicillin/streptomycin at 37°C in a humidified
atmosphere of 5% CO2. p38 chemical inhibitor (SB203580)
and pan-caspase inhibitor Z-VAD-FMK were purchased from
Sigma-Aldrich (St. Louis, MO, USA). MA1 was purchased from AdipoGen
Life Sciences (San Diego, CA, USA).
Cell proliferation assay
SW480 and DKO1 cells were plated onto a 96-well
plate at a density of 1×104 cells/well and were
incubated for 24 h at 37°C before the MA1 treatment. Cells were
then treated with various concentrations of MA1 or DMSO for 24 h.
After the treatment, cell viability was determined by EZ-CyTox
(tetrazolium salts, WST-1) cell viability assay kit (Daeil Lab
Inc., Seoul, Korea), and BrdU cell proliferation assay kit (Cell
Signaling Technology, Inc., Danvers, MA, USA). After the
application, absorbance at 450 nm was measured using a microplate
reader (Infinite M200; Tecan, Austria GmbH, Austria). Each
experiment was done in triplicate wells and was repeated at least
three times.
Flow cytometric analysis
SW480 and DKO1 cells were plated in a 6-well plate
at 5×105 cells/well and were incubated for 24 h at 37°C
before treatment with MA1. MA1 treated cells were collected and
were resuspended in binding buffer (BD Biosciences, San Diego, CA,
USA). These cells were incubated with 7-amino-actinomycin D (7-AAD)
and Annexin V-APC (BD Biosciences) for 20 min at room temperature.
To analyze the number of apoptotic cells, FACSCalibur flow
cytometer (Becton-Dickinson) and WinMDI version 2.9 (The Scripps
Research Institute, San Diego, CA, USA) were used.
DNA fragmentation
MA1-treated cells were collected and incubated with
cell lysate buffer (1% NP-40 in 20 mM EDTA, 50 mM Tris-HCl, pH 7.5)
for 30 min on ice. The samples were then centrifuged at 12,00 × g
for 30 min. RNase A was added to the resulting supernatant and was
incubated for 2 h at 56°C. Proteinase K was then added to the
supernatant and was incubated for 2 h at 37°C. An equal volume of
isopropanol was added and was incubated at −80°C overnight to
precipitate the genomic DNA. The genomic DNA was loaded into 2%
agarose gel and was stained with ethidium bromide. The DNA was
visualized under UV light transilluminator.
Western blotting
MA1-treated cells were lysed in RIPA extraction
solution with Halt™ phosphatase inhibitor and Halt™ protease
inhibitor cocktails (Thermo Fisher Scientific, Rockford, IL, USA)
for 30 min in an ice bath. The protein concentration of each lysate
was measured using BCA™ protein assay (Thermo Fisher Scientific).
Equal amounts of protein were separated using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to a PVDF membrane (Bio-Rad Laboratories, Hercules, CA,
USA). Antibodies against the following proteins were used: cleaved
poly(ADP-ribose) polymerase (PARP), cleaved caspase-3, -7, and -9,
p53 upregulated modulator of apoptosis (PUMA), X-linked inhibitor
of apoptosis protein (XIAP), Survivin, extracellular
signal-regulated kinase1/2 (ERK1/2), phospho-ERK1/2, p38,
phospho-p38, c-Jun NH2-terminal kinase (JNK), phospho-JNK (Cell
Signaling Technology, Inc.), and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH; Santa Cruz Biotechnology, CA, USA). Protein
bands were developed using an ECL reagent (Amersham, Arlington
Heights, IL, USA) and the luminescent image analyzer LAS-4000 (Fuji
Film, Tokyo, Japan).
Wound healing assay
Cell migration was evaluated with a wound-healing
assay using culture inserts (Ibidi, Regensburg, Germany). Cells
were plated into culture-inserts and a wound gap was created by
removing inserts after 24 h of incubation. Wound widths were
measured using images photographed by an inverted microscope at 0,
24 and 48 h time-points.
Transwell invasion assay
SW480 and DKO1 cells were seeded in the upper well
of Transwell filter chambers (8.0-µm pore size; Costar,
Cambridge, MA, USA) with gelatin coating. After 24 h of incubation,
invading cells on the lower surface of the upper chamber were fixed
with 70% ethanol and stained with Hemacolor® Rapid
staining solution (Merck Millipore, Darmstadt, Germany) following
the manufacturer's protocol. The stained cells were counted under a
light microscope.
Results
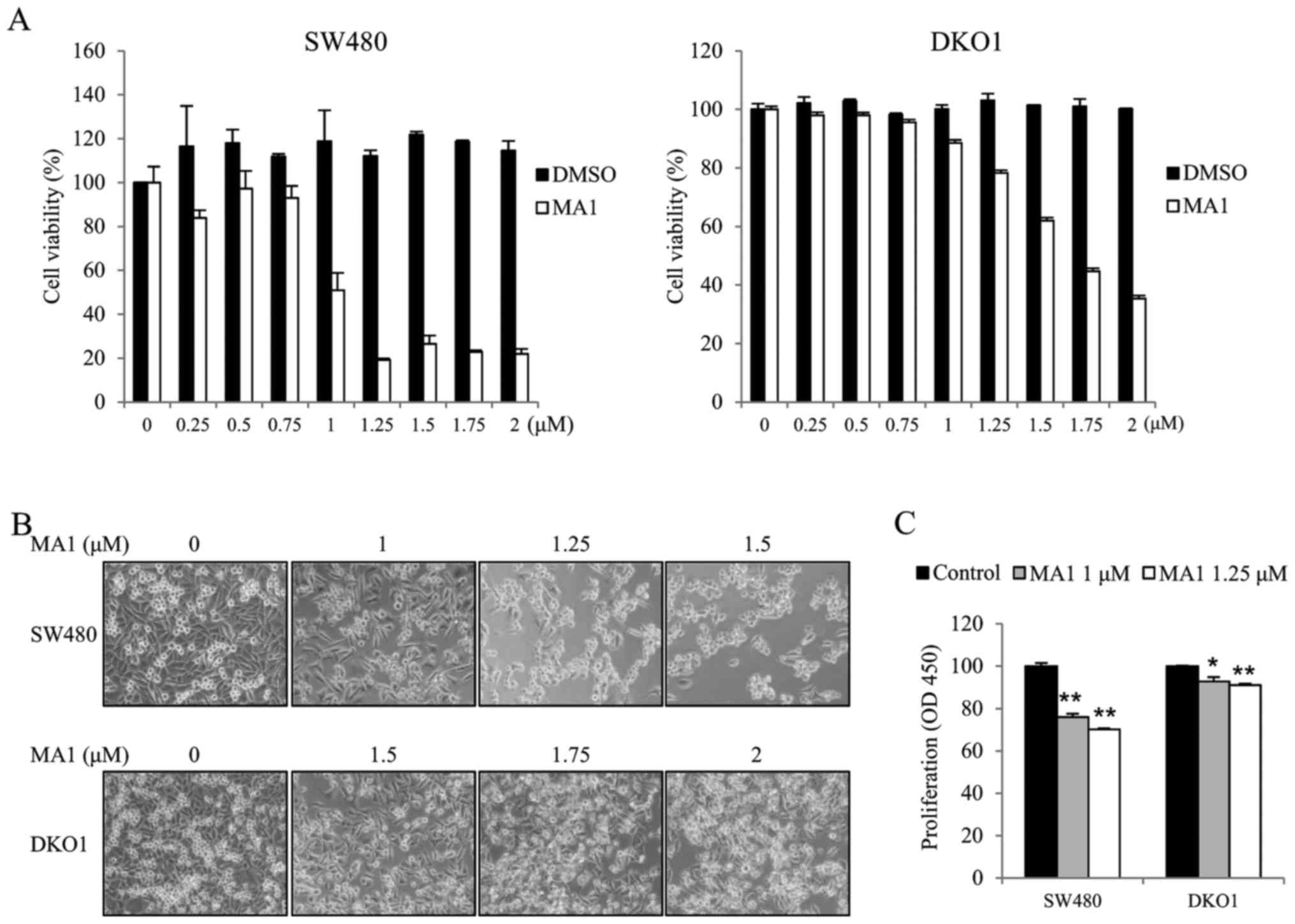
MA1 inhibits the growth of human
colorectal cancer cells
We first investigated the effect of MA1 on the
growth of the human colorectal cancer cell lines, SW480 and DKO1.
Human colorectal cancer cells were exposed to different
concentrations of MA1 (0–2 µM) for 24 h. The effect of MA1
on SW480 and DKO1 cells was determined by the WST-1 cell viability
assay, inverted microscopy and the BrdU incorporation assay. The
WST-1 assays and inverted microscopy showed that MA1 treatment
inhibited the growth of SW480 and DKO1 cells, as compared to
control cells (Fig. 1A and B).
Moreover, the BrdU assays also showed similar results, as 1 and
1.25 µM of MA1 treatment resulted in a slower BrdU
incorporation rate for SW480 and DKO1 cells as compared to control
cells (p<0.05, p<0.01) (Fig.
1C). These results indicate that MA1 inhibits the proliferation
of human colorectal cancer cells.
MA1 induces apoptosis and cell cycle
arrest in human colorectal cancer cells
In order to determine whether MA1-induced human
colorectal cancer cell death is related to apoptosis and cell cycle
arrest, we performed flow cytometric analyses and a DNA
fragmentation assay. Human colorectal cancer cells were exposed to
different concentrations of MA1 (0–1.5 µM) for 24 h. The
proportion of early and late apoptotic cells were dose-dependently
greater in MA1-treated SW480 and DKO1 cells compared to non-treated
control cells (Fig. 2A).
Additionally, MA1 treatment induced cell cycle arrest at the sub-G1
phase in SW480 and DKO1 cells (Fig.
2B). MA1 treatment (0.75–1.5 µM) induced an increase in
DNA fragmentation in human colorectal cancer cells as compared to
the non-treated control cells (Fig.
2C). To determine the activation of caspases, key enzymes in
apoptosis, we further investigated caspase-specific activities. The
cleaved PARP, caspase-3, -7, and -9 expressions were upregulated
dose-dependently in SW480 and DKO1 cells after MA1 treatment
(Fig. 2D). We further examined
whether MA1 treatment-induced apoptosis is associated with the
modulation of apoptosis regulatory proteins. As shown in Fig. 2D, MA1 treatment led to the increase
in the pro-apoptotic protein, PUMA and the decrease in
anti-apoptotic proteins, XIAP and Survivin in SW480 and DKO1
cells.
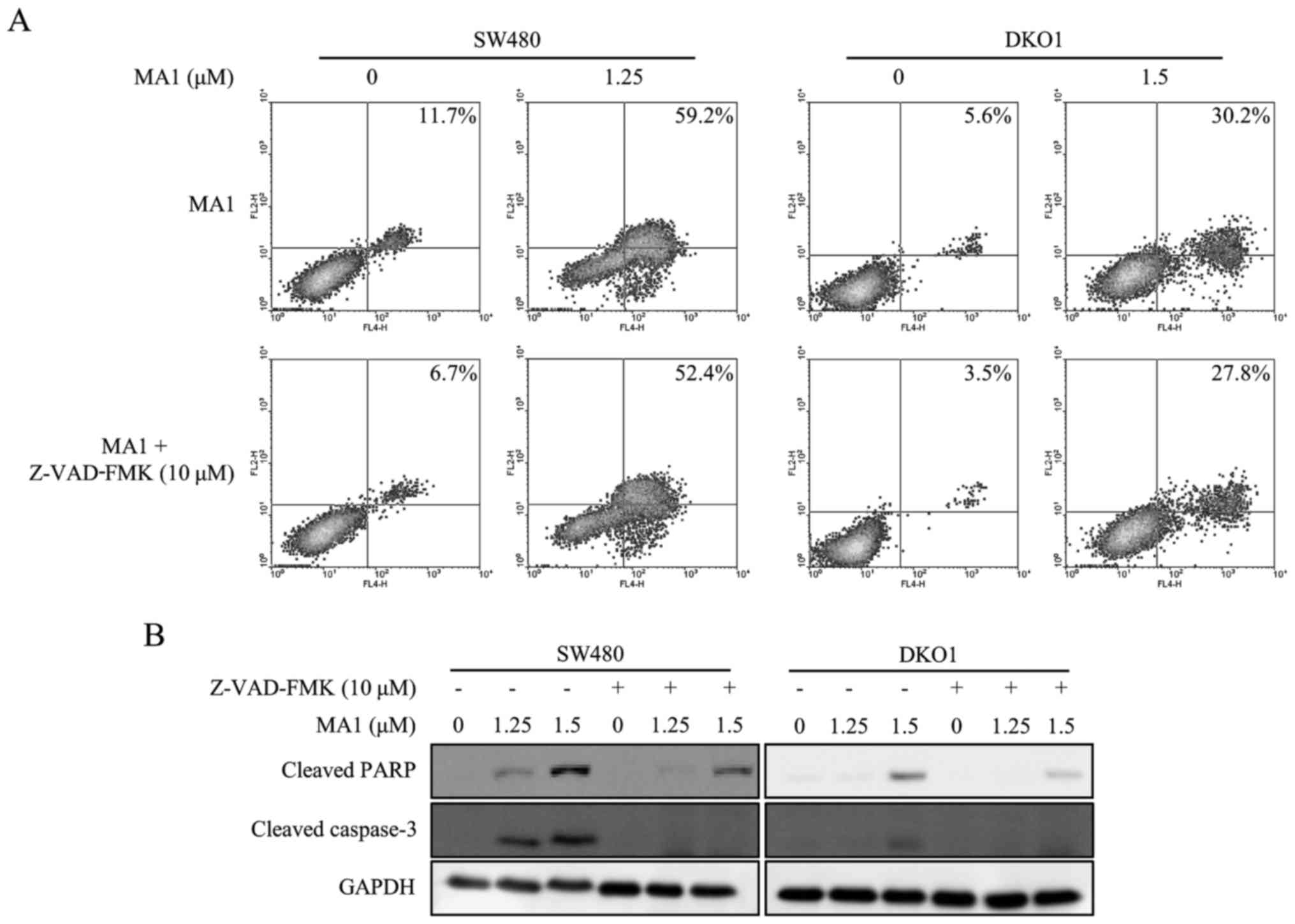
Pan-caspase inhibitor attenuates the
MA1-induced apoptotic effect on human colorectal cancer cells
The pan-caspase inhibitor, Z-VAD-FMK (10 µM),
was used to determine whether the apoptosis was induced by MA1
treatment. The increase in early and late stage apoptotic cells by
MA1 treatment was decreased in SW480 and DKO1 cells when treated
with Z-VAD-FMK (Fig. 3A).
Furthermore, Z-VAD-FMK abrogated the MA1-induced caspase-3 and PARP
activation (Fig. 3B). Therefore,
MA1 treatment works by inducing apoptosis by directly activating
caspases in human colorectal cancer cells.
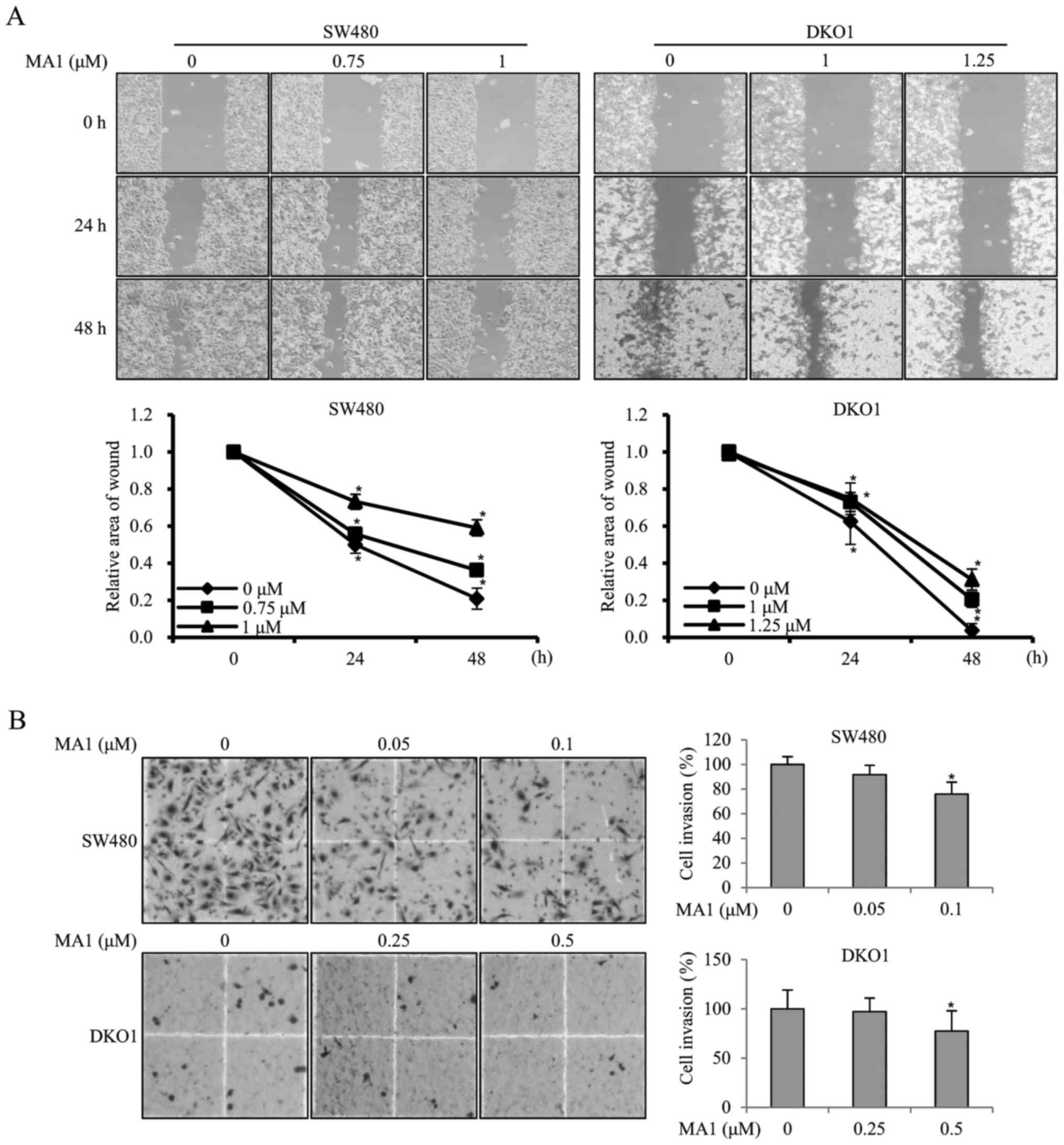
MA1 inhibits the migration and invasion
of human colorectal cancer cells
To investigate the effect of MA1 on the migration
ability of human colorectal cancer cells, SW480 and DKO1 cells were
treated with different concentrations of MA1 (0–1.25 µM) for
24 and 48 h, followed by the scratch wound healing motility assay.
The results showed that SW480 and DKO1 cells treated with MA1 for
24 and 48 h migrated significantly slower than non-treated control
cells (p<0.05, p<0.01) (Fig.
4A), suggesting that MA1 could significantly decrease cell
migration. The effect of MA1 on the invasiveness of SW480 and DKO1
cells was further detected by using a Transwell invasion assay.
Compared to the non-treated control cells, the number of invasive
SW480 and DKO1 cells treated with 0.1 and 0.5 µM MA1
decreased significantly (p<0.05) (Fig. 4B), indicating that MA1 could
significantly decrease the invasiveness of human colorectal cancer
cells.
Impact of MA1 on oncogenic signaling
pathways in human colorectal cancer cells
To examine whether MA1 affects oncogenic signaling
pathways in human colorectal cancer cells, we determined the
phosphorylation levels of MAPK signaling proteins. For these
experiments, the human colorectal cancer cells were exposed to MA1
(1.5 µM) for different times (0–6 h). The phosphorylation
levels of p38 was upregulated by MA1 treatment in SW480 and DKO1
cells but the phosphorylation levels of JNK and ERK1/2 were not
changed by MA1 treatment (Fig.
5A). Subsequently, the inhibitor of the p38 signaling pathway,
SB203580, was used to determine whether this pathway was involved
in MA1 action. SB203580 attenuated the MA1-induced p38
phosphorylation, as well as caspase-3 and PARP activation (Fig. 5B). We also examined whether
SB203580 affects the modulation of apoptosis regulatory proteins.
As shown in Fig. 5B, SB203580 led
to the decrease in the pro-apoptotic protein, PUMA and increase in
the anti-apoptotic proteins, XIAP and Survivin. These results
suggest that the apoptotic effect of MA1 on human colorectal cancer
cells is mediated by the activation of p38 signaling pathway.
 | Figure 5Impact of MA1 on oncogenic signaling
pathways in human colorectal cancer cells. (A) SW480 and DKO1 cells
were treated for the indicated times (0–6 h) with 1.5 µM of
MA1. Cell lysates were prepared and subjected to western blotting
using phospho-ERK1/2, phospho-p38, and phospho-JNK antibodies. (B)
SW480 and DKO1 cells were pretreated with SB203580 (a p38
inhibitor) and then exposed to MA1 for 24 h. Cell lysates were
prepared and subjected to western blotting using cleaved PARP,
cleaved caspase-3, PUMA, XIAP, and Survivin antibodies. MA1,
Malformin A1; ERK1/2, extracellular signal-regulated kinase1/2;
JNK, c-Jun NH2-terminal kinase; PARP, poly(ADP-ribose) polymerase;
PUMA, p53 upregulated modulator of apoptosis; XIAP, X-linked
inhibitor of apoptosis protein; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
Discussion
Plant-associated microorganisms are known to produce
a variety of metabolites with novel structures and interesting
biological activities (4–8). MA1 isolated from the fungus A.
niger has been found to possess a range of bioactive
properties, which includes enhancing fibrinolytic activity, and
acting as an antibacterial/viral/fungal agent (13–18).
Furthermore, MA1 exhibits a strong cytotoxic effect against various
human cancer cell lines (9,22–24).
This activity is believed to be related to its anticancer
properties such as inhibiting cell proliferation, inducing
apoptosis, arresting the cell cycle and inhibiting cell migration
and invasion (25,26). Therefore, searching for the
antitumor targets of MA1 and its signal transduction mechanisms is
crucial for the development of natural drugs and the treatment of
cancer.
In our study, MA1 inhibited cell proliferation and
induced apoptosis and cell cycle arrest in human colorectal cancer
cells. These results suggest that MA1 can alter the oncogenic
phenotypes of human colorectal cancer cells.
Apoptosis is a type of cell death that results in
the orderly and efficient removal of damaged cells. However,
deregulation of apoptosis is responsible for cancer development,
progression, and treatment resistance (27,28).
Apoptosis is a complex process that results from multiple genetic
alterations in apoptotic regulatory genes, and thus, we studied the
effects of MA1 on apoptosis in human colorectal cancer cells. Our
study showed that caspase-3, -7, -9, and PARP in human colorectal
cancer cells were activated by MA1 treatment. Furthermore, MA1
treatment led to the increase in the pro-apoptotic protein, PUMA
and a decrease in anti-apoptotic protein, XIAP and Survivin.
Additionally, we used the pan-caspase inhibitor, Z-VAD-FMK, to
determine whether MA1-induced apoptosis was related to caspase
activation. Our study showed that the pan-caspase inhibitor
abrogated the MA1-induced increase in early and late stage
apoptotic cells, as well as the activation of caspase-3 and PARP.
Therefore, MA1 treatment exerts its effect on apoptosis by directly
inducing caspase activity in human colorectal cancer cells. Our
study also demonstrated that MA1 treatment induced cell cycle
arrest at the sub-G1 phase in human colorectal cancer cells.
Previously, MA1 and Malformin C were found to abrogate
bleomycin-induced G2 arrest, resulting in a drastic decrease in
cells at the G2 phase and increase in cells in the sub-G1 phase
(21,22).
The regulation of cell migration and invasion is
crucial for various physiological processes including embryonic
development, angiogenesis, tissue repair, and the immune response.
Its loss is also a principal hallmark of cancer cells (25,26).
Our study showed that MA1 treatment suppressed tumor cell migration
and invasion in human colorectal cancer cells.
Given the effects of MA1 on cancer cell behavior, we
studied the effects of MA1 treatment on the activation of
intracellular signaling pathways involved in the alteration of the
oncogenic phenotype of colorectal cancer cells. Previously, MA1
induced apoptosis, necrosis, and autophagy through the activation
of AMPK/mTOR signaling pathway in prostatic cancer cells (24). Furthermore, the inhibitor of the
phosphatidylinositol 3-kinase signaling pathway abolished the
enhancement of cellular fibrinolytic activity by MA1 in human
leukemia U937 cells. That said, the inhibitor of MAPK signaling
showed minimal effects on MA1 treated leukemia cells (13). MAPK signaling pathways, including
ERK1/2, JNK, and p38 MAPK, play a critical role in many physiologic
processes, including cell growth, differentiation, migration,
proliferation, apoptosis, and cell cycle progression (29,30).
Moreover, MAPK signaling pathways play important roles in cancer
progression, and particularly in determining the outcome and
sensitivity to anticancer therapies (29,30).
Many chemotherapeutic agents such as cyclophosphamide and
oxaliplatin induce apoptosis through activating p38 MAPK pathway
(31,32). We observed that MA1 treatment
increased the phosphorylation level of p38, but the phosphorylation
levels of JNK and ERK1/2 were unchanged. The inhibitor of the p38
signaling pathway, SB203580, was used to determine whether this
pathway was involved in MA1 action. SB203580 abrogated the
MA1-induced p38 phosphorylation, as well as the activation of
caspase-3 and PARP. Moreover, SB203580 led to a decrease in the
pro-apoptotic protein, PUMA and an increase in the anti-apoptotic
proteins, XIAP and Survivin. Thus, these results confirm that MA1
promotes apoptosis by caspase activation and the modulation of
apoptosis regulatory proteins through the stimulation of the p38
signaling pathway in human colorectal cancer cells.
Taken together, our results indicate that MA1
treatment suppresses tumor progression by the inhibition of
proliferation and induction of apoptosis through the activation of
the p38 signaling pathway in human colorectal cancer cells.
Acknowledgments
This study was supported by a grant (GRI16075-3) of
the Chonnam National University Hospital-Gwangju Institute of
Science and Technology (CNUH-GIST).
References
|
1
|
Kim ER and Kim YH: Clinical application of
genetics in management of colorectal cancer. Intest Res.
12:184–193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park SH, Song CW, Kim YB, Kim YS, Chun HR,
Lee JH, Seol WJ, Yoon HS, Lee MK, Bhang CS, et al:
Clinicopathological characteristics of colon cancer diagnosed at
primary health care institutions. Intest Res. 12:131–138. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin OS, Kozarek RA and Cha JM: Impact of
sigmoidoscopy and colonoscopy on colorectal cancer incidence and
mortality: An evidence-based review of published prospective and
retrospective studies. Lancet. 383:1490–1502. 2014.
|
|
4
|
Gunatilaka AAL: Natural products from
plant-associated microorganisms: Distribution, structural
diversity, bioactivity, and implications of their occurrence. J Nat
Prod. 69:509–526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schulz B, Boyle C, Draeger S, Römmert AK
and Krohn K: Endophytic fungi: A source of novel biologically
active secondary metabolites. Mycol Res. 106:996–1004. 2002.
View Article : Google Scholar
|
|
6
|
Strobel G, Daisy B, Castillo U and Harper
J: Natural products from endophytic microorganisms. J Nat Prod.
67:257–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan RX and Zou WX: Endophytes: A rich
source of functional metabolites. Nat Prod Rep. 18:448–459. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chomcheon P, Wiyakrutta S, Sriubolmas N,
Ngamrojanavanich N, Isarangkul D and Kittakoop P: 3-Nitropropionic
acid (3-NPA), a potent antimycobacterial agent from endophytic
fungi: Is 3-NPA in some plants produced by endophytes? J Nat Prod.
68:1103–1105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li XB, Xie F, Liu SS, Li Y, Zhou JC, Liu
YQ, Yuan HQ and Lou HX: Naphtho-γ-pyrones from Endophyte
Aspergillus niger occurring in the liverwort Heteroscyphus tener
(Steph.) Schiffn. Chem Biodivers. 10:1193–1201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Curtis RW, Stevenson WR and Tuite J:
Malformin in Aspergillus niger-infected onion bulbs (Allium cepa).
Appl Microbiol. 28:362–365. 1974.PubMed/NCBI
|
|
11
|
Kim KW, Sugawara F, Yoshida S, Murofushi
N, Takahashi N and Curtis RW: Structure of malformin B, a
phytotoxic metabolite produced by Aspergillus niger. Biosci
Biotechnol Biochem. 57:787–791. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim KW, Sugawara F, Yoshida S, Murofushi
N, Takahashi N and Curtis RW: Structure of Malformin A, a
phytotoxic metabolite produced by Aspergillus niger. Biosci
Biotechnol Biochem. 57:240–243. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma YM, Liang XA, Zhang HC and Liu R:
Cytotoxic and antibiotic cyclic pentapeptide from an endophytic
Aspergillus tamarii of Ficus carica. J Agric Food Chem.
64:3789–3793. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou X, Fang W, Tan S, Lin X, Xun T, Yang
B, Liu S and Liu Y: Aspernigrins with anti-HIV-1 activities from
the marine-derived fungus Aspergillus niger SCSIO Jcsw6F30. Bioorg
Med Chem Lett. 26:361–365. 2016. View Article : Google Scholar
|
|
15
|
Tan QW, Gao FL, Wang FR and Chen QJ:
Anti-TMV activity of malformin A1, a cyclic penta-peptide produced
by an endophytic fungus Aspergillus tubingensis FJBJ11. Int J Mol
Sci. 16:5750–5761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koizumi Y, Nagai K, Hasumi K, Kuba K and
Sugiyama T: Structure-activity relationship of cyclic pentapeptide
malformins as fibrinolysis enhancers. Bioorg Med Chem Lett.
26:5267–5271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koizumi Y, Fukudome H and Hasumi K:
Fibrinolytic activation promoted by the cyclopentapeptide
malformin: Involvement of cytoskeletal reorganization. Biol Pharm
Bull. 34:1426–1431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koizumi Y and Hasumi K: Enhancement of
fibrinolytic activity of U937 cells by malformin A1. J Antibiot
(Tokyo). 55:78–82. 2002. View Article : Google Scholar
|
|
19
|
Herbert JM, Savi P, Lalé A, Laplace MC,
Baudry N, Pereillo JM and Emonds-Alt X: Malformin-A1 inhibits the
binding of interleukin-1 beta (IL1 beta) and suppresses the
expression of tissue factor in human endothelial cells and
monocytes. Biochem Pharmacol. 48:1211–1217. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bannon PG, Dawes J and Dean RT: Malformin
A prevents IL-1 induced endothelial changes by inhibition of
protein synthesis. Thromb Haemost. 72:482–483. 1994.PubMed/NCBI
|
|
21
|
Kojima Y, Sunazuka T, Nagai K, Julfakyan
K, Fukuda T, Tomoda H and Omura S: Total synthesis of malformin C,
an inhibitor of bleomycin-induced G2 arrest. J Antibiot (Tokyo).
61:297–302. 2008. View Article : Google Scholar
|
|
22
|
Hagimori K, Fukuda T, Hasegawa Y, Omura S
and Tomoda H: Fungal malformins inhibit bleomycin-induced G2
checkpoint in Jurkat cells. Biol Pharm Bull. 30:1379–1383. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhan J, Gunaherath GM, Wijeratne EM and
Gunatilaka AA: Asperpyrone D and other metabolites of the
plant-associated fungal strain Aspergillus tubingensis.
Phytochemistry. 68:368–372. 2007. View Article : Google Scholar
|
|
24
|
Liu Y, Wang M, Wang D, Li X, Wang W, Lou H
and Yuan H: Malformin A1 promotes cell death through induction of
apoptosis, necrosis and autophagy in prostate cancer cells. Cancer
Chemother Pharmacol. 77:63–75. 2016. View Article : Google Scholar
|
|
25
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brábek J, Mierke CT, Rösel D, Veselý P and
Fabry B: The role of the tissue microenvironment in the regulation
of cancer cell motility and invasion. Cell Commun Signal. 8:222010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kiechle FL and Zhang X: Apoptosis:
Biochemical aspects and clinical implications. Clin Chim Acta.
326:27–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Haagenson KK and Wu GS: Mitogen activated
protein kinase phosphatases and cancer. Cancer Biol Ther.
9:337–340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pang H, Cai L, Yang Y, Chen X, Sui G and
Zhao C: Knockdown of osteopontin chemosensitizes MDA-MB-231 cells
to cyclophosphamide by enhancing apoptosis through activating p38
MAPK pathway. Cancer Biother Radiopharm. 26:165–173. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiu SJ, Chao JI, Lee YJ and Hsu TS:
Regulation of gamma-H2AX and securin contribute to apoptosis by
oxaliplatin via a p38 mitogen-activated protein kinase-dependent
pathway in human colorectal cancer cells. Toxicol Lett. 179:63–70.
2008. View Article : Google Scholar : PubMed/NCBI
|