Introduction
The cancer stem cell (CSC) model is an attractive
hypothesis that translates properties of normal stem cells into the
cancer field, and explains some of the most lethal features of
cancer. The CSC model proposes that the cells within the tumor are
hierarchically organized and predicts the existence of the
subpopulation of cells with high tumorigenicity that are able to
both self-renew and to generate differentiated cancer cells
(non-CSCs) (1,2). CSCs are thought also to be
responsible for relapses of disease, sometimes years after chemo-
or radiotherapy (3). Therefore,
elucidating the mechanisms of CSC maintenance is important for
understanding tumor cell persistence and relapses and may enable
specific targeting of CSCs as a potential therapeutic strategy to
stably eradicate cancer (4,5).
The most established pro-apoptotic activity of FasR
(CD95, Apo-1) is to mediate the apoptosis of either virus-infected
cells, useless/autoreactive T cells or cancer cells when engaged by
a CD8+ cytotoxic T lymphocytes (6). The fact that almost all known cancer
cells express FasR, and the observation that the most cancer cells
are resistant to apoptosis induction, suggests that stimulation of
FasR may not be an effective approach to eliminate cancer cells
comprising the main mass of tumor. In addition, stimulation of FasR
could never be used in targeted therapy because of major side
effects such as massive apoptosis induced in the liver (7).
FasR signaling has also been reported to have
non-apoptotic activities (8,9),
important, for example, for liver regeneration (10), migration of renal epithelial cells
(11) and development of neurons
(12,13). Functional evidence of a
pro-survival function of FasR/FasL signaling in normal stem cells
came from experiments which showed that the stimulation of FasR in
neuronal stem cells did not cause cell death but rather increased
cell survival. Additionally, deletion of FasR resulted in reduced
neurogenesis. Because cancer stem cells are supposed to originate
from normal stem cells, FasR was found to gain pro-survival
functions within tumors, improving growth, proliferation and
invasion due to induction of cancer-associated signaling pathways
such as: NF-κB and MAP-kinases (8,14,15).
The meaning of FasR/FasL signaling for CSC survival
and maintenance of their specific features was the goal of
experimental analysis conducted on various cancer cell lines,
including colorectal, breast, ovarian, glioblastoma and renal
cancer. The stimulation of FasR induced a conversion from non-CSCs
to CSCs what was suggested to depend on retro-differentiation
mechanism (16). Additionally, the
inhibition of FasR activity with recombinant trimerized FasL
(CD95L-T4) reduced cancer cell growth and metastasis after
xenotransplantation of pancreatic ductal adenocarcinoma collected
from 35 patients into a mouse model (17). The glioblastoma tumor cells
collected from patients show the correlation between FasR
expression and the presence of stem cell-like markers.
FasRhigh cells revealed high self-renewal in
vitro and high tumorigenic potential in vivo (18). CSCs were found to be almost
completely resistant to CD95-mediated apoptosis and stimulation of
FasR increased the number of CSCs and also prevented
differentiation of these cells, suggesting that FasR expression on
cancer cells maintains the CSC pool (6,16).
It has been shown that the expression of FasR/FasL
in colorectal cancer (CRC) is associated with worse prognosis,
metastasis and recurrence of disease (19–24).
It has been presented that the elimination of either FasR or FasL
cause the cancer cells to die (in vitro and in vivo)
through a process termed as DICE (death induced by CD95 or CD95L
elimination). DICE is a necrotic form of mitotic catastrophe
characterized by cell swelling, ROS production causing DNA damage
and activation of caspase-2 following mitochondrial outer membrane
permabilization (6,25). The inhibition of DICE seems to be
hardly possible because many different death pathways are induced.
These observations suggest that DICE is a naturally occurring
antitumor defense mechanism, which are able to eliminate cancer
cells devoid of proper pro-apoptotic FasR/FasL signaling. The
activity of FasR as a pro-survival factor seems to be mostly
relevant to cancer stem cells, since cancer cells rarely or even
never have mutated or deleted both alleles of FasR. Additionally,
such potential therapeutic strategy seems to be safe since it was
shown that none of the normal tissues during embryonic development
in FASR/FASL-knockout mice showed growth defects or signs of cell
death (6,25).
It is well accepted that spheroid cultures preserve
more faithfully the characteristics of original tumor, including
gene expression profiles, cellular heterogeneity, morphology and
distribution depending on the access to oxygen, nutrients and
growth factors, in comparison to adherent cultures (26). Spheroids mirror more reliably the
three dimensional cellular context and relevant pathophysiological
gradients of in vivo tumor, however, to which extent sphere
formation assays favor the enrichment of CSCs is not fully clear
yet. Spheres contain also differentiated/differentiating tumor
cells which after leaving the original sphere cannot survive in
in vitro environment and undergo anoikis related to
cessation of epithelial-like adhering properties.
Importantly, most of the studies demonstrating CSC
properties expanded in a spherical form were conducted on cancer
cell lines of different origin, for example breast, lung, ovarian
and colon (26–31). Most of our attempts to characterize
spheroid cultures derived from fresh surgical CRC specimens and to
compare them with commercially available CRC lines (HCT116 and
HT29) seem to be very advantageous. In our study we focused on the
analysis of the presence of both Fas ligand and its receptor on CRC
stem cells in two cell lines: HCT116 and HT29, which appeared to be
very useful for analyzing the resemblance of in vitro
settings to in vivo environment after we modified their
expansion model to SC-promoting. We wished to determine the
relationships between the expression of FASR/FASL and other
CSC features crucial/necessary for CRC stem cell success. Moreover,
we also examined the general expression of both proteins in CRC
samples to find the relationships between FASR/FASL mRNA
level and the disease progression level. We hoped to add to the
knowledge concerning the usefulness of cancer cell lines for
chemotherapeutics activity/effectiveness analysis for potential
clinical applications. FasR/FasL activity is still highly
controversial since it is suspected to be engaged in the regulation
of apoptosis, senescence and survival depending on the entire niche
environment (6,15,32).
Further studies are, however, needed to clarify the detailed
signaling relationships between proteins from FasR/FasL-induced
pathways.
Materials and methods
All human samples used in the scope of this study
were donated freely and written informed consent was obtained from
the donor for the use of its sample in particular research part.
Ethics approval was obtained from The Independent Bioethics
Commission for Research of the Medical University of Gdansk.
Isolation and expansion of CRC primary
cell lines
Freshly resected colon tumor tissue from 20 CRC
patients from Department of General, Endocrine and Transplant
Surgery, Medical University of Gdansk, Invasive Medicine Centre,
Gdansk, Poland was collected and immediately processed in culture.
All experimental chemicals were purchased from Sigma-Aldrich,
Poznan, Poland, except for growth factors, which were purchased
from R&D Systems, Biokom, Warszawa, Poland. Tissues were washed
several times in serum-free Dulbecco's modified Eagle's medium
(DMEM)-F12 supplemented with antibiotic-antimycotic agent. The
specimens were minced into 1–2-mm3 pieces followed by
incubation in collagenase (20 ng/ml) and hyaluronidase (20 ng/ml)
for 1.5 h at 37°C. Single cell suspension was obtained by mixing
every 15 min and by filtration through a 70-µm cell
strainer. Primary colon spheroid culture (SC) were maintained in
serum-free stem cell medium containing DMEM-F12 supplemented with
ITS Liquid Media Complement (1X), BSA (4 mg/ml), glucose (3 ml/ml),
HEPES (5 mM), L-glutamine (2 nM), progesterone (20 nm), putrescine
(9.6 µg/ml), heparin (4 µmg/ml), EGF (20 ng/ml), bFGF
(20 ng/ml), and antibiotic-antimycotic solution (1X). This medium
will further be referred to as stem cell medium (SCM). For the need
of this study, we used only early passaged SCs for analysis.
Expansion of HCT116 and HT29 cell lines
in adherent and spheroid cultures
The HT29 and HCT116 human colon adenocarcinoma cell
lines [obtained originally from the American Type Culture
Collection (ATCC), Manassas, VA, USA] were employed in this study.
The cells were cultured routinely as a monolayer in Dulbecco's
modified Eagle's medium (DMEM), supplemented with 10% fetal bovine
serum and 1% penicillin-streptomycin and 2 mM L-glutamine and
incubated at 37°C under a humidified atmosphere of 5%
CO2. The cells were serially subcultured by trypsin
treatment when they achieved 80% confluency and the medium was
renewed 2–3 times/week.
For the culture of spheroid forms of HCT116 and HT29
cell lines, they were grown in SCM characterized as indicated
above. These cultures were maintained under these conditions for
5–6 passages before being used for experiments.
Cell death assay (7AAD)
For cell death evaluation the 7AAD Via Probe (BD
Biosciences, USA) was used. After adding 10 µm of Via Probe
samples were incubated for 30 min, washed and resuspended in PBS
prior to cytometric analysis.
Flow cytometric analysis of cell
phenotype
CRC lines and cells separated from human tumor
fragments were stained with the following cocktail of monoclonal
antibodies purchased from BD Biosciences: anti-CD29-APC (clone
MAR4, IgG1κ), anti-CD44-FITC (clone C26, IgG2bκ), anti-CD95-PE
(clone DX2, C3H/Bi IgG1κ), anti-FasL Biotin (clone NOK-1, IgG1)
coupled with streptavidin-APC. Anti-CD133/2-PE (clone 293C3,
IgG2bκ) monoclonal antibodies were purchased from Miltenyi Biotec.
After 30-min incubation in the dark, samples were fixed with 1% PFA
on ice and prepared for further analysis. Flow cytometric analysis
was performed using FACSCalibur flow cytometer (BD
Biosciences).
Measurements of cytokine
concentrations
The level of soluble FasL was analyzed in
supernatants from cultures of CRC lines and CSCs isolated from CRC
patient tissues using BD Cytometric Bead Array Flex Set system kits
(BD Biosciences) according to the manufacturer's instructions.
Briefly, capture beads were transferred into all tubes and samples
were incubated for 1 h at RT. Afterwards, PE detection reagent was
added to all tubes, which were incubated for 2 h at RT. The
captured beads, detection reagent (reporter antibodies) and samples
were incubated together to form sandwich complexes. After double
washing the samples were resuspended in washing buffer and analyzed
in a flow cytometer. Fluorescence intensity was proportional to the
amount of a given cytokine in a vial and estimated according to the
standard curves acquired after analysis of standard dilutions. Data
from cytometric analysis were transformed into graphical and
tabular formats using FCAP Array Software. Finally, results were
presented as pg/ml.
Analysis of apoptosis
Levels of cell apoptosis were measured using an
Annexin V-FITC Apoptosis Detection Kit™ (BD Biosciences), according
to the manufacturer's instructions. Briefly, 5×105 cells
were suspended in a staining mixture comprised of 100 µl
binding buffer, 5 µl Annexin V-FITC and 5 µl
propidium iodide. After 15-min incubation in RT, in the dark,
samples were diluted in binding buffer and prepared for further
analysis. Flow cytometric analysis was performed within 30 min
using FACSCalibur flow cytometer (BD Biosciences).
Incubation of cells with anti-FasR
antibodies
Cells were seeded into the wells and stimulated with
the usage of anti-FasR (BD, IgM, clone EOS9.1) or concomitant
control antibodies (Thermo Fisher Scientific) in the concentration
of 200 ng/ml. The medium was replaced every 2–3 days to keep the
concentration of antibodies at equally high level. After 14 days of
the culture the cells were analysed.
Quantification of sphere sizes
Analysis of sphere sizes obtained from cells
cultured in sphere-forming media after 2 weeks of continuous
treatment with either anti-FasR monoclonal antibodies or IgM
control antibodies with the using of inverted microscope
Olympus-CKX53 coupled with digital camera Olympus SC50. At least 50
spheres of each experimental option were measured.
Material used for real-time PCR
The specimens were obtained from Department of
General, Endocrine and Transplant Surgery, Medical University of
Gdansk, Invasive Medicine Centre, Gdansk, Poland and Department of
Hepatology and Gastroenterology, Faculty of Medicine, Medical
University of Gdansk, Poland from 2012 to 2014. Clinical data were
collected at the time of enrollment (Table II). The study included 65 patients
with CRC; 32 males and 33 females (mean age ± SD 68.1±11.8; range,
31–91 years). The exclusion criteria were: development of a second
neoplastic disease, previous chemo- and/or radiotherapy, tumors
located in anal canal and anus. The control group comprised 20
healthy subjects, 7 males and 13 females (mean age 57±14.2; range,
21–76 years) who underwent colonoscopy as a part of a routine
screening for CRC. None of the sample donors suffered from
inflammatory bowel disease, had a family history of CRC or were
taking medications.
 | Table IIClinicopathological characteristics
of CRC patients included into the real-time PCR analysis of
FASL and FASR mRNA levels. |
Table II
Clinicopathological characteristics
of CRC patients included into the real-time PCR analysis of
FASL and FASR mRNA levels.
CRC patients,
n=65
Control, n=20 | Subgroups | FASL
n (% of all CRC patients)
| FASR
n (% of all CRC patients)
|
|---|
| Low (≤0.985) | High
(>0.985) | P-va1ueb | Low (≤2.99) | High
(>2.99) | P-va1ueb |
|---|
| Age (years) | ≤68 n=26 | 5 (8) | 21 (32) | 0.39 | 15 (23) | 11 (17) | 0.40 |
| 68.1±11.06 (Mean ±
SD) | >68 n=39 | 10 (15) | 29 (45) | | 20 (31) | 19 (29) | |
| Range, 31–91 | | | | | | | |
| Sex | Female n=33 | 8 (12) | 25 (38) | 1.00 | 18 (18) | 15 (23) | 1.00 |
| Male n=32 | 7 (11) | 25 (38) | | 17 (26) | 15 (23) | |
| Tumor size
(cm) | ≤5 cm n=39 | 12 (18) | 27 (42) | 0.08 | 23 (35) | 16 (25) | 0.32 |
| >5 cm n=26 | 3 (5) | 23 (35) | | 12 (18) | 14 (22) | |
| Histological
differentiation G stage | G2 n=50 | 13 (21) | 37 (61) | 0.43 | 28 (46) | 22 (36) | 0.73 |
| G3 n=11 | 1 (2) | 10 (16) | | 5 (8) | 6 (10) | |
| TNM stages | | | | | | | |
|
Non-metastatic | TNM I±II n=34 | 6 (9) | 28 (43) | 0.38 | 17 (26) | 17 (26) | 0.62 |
| Metastatic | TNM III±IV
n=31 | 9 (14) | 22 (34) | | 18 (28) | 13 (20) | |
All steps regarding sample collection and proceeding
were previously described (33).
Briefly, CRC samples were obtained during surgical hemicolectomy
(5×5×5 mm), whereas control group specimens were collected during
colonoscopy (2×2×2 mm). Collected material was divided for
histopathologic examination and molecular studies and tissues were
processed within 20 min. After tumor resection. In control
patients, one biopsy (2×2×2 mm) was fixed in 10% buffered neutral
formalin for routine histological examination, whereas two
specimens from the nearest location were collected for nucleic acid
analyzes. Both tumor samples and mucosal biopsies were immediately
placed in sterile vials containing RNAlater (Ambion-Life
Technologies, Grand Island, NY, USA), left for 6 h at 4°C and then
stored at −25°C until further analyses.
Nucleic acids extraction and reverse
transcription
Total RNA was extracted from part of tumor samples
(3×5×5 mm) and whole-sized mucosal biopsies of control patients
using Total RNA kit (A&A Biotechnology, Gdynia, Poland)
according to the manufacturer's protocol. Isolated RNA was
quantified by spectrophotometry (Nanodrop ND 1000; Thermo Fisher
Scientific, Fitchburg, WI, USA). DNA was digested with RNase-free
DNase I (Fermentas; Thermo Fischer Scientific, Fitchburg, WI, USA)
for 30 min at 37°C; afterwards DNase was inactivated by addition of
EDTA and incubation at 65°C for 10 min. Before storing at −85°C,
RNA integrity was analyzed by agarose gel electrophoresis. Total
RNA (2 µg) were reverse-transcribed using 0.5 µg
oligo(dT)18 primers (Sigma-Aldrich, Munich, Germany) and 200 U of
RevertAid M-MuLV Reverse Transcriptase (Fermentas; Thermo Fischer
Scientific) in a total volume of 20 µl and resulting cDNA
was stored at −25°C.
Quantitative PCR assay for FASL and FASR
mRNA level
Quantification of FASL and FASR gene expression was
carried out using StepOne Plus (Applied Biosystems, CA, USA) with
SYBR® Green I as a fluorophore. FASL and FASR expression
rates were determined by the comparative method 2−ΔΔCT
(34) in relation to the geometric
mean of the expression level of PGK1 gene (normalization study
results have not been published yet). QPCR conditions were
validated, showing 90–100% efficiency for all assays. The
amplification primer pairs were: 5′-GTTGACCGAATCACCGACCTCTC and
5′-AGAACAGAACATCCTTGCCCAGC for PGK1, 5′-TTCCACCTACAGAAGGAGCTGGC and
5′-AGGTGTCTTCCCATTCCAGAGGC for FASL, 5′-GTGAACACTGTGACCCTTGCACC and
5′-CCTCTTTGCACTTGGTGTTGCTG for FASR, respectively.
The reaction mixture (15 µl) included 0.15
µl of four times diluted reverse transcription product
(cDNA), 0.2 µm forward and reverse primers each, and
SensiFast NoRox SYBR Green (with EvaGreen fluorophore) (BioLine,
London, UK). All reactions were performed in duplicate. For the
studied genes the amplification profile was: 300-sec denaturation
at 95°C, followed by 38 cycles of 5-sec denaturation at 95°C, 10
sec annealing at 58°C for 10 sec, elongation at 72°C for 15 sec and
5 sec fluorescence reading at 77–80°C. Dynamic melt curve analysis
was applied to all reactions. Data were automatically collected and
analyzed by StepOne Plus software ver. 2.2 (Applied
Biosystems).
Statistical analysis
All data obtained during the study were analyzed
with the use of GraphPad Prism ver. 6.05 (GraphPad Software, San
Diego, CA, USA) and the software Statistica 12 (Statsoft, Poland)
according to some non-parametric tests: U Mann-Whitney,
Kruskal-Wallis ANOVA, Fisher's 2×2 exact test, Spearman's
correlation. Values of p<0.05 were considered as statistically
significant. The results are presented as average value ± SD.
Results
Cytometric analysis of CRC cells
We evaluated the CSC enrichment of CRC lines
cultured under two distinct modes: spherical and adherent with the
use of cytometric analysis of commonly used stem cell surface
markers. We could observe that the adherent HCT116 and HT29 lines
contained 100% of CD133+ cells, whereas the conversion
of culture conditions from adhering into sphere-forming was
accompanied by the decrease of the CD133+ cell
proportion to 70±11 and 41±4% for mentioned CRC lines,
respectively. Our analysis revealed that spherical HT29 cells
presented higher content of CD133− cells in comparison
to HCT116 line. Generally, adherent cells were more enriched in
CD133+CD44+CD29+ CSCs, whereas
their spherical counterparts presented major heterogeneity, so
HCT116 contained more
CD133+CD44+CD29+ (61±8%) and
HT29− more
CD133−CD44-CD29+ (41%±8) in
general populations (Fig. 1).
Additionally, adherent CRC lines (HCT116 and HT29) presented higher
proportion of cells carrying FasR and FasL on their surface in
comparison to their spherical counterparts (Fig. 2).
Because of high diversity of patient-derived
spherical cultures, which appeared to contain varying proportion of
CD133+ cells (ranging from 9 to 98%), we divided our
samples according to CD133 average number (average value was 64%)
into 2 groups: with high (85±10%) and low (28±1.4%)
CD133+ cells proportions. We found these groups
significantly different (Fig. 1B).
Further analysis indicated the strong correlation between the time
of their survival in vitro (R=0.9) and the number of
CD133+ cells. The cells expanded in vitro for at
least 5 passages revealed to be highly enriched in
CD133+ cells in contrast to short-lasting expansions
(p<0.001, Kruskal-Wallis ANOVA) which we could maintain only for
2–4 days in vitro.
It has been shown that FasL can be found in two
different forms: a membrane-bound (mFasL) and a soluble form
(sFasL) that is generated through cleavage of mFasL by
metalloproteinases (35). To test
that issue in our experimental settings, we evaluated the presence
of sFasL in tumor conditioned media from tumor cells expanded in
vitro of both CRC lines and patient-derived samples.
Surprisingly, we found that none of the analyzed cell populations
released soluble FasL, whereas control leukocytes released up to
120 pg/ml of sFasL, representing unambiguous proof of
methodological effectiveness.
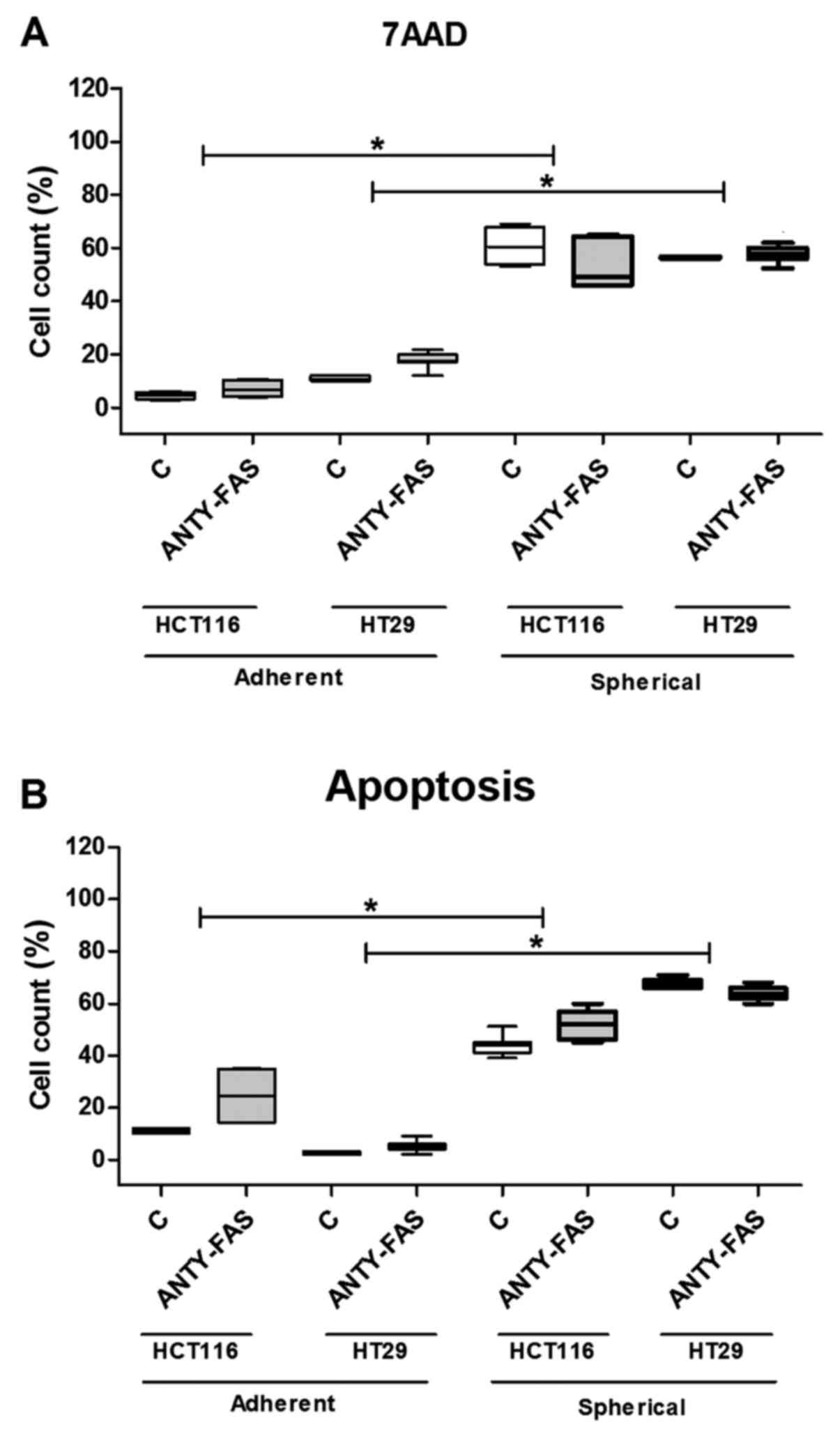
The cytometric analysis of cell death
and/or apoptosis
Because the Fas signaling in its canonical form is
pro-apoptotic, we decided to correlate the dying/apoptosis rate
with all previously presented parameters. The proportion of
dying/apoptotic cells was assessed with the use of flow cytometric
methodology utilizing different dyes: 7AAD and Annexin V-FITC+PI
(Fig. 3). The cell death was
quantified during each passage to test if it changed along the
expansion time. Our analyses confirmed that HCT116 and HT29 cells
expanded in adhering forms had much lower tendency for apoptosis as
we found up to 5% of 7AAD+ cells. The increase of both
Anexin+/PI+ and 7AAD+ cell
proportion during sphere-forming culture was ranging from 60 to
76%. Although, a substantial number of cells died, the overall cell
number maintained at the same level or even slightly increased as
we were measuring at each passage. Surprisingly, when we compared
the CSC-enriched cultures derived from patient samples, we found
that these cells were resistant to apoptosis thus the proportion of
dying/apoptotic cells was very low and was ranging from 2 to 5%
(Fig. 3). The number of
patient-derived cells undergoing apoptosis was statistically
significantly lower in comparison to HCT116 and HT-29-derived
spherical cultures (p<0.001, Kruskal-Wallis ANOVA). We found
some significant correlations between the proportion of cancer
cells CD29+ and the proportion of dying cells during
expansion in all experimental sets, the highest correlation was
observed for SC (R=0.8) (Table I).
At the same time, the presence of FasR significantly negatively
correlated with CD29+ cancer cells (R=−0.8) (Table I).
 | Table ICorrelations between the proportion
of cells carrying different surface markers measured during each
passage of spherical cells of both CRC lines (HCT116, HT29, n=14
for each line) and cells from CRC patients (n=11) (Spearman's rank
correlation coefficients, p<0.05). |
Table I
Correlations between the proportion
of cells carrying different surface markers measured during each
passage of spherical cells of both CRC lines (HCT116, HT29, n=14
for each line) and cells from CRC patients (n=11) (Spearman's rank
correlation coefficients, p<0.05).
| Correlated markers
p<0.05 | R-values | p-values |
|---|
| FasR |
| CD133 | 0.8 | 0.001 |
| CD44 | 0.7 | 0.001 |
| CD29 | −0.8 | 0.0191 |
| FasL |
| CD133 | 0.3 | 0.0351 |
| CD44 | 0.46 | 0.0020 |
| CD29 | 0.6 | 0.001 |
| 7AAD |
| CD133 | −0.6 | 0.001 |
| CD44 | 0.0 | 0.87 |
| CD29 | 0.9 | 0.001 |
| FasR | −0.7 | 0.001 |
| FasL | 0.3 | 0.0280 |
The analysis of cells after incubation
with anti-FasR antibodies
After we exposed cancer lines to anti-FasR agonistic
antibodies which were supposed to activate canonical apoptotic
signaling, we expected this treatment would significantly eradicate
FasR+ cells in particular culture type. However, along
the incubation time of adherent cells, according to the analysis
conducted at each passage, we found that anti-FasR stimulation did
not induce any significant differences concerning phenotype except
the higher proportion of
CD133+CD44−CD29+ cells in
comparison to control cells (Fig.
4B). The analysis of apoptosis rate did not show either any
significant influence of such incubation (Fig. 5). Despite the luck of major
phenotypic differences the anti-Fas stimulation seemed to inhibit
the proliferation of both CRC cell lines in the adherent form
(Fig. 6A).
When we examined the features of SCs we observed
that HCT116 and HT29 cells behaved in distinct, line-specific way.
Although we found that such prolonged FasR stimulation resulted in
expansion of CD133+ cells during SCs, the only
difference for HCT116 was statistically significant. Moreover, the
detailed phenotypic analysis presented altered number of cells
bearing other CSC-like markers nevertheless the change had opposite
direction for the cell lines (Fig. 4B
and C). HT29 cells answered more effectively to our stimulation
since the proportion of specific cell populations significantly
changed. Additionally, anti-FasR treatment seemed to preferentially
influence CD44+ cells since the lowered count of these
cells among both CD133+ and CD133− was
observed. Using flow cytometry to analyze dying cells we confirmed
that the anti-FasR antibodies could not influence the apoptosis
susceptibility of cells during SCs. At the same time such
stimulation increased spherogenicity of CRC cells as the
significantly increased spheres were found in comparison to control
culture (Fig. 6B).
The expression of the FASL and FASR genes
at the mRNA level
As shown in Fig.
7A, the FASL mRNA expression ratio was approximately 80
times higher in tumor CRC samples in comparison to level in 20
biopsies taken from control group of non-CRC patients. Although we
observed a tendency for elevated mRNA ratio of FASR gene in tumor
samples, the difference was not statistically significant (Fig. 7B). When mRNA median ratios of
either FASL or FASR in control groups were taken into
consideration as the threshold, we found that 50 of 65 tumor CRC
samples shown increased FASL mRNA content; for FASR
such difference was not statistically significant (in 30 samples we
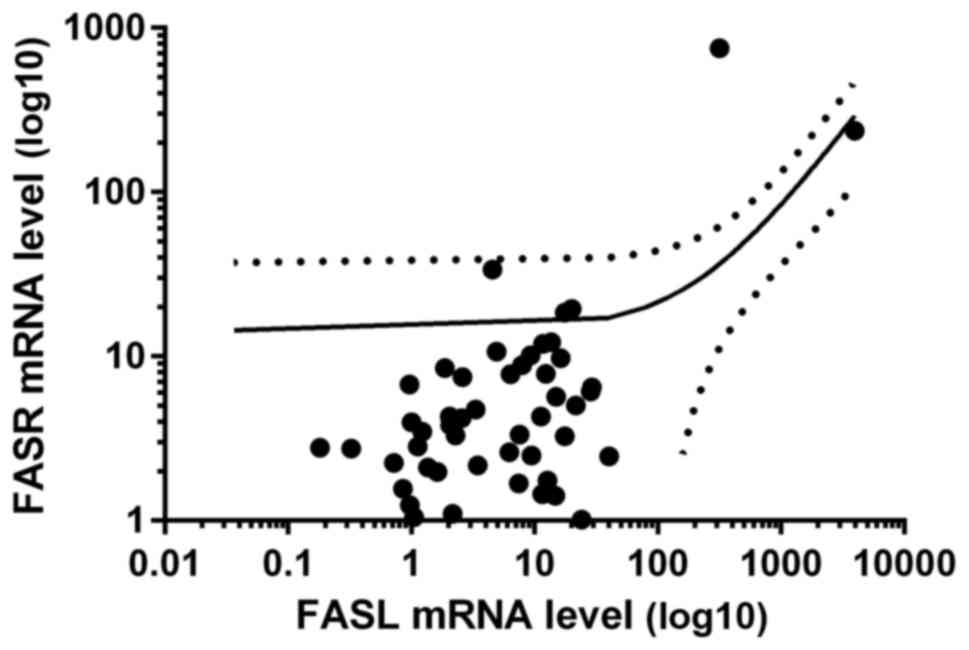
found increased and in 35 decreased FASR mRNA ratio, Table II). Interestingly, we observed
positive correlation between mRNA levels of FASL and
FASR in tumor samples of CRC patients (R = 0.54, p<0.05,
Fig. 8).
Furthermore, regarding the clinicopathological data,
we found that patients with advanced CRC (TNM stages III and IV)
were characterized by ~10-fold increased FASL mRNA content
in comparison to patients with early CRC (TNM stages I and I,
Fig. 1C). We found no
statistically significant differences between the expression of
FASR in biopsies from CRC in I and II TNM stages in
comparison to samples collected from CRC patients in III and IV
stages. When we compared the data to the FASR expression in
healthy tissue we also found no significant difference.
Discussion
Colorectal cancer is one of the most common solid
organ cancers prevalent worldwide causing, in spite of advances in
therapeutic methodology, high rate of patient mortality, especially
due to metastasis development. The cancer stem cell theory of tumor
growth indicates that CSCs within the tumor mass have great
capacity to initiate and sustain tumor growth. According to current
state of knowledge, CSCs are said to be responsible for metastasis,
recurrence, relapse and resistance to conventional chemotherapy.
Taking all these pessimistic facts the CSC biology decipherment
seems to be necessary to find some new efficient therapeutic
strategies (36).
The crucial role of FasR/FasL signaling for the
regulating of cancer cell death/fate has been revealed recently
since it was associated with not only pro-apoptotic pathways but
also with some death-independent activities. Despite the canonical
apoptotic pathway is well described, the signaling of opposite
activities is less understood. Especially DICE (death induced by
CD95 or CD95L elimination) deserves special attention, as it seems
to be a unique protecting mechanism of immune system against cancer
cells devoid of FasR (6).
Conversion of non-CSCs into CSCs resulted in a loss of sensitivity
to Fas-mediated apoptosis and a concomitant increase in the
sensitivity of cells to DICE (16). In fact, DICE was found to
preferentially target CSCs demonstrating that HCT116 cells with the
use of tetracycline analog (Dox) knocked down FasR and decreased
the spherogenicity (16). Similar
results were achieved with the human breast cancer MCF7 and T4F7,
ovarian cancer HeyA8 and mouse colon cancer CT26L cell lines
(16,25). When DICE was induced in the
mentioned cancer cell lines, the number of CSCs and effectiveness
of sphere formation were lowered and finally CSCs became depleted
from experimental populations (37).
In this study we demonstrated that the level of FasR
was significantly reduced in cells cultured under sphere-forming
conditions in comparison to cells expanded in adherent form of both
examined cell lines. This observation suggested that the decreasing
of FasR level (96 to 16% for HCT116 and 92 to 1% for HT29)
(Fig. 1) must have the association
with the culture mode and higher diversity of cells since the
strong (R=0.8) correlation between CD133 and FasR was found.
Moreover, the lower level of FasR in SCs seemed to confirm the
higher resemblance of 3D culture system to in vivo
environment of tumor development. CSC maintenance depends on the
presence of FasR on their surface at a minimal level sufficient to
promote their optimal growth (6,9,16,25),
although, the lower FasR proportion can be interpreted in the
context of adaptation of cancer cells to co-existence with
immunological cells to reduce the risk of undergoing apoptosis
while benefiting from tumorigenic activities.
We demonstrated that spherical forms of CRC lines
diminished the level of FasL to similar level as CSCs isolated from
tumor fragments of CRC patients (Fig.
1), although only for HT29 cells that change were statistically
significant. FasL was described as a positive prognostic marker for
colorectal carcinoma (19,20,22,38)
and the vast majority of reports show that disease progression is
associated with progressively increasing expression of FasL and/or
FasR what seems to be in agreement with some of our results. Taking
into consideration that HCT116 cells originate from a poorly
differentiated (high-grade, more aggressive) colon cancer, whereas
HT29 cell line is derived from well-differentiated (low-grade, less
aggressive) CRC, the higher proportion of FasR+ HCT116
cells in comparison to HT29 cells (15±9 versus 1±0.5, respectively)
in their spherical forms seemed to confirm such attitude, whereas
the analogous difference was not found for FasL (87±6 versus 94±5,
respectively). The analysis of FASR/FASL expression profiles
showed their compatibility with our in vitro experimental
data. The increased expression of both genes was found in tumor
tissue in comparison to healthy control samples, however, only for
FASL the difference reached statistically significant level.
Additionally, depending on the progression status of CRC the
elevated level of FASR/FASL expression was shown (Fig. 2). The samples collected from
patients representing higher-grade tumor (TNM III and IV) indicated
higher expression of both genes, but only for FASL this
difference was statistically significant, however, FASR
revealed substantial tendency.
The cytometric analysis of phenotype indicated some
interesting relationships between measured protein markers on the
surface of analyzed cell populations. Decreasing of the FasR and/or
FasL was suggested to be associated with the elevating number of
7AAD-positively stained cells and decreasing of
CD133+CD29+CD44+ CSC proportion.
The only exception were CD29+ cells
(CD29+CD133−CD44−) which
proportion increased after the culture conditions were modified and
that was accompanied by markedly increased apoptosis rate. We
concluded that CD133 marker is indirectly associated with
maintenance and higher proliferative properties of cells, and CD29−
with higher differentiation and/or dying as we found the
significant phenotypic correlations (Fig. 1 and table I). Moreover, the level of CD133 and
CD44 (Table I) strongly correlated
with the presence of FasR on the SCs cell surface (R=0.7 and R=0.8,
respectively), whereas we could not find such correlations for
adherent cells.
The phenotypic changes observed in our experimental
settings suggested that the death type possessed some features of
DICE because DICE was earlier demonstrated to preferentially target
CSCs (6,16). We found significant decline of
CD44+ cells when expanded in spherical form in
comparison to their adherent counterparts (Fig. 1) and lowered proportion of
CD44+ cells after blockade of the FasR by agonistic
antibodies (Fig. 4). As previously
shown (16), DICE is able to
eliminate CD44+ CSCs of breast cancer MCF-7 and T47D
cells. We could observe during cytometric analysis that cells of
the highest values of FSC and SSC parameters represented
simultaneously cells with higher proportion of apoptosis (60 versus
85% for FSClowSSClow cells and
FSChighSSChigh, respectively, data not shown)
this seemed to confirm the presence of swelling cells
characteristic for DICE (6,25).
Significant correlations were found between FasR and some CSC-like
markers, which seem to indicate the cancer progression promoting
role of FasR/FasL signaling, however the correlations for FasL were
not so substantial (Table I).
We further investigated the function of Fas
signaling in our CRC lines depending on the culture mode as we
incubated HCT116 and HT29 cells with anti-FasR agonistic
antibodies. We found that adherent cells only slightly answered to
that stimulation (Figs. 4 and
5), except the markedly decreased
proliferation rate (Fig. 6A). That
seems to proof the engagement of FasR in the senescence induction
accompanied by the cell cycle arrest as was suggested earlier by
Raats et al (32).
Surprisingly, the Raats group (32) correlated the presence of wild KRAS
gene with FasR inhibitory effects. Although HCT116 cells are known
to have mutated form of KRAS (according to ATCC specification)
(39), they answered efficiently
to anti-FasR antibodies during expansion. Thus the relationships
between Fas signaling cytoplasmic effectors must be much more
complex and require more efforts. The lower number of cells could
not be linked to increased apoptosis as the cytometric analysis did
not show any significant differences in comparison to control
cells, anyway some minor tendencies could be seen (Fig. 5). The assessment of SCs revealed
that the effects are cancer line-dependent and additionally the
prolonged incubation with anti-FasR antibodies could induce
different effects in comparison to these observed for adherent
cells. We found that such FasR stimulation resulted in increased
expansion of CD133+ cells during SC indicating specific
clonal selection of cells sensitive to Fas-mediated supporting
stimulation. During SC with anti-Fas antibodies no substantial
apoptosis rate increase was observed, similarly to data obtained
during adherent cultures. Our results are in line with findings of
other groups suggesting that the Fas pathway have rather
pro-survival activity inducing growth, invasion and metastasis of
cancer cells, especially CSCs (16,25,32,40).
The increased sphere sizes following 2-week incubation with
anti-FasR antibodies agreed with mentioned tumor-promoting activity
of Fas signaling (Fig. 6B) because
that feature might be directly associated with increased
spherogenicity and, by extension-aggressiveness of tumor cells.
Recently it has been demonstrated that expression of
FasL by apoptosis-resistant tumor cells enables a powerful
'counterattack' against antitumor immune effectors which are
themselves sensitive to Fas-mediated apoptosis (41,42).
However, while there is some evidence for the occurrence of this
counterattack, its existence remains controversial (6,41,43).
The reported increased concentration of soluble FasL (sFasL) in the
serum of many cancer patients was often interpreted in the context
of the FasL counterattack theory and would suggest a possible
immunosuppressive role of this molecule. However, the generalized
immune suppression that would be expected from this situation could
not be confirmed in cancer patients; thus, perhaps the increased
FasL expression in tumor tissues has a more direct tumor promoting
role (6,41,43).
The negative results concerning sFasL in media from over the
expanded CRC cells from our experiments were surprising in this
context; the more so because that was shown to be a prognostic
factor for different types of cancers (44,45).
The levels presented in the mentioned manuscripts (from 0.16–0.17
to 21 ng/ml) were much higher that the sensitivity level of our
methodology which was 2.6 pg/ml therefore this issue needs deeper
analysis.
In conclusion, FasR/FasL signaling as the target of
CRC therapy is not utilized nowadays because of its toxic side
effects especially observed in liver. Moreover, since DICE was
suggested being a naturally-occurring antitumor defense mechanism,
which enable elimination of CSCs devoid of FasR/FasL (6), it is reasonable to broaden our
knowledge concerning Fas-signaling and its cytoplasmic effectors.
Our study hopefully enriched the knowledge concerning this issue.
The next advantage of our study seems to be the comparison of
different modes of expansion of cancer cell lines and contrast
these data with information obtained from analysis of cancer cells
separated from CRC patients. Our observation seems to confirm that
spherical model of cancer lines is more reliable for some
sophisticated analysis because of their greater resemblance to the
CSCs from human CRC samples in comparison to commonly used adherent
cells, at least according to aspects of their biology analyzed in
this study, and can be extended to the resemblance of in
vitro sphere forming conditions to the in vivo
environment. However, the greatest difference concerns the level of
apoptosis thus this issue require further experimental
analysis.
Acknowledgments
This study was supported by Polish Ministry of
Science grants nos. N N402 684040 and N N402 683940.
References
|
1
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Charafe-Jauffret E, Ginestier C and
Birnbaum D: Breast cancer stem cells: Tools and models to rely on.
BMC Cancer. 9:2022009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Creighton CJ, Li X, Landis M, Dixon JM,
Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A,
Herschkowitz JI, et al: Residual breast cancers after conventional
therapy display mesenchymal as well as tumor-initiating features.
Proc Natl Acad Sci USA. 106:13820–13825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta PB, Chaffer CL and Weinberg RA:
Cancer stem cells: Mirage or reality? Nat Med. 15:1010–1012. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frank NY, Schatton T and Frank MH: The
therapeutic promise of the cancer stem cell concept. J Clin Invest.
120:41–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peter ME, Hadji A, Murmann AE, Brockway S,
Putzbach W, Pattanayak A and Ceppi P: The role of CD95 and CD95
ligand in cancer. Cell Death Differ. 22:885–886. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogasawara J, Watanabe-Fukunaga R, Adachi
M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T and Nagata S:
Lethal effect of the anti-Fas antibody in mice. Nature.
364:806–809. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martin-Villalba A, Llorens-Bobadilla E and
Wollny D: CD95 in cancer: Tool or target? Trends Mol Med.
19:329–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L, Park SM, Tumanov AV, Hau A, Sawada
K, Feig C, Turner JR, Fu YX, Romero IL, Lengyel E, et al: CD95
promotes tumour growth. Nature. 465:492–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Desbarats J and Newell MK: Fas engagement
accelerates liver regeneration after partial hepatectomy. Nat Med.
6:920–923. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jarad G, Wang B, Khan S, DeVore J, Miao H,
Wu K, Nishimura SL, Wible BA, Konieczkowski M, Sedor JR, et al: Fas
activation induces renal tubular epithelial cell beta 8 integrin
expression and function in the absence of apoptosis. J Biol Chem.
277:47826–47833. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Desbarats J, Birge RB, Mimouni-Rongy M,
Weinstein DE, Palerme JS and Newell MK: Fas engagement induces
neurite growth through ERK activation and p35 upregulation. Nat
Cell Biol. 5:118–125. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zuliani C, Kleber S, Klussmann S, Wenger
T, Kenzelmann M, Schreglmann N, Martinez A, del Rio JA, Soriano E,
Vodrazka P, et al: Control of neuronal branching by the death
receptor CD95 (Fas/Apo-1). Cell Death Differ. 13:31–40. 2006.
View Article : Google Scholar
|
|
14
|
Corsini NS, Sancho-Martinez I, Laudenklos
S, Glagow D, Kumar S, Letellier E, Koch P, Teodorczyk M, Kleber S,
Klussmann S, et al: The death receptor CD95 activates adult neural
stem cells for working memory formation and brain repair. Cell Stem
Cell. 5:178–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siegmund D, Lang I and Wajant H: Cell
death-independent activities of the death receptors CD95, TRAILR1,
and TRAILR2. FEBS J. 284:1131–1159. 2017. View Article : Google Scholar
|
|
16
|
Ceppi P, Hadji A, Kohlhapp FJ, Pattanayak
A, Hau A, Liu X, Liu H, Murmann AE and Peter ME: CD95 and CD95L
promote and protect cancer stem cells. Nat Commun. 5:52382014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teodorczyk M, Kleber S, Wollny D, Sefrin
JP, Aykut B, Mateos A, Herhaus P, Sancho-Martinez I, Hill O,
Gieffers C, et al: CD95 promotes metastatic spread via Sck in
pancreatic ductal adenocarcinoma. Cell Death Differ. 22:1192–1202.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Drachsler M, Kleber S, Mateos A, Volk K,
Mohr N, Chen S, Cirovic B, Tüttenberg J, Gieffers C, Sykora J, et
al: CD95 maintains stem cell-like and non-classical EMT programs in
primary human glioblastoma cells. Cell Death Dis. 7:e22092016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Fan X, Stoicov C, Liu JH, Zubair S,
Tsai E, Ste Marie R, Wang TC, Lyle S, Kurt-Jones E, et al: Human
and mouse colon cancer utilizes CD95 signaling for local growth and
metastatic spread to liver. Gastroenterology. 137:934–944.
944.e931–934. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoogwater FJ, Nijkamp MW, Smakman N,
Steller EJ, Emmink BL, Westendorp BF, Raats DA, Sprick MR, Schaefer
U, Van Houdt WJ, et al: Oncogenic K-Ras turns death receptors into
metastasis-promoting receptors in human and mouse colorectal cancer
cells. Gastroenterology. 138:2357–2367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kykalos S, Mathaiou S, Karayiannakis AJ,
Patsouras D, Lambropoulou M and Simopoulos C: Tissue expression of
the proteins fas and fas ligand in colorectal cancer and liver
metastases. J Gastrointest Cancer. 43:224–228. 2012. View Article : Google Scholar
|
|
22
|
Nijkamp MW, Hoogwater FJ, Steller EJ,
Westendorp BF, van der Meulen TA, Leenders MW, Borel Rinkes IH and
Kranenburg O: CD95 is a key mediator of invasion and accelerated
outgrowth of mouse colorectal liver metastases following
radiofrequency ablation. J Hepatol. 53:1069–1077. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sträter J, Hinz U, Hasel C, Bhanot U,
Mechtersheimer G, Lehnert T and Möller P: Impaired CD95 expression
predisposes for recurrence in curatively resected colon carcinoma:
Clinical evidence for immunoselection and CD95L mediated control of
minimal residual disease. Gut. 54:661–665. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang W, Ding EX, Wang Q, Zhu DQ, He J, Li
YL and Wang YH: Fas ligand expression in colon cancer: A possible
mechanism of tumor immune privilege. World J Gastroenterol.
11:3632–3635. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hadji A, Ceppi P, Murmann AE, Brockway S,
Pattanayak A, Bhinder B, Hau A, De Chant S, Parimi V, Kolesza P, et
al: Death induced by CD95 or CD95 ligand elimination. Cell Rep.
7:208–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weiswald LB, Bellet D and Dangles-Marie V:
Spherical cancer models in tumor biology. Neoplasia. 17:1–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hirschhaeuser F, Menne H, Dittfeld C, West
J, Mueller-Klieser W and Kunz-Schughart LA: Multicellular tumor
spheroids: An underestimated tool is catching up again. J
Biotechnol. 148:3–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chandrasekaran S, Marshall JR, Messing JA,
Hsu JW and King MR: TRAIL-mediated apoptosis in breast cancer cells
cultured as 3D spheroids. PLoS One. 9:e1114872014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Endo H, Okami J, Okuyama H, Kumagai T,
Uchida J, Kondo J, Takehara T, Nishizawa Y, Imamura F, Higashiyama
M, et al: Spheroid culture of primary lung cancer cells with
neuregulin 1/HER3 pathway activation. J Thorac Oncol. 8:131–139.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tong JG, Valdes YR, Barrett JW, Bell JC,
Stojdl D, McFadden G, McCart JA, DiMattia GE and Shepherd TG:
Evidence for differential viral oncolytic efficacy in an in vitro
model of epithelial ovarian cancer metastasis. Mol Ther Oncolytics.
2:150132015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qureshi-Baig K, Ullmann P, Rodriguez F,
Frasquilho S, Nazarov PV, Haan S and Letellier E: What do we learn
from spheroid culture systems? Insights from tumorspheres derived
from primary colon cancer tissue. PLoS One. 11:e01460522016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Raats DA, Frenkel N, van Schelven SJ,
Rinkes IH, Laoukili J and Kranenburg O: CD95 ligand induces
senescence in mismatch repair-deficient human colon cancer via
chronic caspase-mediated induction of DNA damage. Cell Death Dis.
8:e26692017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wierzbicki PM, Adrych K, Kartanowicz D,
Stanislawowski M, Kowalczyk A, Godlewski J, Skwierz-Bogdanska I,
Celinski K, Gach T, Kulig J, et al: Underexpression of LATS1 TSG in
colorectal cancer is associated with promoter hypermethylation.
World J Gastroenterol. 19:4363–4373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
O'Reilly LA1, Tai L, Lee L, Kruse EA,
Grabow S, Fairlie WD, Haynes NM, Tarlinton DM, Zhang JG, Belz GT,
et al: Membrane-bound Fas ligand only is essential for Fas-induced
apoptosis. Nature. 461:659–663. 2009. View Article : Google Scholar
|
|
36
|
Todaro M, Francipane MG, Medema JP and
Stassi G: Colon cancer stem cells: Promise of targeted therapy.
Gastroenterology. 138:2151–2162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schickel R, Park SM, Murmann AE and Peter
ME: miR-200c regulates induction of apoptosis through CD95 by
targeting FAP-1. Mol Cell. 38:908–915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Steller EJ, Ritsma L, Raats DA, Hoogwater
FJ, Emmink BL, Govaert KM, Laoukili J, Rinkes IH, van Rheenen J and
Kranenburg O: The death receptor CD95 activates the cofilin pathway
to stimulate tumour cell invasion. EMBO Rep. 12:931–937. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hong S, Kim S, Kim HY, Kang M, Jang HH and
Lee WS: Targeting the PI3K signaling pathway in KRAS mutant colon
cancer. Cancer Med. 5:248–255. 2016. View Article : Google Scholar :
|
|
40
|
Élez E, Kocáková I, Höhler T, Martens UM,
Bokemeyer C, Van Cutsem E, Melichar B, Smakal M, Csőszi T, Topuzov
E, et al: Abituzumab combined with cetuximab plus irinotecan versus
cetuximab plus irinotecan alone for patients with KRAS wild-type
metastatic colorectal cancer: The randomised phase I/II POSEIDON
trial. Ann Oncol. 26:132–140. 2015. View Article : Google Scholar
|
|
41
|
Igney FH and Krammer PH: Tumor
counterattack: Fact or fiction? Cancer Immunol Immunother.
54:1127–1136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
O'Connell J, O'Sullivan GC, Collins JK and
Shanahan F: The Fas counterattack: Fas-mediated T cell killing by
colon cancer cells expressing Fas ligand. J Exp Med. 184:1075–1082.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Igney FH and Krammer PH: Death and
anti-death: Tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hoogwater FJ, Snoeren N, Nijkamp MW,
Gunning AC, van Houdt WJ, DE Bruijn MT, Voest EE, van Hillegersberg
R, Kranenburg O and Rinkes IH: Circulating CD95-ligand as a
potential prognostic marker for recurrence in patients with
synchronous colorectal liver metastases. Anticancer Res.
31:4507–4512. 2011.PubMed/NCBI
|
|
45
|
Konno R, Takano T, Sato S and Yajima A:
Serum soluble fas level as a prognostic factor in patients with
gynecological malignancies. Clin Cancer Res. 6:3576–3580.
2000.PubMed/NCBI
|