Introduction
Melanoma has been recognized to be one of the most
malignant tumors with the most aggressive and treatment-resistant
form of human skin cancer. Currently its incidence is still
increasing worldwide. The treatment for this disease after it
spread beyond the primary site is difficult (1). Melanoma has rapid proliferation rate
(2), however, the exact mechanisms
of the rapid proliferation of melanoma cells was unclear (3). Melanoma cell metastasis is also a
cause for difficulty in curing this disease. Although melanoma
treatment has shown some breakthroughs in targeted and
immunotherapy (4), it is still
urgently needed to identify new targets for melanoma treatment.
PEITC, a member of isothiocyanates (ITCs), have been
shown to induce cell cycle arrest PC-3 human prostate cancer cells
(5), oral cancer cells (6), gastric cancer cells (7) and gastric cancer cells (8). Furthermore, several studies have
shown that PEITC induced human cancer cell apoptosis (9–11)
and it also inhibited nuclear factor-κB (NF-κB)-regulated gene
expression (12) and activation of
Atg5-mediated autophagy (13) in
human prostate cancer cells. PEITC is also used in clinical trials
for lung cancer (14). It was
reported that PEITC inhibits the invasion of EGF-stimulated SAS
oral cancer cells via targeting EGFR and also to induce its
downstream signaling molecules for reducing the expression and
enzymatic activities of both matrix metalloproteinase-2 (MMP-2) and
MMP-9 (15).
BITC, also one of ITCs, has been shown to induce
cell apoptosis in many human cancer cell lines such as bladder
cancer cells (16), breast cancer
cells (17), ovary cancer cells
(18), prostate cancer cells
(19) and melanoma A375.S2 cells
(20). Furthermore, BITC induced
cell cycle arrest and apoptosis in human leukemia cells through the
downregulation of myeloid cell leukemia-1 (Mcl-1) (21) and alters the gene expression with
cell cycle regulation and cell death in human brain glioblastoma
GBM 8401 cells (22).
Metastasis, a multistep process, which is often
resistant to conventional therapies such as chemotherapy and
radiation therapy, is involved in cell motility, cellular adhesion
and invasiveness, entry to blood circulation, and stays in other
tissues for new colonization of a distant site (23). One of the major steps for cancer
cell metastasis is the breakdown of connective tissue barriers
which is involved with proteolytic enzymes such as MMPs to mediate
ECM breakdown and facilitate invasion (24). ITCs inhibit the invasion and
migration via blocking FAK/JNK-mediated MMP-9 expression in mouse
C6 glioma cells (25). In lung
cancer cells, BITC and PEITC inhibit cell metastasis potential
via the modulation of metastasis-related gene expression and
the inhibition of Akt/NF-κB pathway (26). However, there is no available
report to show BITC and PEITC suppressing the migration and
invasion of mouse melanoma cells. Thus, we investigated the effects
of BITC and PEITC on the B16F10 cell metastasis and we found that
BITC and PEITC suppressed the migration and invasion of B16F10
cells in vitro.
Materials and methods
Test compound, reagents and culture
medium
Benzyl isothiocyanate (BITC), phenethyl
isothiocyanate (PEITC), dimethyl sulfoxide (DMSO), propidium iodide
(PI), Tris-HCl, Trypsin and Trypan blue were purchased from Sigma
Chemical Co. (St. Louis, MO, USA). BITC and PEITC were dissolved in
DMSO as a carrier solvent and control cultures 0.5% DMSO. DMEM
medium, fetal bovine serum (FBS) and penicillin-streptomycin were
purchased from Invitrogen (Carlsbad, CA, USA).
Cell culture
Murine melanoma B16F10 cell line was obtained from
the Food Industry Research and Development Institute (Hsinchu,
Taiwan). Cells were cultured in 75 cm2 flask with DMEM
supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin in 5% CO2 humidified incubators at
37°C.
Cell viability assay
B16F10 cells (1×105 cells/well) were
maintained in 12-well plates with DMEM for 48 h and then PEITC were
added to cells at final concentrations (0, 1, 2.5, 5, 10 and 15
µM) and BITC were added to cells at final concentrations (0,
1, 2.5, 5 and 10 µM) for 48 h. After incubation, cells were
collected from each treatment, washed with PBS and were stained
with PI (5 µg/ml). All samples were analyzed by flow
cytometry (Becton-Dickinson, San Jose, CA, USA) for percentage of
viable cells as previously described (27).
Scratch wound healing assay
To investigate the wound healing effect of PEITC and
BITC on murine melanoma cells, B16F10 cells (2×105
cells/well) were placed in 6-well plate for 24 h and after the
cells formed a confluent monolayer, they were scratched using a
sterile pipette tip to create a wound at confluence and washed in
PBS to remove cell debris. Cells in each well were incubated with
PEITC and BITC at the final concentrations (0, 1, 2.5 and 5
µM) at 37°C with 5% CO2 at time = 0 and 24 h and
were photographed by phase contrast microscopy. The relative wound
size at each time point of treatment was quantified by ImageJ
software. Cell migration inhibition rate (%) = new scratch
width/original scratch width × 100% as previously described
(28,29).
Cell migration and invasion assay
Matrigel Cell Migration Assay and Invasion System
were used for measuring cell migration and invasion in vitro
as previously described (30).
Cell migration was performed with Transwell cell culture chambers
(8-mm pore size; Millipore, Temecula, CA, USA). B16F10 cells
(5×104 cells/well) were added in serum-free DMEM and
were placed in the upper chamber which was coated with collagen of
the Transwell insert and incubated with PEITC and BITC (0, 1, 2.5
and 5 µM). DMEM with 10% FBS was placed in the lower chamber
and were incubated for 48 h. The invasive cells (penetrated the
filter in the lower surface) were fixed with 4% formaldehyde in PBS
and stained with 2% crystal violet and were examined and
photographed under light microscopy at ×200 followed by counting
for the percentage of inhibition (30). Cell invasion experiment was
performed similarly to cell migration assay, using matrigel
collagen to replace collagen on the filter membrane (30).
Gelatin zymography assay
B16F10 cells (5×105 cells/well) were
maintained in 6-well culture plates for approximately 80%
confluency. The serum-free medium with PEITC or BITC was added to
each dish for 24 h culture. After incubation, the conditioned
medium was collected from each treatment for measuring the total
proteins; a 50 µg of protein from each treatment was
electrophoresis on 10% SDS-PAGE containing 0.2% gelatin. Gel was
washed and soaked in 2.5% Triton X-100 in dH2O twice at
25°C for 30 min twice. Gels were soaked in substrate buffer (50 mM
Tris HCl, 5 mM CaCl2, 0.02% NaN3 and 1%
Triton X-100, pH 8.0) while shaking for 18 h at 37°C. Gels were
stained with 0.2% Coomassie blue (Bio-Rad, Hercules, CA, USA) in
10% acetic acid and 50% methanol (30,31)
and were photographed on a light box. Proteolysis was detected as a
white zone (MMP-2 gelatinolytic activities) in a dark blue
field.
Protein extraction and western blot
analysis
B16F10 cells (1×106 cells) in 10-cm dish
were incubated with PEITC or BITC (0, 1, 2.5 and 5 µM) for
24 and 48 h. After incubation, cells were collected and lysed in
ice-cold potassium phosphate buffer (pH 7.4) containing 2 mM EDTA
and 0.1% Triton X-100 for sonication and centrifuged and total
protein was measured by Bio-Rad protein assay kit (Bio-Rad) with
bovine serum albumin (BSA) as the standard as previously described
(30). The protein from each
treatment of total cells was separated by 12% SDS-polyacrylamide
gel electrophoresis, and transferred onto PVDF nitrocellulose
membrane (Millipore, Bedford, MA, USA). The membrane was blocked
with 5% non-fat milk in TBS-T buffer (10 mmol/l Tris-HCl, 150
mmol/l NaCl, and 0.05% Tween-20, pH 7.8) at room temperature fort 1
h followed by washing in TBS-T buffer. The membrane was incubated
with monoclonal antibodies such as anti-p-ERK1/2, anti-p-p38,
anti-p-JNK1/2, anti-PKC, anti-phosphatidylinositol 3 kinase (PI3K),
anti-p-AKT (Thr308), anti-p-AKT(Ser473), anti-PCAN anti-p-FAK,
anti-RhoA, anti-Ras, anti-GRB2, anti-SOS-1, anti-MMP-2, anti-MMP-9,
anti-uPA, anti-TIMP-1, anti-NF-κBp65, anti-NF-κBp50 and
anti-E-cadherin. Membranes were washed and incubated with the
diluted secondary antibodies (goat anti-mouse immunoglobulin G
(IgG), diluted 1:5000, Santa Cruz Biotechnology Inc., Dallas, TX,
USA; goat anti-rabbit IgG, diluted, 1:5,000, Santa Cruz
Biotechnology Inc.).
Investigated proteins on the membrane were
visualized using the enhanced chemiluminescence detection system
(ECL®, Millipore, Temecula, CA, USA) (30,32).
Electrophoretic mobility shift assay
(EMSA)
A 5×105 cells/well of B16F10 cells were
placed in a 12-well and were treated with 0, 1, 2.5 and 5 µM
of PEITC and BITC for 48 h. Cells were collected for nuclear
extracts by the NE-PER Nuclear and Cytoplasmic Extraction kit
(Pierce, Rockford, IL, USA). Nuclear extract protein (5 µg)
was performed for EMSA with a LightShift Chemiluminescent EMSA kit
(Pierce) according to the manufacturer's protocol.
5′-Biotin-GATCCAGGGGACTTTCCCTAGC-3′ (biotin end-labeled
oligonucleotide sequences) corresponding to the consensus of NF-κB
was developed as previously described (33). Both biotin end-labeled duplex DNA
were then incubated with a nuclear extract for further
electrophoresis in 6% polyacrylamide native gel and then a 100-fold
excess of unlabeled double stranded oligonucleotide was added to
the reaction for competition. Both samples (DNA) were transferred
to a positive nylon membrane. They were UV cross-linked and probed
with biotin-HRP conjugate for incubating with the substrate of ECL
kit (Millipore) as previously described (33).
Statistical analysis
All data represent at least 3 independent
experiments and are expressed as mean ± SD. A significant
difference between the PEITC and BITC-treated and control groups
were compared by Student's t-test. *P<0.05 and
***P<0.001 were considered as an indication of
statistical significance.
Results
PEITC and BITC decrease the viability of
B16F10 cells
In order to understand the possible concentrations
for inhibiting cell migration and invasion of B16F10 cells, cells
were incubated with PEITC (0–15 µM) and BITC (0–10
µM) for 48 h. After incubation, cells from each treatment
were collected for measuring cell viability by flow cytometric
assay and results are shown in Fig.
1. Results indicated that PEITC and BITC significantly reduced
total cell viability from 5 to 15 µM and 1 to 10 µM,
respectively in B16F10 cells. Therefore, we selected 1, 2.5, and 5
µM for scratch wound healing assay, cell migration and
invasion experiments.
PEITC and BITC inhibit cell mobility in
B16F10 cells
Scratch wound healing assay was used to investigate
the inhibition of PEITC and BITC on cell mobility of B16F10 cells
in vitro and results are shown in Fig. 2. One of the representative figures
was present and wound healing images (cell mobile capabilities)
were undertaken at the same magnification and time (0 and 24 h)
after PEITC and BITC treatments in B16F10 cells. PEITC and BITC
decreased the closure rate of the scratch, dose-dependently, when
compared to the control group at 24 h treatment. Based on the
results, it indicated that BITC at 1–2.5 µM has higher
inhibition of cell mobility than that of PEITC (Fig. 2).
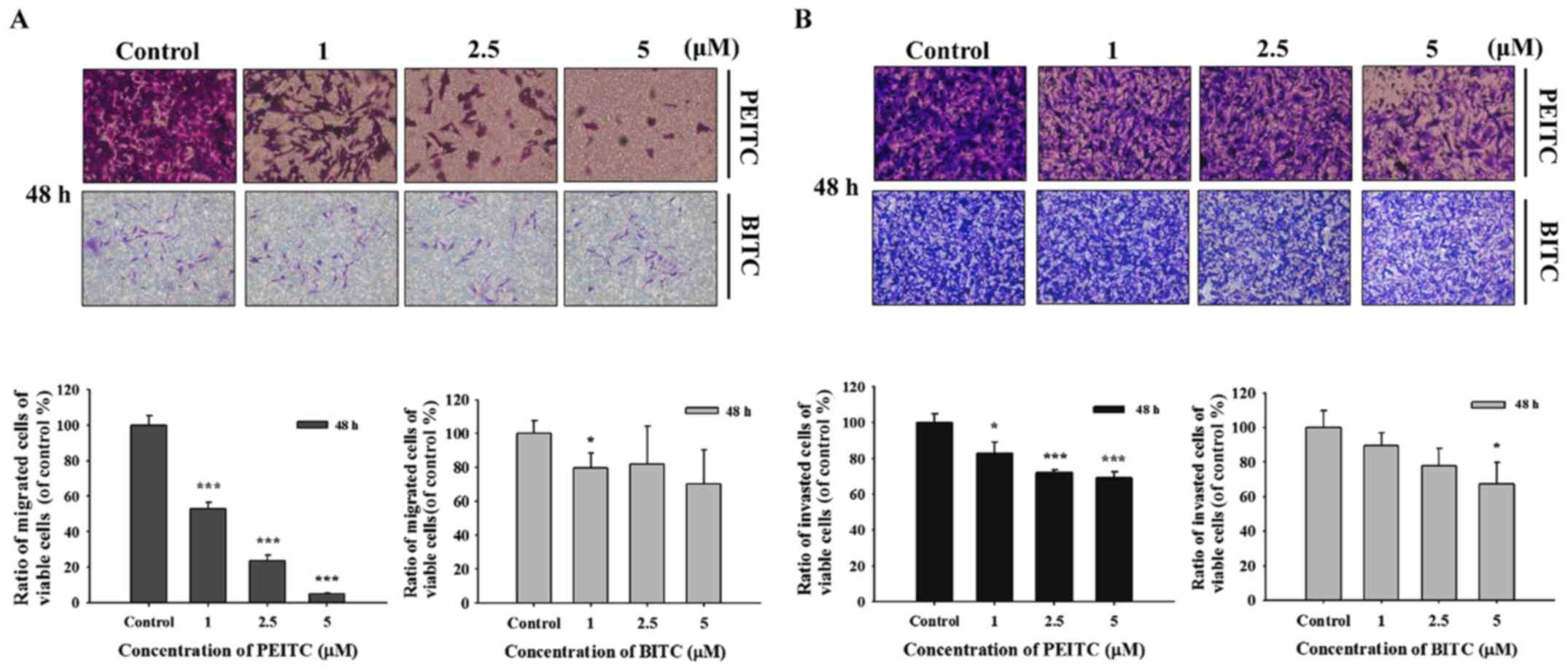
PEITC and BITC suppress migration and
invasion of B16F10 cells
In order to further investigate PEITC and BITC
suppressed cell migration and invasion in B16F10 cells, the
Transwell chamber coated with collagen for cell migration and
coated with matrigel for cell invasion were performed and the
results are shown in Fig. 3.
Fig. 3A indicated that PEITC (1–5
µM) significantly suppressed the migration of B16F10 cells
dose-dependently; however, BITC only at 1 µM induced the
inhibition of cell migration. PEITC suppressed cell migration was
greater than that of BITC. Fig. 3B
indicated that PEITC (1–5 µM) significantly suppressed the
invasion of B16F10 cells dose-dependently; however, BITC only at 5
µM induced the inhibition of cell invasion. PEITC suppressed
cell invasion was greater than that of BITC.
PEITC and BITC inhibit MMP-2 activities
in B16F10 cells
B16F10 cells were incubated with various
concentrations of PEITC and BITC for 24 and 48 h and were collected
for MMP-2 activities by using the gelatin zymography assay and the
results are shown in Fig. 4.
Results indicated that PEITC and BITC suppressed the activities of
MMP-2 in B16F10 cells. The inhibition rate between PEITC and BITC
are not significantly different; however both compounds are
significantly different when compared to control groups.
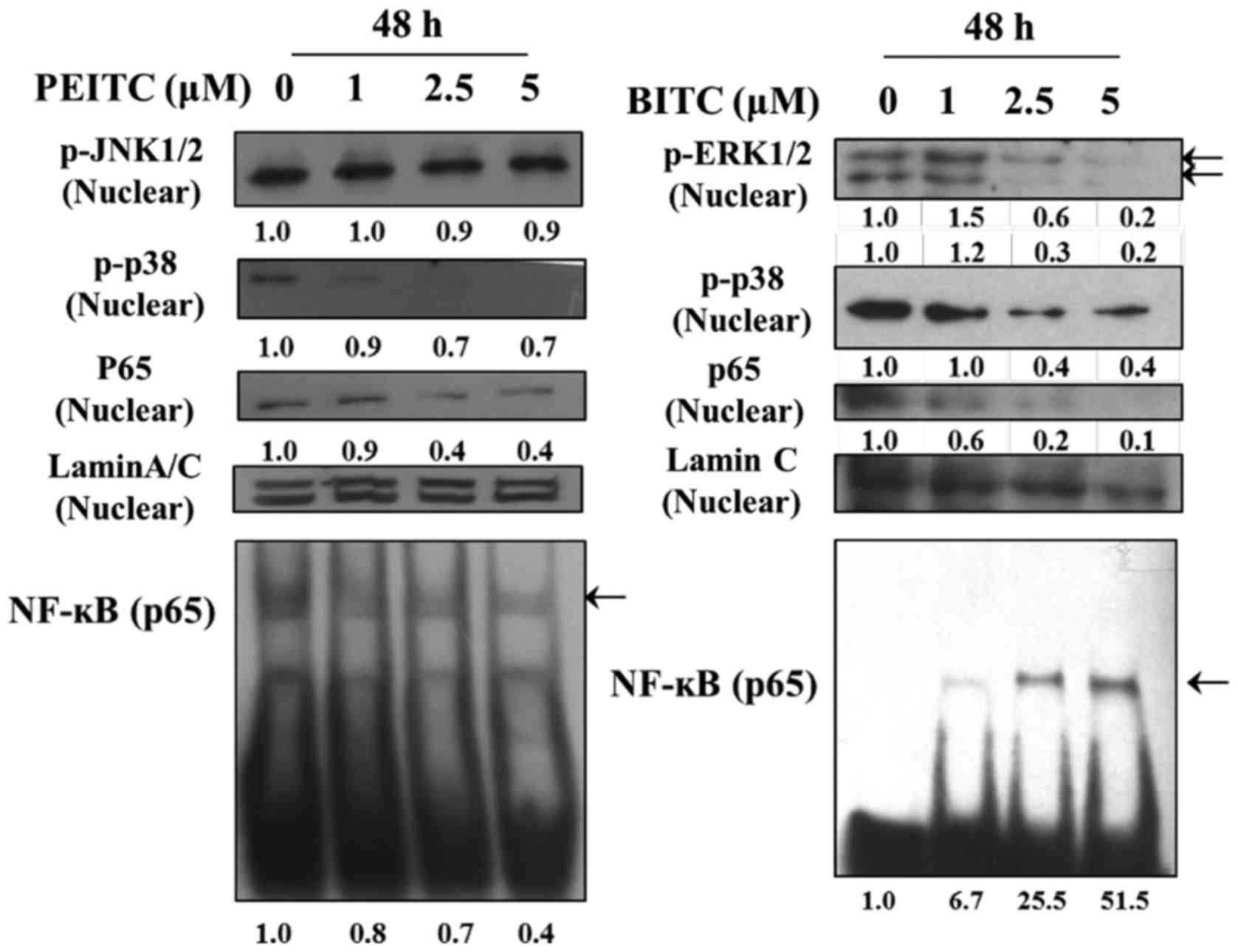
PEITC and BITC affect key
metastasis-related proteins in B16F10 cells
For further investigating PEITC and BITC suppressed
cell invasion and migration involved in the inhibition of
metastasis-associated protein expression in B16F10 cells, cells
after incubation with PEITC and BITC (0, 1, 2.5 and 5 µM)
for 24 and 48 h were harvested for western blotting and the results
are shown in Fig. 5. The results
revealed several depressed key metastasis-related proteins, such as
p-ERK1/2, P-p38 and p-JNK1/2 underwent significant reduction at 24
and 48 h treatment by PEITC (Fig.
5A). However, BITC treatment occurred at both time periods and
only p-ERK1/2 was significantly reduced (Fig. 5A). At PEITC and BITC treatment at
24 and 48 h, the p-AKT (Thr308), p-AKT (Ser473) and PCNA were
significantly reduced when compared to control, however, PKC and
PI3K were significantly increased when compared to control in PEITC
treatment, and PKC and PI3K were reduced at 24 h treatment of BITC
but it increased the expression of PKC and PI3K at 48 h treatment
of BITC (Fig. 5B). The RhoA, Ras
and SOS-1 were reduced at 24 and 48 h treatment of PEITC, p-FAK was
reduced at 24 h treatment of PEITC, GRB2 was increased at 48 h
treatment but p-FAK was increased at 48 h treatment of PEITC
(Fig. 5C). However, for BITC
treatment only GRB2 at 24 h treatment was increased and p-FAK,
RhoA, Ras and SOS-1 were decreased at 24 and 48 h treatment
including GRB2 at 48 h treatment of BITC (Fig. 5C). The MMP-2, MMP-9, uPA and TIMP
were reduced at 24 and 48 h treatment of PEITC (Fig. 5D), however, at BITC treatment for
24 and 48 h, MMP-2 and MMP-9 were reduced, the TIMP-1 was reduced
at 24 h treatment of BITC, uPA was increased at both time periods
of treatment and TIMP-1 was increased at 48 h treatment of BITC
(Fig. 5D). The NF-κBp65 and
NF-κBp50 were reduced at 24 and 48 h treatment of PEITC, however,
BITC at both treatment periods, NF-κBp65 were increased but
NF-κBp50 was reduced at 48 h treatment but 24 h treatment was
increased (Fig. 5E). The
E-cadherin was increased at 24 and 48 h treatment of PEITC, however
BITC treatment at both time periods were increased (Fig. 5F).
 | Figure 5PEITC and BITC affect the levels of
associated proteins in migration and invasion of B16F10 cells.
Cells (1×106 cells/dish) were treated with PEITC and
BITC (0, 1, 2.5 and 5 µM) for 24 and 48 h. Cells were
collected and total protein was determined and for SDS page gel
electrophoresis as described in the Materials and methods. The
levels of p-ERK1/2, p-p38 and p-JNK1/2 (A), PKC, PI3K, p-AKT
(Thr308), p-AKT (Ser473) and PCNA (B), p-FAK, RhoA, Ras, GRB2 and
SOS-1 (C), MMP-2, MMP-9, uPA and TIMP-1 (D), NF-κBp65 and NF-κBp50
(E) and E-cadherin (F) expression levels were estimated by western
blotting as described in Materials and methods. |
PEITC and BITC decreased the binding of
NF-κBp65 on DNA in B16F10 cells
In order to understand the effects of PEITC and BITC
on the binding of NF-κBp65 on DNA in B16F10 cells, cells were
treated with 0, 1, 2.5 and 5 µM of PEITC and BITC for 48 h
and were assayed by using EMSA. The results are shown in Fig. 6. The results indicated that
NF-κBp65 bind on DNA was decreased in PEITC treatment, however,
BITC treatment was increased.
Discussion
Numerous studies have shown that phenethyl
isothiocyanate (PEITC) and benzyl isothiocyanate (BITC)
significantly induced cytotoxic effects on many human cancer cell
lines. The cytotoxic effects include decreased percentage of viable
cell number through cell cycle arrest and apoptosis. However,
currently how PEITC and BITC affect mouse melanoma B16F10 cell
migration and invasion are unclear. Thus, in the present studies,
we investigated PEITC and BITC effect on cell migration and
invasion in B16F10 cells in vitro and we found that 1) PEITC
has lower cytotoxic effects than that of BITC (Fig. 1), thus, we selected 1, 2.5 and 5
µM for further experiments; 2) wound healing assay showed
that PEITC have lower inhibition of wound healing (mobility) than
that of BITC except at high dose (5 µM) PEITC inhibited cell
mobility more than that of BITC (Fig.
2); 3) results obtained from Transwell chamber coated with
collagen or matrigel for cell migration and invasion, respectively,
PEITC inhibited cell migration more than that of BITC (Fig. 3A) and PEITC inhibited cell invasion
more than that of BITC (Fig. 3B);
4) the inhibition of MMP-2 activity was not significantly different
between PEITC and BITC (Fig. 4)
western blot examination demonstrated that PEITC has reduced MAPK
signaling-associated proteins such as p-ERK1/2, p-p38 and p-JNK1/2
(Fig. 5A), but BITC treatment
increased all those MAPK signaling associated proteins (Fig. 5A).
The PEITC increased PI3K but BITC decreased PI3K at
both time periods of treatment (Fig.
5B). PEITC and BITC both suppressed the expression of RhoA,
Ras, and SOS-1, however, PEITC increased FAK and GRB2, but BITC
increased FAK at 48 h (Fig. 5C).
PEITC decreased the expression of matrix metalloproteinase-2
(MMP-2) and tissue inhibitors of matrix metalloproteinase-1
(TIMP-1) but BITC increased them (Fig.
5D). At 48 h treatment, PEITC decreased NF-κBp65 and NF-κBp50
but BITC increased both (Fig. 5E)
and both increased E-cadherin (Fig.
5F); 5) EMSA also confirmed that PEITC inhibited NF-κB binding
DNA but BITC increased NF-κB binding to DNA in B16F10 cells
(Fig. 6). All these observations
are compatible with PEITC and BITC have anti-metastasis
capabilities. Thus, our findings may prove that PEITC and BITC have
potential as anti-metastatic in melanoma.
It is well known that approximately 90% of cancer
deaths are caused by metastasis but the exact pathogenesis and
mechanisms involved are not completely clear (34). Herein, we used wound healing and
Transwell filter to show B16F10 cell suppression of the cell
migration and invasion (Figs. 2
and 3). It is well documented that
matrix metalloproteinases (MMPs) are involved in cell metastasis
(24) and inhibition of MMPs can
lead to suppression of cancer cell metastasis (35). Thus, one of the strategies for
inhibiting cancer metastasis is to suppress the regulation of MMP
proteins. Increased MMP-2 and MMP-9 activities and expression
levels are correlated with reduced survival and poor prognosis in
human malignancies (36,37) and in many pathological processes
including metastatic cancer and tumor-induced angiogenesis
(38). In the present study, we
used gelatin zymography assay which demonstrated that PEITC and
BITC inhibited activities of MMP-2 in B16F10 cells (Fig. 4). PEITC and BITC inhibited MMP-2
production (Fig. 5D) and activity
(Fig. 4) is also evident from the
inhibition of collagen matrix invasion in B16F10 cells in
vitro. MMP-2 (gelatinases) involved in tumor invasion and
angiogenesis and chemical suppression of MMP-2 may lead to the
inhibition of tumor metastasis (39,40).
Results from western blotting indicated that PEITC and BITC at 24
and 48 h treatment significantly reduced the protein levels of
MMP-2 (Fig. 5D) and PEITC
inhibited TIMP-1 at 24 and 48 h treatment but BITC at 48 h
treatment led to increased TIMP-1 (Fig. 5D). PEITC suppressed urokinase-type
plasminogen activator (uPA) at 24 and 48 h treatment but BITC
increased it at both treatment times (Fig. 5D). The uPA protein expression
levels have also been considered as promising targets of anticancer
drugs (41) because it is involved
in cell invasion and metastasis. It was reported that uPA gene
transcription is involved in motifs that upstream sequences which
correspond to NF-κB, AP-1, and PEA3-binding sites (42,43).
In the current study, the effects of PEITC and BITC
on NF-κB transcription activity (DNA binding) were examined by
using EMSA assay and results (Fig.
6) indicated that PEIT reduce the binding of NF-κB to DNA in
DNA-binding domains but BITC elevated the binding of NF-κB to DNA
in DNA-binding domains (Fig. 6).
Based on the results from western blotting indicated that PEITC
suppressed the expression of MMP-2 and TIMP-1 but BITC increased
them (Fig. 5D) and TIMPs act as
natural inhibitors of MMPs by tightly binding the MMP in a 1:1
stoichiometric ratio (44). At 48
h treatment of PEITC, it decreased NF-κBp65 and NF-κBp50 but BITC
increased both (Fig. 5E) and both
increased E-cadherin (Fig. 5F).
This reduced binding activity was accompanied by inhibition of the
nuclear protein expression of this factor in B16F10 cells that was
confirmed by western blotting. These results indicated that NF-κB
binding activity suppression was also possibly implicated in the
inhibition of MMP or uPA synthesis. It is well known that cancer
cells can express high levels of MMPs, cathepsins and uPA, which
degrade tissue extracellular matrix (ECM) and facilitate cancer
invasion and metastasis (45).
NF-κB is a complex family, thus, further investigations are needed
in the future. These MMPs have been shown to be present in
different types of cancer cells, including melanoma, lung and
breast (46).
Numerous studies have shown that in many
physiological and pathological settings, the mitogen-activated
protein kinase (MAPK) pathway involved in regulating cell death and
survival (47,48) plays a central role in regulating
the expression of MMP-2 and MMP-9 (49). MMPs are partly mediated by the MAPK
pathway (50,51). MAPK includes ERK1/2, c-Jun
NH2-terminal kinase, and p38 and other factors. The MAPK pathway
regulated various cellular activities such as proliferation,
invasion, metastasis, and death (52). It was suggested that agents to
inhibit the MAPK pathway might lead to prevent cancer angiogenesis,
proliferation, invasion, and metastasis including melanoma
(49,53). Results from western blotting
indicated that PEITC and BITC treatment at 24 and 48 h
significantly reduced the expression of p-ERK1/2, p-p38 and
p-JNK1/2 when compared to control (Fig. 5A) which means PEITC and BITC
suppressed MAPK signal pathway in B16F10 cells. Fig. 5B indicated that p-AKT(Thr308),
p-AKT(Ser473) and PCAN were reduced in PEITC and BITC treatment,
however, both agents increased AKT expression in B16F10 cells.
Activated PI3K and its downstream target Akt have been recognized
to be involved with tumor cell invasion, and oncogenesis (54,55).
The downregulation of the PI3k/Akt pathway have been reported to
decrease the invasion of melanoma cells (53,56).
In melanoma cells, the activation of the PI3K-Akt signaling pathway
promoting cell invasion has been shown (57). AKT, downstream of PI3K has been
reported to be suppressed by the transcription of the E-cadherin
gene (58). Ras regulating RhoA
has been recognized to affect tumor cells transmigration through
mesothelial monolayer (59).
Results (Fig. 5C) indicated that
PEITC and BITC significantly reduced the expression of RhoA and Ras
in B16F10 cells at both treatment times. Thus, PEITC and BITC
inhibited B16F10 cell migration and invasion may also be via the
inhibition of RhoA and Ras.
In conclusion, our results indicated that PEITC and
BITC reduced the viable cell number of B16F10 cells. We selected
the low concentrations (1, 2.5 and 5 µM) of PEITC and BITC
bufalin for examining the effects of cancer cell metastasis and we
found that PEITC and BITC significantly inhibited cell mobility,
migration and invasion of B16F10 cells in vitro. We also
used western blot assay and found that PEITC and BITC inhibited
many metastasis-associated protein molecules including MMP-2,
MAPKs, E-cadherin, Ras, RhoA and NF-κB. NF-κB also was confirmed by
confocal laser microscopy examination. This finding suggests that
PEITC and BITC are potential candidates for the development of
chemotherapeutic treatments for melanoma cells in the future.
Acknowledgments
The present study was supported by grants
NSC103-2320-B-039-037 from the National Science Council, Taipei,
Taiwan.
References
|
1
|
Soengas MS and Lowe SW: Apoptosis and
melanoma chemo-resistance. Oncogene. 22:3138–3151. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hersey P, Zhuang L and Zhang XD: Current
strategies in overcoming resistance of cancer cells to apoptosis
melanoma as a model. Int Rev Cytol. 251:131–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sykes EK, Mactier S and Christopherson RI:
Melanoma and the Unfolded Protein Response. Cancers (Basel).
8:82016. View Article : Google Scholar
|
|
4
|
Sharma A, Shah SR, Illum H and Dowell J:
Vemurafenib: Targeted inhibition of mutated BRAF for treatment of
advanced melanoma and its potential in other malignancies. Drugs.
72:2207–2222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao D, Johnson CS, Trump DL and Singh SV:
Proteasome-mediated degradation of cell division cycle 25C and
cyclin-dependent kinase 1 in phenethyl isothiocyanate-induced
G2-M-phase cell cycle arrest in PC-3 human prostate cancer cells.
Mol Cancer Ther. 3:567–575. 2004.PubMed/NCBI
|
|
6
|
Yeh YT, Yeh H, Su SH, Lin JS, Lee KJ, Shyu
HW, Chen ZF, Huang SY and Su SJ: Phenethyl isothiocyanate induces
DNA damage-associated G2/M arrest and subsequent apoptosis in oral
cancer cells with varying p53 mutations. Free Radic Biol Med.
74:1–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Øverby A, Zhao CM, Bones AM and Chen D:
Naturally occurring phenethyl isothiocyanate-induced inhibition of
gastric cancer cell growth by disruption of microtubules. J
Gastroenterol Hepatol. 29(Suppl 4): 99–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chou YC, Chang MY, Wang MJ, Liu HC, Chang
SJ, Harnod T, Hung CH, Lee HT, Shen CC and Chung JG: Phenethyl
isothiocyanate alters the gene expression and the levels of protein
associated with cell cycle regulation in human glioblastoma GBM
8401 cells. Environ Toxicol. 32:176–187. 2017. View Article : Google Scholar
|
|
9
|
Huong D, Shim JH, Choi KH, Shin JA, Choi
ES, Kim HS, Lee SJ, Kim SJ, Cho NP and Cho SD: Effect of
β-phenylethyl isothiocyanate from cruciferous vegetables on growth
inhibition and apoptosis of cervical cancer cells through the
induction of death receptors 4 and 5. J Agric Food Chem.
59:8124–8131. 2011. View Article : Google Scholar
|
|
10
|
Hwang ES and Lee HJ: Effects of
phenylethyl isothiocyanate and its metabolite on cell-cycle arrest
and apoptosis in LNCaP human prostate cancer cells. Int J Food Sci
Nutr. 61:324–336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan H, Zhu Y, Liu B, Wu H, Li Y, Wu X,
Zhou Q and Xu K: Mitogen-activated protein kinase mediates the
apoptosis of highly metastatic human non-small cell lung cancer
cells induced by isothiocyanates. Br J Nutr. 106:1779–1791. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu C, Shen G, Chen C, Gélinas C and Kong
AN: Suppression of NF-kappaB and NF-kappaB-regulated gene
expression by sulforaphane and PEITC through IkappaBalpha, IKK
pathway in human prostate cancer PC-3 cells. Oncogene.
24:4486–4495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bommareddy A, Hahm ER, Xiao D, Powolny AA,
Fisher AL, Jiang Y and Singh SV: Atg5 regulates phenethyl
isothiocyanate-induced autophagic and apoptotic cell death in human
prostate cancer cells. Cancer Res. 69:3704–3712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kelloff GJ, Crowell JA, Hawk ET, Steele
VE, Lubet RA, Boone CW, Covey JM, Doody LA, Omenn GS, Greenwald P,
et al: Strategy and planning for chemopreventive drug development:
Clinical development plans II. J Cell Biochem. (Suppl 26): 54–71.
1996. View Article : Google Scholar
|
|
15
|
Chen HJ, Lin CM, Lee CY, Shih NC, Amagaya
S, Lin YC and Yang JS: Phenethyl isothiocyanate suppresses
EGF-stimulated SAS human oral squamous carcinoma cell invasion by
targeting EGF receptor signaling. Int J Oncol. 43:629–637.
2013.PubMed/NCBI
|
|
16
|
Tang L and Zhang Y: Dietary
isothiocyanates inhibit the growth of human bladder carcinoma
cells. J Nutr. 134:2004–2010. 2004.PubMed/NCBI
|
|
17
|
Xiao D, Powolny AA and Singh SV: Benzyl
isothiocyanate targets mitochondrial respiratory chain to trigger
reactive oxygen species-dependent apoptosis in human breast cancer
cells. J Biol Chem. 283:30151–30163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Batra S, Sahu RP, Kandala PK and
Srivastava SK: Benzyl isothiocyanate-mediated inhibition of histone
deacetylase leads to NF-kappaB turnoff in human pancreatic
carcinoma cells. Mol Cancer Ther. 9:1596–1608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu KC, Huang YT, Wu PP, Ji BC, Yang JS,
Yang JL, Chiu TH, Chueh FS and Chung JG: The roles of AIF and Endo
G in the apoptotic effects of benzyl isothiocyanate on DU 145 human
prostate cancer cells via the mitochondrial signaling pathway. Int
J Oncol. 38:787–796. 2011.PubMed/NCBI
|
|
20
|
Huang SH, Wu LW, Huang AC, Yu CC, Lien JC,
Huang YP, Yang JS, Yang JH, Hsiao YP, Wood WG, et al: Benzyl
isothiocyanate (BITC) induces G2/M phase arrest and apoptosis in
human melanoma A375.S2 cells through reactive oxygen species (ROS)
and both mitochondria-dependent and death receptor-mediated
multiple signaling pathways. J Agric Food Chem. 60:665–675. 2012.
View Article : Google Scholar
|
|
21
|
Zhou T, Li G, Cao B, Liu L, Cheng Q, Kong
H, Shan C, Huang X, Chen J and Gao N: Downregulation of Mcl-1
through inhibition of translation contributes to benzyl
isothiocyanate-induced cell cycle arrest and apoptosis in human
leukemia cells. Cell Death Dis. 4:e5152013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang NY, Chueh FS, Yu CC, Liao CL, Lin JJ,
Hsia TC, Wu KC, Liu HC, Lu KW and Chung JG: Benzyl isothiocyanate
alters the gene expression with cell cycle regulation and cell
death in human brain glioblastoma GBM 8401 cells. Oncol Rep.
35:2089–2096. 2016.PubMed/NCBI
|
|
23
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cruz-Munoz W and Khokha R: The role of
tissue inhibitors of metalloproteinases in tumorigenesis and
metastasis. Crit Rev Clin Lab Sci. 45:291–338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee CS, Cho HJ, Jeong YJ, Shin JM, Park
KK, Park YY, Bae YS, Chung IK, Kim M, Kim CH, et al:
Isothiocyanates inhibit the invasion and migration of C6 glioma
cells by blocking FAK/JNK-mediated MMP-9 expression. Oncol Rep.
34:2901–2908. 2015.PubMed/NCBI
|
|
26
|
Wu X, Zhu Y, Yan H, Liu B, Li Y, Zhou Q
and Xu K: Isothiocyanates induce oxidative stress and suppress the
metastasis potential of human non-small cell lung cancer cells. BMC
Cancer. 10:2692010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shih YL, Chou J, Yeh MY, Chou HM, Chou HC,
Lu HF, Shang HS, Chueh FS, Chu YL, Hsueh SC, et al: Casticin
induces DNA damage and inhibits DNA repair-associated protein
expression in B16F10 mouse melanoma cancer cells. Oncol Rep.
36:2094–2100. 2016.PubMed/NCBI
|
|
28
|
Chen YY, Lu HF, Hsu SC, Kuo CL, Chang SJ,
Lin JJ, Wu PP, Liu JY, Lee CH, Chung JG, et al: Bufalin inhibits
migration and invasion in human hepatocellular carcinoma SK-Hep1
cells through the inhibitions of NF-κB and matrix
metalloproteinase-2/-9-signaling pathways. Environ Toxicol.
30:74–2. 2015. View Article : Google Scholar
|
|
29
|
Huang YP and Chang NW: PPARα modulates
gene expression profiles of mitochondrial energy metabolism in oral
tumorigenesis. Biomedicine (Taipei). 6:32016. View Article : Google Scholar
|
|
30
|
Huang AC, Yang MD, Hsiao YT, Lin TS, Ma
YS, Peng SF, Hsia TC, Cheng YD, Kuo CL and Chung JG: Bufalin
inhibits gefitinib resistant NCI-H460 human lung cancer cell
migration and invasion in vitro. J Ethnopharmacol. 194:1043–1050.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chan CY, Lien CH, Lee MF and Huang CY:
Quercetin suppresses cellular migration and invasion in human head
and neck squamous cell carcinoma (HNSCC). Biomedicine (Taipei).
6:152016. View Article : Google Scholar
|
|
32
|
Lin M-C, Tsai S-Y, Wang F-Y, Liu F-H, Syu
J-N and Tang F-Y: Leptin induces cell invasion and the upregulation
of matrilysin in human colon cancer cells. BioMedicine. 3:pp.
174–180. 2013, http://biomedicine.cmu.edu.tw/doc/9-5.pdf.
View Article : Google Scholar
|
|
33
|
Kuo TC, Yang JS, Lin MW, Hsu SC, Lin JJ,
Lin HJ, Hsia TC, Liao CL, Yang MD, Fan MJ, et al: Emodin has
cytotoxic and protective effects in rat C6 glioma cells: Roles of
Mdr1a and nuclear factor kappaB in cell survival. J Pharmacol Exp
Ther. 330:736–744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu Y and Zhou BP: New insights of
epithelial-mesenchymal transition in cancer metastasis. Acta
Biochim Biophys Sin (Shanghai). 40:643–650. 2008. View Article : Google Scholar
|
|
35
|
Jia W, Gao XJ, Zhang ZD, Yang ZX and Zhang
G: S100A4 silencing suppresses proliferation, angiogenesis and
invasion of thyroid cancer cells through downregulation of MMP-9
and VEGF. Eur Rev Med Pharmacol Sci. 17:1495–1508. 2013.PubMed/NCBI
|
|
36
|
Kallakury BV, Karikehalli S, Haholu A,
Sheehan CE, Azumi N and Ross JS: Increased expression of matrix
metalloproteinases 2 and 9 and tissue inhibitors of
metalloproteinases 1 and 2 correlate with poor prognostic variables
in renal cell carcinoma. Clin Cancer Res. 7:3113–3119.
2001.PubMed/NCBI
|
|
37
|
Yoshizaki T, Maruyama Y, Sato H and
Furukawa M: Expression of tissue inhibitor of matrix
metalloproteinase-2 correlates with activation of matrix
metalloproteinase-2 and predicts poor prognosis in tongue squamous
cell carcinoma. Int J Cancer. 95:44–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hsieh YS, Chu SC, Hsu LS, Chen KS, Lai MT,
Yeh CH and Chen PN: Rubus idaeus L. reverses
epithelial-to-mesenchymal transition and suppresses cell invasion
and protease activities by targeting ERK1/2 and FAK pathways in
human lung cancer cells. Food Chem Toxicol. 62:908–918. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li D, Qu X, Hou K, Zhang Y, Dong Q, Teng
Y, Zhang J and Liu Y: PI3K/Akt is involved in bufalin-induced
apoptosis in gastric cancer cells. Anticancer Drugs. 20:59–64.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rajoria S, Suriano R, George A, Shanmugam
A, Schantz SP, Geliebter J and Tiwari RK: Estrogen induced
metastatic modulators MMP-2 and MMP-9 are targets of
3,3′-diindolylmethane in thyroid cancer. PLoS One. 6:e158792011.
View Article : Google Scholar
|
|
41
|
Chu SC, Yu CC, Hsu LS, Chen KS, Su MY and
Chen PN: Berberine reverses epithelial-to-mesenchymal transition
and inhibits metastasis and tumor-induced angiogenesis in human
cervical cancer cells. Mol Pharmacol. 86:609–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
D'Orazio D, Besser D, Marksitzer R, Kunz
C, Hume DA, Kiefer B and Nagamine Y: Cooperation of two PEA3/AP1
sites in uPA gene induction by TPA and FGF-2. Gene. 201:179–187.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mori Y, Akita K, Tanida S, Ishida A, Toda
M, Inoue M, Yashiro M, Sawada T, Hirakawa K and Nakada H: MUC1
protein induces urokinase-type plasminogen activator (uPA) by
forming a complex with NF-κB p65 transcription factor and binding
to the uPA promoter, leading to enhanced invasiveness of cancer
cells. J Biol Chem. 289:35193–35204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Offenberg H, Brünner N, Mansilla F,
Orntoft Torben F and Birkenkamp-Demtroder K: TIMP-1 expression in
human colorectal cancer is associated with TGF-B1, LOXL2, INHBA1,
TNF-AIP6 and TIMP-2 transcript profiles. Mol Oncol. 2:233–240.
2008. View Article : Google Scholar
|
|
45
|
Pulyaeva H, Bueno J, Polette M, Birembaut
P, Sato H, Seiki M and Thompson EW: MT1-MMP correlates with MMP-2
activation potential seen after epithelial to mesenchymal
transition in human breast carcinoma cells. Clin Exp Metastasis.
15:111–120. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Heppner KJ, Matrisian LM, Jensen RA and
Rodgers WH: Expression of most matrix metalloproteinase family
members in breast cancer represents a tumor-induced host response.
Am J Pathol. 149:273–282. 1996.PubMed/NCBI
|
|
47
|
Eisenmann KM, VanBrocklin MW, Staffend NA,
Kitchen SM and Koo HM: Mitogen-activated protein kinase
pathway-dependent tumor-specific survival signaling in melanoma
cells through inactivation of the proapoptotic protein bad. Cancer
Res. 63:8330–8337. 2003.PubMed/NCBI
|
|
48
|
Junttila MR, Li SP and Westermarck J:
Phosphatase-mediated crosstalk between MAPK signaling pathways in
the regulation of cell survival. FASEB J. 22:954–965. 2008.
View Article : Google Scholar
|
|
49
|
Lee KR, Lee JS, Kim YR, Song IG and Hong
EK: Polysaccharide from Inonotus obliquus inhibits migration and
invasion in B16-F10 cells by suppressing MMP-2 and MMP-9 via
down-regulation of NF-κB signaling pathway. Oncol Rep.
31:2447–2453. 2014.PubMed/NCBI
|
|
50
|
Mori T, Wang X, Aoki T and Lo EH:
Downregulation of matrix metalloproteinase-9 and attenuation of
edema via inhibition of ERK mitogen activated protein kinase in
traumatic brain injury. J Neurotrauma. 19:1411–1419. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shin DY, Lu JN, Kim GY, Jung JM, Kang HS,
Lee WS and Choi YH: Anti-invasive activities of anthocyanins
through modulation of tight junctions and suppression of matrix
metalloproteinase activities in HCT-116 human colon carcinoma
cells. Oncol Rep. 25:567–572. 2011.
|
|
52
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lin BW, Jiao ZL, Fan JF, Peng L, Li L,
Zhao ZG, Ding XY and Li HJ: Inhibitory effect of melanoma
differentiation associated gene-7/interleukin-24 on invasion in
vitro of human melanoma cancer cells. J Korean Med Sci. 28:833–839.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim D, Kim S, Koh H, Yoon SO, Chung AS,
Cho KS and Chung J: Akt/PKB promotes cancer cell invasion via
increased motility and metalloproteinase production. FASEB J.
15:1953–1962. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shukla S, Maclennan GT, Hartman DJ, Fu P,
Resnick MI and Gupta S: Activation of PI3K-Akt signaling pathway
promotes prostate cancer cell invasion. Int J Cancer.
121:1424–1432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shi H, Wu Y, Wang Y, Zhou M, Yan S, Chen
Z, Gu D and Cai Y: Liquiritigenin potentiates the inhibitory
effects of cisplatin on invasion and metastasis via downregulation
MMP-2/9 and PI3K/AKT signaling pathway in B16F10 melanoma cells and
mice model. Nutr Cancer. 67:761–770. 2015. View Article : Google Scholar
|
|
57
|
Thang ND, Yajima I, Kumasaka MY, Iida M,
Suzuki T and Kato M: Deltex-3-like (DTX3L) stimulates metastasis of
melanoma through FAK/PI3K/AKT but not MEK/ERK pathway. Oncotarget.
6:14290–14299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Qiao M, Sheng S and Pardee AB: Metastasis
and AKT activation. Cell Cycle. 7:2991–2996. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Narumiya S, Tanji M and Ishizaki T: Rho
signaling, ROCK and mDia1, in transformation, metastasis and
invasion. Cancer Metastasis Rev. 28:65–76. 2009. View Article : Google Scholar : PubMed/NCBI
|