Introduction
World-wide head and neck cancer accounts for
~550,000 new cases annually with ~290,000 deaths (1). Over the past five decades, a
considerable amount of effort has been made to improve the
treatment of patients with head and neck squamous cell carcinoma
(HNSCC), be it surgery (2),
chemotherapy (3), radiotherapy
(4), non-surgical
checkpoint-inhibition immunotherapy (5,6) or a
combination of these (7).
Radiotherapy is the primary treatment for early-stage laryngeal
cancer (T1 and T2) and is commonly used in conjunction with
chemotherapy in T3 laryngeal and oropharyngeal squamous cell
carcinoma patients. However, despite the improvements in treatment
modalities, the overall 5-year survival rate for patients with head
and neck cancer remains low, achieving only 66% for oral cavity
cancers and 63% for cancers of the pharynx and larynx (8), which is thought to be largely due to
the recurrence of the primary tumour as well as intrinsic tumour
radioresistance (9–11). Still, what is lacking is a
comprehensive understanding of the biological mechanisms of
radiotherapy sensitivity, resistance and associated biomarkers.
Numerous studies have focused on the identification of predictive
biomarkers for radioresistance by studying biopsies removed from
treated patients (12–16). In contrast, only a few studies have
taken pieces of the actual patient tumour and subjected them to
in vitro irradiation with the aim of predicting patient
specific tumour sensitivity (17,18),
due to the inability to maintain the tumour tissue ex vivo.
The evaluation of tumour responses to irradiation ex vivo
prior to commencement of the therapeutic intervention, would mean
that treatment regimens could be designed on a rational rather than
an empirical basis, leading to improved quality of life with less
side-effects and associated morbidity.
Microfluidic devices provide a platform on which a
biomimetic microenvironment for human tissues can be maintained,
allowing the culture of biopsies under pseudo in vivo
conditions (19). These are
simple, reproducible, and highly versatile systems for tissue
culture with the preservation of 3-dimensional architecture
(20). Microfluidic-based tissue
culture mimics the systems of the human body with continuous
perfusion, permitting the constant supply of nutrients to, and
removal of waste from, multiple pieces of the same patient tissue
in parallel (21).
Microfluidic culture of head and neck tumour tissue
has been demonstrated previously by colleagues, in which the
viability of the cultured tissues was confirmed by the relatively
low release of cytosolic enzymes (LDH and cytochrome c) and
high release of proliferation markers (metabolised tetrazolium
salts) (18,22–24).
A report by Carr et al (18) is the only study so far
investigating the response of HNSCC to on-chip X-ray irradiation
and showed that administration of fractionated irradiation doses
(5×2 Gy over a 5-day period) demonstrated an enhanced level of
apoptotic cell death compared to non-irradiated control tissue
based on the increased expression of caspase-cleaved cytokeratin 18
(cCK18); also the study showed increased LDH release following high
doses of irradiation.
DNA repair pathways remove radiation-induced DNA
lesions and protect tumour cells from death. The evidence for the
radio-protective effect of cellular DNA repair has been confirmed
in cellular, animal and human studies (25–27);
individuals with defects in DNA repair pathways often display
hypersensitivity to radiation (28,29).
Building on the results of previous studies, the present study
aimed to determine the effects of single-dose external beam
irradiation on microfluidic-perfused HNSCC biopsies using an
extended panel of biological markers and expression profiles:
caspase-dependent apoptosis (cCK18), caspase-independent necrotic
cell death (LDH), DNA damage repair (phosphorylated H2AX), DNA
fragmentation (TUNEL) and proliferative status (Ki-67). Ultimately
it is hoped that these data could be used to customise patient
treatment on a rational basis.
Materials and methods
HNSCC tissue collection
Samples of HNSCC primary or metastatic node tissue
were obtained from 5 patients undergoing resection surgery at
Castle Hill Hospital (Hull, UK) with no history of previous
treatment (Table I). The project
had approval from the Local Research Ethics Committee
(LREC-10/H1304/6) and Hull and East Yorkshire NHS Trust R&D
(R0987), and all patients provided written, informed consent.
Tissue samples were transported to the laboratory in complete
Dulbecco's modified Eagle's medium [DMEM; supplemented with 10%
(v/v) foetal bovine serum (FBS); Biosera, East Sussex, UK), 100
U/ml penicillin, 100 µg/ml streptomycin, 2 mM (v/v)
L-glutamine and 2.5 µg/ml fungizone (Thermo Fisher, Paisley,
UK)] and snap-frozen in liquid nitrogen before storage at −80°C
prior to microfluidic culture.
 | Table IClinicopathological details of five
HNSCC patients, clinical treatment received and outcome. |
Table I
Clinicopathological details of five
HNSCC patients, clinical treatment received and outcome.
| Sample | Subsite | Stage | Age; gender | Therapy received
and datea | Treatment outcome
(updated June 2016) |
|---|
| LN 1 | Unknown
primary | TxN2aM0 | 61; M | Surgery and
CRT | No local or
regional recurrence |
| LN 2 | Laryngeal
primary | T4N2bM0 | 58; M | Surgery and
CRT | No information |
| PT 1 | Oral cavity floor
of mouth I | T3N0M0 | 49; M | Surgery | Passed away due to
alcoholic liver disease |
| PT 2 | Oral cavity floor
of mouth II | T2N0 | 64; M | Surgery | No evidence of
recurrence |
| PT 3 | Laryngeal
supraglottis | T2N0M0 | 68; F | RT | No evidence of
loco-regional recurrence |
Incubation of HNSCC tissue in a
microfluidic device
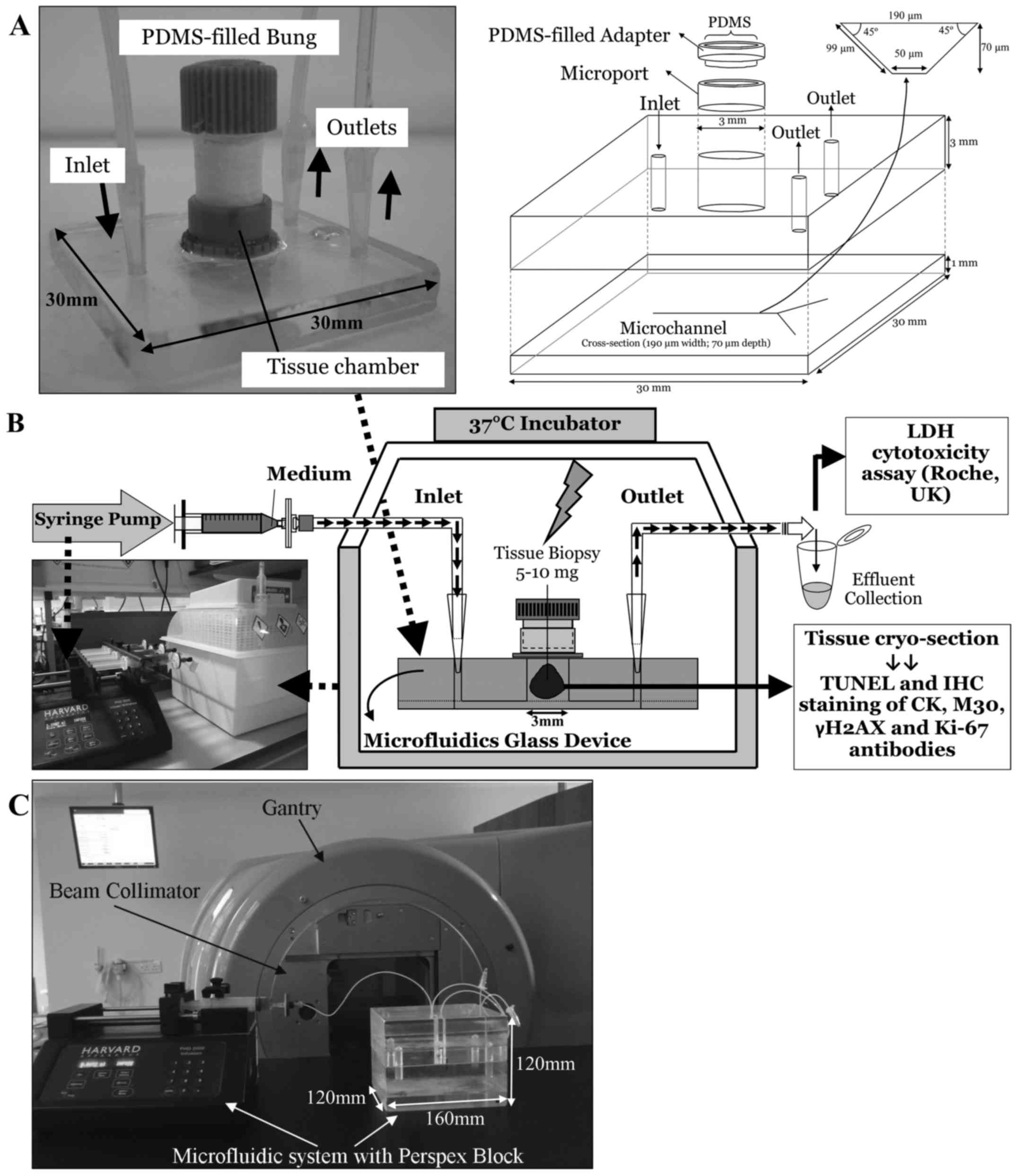
The microfluidic devices were manufactured in-house
in the Department of Chemistry (University of Hull) and consisted
of two thermally bonded layers of glass with micro-etched channels
as described previously [(18,22);
Fig. 1A)]. As the etching process
occurs both horizontally as well as vertically it creates channels
with a trapezoidal cross-section (30).
A piece of HNSCC tissue (5–10 mg) was placed in the
central tissue chamber (Fig. 1)
and perfused with complete DMEM (supplemented with 30 mM HEPES and
0.1 mM NEAA; all medium and supplements were from PAA, Somerset,
UK, unless otherwise stated) using tubing connected to a syringe
mounted in a Harvard PhD 2000 syringe pump (Harvard, Kent, UK),
delivering medium at a rate of 2 µl/min. The microfluidic
device was placed in a 37°C incubator (Novital, Italy; Fig. 1B) and the effluent was collected at
2-h intervals and overnight before storage for up to 6 days at 4°C
for analysis of LDH content.
The fluid flow pattern in the microfluidic device
was laminar with a flow velocity of 3.96×10−3 m/sec and
a Reynolds number within the microchannel of 0.386. Diffusion
becomes a crucial transport mechanism between the fluid flow and
tissue, thus allowing the tissue to access nutrients and reagents
supplied via culture medium as well as disposing of cellular waste.
This system mimics the nutrient exchange between capillaries and
tissue in vivo. When the culture medium is perfused into the
microchannel, the fluid layer near the channel is renewed rapidly
while the liquid within the chamber mostly recirculates (31). The nutrients from the channels
gradually diffuse into the chamber while the waste products diffuse
out from the chamber.
In vitro irradiation of tissue in a
microfluidic device
Ten parallel microfluidic devices perfusing HNSCC
tissue from the same patient were set up each time and following 24
h of incubation were subjected to single-dose irradiation in
duplicate (0, 5, 10, 15 and 20 Gy). Irradiation was applied to the
tissue under the guidance of medical radiation physicists, using a
6MV X-ray beam from a Varian Clinical Linear Accelerator. During
irradiation, the microfluidic device was housed inside a perspex
block which served as a surrogate for the tissue around the tumour
(Fig. 1C). At a dose rate of 600
MU/min, computerised tomography planning calculated that each beam
delivered 53 MU, producing a dose of 1 Gy to the centre of the
tissue at gantry angles of 90° and 270° with an 8×8 cm field.
Following a further 24 h of culture post-irradiation, tissue was
embedded in OCT embedding medium (CellPath Ltd., UK) and
snap-frozen in liquid nitrogen-cooled 2-methyl-butane (Sigma, UK)
prior to cryosectioning for IHC analysis.
Measurement of lactate dehydrogenase
(LDH)
The release of LDH was measured using the LDH
Cytotoxicity Kitplus (Roche Diagnostics, Hertfordshire,
UK) according to the manufacturer's instructions. Medium alone
values were subtracted from experimental readings before
normalising by the weight of tissue to give LDH released/mg. Values
were grouped according to a 4-h interval to give a mean before and
after irradiation.
Immunohistochemistry (IHC)
Tissue sections (8 µm) were cut using a
cryotome (Leica CM 1100) and mounted onto StarFrost®
glass slides (SLS, Nottingham, UK). IHC staining was carried out as
described previously (18). The
primary antibodies used in the present study were monoclonal
primary mouse anti-human antibodies [M30 (cCK18; Peviva,
Tewkesbury, UK), CK (Clone MNF116; Dako, Denmark), phosphorylated
H2AX (Clone 2F3; γH2AX; BioLegend, UK) and Ki-67; (Clone MIB-1;
Dako)] at a 1:100 dilution for 1 h at room temperature. Matched
isotype control antibodies at the same concentration provided a
non-specific binding control. Antibody binding was detected with
biotinylated horse anti-mouse secondary antibody and an
avidin/biotin system linked to horse peroxidase (vector
Laboratories Ltd., Peterborough, UK), and subsequent reaction with
3, 3′-diaminobenzidine (DAB; Sigma). Sections were counterstained
with Harris haematoxylin (Sigma), dehydrated through graded
ethanols (70, 90 and 100%), and three changes of Histoclear, before
mounting with Histomount (National Diagnostics, Hessle, UK).
Terminal deoxynucleotidyl transferase
dUTP nick end labelling (TUNEL)
To detect DNA fragmentation, 8 µm tissue
sections were fixed in 4% (w/v) paraformaldehyde for 20 min before
being washed with phosphate-buffered saline (PBS; pH 7.4) for 30
min (32). The cells were
permeabilised (0.1% Triton X-100 and 0.1% sodium citrate), rinsed
twice with PBS and incubated with terminal deoxynucleotidyl
transferase (TdT) and TUNEL label reagent containing fluorescent
dUTP, according to the manufacturer's instructions (Roche
Diagnostics) for an hour in a dark humidified box at 37°C. The
tissue sections were rinsed three times with PBS before being
mounted with Vectashield® mounting medium containing 4′,
6-diamidino-2-phenylindole (DAPI; Vector Laboratories Ltd).
Additional tissue sections were subjected to 3,000 U/l DNase (Roche
Diagnostics) treatment prior to TdT incubation to serve as a
positive control while sections that were not exposed to TdT enzyme
after DNase treatment served as a negative control.
Quantification and statistical
analysis
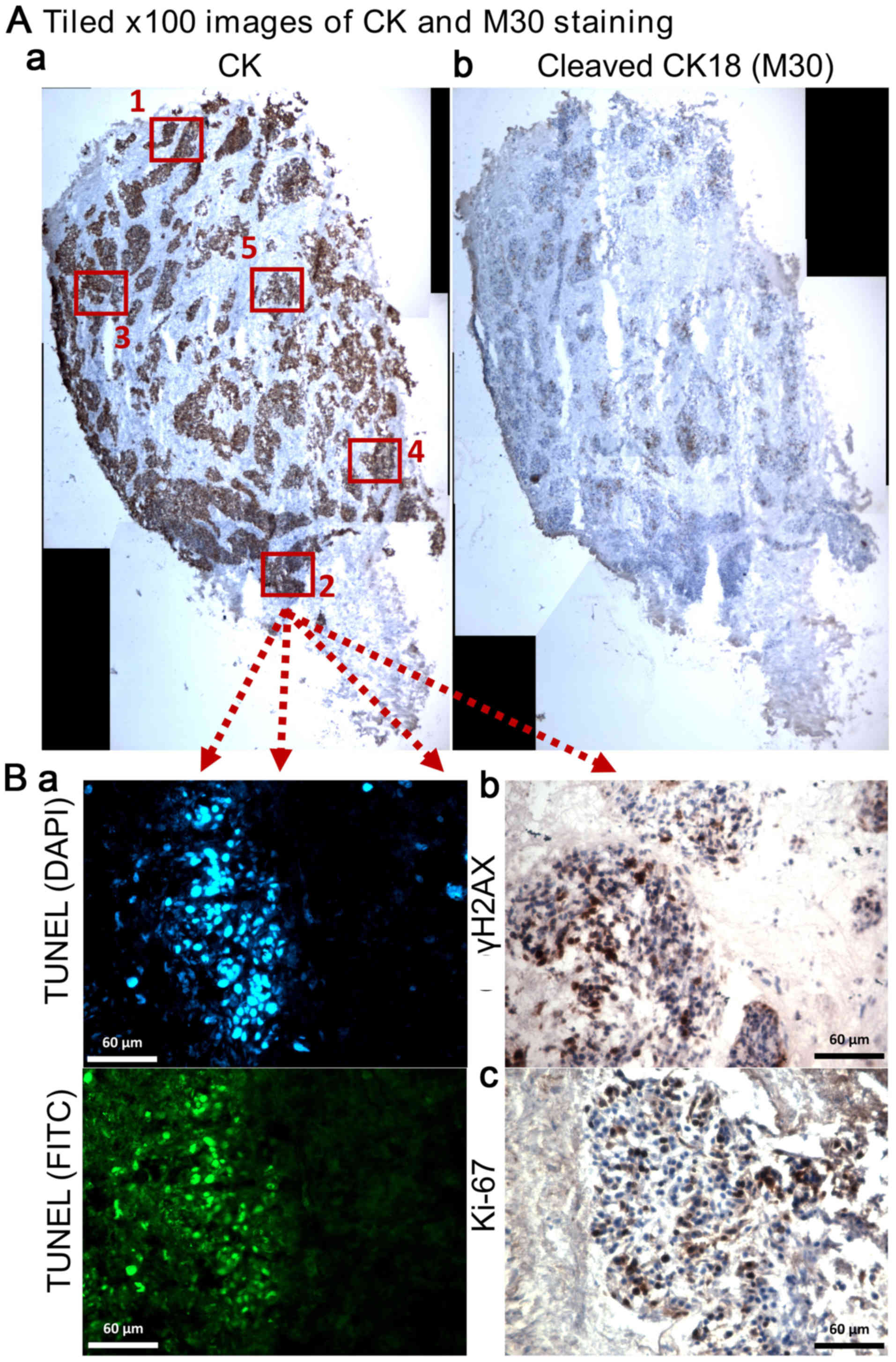
A tiled image of the whole tumour section was
constructed under ×100 magnification and positive staining for M30
and CK on serial sections was evaluated using Image Pro-premier
software (Digital Imaging Systems; V9; Fig. 2A). The labelling index of cCK18 was
determined as the apoptotic area (M30 positive staining) over the
total area of tumour cells (CK positive staining). γH2AX, TUNEL and
Ki-67 were evaluated using five randomly selected tumour fields of
×400 magnification (Fig. 2B). The
number of positively stained nuclei (γH2AX and Ki-67) and total
nuclei in each field were counted manually using the Point or
Multi-point function of ImageJ 1.48v (Java 1.6.0_20 64-bit) and the
percentage positivity determined. The area of FITC (green; DNA
fragmentation) staining over nuclear DAPI area (blue) on each ×400
magnification field was determined and the mean percentage of five
fields obtained (Fig. 2B). The
mean percentage of duplicate tissues at each irradiation dose were
obtained and statistical differences between non-irradiated control
and irradiated tissues were determined using one-way ANOVA followed
by Tukey's multiple comparison test (IBM SPSS Statistics 22).
Results
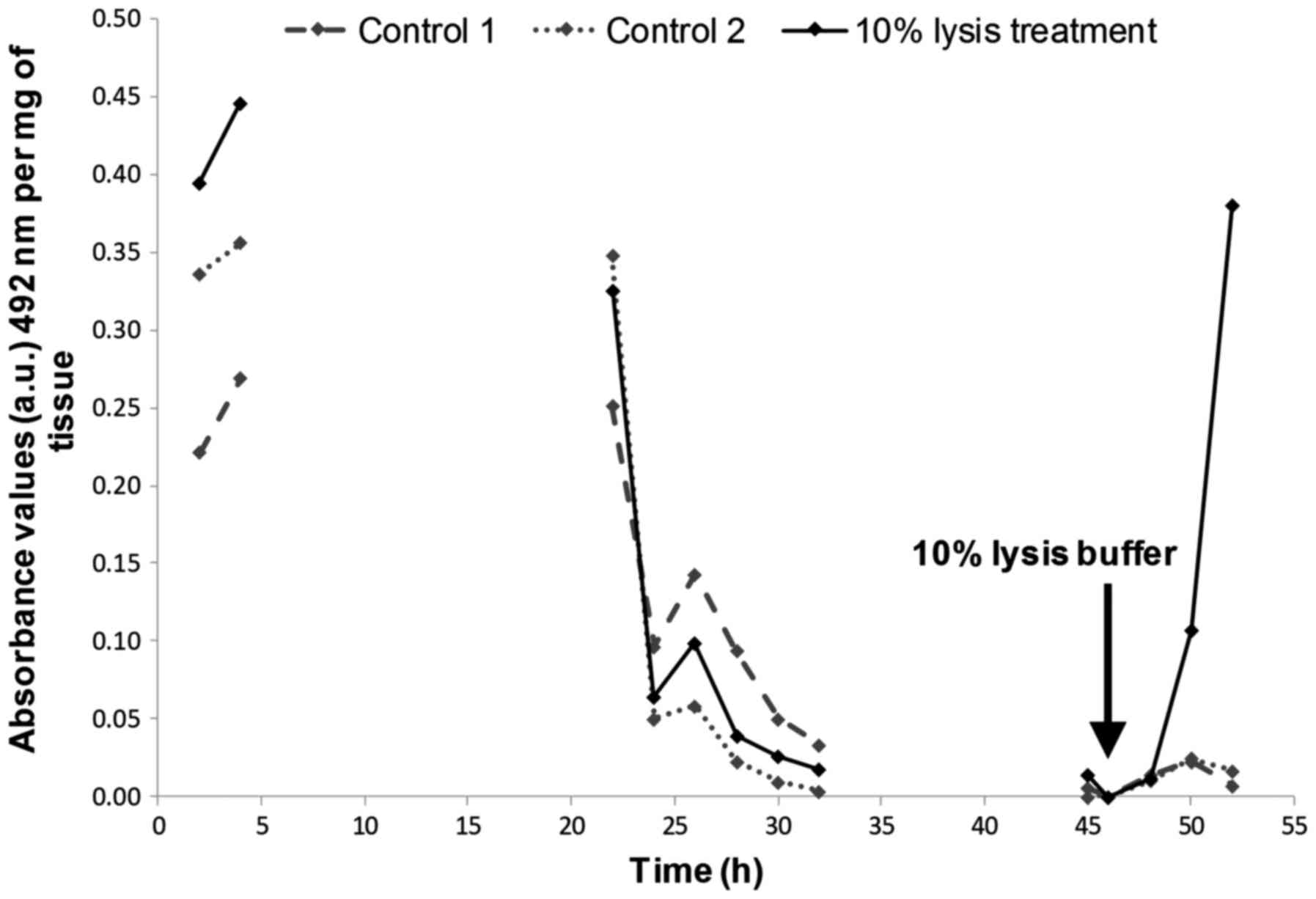
The effect of irradiation on the release
of LDH
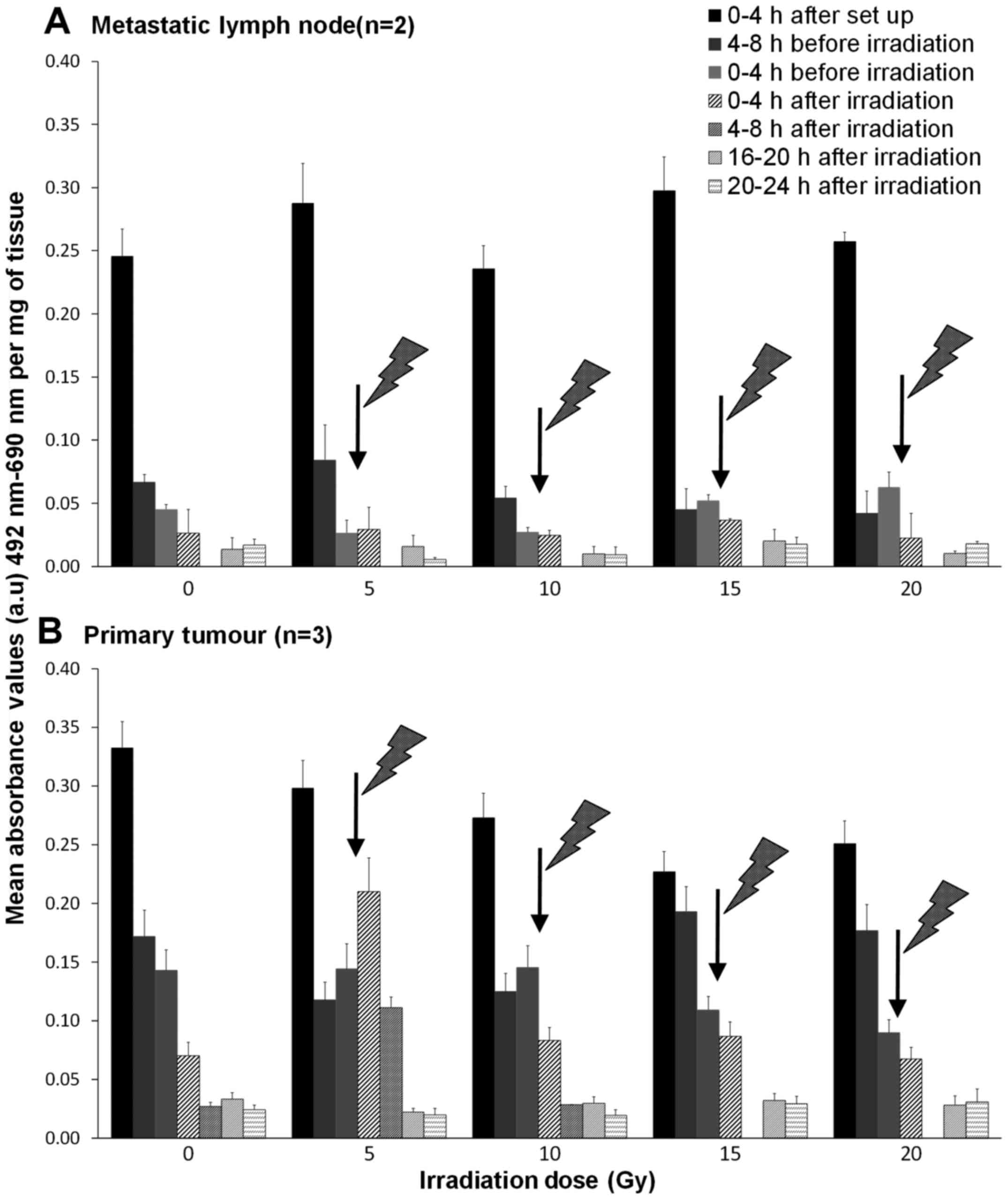
An initial high release of LDH was observed within
the first 4 h after experimental set-up in all tissues (Fig. 3), after which, LDH release
decreased and remained low in control tissues. Unexpectedly, the
same was true for both the metastatic node and the tumour tissues
subjected to irradiation, except for the primary tumours receiving
a 5 Gy irradiation. In the 5 Gy treated biopsies, the LDH release
increased by 45.5% during the first 4-h after irradiation and
decreased to values similar to that of the control thereafter
(Fig. 3B).
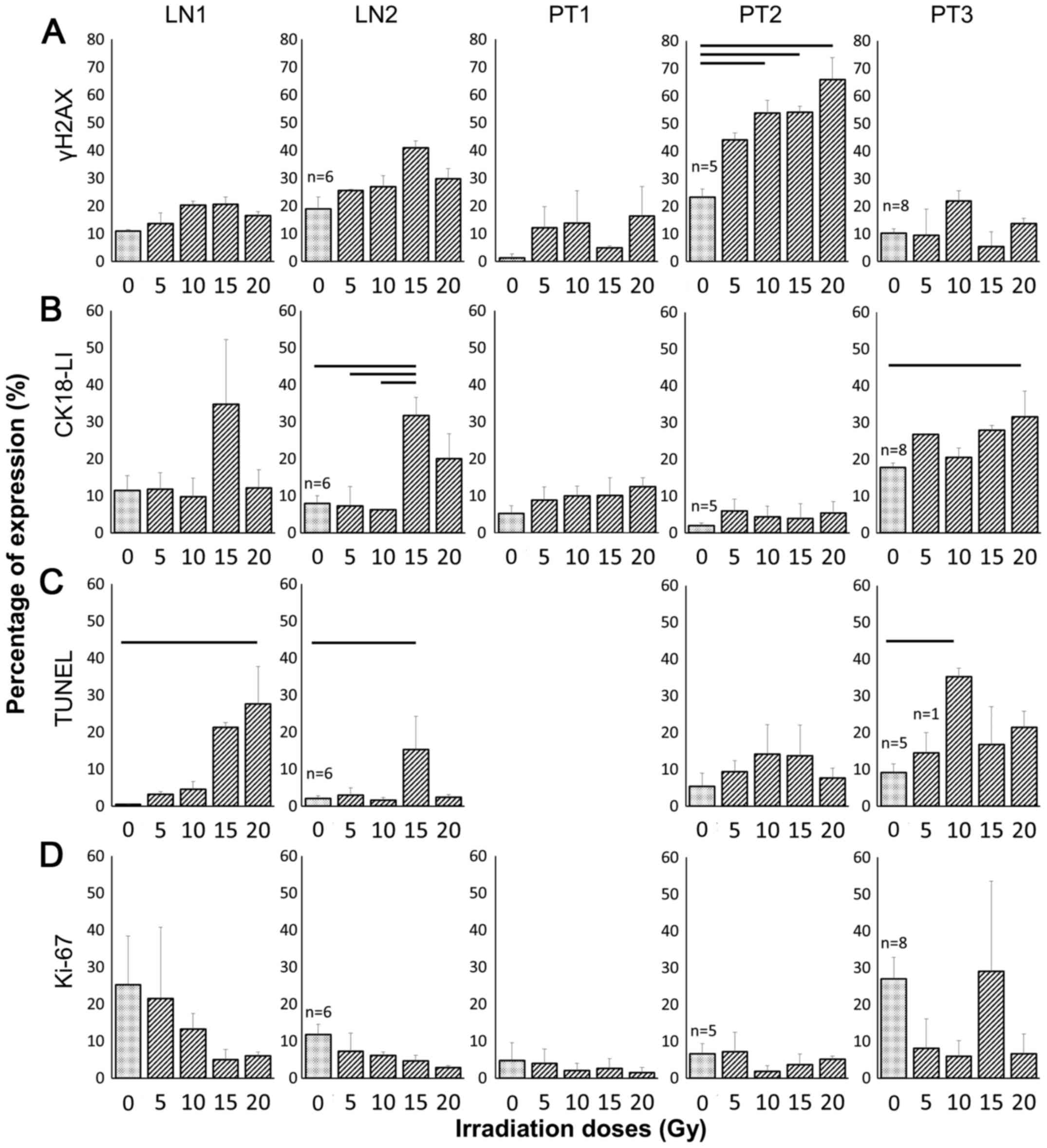
The effect of irradiation on γH2AX
expression
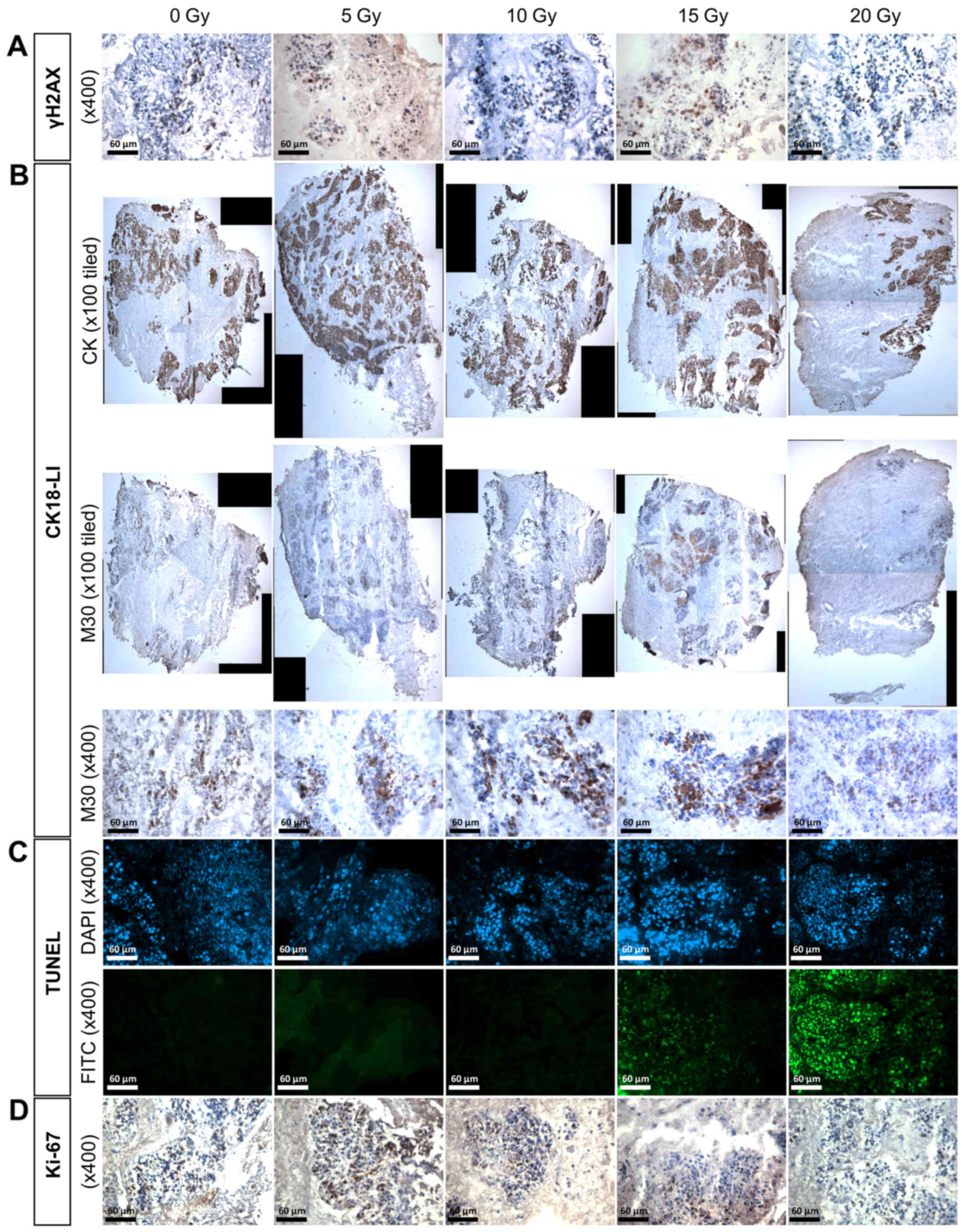
Clear positive brown nuclear staining of γH2AX, but
not individual foci, was detected in the HNSCC tissue (Fig. 4A) and irradiation of the two lymph
node tissues (LN1 and 2) resulted in up to twice as many positive
γH2AX cells compared with the control at both 10 and 15 Gy,
however, the increase was not significant (Fig. 5A). Irradiation of primary tumours 1
and 3 also induced an increase in γH2AX expression at some
irradiation doses, but again, these were not statistically
significant. Despite demonstrating almost twice the basal level of
γH2AX expression in the control compared with the other primary
tumour tissues, PT2 demonstrated a significant dose-dependent
increase in γH2AX expression following treatment with 10, 15 and 20
Gy irradiation (p= 0.022; p=0.020; p=0.003, respectively), reaching
almost three times the percentage of positive nuclei at the 20 Gy
dose (23.3±3.0% control vs. 66.0±8.0% 20 Gy).
The effect of irradiation on CK18-LI
Immunostaining with the M30 antibody demonstrated
apoptotic cells with brown cytoplasm (Fig. 4B). A dose of 15 Gy induced an
elevated CK18-LI in LN1 (34.8±17.4%) and LN2 (31.7±4.9%) compared
with the corresponding controls (11.4±4.0 and 7.9±2.1%,
respectively; Fig. 5B), but the
difference was only significant in LN2 (p<0.05). The
non-irradiated PT3 sample had greater apoptotic cell death
(17.7±1.3%) compared with the corresponding controls in other
primary tumours (5.2±2.2% PT1; 1.9±0.8% PT2) and irradiation
induced a dose-dependent increase in apoptosis >10 Gy, however,
the difference between irradiated tissue and controls was only
significant following 20 Gy irradiation (31.5±7.0% vs. control
17.7±1.3%; p=0.014). No significant increases in CK18-LI were
observed for PT1 or PT2 following irradiation.
The effect of irradiation on DNA
fragmentation
Green fluorescent nuclei demonstrated the presence
of incorporated dUTP in apoptotic cells (Fig. 4C). Insufficient tissue meant that
the evaluation of cells positive for DNA fragmentation in PT1
samples was not possible. The basal fraction of cells demonstrating
fragmented DNA was <10% in all control tissues investigated, and
this increased by >2-fold in the four tumours after irradiation,
but this increase was not consistent across all doses and only
significant following 20 Gy irradiation of LN1 (27.6±10.2%), 15 Gy
irradiation of LN2 (15.3±9.0%) and 10 Gy irradiation of PT3
(35.2±2.3%).
The effect of irradiation on
proliferation
Positive brown nuclei, representative of Ki-67
positivity, were observed in controls as well as 5 Gy irradiated
tissues (Fig. 4D). Although the
percentage of cells positive for the proliferation marker Ki-67
decreased in a dose-dependent manner in both metastatic nodal
tissues, the decrease was not significant compared to the controls
(Fig. 5D). The degree of
proliferation in PT3 decreased by approximately 5-fold following
all irradiation doses except 15 Gy but the decrease was again not
significant. In both the floor of mouth tumours (PT1 and PT2), the
basal level of proliferation was <10% making any effects of
irradiation difficult to observe.
Comparison between in vitro results and
clinical outcome
In order to determine whether the microfluidic
culture of HNSCC tissue and its subsequent irradiation can predict
whether a tumour is radioresistant/responsive, the data obtained
in vitro was compared with the in vivo response to
irradiation (Table I). The samples
were collected between April and August 2013 with a minimum
2.5-year follow-up. At follow-up, no clinical information was
recorded for LN2 and three of the remaining four patients were
alive with no evidence of loco-regional recurrence (LN1, PT2 and
PT3), however, only two of these patients (LN1 and PT3) received
any form of radiotherapy. The patient with a laryngeal supraglottic
tumour (PT3; T2 tumour) who was treated clinically with
radiotherapy had a positive outcome after 3 years with no
loco-regional recurrence diagnosed, this is in agreement with the
in vitro results which showed a positive response to
irradiation in terms of increased cell death (both CK18-LI and
TUNEL). LN1 received chemoradiotherapy with a positive outcome
which was again reflected in the increased cell death levels
(TUNEL) and a trend towards reduced proliferation (Ki-67) observed
following on-chip irradiation.
Discussion
The biological mechanisms present in malignant cells
which confer resistance to ionising radiation are largely unknown.
The use of immortalised cell lines to investigate these mechanisms,
although a good starting point, are not truly representative of the
original in vivo tumours in terms of architectural and
cellular complexity (33,34). The use of patient-derived xenograft
models offers the benefit of more closely reproducing the human
in vivo tumour microenvironment for various therapeutic
testing purposes. However, the process is lengthy (xenograft
generation takes up to six months), costly (tens of thousands of
dollars), uses a large number of animals and the tumour is not
entirely free from the rodent host influences (35–40).
Microfluidic culture of 3-dimensional pieces of patient-derived
tumour tissue (3 mm3) in a pseudo in vivo
microenvironment, has the potential to overcome many, if not all,
of the limitations described above (41). The continuous delivery of nutrients
and removal of waste from a piece of tissue whilst maintaining
complex multicellular architecture, without rodent influence, gives
microfluidic technology unique characteristics to be a platform for
preclinical biological investigations. The ability to run multiple
samples in parallel microfluidic devices is fundamental, to ensure
representative parts of the tumour are responding in the same way
to the treatment supplied, if this methodology is to be transferred
into the clinical setting, whereas the development of multiple
xenograft models for single regimen testing is much more difficult
(37,39).
The present study has demonstrated the novel
application of an in-house designed microfluidic device to
interrogate the response of HNSCC tissues to irradiation, in terms
of cell death and proliferation. Tumour perfusion and irradiation
were carried out on chip, mimicking the microenvironment in
vivo, whilst analysis of the markers was done either from the
effluent collected during the perfusion, or using the tissue
biopsies post-experiment. An initial peak of LDH release was
observed at the beginning of the perfusion which is consistent with
previous studies (18,22,23)
and is most probably a result of cell damage caused during tissue
manipulation. Previous studies which have used the same
microfluidic device have measured increased LDH release from
tissues in response to the addition of lysis buffer (23), when oxygen was removed from the
culture medium (24), and
following exposure to ethanol levels >100 mM (22), which helps to verify the viability
of tissue prior to induction of damage. Elevated LDH serum levels
in patients with nasopharyngeal carcinoma have also been detected
in vivo following treatment with intensity-modulated
radiotherapy (42). In contrast,
during the present study, the four single-dose irradiations ≤20 Gy
had no consistent effect in triggering necrotic cell death measured
by LDH release. These results are in agreement with the results
seen by Carr et al (18)
who only observed a consistent increase in LDH release from HNSCC
tissue following administration of the highest dose of irradiation
given (40 Gy). The lack of LDH release following irradiation was
not due to the fact that the tissue had all died during
manipulation as the addition of lysis reagent to the tissue at the
end of the microfluidic culture, post-irradiation induced a sharp
increase in LDH release confirming the viability of the tissue
(Fig. 6). It is probable that the
lack of LDH is due to the relatively short timeframe over which the
tissue was analysed.
Since radioresistance is multifactorial, the use of
more than one marker to predict HNSCC response is essential
(43). In comparison to the
preliminary study previously conducted by Carr et al
(18), additional markers were
evaluated to measure cell death and proliferation in both
non-irradiated and irradiated samples. One of the impacts of
ionising radiation is the formation of DNA double-strand breaks
(DSBs and the subsequent activation of the DNA-damage response
(DDR) pathway. TUNEL has been extensively used to detect DNA
fragmentation and later stages of apoptosis (44). An increase in the apoptotic rate
following irradiation on HNSCC cells has been demonstrated
previously by Feng et al (45) using TUNEL and the effect was
enhanced when ataxia telangiectasia mutated (ATM), an essential
component of the DNA repair pathway, was inhibited (45,46).
These results are in agreement with the data in the present study
which showed that the apoptotic rate of the HNSCC increased
following irradiation compared to the non-irradiated tissue which
had a low level of DNA-strand breaks.
The phosphorylation of the histone H2AX (γH2AX) is
one of the early events following the generation of DNA-DSBs and is
responsible for the recruitment of other molecules in the DDR
pathway (47). It is thought that
γH2AX levels increase to a peak expression at 1 h following
irradiation and return to normal within 24 h (28,48,49).
However, a higher retention level of γH2AX at 24 h following
irradiation has been observed in a radiosensitive cervical cancer
cell line compared to a non-sensitive one, where the remaining
γH2AX level correlated with the surviving fraction of cells
determined using the clonogenic assay (48), suggesting impaired DNA repair,
extended DNA repair and radiosensitivity (28,48,49).
The majority of the tumours in the current study, observed at 24 h
post-irradiation, had a slight increase in γH2AX following
irradiation compared with the corresponding control tissues,
however, this was only significant in one of the five tissues
(PT2). The increased expression of γH2AX seen in PT2 at 24 h
post-irradiation may suggest that this tumour is more sensitive to
irradiation, unfortunately this patient was treated with surgery
alone so no clinical correlation could be made.
The presence of cCK18, as a marker of activation of
the caspases involved in the apoptotic pathway, has been used
previously to demonstrate increases in apoptosis in tumour tissue
following chemotherapy treatments. Conflicting results have been
reported, however, as the rectal studies showed no prognostic value
for cCK18 (50,51), but in the
gastric/gastro-oesophageal cancer patients exposed to neoadjuvant
chemotherapy 43.6% of patients that had tumours positive for cCK18,
following treatment, had a favourable tumour response compared with
23.8% of patients with tumours negative for cCK18 expression
(52). In the present study, a
varied response to irradiation in terms of the CK18-LI was observed
between HNSCC samples from different sub-sites. For example, the
two oral cavity tumours (PT1 and PT2) showed minimal response to
single-dose irradiation whereas, the laryngeal supraglottic tumour
(PT3) demonstrated a dose-dependent increase following irradiation,
reaching significance compared with the control at 20 Gy; greater
numbers of tumours would be needed to clarify if these differences
are sub-site specific. Unexpectedly, two metastatic lymph nodes
(LN1 and LN2) had a higher CK18-LI (>30%) following 15 Gy
compared with control tissues which decreased again after 20 Gy
possibly due to increased cellular damage following such a high
single acute dose (53). The
varied responses observed among the five tumours confirmed the
inter-tumour variation on the HNSCC response to irradiation and
further emphasised the value for individual analysis of tumours to
determine the patient specific response.
A high pre-treatment proliferation index, measured
using Ki67, has been shown previously to correlate with reduced
survival/increased recurrence in patients with HNSCC following
irradiation (43,54), however, this is the first study to
use microfluidic culture to analyse the proliferation of tumour
cells in response to irradiation ex vivo. As hypothesised,
the expression of Ki-67 in both metastatic lymph nodes in the
present study followed a trend of dose-dependent reduction after
irradiation. This was in agreement with other studies which have
demonstrated a reduction of Ki-67-labelling by 79% in male Wistar
rat tissues that were removed from rats sacrificed 3 weeks
following 10 Gy γ-irradiation (55). In addition, a reduction of the
Ki-67 labelling index was observed during the first week following
five fractions (1.1 Gy per fraction) per week for seven weeks on
human normal skin biopsies (56).
A similar dose-dependent reduction was seen in the laryngeal tumour
(PT3) except in tissues with 15 Gy irradiation. The fact that 15 Gy
irradiation appeared to have little effect in the laryngeal tumour
biopsies, is likely to indicate intra-tumour heterogeneity and
highlights that in future experiments a greater number of
replicates need to be established for each treatment group. In
contrast, no effect of irradiation on proliferation was observed in
PT1 and PT2 samples, who were both treated clinically with surgery,
suggesting that these tumours might not be responsive to
irradiation.
In conclusion, although the authors acknowledge the
microfluidic maintenance of 3-dimensional tumour biopsies has
limitations, principally in the loss of vasculature, this
proof-of-concept study shows the potential of the
microfluidic-irradiation model and the IHC expression profiles to
determine the response of an individual's tumour to irradiation and
provides a system for further investigations of various treatment
regimens using a methodology applicable to all solid tumours. The
variation of the tumour responses detected between different HNSCC
samples and when treating samples from the same patient with
different irradiation doses suggests the existence of both inter-
and intra-patient variation respectively in terms of response to
irradiation in the microfluidic model, highlighting again the need
for such a model to customise treatment on an individual patient
basis prior to clinical intervention. The results show that a
larger scale investigation is the priority, running multiple
repeats so that the 'average' effect can be determined and
correlated with the corresponding patients' clinical behaviour. A
further modification to the approach being developed in our group
is to use precision cut tissue slices in a redesigned tissue device
which gives improved fluid flow dynamics, increasing the perfusion
kinetics, ensuring optimal nutrient delivery and waste removal.
Studies are ongoing comparing these two tissue-bearing devices.
Prediction of response would bring multiple benefits, firstly to
the patients in terms of treatment effectiveness and quality of
life and secondarily to the NHS in terms of cost reduction and
improved patient care.
Acknowledgments
We would like to thank Mr. J. Jose, consultant head
and neck surgeon, and the rest of the surgical team at Castle Hill
Hospital, Hull, United Kingdom, for providing the tissue samples;
Professor A. Beavis, consultant medical physicist and head of
radiation physics for the Hull and East Yorkshire Hospitals NHS
Trust, Mr. C. Horsfield, senior radiotherapy physicist, and other
physicists for their expertise and assistance with the irradiation
treatments.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Grégoire V, Lefebvre JL, Licitra L and
Felip E; EHNS-ESMO-ESTRO Guidelines Working Group: Squamous cell
carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol.
21(Suppl 5): V184–V186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galbiatti AL, Padovani-Junior JA, Maníglia
JV, Rodrigues CD, Pavarino EC and Goloni-Bertollo EM: Head and neck
cancer: Causes, prevention and treatment. Braz J Otorhinolaryngol.
79:239–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
The Royal College of Radiologists:
Radiotherapy Dose-fractionation. 2006, https://www.rcr.ac.uk/publication/radiotherapy-dose-fractionation.
Accessed December, 2013.
|
|
5
|
Burtness B: Moving forward in the
management of squamous cell carcinoma of the head and neck:
Promising immuno-oncology approaches. Am J Hematol Oncol. 11:28–31.
2015.
|
|
6
|
Sadraei NH, Sikora AG and Brizel DM:
Immunotherapy and checkpoint inhibitors in recurrent and metastatic
head and neck cancer. Am Soc Clin Oncol Educ Book. 35:e277–282.
2016. View Article : Google Scholar
|
|
7
|
Head and Neck NSSG; Head and Neck Network
Group: Head and Neck Cancer Treatment Guidelines. NHS; UK: 2014
|
|
8
|
American Cancer Society: Cancer Facts and
Figures 2015. American Cancer Society Inc; Atlanta, GA: 2015
|
|
9
|
Scaife L, Hodgkinson VC, Drew PJ, Lind MJ
and Cawkwell L: Differential proteomics in the search for
biomarkers of radiotherapy resistance. Expert Rev Proteomics.
8:535–552. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biau J, Chautard E, Miroir J and Lapeyre
M: Radioresistance parameters in head and neck cancers and methods
to radiosensitize. Cancer Radiother. 19:337–346. 2015. View Article : Google Scholar
|
|
11
|
Guy JB, Rancoule C, Méry B, Espenel S,
Wozny AS, Simonet S, Vallard A, Alphonse G, Ardail D,
Rodriguez-Lafrasse C, et al: Radiosensitivity and/or
radioresistance of head and neck cancers: Biological angle. Bull
Cancer. 103:41–47. 2016.In French. View Article : Google Scholar
|
|
12
|
Ataman OU, Bentzen SM, Wilson GD, Daley
FM, Richman PI, Saunders MI and Dische S: Molecular biomarkers and
site of first recurrence after radiotherapy for head and neck
cancer. Eur J Cancer. 40:2734–2741. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar B, Cordell KG, Lee JS, Worden FP,
Prince ME, Tran HH, Wolf GT, Urba SG, Chepeha DB, Teknos TN, et al:
EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as
indicators of response to therapy and survival in oropharyngeal
cancer. J Clin Oncol. 26:3128–3137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moeller BJ, Yordy JS, Williams MD, Giri U,
Raju U, Molkentine DP, Byers LA, Heymach JV, Story MD, Lee JJ, et
al: DNA repair biomarker profiling of head and neck cancer: Ku80
expression predicts locoregional failure and death following
radiotherapy. Clin Cancer Res. 17:2035–2043. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akervall J, Nandalur S, Zhang J, Qian CN,
Goldstein N, Gyllerup P, Gardinger Y, Alm J, Lorenc K, Nilsson K,
et al: A novel panel of biomarkers predicts radioresistance in
patients with squamous cell carcinoma of the head and neck. Eur J
Cancer. 50:570–581. 2014. View Article : Google Scholar
|
|
16
|
Kilic S, Cracchiolo B, Gabel M, Haffty B
and Mahmoud O: The relevance of molecular biomarkers in cervical
cancer patients treated with radiotherapy. Ann Transl Med.
3:2612015.PubMed/NCBI
|
|
17
|
Sharma A, Bode B, Wenger RH, Lehmann K,
Sartori AA, Moch H, Knuth A, Boehmer L and Broek M: γ-Radiation
promotes immunological recognition of cancer cells through
increased expression of cancer-testis antigens in vitro and in
vivo. PLoS One. 6:e282172011. View Article : Google Scholar
|
|
18
|
Carr SD, Green VL, Stafford ND and
Greenman J: Analysis of radiation-induced cell death in head and
neck squamous cell carcinoma and rat liver maintained in
microfluidic devices. Otolaryngol Head Neck Surg. 150:73–80. 2014.
View Article : Google Scholar
|
|
19
|
Ma H, Xu H and Qin J: Biomimetic tumor
microenvironment on a microfluidic platform. Biomicrofluidics.
7:115012013. View Article : Google Scholar
|
|
20
|
Halldorsson S, Lucumi E, Gómez-Sjöberg R
and Fleming RM: Advantages and challenges of microfluidic cell
culture in polydimethylsiloxane devices. Biosens Bioelectron.
63:218–231. 2015. View Article : Google Scholar
|
|
21
|
van der Meer AD and van den Berg A:
Organs-on-chips: Breaking the in vitro impasse. Integr Biol.
4:461–470. 2012. View Article : Google Scholar
|
|
22
|
Hattersley SM, Sylvester DC, Dyer CE,
Stafford ND, Haswell SJ and Greenman J: A microfluidic system for
testing the responses of head and neck squamous cell carcinoma
tissue biopsies to treatment with chemotherapy drugs. Ann Biomed
Eng. 40:1277–1288. 2012. View Article : Google Scholar
|
|
23
|
Hattersley SM, Dyer CE, Greenman J and
Haswell SJ: Development of a microfluidic device for the
maintenance and interrogation of viable tissue biopsies. Lab Chip.
8:1842–1846. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheah LT, Dou YH, Seymour AM, Dyer CE,
Haswell SJ, Wadhawan JD and Greenman J: Microfluidic perfusion
system for maintaining viable heart tissue with real-time
electrochemical monitoring of reactive oxygen species. Lab Chip.
10:2720–2726. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hakem R: DNA-damage repair; the good, the
bad, and the ugly. EMBO J. 27:589–605. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mahaney BL, Meek K and Lees-Miller SP:
Repair of ionizing radiation-induced DNA double-strand breaks by
non-homologous end-joining. Biochem J. 417:639–650. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Willers H, Azzoli CG, Santivasi WL and Xia
F: Basic mechanisms of therapeutic resistance to radiation and
chemotherapy in lung cancer. Cancer J. 19:200–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Olive PL and Banáth JP: Kinetics of H2AX
phosphorylation after exposure to cisplatin. Cytometry B Clin
Cytom. 76:79–90. 2009. View Article : Google Scholar
|
|
29
|
Taneja N, Davis M, Choy JS, Beckett MA,
Singh R, Kron SJ and Weichselbaum RR: Histone H2AX phosphorylation
as a predictor of radiosensitivity and target for radiotherapy. J
Biol Chem. 279:2273–2280. 2004. View Article : Google Scholar
|
|
30
|
McCreedy T and Wilson NG: Microfabricated
reactors for on-chip heterogeneous catalysis. Analyst (Lond).
126:21–23. 2001. View
Article : Google Scholar
|
|
31
|
Astolfi M, Péant B, Lateef MA, Rousset N,
Kendall-Dupont J, Carmona E, Monet F, Saad F, Provencher D,
Mes-Masson AM, et al: Micro-dissected tumor tissues on chip: An ex
vivo method for drug testing and personalized therapy. Lab Chip.
16:312–325. 2016. View Article : Google Scholar
|
|
32
|
Loo DT: TUNEL assay. An overview of
techniques. Methods Mol Biol. 203:21–30. 2002.PubMed/NCBI
|
|
33
|
Richmond A and Su Y: Mouse xenograft
models vs GEM models for human cancer therapeutics. Dis Model Mech.
1:78–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stein AP, Swick AD, Smith MA, Blitzer GC,
Yang RZ, Saha S, Harari PM, Lambert PF, Liu CZ and Kimple RJ:
Xenograft assessment of predictive biomarkers for standard head and
neck cancer therapies. Cancer Med. 4:699–712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang J, Liu A, Dougherty C, Chen X, Guzman
R and Nandi S: Beware of contaminating mouse cells in human
xenografts from nude mice. Anticancer Res. 20A:1635–1639. 2000.
|
|
36
|
Garrido-Laguna I, Uson M, Rajeshkumar NV,
Tan AC, de Oliveira E, Karikari C, Villaroel MC, Salomon A, Taylor
G, Sharma R, et al: Tumor engraftment in nude mice and enrichment
in stroma- related gene pathways predict poor survival and
resistance to gemcitabine in patients with pancreatic cancer. Clin
Cancer Res. 17:5793–5800. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kahn J, Tofilon PJ and Camphausen K:
Preclinical models in radiation oncology. Radiat Oncol. 7:2232012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kimple RJ, Harari PM, Torres AD, Yang RZ,
Soriano BJ, Yu M, Armstrong EA, Blitzer GC, Smith MA, Lorenz LD, et
al: Development and characterization of HPV-positive and
HPV-negative head and neck squamous cell carcinoma tumor-grafts.
Clin Cancer Res. 19:855–864. 2013. View Article : Google Scholar
|
|
39
|
Malaney P, Nicosia SV and Davé V: One
mouse, one patient paradigm: New avatars of personalized cancer
therapy. Cancer Lett. 344:1–12. 2014. View Article : Google Scholar :
|
|
40
|
Stebbing J, Paz K, Schwartz GK, Wexler LH,
Maki R, Pollock RE, Morris R, Cohen R, Shankar A, Blackman G, et
al: Patient-derived xenografts for individualized care in advanced
sarcoma. Cancer. 120:2006–2015. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dawson AL, Green VL, Bower R and Greenman
J: Microfluidics: The fur-free way towards personalised medicine in
cancer therapy. Univ Hull. 3:12–17. 2016.
|
|
42
|
Zhou GQ, Ren XY, Mao YP, Chen L, Sun Y,
Liu LZ, Li L, Lin AH, Mai HQ and Ma J: Prognostic implications of
dynamic serum lactate dehydrogenase assessments in nasopharyngeal
carcinoma patients treated with intensity-modulated radiotherapy.
Sci Rep. 6:223262016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Raybaud H, Fortin A, Bairati I, Morency R,
Monteil RA and Têtu B: Nuclear DNA content, an adjunct to p53 and
Ki-67 as a marker of resistance to radiation therapy in oral cavity
and pharyngeal squamous cell carcinoma. Int J Oral Maxillofac Surg.
29:36–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sundquist T, Moravec R, Niles A, O'Brien M
and Riss T: Timing your apoptosis assays. Cell Notes. 16:18–21.
2006.
|
|
45
|
Feng J, Zou J, Li L, Zhao Y and Liu S:
Antisense oligodeoxy-nucleotides targeting ATM strengthen apoptosis
of laryngeal squamous cell carcinoma grown in nude mice. J Exp Clin
Cancer Res. 30:432011. View Article : Google Scholar
|
|
46
|
Zou J, Qiao X, Ye H, Yang Y, Zheng X, Zhao
H and Liu S: Antisense inhibition of ATM gene enhances the
radiosensitivity of head and neck squamous cell carcinoma in mice.
J Exp Clin Cancer Res. 27:562008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Valdiglesias V, Giunta S, Fenech M, Neri M
and Bonassi S: γH2AX as a marker of DNA double-strand breaks and
genomic instability in human population studies. Mutat Res.
753:24–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Banáth JP, Macphail SH and Olive PL:
Radiation sensitivity, H2AX phosphorylation, and kinetics of repair
of DNA strand breaks in irradiated cervical cancer cell lines.
Cancer Res. 64:7144–7149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Clingen PH, Wu JY, Miller J, Mistry N,
Chin F, Wynne P, Prise KM and Hartley JA: Histone H2AX
phosphorylation as a molecular pharmacological marker for DNA
interstrand crosslink cancer chemotherapy. Biochem Pharmacol.
76:19–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Debucquoy A, Goethals L, Libbrecht L,
Perneel C, Geboes K, Ectors N, McBride WH and Haustermans K:
Molecular and clinico-pathological markers in rectal cancer: A
tissue microarray study. Int J Colorectal Dis. 24:129–138. 2009.
View Article : Google Scholar
|
|
51
|
Debucquoy A, Libbrecht L, Roobrouck V,
Goethals L, McBride W and Haustermans K: Morphological features and
molecular markers in rectal cancer from 95 patients included in the
European Organisation for Research and Treatment of Cancer 22921
trial: Prognostic value and effects of preoperative radio (chemo)
therapy. Eur J Cancer. 44:791–797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fareed KR, Soomro IN, Hameed K, Arora A,
Lobo DN, Parsons SL and Madhusudan S: Caspase-cleaved
cytokeratin-18 and tumour regression in gastro-oesophageal
adenocarcinomas treated with neoadjuvant chemotherapy. World J
Gastroenterol. 18:1915–1920. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Koukourakis MI: Radiation damage and
radioprotectants: New concepts in the era of molecular medicine. Br
J Radiol. 85:313–330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Buffa FM, Bentzen SM, Daley FM, Dische S,
Saunders MI, Richman PI and Wilson GD: Molecular marker profiles
predict locoregional control of head and neck squamous cell
carcinoma in a randomized trial of continuous hyperfractionated
accelerated radiotherapy. Clin Cancer Res. 10:3745–3754. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kee N, Sivalingam S, Boonstra R and
Wojtowicz JM: The utility of Ki-67 and BrdU as proliferative
markers of adult neurogenesis. J Neurosci Methods. 115:97–105.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Turesson I, Bernefors R, Book M, Flogegård
M, Hermansson I, Johansson KA, Lindh A, Sigurdardottir S, Thunberg
U and Nyman J: Normal tissue response to low doses of radiotherapy
assessed by molecular markers - a study of skin in patients treated
for prostate cancer. Acta Oncol. 40:941–951. 2001. View Article : Google Scholar
|