Introduction
Ewing's sarcoma (ES), characterized by small round
cells, is the second most common primary bone and soft tissue
malignant tumor that mainly affects children and adolescents
(1–3). The initial symptom of the primary ES
was usually intermittent pain that was not frequently felt during
night. Usually the most important clinical feature was a palpable
mass, which was found at the first visit in over one-third of the
patients (4). Owing to the
development of multiagent systemic chemotherapy and local control
methods over the past few decades, overall survival of the patients
with localised ES has risen to approximately 75% (5). However, the multimodality
chemotherapy inevitably results in serious toxicity and severe
side-effects, such as cardiotoxicity and nephrotoxicity.
Furthermore, nearly 25% of patients with ES have metastatic disease
at the time of diagnosis (1). For
these patients, cytotoxic chemotherapy has had much less effect on
the survival of patients with metastases at diagnosis (5). Furthermore, the effectiveness of
chemotherapy is seriously limited by the occurrence of drug
resistance (6). Thus, safer and
more effective anticancer drugs are urgently needed in clinical
practice.
Great efforts have been made to develop new
therapeutic agents against cancer using novel bioactive compounds
extracted from plants and other natural sources (7–9). Scutellaria baicalensis (SB),
known as Huang Qin in China, is widely used to treat various
diseases including inflammation, hypertension and bacterial and
viral infections with low side-effects as a traditional Chinese
medicine (10). Among over 50
different kinds of flavonoids currently derived and identified from
the root of SB, baicalein has been shown to exert a potent
antitumor and/or pro-apoptotic activity against different types of
cancers. Baicalein has been reported to suppress adhesion,
migration and invasion of MDA-MB-231 human breast cancer cells
(11). Baicalein can also
preferentially inhibit HCC tumor growth through inhibition of
MEK-ERK signaling and by inducing intrinsic apoptosis (12). Besides, baicalein can lead to
suppression of proliferation and induction of apoptosis in human
myeloma cells (13). Moreover,
baicalein has been demonstrated to suppresses colorectal carcinoma
cell invasion via inhibition of the ERK signaling pathways
(14). However, there have not yet
been any studies on the effects of baicalein on ES.
We therefore assumed that baicalein may also possess
an antitumor and/or pro-apoptotic activity in ES. Subsequently, we
investigated the effects of baicalein on viability, apoptosis,
migration and invasion of human ES SK-ES-1 cells and further
expound the related molecular mechanisms.
Materials and methods
Chemicals
Baicalein was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Baicalein was freshly prepared before each
experiment and was solubilized with dimethyl sulfoxide (DMSO). The
final concentration of DMSO in the medium was <0.1% (v/v) in the
treatment range (20–40 μM) and showed no influence on cell
growth (data not shown).
Reagents
RPMI-1640 medium, phosphate-buffered saline (PBS),
DMSO, bovine serum albumin (BSA) and Cell Counting kit-8 (CCK-8)
were purchased from TransGen Biotech, Inc. (Beijing, China). Fetal
bovine serum (FBS) was purchased from HyClone Laboratoriess (Thermo
Fisher Scientific, Waltham, MA, USA). An Annexin V-FITC/PI
(propidium iodide) Apoptosis Detection kit and Matrigel were
purchased from Becton-Dickinson (San Jose, CA, USA). The Transwell
invasion chambers were purchased from Costar (Cambridge, MA, USA).
Crystal violet staining solution and methanol were purchased from
Beyotime Institute of Biotechnology (Haimen, China). Hoechst 33258
staining kit was purchased from Keygen Nanjing KeyGen Biotech Co.,
Ltd. (Nanjing, China). Antibodies against Bax, Bcl-2, cytochrome
c, caspase-3, procaspase-9, PARP and β-actin were purchased
from Abcam (Cambridge, UK). Antibodies against cleaved caspase-8,
MMP-2 and MMP-9 were purchased from Wuhan Boster Biological
Technology, Ltd. (Wuhan, China). Horseradish peroxidase
(HRP)-conjugated secondary antibodies were purchased from TransGen
Biotech.
Cell culture
Human ES cell lines, SK-ES-1 and RD-ES, were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). Cells were cultured in RPMI-1640 medium supplemented with
10% (v/v) FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin. The cells were maintained in a humidified atmosphere
containing 5% CO2 at 37°C. The cells used in the present
study were subjected to <20 passages, and all cells used in this
study were in the logarithmic phase.
Cell viability by CCK-8 assay
Cell viability was determined using CCK-8 assay. The
cells were cultured in 96-well plates (5×103
cells/well). The cells were treated with baicalein at different
final concentrations (5, 10, 20, 40, 80 and 160 μM) for 24,
36 and 48 h, respectively, and the control cells were treated with
DMSO <0.1% (v/v). After indicated cultivation time, the medium
was changed to normal culture medium containing 10% CCK-8 solution
at 37°C for 1 h. Subsequently, the absorbance was measured at 450
nm using a Universal microplate reader (EL800; Bio-Tek Instruments,
Inc., Winooski, VT, USA). Cell viability as percent viability was
calculated by comparing the absorbance of treated cells vs. the
untreated ones.
Hoechst 33258 staining of SK-ES-1
cells
Cells were incubated with 0, 20 and 40 μM of
baicalein in 6-well plates for 24 h. Then cells were fixed with 4%
paraformaldehyde for 30 min at 25°C. Later on, the cells were
washed three times with ice-cold phosphate-buffered saline (PBS)
and stained with 10 mg/l Hoechst 33258 solution for 10 min in the
dark at 25°C. Subsequently, the stained nuclei were observed under
a fluorescence microscope (Olympus Corp., Tokyo, Japan) with
excitation at 350 nm and emission at 460 nm (original
magnification, ×200).
Analysis of cell apoptosis by Annexin
V-FITC/PI staining assay
Flow cytometry was conducted to assess the apoptosis
induced by baicalein. SK-ES-1 cells incubated with 0, 20 and 40
μM of baicalein for 24 h were collected, washed twice with
ice-cold PBS, and resuspended in 1X binding buffer at a
concentration of 1×106 cells/ml. The cell suspension
(100 μl) was incubated with 1 μl of Annexin V-FITC
and 2 μl of a PI solution in the dark for 15 min at 25°C.
The samples were analyzed on a FACSVerse flow cytometer (BD
Biosciences, San Jose, CA, USA) after the addition of 150 μl
of 1X binding buffer. The apoptosis rates were analyzed using the
FlowJo 7.6 software (Tree Star, Inc., Ashland, OR, USA).
Western blot analysis
SK-ES-1 cells were cultured in 6-well plates at a
concentration of 3×105 cells/well. After treatment with
0, 20 and 40 μM of baicalein for 24 h, the cells were
collected and lysed in RIPA buffer containing protease inhibitor
cocktail (Sigma-Aldrich). Subsequently the samples were centrifuged
at 17,105.6 × g for 10 min at 4°C to remove cell debris using a
Universal 320R centrifuge (Hettich Corp., Germany). Then the
supernatants were collected, and the protein concentrations were
determined by a BCA protein assay kit (Thermo Fisher Scientific).
Identical quantities of proteins were loaded, separated by sodium
dodecyl sulfate polyacrylamide gel electrophoresis, and then
transferred onto polyvinylidene difluoride membranes. The membranes
were incubated with 5% skim milk for 2 h. Then the membranes were
incubated overnight at 4°C with the primary antibodies.
Subsequently, the membranes were washed three times for 10 min with
1X TBST buffer and incubated for 2 h with horseradish
peroxidase-conjugated secondary antibodies at 25°C for 2 h.
Finally, antigenantibody complexes were detected using an enhanced
chemiluminescence detection system (Amersham Life Science, Inc.,
Pittsburg, PA, USA). The gray values of the bands were analyzed
using the ImageJ software (National Institutes of Health, Bethesda,
MD, USA).
Cell migration assay
Migration of SK-ES-1 cells was measured using wound
healing assays. SK-ES-1 cells were seeded in 6-well culture plates
(3×105 cells/well) to form a confluent monolayer, and
then cells were wounded with a sterile 100-μl pipette tip.
The cells in the plates were treated with baicalein at final
concentrations of 0, 20 and 40 μM, and then incubated in
fresh RPMI-1640 medium without FBS for 24 h. Scratch wounds were
then inspected using a phase-contrast microscope (Olympus) and
images of each wound were taken (original magnification, ×40).
Cell invasion assay
Invasion of SK-ES-1 cells was examined using
Matrigel-coated Transwell cell culture chambers (8 μm pore
size). Briefly, the membranes in each chamber were coated with 100
μg/ml Matrigel, after which 5×104 cells were
seeded into the upper chamber and treated with baicalein (0, 20 and
40 μM), and the lower wells were filled with RPMI-1640
medium supplemented with 20% (v/v) FBS in 24-well culture plates.
All the cells were incubated for 24 h at 37°C in an incubator
containing 5% CO2. Subsequently, the non-invaded cells
in the upper chamber were gently removed with a cotton swab,
whereas the cells attached to the lower surface were fixed with
precooled methanol and stained with 0.1% crystal violet solution.
Five fields of each chamber were randomly selected, and the cell
numbers were counted under a microscope (original magnification,
×100). The numbers of invaded cells were analyzed by the ImageJ
software.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD) of three independent experiments. Statistical analysis was
performed using the SPSS 19.0 software (SPSS, Inc., Chicago, IL,
USA). Student's t-test (two-tailed) was used to analyze the
differences between the two groups. P<0.05 was considered to be
statistically significant.
Results
Baicalein inhibits the viability of ES
cells
To study the effects of baicalein on the viability
of ES cells, SK-ES-1 and RD-ES cells were exposed to different
concentrations of baicalein for 24 h, and their viability was
determined by CCK-8 assay. As shown in Fig. 1A, baicalein significantly repressed
the viability of SK-ES-1 and RD-ES cells in a dose-dependent manner
(P<0.05). Besides, SK-ES-1 cells were more sensitive to
baicalein. The IC50 value for the SK-ES-1 cells treated
with baicalein was 28.1 μM at 24 h. Furthermore, it was
observed that baicalein suppressed the viability of SK-ES-1 in a
time- and dose-dependent manner (P<0.01) (Fig. 1B). Subsequently, SK-ES-1 cells were
treated with baicalein at the concentrations of 0, 20 and 40
μM for 24 h in the following assays.
Baicalein causes the nuclear changes of
SK-ES-1 cells
SK-ES-1 cells were treated with baicalein (0, 20 and
40 μM) for 24 h. It was found that baicalein produced
nuclear chromosomal condensation and fragmentation, which are the
typical morphological features of apoptotic cells, in SK-ES-1 cells
stained with Hoechst 33258 in a dose-dependent manner. These
findings suggested that cell death occurred through apoptosis
(Fig. 2). The arrows in Fig. 2 indicated the nuclear changes in
cells. Baicalein induces apoptosis in SK-ES-1 cells. Cell apoptosis
was measured by flow cytometry by double labeling with Annexin
V-FITC/PI. Representative graphs, which were obtained by flow
cytometric analysis of SK-ES-1 cells treated with baicalein at 0,
20 and 40 μM for 24 h, are shown in Fig. 3A. The apoptosis rate (the sum of
apoptotic rates of both early stage and late stage) in the control
group and SK-ES-1 cells treatment with baicalein at 20 and 40
μM for 24 h, was 6.7±1.5, 17.4±2.2 and 38.9±3.8%,
respectively. Compared with the control group, the apoptosis rate
in the treatment group significantly increased (P<0.01). The
treatment group was dose-dependent (Fig. 3B).
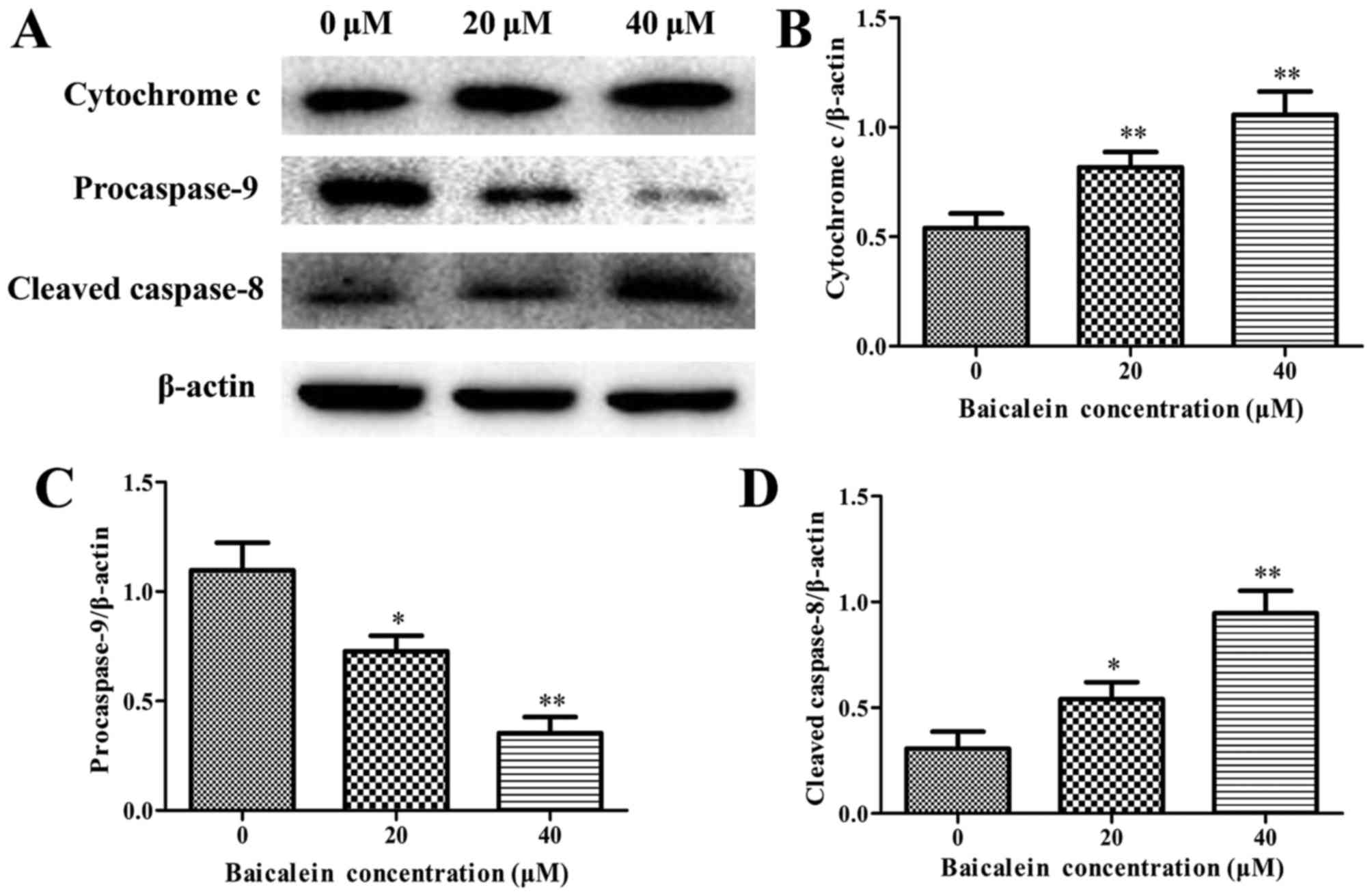
Effects of baicalein on the expression of
cell apoptosis-related proteins
The expression levels of anti-apoptotic Bcl-2,
proapoptotic Bax, cytochrome c, caspase-3, caspase-8,
caspase-9, and PARP were assessed by western blot analysis to
determine the molecular mechanism on baicalein induced apoptosis of
SK-ES-1 cells. The results showed that baicalein treatment caused a
remarkable increase in the expression of Bax and the release of
cytochrome c, whereas a decrease in Bcl-2 expression when
compared to in the control group (P<0.05) (Figs. 4 and 5A and B). Besides, the expression level
of procaspase-9 was downregulated, whereas the cleaved caspase-8
and the cleaved caspase-3 markedly increased in a dose-dependent
manner (P<0.05) (Figs. 5 and
6). The cleavage of PARP, a key
substrate of activated caspase-3, remarkably increased in a
dose-dependent manner (Fig. 6).
These findings revealed that baicalein induced ES cell apoptosis by
activation of caspase-3, caspase-8 and caspase-9.
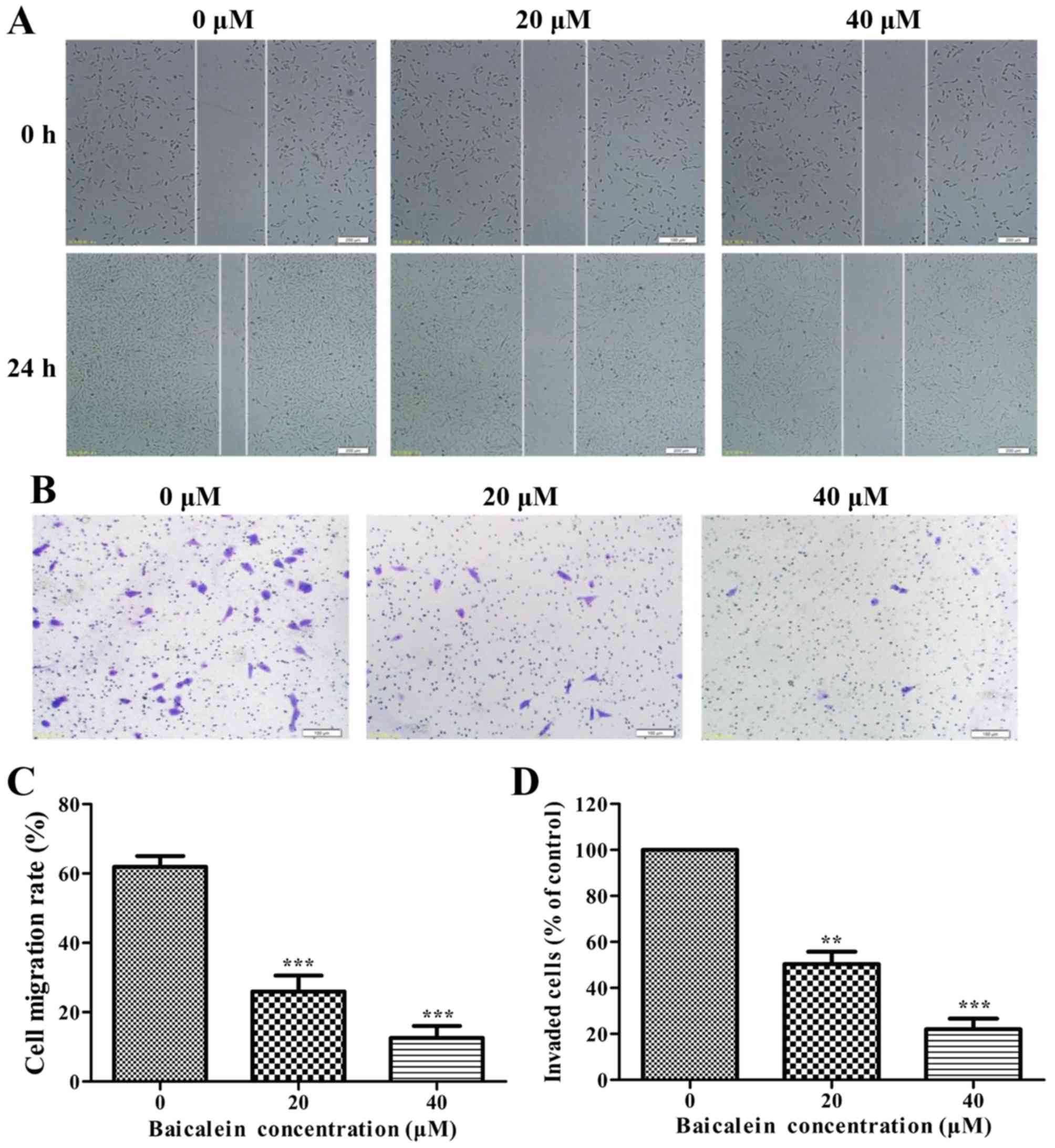
Baicalein inhibits cell migration and
invasion in SK-ES-1 cells
The effects of baicalein on the migration and
invasion of SK-ES-1 cells were detected by wound healing assays and
Boyden chamber Transwell assays, respectively. As shown in Fig. 7A, baicalein significantly repressed
the migration and invasion of SK-ES-1 cells in a dose-dependent
manner (P<0.01).
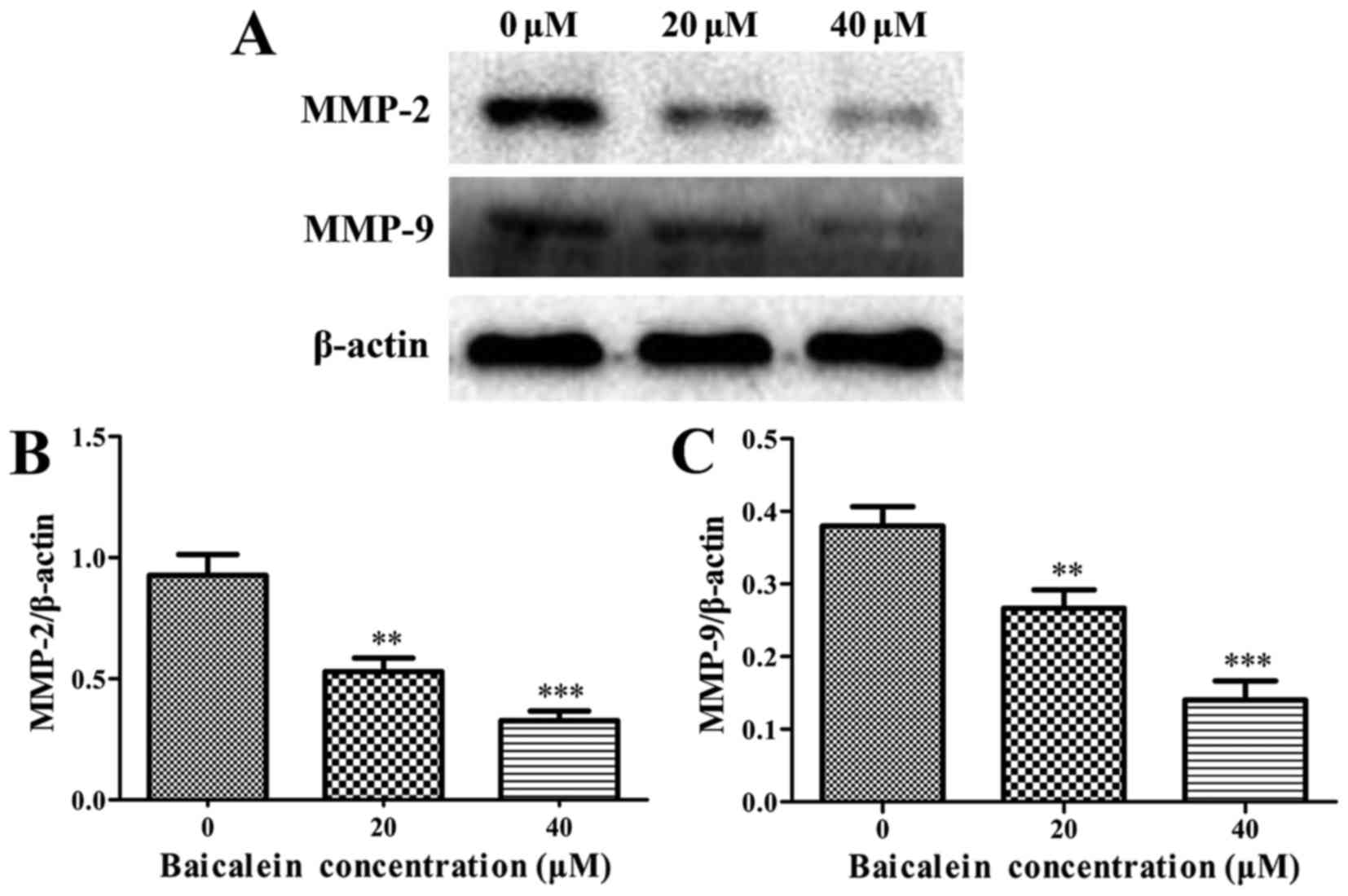
Baicalein decreases the expression levels
of MMP-2 and MMP-9
It is well-known that matrix metalloproteinases
(MMPs), among which MMP-2 and MMP-9 play the leading roles, can
facilitate the invasion and migration of tumor cells. It was found
that baicalein treatment group caused a significant decrease in
MMP-2 and MMP-9 when compared with the control group (P<0.01)
(Fig. 8). These results indicated
that baicalein inhibited migration and invasion in SK-ES-1 cells
through downregulating the expression of MMP-2 and MMP-9.
Discussion
Apoptosis (a programmed cell death), is an innate
process to eliminate abnormal or redundant cells in mammals and is
considered to be an important mechanism in the action of many
anticancer drugs (15). Growing
evidence shows that various kinds of herbal medicines and compounds
derived from natural products with antitumor effects can induce
apoptosis in various tumor cells (15–17).
Accumulating evidence demonstrates that baicalein can induce
apoptosis in a wide variety of cancer cell lines. Baicalein can
induce apoptosis of the human hepatoblastoma G2 cell line by
mitochondrial dysfunction and Bcl-2 regulation (18). Besides, as a lipoxygenase
inhibitor, baicalein can block both the 5-LOX and 12-LOX pathways
and therefore induce apoptosis in breast cancer cells through the
cytochrome c release and caspase-9 activation, with changes
in the levels of Bcl-2 family proteins (19). Furthermore, it has been reported
that baicalein is an effective anti-HCC agent with low cytotoxicity
to normal liver cells (12). Given
the above, baicalein is likely to exert potent antitumor effects
with few side-effects. Hence, we tried to explore the exact effects
of baicalein on ES and the related molecular mechanisms, which have
remained obscure.
It was seen from the present study that baicalein
significantly inhibited SK-ES-1 cell viability of human ES and
induced apoptosis in SK-ES-1 cells in a time- and dose-dependent
manner. Besides, baicalein can suppress the migration and invasion
of SK-ES-1 cells in a dose-dependent manner. All these findings are
consistent with the reported literature in other types of tumors,
which consider baicalein as an anticancer substance. To the best of
our knowledge, this is the first study to explore the effects of
baicalein on ES in vitro.
Then, we further explored the possible mechanisms of
apoptosis, which is the most important process in the function of
many anticancer drugs. Apoptosis occurs through two different
pathways: the intrinsic pathway and extrinsic pathway, which was
regulated by caspase-9 and caspase-8, respectively (20,21).
As a pivotal process in apoptosis, caspase activation is critical
for both extrinsic and intrinsic pathways. In the intrinsic pathway
(also known as the mitochondrial pathway), the activation of
downstream caspases is regulated by members of the Bcl-2 family.
Pro-apoptotic Bax-like proteins in the regulation of the formation
of pores in the mitochondria, which results in the release of
cytochrome c. Anti-apoptotic Bcl-2-like proteins exert the
completely opposite effect. Following the increase of the ratio of
Bax/Bcl-2, many apoptogenic proteins are released from the
mitochondrial intermembrane space, such as cytochrome c that
can further activate caspase-9. Caspase-3, an important executioner
caspase, is activated after the activation of caspase-8 and
caspase-9. Subsequently, active caspase-3 leads to the cleavage or
degradation of some key cellular substrates including PARP, which
results in the subsequent apoptosis (22–28).
The extrinsic pathway (also known as the death receptor pathway),
functioning in other ways, activates the death receptor (known as
Fas/FasL) on cell surface and then induces caspase-8 activation
(29–31). Our results indicated that
baicalein-induced apoptosis was accompanied with the increase of
the release of cytochrome c, Bax/Bcl-2 ratio, and the
activation of caspase-3, caspase-8 and caspase-9. Moreover, the
cleavage of PARP significantly increased in a dose-dependent
manner. The present data suggests that baicalein induces apoptosis
in ES cells through the mitochondrial apoptotic (intrinsic) pathway
and the death receptor (extrinsic) pathway.
Nearly 25% of patients with ES have metastatic
disease at the time of diagnosis. Besides, early metastasis also
contributes to the poor prognosis of ES. Therefore, we decided to
test also migration and invasion after apoptosis. Besides, the
relationship between the inhibition of migration/invasion and
increased apoptosis could be negatively correlated as we
speculated. It was found that baicalein inhibited the migration and
invasion of SK-ES-1 cells in a dose-dependent manner. Western blot
assays showed a marked reduction in the expression levels of MMP-2
and MMP-9, which are closely related with tumor invasion and
metastasis (32–34). Hence, it was demonstrated that
baicalein inhibits ES cell migration and invasion by decreasing
MMP-2 and MMP-9 expression.
There are some limitations to the present study.
First, the study included only in vitro experiments. To
further clarify the effects of baicalein on ES, studies on the
in vivo effects of baicalein on SK-ES-1 xenograft tumors in
nude mice are necessary. To finally assess the involvement of
caspase in cell death, experiments in the presence of caspase
inhibitor Z-VAD-FMK should be performed. Since this study focused
on merely SK-ES-1 cell line, we need to conduct the experiments on
other ES cell lines. It has been shown that baicalein can inhibit
tumors by targeting different proteins or signal pathways, such as
c-MYC, Wnt signaling pathway and TGF-β signaling pathway (35,36).
However, we did not try to discover a target of baicalein in
ES.
In summary, baicalein inhibits ES cell viability and
induces apoptosis through both the mitochondrial apoptotic pathway
and the death receptor pathway. Furthermore, baicalein can suppress
ES cell migration and invasion by decreasing MMP-2 and MMP-9
expression. Taken together, the conclusion from this study provides
in vitro evidence to support baicalein as an efficient
candidate agent for the chemoprevention and/or treatment of ES
progression. In addition, studies on the in vivo effect of
baicalein on SK-ES-1 xenograft tumors in nude mice are in
progress.
Acknowledgments
The present study was supported by the Natural
Science Foundation of Jiangxi Province (20171BAB205059), the
Foundation of the Health Department of Jiangxi Province on
Traditional Chinese Medicine (2016A073) and the Innovation Fund
Designated for Graduate Students of Jiangxi Province
(YC2016-S056).
References
|
1
|
Esiashvili N, Goodman M and Marcus RB Jr:
Changes in incidence and survival of Ewing sarcoma patients over
the past 3 decades: Surveillance Epidemiology and End Results data.
J Pediatr Hematol Oncol. 30:425–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gaspar N, Hawkins DS, Dirksen U, Lewis IJ,
Ferrari S, Le Deley MC, Kovar H, Grimer R, Whelan J, Claude L, et
al: Ewing sarcoma: Current management and future approaches through
collaboration. J Clin Oncol. 33:3036–3046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwamoto Y: Diagnosis and treatment of
Ewing's sarcoma. Jpn J Clin Oncol. 37:79–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Widhe B and Widhe T: Initial symptoms and
clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint
Surg Am. 82:667–674. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Balamuth NJ and Womer RB: Ewing's sarcoma.
Lancet Oncol. 11:184–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Newman DJ, Cragg GM, Gao Y, Du Z, Wang Y,
Cheng P, Chen A and Huang H: Natural products as sources of new
drugs over the last 25 years. J Nat Prod. 70:461–477. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cassileth B, Yeung KS and Gubili J: Herbs
and other botanicals in cancer patient care. Curr Treat Options
Oncol. 9:109–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu X, Zhou X, Fu C, Wang Q, Nie T, Zou F,
Guo R, Liu H, Zhang B and Dai M: Celastrol induces apoptosis of
human osteosarcoma cells via the mitochondrial apoptotic pathway.
Oncol Rep. 34:1129–1136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li-Weber M: New therapeutic aspects of
flavones: The anticancer properties of Scutellaria and its main
active constituents Wogonin, Baicalein and Baicalin. Cancer Treat
Rev. 35:57–68. 2009. View Article : Google Scholar
|
|
11
|
Wang L, Ling Y, Chen Y, Li CL, Feng F, You
QD, Lu N and Guo QL: Flavonoid baicalein suppresses adhesion,
migration and invasion of MDA-MB-231 human breast cancer cells.
Cancer Lett. 297:42–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang RR, Zhang S, Qi JA, Wang ZD, Li J,
Liu PJ, Huang C, Le XF, Yang J and Li ZF: Preferential inhibition
of hepatocellular carcinoma by the flavonoid Baicalein through
blocking MEK-ERK signaling. Int J Oncol. 41:969–978. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma Z, Otsuyama K, Liu S, Abroun S,
Ishikawa H, Tsuyama N, Obata M, Li FJ, Zheng X, Maki Y, et al:
Baicalein, a component of Scutellaria radix from
Huang-Lian-Jie-Du-Tang (HLJDT), leads to suppression of
proliferation and induction of apoptosis in human myeloma cells.
Blood. 105:3312–3318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chai Y, Xu J and Yan B: The
anti-metastatic effect of baicalein on colorectal cancer. Oncol
Rep. 37:2317–2323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang K, Wang X, Wang C, Zheng H, Li T,
Xiao S, Wang M, Fei C, Zhang L and Xue F: Investigation of
quinocetone-induced mitochondrial damage and apoptosis in HepG2
cells and compared with its metabolites. Environ Toxicol Pharmacol.
39:555–567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Song J, Wu D, Wang J and Dong W:
Hesperetin induces the apoptosis of hepatocellular carcinoma cells
via mitochondrial pathway mediated by the increased intracellular
reactive oxygen species, ATP and calcium. Med Oncol. 32:1012015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pieme CA, Santosh GK, Tekwu EM, Askun T,
Aydeniz H, Ngogang JY, Bhushan S and Saxena AK: Fruits and barks
extracts of Zanthozyllum heitzii a spice from Cameroon induce
mitochondrial dependent apoptosis and Go/G1 phase arrest in human
leukemia HL-60 cells. Biol Res. 47:542014. View Article : Google Scholar
|
|
18
|
Chang WH, Chen CH, Gau RJ, Lin CC, Tsai
CL, Tsai K and Lu FJ: Effect of baicalein on apoptosis of the human
Hep G2 cell line was induced by mitochondrial dysfunction. Planta
Med. 68:302–306. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tong WG, Ding XZ and Adrian TE: The
mechanisms of lipoxygenase inhibitor-induced apoptosis in human
breast cancer cells. Biochem Biophys Res Commun. 296:942–948. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lorenzo HK and Susin SA: Therapeutic
potential of AIF-mediated caspase-independent programmed cell
death. Drug Resist Updat. 10:235–255. 2007. View Article : Google Scholar
|
|
22
|
Chang HY and Yang X: Proteases for cell
suicide: Functions and regulation of caspases. Microbiol Mol Biol
Rev. 64:821–846. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao X, Bennett RL and May WS: c-Myc and
caspase-2 are involved in activating Bax during cytotoxic
drug-induced apoptosis. J Biol Chem. 283:14490–14496. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stennicke HR and Salvesen GS: Properties
of the caspases. Biochim Biophys Acta. 1387:17–31. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hui KK, Kanungo AK, Elia AJ and Henderson
JT: Caspase-3 deficiency reveals a physiologic role for Smac/DIABLO
in regulating programmed cell death. Cell Death Differ.
18:1780–1790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gillies LA and Kuwana T: Apoptosis
regulation at the mitochondrial outer membrane. J Cell Biochem.
115:632–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jourdain A and Martinou JC: Mitochondrial
outer-membrane permeabilization and remodelling in apoptosis. Int J
Biochem Cell Biol. 41:1884–1889. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wood WG, Igbavboa U, Muller WE and Eckert
GP: Statins, Bcl-2, and apoptosis: Cell death or cell protection?
Mol Neurobiol. 48:308–314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Villa-Morales M and Fernández-Piqueras J:
Targeting the Fas/FasL signaling pathway in cancer therapy. Expert
Opin Ther Targets. 16:85–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gordon N and Kleinerman ES: Aerosol
therapy for the treatment of osteosarcoma lung metastases:
Targeting the Fas/FasL pathway and rationale for the use of
gemcitabine. J Aerosol Med Pulm Drug Deliv. 23:189–196. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li H, Zhang K, Liu LH, Ouyang Y, Bu J, Guo
HB and Xiao T: A systematic review of matrix metalloproteinase 9 as
a biomarker of survival in patients with osteosarcoma. Tumour Biol.
35:5487–5491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Shi Q, Yuan TX, Song QL, Zhang Y,
Wei Q, Zhou L, Luo J, Zuo G, Tang M, et al: Matrix
metalloproteinase 9 (MMP-9) in osteosarcoma: Review and
meta-analysis. Clin Chim Acta. 433:225–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shang HS, Chang JB, Lin JH, Lin JP, Hsu
SC, Liu CM, Liu JY, Wu PP, Lu HF, Au MK, et al: Deguelin inhibits
the migration and invasion of U-2 OS human osteosarcoma cells via
the inhibition of matrix metalloproteinase-2/-9 in vitro.
Molecules. 19:16588–16608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He N and Zhang Z: Baicalein suppresses the
viability of MG-63 osteosarcoma cells through inhibiting c-MYC
expression via Wnt signaling pathway. Mol Cell Biochem.
405:187–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen F, Zhuang M, Peng J, Wang X, Huang T,
Li S, Lin M, Lin H, Xu Y, Li J, et al: Baicalein inhibits migration
and invasion of gastric cancer cells through suppression of the
TGF-β signaling pathway. Mol Med Rep. 10:1999–2003. 2014.
View Article : Google Scholar : PubMed/NCBI
|