Introduction
Colorectal cancer (CRC) is a common malignant tumor,
5-fluorouracil (5-Fu) and cisplatin (DDP) are commonly used in the
chemotherapy of CRC, but the resistance to chemotherapy is one of
the main obstacles in CRC treatment, which leads to the failure of
chemotherapy (1,2). WNT signaling pathway effector
β-catenin is the main oncoprotein in CRC subtypes CMS2 (consensus
molecular subtypes 2) (3).
Numerous studies indicate that the drug resistance abilities
enhancing, angiogenesis, presence of highly resistant cancer stem
cells (CSCs), dysregulation of cell cycle and apoptosis in CRC
cells were mainly due to WNT/β-catenin overactivation (4–7).
Genetic mutations in the β-catenin destruction
complex, which consists of Apc, Axin, Ck1 and GSK-3β, led to
β-catenin transportation to the nucleus and over activation in most
CRC cases (8–10). These genes may be the potential
anti-multidrug resistance targets for CRC, for their demonstrable
effect on β-catenin polyubiquitination and degradation (11,12).
The serine/threonine kinase GSK-3 was initially identified in rat
skeletal muscle as a kinase that phosphorylated and inactivated
glycogen synthase (GS) (13,14).
The two known GSK-3 family members, GSK-3α and GSK-3β, were
ubiquitously expressed and highly conserved. GSK-3β activity is
regulated by phosphorylation at serine 9, threonine 216 and other
residues. GSK-3β regulates a variety of physiological functions,
including cell proliferation, differentiation, motility, cycle
progression, apoptosis, and has become an attractive target for
chemotherapy (15–18). Therefore, numerous studies focus on
the biological behavior alteration of CRC cells after
activation/inhibition of GSK-3β by small molecule compounds.
Small-molecule ATP competitive BIO is regarded as a
classical GSK-3 inhibitor (19,20).
It inhibits not only GSK-3β, but also GSK-3α. More recent research
suggest that BIO inhibits also JAK1 and TYK2 (21). Importantly, the role of BIO in the
biological properties of cells, which joins two or more metabolic
and signal transduction pathways, may vary between cell types.
Studies reported that BIO suppressed the proliferation of ovarian
cancer cells (22) and induces
apoptosis of human melanoma cells (21), but enhanced the proliferation
capacity of rat marrow-derived mesenchymal stem cells and
cardiomyocytes (23,24).
In the present study, we evaluated the effects of
BIO on stemness, adhesive and drug resistance activities of CRC
SW480 and SW620 cells. In addition, the effects of BIO on GSK-3β
phosphorylation and WNT/β-catenin activity were studied. Our
results indicated that BIO affects stemness, adhesive and
chemoresistance abilities of CRC cells. The biological behavior
alteration in BIO treated CRC cells may partly attribute to
WNT/β-catenin activation.
Materials and methods
Chemicals
BIO was purchased from Sigma-Aldrich (St. Louis, MO,
USA) and 5-fluorouracil (5-Fu) was from Shanghai Xudong Haipu
Pharmaceutical Co., Ltd. (Shanghai, China). Cisplatin (DDP) was
purchased from Yunnan Biovalley Dengzhanhua Pharmaceutical Co.,
Ltd. (Kunming, China).
Cell culture
Human colon adenocarcinoma SW480 and SW620 cells
were grown as monolayers and routinely cultured as previously
described (12). Cell lines were
subjected to DNA tests and authenticated in our previous studies
(12). The cells were cultured in
RPMI-1640 medium (Gibco, Grand Island, NY, USA) with 10% fetal
bovine serum [FBS; Tianjin Institute of Blood (TBD), Tianjin,
China] at 37°C under an atmosphere of 5% CO2. Both cell
lines are regularly authenticated on the basis of viability,
recovery, growth, morphology and chemical response as well, were
most recently confirmed 3–6 months before use by using a short
tandem repeat method.
Microarray analysis
The main observation target after BIO treatment is
the expression level of WNT signaling pathway effector β-catenin.
The CRC SW480 and SW620 cells were treated for 24 h with 4
μM BIO in our experiments. Total RNA (3 repeated) was
extracted from CRC SW480 and SW620 cells. Integrity and
concentration of RNA was assessed after RNA extraction and prior to
sample labeling. NimbleGen One-Color DNA Labeling kit was used for
sample labeling. Hybridization was performed in NimbleGen
Hybridization System (Roche). After washing, slides were scanned
with Axon GenePix 4000B scanner. Data were extracted and normalized
using NimbleScan v2.5 software. Results are provided in the
NimbleScan Generated Data Folder. Further data analysis was
performed using Agilent GeneSpring GX v11.5 software and the
GenCLiP 2.0 online tool (http://ci.smu.edu.cn/).
Apoptosis analysis
The cells were treated for 24 h with BIO (4
μmol/l), 5-Fu (50 μmol/l), DDP (8 μmol/l), BIO
(4 μmol/l) + DDP (8 μmol/l) and BIO (4 μmol/l)
+ 5-Fu (50 μmol/l), separately. The cells were measured
using FACSAria flow cytometer (BD Biosciences, San Jose, CA, USA),
and Annexin V (+) cells were counted for apoptotic cells after
Annexin V/fluorescein isothiocyanate/propidium iodide (FITC/PI) (BD
Biosciences) double staining. The experiment was performed in
triplicate.
Cell cycle analysis
The cells were treated for 24 h with BIO (4
μmol/l). The cells were harvested and fixed in 70% ice-cold
ethanol at 4°C for 30 min. After washed with phosphate-buffered
saline (PBS), the cells were incubated with propidium iodide
staining buffer (BD Biosciences) for 15 min and analyzed by flow
cytometer. The experiment was performed in triplicate.
Flow cytometric analysis of
CD133-positive cell population
Human colon adenocarcinoma SW480 and SW620 cell
lines (1×106) were detached by treatment with 0.25%
trypsin/EDTA and washed twice with PBS. The cells were then
resuspended in 100 μl of staining buffer containing 1% FBS
and place on ice for 20 min to block Fc receptors. After incubating
with primary phycoerythrin antihuman CD133 antibody (Miltenyi
Biotec GmbH, Bergisch Gladbach, Germany) for another 10 min on ice
in the dark, the cells were washed twice with 1 ml of ice-cold
staining buffer and centrifuged (300 × g) for 10 min at 4°C. Cells
resuspended in 0.3 ml of 2% formaldehyde fixation buffer were
analyzed using a FACSAria flow cytometer and CellQuest software (BD
Biosciences). All flow cytometric results were obtained from two
independent experiments performed in triplicate.
Transwell migration and boyden chamber
invasion assays
For the Transwell migration assay, 105
cells in 100 ml of serum-free DMEM media were seeded in triplicate
in each fibronectin-coated polycarbonate membrane insert in a
Transwell apparatus (Corning, Inc., Corning, NY, USA). A total of
600 μl of 10% NCS in DMEM was added to the bottom chamber.
SW480 and SW620 cells were incubated at 37°C for 12 h. The inserts
were washed twice with prewarmed PBS. Cells adhered on the lower
surface were fixed with 100% methanol at RT for 15 min and stained
with hematoxylin for 15 min. Cell numbers in six predetermined
fields in each replicate were counted under the microscope (Nikon
Eclipse 80i system; ×200). All assays were independently repeated
at least three times. Cell invasion assays were performed as the
migration assay except the Transwell membrane was precoated with 24
mg/ml−1 Matrigel (R&D Systems, Minneapolis, MN, USA)
and the cells were incubated for 24 and 18 h, respectively.
Western blot analysis
Following treatment with different drugs for 24 h,
the cells were collected and lysed. Protein content was measured by
the BCA protein assay kit (Beyotime Institute of Biotechnology,
Shanghai, China) and 20 μg proteins per lane was separated
by 8–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred onto nitrocellulose membranes. Specific
protein bands were achieved with an ECL detection reagent (Pierce,
Rockford, IL, USA). Anti-β-catenin (Cell Signaling Technology,
Danvers, MA, USA) dilutions were 1:1,000. Anti-DCAMKL-1 and
anti-TERT (Abcam, Cambridge, MA, USA) dilutions were 1:300 and
1:800. Anti-EpCAM, anti-GAPDH and anti-α-tubulin (Cell Signaling
Technology) dilutions were 1:500, 1:1,000 and 1:1,000,
respectively. Anti-CD44v6 (Cell Signaling Technology) dilution was
1:1,000. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit
and goat anti-mouse IgG antibodies (ProteinTech Group, Chicago, IL,
USA) dilutions were 1:3,000. α-tubulin was used as a protein
loading control. The images were captured with ChemiDoc™ CRS+
Molecular Imager (Bio-Rad Laboratories, Hercules, CA, USA). The
density of the protein band was quantitated using the Quantity One
software (Bio-Rad Laboratories). The experiment was performed in
triplicate.
Statistical analysis
Statistical analysis was performed with the SPSS
13.0 software package (SPSS, Inc., Chicago, IL, USA). Two-class
significance analysis of microarrays (SAM) was used to identify
genes that were differentially expressed in before and after
BIO-treated CRC SW480 and SW620 cells, the statistical significance
was assessed by a false discovery rate (FDR). Data are presented as
mean ± SD. One way analysis of variance (ANOVA) was used for
apoptosis, CD133, cell cycle and western blot data analyses.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Screening differentially expressed genes
before and after BIO treatment in SW480 and SW620 cells
In order to survey the biological behavior
alteration induced by BIO in CRC cells, we conducted cDNA
microarrays (Roche Nimble Gen) to pick out the differentially
expressed genes before and after BIO-treated in CRC cells (Fig. 1A). CRC cell lines SW480 and SW620
were submitted to the gene chip experiment in the present study.
The SAM supervised analysis identified differentially expressed
genes of SW480 and SW620 cells after treatment with BIO,
respectively. The 545 differentially expressed genes consistently
upregulated or downregulated in SW480 and SW620 cells after
treatment with BIO were selected and used for further functional
annotation and enrichment analysis. As shown in Fig. 1B, functional annotations of a total
545 differentially expressed genes, which were jointly upregulated
or downregulated in SW480 and SW620 cells after treatment with BIO,
were generated using the GenCLiP 2.0 online tool. As shown in
Fig. 1C, heatmap showing the
clustering result of differentially expressed genes and literature
profile keywords based on GenCLiP 2.0 online tool analyzed between
before and after BIO-treated in SW480 and SW620 cells.
The key literature profile-based keywords before and
after BIO-treatment in SW480 and SW620 cells are extracellular
matrix, basement membrane, transforming growth factor, endothelial
growth factor, mesenchymal stem cell, cell differentiation, cell
adhesion and cell migration. Gene-gene interaction network of 41
selected genes were generated and shown in Fig. 1D using GenCLiP 2.0 online tool. The
differential signaling pathway comparisons between before and after
BIO-treatment are shown in Fig.
1E.
Effect of BIO on cell migration, invasion
and GSK-3β activity in SW480 and SW620 cells
Functional annotations of the differentially
expressed genes indicated that cell migration and invasion related
genes were changed significantly after treatment with BIO in SW480
and SW620 cells. The migration and invasion abilities were
determined through Transwell assays and boyden chamber assays
(Fig. 2A). As shown in Fig. 2B and C, the migration and invasion
abilities of SW480 and SW620 cells were significantly decreased
after treatment with BIO (P<0.05).
Detection the key components of cell migration and
invasion relative proteins by western blotting demonstrated that
the expression level of MMP-9 was decreased, while the level of
E-cadherin was elevated after BIO treatment in SW480 and SW620
cells (Fig. 2D–F; P<0.05), but
the expression level of CD44V6 was not altered significantly
(Fig. 2D–F; P>0.05). In
addition, BIO treatment in SW480 and SW620 cells downregulated the
expression level of p-GSK-3β (Fig.
2D–F; P<0.05), whereas the effects were slight in total
GSK-3β expression (Fig. 2D–F;
P>0.05).
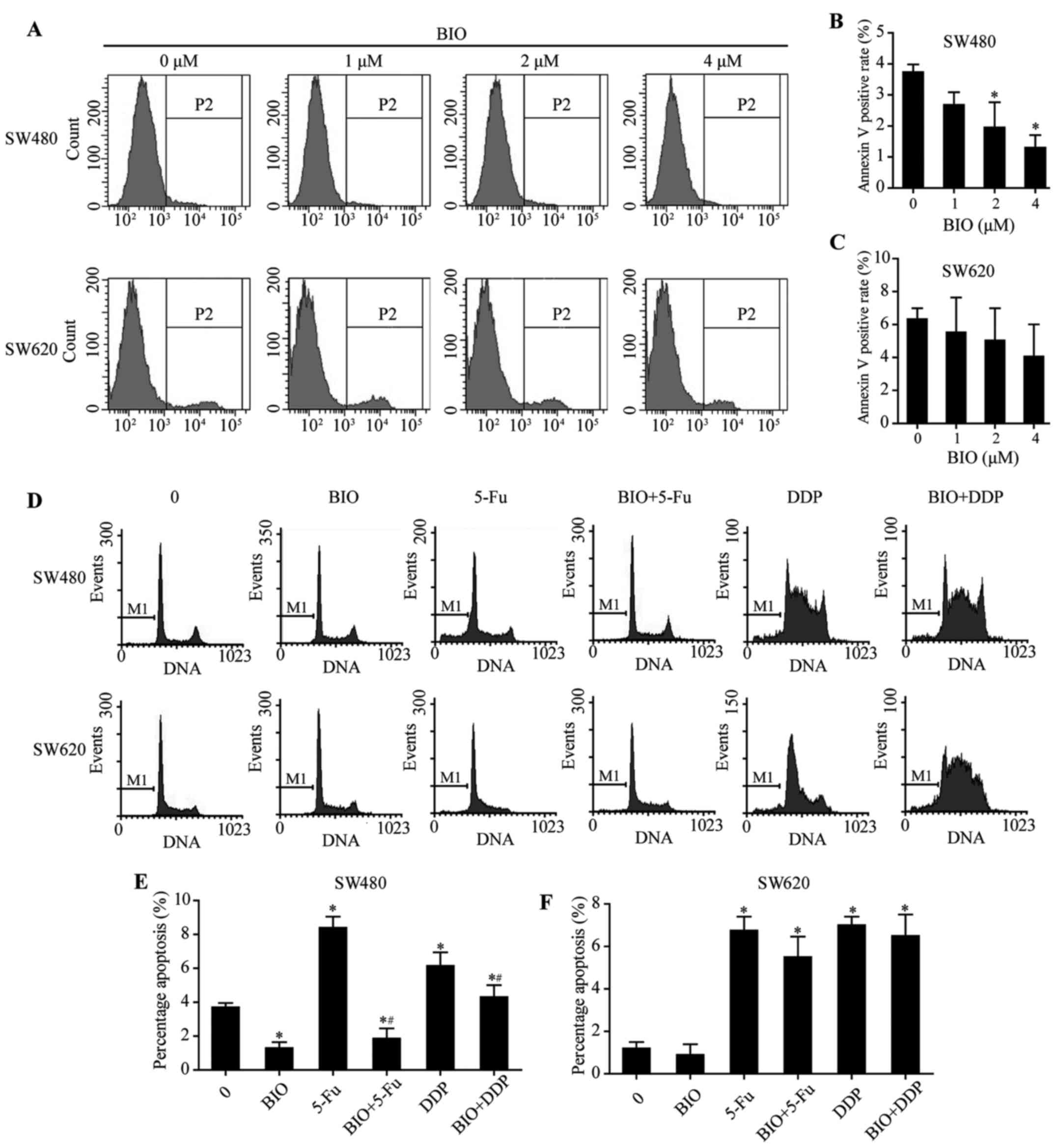
Effect of BIO on apoptosis in SW480 and
SW620 cells
After treatment with different concentrations of BIO
(0, 1, 2 and 4 μM), apoptosis of SW480 and SW620 cells were
examined using flow cytometry (Fig.
3A). When BIO concentration increased, apoptosis of SW480 cells
was decreased (Fig. 3B;
P<0.05), whereas the effects were slight in SW620 cells
(Fig. 3C; P>0.05). After
incubation with either BIO/5-Fu/DDP alone or combined 5-Fu/DDP with
BIO, cell apoptosis was examined using flow cytometry (Fig. 3D). Apoptosis was decreased in
BIO-treated SW480 cells (Fig. 3E;
P<0.05). In addition, BIO treatment significantly decreased
apoptosis induced by 5-FU/DDP in SW480 cells (Fig. 3E; P<0.05), whereas the effects
were slight in SW620 cells (Fig.
3F; P>0.05).
Effect of BIO on cell cycle progression
in SW480 and SW620 cells
Cell cycle distribution was determined by flow
cytometry after BIO treatment (Fig.
4A). There was no significant difference in the cell cycle
distribution after treatment with BIO/5-Fu alone or combining 5-Fu
with BIO in SW480 and SW620 cells (Fig. 4B; P>0.05).
In SW480 cells, the percentage of G0/G1 phase cells
were decreased, S+G2/M phase cells were increased after treatment
with DDP alone or combining DDP with BIO (Fig. 4B; P<0.05), but no significant
difference existed between the two groups (Fig. 4B; P>0.05). In SW620 cells, no
significant difference was found in the cell cycle distribution
after treatment with DDP alone or combining DDP with BIO (Fig. 4C; P>0.05). Collectively, these
results suggest that BIO has no significant effect on
5-Fu/DDP-induced cell cycle distribution.
Effect of BIO on proportion of
CD133+ SW480 and SW620 cells
CD133+ cells were examined using flow
cytometry after BIO treatment (Fig.
5A). As shown in Fig. 5B, the
proportions of CD133+ SW480 cells were decreased when
either 5-Fu or DDP was added (P<0.05). There was no significant
difference in the proportions of CD133+ SW480 cells
between treated with 5-Fu/DDP alone and combining 5-Fu/DDP with BIO
(Fig. 5B; P>0.05). In SW620
cells, after treated with 5-FU, the proportions of
CD133+ cells were decreased (Fig. 5C; P<0.05). BIO combining 5-Fu
treatment reversed the CD133+ cell downregulation trend
compared with treatment with 5-Fu alone (Fig. 5C; P<0.05). In addition, either
treated with DDP alone or combining DDP with BIO has no
significantly effect on the proportions of CD133+ SW620
cells (Fig. 5C; P>0.05).
Effect of BIO on CSC marker proteins in
SW480 and SW620 cells
To further investigate the effect of BIO on CSC
marker protein expression, total protein lysates were prepared and
analyzed by western blotting (Fig. 6A
and B). As shown in Fig. 6C–F,
following treatment of BIO, the expression levels of EpCAM, TERT
and DCAMKL-1 were increased in SW480 cells, EpCAM was increased in
SW620 cells (P<0.05), respectively.
In addition, BIO treatment combining 5-Fu/DDP
upregulated the expression levels of EpCAM, TERT and DCAMKL-1 in
SW480 cells (Fig. 6C and E;
P<0.05). In SW620 cells, BIO treatment upregulated the
expression levels of DCAMKL-1 in combining with 5-Fu, and
upregulated the expression levels of EpCAM in combining with DDP
(Fig. 6D and F; P<0.05).
Effect of BIO on β-catenin expression in
SW480 and SW620 cells
To further investigate the effect of BIO on proteins
involved in WNT/β-catenin signaling pathway expression, total
protein lysates were prepared and analyzed by western blotting. As
shown in Fig. 7A, following
treated with different doses of BIO, the expression levels of total
β-catenin increased in SW480 and SW620 cells (Fig. 7B; P<0.05), respectively. The
effect of BIO on β-catenin in SW480 and SW620 cells is
concentration-dependent. Western blot analysis of β-catenin protein
both nuclear and cytoplasmic (Fig.
7C) displayed that β-catenin was upregulated in the nuclei of
SW480 (Fig. 7D; P<0.05) and
SW620 cells (Fig. 7E; P<0.05)
after treatment with BIO, respectively.
Discussion
In the present study, to survey the tangible
biological behavior alteration induced by BIO in CRC cells, we
conducted cDNA microarrays to pick out the differentially expressed
genes after BIO-treatment in SW480 and SW620 cells. The cDNA
microarrays identified various genes, including mesenchymal stem
cell and cell migration relative genes, significantly aberrant
expression in BIO-treated SW480 and SW620 cells. In vitro
experimental results indicated that the apoptosis was decreased in
BIO-treated SW480 cells. More importantly, the apoptosis induced by
5-Fu/DDP was also decreased in BIO-treated SW480 cells. The
Transwell and boyden chamber experimental results demonstrated that
the migration and invasion abilities of SW480 and SW620 cells were
decreased after treatment with BIO, accompanying with MMP-9
downregulation and E-cadherin upregulation in these cells. The
MMP-9 has been widely documented in many physiological and
pathological processes including cell migration, invasion and
growth (25,26). E-cadherin, an important adhesive
factor, can produce a marked effect on mediating tissue integration
and cellular adhesiveness in form of complex with catenin (27). Therefore, cell motility related
protein alteration after BIO treatment may result in adhesive
activity enhancing the CRC cells.
We found that BIO treatment promotes both adhesive
activity and drug resistance in CRC cells, interestingly, in these
biological behaviors exist certain correlation in the mechanism. An
important theory indicated that cell adhesion mediated drug
resistance (CAM-DR) through suppressed drug-induced apoptosis
(28). Tumor cells grown as
multicellular spheroids were more resistant to chemotherapeutic
drugs compared to the same cells grown as dispersed monolayer cell
cultures (29). Possible
mechanisms have been put forward to account for CAM-DR.
E-cadherinmediated intracellular adhesion causing lack of
sufficient drug penetration into solid tumors is one of the
important molecular mechanisms for CAM-DR (30). In addition, various research has
suggested that with the adhesive activity increasing, drug
resistance protein such as P-gp was induced to express in cancer
cells (31). Our previous research
shows that treatment with different concentrations of BIO, the
expression level of P-gp and cellular efflux ability were
dose-dependently elevated in SW480 and SW620 cells (32). In the present study, our
experimental results suggested that BIO treatment promotes adhesive
activity through altering the expression levels of E-cadherin and
MMP-9 in SW480 and SW620 cells. Therefore, the aberrant expression
of E-cadherin and MMP-9 may also contribute to CAM-DR in
BIO-treated CRC cells.
Besides adhesiveness promotes drug resistance in
BIO-treated cells, we found that the stem cell relative proteins
had significant aberrant expression in BIO-treated SW480 and SW620
cells. It is generally known that CSCs are in dormant or
slow-growing phase of the cell cycle, such features contributing
for their therapeutic refractoriness to chemo/radiation therapy and
tumor relapse (33–35). Colorectal CSCs are characterized by
different types of CSC markers such as CD133, EpCAM, TERT and
DCAMKL-1 (36–39). In the present study, BIO treatment
upregulated the expression levels of EpCam, TERT and DCAMKL-1
proteins in SW480 or SW620 cells at various degrees. In addition,
treatment with BIO reversed the 5-FU-induced CD133+ cell
downregulation trend in SW620 cells. These results suggested that
the drug resistance ability enhancing after BIO treatment was
partly attributed to stemness increasing in CRC cells.
In the present study, we found that the biological
behavior alterations of SW480 and SW620 cells after treatment with
BIO were not entirely consistent. The reason may be that
heterogeneity was present between these two cell lines. Originally,
CRC cell line SW480 was isolated from a high-grade primary colon
tumor, CRC cell line SW620 was isolated from the same patient's
metastatic lymph node at the time of clinical relapse one year
later (40). In our previous
study, experimental results suggested that heterogeneity of drug
resistance was present between metastatic and primary CRC specimens
and cell lines (41). We found
that the ability of drug resistance in metastatic SW620 cells was
greater than primary colorectal cancer cells SW480 owing to cancer
stem cells and drug resistance relative protein activation.
Therefore, we believe that different reaction after BIO treatment
may partly attribute to heterogeneity between SW480 and SW620
cells.
As mentioned above, the effect of BIO on biological
behavior of CRC cells was complicated. There are a number of
independent studies reporting that BIO could evidently inhibit the
activity of GSK-3β through competing with ATP and influencing the
phosphorylation of GSK-3β (24).
The full activity of GSK-3β generally requires phosphorylation at
tyrosine 216, and conversely, phosphorylation at serine 9 inhibits
GSK-3β activity (42–44). Recently, the biological effects of
small molecule compounds on GSK-3β were widely studied for its
potential value on CRC treatment. It is reported that activation of
GSK-3β while downregulating the PI3-K/Akt oncogenic pathway by
non-steroidal anti-inflammatory drugs (NSAIDs), results uniformly
in the chemopreventive and anti-neoplastic effects in the early
stage of colon cancer (45). In
the present study, treated with BIO in CRC cells, the expression
levels of p-GSK-3β (Tyr 216) were decreased both in CRC SW480 and
SW620 cells, demonstrating that GSK-3β activity was significantly
inhibited by BIO. GSK-3β is the key component of the β-catenin
destruction complex in WNT pathway, and promotes
proteasome-mediated proteolysis of phosphorylated β-catenin
(46). As expected, subsequent
experimental results showed that BIO treatment upregulation and
transportation of β-catenin to the nucleus of SW480 and SW620
cells. It is generally believed that WNT signaling pathway effector
β-catenin plays an important role in the maintenance of stemness
and drug resistance ability of CSCs (47–49),
the chemoresistance increasing and stemness enhancing in
BIO-treated CRC cells may partly attribute to β-catenin activation.
Importantly, GSK-3β is a critical regulatory component of
WNT/β-catenin, NF-κB, phosphoinositide 3-kinase (PI3K)/AKT and
other signaling pathways (50–53).
The present study mainly focused on the effect and mechanism of BIO
on WNT/β-catenin signaling pathway. However, the important role of
other signaling pathways in BIO treated CRC cells, such as NF-κB
and PI3K/AKT, still need to be further evaluated.
Taken together, the results presented here
demonstrated that BIO upregulated stemness, adhesive and
chemoresistance of CRC cells. In addition, BIO treatment
downregulated the activity of GSK-3β, upregulated and transported
β-catenin to the nucleus, which may partly result in these
biological behavior alterations in CRC cells (Fig. 8). Of note, BIO is a multi-target
inhibitor. As proven by various research, BIO inhibits not only
GSK-3β activity, but also GSK-3α, JAK1 activities and possbly TYK2
(23–25). In the present study, the cDNA
microarray analysis results also suggested that BIO treatment
impact various signaling pathways. Therefore, using more than one
inhibitor and specific RNA interference will be a better way to
evaluate the role of GSK-3β inhibition in CRC cells for further
work. In addition, the activity alterations of these signaling
pathways in BIO treated CRC cells were not evaluate in present
study. The underlying molecular mechanism causing stemness,
adhesive and chemoresistance increase in CRC cells after BIO
treatment still need to be further evaluated.
Acknowledgments
The present study was sponsored by the Guangdong
Natural Science Foundation (nos. 2014A030307007 and no.
2017A030307005) and the Sci-Tech Project Foundation of Qingyuan
City (no. 2013A009).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peters GJ, Backus HH, Freemantle S, van
Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J,
Calvert AH, Marsh S, et al: Induction of thymidylate synthase as a
5-fluorouracil resistance mechanism. Biochim Biophys Acta.
1587:194–205. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guinney J, Dienstmann R, Wang X, de
Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda
G, Angelino P, et al: The consensus molecular subtypes of
colorectal cancer. Nat Med. 21:1350–1356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Z, Zhou C, Chang Y, Zhang Z, Hu Y,
Zhang F, Lu Y, Zheng L, Zhang W, Li X, et al: Long non-coding RNA
CASC11 interacts with hnRNP-K and activates the WNT/β-catenin
pathway to promote growth and metastasis in colorectal cancer.
Cancer Lett. 376:62–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng K, Zhou X, Yu J, Li Q, Wang H, Li M,
Shao Z, Zhang F, Luo Y, Shen Z, et al: Epigenetic silencing of
miR-490-3p promotes development of an aggressive colorectal cancer
phenotype through activation of the Wnt/β-catenin signaling
pathway. Cancer Lett. 376:178–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao Y, Liu Z, Zhang X, He J, Pan Y, Hao F,
Xie L, Li Q, Qiu X and Wang E: Inhibition of cytoplasmic GSK-3β
increases cisplatin resistance through activation of Wnt/β-catenin
signaling in A549/DDP cells. Cancer Lett. 336:231–239. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vermeulen L, De Sousa E, Melo F, van der
Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M,
Merz C, Rodermond H, et al: Wnt activity defines colon cancer stem
cells and is regulated by the microenvironment. Nat Cell Biol.
12:468–476. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morin PJ, Sparks AB, Korinek V, Barker N,
Clevers H, Vogelstein B and Kinzler KW: Activation of
beta-catenin-Tcf signaling in colon cancer by mutations in
beta-catenin or APC. Science. 275:1787–1790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kimelman D and Xu W: beta-catenin
destruction complex: Insights and questions from a structural
perspective. Oncogene. 25:7482–7491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prunier C, Hocevar BA and Howe PH: Wnt
signaling: Physiology and pathology. Growth Factors. 22:141–150.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cui J, Jiang W, Wang S, Wang L and Xie K:
Role of Wnt/β-catenin signaling in drug resistance of pancreatic
cancer. Curr Pharm Des. 18:2464–2471. 2012. View Article : Google Scholar
|
|
12
|
Wu X, Luo F, Li J, Zhong X and Liu K:
Tankyrase 1 inhibitior XAV939 increases chemosensitivity in colon
cancer cell lines via inhibition of the Wnt signaling pathway. Int
J Oncol. 48:1333–1340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Woodgett JR: Molecular cloning and
expression of glycogen synthase kinase-3/factor A. EMBO J.
9:2431–2438. 1990.PubMed/NCBI
|
|
14
|
Embi N, Rylatt DB and Cohen P: Glycogen
synthase kinase-3 from rabbit skeletal muscle. Separation from
cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J
Biochem. 107:519–527. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lucas JJ, Hernández F, Gómez-Ramos P,
Morán MA, Hen R and Avila J: Decreased nuclear beta-catenin, tau
hyperphosphorylation and neurodegeneration in GSK-3beta conditional
transgenic mice. EMBO J. 20:27–39. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takahashi-Yanaga F and Sasaguri T:
GSK-3beta regulates cyclin D1 expression: A new target for
chemotherapy. Cell Signal. 20:581–589. 2008. View Article : Google Scholar
|
|
17
|
Grassilli E, Narloch R, Federzoni E,
Ianzano L, Pisano F, Giovannoni R, Romano G, Masiero L, Leone BE,
Bonin S, et al: Inhibition of GSK3B bypass drug resistance of
p53-null colon carcinomas by enabling necroptosis in response to
chemotherapy. Clin Cancer Res. 19:3820–3831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Smith KS, Murphy M, Piloto O,
Somervaille TC and Cleary ML: Glycogen synthase kinase 3 in MLL
leukaemia maintenance and targeted therapy. Nature. 455:1205–1209.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Forde JE and Dale TC: Glycogen synthase
kinase 3: A key regulator of cellular fate. Cell Mol Life Sci.
64:1930–1944. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato N, Meijer L, Skaltsounis L, Greengard
P and Brivanlou AH: Maintenance of pluripotency in human and mouse
embryonic stem cells through activation of Wnt signaling by a
pharmacological GSK-3-specific inhibitor. Nat Med. 10:55–63. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu L, Nam S, Tian Y, Yang F, Wu J, Wang
Y, Scuto A, Polychronopoulos P, Magiatis P, Skaltsounis L, et al:
6-Bromoindirubin-3′-oxime inhibits JAK/STAT3 signaling and induces
apoptosis of human melanoma cells. Cancer Res. 71:3972–3979. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu AS and Zhao L: Effects of the GSK-3β
inhibitor (2Z,3E)-6-bromoindirubin-3′-oxime upon ovarian cancer
cells. Tumour Biol. 37:4857–4864. 2016. View Article : Google Scholar
|
|
23
|
Eslaminejad MB, Salami F, Mehranjani MS,
Abnoosi M and Eftekhari-yazdi P: BIO treatment enhances rat
marrow-derived mesenchymal stem cell in vitro proliferation and
viability. Physiol Pharmacol. 13:121–126. 2009.
|
|
24
|
Tseng AS, Engel FB and Keating MT: The
GSK-3 inhibitor BIO promotes proliferation in mammalian
cardiomyocytes. Chem Biol. 13:957–963. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu L, Zhang Q, Wu K, Chen X, Zheng Y, Zhu
C and Wu J: Hepatitis C virus NS3 protein enhances cancer cell
invasion by activating matrix metalloproteinase-9 and
cyclooxygenase-2 through ERK/p38/NF-κB signal cascade. Cancer Lett.
356:470–478. 2015. View Article : Google Scholar
|
|
26
|
Che YL, Luo SJ, Li G, Cheng M, Gao YM, Li
XM, Dai JM, He H, Wang J, Peng HJ, et al: The C3G/Rap1 pathway
promotes secretion of MMP-2 and MMP-9 and is involved in serous
ovarian cancer metastasis. Cancer Lett. 359:241–249. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou BP, Deng J, Xia W, Xu J, Li YM,
Gunduz M and Hung MC: Dual regulation of Snail by
GSK-3beta-mediated phosphorylation in control of
epithelial-mesenchymal transition. Nat Cell Biol. 6:931–940. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Petitclerc E, Deschesnes RG, Côté MF,
Marquis C, Janvier R, Lacroix J, Miot-Noirault E, Legault J,
Mounetou E, Madelmont JC, et al: Antiangiogenic and antitumoral
activity of phenyl-3-(2-chloroethyl)ureas: A class of soft
alkylating agents disrupting microtubules that are unaffected by
cell adhesion-mediated drug resistance. Cancer Res. 64:4654–4663.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shain KH and Dalton WS: Cell adhesion is a
key determinant in de novo multidrug resistance (MDR): New targets
for the prevention of acquired MDR. Mol Cancer Ther. 1:69–78.
2001.
|
|
31
|
Damiano JS, Cress AE, Hazlehurst LA, Shtil
AA and Dalton WS: Cell adhesion mediated drug resistance (CAM-DR):
Role of integrins and resistance to apoptosis in human myeloma cell
lines. Blood. 93:1658–1667. 1999.PubMed/NCBI
|
|
32
|
Liu KP, Luo F, Xie SM, Tang LJ, Chen MX,
Wu XF, Zhong XY and Zhao T: Glycogen synthase kinase 3β inhibitor
(2′Z,3′E)-6-Bromo-indirubin-3′-oxime enhances drug resistance to
5-fluorouracil chemotherapy in colon cancer cells. Chin J Cancer
Res. 24:116–123. 2012. View Article : Google Scholar
|
|
33
|
Yang HZ, Ma Y, Zhou Y, Xu LM, Chen XJ,
Ding WB and Zou HB: Autophagy contributes to the enrichment and
survival of colorectal cancer stem cells under oxaliplatin
treatment. Cancer Lett. 361:128–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
De Angelis ML, Zeuner A, Policicchio E,
Russo G, Bruselles A, Signore M, Vitale S, De Luca G, Pilozzi E,
Boe A, et al: Cancer stem cell-based models of colorectal cancer
reveal molecular determinants of therapy resistance. Stem Cells
Transl Med. 5:511–523. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McCubrey JA, Steelman LS, Abrams SL,
Misaghian N, Chappell WH, Basecke J, Nicoletti F, Libra M, Ligresti
G, Stivala F, et al: Targeting the cancer initiating cell: The
ultimate target for cancer therapy. Curr Pharm Des. 18:1784–1795.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakanishi Y, Seno H, Fukuoka A, Ueo T,
Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, et
al: Dclk1 distinguishes between tumor and normal stem cells in the
intestine. Nat Genet. 45:98–103. 2013. View Article : Google Scholar
|
|
37
|
Imrich S, Hachmeister M and Gires O: EpCAM
and its potential role in tumor-initiating cells. Cell Adhes Migr.
6:30–38. 2012. View Article : Google Scholar
|
|
38
|
Merlos-Suárez A, Barriga FM, Jung P,
Iglesias M, Céspedes MV, Rossell D, Sevillano M, Hernando-Momblona
X, da Silva-Diz V, Muñoz P, et al: The intestinal stem cell
signature identifies colorectal cancer stem cells and predicts
disease relapse. Cell Stem Cell. 8:511–524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Elsaba TM, Martinez-Pomares L, Robins AR,
Crook S, Seth R, Jackson D, McCart A, Silver AR, Tomlinson IP and
Ilyas M: The stem cell marker CD133 associates with enhanced colony
formation and cell motility in colorectal cancer. PLoS One.
5:e107142010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Leibovitz A, Stinson JC, McCombs WB III,
McCoy CE, Mazur KC and Mabry ND: Classification of human colorectal
adenocarcinoma cell lines. Cancer Res. 36:4562–4569.
1976.PubMed/NCBI
|
|
41
|
Luo F, Li J, Wu S, Wu X, Chen M, Zhong X
and Liu K: Comparative profiling between primary colorectal
carcinomas and metastases identifies heterogeneity on drug
resistance. Oncotarget. 7:63937–63949. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang H, Guo W, Liang X and Rao Y: Both
the establishment and the maintenance of neuronal polarity require
active mechanisms: Critical roles of GSK-3beta and its upstream
regulators. Cell. 120:123–135. 2005.PubMed/NCBI
|
|
43
|
Doble BW and Woodgett JR: GSK-3: Tricks of
the trade for a multi-tasking kinase. J Cell Sci. 116:1175–1186.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin CL, Wang JY, Huang YT, Kuo YH,
Surendran K and Wang FS: Wnt/beta-catenin signaling modulates
survival of high glucose-stressed mesangial cells. J Am Soc
Nephrol. 17:2812–2820. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jain S, Ghanghas P, Rana C and Sanyal SN:
Role of GSK-3β in regulation of canonical Wnt/β-catenin signaling
and PI3-K/Akt oncogenic pathway in colon cancer. Cancer Invest.
35:473–483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ikeda S, Kishida S, Yamamoto H, Murai H,
Koyama S and Kikuchi A: Axin, a negative regulator of the Wnt
signaling pathway, forms a complex with GSK-3beta and beta-catenin
and promotes GSK-3beta-dependent phosphorylation of beta-catenin.
EMBO J. 17:1371–1384. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang WJ, Wu MY, Shen M, Zhi Q, Liu ZY,
Gong FR, Tao M and Li W: Cantharidin and norcantharidin impair
stemness of pancreatic cancer cells by repressing the β-catenin
pathway and strengthen the cytotoxicity of gemcitabine and
erlotinib. Int J Oncol. 47:1912–1922. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Todaro M, Francipane MG, Medema JP and
Stassi G: Colon cancer stem cells: Promise of targeted therapy.
Gastroenterology. 138:2151–2162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Buss H, Dörrie A, Schmitz ML, Frank R,
Livingstone M, Resch K and Kracht M: Phosphorylation of serine 468
by GSK-3beta negatively regulates basal p65 NF-kappaB activity. J
Biol Chem. 279:49571–49574. 2004. View Article : Google Scholar : PubMed/NCBI
|