Introduction
E-cadherin, a 120-kDa transmembrane glycoprotein
encoded by the CDH1 gene located on 16q 22.1, is a prime
mediator of calcium dependent cell-cell adhesion and forms the key
functional component of adherens junctions between neighboring
homozygous cells (1). There is
increasing evidence that modulation of this complex by different
mechanisms, such as gene mutation (2,3),
loss of heterozygosity (LOH) (4,5) and
epigenetic and micro-RNA alternations (6–8), is
an important step in the initiation and propagation of human
cancers. Promoter methylation, a type of epigenetic alteration, is
considered to be the predominant mechanism of CDH1
inactivation. This mechanism has been recognized in many solid
tumors, including salivary adenoid cystic carcinoma (ACC) (6), eyelid and oral squamous cell
carcinoma (SCC) (9), gastric
cancer (8), breast cancer
(7,10), bladder cancer (11) and colorectal adenocarcinoma
(12,13).
The carcinoma ex pleomorphic adenoma (CXPA) is a
malignant tumor of the salivary gland that develops in or from a
recurrent or long-lasting benign pleomorphic adenoma (PA). This
tumor type comprises ~4% of all salivary tumors and 12% of all
salivary malignancies (14). Based
on the data of our department, CXPA is the second most common (tied
with acinic cell carcinoma) malignancy of the salivary gland in the
Chinese population, accounting for 8% of all salivary malignancies
(15). To date, however, the
expression of E-cadherin in human salivary CXPA has been
infrequently studied (16–18). Moreover, there are no reports
describing the relationship between CDH1 promoter
methylation and E-cadherin expression in salivary CXPA.
Furthermore, the association between molecular changes to the
CDH1 gene and tumor progression remains to be clarified.
In the present study, we evaluated CDH1
promoter methylation status and E-cadherin expression levels in 37
CXPA samples. We also correlated the promoter methylation status in
these tumors with clinical and pathological parameters to determine
the role of CDH1 methylation in the development and
progression of salivary CXPA. In addition, we analyzed the promoter
methylation status as well as the messenger RNA (mRNA) and protein
expression levels of CDH1 in 2 CXPA cell lines: SM-AP1 and
SM-AP4. To our knowledge, this is the first report of a
comprehensive analysis of CDH1 methylation in salivary CXPA
samples.
Materials and methods
Tissue samples and cell lines
Formalin-fixed and paraffin-embedded tissues from 37
cases of salivary CXPA with complete clinical and pathological data
were retrieved from the Department of Oral Pathology at Shanghai
Ninth People's Hospital, Shanghai Jiao Tong University in Shanghai,
China. Tissue sections (4 µm) were stained with hematoxylin
and eosin (H&E) and were reviewed by two investigators. The
tumors were histologically examined and classified as high or low
grade (19). High-grade tumors
exhibited ≥2 of the following features: i, anaplasia with nuclear
pleomorphism and prominent nucleolus; ii, frequent mitoses: ≥5 per
10 high-power fields; iii, atypical mitosis; and iv, extensive
coagulative tumor necrosis. The clinical stage of each patient's
disease was determined according to criteria of the tumor-lymph
node-metastasis (TNM) classification system (2002) International
Union Against Cancer (20). CXPAs
could be classified into 2 main subtypes according to their
morphological and immunohistochemical features. The classification
of CXPA-L and CXPA-NL in our study followed the methods detailed by
Kim et al (19). This study
was approved by the ethics committee of Shanghai Jiao Tong
University.
For our in vitro experiments, 2 CXPA cell
lines (SM-AP1 and SM-AP4) (21)
were cultured in DMEM supplemented with 100 U/ml penicillin, 100
mg/ml streptomycin, 2 mM glutamine and 10% fetal bovine serum (FBS)
and were incubated at 37°C in a humidified atmosphere containing 5%
CO2. Induction of CDH1 promoter hypermethylation
in SM-AP1 cancer cells was initiated by the addition of 10 ng/ml
TGF-β1 (Peprotech, NJ, USA) to the medium for up to 72 h. Induction
of CDH1 promoter demethylation in SM-AP4 cancer cells was
initiated by the addition of a demethylation agent,
5-Aza-2′-deoxycytidine (5-Aza-dC; Selleck, TX, USA).
DNA extraction and bisulfite-treated DNA
polymerase chain reaction (BSP) amplification and direct
sequencing
Genomic DNA was extracted from paraffin-embedded
tumor tissues and cultured cells using the QIAamp DNA
formalin-fixed paraffin-embedded (FFPE) tissue kit (Qiagen,
Duesseldorf, Germany) and the QIAamp DNA Mini kit (Qiagen),
respectively, according to the manufacturer's instructions. We
selected regions of malignant tumor as much as possible when
extracting DNA from tissue sections. Extracted DNA was treated with
sodium bisulfite using the EpiTect Bisulfite kit (Qiagen) according
to the manufacturer's protocol. Nested PCR primer sequences were as
follows: first round, 5′-GGTAAAAGAAAAAAAAATTAGTTTG-3′ and
5′-AATACCTACAACAACAACAACAA-3′; second round,
5′-TAGAGAGGTTGGGGTTAGAG-3′ and 5′-AACCCCTCCCCAAAACRAAACTAA-3′.
Methylation status was assessed at the CDH1 promoter region
by sequencing the PCR-amplified bisulfite-treated DNA using the
automated ABI PRISM 3730XL DNA sequencer (Applied Biosystems, USA),
as previously described (22). The
CDH1 promoter region studied here contains 10 CpG
dinucleotides located in the -571- to -230-bp fragment upstream of
the transcription start site. Ten random clones were selected from
each sample for sequencing. Methylation status was defined as low
[methylation rate (MR)≤20%], medium (20%<MR≤40%), or high
(MR≥40%).
Immunohistochemistry and evaluation
Immunohistochemistry (IHC) was performed on
4-µm paraffin-embedded sections according to the protocol.
An anti-E-cadherin receptor antibody (monoclonal mouse anti-human,
dilution 1:200; Life Technologies, USA) was applied as the primary
antibody for IHC detection. The IHC procedure was performed by the
Envision™ method (Dako, Glostrup, Denmark) according to the
manufacturer's protocol. In the negative control samples, primary
antibodies were replaced by PBS. Normal salivary gland tissue
slices served as a positive control. In the CXPA samples,
E-cadherin was located on the cell membrane and in the cytoplasm.
Five random high-power fields were chosen from every slice to
assess the E-cadherin score. The score of each slice was based on
the percentage and intensity of positively stained cells. The
percentage scoring system was as follows: no positive cells (0),
<50% positive tumor cells (1),
50–75% positive tumor cells (2),
and >75% positive tumor cells (3). The intensity scoring system was as
follows: no staining (0), light yellow (1), yellow brown (2), and dark brown (3). The percentage score was multiplied by
the intensity score and sections were divided into 2 groups based
on the resulting product, as follows: low expression (score ≤6) and
high expression (score >6). IHC slides were scored by two
pathologists without knowledge of the clinical data in order to
eliminate bias. Discrepancies were eliminated by consensus.
Western blotting and quantitative RT-PCR
(qRT-PCR)
Western blotting and qRT-PCR were carried out as
previously described (23,24). Protein lysates were separated by
10% SDS-PAGE and electrophoretically transferred to a PVDF membrane
(Millipore, MA, USA). Subsequently, the membrane was incubated with
a primary monoclonal antibody followed by a fluorescent secondary
antibody. β-tubulin was used as a protein loading control. Primary
antibodies used for western blotting included those against
E-cadherin (Abcam), vimentin (Abcam), and β-tubulin (Santa Cruz,
CA, USA). Western blot bands were visualized using Imaging system
(LI-COR Biosciences, Lincoln, NE, USA), and protein density was
quantified using Odyssey version 1.2 software (LI-COR Biosciences).
qRT-PCR was performed using SYBR-Green PCR master mix (Applied
Biosystems) on an ABI 7300 system. PCR primers were as follows:
CDH1 (5′-AGAACAGCACGTACACAGCCCTAA-3′ and
3′-ATCAGCAGAAGTGTCCCTGTTCCA-5′) and β-actin
(5′-CTCCATCCTGGCCTCGCTGT-3′ and 3′-GCTGTCACCTTCACCGTTCC-5′).
Statistical analysis
The data were analyzed using version 13 of the
Statistical Package for Social Sciences (SPSS Inc., Chicago, IL,
USA). Quantitative data were summarized using the means and
standard deviations and were compared using the Student's t-test.
Qualitative and ranked data were compared using the χ2
test. Associations between clinicopathological variables and
CDH1 promoter methylation status were evaluated using
Pearson's χ2 test. Patient survival analysis was
performed by the Kaplan-Meier method, and differences were
evaluated with the log-rank test. Hazard ratios (HR) and their 95%
confidence intervals (CI) were estimated using univariate and
multivariate Cox proportional hazard models. All statistical
analyses were considered significant when the P-value was
≤0.05.
Results
Clinical and pathological
characteristics
A total of 37 CXPAs from 26 males (70.27%) and 11
females (29.73%) were investigated in this study. The male to
female ratio was 2.36. The age range of the patients was 26–83
years, and the mean age was 61.62 years. Twenty-nine tumors
(78.38%) originated from the major salivary glands, and 8 tumors
(21.62%) originated from the minor salivary glands. Histologically,
16 (43.24%) tumors were classified as low grade, and 21 (56.76%)
were classified as high grade. Perineural invasion was observed in
11 of 37 patients (29.73%). Sixteen patients (43.24%) developed
lymph node metastases. The mean follow-up time was 28.86 months.
There were 17 deaths, 16 patients died of CXPA and 1 of another
disease. Of those that survived, 17 patients survived without
tumors and 3 survived with tumors.
Promoter methylation status of CDH1 and
its correlation with E-cadherin expression
The methylation status of CDH1 was analyzed
in 37 salivary CXPA tissues using BSP. As shown in Table I, low, medium and high methylation
of the CDH1 promoter (Fig.
1) was found in 16 (43.24%), 9 (24.32%) and 12 (32.43%) CXPA
samples, respectively. IHC analysis was performed to investigate
E-cadherin expression. The results showed that 13 (35.14%) of 37
cases had low E-cadherin expression, while 24 (64.86%) cases showed
high E-cadherin expression (Table
I and Fig. 2). As shown in
Table I, we found that CDH1
promoter methylation was significantly lower in the high E-cadherin
expression group as compared with the low E-cadherin expression
group (P<0.01). This result indicates that the methylation
status of CDH1 strongly correlates with E-cadherin
expression.
 | Table ICorrelation of E-cadherin expression
with CDH1 methylation. |
Table I
Correlation of E-cadherin expression
with CDH1 methylation.
| Group | Caterogy (n) | Expression of
E-cadherin
| χ2 | P-value |
|---|
| Low (%) | High (%) |
|---|
| Methylation
status | | | | | |
| Low | 16 | 1 (7.7) | 15 (62.5) | | |
| Medium | 9 | 1 (7.7) | 8 (33.3) | | |
| High | 12 | 11 (84.6) | 1 (4.2) | | |
| Total | 37 | 13 | 24 | 24.964 | <0.01a |
CDH1 promoter methylation and mRNA and
protein expression in CXPA cell lines
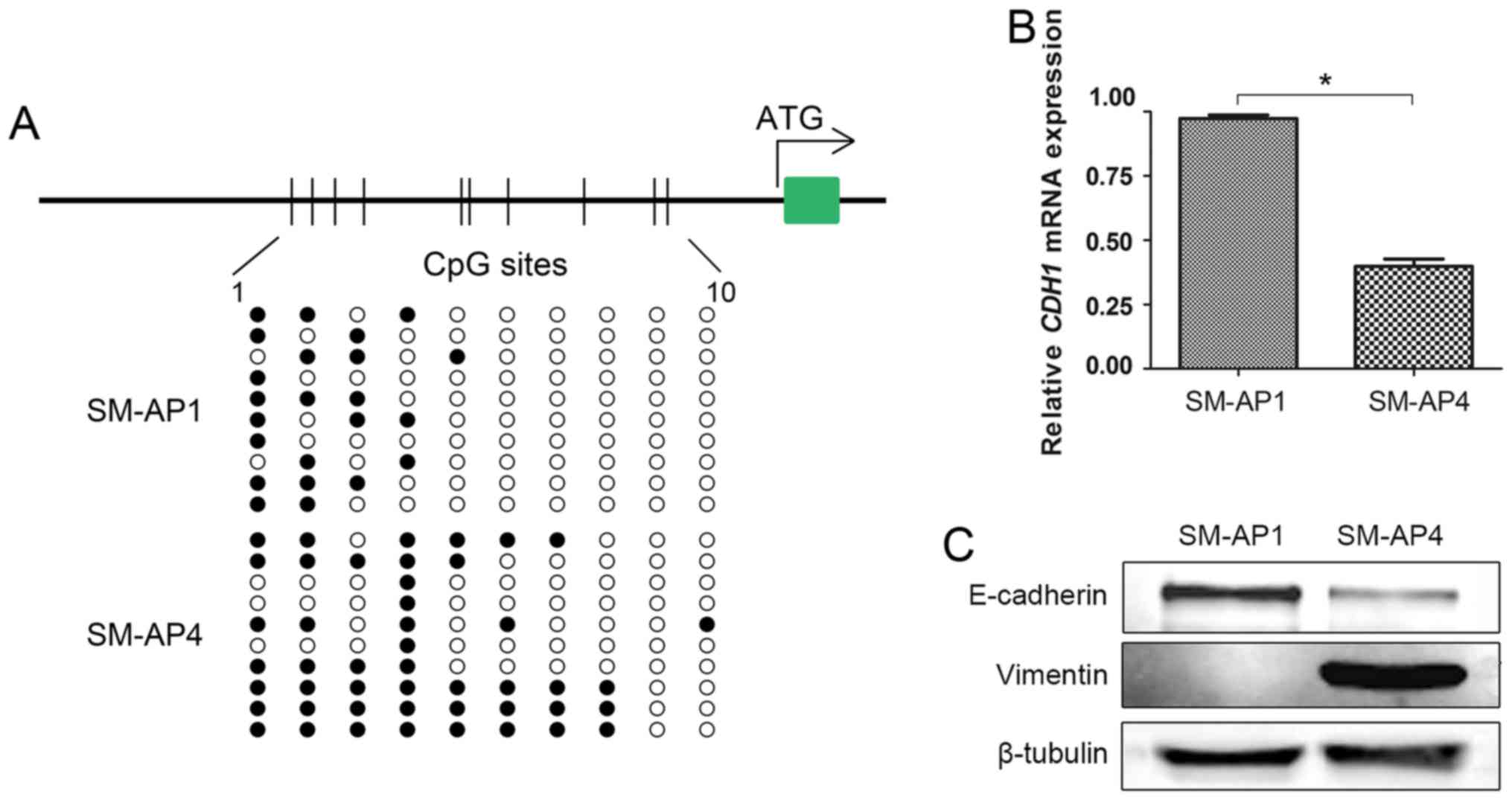
CDH1 promoter methylation was detected in
both SM-AP1 and SM-AP4 cell lines (Fig. 3A). Consistent with the notion that
methylation contributes to gene inactivation, CDH1
hypermethylation (MR=48%) status resulted in lower CDH1 mRNA
and protein expression in SM-AP4 cell lines than (MR=23%) in SM-AP1
cell lines (Fig. 3B and C). When
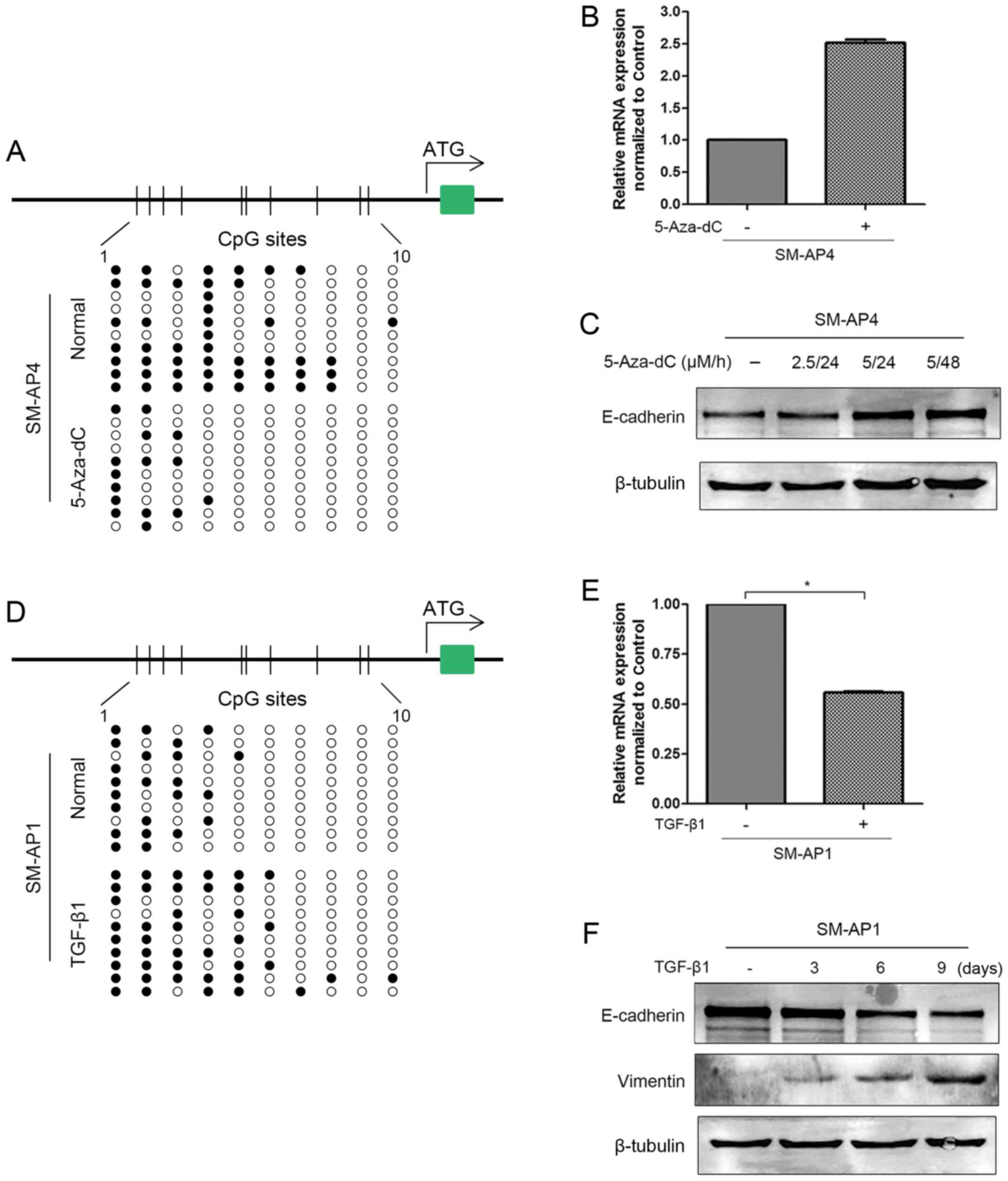
treated with 5-Aza-dC, a demethylating agent, SM-AP4 cells showed
increased CDH1 mRNA and protein expression and decreased
CDH1 promoter methylation (Fig.
4A–C). However, SM-AP1 cells showed decreased CDH1 mRNA
and E-cadherin expression but an elevated methylation status after
TGF-β1 treatment (Fig. 4D–F).
Taken together, we suggest that promoter methylation is a
predominant factor regulating CDH1 expression in CXPA cell
lines.
Associations between CDH1 promoter
methylation and clinicopathological parameters
To evaluate the clinical significance of CDH1
promoter methylation, we investigated the association between
methylation status and clinicopathological features in CXPA
patients. As presented in Table
II, CDH1 methylation status was differentially detected
according to sex, histological subtype, histological grade, and
tumor N-stage and TNM-stage. CXPA cases with high histological
grade (42.9% versus 7.1%, P=0.005), lymph node metastasis (56.2%
versus 14.3%, P=0.024), or advanced TNM-stage (41.7% versus 7.7%,
P=0.038) were more likely to display high CDH1 promoter
methylation, which indicates that promoter methylation may be a
prognostic factor in CXPA. Interestingly, compared with males,
females tended to present with higher CDH1 methylation rates
(P=0.028). CDH1 methylation status was not significantly
correlated with other clinicopathological parameters, such as age,
tumor site or neural invasion.
 | Table IIAssociations between the promoter
methylation status of CDH1 and clinicopathological
characteristics. |
Table II
Associations between the promoter
methylation status of CDH1 and clinicopathological
characteristics.
| Characteristic | Category (no.) | Low (%) | Methylation status
| χ2 | P-value |
|---|
| Medium (%) | High (%) |
|---|
| Age (years) | <60 (14) | 6 (42.9) | 2 (14.3) | 6 (42.9) | 1.688 | 0.430 |
| ≥60 (23) | 10 (43.5) | 7 (30.4) | 6 (26.1) | | |
| Sex | Male (26) | 13 (50.0) | 8 (30.8) | 5 (19.2) | 7.116 | 0.028a |
| Female (11) | 3 (27.3) | 1 (9.1) | 7 (63.6) | | |
| Subtype | ICXPA-L (23) | 12 (52.2) | 8 (34.8) | 3 (13.0) | 10.900 | 0.004a |
| ICXPA-NL (14) | 4 (28.6) | 1 (7.1) | 9 (64.3) | | |
| Tumor site | Major gland
(29) | 12 (41.4) | 8 (27.6) | 9 (31.0) | 0.775 | 0.679 |
| Minor gland
(8) | 4 (50.0) | 1 (12.5) | 3 (37.5) | | |
| Neural
invasion | Yes (11) | 5 (42.3) | 4 (36.4) | 2 (18.2) | 1.931 | 0.381 |
| No (26) | 11 (42.3) | 5 (19.2) | 10 (38.5) | | |
| Histological
grade | Low (16) | 11 (78.6) | 2 (14.3) | 1 (7.1) | 10.446 | 0.005a |
| High (21) | 5 (23.8) | 7 (33.3) | 9 (42.9) | | |
| N-stage | N−
(21) | 12 (57.1) | 6 (28.6) | 3 (14.3) | 7.461 | 0.024a |
| N+
(16) | 4 (25.0) | 3 (18.8) | 9 (56.2) | | |
| TNM-stage | I+II (13) | 9 (69.2) | 3 (23.1) | 1 (7.7) | 6.520 | 0.038a |
| III+IV (24) | 7 (29.2) | 7 (29.2) | 10 (41.7) | | |
Survival analysis
Survival curves were generated for all 37 salivary
CXPA cases. Methylation of the CDH1 promoter was significantly
associated with overall survival (log-rank test, P=0.026) (Fig. 5). In univariate analyses, lymph
node metastasis (P= 0.004) and CDH1 promoter hypermethylation (P=
0.030) were significantly associated with poor overall survival
(Table III). To determine
whether the association between CDH1 promoter methylation and
survival was independent of other parameters, a multivariate
analysis was performed including N-stage and CDH1 promoter
methylation as co-factors. The multivariate analysis showed that
lymph node metastasis (P=0.010) is independently associated with
overall survival (Table III) and
is an independent prognostic factor in CXPA.
 | Table IIISummary of Cox proportional hazard
models for the overall survival of salivary CXPAs. |
Table III
Summary of Cox proportional hazard
models for the overall survival of salivary CXPAs.
| Characteristic | Hazard ratio | Hazard ratio (95%
CI)
| P-value |
|---|
| Lower | Upper |
|---|
| Univariate | | | | |
| Age <60 vs.
≥60 | 3.011 | 0.976 | 9.296 | 0.055 |
| Sex, male vs.
female | 1.116 | 0.411 | 3.032 | 0.830 |
| Subtype CXPA-L vs.
CXPA-NL | 0.394 | 0.136 | 1.143 | 0.087 |
| Location major vs.
minor | 1.260 | 0.407 | 3.903 | 0.689 |
| Neural invasion
positive vs. negative | 1.738 | 0.634 | 4.764 | 0.283 |
| Grade low vs.
high | 34.266 | 0.329 | 71.975 | 0.136 |
| T-stage T1/T2 vs.
T3/T4 | 1.063 | 0.402 | 2.812 | 0.901 |
| N-stage
N− vs. N+ | 5.573 | 1.727 | 17.982 | 0.004a |
| TMN-stage I/II vs.
III/IV | 3.043 | 0.862 | 10.743 | 0.084 |
| E-cadherin low vs.
high | 1.380 | 0.478 | 3.989 | 0.552 |
| Methylation low
vs. medium/high | 2.761 | 0.945 | 8.061 | 0.030a |
| Multivariate | | | | |
| N-stage
N− vs. N+ | 4.739 | 1.446 | 15.528 | 0.010a |
| Methylation low
vs. medium/high | 2.043 | 0.680 | 6.137 | 0.053a |
Discussion
Generally, alterations in gene expression are mainly
achieved by genetic and epigenetic methods. Genetic alternations
primarily change the structure or number of a certain gene, whereas
epigenetic alternations occur at the transcriptional level
(9). CpG island methylation in the
promoter region is a common epigenetic method of modifying gene
expression. CpG methylation has been shown to modulate tumor
progression in various cancer types, including esophageal squamous
cell carcinoma (25,26), oral squamous cell carcinoma
(27), salivary CXPA (28) and ACC (29). This modulation occurs mainly via
the inactivation of tumor suppressor genes such as p16, MGMT,
DAPK and RASSF1A. Altered CDH1 promoter
methylation status has been shown to be the key factor in
E-cadherin silencing in many tumors (7,9,11).
CDH1 silencing is directly related to advanced tumor stage
and an aggressive phenotype (7).
This is the first study to evaluate CDH1 promoter
methylation status in salivary CXPA. In this study, we have also
demonstrated the relationship between E-cadherin expression and
CDH1 promoter methylation.
In our study, an absence of E-cadherin expression
was found in 35.14% (13/37) of CXPA cases. This is similar to a
study by Zhang et al (6),
which reported a negative E-cadherin detection rate of 38.33%
across 60 ACC cases. However, negative E-cadherin expression was
found in 68.42% (26/38) of eyelid SCC cases and 87.26% (18/23) of
oral SCC cases. A study (7) in
breast cancer showed a 42.33% (58/137) rate of reduced E-cadherin
expression. It seems that E-cadherin reduction occurs with varying
frequencies in different tumor types and at a relatively low
frequency in salivary gland tumors specifically. In the meantime,
we detected CDH1 promoter methylation using the BSP method,
which is considered the 'gold standard' for determining DNA
methylation and has the advantage of detecting methylation at each
CpG site individually. Our study indicated that the CDH1
methylation rate in CXPA was 67.57% (25/37). This rate is similar
to that of many other tumors, including primary lung cancer (88%)
(30), breast carcinoma (65–95%)
(7,10,31,32)
and colorectal carcinoma (52%) (33). We found that DNA methylation
preferentially occurred in the first four CpG islands compared with
the other CpG islands.
We then analyzed the association between CDH1
methylation status and E-cadherin expression in CXPA patients. This
analysis demonstrated that CDH1 methylation was
significantly correlated with decreased E-cadherin expression
(P<0.001) in clinical specimens. In addition, we evaluated the
CDH1 methylation status and the corresponding CDH1
mRNA and protein levels in SM-AP1 and SM-AP4 cell lines. Consistent
with the above results, cells with higher CDH1 methylation
levels showed lower E-cadherin expression. Furthermore, to
demonstrate that methylation is the critical factor in the
silencing of E-cadherin expression, a dynamic experiment was
performed in vitro. The demethylating agent 5-Aza-dC
restored CDH1 mRNA and protein expression levels by
reversing the high methylation status of SM-AP4 cell lines.
Conversely, upregulation of CDH1 methylation levels via
TGF-β1 treatment resulted in a repression of CDH1 mRNA and
protein levels in SM-AP1 cells. TGF-β1-induced CDH1 promoter
methylation was achieved by inducing the expression of the Snail
protein, a transcriptional factor that binds the CDH1
promoter region and recruits DNA methyltransferases (DNMT), which
subsequently methylate the DNA fragment (34). TGF-β1 is a signaling molecule that
mediates the epithelial-mesenchymal transition (EMT) (34). The hallmark of EMT is the loss of
E-cadherin expression. In in vitro experiments, TGF-β1
treatment of SM-AP1 cells resulted in the downregulation of the
epithelial marker E-cadherin and upregulation of the mesenchymal
marker vimentin. This indicated that the EMT process might play a
role in the repression of E-cadherin in salivary CXPA.
Despite these results, however, CDH1 promoter
methylation was not associated with the downregulation of
E-cadherin expression levels in each case. As shown in Table I, E-cadherin expression was absent
in one sample in the low-methylation group. Various studies have
demonstrated that CDH1 expression could be repressed by
mechanisms other than promoter methylation, such as changes in
chromatin structure, LOH at 16q22.1, inactivating gene mutations,
specific transcriptional factors, and translational and
post-translational regulation (5,8,35).
Thus, taken together, we suggest that E-cadherin expression levels
are primarily, but not solely, regulated by DNA methylation in CXPA
both in vivo and in vitro. Other regulatory
mechanisms affecting CDH1 in CXPA may be investigated in
further studies.
Consistent with similar studies in eyelid SCC
(9), colorectal cancer (13) and breast cancer (32), the association of CDH1
methylation with cervical lymph node metastasis, histological grade
and advanced tumor stage suggests that the CDH1 gene may be
particularly important in salivary CXPA tumor progression.
Consequently, CDH1 methylation, as well as N stage, is a strong
predicator of overall survival in patients with CXPA in univariate
survival analyses. However, in multivariate survival analyses,
lymph node metastasis was shown to be an independent prognostic
factor of overall survival for CXPA patients. Our findings provide
evidence of the potential usefulness of CDH1 methylation
status as an informative prognostic biomarker in patients with
CXPA.
Reduction in E-cadherin expression is reportedly
correlated with invasion, metastasis and recurrence of tumors in
patients with oral squamous cell (36), bladder (37), and breast carcinomas (32). However, we observed no association
between E-cadherin expression and any of the clinicopathological
parameters that were investigated in the present study (data not
shown). This discrepancy may be due to the smaller sample size of
our study.
In conclusion, the present study indicates that DNA
promoter methylation is the most common molecular abnormality of
the CDH1 gene in salivary CXPA. Moreover, CDH1
promoter methylation is associated with histological
differentiation, histological grade, tumor N stage and TNM stage.
Methylation status may play a significant role in CXPA
carcinogenesis and tumor progression and may be a reliable
prognostic biomarker of poor patient survival. Further study of the
correlation between abnormalities in the CDH1 gene and
protein expression as well as interactions with other genes in
salivary CXPA is therefore warranted.
Acknowledgments
This study was supported by the Chinese Nature
Science Foundation (grant nos. 81272976 and 81372910).
References
|
1
|
Wijnhoven BP, Dinjens WN and Pignatelli M:
E-cadherin-catenin cell-cell adhesion complex and human cancer. Br
J Surg. 87:992–1005. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Corso G, Intra M, Trentin C, Veronesi P
and Galimberti V: CDH1 germline mutations and hereditary lobular
breast cancer. Fam Cancer. 15:215–219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Corso G, Figueiredo J, Biffi R, Trentin C,
Bonanni B, Feroce I, Serrano D, Cassano E, Annibale B, Melo S, et
al: E-cadherin germline mutation carriers: Clinical management and
genetic implications. Cancer Metastasis Rev. 33:1081–1094. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carter BS, Ewing CM, Ward WS, Treiger BF,
Aalders TW, Schalken JA, Epstein JI and Isaacs WB: Allelic loss of
chromosomes 16q and 10q in human prostate cancer. Proc Natl Acad
Sci USA. 87:8751–8755. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sato T, Tanigami A, Yamakawa K, Akiyama F,
Kasumi F, Sakamoto G and Nakamura Y: Allelotype of breast cancer:
Cumulative allele losses promote tumor progression in primary
breast cancer. Cancer Res. 50:7184–7189. 1990.PubMed/NCBI
|
|
6
|
Zhang CY, Mao L, Li L, Tian Z, Zhou XJ,
Zhang ZY and Li J: Promoter methylation as a common mechanism for
inactivating E-cadherin in human salivary gland adenoid cystic
carcinoma. Cancer. 110:87–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shargh SA, Sakizli M, Khalaj V, Movafagh
A, Yazdi H, Hagigatjou E, Sayad A, Mansouri N, Mortazavi-Tabatabaei
SA and Khorram Khorshid HR: Downregulation of E-cadherin expression
in breast cancer by promoter hypermethylation and its relation with
progression and prognosis of tumor. Med Oncol. 31:2502014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cui H, Wang L, Gong P, Zhao C, Zhang S,
Zhang K, Zhou R, Zhao Z and Fan H: Deregulation between miR-29b/c
and DNMT3A is associated with epigenetic silencing of the CDH1
gene, affecting cell migration and invasion in gastric cancer. PLoS
One. 10:e01239262015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang YQ, Yuan Y, Jiang S and Jiang H:
Promoter methylation and expression of CDH1 and susceptibility and
prognosis of eyelid squamous cell carcinoma. Tumour Biol.
37:9521–9526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lombaerts M, van Wezel T, Philippo K,
Dierssen JW, Zimmerman RM, Oosting J, van Eijk R, Eilers PH, van de
Water B, Cornelisse CJ, et al: E-cadherin transcriptional
downregulation by promoter methylation but not mutation is related
to epithelial-to-mesenchymal transition in breast cancer cell
lines. Br J Cancer. 94:661–671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li G, Liu Y, Yin H, Zhang X, Mo X, Tang J
and Chen W: E-cadherin gene promoter hypermethylation may
contribute to the risk of bladder cancer among Asian populations.
Gene. 534:48–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Michailidi C, Theocharis S, Tsourouflis G,
Pletsa V, Kouraklis G, Patsouris E, Papavassiliou AG and Troungos
C: Expression and promoter methylation status of hMLH1, MGMT, APC,
and CDH1 genes in patients with colon adenocarcinoma. Exp Biol Med
(Maywood). 240:1599–1605. 2015. View Article : Google Scholar
|
|
13
|
Kim SA, Inamura K, Yamauchi M, Nishihara
R, Mima K, Sukawa Y, Li T, Yasunari M, Morikawa T, Fitzgerald KC,
et al: Loss of CDH1 (E-cadherin) expression is associated with
infiltrative tumour growth and lymph node metastasis. Br J Cancer.
114:199–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gnepp D, Brandwein-Gensler M, El-Naggar A
and Nagao T: Carcinoma ex pleomorphic adenoma. World Health
Organization Classification of Tumours: Pathology and Genetics of
Head and Neck Tumours. Barnes L, Eveson JW, Reichart P and
Sidransky D: IARC Press; Lyon: pp. 242–243. 2005
|
|
15
|
Tian Z, Li L, Wang L, Hu Y and Li J:
Salivary gland neoplasms in oral and maxillofacial regions: A
23-year retrospective study of 6982 cases in an eastern Chinese
population. Int J Oral Maxillofac Surg. 39:235–242. 2010.
View Article : Google Scholar
|
|
16
|
Prabhu S, Kaveri H and Rekha K: Benign,
malignant salivary gland tumors: Comparison of immunohistochemical
expression of e-cadherin. Oral Oncol. 45:594–599. 2009. View Article : Google Scholar
|
|
17
|
Economopoulou P, Hanby A and Odell EW:
Expression of E-cadherin, cellular differentiation and polarity in
epithelial salivary neoplasms. Oral Oncol. 36:515–518. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xin W and Paulino AF: Prognostic factors
in malignant mixed tumors of the salivary gland: Correlation of
immunohistochemical markers with histologic classification. Ann
Diagn Pathol. 6:205–210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JW, Kwon GY, Roh JL, Choi SH, Nam SY,
Kim SY and Cho KJ: Carcinoma ex pleomorphic adenoma of the salivary
glands: Distinct clinicopathologic features and immunoprofiles
between subgroups according to cellular differentiation. J Korean
Med Sci. 26:1277–1285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sobin LHWC: TNM Classification of
Malignant Tumors. 6th edition. John Wiley & Sons, Inc; New
York, NY: 2002
|
|
21
|
Maruyama S, Cheng J, Shingaki S, Tamura T,
Asakawa S, Minoshima S, Shimizu Y, Shimizu N and Saku T:
Establishment and characterization of pleomorphic adenoma cell
systems: An in-vitro demonstration of carcinomas arising
secondarily from adenomas in the salivary gland. BMC Cancer.
9:2472009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rotondo JC, Selvatici R, Di Domenico M,
Marci R, Vesce F, Tognon M and Martini F: Methylation loss at H19
imprinted gene correlates with methylenetetrahydrofolate reductase
gene promoter hypermethylation in semen samples from infertile
males. Epigenetics. 8:990–997. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu H, Xu J, Zhou L, Yun X, Chen L, Wang
S, Sun L, Wen Y and Gu J: Hepatitis B virus large surface antigen
promotes liver carcinogenesis by activating the Src/PI3K/Akt
pathway. Cancer Res. 71:7547–7557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang YQ, Wei XL, Liang YK, Chen WL, Zhang
F, Bai JW, Qiu SQ, Du CW, Huang WH and Zhang GJ: Over-expressed
Twist associates with markers of epithelial mesenchymal transition
and predicts poor prognosis in breast cancers via ERK and Akt
activation. PLoS One. 10:e01358512015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo W, Cui L, Wang C, Guo Y, Shen S, Kuang
G and Dong Z: Decreased expression of RASSF1A and up-regulation of
RASSF1C is associated with esophageal squamous cell carcinoma. Clin
Exp Metastasis. 31:521–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bai J, Zhang X, Hu K, Liu B, Wang H, Li A,
Lin F, Zhang L, Sun X, Du Z, et al: Silencing DNA methyltransferase
1 (DNMT1) inhibits proliferation, metastasis and invasion in ESCC
by suppressing methylation of RASSF1A and DAPK. Oncotarget.
7:44129–44141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pannone G, Santoro A, Feola A, Bufo P,
Papagerakis P, Lo Muzio L, Staibano S, Ionna F, Longo F, Franco R,
et al: The role of E-cadherin down-regulation in oral cancer: CDH1
gene expression and epigenetic blockage. Curr Cancer Drug Targets.
14:115–127. 2014. View Article : Google Scholar
|
|
28
|
Hu YH, Zhang CY, Tian Z, Wang LZ and Li J:
Aberrant protein expression and promoter methylation of p16 gene
are correlated with malignant transformation of salivary
pleomorphic adenoma. Arch Pathol Lab Med. 135:882–889.
2011.PubMed/NCBI
|
|
29
|
Li J, El-Naggar A and Mao L: Promoter
methylation of p16INK4a, RASSF1A, and DAPK is frequent in salivary
adenoid cystic carcinoma. Cancer. 104:771–776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan F, Shen N, Pang J, Molina JR, Yang P
and Liu S: The DNA methyltransferase DNMT1 and tyrosine-protein
kinase KIT cooperatively promote resistance to
5-Aza-2′-deoxycytidine (Decitabine) and Midostaurin (PKC412) in
lung cancer cells. J Biol Chem. 290:18480–18494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nass SJ, Herman JG, Gabrielson E, Iversen
PW, Parl FF, Davidson NE and Graff JR: Aberrant methylation of the
estrogen receptor and E-cadherin 5′ CpG islands increases with
malignant progression in human breast cancer. Cancer Res.
60:4346–4348. 2000.PubMed/NCBI
|
|
32
|
Liu J, Sun X, Qin S, Wang H, Du N, Li Y,
Pang Y, Wang C, Xu C and Ren H: CDH1 promoter methylation
correlates with decreased gene expression and poor prognosis in
patients with breast cancer. Oncol Lett. 11:2635–2643.
2016.PubMed/NCBI
|
|
33
|
Li YX, Lu Y, Li CY, Yuan P and Lin SS:
Role of CDH1 promoter methylation in colorectal carcinogenesis: A
meta-analysis. DNA Cell Biol. 33:455–462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong C, Wu Y, Yao J, Wang Y, Yu Y,
Rychahou PG, Evers BM and Zhou BP: G9a interacts with Snail and is
critical for Snail-mediated E-cadherin repression in human breast
cancer. J Clin Invest. 122:1469–1486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dong C, Wu Y, Wang Y, Wang C, Kang T,
Rychahou PG, Chi YI, Evers BM and Zhou BP: Interaction with Suv39H1
is critical for Snail-mediated E-cadherin repression in breast
cancer. Oncogene. 32:1351–1362. 2013. View Article : Google Scholar :
|
|
36
|
Nakayama S, Sasaki A, Mese H, Alcalde RE,
Tsuji T and Matsumura T: The E-cadherin gene is silenced by CpG
methylation in human oral squamous cell carcinomas. Int J Cancer.
93:667–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kashibuchi K, Tomita K, Schalken JA, Kume
H, Takeuchi T and Kitamura T: The prognostic value of E-cadherin,
alpha-, beta-and gamma-catenin in bladder cancer patients who
underwent radical cystectomy. Int J Urol. 14:789–794. 2007.
View Article : Google Scholar : PubMed/NCBI
|