Introduction
Lung cancer is the leading cause of cancer-related
mortality among males and the second among females worldwide
(1). Platinum-based chemotherapy
is still an important cornerstone of most combination regimens
applied in lung cancer (2). Lung
cancer patients have the highest incidence of anemia among patients
with solid tumors (3). Anemia is a
major cause of cancer-related fatigue that largely decreases the
health-related quality of life (3)
and is associated with the decreased survival of lung cancer
patients (4). The causes of
cancer-related anemia are multifactorial and may be for one
attributed to malignancy itself through suppressed hematopoiesis
(infiltration of bone marrow and functional iron deficiency),
enhanced destruction (hemolysis) and blood loss (5). In addition, myelosuppressive therapy
regimens, such as chemotherapy or ionizing radiation may cause
considerable anemia in cancer patients. Apart from the correction
of nutritional deficiencies and red blood cell transfusion, the use
of recombinant human erythropoietin (Epo) has consistently been
shown to increase hemoglobin levels and to thus reduce the need for
transfusions in lung cancer patients with chemotherapy-related
anemia (3,6,7).
In hematopoietic progenitor cells, Epo binds to its
cytokine receptor, EpoR, that forms a homodimer and leads to
conformational changes in the proximal cytoplasmic tail of EpoR.
Since EpoR has no intrinsic tyrosine kinase activity to initiate
receptor-mediated signaling, receptor phosphorylation and thus
activation is usually mediated by the adapter protein Janus kinase
2 (Jak2). Conformational changes of the cytoplasmic tail of EpoR
homodimer after the binding of Epo leads to the autophosphorylation
of Jak2 and the transphosphorylation of cytoplasmic tyrosine
residues of EpoR. In turn, this activates four key signal
transduction pathways of EpoR (8).
In detail, they consist principally of pathways involving signal
transducer and activator of transcription 5 (STAT5),
mitogen-activated protein (MAP) kinase, phosphatidylinositol
3-kinase (PI3K) and protein kinase C, whose effects are summarized
by the promotion of proliferation and differentiation, and the
suppression of apoptosis (8).
However, over the past decade, there has been
growing concern from both in vitro and in vivo
studies that Epo may promote the growth and survival not only of
hematopoietic progenitor cells, but also that of solid tumor cells
(9,10). Due to the observed increased
mortality in cancer patients, including lung cancer patients
(11), who were treated with
recombinant human Epo (12), the
recommendations of the American Society of Hematology/Clinical
Oncology in 2008 (13) and 2010
(7) and the Clinical Practice
Guidelines in Oncology of the National Comprehensive Cancer Network
on cancer-and chemotherapy-induced anemia in 2016 (14) approved the use of recombinant human
Epo for patients receiving chemotherapy for palliative intent only.
It remains to be determined whether there are direct effects of Epo
on tumor cells, which are responsible for the increased
mortality.
EpoR mRNA and protein have been identified in
several cancer cell lines and in different types of cancer
(9,15). In some cell lines, Epo treatment is
responsible for a statistically significant growth advantage
(16,17), the reduced initiation of apoptosis
(18,19), or increased angiogenesis (20,21).
Both Epo and its receptor, EpoR, have been shown to be expressed in
non-small cell lung cancer (NSCLC) specimens (22). Furthermore, NSCLC cells have been
demonstrated to have a functional, Epo-dependent receptor that is
able to activate three key Epo-signaling pathways (23). The presence of EpoR, its immunoblot
detection, and its effects on cancer cells are still a cause of
controversy. Another aspect is hypoxia, which is a common feature
of solid tumors (24), a
well-known inducer of Epo and EpoR (25) and is also responsible for the
induction of resistance to chemotherapy (26). For these reasons, in the present
study, we have paid particular attention to the cellular effects of
exogenous Epo in an oxygen-reduced atmosphere (1% O2,
referred to as hypoxia).
In the present in vitro study, we
investigated the presence and activity of EpoR, as well as its
signal transduction pathways, and focused on its effects on the
proliferation and survival of three NSCLC cell lines (A427, A549
and NCI-H358). We mimicked the hypoxic tumor microenvironment by
performing experiments under hypoxic conditions. As a proof of
principle, we utilized a well-established EpoR-positive,
Epo-dependent cell line, UT-7/Epo. The findings of this study may
enhance our understanding of the expression of EpoR and the
cellular effects of Epo on NSCLC cells, particularly in the context
of hypoxia. Since safety issues are a matter of recent debate
regarding the use of Epo in anemic cancer patients due to its
possible direct effects on cancer cells, this study adds important
information to this field.
Materials and methods
Tumor cell lines and culture
The human NSCLC cell lines, A427 and A549, were
purchased from Cell Lines Service (Eppelheim, Germany) and cultured
in Dulbecco's modified Eagle's medium (DMEM)-F12 culture medium
(Gibco, Paisley, UK). The human NSCLC cell line, NCI-H358, was
ordered from the American Type Culture Collection (ATCC, Manassas,
VA, USA) and cultured in RPMI-1640 culture medium (Roswell Park
Memorial Institute medium, ATCC). The Epo-dependent human
megakaryoblastic leukemia cell line, UT-7/Epo, which served as a
positive control for EpoR (27),
was a generous gift from Professor Norio Komatsu (Department of
Hematology, Juntendo University, School of Medicine, Tokyo, Japan)
and was cultured in Iscove's modified Dulbecco's medium (IMDM;
Gibco). They were supplemented with 0.4 U/ml recombinant hyman Epo
(rHuEpo; Epoetin alfa, Erypo 10,000 U/ml; Janssen-Cilag Pharma,
Vienna, Austria) every 3–4 days following a medium change,
following the protocol described in the study by Erickson-Miller
et al (28). The culture
media were supplemented with 2 mM L-glutamine (Gibco) if absent,
10% fetal calf serum (FCS; Biowest, Nuaillé, France), 100 U/ml
penicillin and 100 µg/ml streptomycin (Gibco). All 4 cell
lines were cultured in a humidified incubator providing 21%
O2 and 5% CO2 at 37°C referred to as the
normoxic or ambient condition.
Hypoxic conditions
The cells were cultured in a humidified incubator at
37°C in an atmosphere containing 1% O2 and 5%
CO2 regulated through a N2 and a
CO2 gas mixture (Air Liquide, Paris, France) in the
automated Xvivo system G300CL (BioSpherix, Ltd., Lacona, NY, USA),
which served as a hypoxic processing chamber. Unless stated
otherwise, the cells were pre-incubated for 3 days under hypoxic
conditions prior to being used in the experiments.
RNA isolation and quantitative
(real-time) PCR
The cells were harvested, centrifuged and the
pellets were resuspended in RLT buffer (Qiagen, Hilden, Germany)
for cell lysis. Total RNA was extracted using the RNeasy Mini kit
(Qiagen) including DNA digestion with RNase-Free DNase Set (Qiagen)
according to the manufacturer's instructions. Complementary DNA was
synthesized using the Revert-Aid™ H Minus First Strand cDNA
Synthesis kit (Fermentas GmbH, St. Leon-Rot, Germany). Quantitative
PCR was conducted with cDNA from at least 3 independent experiments
and carried out in triplicate. The amplification and detection of
cDNA was performed using the AB 7900 Detection system (Applied
Biosystems, Carlsbad, CA, USA). PCR reaction mix (10 µl)
contained 5.0 µl TaqMan Gene Expression Master Mix, 0.5
µl Assay-on-Demand TaqMan Gene Expression Assay (Applied
Biosystems), forward and reverse primer for Epo
(Hs00171267_m1), EpoR (Hs00959427_m1), and β-actin
(ACTB, Hs99999903_m1), 0.5 µl cDNA and 4.0 µl
distilled H2O. The cycling protocol was as follows: One
cycle at 50°C for 2 min and 95°C for 10 min followed by 45 cycles
consisting of denaturation at 95°C for 15 sec, annealing of primers
and elongation at 60°C for 1 min. The mRNA results were displayed
relative to β-actin as a reference gene. Calculations were
performed using the relative expression software tool REST version
2.0.7 and the comparative CT method (29).
Western blot analysis
The cells were harvested and lysed with ice-cold
RIPA buffer (Sigma-Aldrich, St. Louis, MO, USA) supplemented with
protease inhibitor cocktail (Complete mini; Roche Diagnostics,
Indianapolis, IN, USA) and phosphatase inhibitor cocktail
(PhosSTOP; Roche Diagnostics). For the analysis of EpoR and its
downstream signaling, all cell lines were treated with 100 U/ml
rHuEpo in a time course manner (0, 5, 15, 30 and 60 min). In
detail, 2×105 cells/dish were seeded out in 35-mm Petri
dishes with 2 ml medium containing 10% heat-inactivated FCS under
normoxic conditions. After 72 h, the cells were subjected to
starvation medium containing 0% FCS for 24 h. The starvation medium
was renewed and after 4 h the cells were treated with 100 U/ml
rHuEpo and harvested at the time-points indicated above. Protein
concentrations were determined using a BCA Protein Assay kit (Merck
KGaA, Darmstadt, Germany) according to the manufacturer's
instructions. In total, 10–40 µg of total protein were
separated via 8% SDS-PAGE and transferred onto nitrocellulose
membranes (Bio-Rad Laboratories, Hercules, CA, USA). The membranes
were blocked in 5% milk TBS-T (20 mM Tris-HCl, 140 mM NaCl, 0.1%
Tween-20, pH 7.6) for 1 h at room temperature, then incubated
overnight with primary antibodies diluted in 1% milk or 5% bovine
serum albumin (BSA). The primary antibodies used were as follows:
Rabbit polyclonal antibody against EpoR (1:500; M-20; Sc-697; Santa
Cruz Biotechnology Inc., Santa Cruz, CA, USA), rabbit polyclonal
antibody against p-EpoR (Tyr456; 1:1,000; Sc-20236-R; Santa Cruz
Biotechnology), purified mouse anti-human hypoxia-inducible factor
(HIF)-1α antibody (1:500; clone 54/HIF-1α; BD Biosciences, San
Diego, CA, USA), monoclonal antibody against STAT-5 alpha c-term
(1:500; AJ1741a; Abgent, Oxfordshire, UK), poly-clonal antibody
against p-STAT-5a-Y694 (1:500; AP3268a; Abgent), mouse monoclonal
antibody against β-actin (1:2,000; Sc-47778; Santa Cruz
Biotechnology), rabbit polyclonal antibody against Akt (1:1,000;
#9272), p-Akt (Ser473; 1:1,000; #9271S), ERK1/2 (1:1,000; #9102)
and p-ERK1/2 (Thr202/Tyr204; 1:1,000; #9101S) (all from Cell
Signaling Technology, Danvers, MA, USA). The membranes were
incubated with specific horseradish peroxidase (HRP)-conjugated
secondary antibodies diluted in 1% milk TBS-T for 1 h at room
temperature. Immunoreactivity was detected using SuperSignal West
Pico or Femto Chemiluminescent Substrate (Thermo Fisher Scientific,
Rockford, IL, USA) and visualized on a Kodak T-MAX G/RA film
(Carestream Health France, Noisy-Le-Grand Cedex, France).
Immunocytochemical staining and confocal
microscopy
A total of 150,000 cells per chamber were seeded out
in 2-well chamber slides (Nunc, Langenselbold, Germany) and
cultured in complete culture medium for 48 h under hypoxic
conditions. Thereafter, the cells were fixed in 4% formaldehyde
containing 2% sucrose for 10 min and permeabilized with
phosphate-buffered saline (PBS) containing 0.5% Triton X-100
(Sigma-Aldrich) for 5 min. Antibodies were diluted in PBS
containing 1% BSA solution and applied for 1 h at room temperature.
The primary antibody (EpoR, sc-697; Santa Cruz Biotechnology) was
diluted 1:50 and the secondary antibody (Alexa Fluor®
555, A21428; Thermo Fisher Scientific) 1:200. Controls included the
omission of the primary antibody for A427 cells, or using an
isotype immunoglobulin G (rabbit polyclonal IgG: ab27478; Abcam,
Cambridge, UK) as a primary antibody for A549 and NCI-H358 cells.
Nuclei were counterstained with a fluorescent mounting medium
containing DAPI (Vector Laboratories, Inc., Burlingame, CA, USA).
The cells were examined using a Zeiss Axiovert 200M microscope
(Carl Zeiss, Oberkochem, Germany) and LSM 510 release version 4.0
software. Images were acquired using a 63X oil immersion objective
with a 1.4 numeric aperture. The optical slice thickness was below
1.4 µm.
Analysis of cell proliferation and
viability
To examine the effects of Epo on cell proliferation,
the cells treated with or without Epo treatment under normoxic and
hypoxic conditions were counted daily for 3 consecutive days using
electronic pulse area analysis (CASY; Innovatis, Reutlingen,
Germany). The cells were equally plated in duplicate
(2×105 cells/dish) in 35-mm Petri dishes. For 24 h, the
culture media were totally deprived of FCS, then refreshed, and
media containing heat-inactivated 10% FCS, plus rHuEpo (100 U/ml)
were added. To achieve the complete inactivation of endogenous Epo,
fetal calf serum (FCS) was incubated for 30 min at 56°C prior to
the addition to the cell medium, according to the protocol
described in the study by Belenkov et al (30). After 3 h of cell settlement, the
cells were incubated under normoxic or hypoxic conditions. An
additional group of UT-7/Epo cells was administered 0.4 U/ml Epo
for basal stimulation. At 24, 48 and 72 h, the cell supernatant was
collected, and the adherent cells were harvested by trypsinization.
Cell number and cell viability were determined using CASY.
Determination of cell apoptosis
To determine apoptosis by the detection of activated
caspase-3, the NSCLC and UT-7/Epo cells were pre-incubated under
normoxic conditions for 48 h in their starvation media containing
1% FCS and rHuEpo (100 U/ml), while the control group received no
Epo. An additional group of UT-7/Epo cells received basal
stimulation of 0.4 U/ml Epo. The cells were split into
5×105 cells per culture flask with Epo-containing medium
(0.4 or 100 U/ml). Cisplatin (8 µM; 1 mM stock in 0.9% NaCl;
Central Pharmacy of the University Hospital, Medical University of
Graz, Graz, Austria) was added after 3 h of cell settlement. This
relatively low cisplatin concentration was used in order to avoid
too potent and rapid apoptotic effects induced by higher cisplatin
concentrations, particularly in the UT-7/Epo cell line (31,32).
After 48 h, the cells were brought into suspension by
trypsinization, collected and stained with the Caspase-3
Intracellular Activity Assay kit I (PhiPhiLux® G1D2;
Merck, Darmstadt, Germany). Therefore, 5×105 cells were
centrifuged, resuspended with 25 µl of PhiPhiLux substrate
and 25 µl of medium. Subsequently, all cells were subjected
to 1 h of incubation under normoxic conditions, washed with 1 ml
ice-cold PBS, resuspended in 400 µl of flow cytometry buffer
(PhiPhiLux) and analyzed for caspase-3 activity by flow cytometry
(FACSCalibur® flow cytometer; BD Biosciences, San Jose,
CA, USA).
Statistical analyses
All experiments were repeated at least 3 times. Data
were compiled and analyzed using the software package GraphPad
Prism version 5.03 (GraphPad Software, Inc., La Jolla, CA, USA) or
SPSS, version 14.0 (IBM SPSS, Chicago, IL, USA). Group differences
were calculated using the Student's t-test and two-way analysis of
variance (ANOVA) with post hoc analysis (Bonferroni correction).
Two-sided P<0.05 were considered to indicate statistically
significant differences. Results are expressed as the means ±
standard deviation (SD).
Results
Expression of EpoR in NSCLC cell
lines
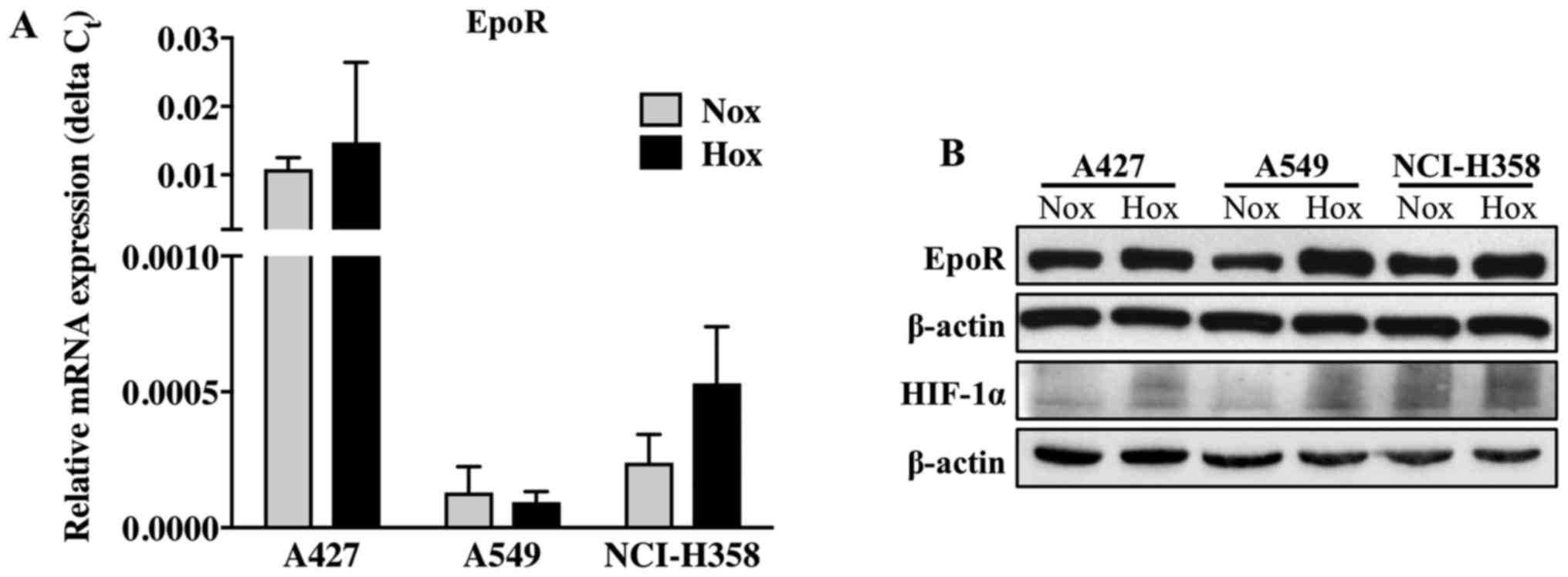
All 3 cell lines expressed EpoR mRNA (Fig. 1A). The EpoR mRNA levels were
highest in the A427 cells, both under normoxic and hypoxic
conditions. The mRNA level of EpoR did not differ in the cells
cultured under normoxic or hypoxic conditions. Western blot
analysis of EpoR revealed that EpoR mRNA was translated into the
mature, full-length form of EpoR protein (59 kDa) in all 3 NSCLC
cell lines (Fig. 1B). Hypoxic
conditions were confirmed by immunoblotting for hypoxia-inducible
factor 1α (HIF-1α), one of the most important and well described
regulatory proteins in hypoxia (Fig.
1B). As expected, HIF-1α protein expression was increased under
hypoxic conditions, particularly in the A427 and A549 cells,
whereas in the NCI-H358 cells, this difference was less prominent.
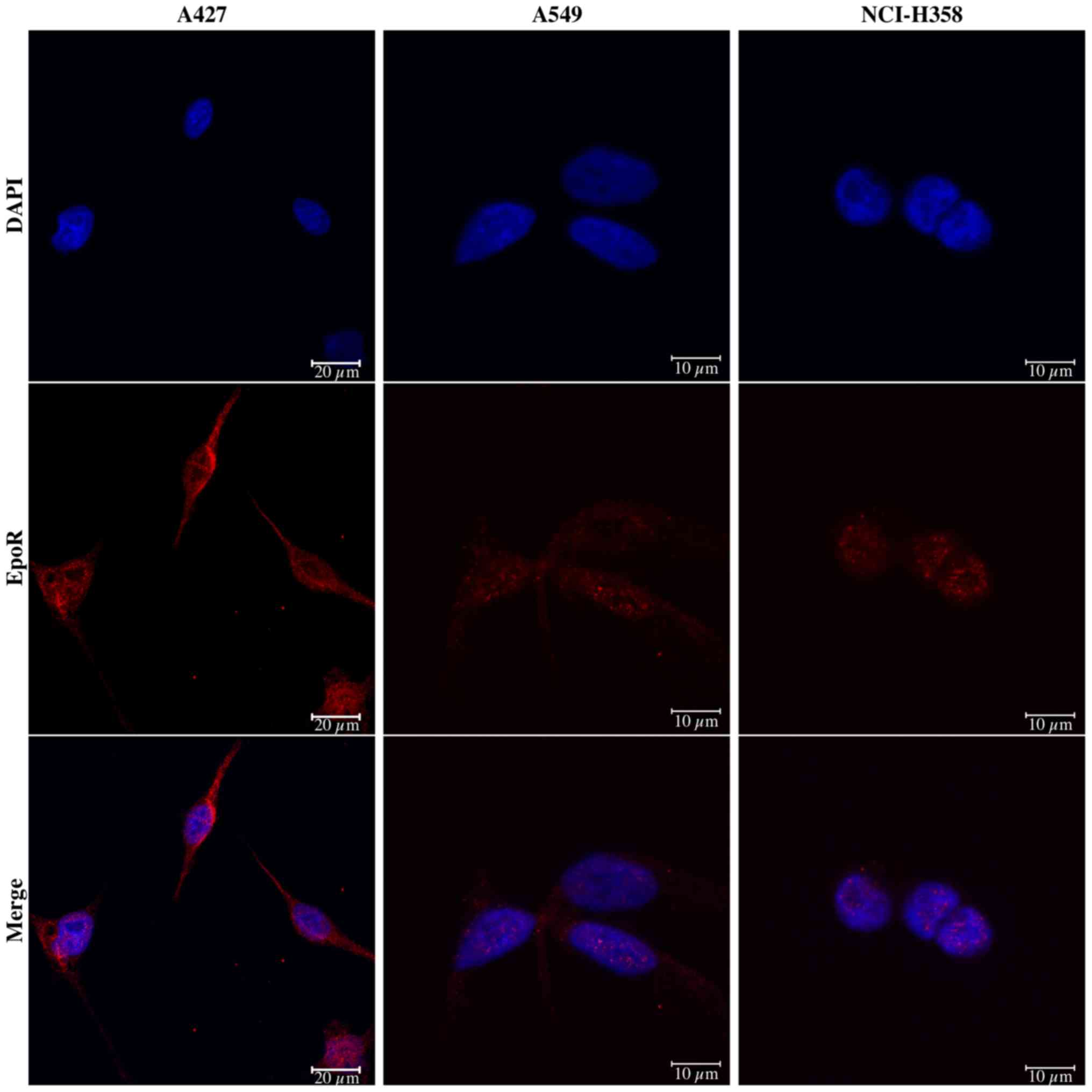
As shown by confocal laser scanning microscopy, all NSCLC cells
exhibited immunofluorescence staining for EpoR protein (Fig. 2), which appeared to be most
prominent in the A427 cells. The localization of EpoR was mainly
cytoplasmic and perinuclear.
Activity and functionality of EpoR in
NSCLC cells
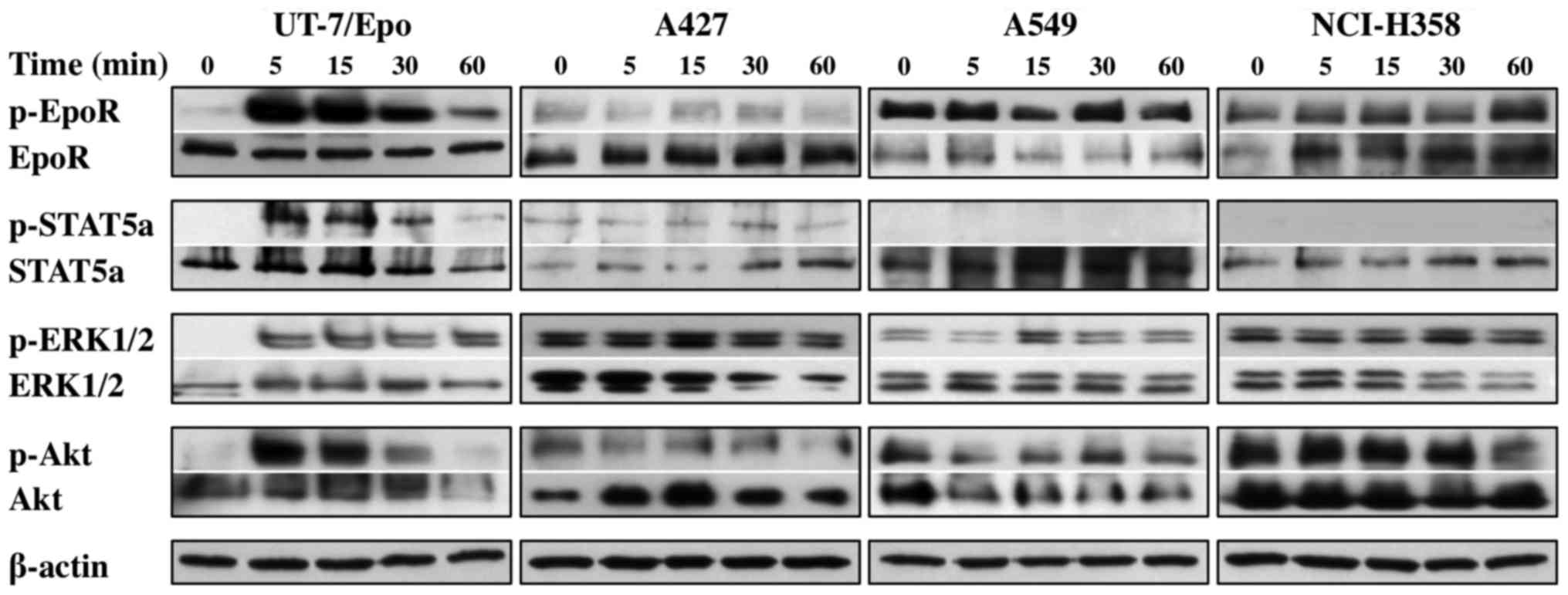
Since we found EpoR protein expression in these
NSCLC cell lines, we explored its functionality via the analysis of
the activating phosphorylation of EpoR and its downstream signaling
pathways. All NSCLC cells exhibited the continuous phosphorylation
of EpoR, ERK1/2 and Akt proteins, and the A427 cells also exhibited
the phosphorylation of STAT5a protein, which was independent of
exogenous Epo administration (Fig.
3). By contrast, the Epo-dependent UT-7/Epo cells exhibited a
clear time-dependent activation of EpoR, STAT5a, ERK1/2 and Akt
proteins, peaking in intensity after 5 min and gradually declining
within 1 h.
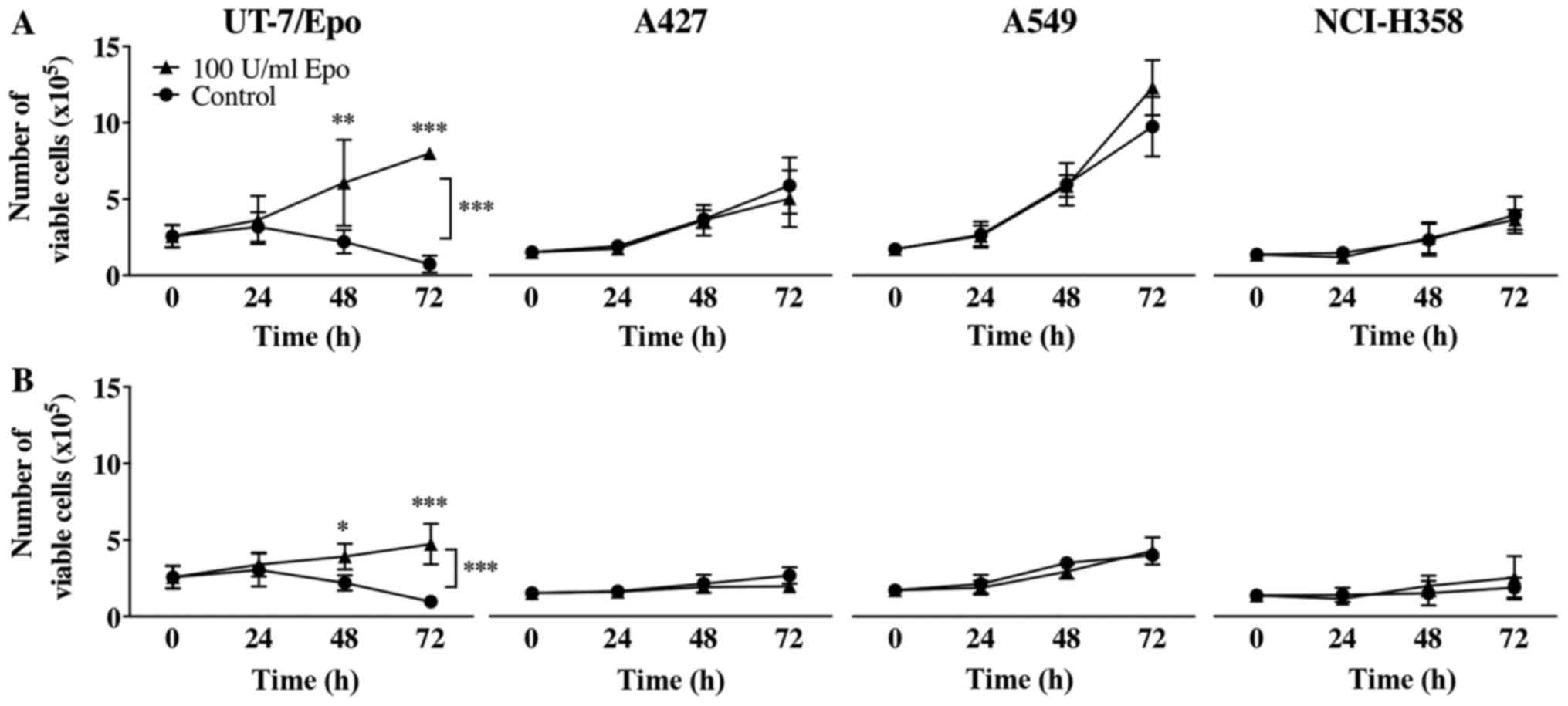
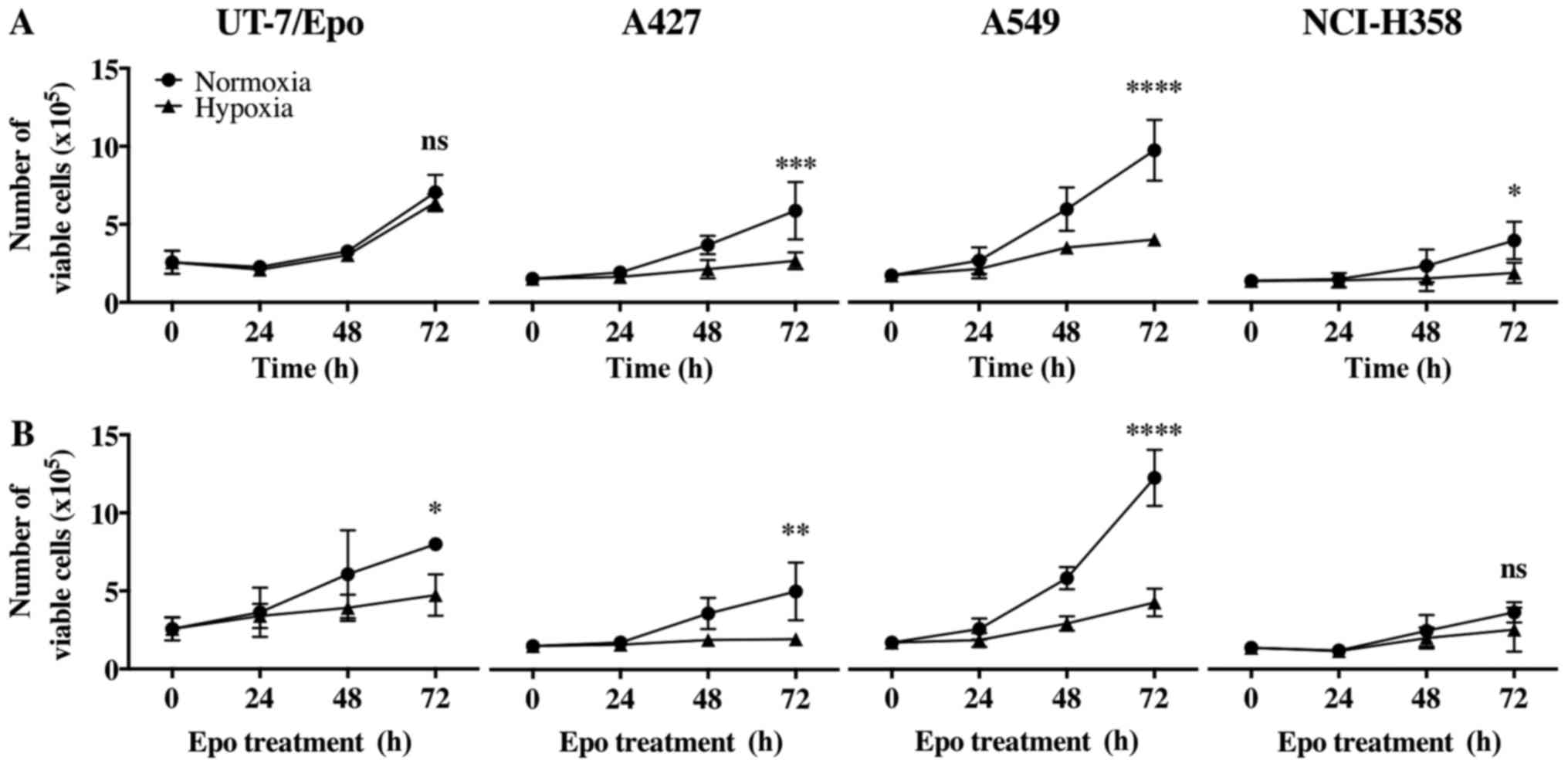
Proliferation of Epo-treated NSCLC cell
lines under hypoxic conditions
To examine the effects of Epo on the proliferation
of the NSCLC cells, electronic pulse area analysis was conducted to
count all 4 cell lines treated with or without Epo (100 U/ml) both
under normoxic and hypoxic conditions for up to 72 h.
Epo did not lead to a statistically significant
growth advantage of the A427, A549 and NCI-H358 cells neither under
normoxic nor under hypoxic conditions (Fig. 4). The UT-7/Epo cells, however,
exhibited a clear Epo-dependent overall increase in cell number
(P<0.0001, two-way ANOVA). In the post hoc subgroup analyses,
the number of viable UT-7/Epo cells under normoxic and hypoxic
conditions differed significantly after 48 h of Epo treatment
(P<0.01 and P<0.05, respectively). Hypoxia itself led to a
statistically significant overall decrease in the number of viable
cells without Epo treatment: A427 cells, P=0.0008; A549 cells,
P<0.0001; NCI-H358 cells, P=0.02; two-way ANOVA (Fig. 5).
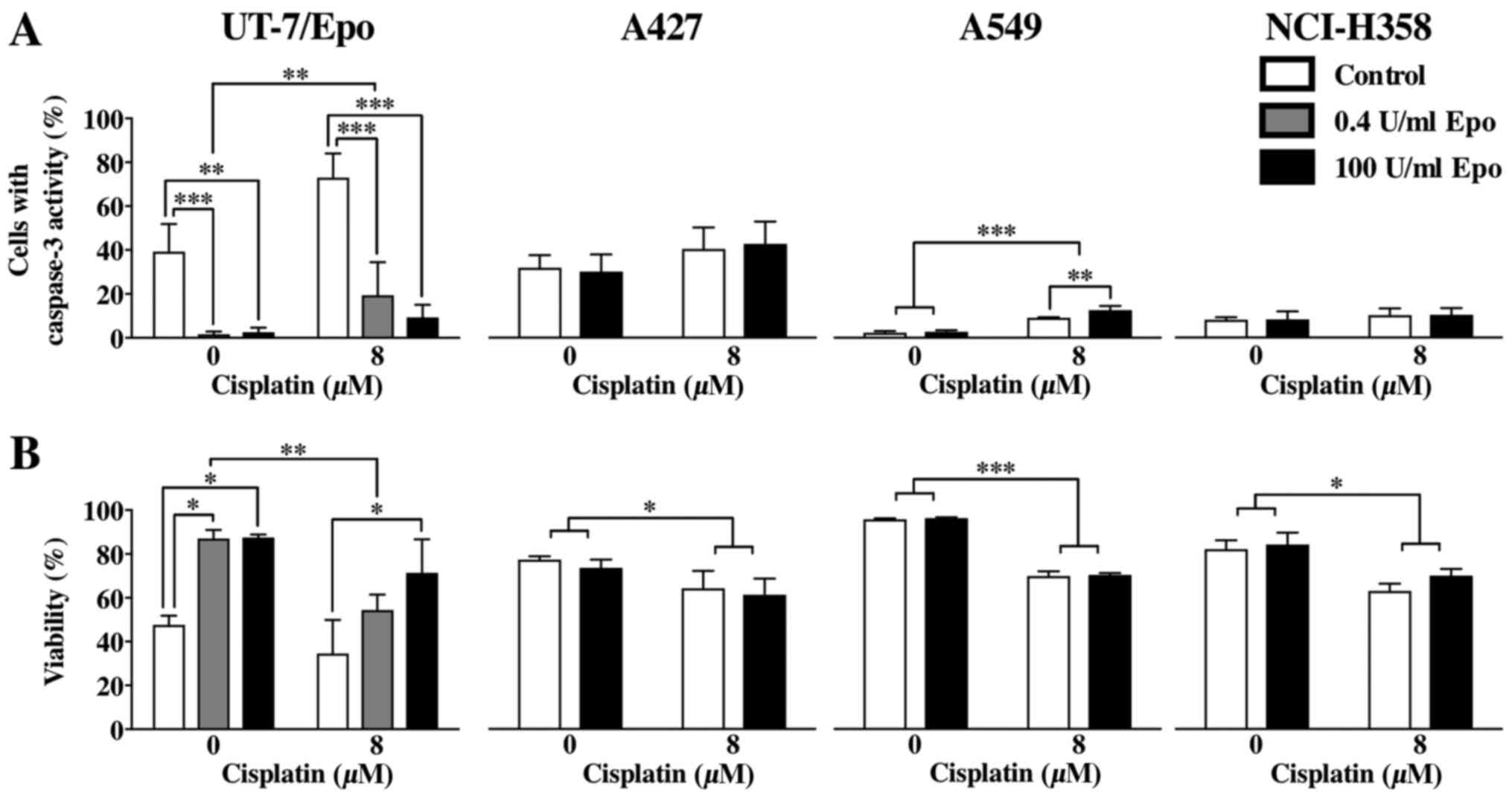
Apoptosis of Epo-treated NSCLC cell
lines
To determine the extent of apoptosis by the
detection activated caspase-3, all cell lines were analyzed for
caspase-3 activity by flow cytometry. Treatment with 8 µM
cisplatin led to a significant decrease in the viability of all
cell lines, which was independent of Epo treatment (UT-7/Epo cells,
P<0.01; A427 cells, P<0.05; A549 cells, P<0.0001; NCI-H358
cells, P<0.01, Fig. 6). An
increased number of cells with caspase-3 activity due to cisplatin
treatment was only found in the UT-7/Epo and A549 cells (P=0.001
and P<0.0001, respectively), but not in the A427 and NCI-H358
cells (P=0.0636 and P=0.2433, respectively). Epo did not lead to a
significant reduction in the number of cells with caspase-3
activity in the A427, A549 and NCI-H358 cells, which was
independent of cisplatin treatment, in contrast to the UT-7/Epo
cells (0.4 and 100 U/ml Epo, overall effect of Epo, P<0.0001).
An analysis of cell viability revealed that cisplatin treatment
induced a significant reduction in the number of viable cells in
all cell lines (Fig. 6B). Epo
treatment did not reverse this cytotoxic effect of cisplatin in the
NSCLC cell lines in contrast to the UT-7/Epo cell line, in which
Epo reversed the effects of cisplatin.
Discussion
Recombinant human Epo is an effective treatment
option for chemotherapy-induced anemia in lung cancer patients.
Results from clinical trials have suggested that adverse effects,
including a higher mortality rate are due to the use of Epo
(12), and have prompted concerns
as to the safety of Epo, and thus its molecular effects on tumor
cells (7,14). Results from cell-based analyses
that have investigated the presence and functionality of EpoR in
tumor cells, as well as the effects of its ligand Epo have been
largely contradictory (33,34).
The present in vitro study aimed to assess
the expression and the functionality of EpoR in selected NSCLC cell
lines, as well as the effects of Epo on cell proliferation and
apoptosis under normoxic and hypoxic conditions. We found that the
NSCLC cell lines A427, A549 and NCI-H358 expressed EpoR mRNA and
protein. Immunocytochemical staining of the receptor protein
identified a mainly cytoplasmic and perinuclear localization. Epo
did not lead to a ligand-dependent activation of neither EpoR nor
further downstream signaling pathways (STAT5, PI3K-Akt and ERK1/2).
EpoR appeared to be rather constitutively active. While Epo
markedly enhanced the proliferation and reduced the apoptosis of
Epo-dependent UT-7/Epo leukemia cells, it did not affect the
proliferation or the cisplatin-induced apoptosis of NSCLC
cells.
Intratumoral hypoxia is known for its ambivalent
effects leading on one hand to restrained proliferation,
differentiation, apoptosis and necrosis. On the other had, it may
indeed result in a more aggressive tumor phenotype, tumor
progression and acquired resistance (26,35).
In this study, to mimic lower oxygen levels that commonly exist in
lung tumors in vivo, we cultured the NSCLC cell lines in all
hypoxic experiments at 1% O2. Hypoxic conditions were
confirmed by immunoblotting for HIF-1α in all 3 NSCLC cell lines.
The A427, A549 and NCI-H358 cells exhibited a statistically
significant decrease in cell growth due to hypoxia. We did not
detect an altered transcription of either EpoR mRNA or its protein
due to hypoxia.
The presence of EpoR in the selected NSCLC cell
lines makes them potentially able to respond to an appropriate
stimulation with Epo. The NSCLC cells showed in confocal laser
scanning microscopy a mainly cytoplasmic and perinuclear
fluorescent signal of EpoR protein, raising the question of how
EpoR can be reached and activated by its ligand. However, the
predominant cytoplasmic localization of EpoR is well known for both
hematopoietic progenitor and cancer cells (34,36).
It has been reported that <10% of total EpoR protein is
expressed on the cell surface of these cells due to highly dynamic
intracellular receptor pools, an incomplete processing in
organelles, and a short half-life of EpoR protein (37,38).
There are still serious concerns as to the specificity of anti-EpoR
antibodies commonly used in recent studies on non-hematopoietic and
in particular malignant cells (33,39).
Nevertheless, the anti-EpoR antibody applied in this study (M-20,
Santa Cruz Biotechnology) has been described to be suitable for the
detection of EpoR with immunoblots (33). This antibody was able to
differentiate Epo-stimulated from unstimulated EpoR in the positive
control UT-7/Epo cells and to detect the full-length form of EpoR
at 59 kDa with immunoblots.
Apart from the expression of EpoR, its functionality
seems to be another crucial factor that may be responsible for the
influence of Epo on cancer cell progression (34,40).
Some studies have provided evidence that EpoR is capable of
transmitting the Epo-driven signal into the cell via cascades of
activating protein phosphorylation in selected cancer cells
(17,23,41–43).
Others have shown that a range of cancer cell lines does not
express a functionally active EpoR (34). In this study, we demonstrated that
EpoR expression was present in all 3 NSCLC cell lines and was
rather constitutively phosphorylated, which was also independent of
exogenous Epo administration. Likewise, the signaling pathways
downstream of EpoR appeared to be continuously activated in these
lung cancer cells, even though these cells had been cultured in
serum-free starvation medium prior to and during the Epo challenge
to reduce disturbing and unspecific signaling. A constitutive
activation of EpoR and its downstream signaling pathways may reduce
the sensitivity of these cells towards growth factor stimulation,
such as Epo. A certain intracellular cross-activation of these
signaling pathways may also explain the downstream phosphorylation.
However, the control cell line, UT-7/Epo, underwent identical
treatment and displayed no baseline phosphorylation of any of the
downstream pathways tested. The possibility that EpoR can be
constitutively phosphorylated and thus activated has already been
described, e.g., in human ovarian carcinoma cells (44). All 3 NSCLC cell lines exhibited an
activating point mutation in codon 12 of the KRAS gene,
leading to an enhanced activation of the MAP kinase pathway and
thus enhanced proliferation (45,46).
It is conceivable that the effect of Epo may not be registered due
to constitutively active KRAS protein. Likewise, the time-course
experiments showed phosphorylation of ERK1/2 without Epo
stimulation in all cell lines, although they were cultured in
starvation medium. The crosstalk between these signaling pathways
(Jak2-STAT5, PI3K-Akt and MAP kinase) may explain a protein
phosphorylation in unstimulated cells.
In our experimental model, 100 U/ml of Epo did not
significantly stimulate the proliferation of the NSCLC cells nor
did it protect them from apoptosis. According to other authors
(9), we consider this
concentration as suprapharmacologic compared with physiological
basal plasma concentrations of Epo ranging from 6 to 32 U/l
(25). The results from the
proliferation and apoptosis experiments on the protective effects
of Epo in tumor cells are, by now, rather contradictory. Some
studies have shown that Epo has the capacity to enhance cancer cell
progression in diverse cancer cell types (16,17,47–49),
while others have demonstrated the opposite (23,40,50–57).
Since EpoR appeared to be constitutively
phosphory-lated in these 3 lung cancer cell lines, the
anti-apoptotic and proliferative effects of exogenous Epo may not
be sufficiently determinable. Nevertheless, the control cell line,
UT-7/Epo, confirmed the principle of the design of this study and
responded to Epo treatment in a clear Epo-dependent manner,
increasing proliferation and reducing apoptosis.
We also assessed the cytotoxic effects of cisplatin
via the induction of apoptosis (caspase-3 activation) and cell
viability. The latter was derived from the proportion of viable
cells, which was calculated in function of the cell volume and
assessed by electronic pulse area analysis. Based on our previous
studies (26,58), we know that higher cisplatin
concentrations induce the prominent and rapid apoptosis of NSCLC
cell lines. We intended to induce apoptosis and cell death in
general with a cisplatin concentration (8 µM) that is low
enough to allow a sufficient amount of viable cells to remain,
which are still able to respond to exogenous Epo. This cisplatin
concentration is similar to values measured in the serum of
patients treated with cisplatin, and is thus clinically relevant.
This concentration was able to induce the irreversible apoptosis of
the A549, but not that of the A427 and NCI-H358 cells. However, the
viability of all these cell lines was significantly reduced by
treatment with 8 µM of cisplatin. Therefore, we can conclude
that the cytotoxic effects of cisplatin in the given concentration
were sufficiently high to universally reduce cell viability, but
not to induce significant apoptosis in all cell lines. This may be
due to the different susceptibilities to cisplatin of each cell
line.
As a limitation of our study, the results derived
from isolated cancer cells and the nature of this study excluded
the investigation of any molecular interplay between the tumor
cells and the surrounding tumor microenvironment, i.e., stromal
cells such as fibroblasts, endothelial or immune cells.
Future experiments are required to focus on the
interplay between stromal and NSCLC cells. For instance, a simple
co-culture system of these cell types would allow the assessment of
the effects of Epo between them in terms of cancer cell survival
and proliferation. Furthermore, a novel and appealing strategy may
be tumor-derived organotypic slice cultures, in which the effects
of both chemotherapeutics and Epo can be analyzed (59–61).
In contrast to pure monoculture experiments, this model allows a
partial preservation of the human tumor microenvironment, the
collection of samples from the media over time, the analysis of the
effect of cytotoxic drugs, and thus, a possible prediction of tumor
response or resistance to therapy (59).
In conclusion, in our experimental setting,
exogenous Epo had no significant effect on the proliferation and
apoptosis of NSCLC cell lines, neither under hypoxic nor under
normoxic conditions despite the expression of EpoR.
Acknowledgments
We are grateful to Dr Zoltán Bálint (Ludwig
Boltzmann Institute for Lung Vascular Research) for the preparation
of the confocal imaging, and to Elisabeth Pöllitzer, BSc and
Alexandra Bertsch, MSc (Division of Pulmonology, Department of
Internal Medicine, Medical University of Graz) for their excellent
technical assistance. In addition, we would like to thank Dr Slaven
Crnkovic and all other members of the Ludwig Boltzmann Institute
for Lung Vascular Research, Graz, Austria, as well as the
core-facilities at the Center for Medical Research (Zentrum für
Medizinische Grundlagenforschung, ZMF) at the Medical University of
Graz for their support. Furthermore, we are very grateful for the
generosity of Professor Dr Norio Komatsu (Department of Hematology,
Juntendo University, Tokyo, Japan) for providing the control cell
line UT-7/Epo. Dr Armin Frille was temporarily supported by the
Federal Ministry of Education and Research (BMBF), Germany, FKZ:
01EO1501 (IFB AdiposityDiseases, MetaRot program).
Abbreviations:
|
Akt
|
protein kinase B
|
|
ANOVA
|
analysis of variance
|
|
Epo
|
erythropoietin
|
|
EpoR
|
erythropoietin receptor
|
|
ERK1/2
|
extracellular signal-regulated kinases
1/2
|
|
Jak2
|
Janus kinase 2
|
|
KRAS gene
|
Kirsten rat sarcoma gene
|
|
MAP kinase
|
mitogen-activated protein kinase
|
|
NSCLC
|
non-small cell lung cancer
|
|
PI3K
|
phosphatidylinositol-3-kinase
|
|
STAT5
|
signal transducer and activator of
transcription 5
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reck M, Popat S, Reinmuth N, De Ruysscher
D, Kerr KM and Peters S; ESMO Guidelines Working Group: Metastatic
non-small-cell lung cancer (NSCLC): ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol.
25(Suppl 3): iii27–iii39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pirker R, Wiesenberger K, Pohl G and Minar
W: Anemia in lung cancer: Clinical impact and management. Clin Lung
Cancer. 5:90–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caro JJ, Salas M, Ward A and Goss G:
Anemia as an independent prognostic factor for survival in patients
with cancer: A systemic, quantitative review. Cancer. 91:2214–2221.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gilreath JA, Stenehjem DD and Rodgers GM:
Diagnosis and treatment of cancer-related anemia. Am J Hematol.
89:203–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crawford J, Demetri GD, Gabrilove JL,
Blasi MV, Sarokhan BJ and Glaspy J: Clinical benefits of epoetin
alfa therapy in patients with lung cancer. Clin Lung Cancer.
3:180–190. 2002. View Article : Google Scholar
|
|
7
|
Rizzo JD, Brouwers M, Hurley P, Seidenfeld
J, Arcasoy MO, Spivak JL, Bennett CL, Bohlius J, Evanchuk D, Goode
MJ, et al American Society of Hematology and the American Society
of Clinical Oncology Practice Guideline Update Committee: American
Society of Hematology/American Society of Clinical Oncology
clinical practice guideline update on the use of epoetin and
darbepoetin in adult patients with cancer. Blood. 116:4045–4059.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jelkmann W, Bohlius J, Hallek M and
Sytkowski AJ: The erythropoietin receptor in normal and cancer
tissues. Crit Rev Oncol Hematol. 67:39–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sinclair AM, Todd MD, Forsythe K, Knox SJ,
Elliott S and Begley CG: Expression and function of erythropoietin
receptors in tumors: Implications for the use of
erythropoiesis-stimulating agents in cancer patients. Cancer.
110:477–488. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szenajch J, Wcislo G, Jeong JY, Szczylik C
and Feldman L: The role of erythropoietin and its receptor in
growth, survival and therapeutic response of human tumor cells:
From clinic to bench - a critical review. Biochim Biophys Acta.
1806:82–95. 2010.PubMed/NCBI
|
|
11
|
Wright JR, Ung YC, Julian JA, Pritchard
KI, Whelan TJ, Smith C, Szechtman B, Roa W, Mulroy L, Rudinskas L,
et al: Randomized, double-blind, placebo-controlled trial of
erythropoietin in non-small-cell lung cancer with disease-related
anemia. J Clin Oncol. 25:1027–1032. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bohlius J, Schmidlin K, Brillant C,
Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke M, Weingart
O, Kluge S, et al: Recombinant human erythropoiesis-stimulating
agents and mortality in patients with cancer: A meta-analysis of
randomised trials. Lancet. 373:1532–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rizzo JD, Somerfield MR, Hagerty KL,
Seidenfeld J, Bohlius J, Bennett CL, Cella DF, Djulbegovic B, Goode
MJ, Jakubowski AA, et al: Use of epoetin and darbepoetin in
patients with cancer: 2007 American Society of Hematology/American
Society of Clinical Oncology clinical practice guideline update.
Blood. 111:25–41. 2008. View Article : Google Scholar
|
|
14
|
Rodgers GM III, Becker PS, Blinder M,
Cella D, Chanan-Khan A, Cleeland C, Coccia PF, Djulbegovic B,
Gilreath JA, Kraut EH, et al: Cancer- and chemotherapy-induced
anemia (Version 1.2018). J Natl Compr Canc Netw. 10:628–653. 2017.
View Article : Google Scholar
|
|
15
|
Osterborg A, Aapro M, Cornes P, Haselbeck
A, Hayward CR and Jelkmann W: Preclinical studies of erythropoietin
receptor expression in tumour cells: Impact on clinical use of
erythropoietic proteins to correct cancer-related anaemia. Eur J
Cancer. 43:510–519. 2007. View Article : Google Scholar
|
|
16
|
Westenfelder C and Baranowski RL:
Erythropoietin stimulates proliferation of human renal carcinoma
cells. Kidney Int. 58:647–657. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feldman L, Wang Y, Rhim JS, Bhattacharya
N, Loda M and Sytkowski AJ: Erythropoietin stimulates growth and
STAT5 phosphorylation in human prostate epithelial and prostate
cancer cells. Prostate. 66:135–145. 2006. View Article : Google Scholar
|
|
18
|
Acs G, Chen M, Xu X, Acs P, Verma A and
Koch CJ: Autocrine erythropoietin signaling inhibits
hypoxia-induced apoptosis in human breast carcinoma cells. Cancer
Lett. 214:243–251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dolznig H, Habermann B, Stangl K, Deiner
EM, Moriggl R, Beug H and Müllner EW: Apoptosis protection by the
Epo target Bcl-XL allows factor-independent
differentiation of primary erythroblasts. Curr Biol. 12:1076–1085.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hardee ME, Cao Y, Fu P, Jiang X, Zhao Y,
Rabbani ZN, Vujaskovic Z, Dewhirst MW and Arcasoy MO:
Erythropoietin blockade inhibits the induction of tumor
angiogenesis and progression. PLoS One. 2:e5492007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Batra S, Perelman N, Luck LR, Shimada H
and Malik P: Pediatric tumor cells express erythropoietin and a
functional erythropoietin receptor that promotes angiogenesis and
tumor cell survival. Lab Invest. 83:1477–1487. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dagnon K, Pacary E, Commo F, Antoine M,
Bernaudin M, Bernaudin JF and Callard P: Expression of
erythropoietin and erythropoietin receptor in non-small cell lung
carcinomas. Clin Cancer Res. 11:993–999. 2005.PubMed/NCBI
|
|
23
|
Dunlop EA, Percy MJ, Boland MP, Maxwell AP
and Lappin TR: Induction of signalling in non-erythroid cells by
pharmacological levels of erythropoietin. Neurodegener Dis.
3:94–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vaupel P and Harrison L: Tumor hypoxia:
Causative factors, compensatory mechanisms, and cellular response.
Oncologist. 9(Suppl 5): 4–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jelkmann W: Regulation of erythropoietin
production. J Physiol. 589:1251–1258. 2011. View Article : Google Scholar :
|
|
26
|
Wohlkoenig C, Leithner K, Deutsch A,
Hrzenjak A, Olschewski A and Olschewski H: Hypoxia-induced
cisplatin resistance is reversible and growth rate independent in
lung cancer cells. Cancer Lett. 308:134–143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Komatsu N, Yamamoto M, Fujita H, Miwa A,
Hatake K, Endo T, Okano H, Katsube T, Fukumaki Y, Sassa S, et al:
Establishment and characterization of an erythropoietin-dependent
subline, UT-7/Epo, derived from human leukemia cell line, UT-7.
Blood. 82:456–464. 1993.PubMed/NCBI
|
|
28
|
Erickson-Miller CL, Pelus LM and Lord KA:
Signaling induced by erythropoietin and stem cell factor in
UT-7/Epo cells: Transient versus sustained proliferation. Stem
Cells. 18:366–373. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Belenkov AI, Shenouda G, Rizhevskaya E,
Cournoyer D, Belzile JP, Souhami L, Devic S and Chow TY:
Erythropoietin induces cancer cell resistance to ionizing radiation
and to cisplatin. Mol Cancer Ther. 3:1525–1532. 2004.
|
|
31
|
Gormley PE, Bull JM, LeRoy AF and Cysyk R:
Kinetics of cisdichlorodiammineplatinum. Clin Pharmacol Ther.
25:351–357. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Himmelstein KJ, Patton TF, Belt RJ, Taylor
S, Repta AJ and Sternson LA: Clinical kinetics on intact cisplatin
and some related species. Clin Pharmacol Ther. 29:658–664. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Elliott S, Busse L, Bass MB, Lu H, Sarosi
I, Sinclair AM, Spahr C, Um M, Van G and Begley CG: Anti-Epo
receptor antibodies do not predict Epo receptor expression. Blood.
107:1892–1895. 2006. View Article : Google Scholar
|
|
34
|
Swift S, Ellison AR, Kassner P, McCaffery
I, Rossi J, Sinclair AM, Begley CG and Elliott S: Absence of
functional EpoR expression in human tumor cell lines. Blood.
115:4254–4263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vaupel P: Hypoxia and aggressive tumor
phenotype: Implications for therapy and prognosis. Oncologist.
13(Suppl 3): 21–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hadland BK and Longmore GD:
Erythroid-stimulating agents in cancer therapy: Potential dangers
and biologic mechanisms. J Clin Oncol. 27:4217–4226. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Neumann D, Wikström L, Watowich SS and
Lodish HF: Intermediates in degradation of the erythropoietin
receptor accumulate and are degraded in lysosomes. J Biol Chem.
268:13639–13649. 1993.PubMed/NCBI
|
|
38
|
Becker V, Schilling M, Bachmann J, Baumann
U, Raue A, Maiwald T, Timmer J and Klingmüller U: Covering a broad
dynamic range: Information processing at the erythropoietin
receptor. Science. 328:1404–1408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brown WM, Maxwell P, Graham AN, Yakkundi
A, Dunlop EA, Shi Z, Johnston PG and Lappin TR: Erythropoietin
receptor expression in non-small cell lung carcinoma: A question of
antibody specificity. Stem Cells. 25:718–722. 2007. View Article : Google Scholar
|
|
40
|
Westphal G, Niederberger E, Blum C,
Wollman Y, Knoch TA, Rebel W, Debus J and Friedrich E:
Erythropoietin and G-CSF receptors in human tumor cells: Expression
and aspects regarding functionality. Tumori. 88:150–159.
2002.PubMed/NCBI
|
|
41
|
Kumar SM, Yu H, Fong D, Acs G and Xu X:
Erythropoietin activates the phosphoinositide 3-kinase/Akt pathway
in human melanoma cells. Melanoma Res. 16:275–283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lester RD, Jo M, Campana WM and Gonias SL:
Erythropoietin promotes MCF-7 breast cancer cell migration by an
ERK/ mitogen-activated protein kinase-dependent pathway and is
primarily responsible for the increase in migration observed in
hypoxia. J Biol Chem. 280:39273–39277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yasuda Y, Fujita Y, Matsuo T, Koinuma S,
Hara S, Tazaki A, Onozaki M, Hashimoto M, Musha T, Ogawa K, et al:
Erythropoietin regulates tumour growth of human malignancies.
Carcinogenesis. 24:1021–1029. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Paragh G, Kumar SM, Rakosy Z, Choi SC, Xu
X and Acs G: RNA interference-mediated inhibition of erythropoietin
receptor expression suppresses tumor growth and invasiveness in
A2780 human ovarian carcinoma cells. Am J Pathol. 174:1504–1514.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yoon YK, Kim HP, Han SW, Oh DY, Im SA,
Bang YJ and Kim TY: KRAS mutant lung cancer cells are
differentially responsive to MEK inhibitor due to AKT or STAT3
activation: Implication for combinatorial approach. Mol Carcinog.
49:353–362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Valenzuela DM and Groffen J: Four human
carcinoma cell lines with novel mutations in position 12 of c-K-ras
oncogene. Nucleic Acids Res. 14:843–852. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Acs G, Zhang PJ, McGrath CM, Acs P,
McBroom J, Mohyeldin A, Liu S, Lu H and Verma A: Hypoxia-inducible
erythropoietin signaling in squamous dysplasia and squamous cell
carcinoma of the uterine cervix and its potential role in cervical
carcinogenesis and tumor progression. Am J Pathol. 162:1789–1806.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pajonk F, Weil A, Sommer A, Suwinski R and
Henke M: The erythropoietin-receptor pathway modulates survival of
cancer cells. Oncogene. 23:8987–8991. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hardee ME, Arcasoy MO, Blackwell KL,
Kirkpatrick JP and Dewhirst MW: Erythropoietin biology in cancer.
Clin Cancer Res. 12:332–339. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Berdel WE, Danhauser-Riedl S, Oberberg D
and Zafferani M: Effects of hematopoietic growth factors on
malignant nonhematopoietic cells. Semin Oncol. 19(Suppl 4): 41–45.
1992.PubMed/NCBI
|
|
51
|
Berdel WE, Oberberg D, Reufi B and Thiel
E: Studies on the role of recombinant human erythropoietin in the
growth regulation of human nonhematopoietic tumor cells in vitro.
Ann Hematol. 63:5–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu WM, Powles T, Shamash J, Propper D,
Oliver T and Joel S: Effect of haemopoietic growth factors on
cancer cell lines and their role in chemosensitivity. Oncogene.
23:981–990. 2004. View Article : Google Scholar
|
|
53
|
Mundt D, Berger MR and Bode G: Effect of
recombinant human erythropoietin on the growth of human tumor cell
lines in vitro. Micro-titertec-tetrazolium assay.
Arzneimittelforschung. 42:92–95. 1992.PubMed/NCBI
|
|
54
|
Rosti V, Pedrazzoli P, Ponchio L, Zibera
C, Novella A, Lucotti C, Della Cuna GR and Cazzola M: Effect of
recombinant human erythropoietin on hematopoietic and
non-hematopoietic malignant cell growth in vitro. Haematologica.
78:208–212. 1993.PubMed/NCBI
|
|
55
|
Rössler J, Stolze I, Frede S, Freitag P,
Schweigerer L, Havers W and Fandrey J: Hypoxia-induced
erythropoietin expression in human neuroblastoma requires a
methylation free HIF-1 binding site. J Cell Biochem. 93:153–161.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gewirtz DA, Di X, Walker TD and Sawyer ST:
Erythropoietin fails to interfere with the antiproliferative and
cytotoxic effects of antitumor drugs. Clin Cancer Res.
12:2232–2238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rosti V, Pedrazzoli P, Ponchio L, Zibera
C, Novella A, Lucotti C, Della Cuna GR and Cazzola M: Effect of
recombinant human erythropoietin on hematopoietic and
non-hematopoietic malignant cell growth in vitro. Haematologica.
78:208–212. 1993.PubMed/NCBI
|
|
58
|
Fischer C, Leithner K, Wohlkoenig C,
Quehenberger F, Bertsch A, Olschewski A, Olschewski H and Hrzenjak
A: Panobinostat reduces hypoxia-induced cisplatin resistance of
non-small cell lung carcinoma cells via HIF-1α destabilization. Mol
Cancer. 14:42015. View Article : Google Scholar
|
|
59
|
Gerlach MM, Merz F, Wichmann G, Kubick C,
Wittekind C, Lordick F, Dietz A and Bechmann I: Slice cultures from
head and neck squamous cell carcinoma: A novel test system for drug
susceptibility and mechanisms of resistance. Br J Cancer.
110:479–488. 2014. View Article : Google Scholar :
|
|
60
|
Koerfer J, Kallendrusch S, Merz F,
Wittekind C, Kubick C, Kassahun WT, Schumacher G, Moebius C, Gaßler
N, Schopow N, et al: Organotypic slice cultures of human gastric
and esophago-gastric junction cancer. Cancer Med. 5:1444–1453.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Merz L, Höbel S, Kallendrusch S, Ewe A,
Bechmann I, Franke H, Merz F and Aigner A: Tumor tissue slice
cultures as a platform for analyzing tissue-penetration and
biological activities of nanoparticles. Eur J Pharm Biopharm.
112:45–50. 2017. View Article : Google Scholar
|