Introduction
As a type of solid tumor, oral squamous cell
carcinoma (OSCC) accounts for ~90% of oral malignancies, and
remains associated with a poor prognosis and low survival rate,
despite advances in diagnosis and therapy (1,2).
Approximately 20% of OSCC cases develop from epithelial dysplasia
lesions, so-called oral potentially malignant disorders (OPMDs),
such as oral leukoplakia and erythroplakia (3). Epidemiological surveys have confirmed
that OPMDs and OSCC are often influenced by continuous high-risk
behaviors, such as the use of tobacco, alcohol consumption and
betel nut use, which can generate chronic oxidative stress
(4,5). The effective early prevention and
detection of OPMDs may play a pivotal role in decreasing the
incidence of OSCC. However, no effective treatment approaches have
been used to prevent the development of dysplasia into cancerous
lesions thus far. Consequently, there is a critical need for the
discovery of novel therapeutic targets for OPMDs for the early
prevention of OSCC (6).
Proteomics analysis has become increasingly powerful
for the elucidation of protein expression and cell signaling
pathways (7). Numerous studies
have described the preliminary application of proteomics in the
identification of biomarkers for OSCC (8–10),
while few have used it to identify the development of OPMDs
(11). This may due to the fact
that it is difficult to trace the progress of oral malignant
transformation in individual patients, since this is often a
lengthy process. A well-established OSCC model, which can mimic
naturally-occurring OSCC within an observable duration, is induced
by the tumorigenic compound, 4-nitroquinoline 1-oxide (4NQO). It is
one of the most extensively studied animal systems due to its close
similarity to human oral malignant transformation at the
histological and molecular levels (12). This animal model has been widely
used in the study of OPMDs (13).
Furthermore, Lan et al (14) demonstrated that 4NQO induced
oxidative DNA damage and activated the nuclear factor
(erythroid-derived 2)-like 2 (Nrf2) pathway in the mouse
tongue.
In the present study, the profile of
differentially-expressed proteins during 4NQO-induced oral
carcinogenesis was investigated by iTRAQ-based proteomics followed
by quantitative verification. The oxidative stress-associated
proteins, such as thioredoxin-1 (Trx-1), glutaredoxin-1 (Grx-1) and
peroxiredoxin-2 (Prx-2), were noted as the proteins with the most
significant changes in expression. Among these proteins, Trx-1,
which is a member of the thioredoxin system and plays an important
role in maintaining redox balance, seemed to be the most
significantly upregulated protein in the precancerous stage. A
delay in tumor formation and a lower cancerization rate was
observed following the inhibition of Trx-1 with an irreversible
inhibitor, 1-methylpropyl 2-imidazolyl disulfide (PX-12) (15), in the animal model, which indicated
that Trx-1 may be a promising intervention target for OSCC
development.
Materials and methods
Established of rat model of 4NQO-induced
oral carcinogenesis
The rat model of 4NQO-induced oral carcinogenesis
was established as described previously (16). A total of 20 male Sprague-Dawley
(SD) rats (4 weeks of age and weighing 75–100 g) were divided into
2 groups. In the experimental group (n=14), rats were fed daily
with 20 ppm 4NQO (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
solution in their drinking water. At weeks 16–24, the experimental
rats were sacrificed, as visible lesions of tongue dysplasia or
squamous cell carcinoma had developed. The normal control group
(n=6) was fed with distilled water only. The rat tongues were
harvested and sagittally cut into 2 halves. One half was fixed in
10% buffered formalin, embedded in paraffin and cut into 4-mm-thick
sections for hematoxylin and eosin staining to confirm the
pathological diagnosis. The other half was collected and stored in
liquid nitrogen. Finally, 6 dysplasia samples (1 mild, 3 moderate
and 2 severe) and 8 well-differentiated carcinoma samples were
collected. All the animal procedures were conducted in accordance
with the Guidelines for the Care and Use of Laboratory Animals and
were approved by the Institutional Animal Care and Use Committee at
Sun Yat-sen University (Guangzhou, China).
PX-12 intervention in the rat model of
4NQO-induced oral carcinogenesis
Another 22 male SD rats (4 weeks of age and 75–100 g
in weight) were divided into 2 groups. In the experimental group
(n=16), the rats were fed daily with 20 ppm 4NQO (Sigma-Aldrich;
Merck KGaA) solution in their drinking water for 24 weeks. The rats
in the normal control group (n=6) were fed with distilled water
only. At week 18, the experimental rats were divided into 2 groups
as visible lesions of tongue dysplasia had appeared. One group was
injected with PX-12 (12 mg/kg) weekly via the tail vein. The other
group and the normal control group were injected with an equal
amount of saline. The rats were sacrificed at week 24. The tongues
were dissected, and a longitudinal mid-lingual incision was made.
The specimens were collected and analyzed as described below.
Proteomics analysis
The normal control, dysplasia and squamous cell
carcinoma specimens (n=6, 6 and 8, respectively) were digested in
1.8 U/ml dispase II (Roche Applied Science, Penzberg, Germany) at
4°C overnight. The epithelial tissues were enzymatically and
mechanically separated from the connective tissues. The samples
were detected using iTRAQ-based proteomic analysis at the Beijing
Genomics Institute (BGI; Shenzhen, China). The protocol for
proteomics analysis was conducted according to the BGI guidelines,
as previously described (7). In
this experiment, the total proteins of the epithelial tissues from
the 3 groups were extracted and digested into peptides,
respectively. The desalted peptides were labeled with iTRAQ
reagents (SCIEX, Framingham, MA, USA). The normal control,
dysplasia and squamous cell carcinoma were labeled with 114, 116
and 119 iTRAQ tags, respectively [normal (N)-114, dysplasia (D)-116
and carcinoma (C)-119]. The LTQ-Orbitrap-Velos hybrid mass
spectrometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
coupled with strong cation exchange chromatography (SCX) and liquid
chromatography (Thermo Fisher Scientific, Inc.) was used to analyze
the mixed peptides.
Database search and quantitative
proteomics analysis
All raw data files were searched using Mascot 2.3.02
(Matrix Science Ltd., London, UK) against the Rattus RefSeq
database (www.ncbi.nlm.nih.gov/protein) and the Uniprot Human
database (www.uniprot.org), containing 29,964
sequences. The following identification parameters were selected:
MS/MS ion search; enzyme, trypsin; fragment mass tolerance, ±0.02
Da; mass values, monoisotopic mass; max missed cleavages, 1;
peptide mass tolerance, 10 ppm; variable modifications, Gln->
pyro-Glu (N-termQ), oxidation (M) and iTRAQ8plex (Y); fixed
modifications, carbamidomethyl (C), iTRAQ8plex (N-term) and
iTRAQ8plex (K). The identified proteins and peptides were filtered
with confidence corresponding to the 1% FDR. The ratios of 116:114
and 119:114 were calculated. A fold change of ≥1.5 or ≤0.67 and a
P-value <0.05 were set as the thresholds for screening
differentially expressed proteins.
Patients and samples
Specimens were obtained from the oral mucosa of 60
subjects, including patients with OSCC (n=35), patients with oral
leukoplakia (n=15) and healthy control individuals (n=10). The
human healthy mucosa tissues were obtained from the cheeks and
gingiva of patients who underwent orthognathic surgery. The
diagnosis was based on clinical appearance and histological
analysis. Each subject provided written consent to participate
after being informed about the aims and protocol of the research.
This study was approved by the Ethics Committee of Guanghua School
of Somatology, Sun Yat-sen University.
Cell culture and induction of
hypoxia
The CAL33 cell line was originally purchased from
the Leibniz Institute DSMZ-German Collection of Microorganisms and
Cell Cultures GmbH (Braunschweig, Germany). The HSC6 cell line was
obtained from the National Cancer Center Research Institute (Tokyo,
Japan). Both cell lines were preserved at the Guangdong Provincial
Key Laboratory of Stomatology. The cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) (both from Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin (Gibco, Grand
Island, NY, USA) at 37°C in a 20% O2 and 5%
CO2 humidified incubator (normoxic conditions). Hypoxic
conditions were induced in a tri-gas incubator at 37°C with 1%
O2, 5% CO2 and 94% N2. The cells
were transferred into the specific incubator where necessary. PX-12
was dissolved in dimethyl sulfoxide (DMSO) at 100 mM as a stock
solution. N-acetyl cysteine (NAC) (all from Sigma-Aldrich; Merck
KGaA) was diluted to 500 mM by filtering. In this study, the cells
were treated with 5 mM NAC for 1 h prior to treatment with PX-12,
and 0.03% DMSO was used as a vehicle control.
Cell proliferation assay
Cell viability was detected by Cell Counting Kit-8
(CCK-8) assay (Dojindo, Kumamoto, Japan). The HSC6
(5×103 cells/well) and CAL33 cells (6×103
cells/well) were seeded into 96-well plates. After 24 h, the cells
were treated with various concentrations of PX-12 (0–50 µM)
for 24 h. Prior to the detection of the absorbance at 450 nm using
a microplate reader (Thermo Fisher Scientific, Inc.) the cells were
incubated with CCK-8 reagent (10 µl/well) for 2 h at 37°C.
All experiments were performed in triplicate.
Cell invasion assay
The invasion assays were performed using 24-well
Transwell units (BD Biosciences, Franklin Lakes, NJ, USA). Matrigel
(50 µl) diluted with serum-free DMEM (1:5) was coated on the
upper chamber. Subsequently, 8×104 cells in 200
µl serum-free medium with or without PX-12 (0, 10, 20 or 40
µM) were seeded in the upper chamber of the system. In the
experiments with NAC, the cells were treated with NAC for 1 h prior
to the addition of PX-12. A total of 600 µl DMEM with 10%
FBS was added to the bottom wells. The cells were incubated for 24
h. The invading cells on the bottom membrane of the upper chamber
were fixed with 4% paraformaldehyde and stained with 0.4% crystal
violet. Invading cell numbers were counted in 5 random fields
(original magnification, ×100).
Cellular apoptosis assay
For apoptosis assay, the Annexin V-FLUOS/propidium
iodide (PI) double-staining apoptosis detection kit (Roche
Diagnostics GmbH, Mannheim, Germany) was used. The OSCC cells were
treated with various concentrations of PX-12 for 24 h, following
pretreatment with or without 5 mM NAC for 1 h. The cells were
collected and stained with 5 µl Annexin V-fluorescein
isothiocyanate and 5 µl PI, according to the manufacturer’s
instructions. The acquisition and analysis of the apoptosis data
were performed using a flow cytometer (FACSCalibur; BD
Biosciences). Basal apoptosis was determined using the same method
in control cells.
Terminal
deoxynucleotidyltransferase-mediated dUTP nick end labelling
(TUNEL) assay
TUNEL assays were used to identify the apoptotic
cells using the FragEL™ DNA Fragmentation Detection kit
(Calbiochem; EMD Chemicals Inc., Gibbstown, NJ, USA) according to
the manufacturer’s instructions. Briefly, the tissue sections were
deparaffinized, rehydrated and incubated with proteinase K for 15
min. Following treatment with 3% H2O2 for 5
min, the sections were incubated with terminal deoxynucleotidyl
transferase (TdT) enzyme with TdT buffer and biotin-tagged
nucleotides in a humidified chamber at 37°C. Tagged nucleotides
were detected using HRP solution. After washing, the sections were
stained with diaminobenzidine (DAB) solution and counterstained
with hematoxylin. For the evaluation of the slides, 100 tumor or
epithelial cells were counted per high-power field (original
magnification, ×400).
Intracellular reactive oxygen species
(ROS) detection
The intracellular ROS levels were measured using a
dichlorofluorescein assay (Beyotime Institute of Biotechnology,
Haimen, China). 2,7-Dichlorodihydrofluorescein diacetate (DCFH-DA)
was used to evaluate the generation of ROS during oxidative damage.
The cells were incubated with 100 µM DCFH-DA for 20 min
after being washed 3 times in serum-free medium. Finally, the cells
were harvested and DCFH-DA fluorescence was assessed using a flow
cytometer (FACSCalibur; BD Biosciences) at 494/525 nm. All
experiments were performed in triplicate.
Immunohistochemistry (IHC)
IHC staining was performed according to the
manufacturer’s instructions. Tissue sections were incubated with
primary antibodies against Trx-1 (1:600, #2429; Cell Signaling
Technology, Inc., Danvers, MA, USA), hypoxia-inducible factor
(HIF)-1α (1:500, ab1) and Bax (1:100, ab32503) (both from Abcam,
Cambridge, MA, USA) overnight at 4°C, after being blocked in normal
goat serum for 20 min. Subsequently, the sections were incubated
with peroxidase-conjugated goat anti-rabbit secondary antibody
(GK600510; Gene Tech, Shanghai, China) for 30 min at room
temperature. Finally, the slides were visualized with DAB (R&D
Systems, Inc., Minneapolis, MN, USA) for antigen detection and
counterstained with hematoxylin. All steps were separated by
phosphate-buffered saline (PBS) or PBST washes. The expression of
proteins was quantified using a visual grading system based on the
extent of staining (percentage of positive cells graded on a scale
from 0–3 as follows: 0, <5%; 1, 5–30%; 2, 30–70%; and 3,
>70%) and the intensity of staining (graded on a scale from 0–3
as follows: 0, none; 1, weak; 2, moderate; and 3, strong) (17). A total of 5 representative fields
at ×400 magnification were evaluated.
Western blot analysis
The cells were lysed with RIPA buffer supplemented
with protease (both from Sigma-Aldrich; Merck KGaA) and phosphatase
inhibitors (Roche Applied Science). A BCA protein assay kit (ComWin
Biotech Co., Ltd., Beijing, China) was used to measured the
concentrations of the lysates. The lysates were then incubated at
99°C for 5 min and mixed with loading buffer (4:1; ComWin Biotech
Co., Ltd.). The samples (30 µg/lane) were separated on 10 or
12% SDS-PAGE gels and electrophoretically transferred onto a PVDF
membrane (EMD Millipore, Billerica, MA, USA). The membrane was
blocked in 5% non-fat milk for 1 h at room temperature and then
incubated with primary antibodies against Trx-1 (1:1,000, #2429;
Cell Signaling Technology, Inc.), HIF-1α (1:1,000, ab1), Bax
(1:1,000, ab32503) (both from Abcam), Bcl-2 (1:2,000, #2870s),
β-actin (1:1,000, #4970s), poly(ADP-ribose) polymerase (PARP) and
cleaved PARP (1:1,000; Cle-PARP; #9532s) (all from Cell Signaling
Technology, Inc.) overnight at 4°C, respectively. Subsequently, the
membrane was washed in TBST 3 times and incubated with
HRP-conjugated secondary antibody (1:3,000, #7074s; Cell Signaling
Technology, Inc.) for 1 h at room temperature. The immunoreactive
bands were visualized with an enhanced chemiluminescence detection
system (EMD Millipore). Immunoreactive bands were quantified by
densitometry with ImageJ 1.48 (National Institutes of Health,
Bethesda, MD, USA). Similar results were obtained from 3
independent experiments.
Statistical analysis
All results shown represent the means ± standard
deviation from triplicate experiments performed in a parallel
manner, unless otherwise indicated. Statistical analyses were
performed using a two-tailed Student’s t-test, Mann-Whitney U test,
Fisher’s exact test and one-way ANOVA, where appropriate. A value
of P<0.05 was considered to indicate a statistically significant
difference.
Results
Proteomics profiles of different stages
of carcinogenesis in rats induced by 4NQO
To explore protein profiles during oral
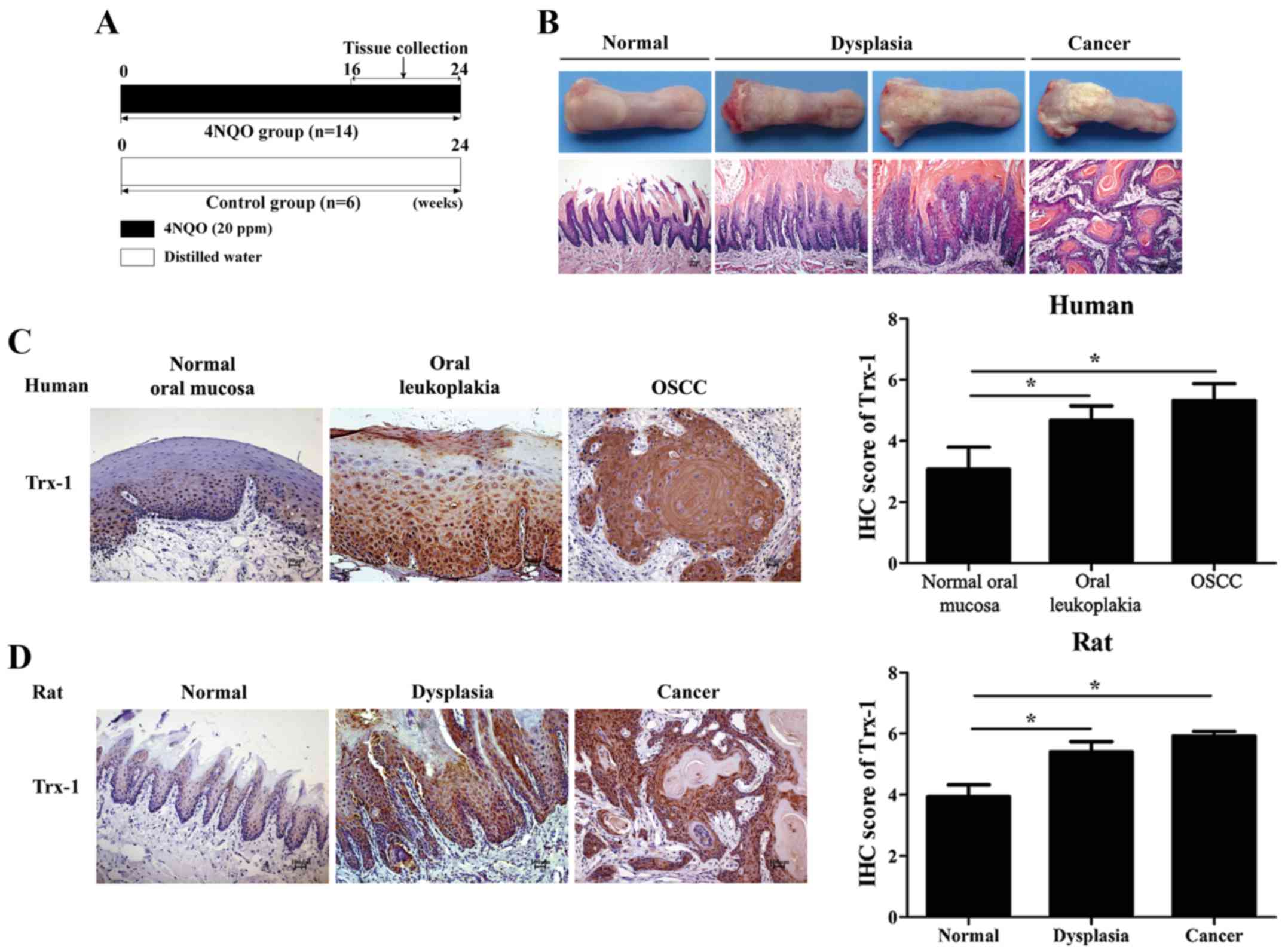
carcinogenesis, a rat model of 4NQO-induced oral carcinogenesis was
established (Fig. 1A and B). The
epithelia from the normal, dysplasia and squamous cell carcinoma
stages were collected for proteomics analysis. In this experiment,
proteins were found to be differentially expressed in the specimens
at the dysplasia stage (74 upregulated and 35 downregulated) and
squamous cell carcinoma stage (119 upregulated and 170
downregulated) compared with the normal group specimens (data not
shown). In total, 15 proteins were upregulated in both the
dysplasia and carcinoma stages (Table
I). Notably, the Trx, Grx and Prx proteins, which have been
characterized as ‘guardians’ of the intracellular redox state
(18), were indicated to be
upregulated during oral carcinogenesis, including Trx-1, Grx-1 and
Prx-2. Among these three oxidation-associated proteins, Trx-1 was
identified to be the most upregulated protein among the 3
oxidation-associated proteins in the dysplasia stage (Table I).
 | Table IDifferentially expressed proteins in
both the dysplasia and carcinoma stages. |
Table I
Differentially expressed proteins in
both the dysplasia and carcinoma stages.
| Protein name | Peptide detection
| Fold change
|
|---|
| Score | Coverage(%) | Unique peptide | D-116/N-114 | C-119/N-114 |
|---|
| Upregulated
proteins | | | | | |
| Thioredoxin-1 | 105 | 31.4 | 4 | 1.869 | 1.503 |
| Repetin | 1237 | 15.1 | 2 | 1.585 | 2.142 |
| Plasminogen
activator inhibitor 1 RNA-binding protein | 109 | 6.1 | 2 | 2.101 | 3.231 |
|
Peroxiredoxin-2 | 522 | 44.7 | 8 | 1.582 | 1.574 |
| Nucleoside
diphosphate kinase A | 184 | 45.4 | 3 | 1.580 | 2.136 |
| Hepatoma-derived
growth factor | 61 | 7.6 | 2 | 1.773 | 2.005 |
| Glutaredoxin-1 | 169 | 16.8 | 2 | 1.658 | 1.555 |
| Fatty acid-binding
protein, epidermal | 496 | 22.2 | 3 | 1.558 | 1.942 |
| Family with
sequence similarity 83, member H | 47 | 0.8 | 1 | 1.866 | 2.178 |
| Eukaryotic
translation elongation factor-1 β2 | 259 | 16.4 | 3 | 2.101 | 1.713 |
| Elongation factor
1-δ | 224 | 4.8 | 3 | 1.629 | 2.042 |
| Cystatin-A | 403 | 20.4 | 2 | 2.392 | 1.883 |
| Clathrin light
chain B | 131 | 10.5 | 3 | 2.028 | 2.055 |
| Calmodulin-like
protein 3 | 332 | 22.1 | 2 | 1.736 | 3.797 |
|
Barrier-to-autointegration factor | 206 | 40.4 | 2 | 1.527 | 1.505 |
| Downregulated
proteins | | | | | |
| Phosphate carrier
protein, mitochondrial | 75 | 6.2 | 2 | 0.635 | 0.362 |
| Myotilin | 130 | 2.8 | 1 | 0.492 | 0.351 |
| MICOS complex
subunit | 229 | 6.3 | 1 | 0.653 | 0.420 |
| Cytochrome c
oxidase subunit 6C-2 | 40 | 17.1 | 2 | 0.444 | 0.382 |
| Cytochrome b-c1
complex subunit 2 | 334 | 14.8 | 5 | 0.646 | 0.359 |
Trx-1 is overexpressed during oral
carcinogenesis
To confirm the expression pattern of Trx-1 during
oral carcinogenesis, IHC was performed. The results demonstrated
that Trx-1 expression gradually increased during oral malignant
transformation, both in the human samples and the rat specimens. As
shown in Fig. 1C, none or weak
Trx-1 staining was detected in the human normal oral mucosa (the
score of Trx-1 positive staining was 3.08±0.72), while the staining
of Trx-1 was particularly prominent in the abundant epithelial
cytoplasm and nuclei in the oral leukoplakia (4.68±0.47) and OSCC
samples (5.32±0.55). These scores were significantly higher
compared with the normal samples (P<0.05). The
clinicopathological characteristics of the patients are shown in
Table II. Moreover, similar
characteristics were observed in the animal model. As shown in
Fig. 1D, Trx-1 expression was
increased in the dysplasia tissues (5.4±0.33) compared with the
control tissues (3.93±0.39; P<0.05), as well as the tongue
carcinogenesis tissues (5.91±0.16), depending on the extent and
intensity of staining.
 | Table IIThe clinicopathological
characteristics of all the patients. |
Table II
The clinicopathological
characteristics of all the patients.
|
Characteristics | Normal oral
mucosa
(n=10) | Oral
leukoplakia
(n=15) | OSCC
(n=35) |
|---|
| Age (years) | 37.8±13.96 | 52.13±11.60 | 55.97±10.29 |
| Sex | | | |
| Male | 4 | 11 | 25 |
| Female | 6 | 4 | 10 |
| Smoking
history | | | |
| Yes | 3 | 10 | 18 |
| No | 7 | 5 | 17 |
| Alcohol
comsumption | | | |
| Yes | 2 | 4 | 9 |
| No | 8 | 11 | 26 |
| Site | | | |
| Tongue | – | 12 | 24 |
| Bucca
cavioris | 6 | 3 | 2 |
| Gingiva | 4 | – | 6 |
| Palate | – | – | 3 |
| Histological grade
of dysplasia | | | |
| Mild | – | 2 | – |
| Moderate | – | 5 | – |
| Severe | – | 8 | – |
| Histological grade
of tumor | | | |
| Well | – | – | 14 |
| Moderate | – | – | 10 |
| Poor | – | – | 11 |
| TNM stage | | | |
| I | – | – | 11 |
| II | – | – | 13 |
| III | – | – | 5 |
| IV | – | – | 6 |
Inhibition of Trx-1 suppresses the
proliferation and invasion, and enhances the apoptosis of OSCC
cells under hypoxic conditions
In solid tumors, hypoxia is a common feature that
leads to oxidative stress and results in redox imbalance (19). It has been demonstrated that
hypoxia is involved in the growth and aggressiveness of many types
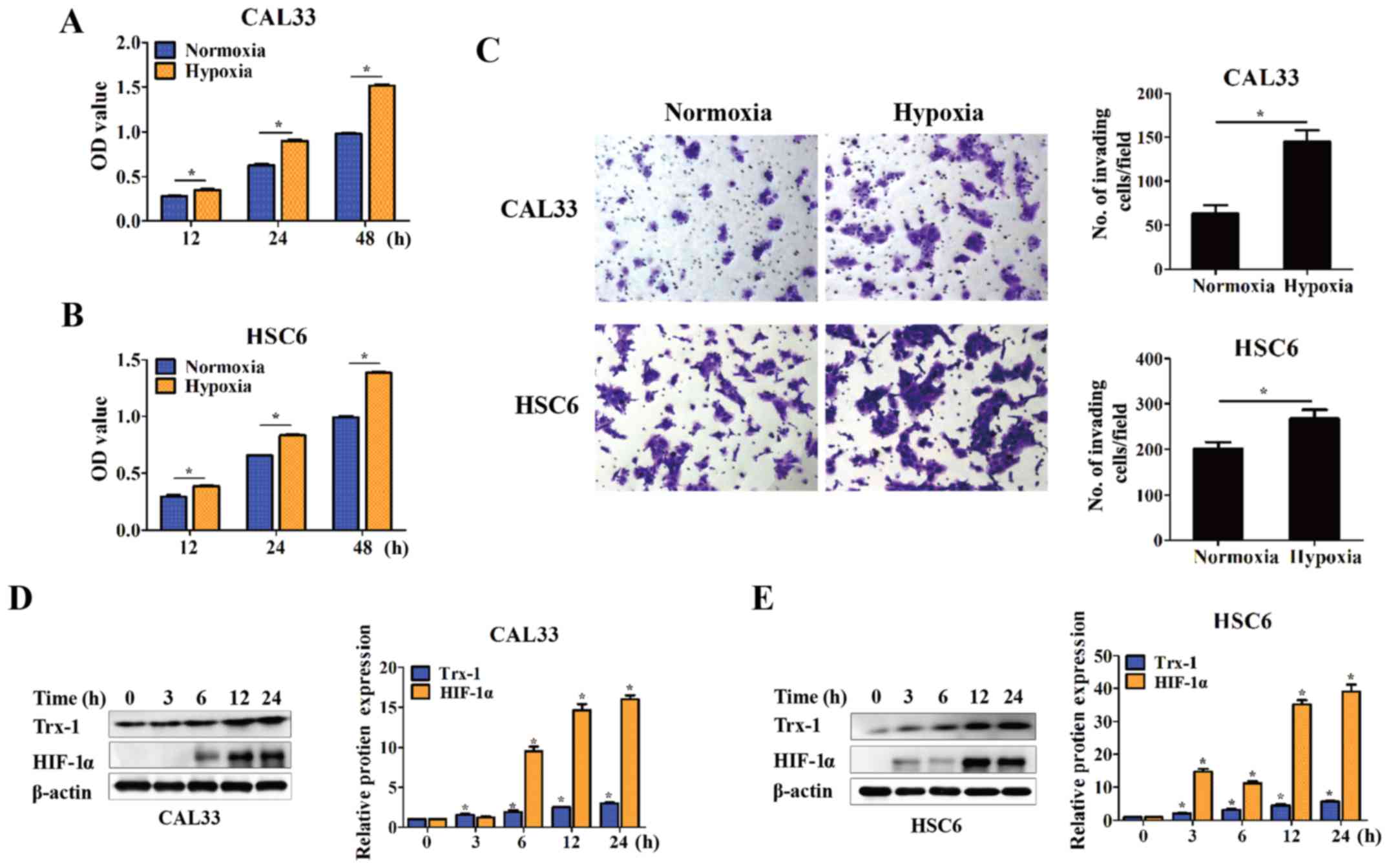
of cancer (20–22). In this study, two OSCC cell lines,
CAL33 and HSC6, were cultured under hypoxic conditions for 12, 24
and 48 h. Cell proliferation was significantly increased compared
with normoxic conditions, as demonstrated by CCK-8 assays
(P<0.05) (Fig. 2A and B). The
cell invasive ability was also promoted at 24 h, as demonstrated by
Transwell assay (P<0.05) (Fig.
2C). Moreover, Trx-1 expression was detected by western blot
analysis. The cells were cultured under hypoxic conditions for 3,
6, 12 and 24 h. Trx-1 expression gradually increased in a
time-dependent manner upon hypoxic stimulation, as was the
expression of HIF-1α (P<0.05) (Fig.
2D and E).
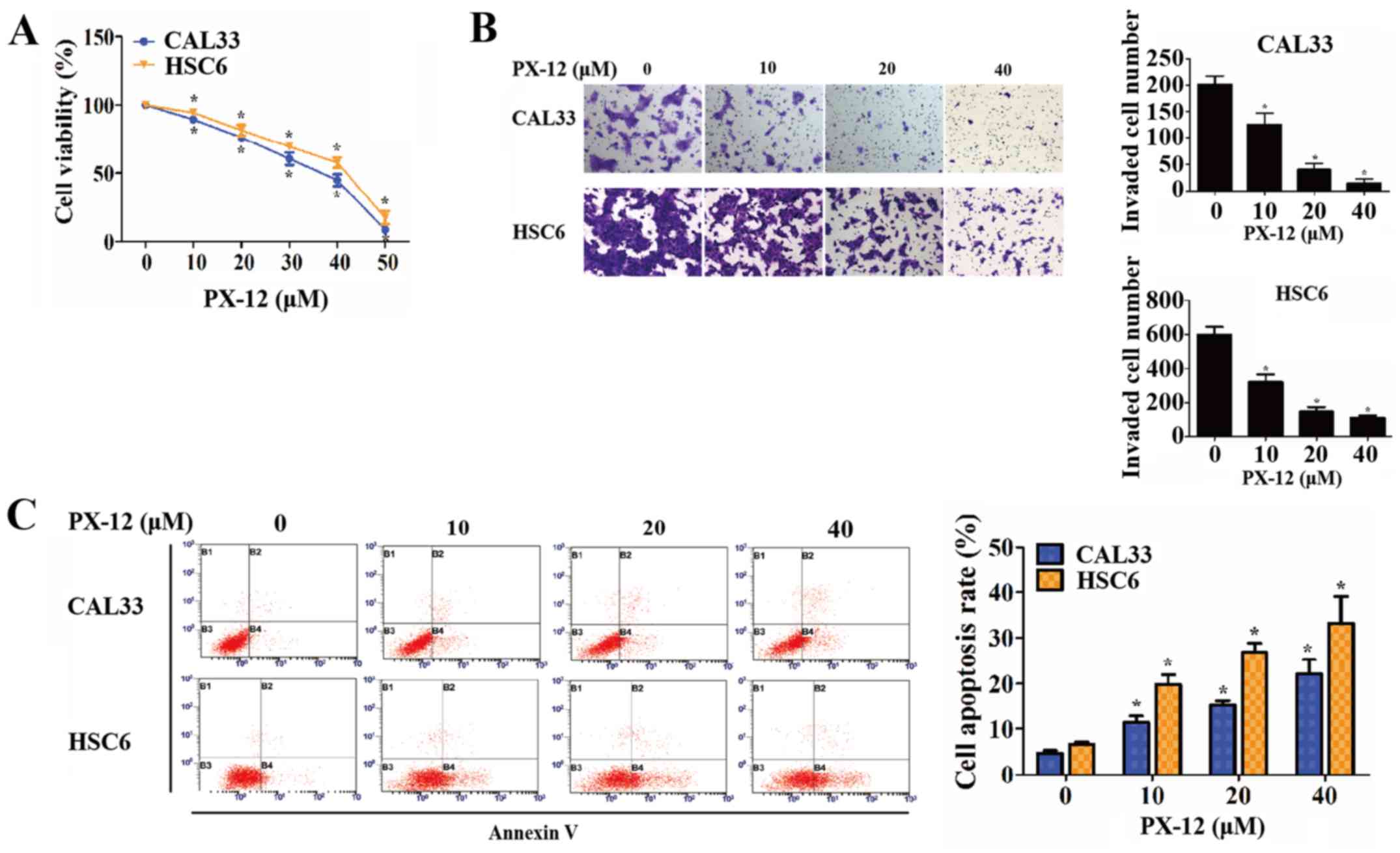
Subsequently, experiments were carried out to
examine the effect of Trx-1 on the phenotypes of OSCC cells under
hypoxic conditions. When Trx-1 was specifically inhibited by PX-12
at various concentrations (data not shown), cell viability and the
cell invasive capacity gradually decreased in a dose-dependent
manner (P<0.05) (Fig. 3A and
B). Moreover, the apoptosis of the OSCC cells was measured by
flow cytometry. As a result, the percentages of OSCC cells
undergoing early apoptosis were increased by treatment with PX-12
in a concentration-dependent manner (P<0.05) (Fig. 3C).
Inhibition of Trx-1 induces the apoptosis
of OSCC cells in a ROS-dependent manner under hypoxic
conditions
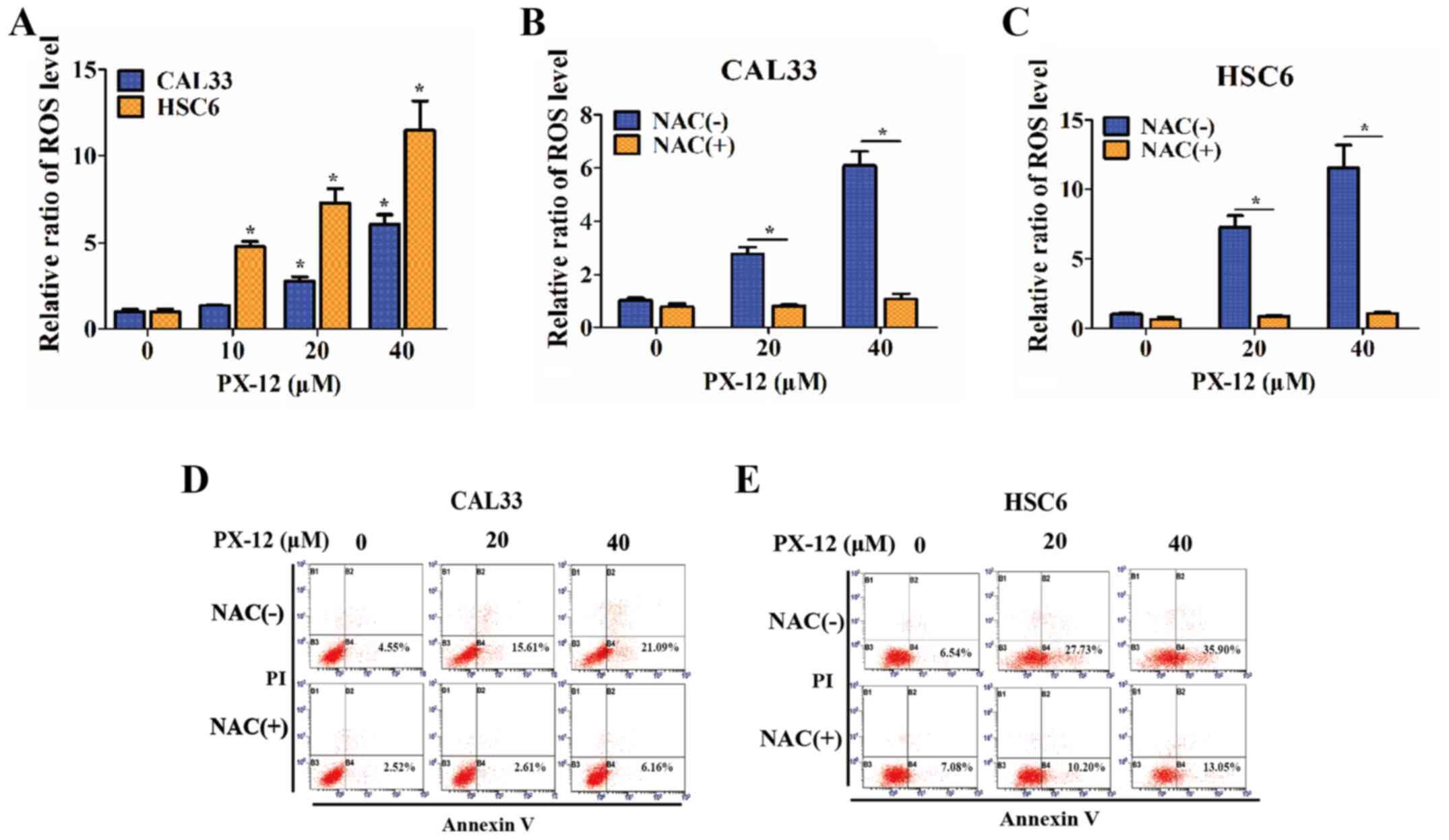
Since the inhibition of the activation of Trx-1 has
been hypothesized to affect the redox state of cells (23), the intracellular ROS levels of OSCC
cells were detected in the presence of PX-12 (10, 20 or 4
µM) for 24 h, and were markedly increased compared with
those of controls under hypoxic conditions (P<0.05) (Fig. 4A). Treatment of the OSCC cells with
the antioxidant agent, NAC (5 mM), 1 h prior to the addition of
PX-12 caused a significant decrease in ROS levels (P<0.05)
(Fig. 4B and C) and markedly
prevented cellular apoptosis (P<0.05) (Fig. 4D and E). Furthermore, the addition
of NAC also attenuated the inhibitory effects of PX-12 on cell
proliferation and invasion (data not shown). These results
demonstrated that the effects of PX-12 were ROS-dependent.
In addition, the mechanisms underlying the
apoptosis-promoting effects of PX-12 in OSCC cells were assessed by
western blot analysis. The levels of apoptosis-related proteins,
such as the anti-apoptotic protein, Bcl-2, the pro-apoptotic
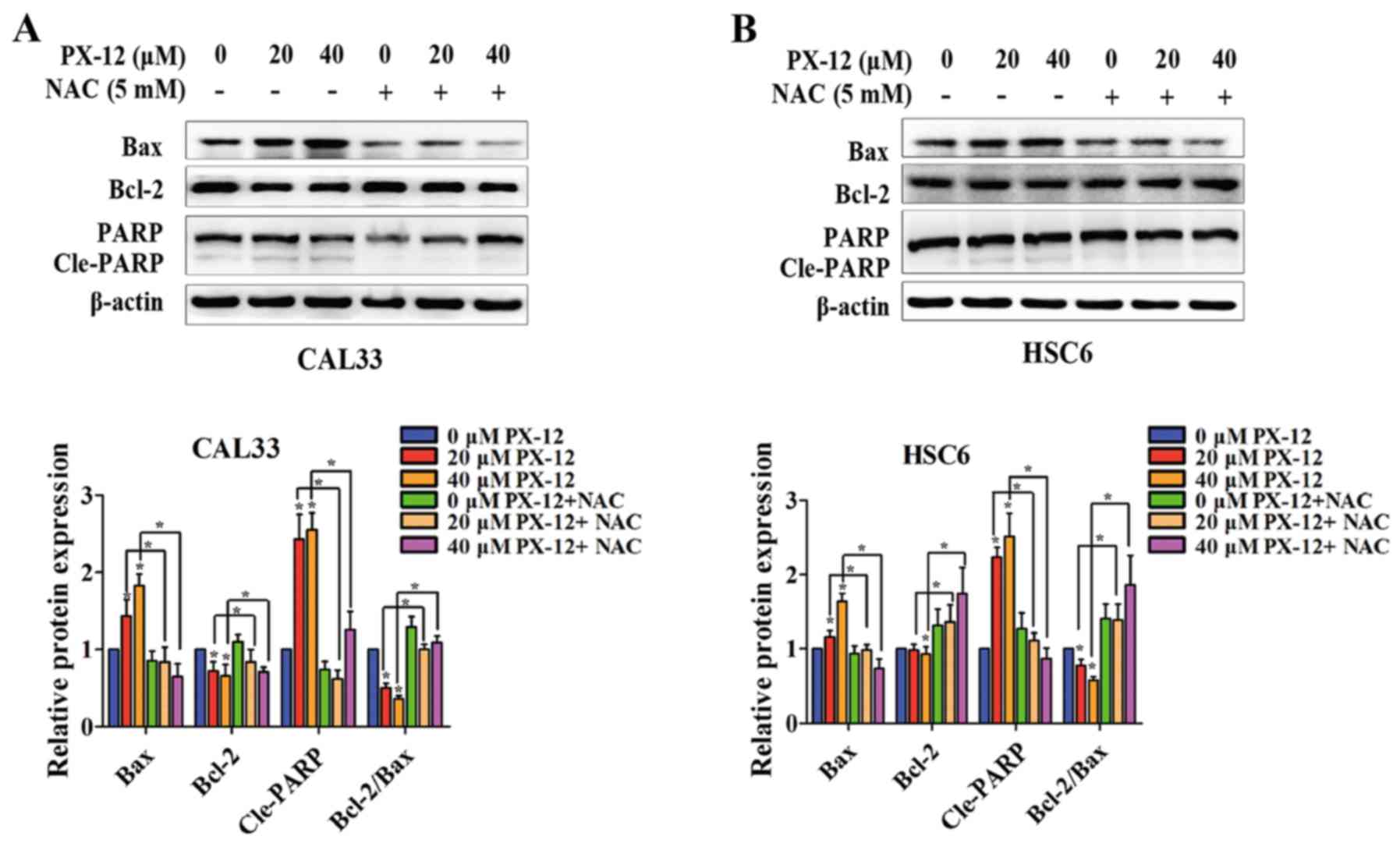
protein, Bax, and PARP cleavage (Cle-PARP) were examined in the
total protein from CAL33 and HSC6 cells. As shown in Fig. 5, the expression of Bax and Cle-PARP
was upregulated and the expression of Bcl-2 was downregulated by
PX-12 under hypoxic conditions. Additionally, the ratio of
Bcl-2/Bax was decreased. However, pretreatment with NAC reversed
the changes in Bax, Bcl-2 and Cle-PARP expression, and in the ratio
of Bcl-2/Bax. Thus, these results suggested that the inhibition of
Trx-1 induced apoptosis via ROS accumulation.
Inhibition of Trx-1 delays 4NQO-induced
oral carcinogenesis in vivo
The in vitro study results indicated that
Trx-1 was a promising therapeutic target for oral carcinogenesis.
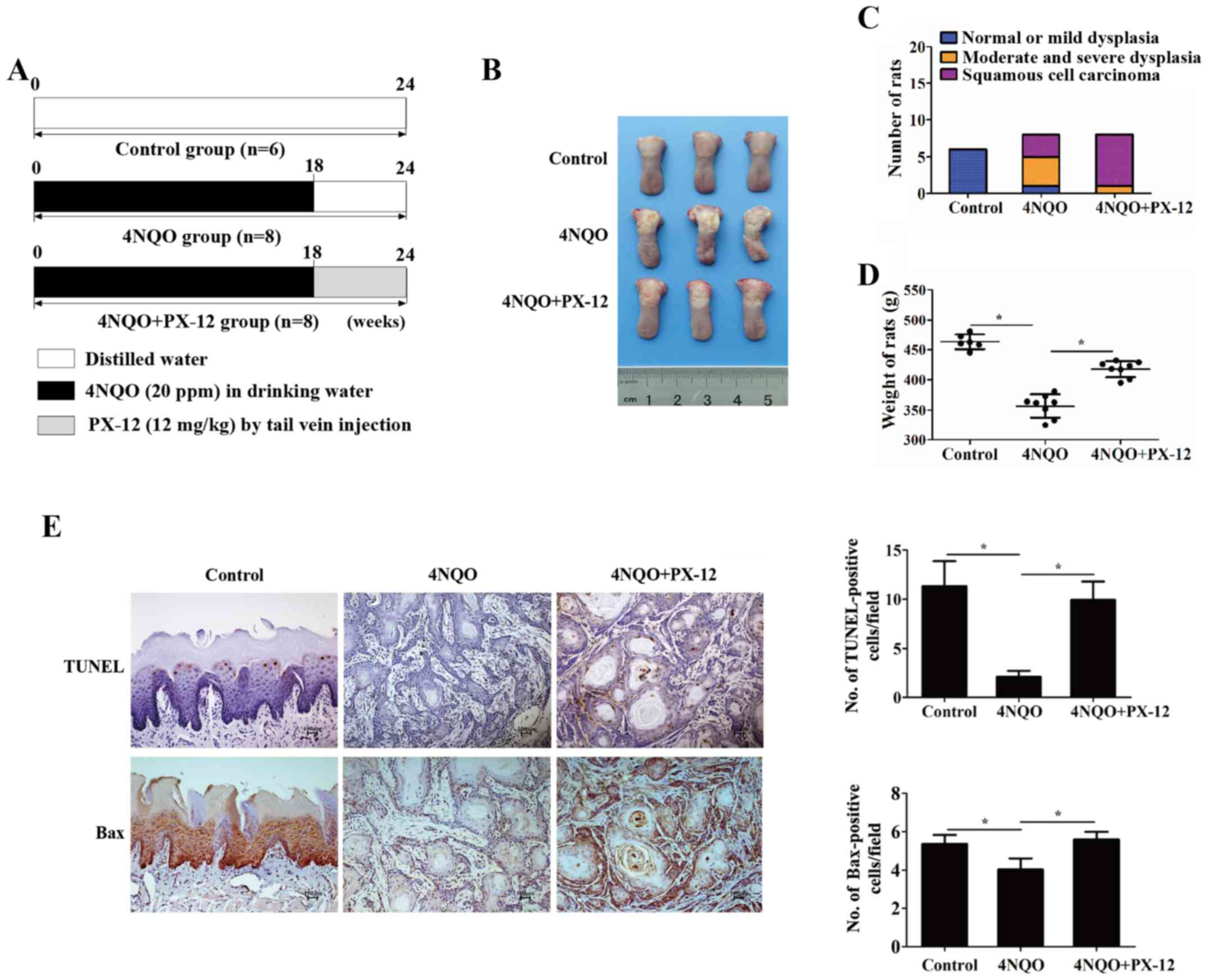
Thus, in our in vivo experiments, we randomly subdivided the
rats exposed to 4NQO into the PX-12 treatment group (n=8) and the
disease-control group (n=8) at week 18, when dysplasia was clearly
observed. The rats were administered PX-12 (12 mg/kg) via tail vein
injection weekly, from week 18 to the end of the experiment
(Fig. 6A). The gross weight and
emergence rate of the tumor was measured periodically. Notably, the
PX-12-treated rats had a lower cancerization rate (3/8) than the
rats in the disease-control group (7/8) (Fig. 6B and C). In addition, the weights
of the rats in the PX-12 treatment group (417.63±13.22 g) were
higher than those of the rats in the disease-control group
(356.38±19.56 g) (P<0.05) (Fig.
6D). Additionally, apoptosis was determined by TUNEL assay and
the expression of Bax was detected by IHC. As shown in Fig. 6E, the apoptotic rate of the PX-12
treatment group appeared higher than that of the disease-control
group (9.93±1.86 vs. 2.09±0.61%, P<0.05). Similarly, the
expression of Bax was increased in the PX-12 treatment group
(5.6±0.4, P<0.05). These results demonstrated that Trx-1 may be
a preventative and therapeutic target during oral epithelial
malignant transformation.
Discussion
Oral carcinogenesis is a complex and multifaceted
process, and the majority of cases begin with epithelial dysplasia
lesions (3,4). However, effective interference
efforts to end or delay oral leukoplakia or erythroplakia from
undergoing malignant transformation remain limited, which may be
due to the complex causes, lengthy course and individual
differences. With the use of a mature animal model, the rat model
of 4NQO-induced oral carcinogenesis, studies are able to include
the whole process of the disease while reducing the individual
differences. In the present study, iTRAQ-labeled quantitative
proteomics analysis was performed to disclose the molecular
alterations at different stages during 4NQO-induced oral
carcinogenesis.
In this study, the high-throughput results
demonstrated that the expression of oxidative stress-associated
proteins was altered significantly, which was consistent with the
experimental results on solid tumors. Several systematic reviews
and meta-analyses have reported that tobacco use and alcohol
consumption are risk factors for oral cancer (24). The characteristic increased levels
of oxidative stress in cancer cells result from an imbalance
between the generation and elimination of ROS (25). It has been reported that oxidative
stress contributes to oral epithelial malignant progression from
dysplasia to carcinogenesis (4).
As a common feature of solid tumors, hypoxia causes oxidative
stress and results in a redox imbalance in the tumor
microenvironment (26). Some
researchers have reported that the dysfunction and deregulation of
hypoxia-related proteins are often an early event during oral
carcinogenesis (27,28).
Human Trx-1 belongs to a family of small redox
proteins that are reduced by thioredoxin reductase and NADPH,
following the reduction of oxidative target proteins (29). Trx-1 plays an important role in
maintaining the redox balance, which is essential for cell
survival, tumor development and angiogenesis (30). It has been found to be upregulated
in many types of cancer (23,31–33)
and is regarded as a target for cancer therapy (34,35).
In this study, we investigated the role of Trx-1 in the development
of oxidative stress-associated oral epithelial malignancy. The
results revealed that the specific inhibition of Trx-1 induced
apoptosis under hypoxic conditions via ROS accumulation in OSCC
cells, which was consistent with results in other solid tumors
(36). The role of ROS in
tumorigenesis has been controversial for several years (37). It may play a dual role in cell
survival. In a previous study, it was found that interleukin
(IL)-1β promoted the invasive ability of OSCC CAL27 cells by
upregulating ROS (38). In the
present study, it was suggested that the excessive accumulation of
intracellular ROS through the specific inhibition of Trx-1 was due
to the apoptosis of the OSCC cell lines, CAL33 and HSC6. These
results suggest that Trx-1 may be a therapeutic target for OSCC
prevention and therapy.
To further validate the hypothesis, the preventive
effect of Trx-1 inhibition was tested with a rat model of
4NQO-induced oral carcinogenesis. A specific inhibitor of Trx-1
(PX-12) was administered via tail vein injection when dysplasia
lesions had developed. In general, by comparing the gross weight
and cancerization rate, the rats treated with PX-12 were in a
better condition than the disease controls when exposed to the same
carcinogen. No obvious injury to the liver or kidneys was observed
in the PX-12 treatment group (data not shown). The results
suggested that the inhibition of Trx-1 may provide a promising
chemoprevention strategy with which to interrupt oral malignant
transformation. Although the antitumor effects of PX-12 have been
previously reported (15,36), the present study investigated the
preventative effect of PX-12 in potentially malignant disorders by
continual observation of the 4NQO rat model in vivo. These
results are hopefully more objective than those from a xenograft
model or in vitro studies (15).
In conclusion, this was a preliminary study
examining the oxidative stress-associated proteins during oral
malignant transformation in vivo and in vitro. In
this study, it was demonstrated that the inhibition of Trx-1 in
OPMDs may be a potential target for delaying hypoxia-induced oral
malignant transformation, and that this chemopreventive effect is
mediated in a ROS-dependent manner. These results open up the
possibility for prevention and early intervention strategies for
OSCC, and are worthy of further research in the future.
Acknowledgments
This study was supported by the Science and
Technology Planning Project of Guangdong Province, China (grant
nos. 2014A020212104 and 2014A020212081).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arduino PG, Bagan J, El-Naggar AK and
Carrozzo M: Urban legends series: Oral leukoplakia. Oral Dis.
19:642–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choudhari SK, Chaudhary M, Gadbail AR,
Sharma A and Tekade S: Oxidative and antioxidative mechanisms in
oral cancer and precancer: A review. Oral Oncol. 50:10–18. 2014.
View Article : Google Scholar
|
|
5
|
Zhang X, Han S, Han HY, Ryu MH, Kim KY,
Choi EJ, Cha IH and Kim J: Risk prediction for malignant conversion
of oral epithelial dysplasia by hypoxia related protein expression.
Pathology. 45:478–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dionne KR, Warnakulasuriya S, Zain RB and
Cheong SC: Potentially malignant disorders of the oral cavity:
Current practice and future directions in the clinic and
laboratory. Int J Cancer. 136:503–515. 2015.
|
|
7
|
Liu F, Zhang Y, Men T, Jiang X, Yang C, Li
H, Wei X, Yan D, Feng G, Yang J, et al: Quantitative proteomic
analysis of gastric cancer tissue reveals novel proteins in
platelet-derived growth factor b signaling pathway. Oncotarget.
8:22059–22075. 2017.PubMed/NCBI
|
|
8
|
Wang Z, Jiang L, Huang C, Li Z, Chen L,
Gou L, Chen P, Tong A, Tang M, Gao F, et al: Comparative proteomics
approach to screening of potential diagnostic and therapeutic
targets for oral squamous cell carcinoma. Mol Cell Proteomics.
7:1639–1650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qing S, Tulake W, Ru M, Li X, Yuemaier R,
Lidifu D, Rouzibilali A, Hasimu A, Yang Y, Rouziahong R, et al:
Proteomic identification of potential biomarkers for cervical
squamous cell carcinoma and human papillomavirus infection. Tumour
Biol. 39:1010428317697547. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dey KK, Pal I, Bharti R, Dey G, Kumar BN,
Rajput S, Parekh A, Parida S, Halder P, Kulavi I, et al:
Identification of RAB2A and PRDX1 as the potential biomarkers for
oral squamous cell carcinoma using mass spectrometry-based
comparative proteomic approach. Tumour Biol. 36:9829–9837. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hung KF, Liu CJ, Chiu PC, Lin JS, Chang
KW, Shih WY, Kao SY and Tu HF: MicroRNA-31 upregulation predicts
increased risk of progression of oral potentially malignant
disorder. Oral Oncol. 53:42–47. 2016. View Article : Google Scholar
|
|
12
|
Kanojia D and Vaidya MM:
4-nitroquinoline-1-oxide induced experimental oral carcinogenesis.
Oral Oncol. 42:655–667. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu T, Hong Y, Jia L, Wu J, Xia J, Wang J,
Hu Q and Cheng B: Modulation of IL-1β reprogrammes the tumor
microenvironment to interrupt oral carcinogenesis. Sci Rep.
6:202082016. View Article : Google Scholar
|
|
14
|
Lan A, Li W, Liu Y, Xiong Z, Zhang X, Zhou
S, Palko O, Chen H, Kapita M, Prigge JR, et al: Chemoprevention of
oxidative stress-associated oral carcinogenesis by sulforaphane
depends on NRF2 and the isothiocyanate moiety. Oncotarget.
7:53502–53514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li GZ, Liang HF, Liao B, Zhang L, Ni YA,
Zhou HH, Zhang EL, Zhang BX and Chen XP: PX-12 inhibits the growth
of hepatocelluar carcinoma by inducing S-phase arrest,
ROS-dependent apoptosis and enhances 5-FU cytotoxicity. Am J Transl
Res. 7:1528–1540. 2015.PubMed/NCBI
|
|
16
|
Hong Y, Yang L, Li C, Xia H, Rhodus NL and
Cheng B: Frequent mutation of p16(CDKN2A) exon 1 during rat tongue
carcinogenesis induced by 4-nitroquinoline-1-oxide. Mol Carcinog.
46:85–90. 2007. View
Article : Google Scholar
|
|
17
|
Jiang X, Wang J, Chen X, Hong Y, Wu T,
Chen X, Xia J and Cheng B: Elevated autocrine chemokine ligand 18
expression promotes oral cancer cell growth and invasion via Akt
activation. Oncotarget. 7:16262–16272. 2016.PubMed/NCBI
|
|
18
|
Hanschmann EM, Godoy JR, Berndt C,
Hudemann C and Lillig CH: Thioredoxins, glutaredoxins, and
peroxiredoxins - molecular mechanisms and health significance: From
cofactors to antioxidants to redox signaling. Antioxid Redox
Signal. 19:1539–1605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Höckel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Hu Y, Xi N, Song J, Huang W, Song
S, Liu Y, Liu X and Xie Y: Partial oxygen pressure affects the
expression of prognostic biomarkers HIF-1 alpha, Ki67, and CK20 in
the microenvironment of colorectal cancer tissue. Oxid Med Cell
Longev. 2016:12047152016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng Y, Ni Y, Huang X, Wang Z and Han W:
Overexpression of HIF-1α indicates a poor prognosis in tongue
carcinoma and may be associated with tumour metastasis. Oncol Lett.
5:1285–1289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Z, Tu K, Wang Y, Yao B, Li Q, Wang L,
Dou C, Liu Q and Zheng X: Hypoxia accelerates aggressiveness of
hepatocellular carcinoma cells involving oxidative stress,
epithelial-mesenchymal transition and non-canonical hedgehog
signaling. Cell Physiol Biochem. 44:1856–1868. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Noike T, Miwa S, Soeda J, Kobayashi A and
Miyagawa S: Increased expression of thioredoxin-1, vascular
endothelial growth factor, and redox factor-1 is associated with
poor prognosis in patients with liver metastasis from colorectal
cancer. Hum Pathol. 39:201–208. 2008. View Article : Google Scholar
|
|
24
|
Mishra R: Glycogen synthase kinase 3 beta:
Can it be a target for oral cancer. Mol Cancer. 9:1442010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou J, Schmid T, Schnitzer S and Brüne B:
Tumor hypoxia and cancer progression. Cancer Lett. 237:10–21. 2006.
View Article : Google Scholar
|
|
27
|
DE Lima PO, Jorge CC, Oliveira DT and
Pereira MC: Hypoxic condition and prognosis in oral squamous cell
carcinoma. Anticancer Res. 34:605–612. 2014.PubMed/NCBI
|
|
28
|
Kujan O, Shearston K and Farah CS: The
role of hypoxia in oral cancer and potentially malignant disorders:
A review. J Oral Pathol Med. 46:246–252. 2017. View Article : Google Scholar
|
|
29
|
Sahaf B, Söderberg A, Spyrou G, Barral AM,
Pekkari K, Holmgren A and Rosén A: Thioredoxin expression and
localization in human cell lines: Detection of full-length and
truncated species. Exp Cell Res. 236:181–192. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kakolyris S, Giatromanolaki A, Koukourakis
M, Powis G, Souglakos J, Sivridis E, Georgoulias V, Gatter KC and
Harris AL: Thioredoxin expression is associated with lymph node
status and prognosis in early operable non-small cell lung cancer.
Clin Cancer Res. 7:3087–3091. 2001.PubMed/NCBI
|
|
31
|
Zhu X, Huang C and Peng B: Overexpression
of thioredoxin system proteins predicts poor prognosis in patients
with squamous cell carcinoma of the tongue. Oral Oncol. 47:609–614.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li C, Thompson MA, Tamayo AT, Zuo Z, Lee
J, Vega F, Ford RJ and Pham LV: Over-expression of Thioredoxin-1
mediates growth, survival, and chemoresistance and is a druggable
target in diffuse large B-cell lymphoma. Oncotarget. 3:314–326.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bhatia M, McGrath KL, Di Trapani G,
Charoentong P, Shah F, King MM, Clarke FM and Tonissen KF: The
thioredoxin system in breast cancer cell invasion and migration.
Redox Biol. 8:68–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Powis G and Kirkpatrick DL: Thioredoxin
signaling as a target for cancer therapy. Curr Opin Pharmacol.
7:392–397. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roh JL, Jang H, Kim EH and Shin D:
Targeting of the glutathione, thioredoxin, and Nrf2 antioxidant
systems in head and neck cancer. Antioxid Redox Signal. 27:106–114.
2017. View Article : Google Scholar
|
|
36
|
You BR, Shin HR, Han BR and Park WH: PX-12
induces apoptosis in Calu-6 cells in an oxidative stress-dependent
manner. Tumour Biol. 36:2087–2095. 2015. View Article : Google Scholar
|
|
37
|
Di Meo S, Reed TT, Venditti P and Victor
VM: Harmful and beneficial role of ROS. Oxid Med Cell Longev.
2016:79091862016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen X, Lv Q, Hong Y, Chen X, Cheng B and
Wu T: IL-1β maintains the redox balance by regulating glutaredoxin
1 expression during oral carcinogenesis. J Oral Pathol Med.
46:332–339. 2017. View Article : Google Scholar
|