Introduction
Head and neck squamous cell carcinoma (HNSCC)
frequently invades the facial bones, and this invasion is a
prognostic factor for poor clinical outcomes (1,2).
Bone resection is a treatment that often leads to the
post-operative disruption of speech and swallowing functions, and
thus poses a significant challenge to the quality of life of
patients with HNSCC presenting with facial bone invasion. Cancer
cells have been demonstrated to secrete significant amounts of
growth factors, which promotes osteoclastogenesis (3). Therefore, it is critical that novel
approaches should be evaluated for the treatment of bone
destruction in advanced HNSCC. Copper is known as a key factor for
cellular metabolism, neuronal transmission and bone remodeling
(4,5). Copper metabolic disorder induces
Wilson's disease, a rare inherited disorder that causes copper to
accumulate in the liver, brain and other vital organs (6). The copper chelator, ammonium
tetrathiomolybdate (TM), is used for the treatment of copper
metabolic disorder and Wilson's disease. In addition, recent
research has revealed that copper chelators exert an antitumor
effect against several cancer types, such as breast cancer with
lung metastases and head and neck cancer (7,8).
Copper is a factor that binds to selected enzymes
and functions to increase their activation. For example, Lysyl
oxidase (LOX) is the prototypical member of copper-dependent
enzymes whose documented function is to oxidize primary amine
substrates to reactive aldehydes (9). The most well-characterized role of
LOX is in the remodeling of the extracellular matrix (ECM) through
the oxidative deamination of peptidyl lysine residues in collagens
and elastin to facilitate covalent cross-linking (10). It has also been reported that LOX
is essential for bone remodeling via the regulation of receptor
activator of nuclear factor-κB ligand (RANKL) expression on bone
marrow stromal cells (11,12). Cancer cells release significant
amounts of LOX (13). This
copper-dependent LOX activation may promote bone resorption;
however, the role of copper in bone resorption in HNSCC remains
unclear and thus requires clarification.
In the present study, we thus aimed to determine the
role of copper in bone resorption in HNSCC. To the best of our
knowledge, we are the first to provide evidence that the copper
chelator, TM, exerts an anti-bone destruction effect against
cancer-induced bone resorption and that TM enhances the antitumor
effect of a clinically validated anticancer agent on HNSCC
associated with bone invasion.
Materials and methods
Cell lines and culture conditions
The human HNSCC cell lines, HSC-2 (#JCRB0622), HSC-3
(#JCRB0523) and SAS (#JCRB0260), were obtained from the Human
Science Research Resources Bank (Osaka, Japan). All cell lines were
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% heat-inactivated fetal bovine serum (FBS). Primary
fibroblasts were obtained from Cosmo Bio (#SCR2620, Tokyo,
Japan).
Primary osteoblasts, osteocytes, bone marrow cells
(obtained as described below), and fibroblasts were cultured in
alpha-modification of minimum essential medium (α-MEM). T cells
(obtained as described below) were cultured in RPMI-1640 medium
supplemented with 10% FBS, 1% penicillin-streptomycin, 10 mM
2-mercaptoethanol (Sigma, St. Louis, MO, USA) and Pyruvic acid
(Wako, Osaka Japan). All of the above-cited cell lines were
characterized by genotyping at cell banks. All cell lines were
cultured in an atmosphere of 10% CO2 at 37°C.
Osteoclastogenesis assay
Bone marrow cells were obtained from the femurs and
tibiae of 4-week-old male C57BL/6 mice (n=2), purchased from
Charles River Laboratories (Yokoyama, Japan). Under anesthesia with
0.4 mg/kg of medetomidine, 4.0 mg/kg of midazolam and 5.0 mg/kg of
butorphanol, the mice were sacrificed by cervical dislocation.
After the leg was cut, muscle and connective tissue was removed
from the tibiae and femurs. Both ends of the femur and tibiae were
then clipped with a scissors. A 5 cc syringe was filled using a 27
gauge needle with PBS, and a needle was inserted into one end and
the bone marrow was flushed out the other end. The flushed bone
marrow cells wre corrected in a 50 cc tube. The bone marrow cells
were washed twice by centrifugation (125 × g, 10 min) in 4°C PBS.
The cells were the nincubated in α-MEM in 10 cm culture dishes in
the presence of macrophage colony-stimulating factor (M-CSF) (10
ng/ml) for 24 h. Non-adherent cells were transferred to 24-well
plates (2×106 cells/well). The cells were treated with
vitamin D3, 1,25-Dihydroxy (10−8 M) and TM
(0.1, 1, 2.5 and 5 μM) for 9 days.
Purification of osteoclast
progenitors
Bone marrow cells were washed twice by
centrifugation (125 × g, 10 min) in 20 ml of 4°C sterile
phosphate-buffered saline (PBS) supplemented with 0.5% bovine serum
albumin. The cell pellet was resuspended, and the cells were
magnetically labeled by the addition of anti-CD11b microbeads
(Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The cells were
then incubated for 30 min on ice and then washed by centrifugation
(125 × g, 10 min) with a volume of 4°C buffer that was 10-fold that
of the labeling volume and resuspended. CD11b+ cells
were depleted using an MD depletion column (Miltenyi Biotec GmbH).
A total of 1×105 murine CD11b+ bone marrow
cells/well were plated in a 24-well plate. The cells were treated
with RANKL (50 ng/ml) and M-CSF (30 ng/ml) and the desired amount
of TM (0.1, 1, 2.5 and 5 μM) for 9 days. Following 9 days of
incubation, the cells were fixed and stained for tartrate-resistant
acid phosphatase activity using the acid phosphatase, leukocyte
(TRAP) kit (#A386A, Merck KGaA, Darmstadt, Germany), and the number
of TRAP-positive multinucleate cells (i.e., a nuclear number >3)
in each well was then counted.
Cell proliferation assay
The HSC-2, HSC-3 and SAS cells were each plated in
6-well plates at a density of 5×103 cells per well and
treated with the TM (1, 5 μM) or with an equivalent volume
of the diluent (DMSO) as a control for 5 days. Osteoblasts,
osteocytes and fibroblasts were each plated in 6-well plates at a
density of 1×104 cells per well and treated with TM (1,
5 μM) for 48 h. CD4+ T cells were isolated by the
magnetic sorting system. The cells from the homozenized spleens of
two C57BL/6J mice were incubated with CD4 antibody-conjugated
microbeads (eBioscience, San Diego, CA, USA) and sorted with MD
depletion column (Miltenyi Biotec). The cells were stimulated with
anti-CD3 monoclonal antibody (Cat. no. 16-0031, 5 μg/ml),
CD28 antibody (Cat. no. 16-0281, 2 μg/ml) (Affymetrix, Santa
Clara, CA, USA) and TM (1, 5 μM) for 48 h. The cell number
was then counted with trypan blue assay. Osteoblasts were obtained
following the method of Teramachi et al (14). After flushing the bone marrow from
the tibiae of 3 C57BL/6J mice, the tibiae were cultured in αMEM for
7–10 days in 60-mm dishes until the cells growing out of the bones
formed a confluent monolayer. The original bone was removed, and
the cells grown out of the the bone were treated with 0.25% trypsin
and 0.05% EDTA for 10 min at 37°C. These cells were used as primary
osteoblas without further passage. Osteocytes were obtained
following the method of Shah et al (15). After flushing the bone marrow from
the tibiae of the 2 C57BL/6J mice, the bones were dissected into
1–2-mm sized sections. The bone sections were then incubated for 25
min in collagenase (300 units/ml) and 0.25% EDTA (5 mM) and the
collagenase was removed and discarded. These processes were
repeated 8 times. The cells from the bone sections were cultured as
osteocytes without further passage. These cells were classified as
osteocytes by measuring dentin matrix acidic phosphoprotein 1
(DMP-1) expression by western blot analysis (data not shown).
Copper concentration measurement
assay
The copper concentrations in the culture medium and
serum, collected from the tail vein of the mice inoculated with the
cancer cells at the time of sacrifice were measured using the
Metallo Assay Copper Assay kit (Funakoshi, Tokyo, Japan). The
samples, buffer and chelate color solution were mixed and incubated
for 10 min at room temperature. Subsequently, the absorbance was
read at a 580 nm wavelength using a microplate reader (SH-1000,
Hitachi, Tokyo, Japan).
LOX activity assay
The HSC-2 and SAS cells were cultured in DMEM with
increased copper ion (10 μM) in the presence or absence of
TM for 24 h. Conditioned medium and serum collected from the mice
inoculated with the cancer cells were tested using a LOX activity
kit (Cat. no. ab112139, Abcam, Cambridge, MA, USA). The samples and
LOX reaction mix solution were mixed and incubated for 30 min at
room temperature. Subsequently, the fluorescence increase was
measured on a microplate reader (Gemini EM microplate reader,
Molecular Devices, Sunnyvale, CA, USA) at excitation and emission
wavelengths of 550 and 600 nm. These conditioned media were used in
the following experiment.
Western blot analysis
The osteocytes and osteoblasts were cultured in the
above-mentioned conditioned media (30%) for 24 h. The cell culture
conditioned media (25 μl) were mixed with 4X Laemmli sample
buffer (Bio-Rad, Hercules, CA, USA) and boiled at 95°C for 5 min.
The samples were electrophoresed in 4–12% SDS-PAGE gels and the
proteins were transferred onto membranes (Immobilon-P; Millipore,
Bedford, MA, USA). The membranes were incubated with primary and
secondary antibodies according to the ECL chemiluminescence
protocol (RPN2109; Amersham Biosciences, Buckinghamshire, UK) to
detect secondary antibody binding. Antibodies against RANKL (Cat.
no. sc-377079, 1:1,000, Santa Cruz Biotechnology (Dallas, TX, USA)
was used as a primary antibody and HRP-conjugated anti-mouse
antibody (Cat. no. 7076, 1:2,000, Cell Signaling Technology,
Danvers, MA, USA) was used as the secondary antibody.
Immunohistochemical analysis
The tibial bone and soft tumor was fixed in 10%
formalin, decalcified and then embedded in paraffin. Serial
sections were then prepared (3-μm-thick). For
immunohistochemical analysis, the specimens were incubated with
antibody (CD-31, 1:50, Cat. no. ab28364, Abcam), (Ki67, 1:400, Cat.
no. 9129, Cell Signaling Technology), (EGFR, 1:50, Cat. no. 4267,
Cell Signaling Technology), (p-EGFR, 1:200, Cat. no. 3777, Cell
Signaling Technology), (RANKL, 1:100, Cat. no. sc-377079, Santa
Cruz Biotechnology) overnight at 4°C, followed by 3 washes with
TBS. The slides were then treated with a streptoavidin-biotin
complex [EnVision System labelled polymer, horseradish peroxidase
(HRP); Dako, Carpinteria, CA, USA] for 60 min at a dilution of
1:100. The immunoreaction was visualized by using a DAB
substrate-chromogen solution (Dako Cytomation Liquid DAB Substrate
Chromogen System; Dako). The cells were counted using a microscope
and evaluated.
Animal experiments
Mouse models of bone invasion by human oral squamous
cell carcinoma were established in 5-week-old female BALB/c nude
mice (each group, n=5; total, n=20, mean body weight, 19.5 g;
Charles River Laboratories) by the inoculation of 1×105
HSC-2 cells into the bone marrow space of the left tibial
metaphysis. At 7 days after tumor cell inoculation, the mice were
divided into 4 groups (control, cetuximab-treated, TM-treated, and
TM- and cetuximab-treated). The cetuximab group was treated with an
intraperitoneal injection of 100 μl of a solution containing
cetuximab (1 mg/kg) in PBS or PBS alone twice a week for 5 weeks.
The TM group was orally administered a 100 μl solution
containing TM (1 mg) in distilled deionized water (DDW) or DDW
alone 5 times a week for 5 weeks. Under anesthesia with 0.4 mg/kg
of medetomidine, 4.0 mg/kg of midazolam and 5.0 mg/kg of
butorphanol, the hind limb long bones of the nude mice that had
been injected with the cancer cells were excised, fixed in 10%
neutral-buffered formalin. Osteolytic bone destruction was assessed
on radiographs. The bones were placed against films (22×27 cm; Fuji
industrial film FR: Fuji Photo Film Co. Ltd., Tokyo, Japan), and
exposed to soft X-rays at 35 kV for 15 sec by the use of a Sofron
apparatus (Sofron, Tokyo, Japan). The radiolucent bone lesions were
observed microscopically (IX81, Olympus, Tokyo, Japan), and the
areas were quantified with Lumina Vision/OL (Mitani, Tokyo, Japan).
A micro-CT image was obtained with SKYSCAN (Bruker Japan, Kanagawa,
Japan). Subsequently, the bone was decalcified and embedded in
paraffin. Serial sections (3 μm-thick) were cut
cross-sectionally, and the sections were stained with TRAP
stain.
Human oral squamous cell carcinoma xenografts were
established in 5-week-old male BALB/c nude mice (each group, n=5;
total, n=20, Charles River Laboratories) by the inoculation of
1×106 HSC-2 cells into the dorsal flank. At 7 days after
tumor cell inoculation, the mice were divided into 4 groups
(control, cetuximab-treated, TM-treated, and TM- and
cetuximab-treated). The mice were treated with an intraperitoneal
injection of 100 μl of solution containing cetuximab (1
mg/kg) in PBS or PBS alone twice a week for 5 weeks. The mice were
treated with orally adminisitration of 100 μl of a solution
containing TM (1 mg) or DDW, 5 times a week for 5 weeks. The tumor
volume (cubic mm) was calculated using the following equation:
4π/3xr1/2xr2/22, where r1 is the longitudinal diameter,
and r2 is the transverse diameter. At 5 weeks after tumor cell
inoculation, all the mice were then sacrificed, and the volume of
tumors was measured.
All the animal experimental protocols were approved
by the Ethics Review Committee for Animal Experimentation of the
Okayama University Graduate School of Medicine and Dentistry with
the following approval numbers: OKU-2016055 (for human oral
squamous cell carcinoma xenografts), OKU2016060 (isolation of
bone-related cells) and OKU-2016056 (inoculation of cancer cells
into bone marrow).
Statistical analysis
The experiments were performed in quadruplicate.
Data were analyzed using an unpaired Student's t-test for the
analysis of two groups, and one-way ANOVA with Bonferroni and
Dunnett's post hoc tests for the analysis of multiple group
comparisons using SPSS statistical software. The results are
expressed as the means ± SD. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
TM suppresses the growth of oral squamous
cell carcinoma cells
To examine the antitumor effects of TM against oral
squamous cell carcinoma in vitro, we performed a trypan blue
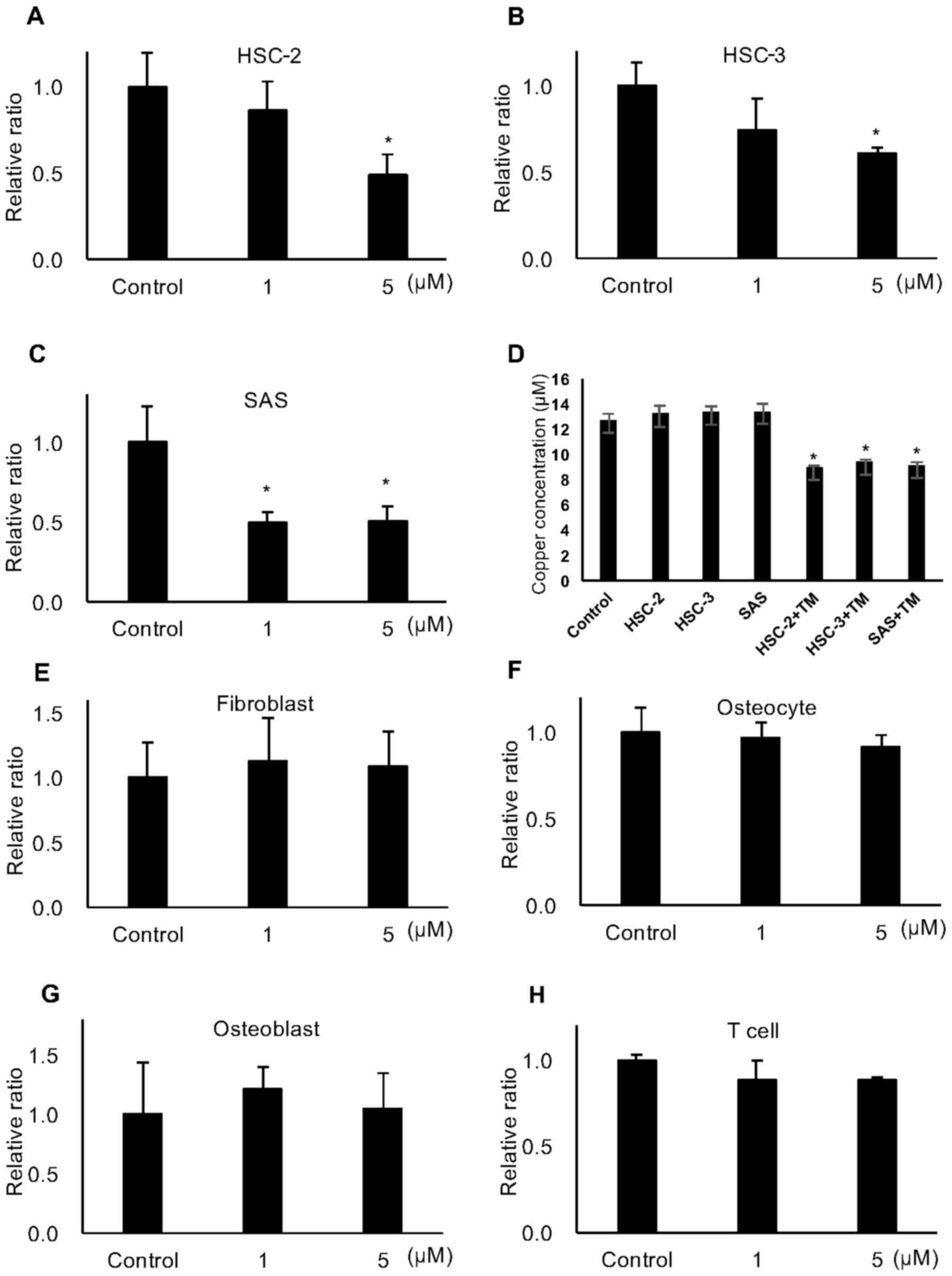
staining assay. As shown in Fig.
1A–C, TM significantly reduced the number of viable HSC-2,
HSC-3 and SAS cells proportionately with the increasing
concentrations 5 days after treatment. In the same experiment, the
concentrations of copper ion in the conditioned media of the HSC-2,
HSC-3 and SAS cells treated with 5 μM TM for 72 h was
decreased by approximately 30% (Fig.
1D). By contrast, TM did not affect the proliferation of
fibroblasts, osteoblasts, osteocytes and T cells, which are
components of the bone microenvironment (Fig. 1E–H).
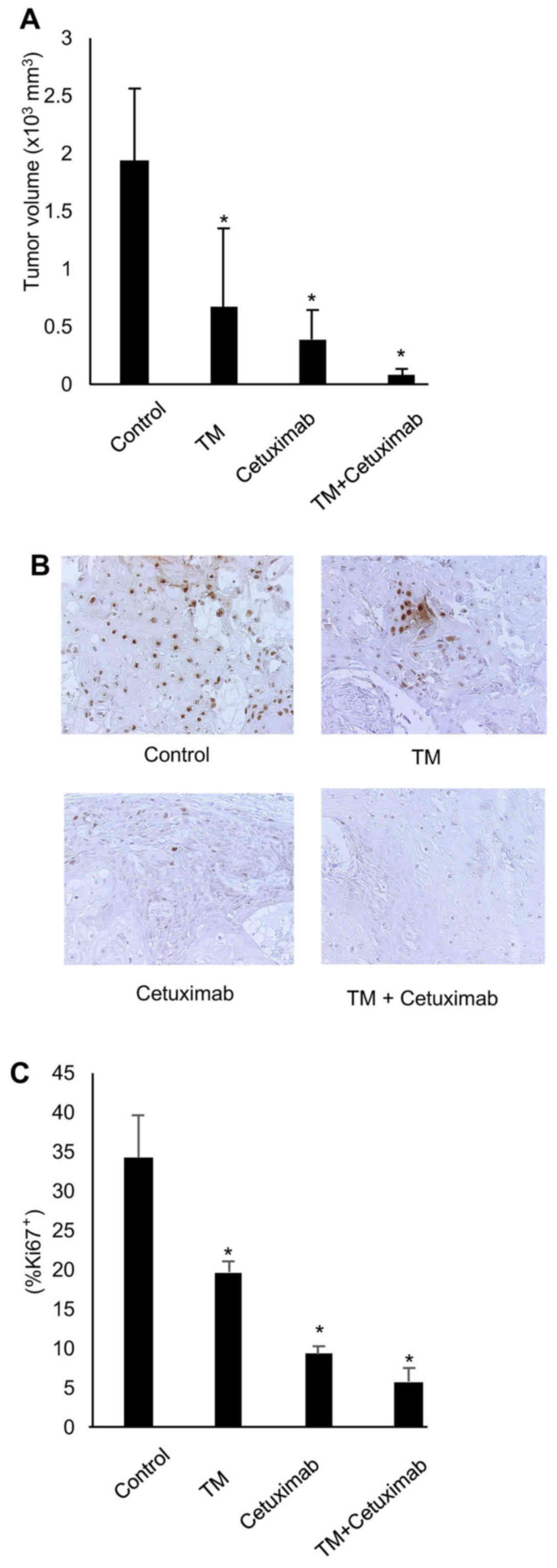
To examine the antitumor effects of TM in
vivo, we established an HNSCC xenograft tumor derived from
HSC-2 cells in nude mice. The mice were treated with TM (1 mg; 5
times a week) and/or cetuximab (1 mg/kg; twice a week) for 5 weeks
beginning at 7 days after tumor cell inoculation, and the tumor
volume was measured at day 35. As shown in Fig. 2A, the HSC-2 xenograft tumor volumes
were significantly decreased in the TM-or cetuximab-treated mice
compared to the untreated mice. No significant toxicity was
observed during the treatment period. At sacrifice after the
treatment period with TM or cetuximab, the tumors were excised and
examined histologically. Immunohistochemical analysis revealed a
significant decrease in the number of Ki67-positive tumor cells in
the HSC-2 tumor sections from the TM-treated and cetuximab-treated
mice (Fig. 2B and C). TM treatment
led to a positive trend by enhancing the antitumor effects of
cetuximab (cetuximab single treatment vs. combined treatment,
P=0.057). TM or cetuximab did not cause any body weight loss at the
end of experiment [mean body weight (g): control, 23.01; TM, 23.15;
cetuximab, 24.15; and TM + cetuzimab, 22.8]. None of the animals
experienced >20% decrease in body weight during the experiment.
However, no statistically significant differences were observed
between single treatment and combination treatment with TM and
cetuximab as regards by tumor volume in this soft tissue tumor
model. These results suggest that antitumor effects of TM require
further evaluation in soft tissue HNSCC models in order to
definitively evaluate its direct anticancer activity.
TM suppresses osteoclast formation
Copper ions are known to play a key role in bone
remodeling (5); however, the
effects of copper chelating on bone cancer remain unknown. To
examine the effects of TM on osteoclast formation, we treated
murine total bone marrow cells harvested from mouse tibias with
vitamin D3 (1×10−8 M) in the presence or
absence of TM for 5 days. TM inhibited the number of TRAP-positive
multinucleated osteoclasts in a dose-dependent manner (Fig. 3A and B). The copper concentration
in the cell conditioned media and RANKL expression in bone marrow
cells were decreased by TM treatment in a dose-dependent manner
(Fig. 3C and D). By contrast, TM
did not affect RANKL-induced osteoclast differentiation on
CD11b-positive bone marrow cells (Fig.
3E). These results indicate that copper chelating by TM
suppresses osteoclast formation via indirect osteoclast
differentiation, such as that represented by RANKL expression in
osteoblasts.
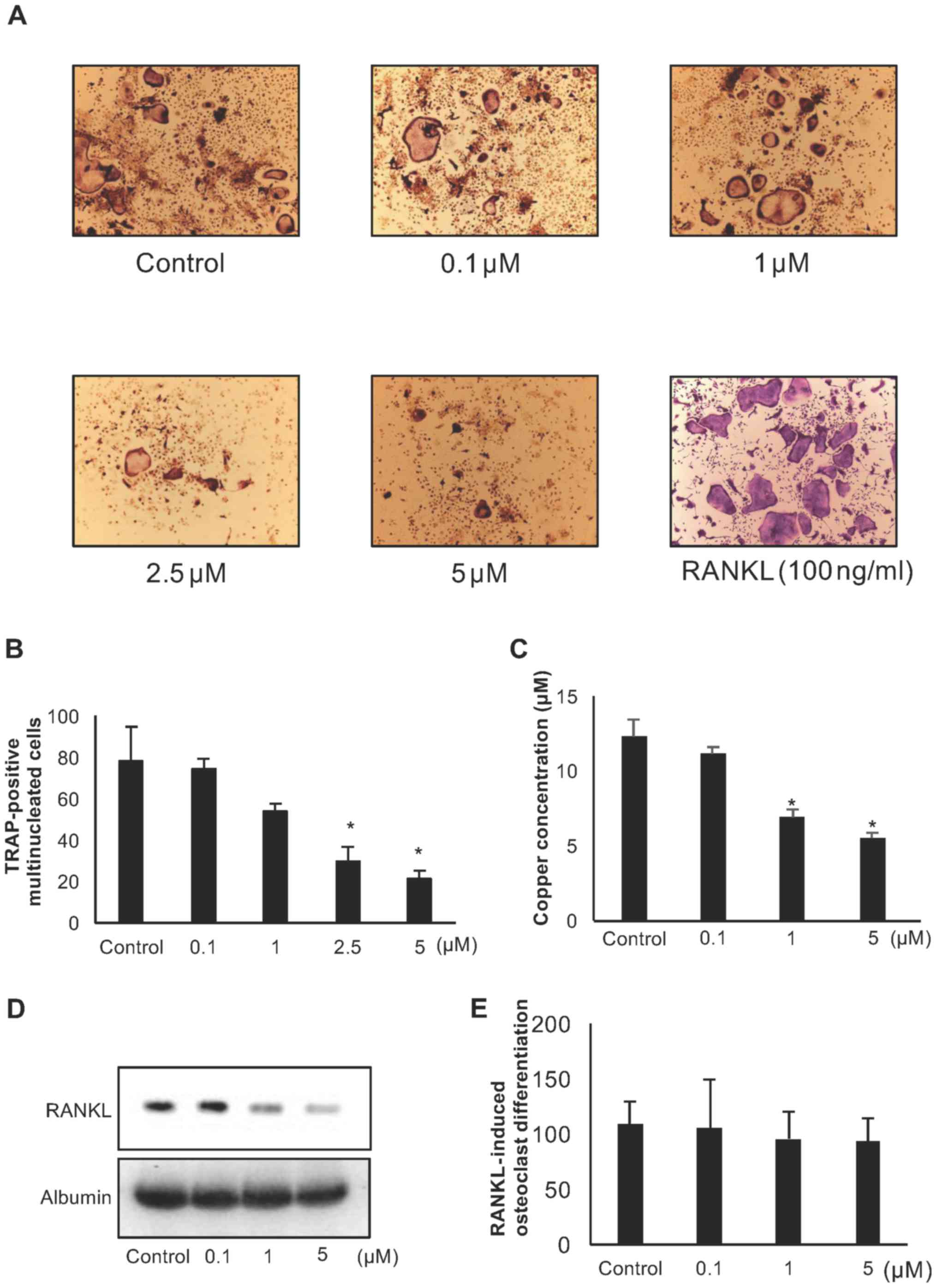 | Figure 3Effect of ammonium tetrathiomolybdate
(TM) on osteoclastogenesis. (A and B) Total bone marrow cells were
cultured with TM (0, 0.1, 1, 2.5, or 5 μM) in the presence
of vitamin D3, 1,25-Dihydroxy (10−8 M) in a
24-well plate for 9 days. (B) The numbers of TRAP-positive
multinucleated cells (nuclear number >3) were counted as
osteoclasts (y-axis). Data are the means ± SD;
*P<0.05 between the indicated groups. (C) Copper
concentration in bone marrow cell cultured medium in the presence
of vitamin D3, 1,25-Dihydroxy (10−8 M) with
or without TM (0.1, 1, 5 μM) for 3 days. Data are means ±
SD; *P<0.05 vs. control (D) RAKNL expression in bone
marrow cell culture medium in the presence of vitamin
D3, 1,25-Dihydroxy (10−8 M) with or without
TM (0.1, 1, 5 μM) for 3 days. (E) CD11b-positive
pre-osteoclasts were cultured with TM (0, 0.1, 1, 5 μM) in
the presence of 30 ng/ml M-CSF and 50 ng/ml RANKL in a 24-well
plate for 9 days. TRAP-positive multinucleated cells (nuclear
number >3) were counted as osteoclasts. Data are the means ±
SD. |
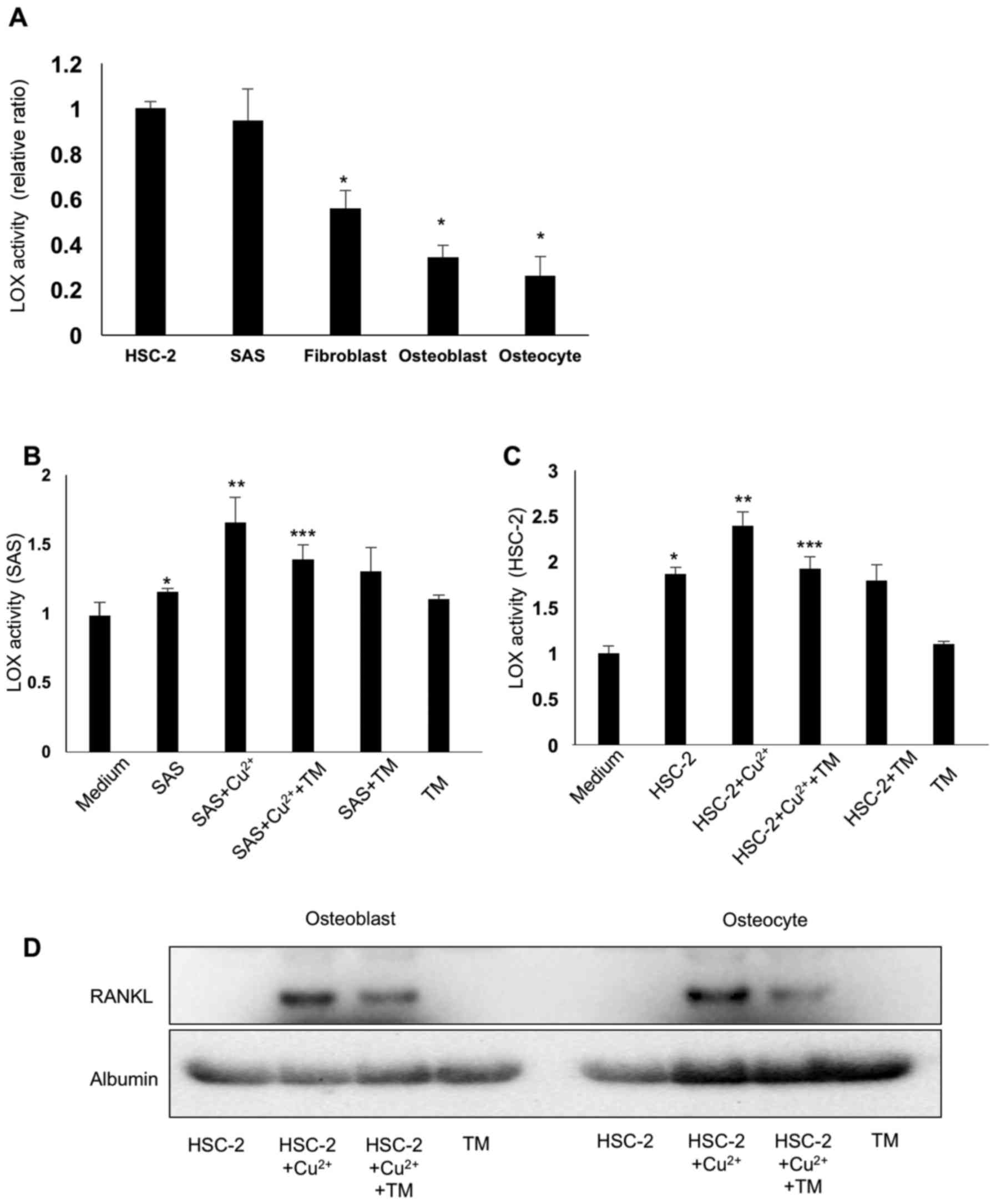
TM suppresses cancer cell-derived LOX
activation via copper chelating
The enzyme activity of LOX was activated by copper
binding to pro-LOX. Fig. 4A
illustrates soluble LOX activation increased by copper ion (10
μM) from the conditioned media of the cancer cells and bone
microenvironment cells. Both the SAS and HSC-2 cells released a
large amount of activated LOX compared to the bone microenvironment
cells, i.e., the fibroblasts, osteoblasts and osteocytes. As
expected, TM suppressed LOX activation in the conditioned medium of
HNSCC cells via copper chelating (Fig.
4B and C).
TM suppresses RANKL expression in
osteoblasts and osteocytes in vitro
To examine the effects of copper-induced LOX
activation on RANKL expression in bone marrow cells, we treated
osteoblasts and osteocytes with HSC-2 conditioned medium and
cultured the cells in increased copper ion concentrations with or
without TM for 24 h. The HSC-2 conditioned medium and the presence
of increased copper ions promoted RANKL expression in the
osteoblasts and osteocytes. TM treatment decreased this effect via
copper chelating (Fig. 4D).
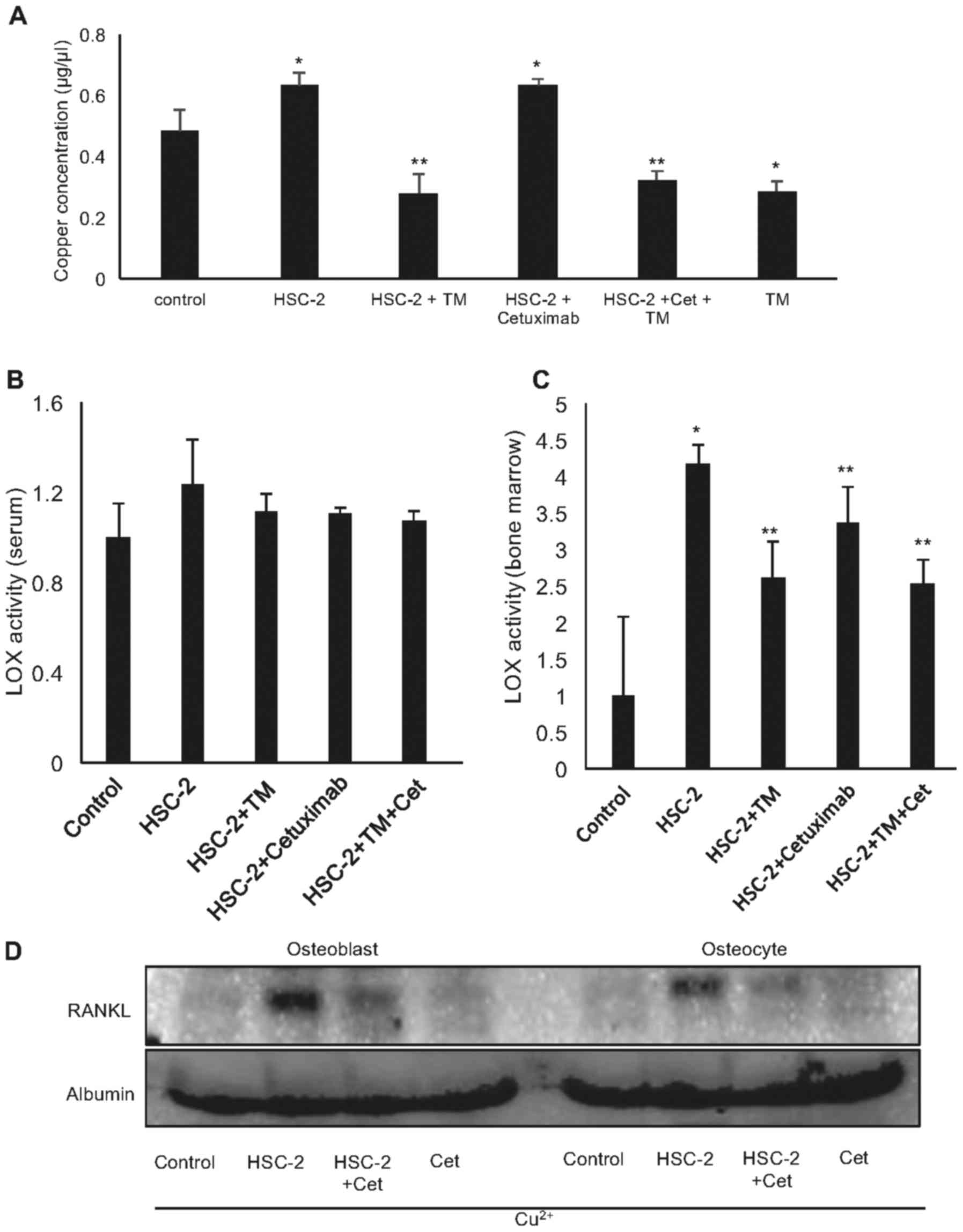
TM decreases the copper levels and
cancer-induced LOX activity in vivo
To build on these findings, we evaluated the effects
of TM on bone destruction and resorption induced by the injection
of HSC-2 cells into mouse tibiae. The results demonstrated that the
serum copper levels were increased in the mice injected with the
HSC-2 into the tibiae, and this was suppressed by treatment with TM
(Fig. 5A).
As expected, HSC-2 cell inoculation into the tibiae
increased LOX activity in bone marrow, and treatment with TM and
cetuximab significantly decreased this activity (Fig. 5C). However, TM did not affect the
whole serum LOX activity (Fig.
5B). These data thus demonstrated that TM and cetuximab
suppressed the local LOX activation in bone by chelating copper
ion.
Cetuximab reduced LOX activation in bone marrow
in vivo (Fig. 5C). We also
evaluated the effects cetuximab treatment on RANKL expression in
osteoblasts and osteocytes in vitro. Surprisingly,
conditioned medium from the HSC-2 cells treated with cetuximab
markedly decreased RANKL expression in osteoblasts and osteocytes
(Fig. 5D). Cetuximab decreased the
number of HNSCC cells in vitro and thereby cetuximab may
suppress the amount of LOX in media.
TM enhances the anticancer effects of
cetuximab and prevents bone resorption in vivo
We examined the in vivo effects of TM on
osteolytic bone destruction induced by oral squamous carcinoma by
conducting soft X-ray and micro-CT examinations. As shown in
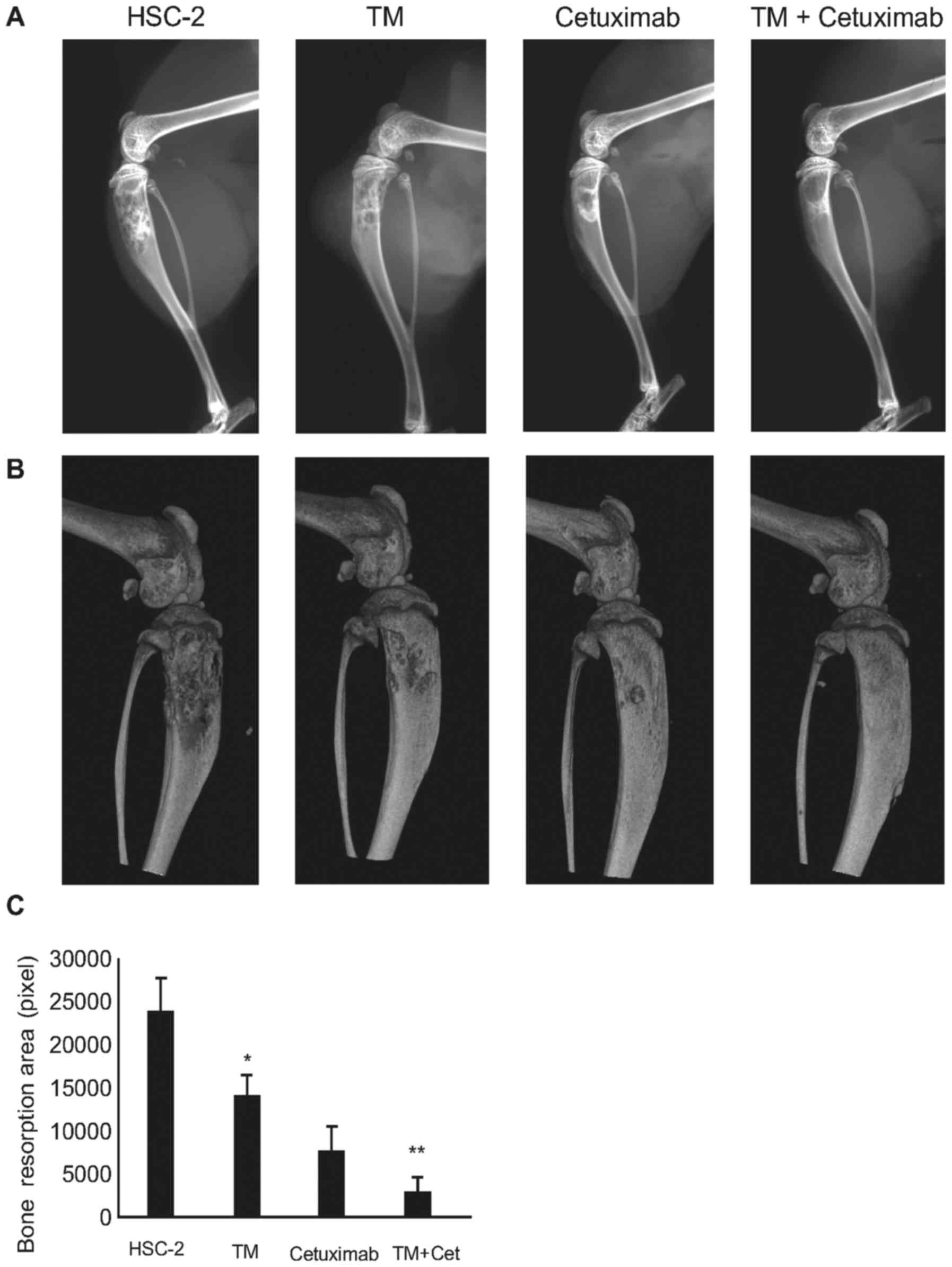
Fig. 6A and B, the osteolytic
lesions were clearly visible in the tibiae of mice with bone
invasion induced by HSC-2 cells treated with the vehicle only.
Surprisingly, few destructive lesions were detected in the tibiae
of mice treated with TM. The total area of radiographic osteolytic
lesions from all tibiae was significantly suppressed by TM
treatment compared to the controls (P<0.05). Cetuximab is a
standard of care agent for the treatment of human head and neck
cancers (16), and it is well
known to suppress HSC-2 cell growth. Surprisingly, TM enhanced the
antitumor effects of cetuximab in bone (Figs. 6C and 7A). Treatment with both TM and cetuximab
alone decreased cancer cell proliferation in bone marrow.
Furthermore, combination treatment with TM and cetuximab
intensively suppressed tumor cell proliferation in bone marrow
compared to treatment with each agent alone.
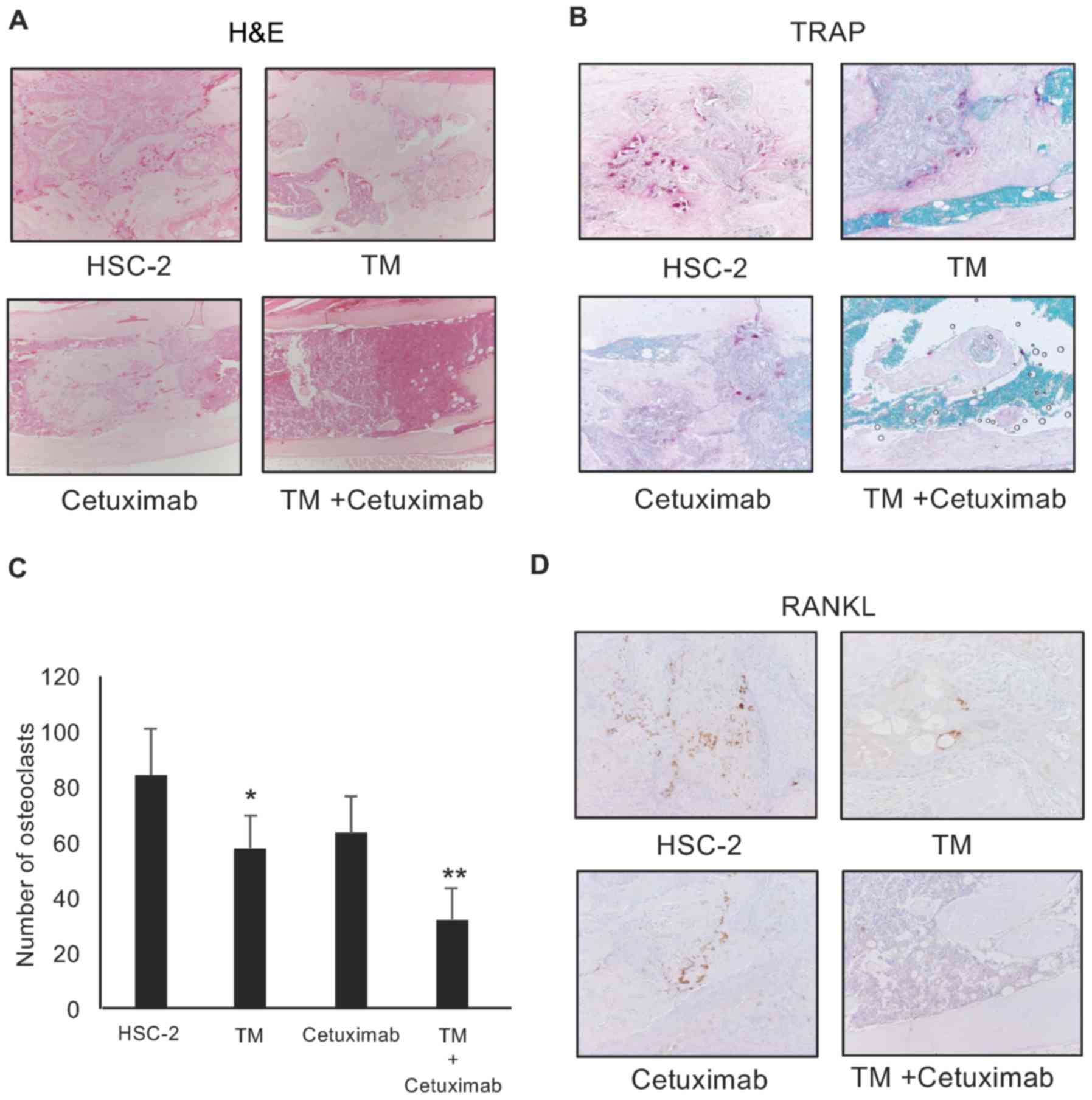
In addition, the numbers of RANKL-positive cells and
TRAP-positive osteoclasts were significantly decreased in the
tibiae of the mice treated with both agents compared with the mice
treated with single treatment (TM or cetuximab only) (P<0.05;
Fig. 7B–D). To build on these
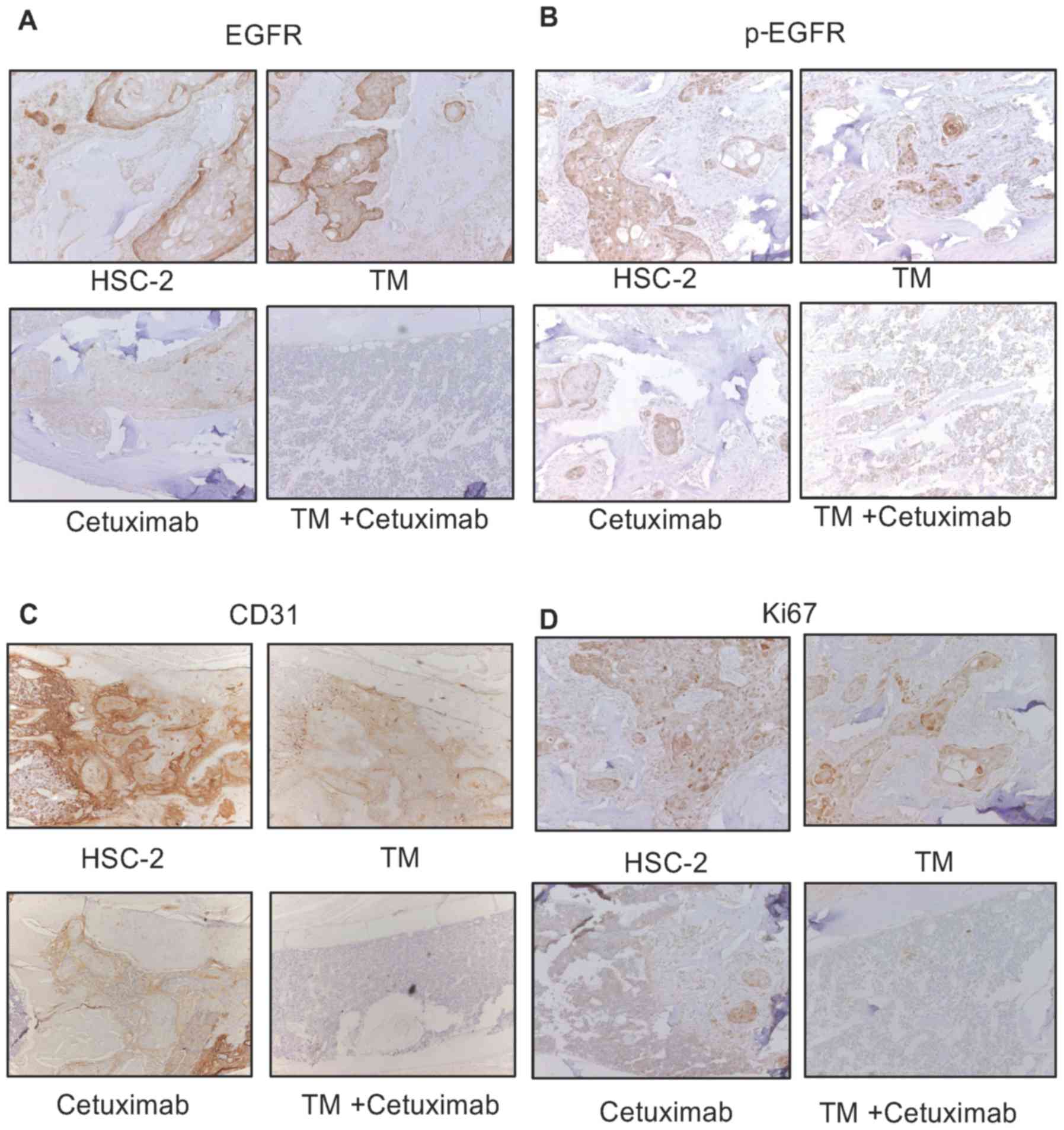
findings, we examined the activity of cetuximab in this model.
Cetuximab suppressed EGFR levels and phosphorylated EGFR expression
in these bone marrow tumors. In addition, combined treatment with
cetuximab and TM enhanced the suppressive effects on the epression
of EGFR, phosphorylated EGFR, CD31 and Ki67 compared with single
treatment (Fig. 8A).
These results suggest the following: i) TM
significantly suppresses oral squamous cell carcinoma via the
suppression of osteoclastogenesis and angiogenesis in osteolytic
bone destruction that is associated with the invasion of oral
squamous cell carcinoma; and ii) TM may enhance the effectiveness
of cetuximab.
Discussion
Copper chelators have been previously reported to
inhibit cancer cell growth in vitro and in vivo
(7,16,17).
LOXs are copper-dependent enzymes. Copper ion binding to pro-LOX is
necessary for LOX activation (9,18).
The most well-studied roles of LOX enzymes are in the remodeling of
the ECM and angiogenesis. It has been reported that cancer-derived
LOX induces bone destruction in HNSCC and other malignancies
(19–21); however, the role of copper ion
involvement in bone destruction induced by HNSCC is not yet well
understood. To the best of our knowledge, the present findings are
the first to demonstrate that the chelation of copper ions by TM,
inhibited LOX activation from HSC-2 head and neck cancer cell
models. In an earlier study, LOX was shown to increase RANKL
expression in osteoblasts and consequently promote
osteoclastogenesis (11). In this
study, we observed that copper chelation by TM inhibited RANKL
expression in osteoblasts and osteocytes via LOX suppression,
resulting in an inhibition of the bone destruction associated with
HNSCC invasion. These results indicate that copper ions are a
critical mediator of osteolytic bone destruction in the bone tumor
microenvironment.
Our experiments revealed that the oral squamous cell
carcinoma HSC-2 and SAS cells were potently and effectively
inhibited by TM at the level of proliferation. TM inhibited HSC-2
and SAS cell growth at an IC50 of 1–5 μM, whereas
TM did not inhibit the growth of the fibroblasts, osteocytes,
osteoblasts or T cells at the IC50 of 5 μM
(Fig. 1D–G), which is consistent
with previous findings (22). The
discrepancy in IC50 results may be due to differences in
the cellular systems, and cancer cells may be more sensitive to
copper metabolism than normal cells. We also observed that the
administration of TM exhibited efficacy in the HSC-2 xenograft
model, where TM inhibited tumor growth in vivo. Furthermore,
TM demonstrated a positive trend by enhancing the antitumor effects
of cetuximab, which is an EGFR receptor inhibitor used to treat
head and neck cancer patients (cetuximab single treatment vs.
combined treatment, P=0.057). However, there no statistically
significant differences were observed between single treatment and
combined treatment with TM and cetuximab as regards tumor volume in
this soft tissue tumor model. These results suggest that the
antitumor effects of TM require further evaluation in soft tissue
HNSCC tumor models in vivo.
In our investigation of the molecular mechanisms of
action of TM in osteoclastogenesis in total bone marrow cells and
CD11b-positive bone marrow cells, the data indicated that TM
inhibited osteoclastogenesis from total bone marrow cells induced
by vitamin D3 (Fig. 3A and
B). However, TM did not affect RANKL-induced osteoclast
differentiation from CD11b-positive cells (Fig. 3E). These data indicated that TM did
not have a direct effect on osteoclast precursor cells, but did
have an effect on other cells of the bone microenvironment.
TM reduced LOX activation in the HSC-2 and SAS cells
following copper ion treatment. It has been previously reported
that LOX induces RANKL expression in osteoblasts (11). Consistently, the present findings
demonstrated that the suppression of LOX downregulated RANKL
expression in osteoblasts and osteocytes, resulting in the
suppression of the differentiation of osteoclasts. The HSC-2 and
SAS head and neck cancer cells released significant amounts of LOX
(Fig. 4A). Copper ions increased
LOX activation and TM suppressed it. These data indicated that TM
may have a potent antitumor effect in bone-invasive HNSCC cells by
not only suppressing tumor growth, but also by suppressing bone
resorption by osteoclasts. To test this hypothesis, we created an
HNSCC bone destruction mouse model, and treated the mice with TM.
As expected, the serum copper levels in the mice injected with
HSC-2 cells in their tibiae that were treated with TM were
decreased compared to those of the untreated mice. The intratibial
LOX activation in these mice was decreased similar to the serum
copper levels. By contrast, there were no differences in LOX
activation in blood serum. These data indicated that pro-LOX from
cancer cells and copper ions are necessary for LOX activation and
bone destruction.
Studies have reported that copper and LOX promote
EGFR activation (22,23). In the present study, TM inhibited
EGFR and phosphorylated EGFR expression in HSC-2 tumors in
vivo. This mechanism may result in reduced Ki67 expression and
consequent tumor growth suppression. To test the effects of TM
in vivo, we treated the mice in the bone-destructive HNSCC
model with TM, cetuximab or both, and the results indicated that
single treatment with TM and single treatment with cetuximab
reduced tumor growth in the bone (Fig.
6), and the combination treatment significantly decreased tumor
growth and bone resorption compared to single treatment with either
agent alone. This additive effect was due to the suppression of
osteoclast formation, angiogenic potential and EGFR activity
(Figs. 7B–D, and 8A–C).
In conclusion, copper may be a target for the
treatment of bone osteolysis induced by HNSCC, and our findings
strongly suggest that the single use of TM or combination treatment
with TM and approved agents, such as cetuximab requires further
evaluation as a potential novel therapy for the treatment of
advanced bone invasive HNSCC.
Acknowledgments
This study was supported by a Grant-in-Aid for
Scientific Research (B) (JSPS KAKENHI grant no. 17H04405) to A.
Sasaki and a Grant-in-Aid for Research Activity start-up (JSPS
KAKENHI grant no. 16H06992) to T. Okui from the Ministry of
Education, Culture, Sports, Science, and Technology of Japan.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Brown JS, Lowe D, Kalavrezos N, D'Souza J,
Magennis P and Woolgar J: Patterns of invasion and routes of tumor
entry into the mandible by oral squamous cell carcinoma. Head Neck.
24:370–383. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shaw RJ, Brown JS, Woolgar JA, Lowe D,
Rogers SN and Vaughan ED: The influence of the pattern of
mandibular invasion on recurrence and survival in oral squamous
cell carcinoma. Head Neck. 26:861–869. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takada H, Ibaragi S, Eguchi T, Okui T,
Obata K, Masui M, Morisawa A, Takabatake K, Kawai H, Yoshioka N, et
al: Semaphorin 4D promotes bone invasion in head and neck squamous
cell carcinoma. Int J Oncol. 51:625–632. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spence JA, Suttle NF, Wenham G, El-Gallad
T and Bremner I: A sequential study of the skeletal abnormalities
which develop in rats given a small dietary supplement of ammonium
tetrathiomolybdate. J Comp Pathol. 90:139–153. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith BJ, King JB, Lucas EA, Akhter MP,
Arjmandi BH and Stoecker BJ: Skeletal unloading and dietary copper
depletion are detrimental to bone quality of mature rats. J Nutr.
132:190–196. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roberts EA and Schilsky ML; American
Association for Study of Liver Diseases (AASLD): Diagnosis and
treatment of Wilson disease: An update. Hepatology. 47:2089–2111.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan N, Willis A, Kornhauser N, Ward MM,
Lee SB, Nackos E, Seo BR, Chuang E, Cigler T, Moore A, et al:
Influencing the tumor microenvironment: A phase II study of copper
depletion using tetrathiomolybdate in patients with breast cancer
at high risk for recurrence and in preclinical models of lung
metastases. Clin Cancer Res. 23:666–676. 2017. View Article : Google Scholar
|
|
8
|
Hassouneh B, Islam M, Nagel T, Pan Q,
Merajver SD and Teknos TN: Tetrathiomolybdate promotes tumor
necrosis and prevents distant metastases by suppressing
angiogenesis in head and neck cancer. Mol Cancer Ther. 6:1039–1045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhuvanasundar R, John A, Sulochana KN,
Coral K, Deepa PR and Umashankar V: A molecular model of human
Lysyl Oxidase (LOX) with optimal copper orientation in the
catalytic cavity for induced fit docking studies with potential
modulators. Bioinformation. 10:406–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rucker RB, Kosonen T, Clegg MS, Mitchell
AE, Rucker BR, Uriu-Hare JY and Keen CL: Copper, lysyl oxidase, and
extracellular matrix protein cross-linking. Am J Clin Nutr.
67(Suppl): S996–S1002. 1998. View Article : Google Scholar
|
|
11
|
Tsukasaki M, Hamada K, Okamoto K,
Nagashima K, Terashima A, Komatsu N, Win SJ, Okamura T, Nitta T,
Yasuda H, et al: LOX fails to substitute for RANKL in
osteoclastogenesis. J Bone Miner Res. 32:434–439. 2017. View Article : Google Scholar
|
|
12
|
Cox TR, Rumney RMH, Schoof EM, Perryman L,
Høye AM, Agrawal A, Bird D, Latif NA, Forrest H, Evans HR, et al:
The hypoxic cancer secretome induces pre-metastatic bone lesions
through lysyl oxidase. Nature. 522:106–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reynaud C, Ferreras L, Di Mauro P, Kan C,
Croset M, Bonnelye E, Pez F, Thomas C, Aimond G, Karnoub AE, et al:
Lysyl oxidase is a strong determinant of tumor cell colonization in
bone. Cancer Res. 77:268–278. 2017. View Article : Google Scholar
|
|
14
|
Teramachi J, Nagata Y, Mohammad K, Inagaki
Y, Ohata Y, Guise T, Michou L, Brown JP, Windle JJ, Kurihara N, et
al: Measles virus nucleocapsid protein increases osteoblast
differentiation in Paget's disease. J Clin Invest. 126:1012–1022.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shah KM, Stern MM, Stern AR, Pathak JL,
Bravenboer N and Bakker AD: Osteocyte isolation and culture
methods. Bonekey Rep. 5:8382016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park SJ, Kim MJ, Kim YK, Kim SM, Park JY
and Myoung H: Combined cetuximab and genistein treatment shows
additive anti-cancer effect on oral squamous cell carcinoma. Cancer
Lett. 292:54–63. 2010. View Article : Google Scholar
|
|
17
|
Chisholm CL, Wang H, Wong AH,
Vazquez-Ortiz G, Chen W, Xu X and Deng CX: Ammonium
tetrathiomolybdate treatment targets the copper transporter ATP7A
and enhances sensitivity of breast cancer to cisplatin. Oncotarget.
7:84439–84452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krebs CJ and Krawetz SA: Lysyl oxidase
copper-talon complex: A model. Biochim Biophys Acta. 1202:7–12.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shih YH, Chang KW, Chen MY, Yu CC, Lin DJ,
Hsia SM, Huang HL and Shieh TM: Lysyl oxidase and enhancement of
cell proliferation and angiogenesis in oral squamous cell
carcinoma. Head Neck. 35:250–256. 2013. View Article : Google Scholar
|
|
20
|
Cox TR, Gartland A and Erler JT: Lysyl
oxidase, a targetable secreted molecule involved in cancer
metastasis. Cancer Res. 76:188–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gartland A, Erler JT and Cox TR: The role
of lysyl oxidase, the extracellular matrix and the pre-metastatic
niche in bone metastasis. J Bone Oncol. 5:100–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim KK, Han A, Yano N, Ribeiro JR, Lokich
E, Singh RK and Moore RG: Tetrathiomolybdate mediates
cisplatin-induced p38 signaling and EGFR degradation and enhances
response to cisplatin therapy in gynecologic cancers. Sci Rep.
5:159112015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang H, Leung L, Saturno G, Viros A, Smith
D, Di Leva G, Morrison E, Niculescu-Duvaz D, Lopes F, Johnson L, et
al: Lysyl oxidase drives tumour progression by trapping EGF
receptors at the cell surface. Nat Commun. 8:149092017. View Article : Google Scholar : PubMed/NCBI
|