Introduction
Although the mortality rate of patients with gastric
cancer has declined significantly over the past 10 years, gastric
cancer remains a leading cause of cancer-related mortality
worldwide (1). In recent years,
considerable advances have been made in chemotherapy and
personalized treatment, including combined chemotherapy with
trastuzumab, a monoclonal antibody against human epidermal growth
factor receptor-2 (HER-2), which is now considered as a standard
treatment modality for patients with HER-2-positive advanced
gastric cancer (2). c-MET
inhibitors, such as crizotinib and foretinib, have also received
increasing attention (3,4). Despite these developments, the
prognosis of patients with advanced gastric cancer remains poor.
Therefore, the identification of effective prognostic markers and
therapeutic targets is essential to improve the clinical
outcomes.
B7-H4, also known as B7x or B7S1, is a member of the
B7 superfamily. B7-H4 is a transmembrane protein. A high expression
of B7-H4 has been reported in tumor tissues of various types of
human cancer, including gastric cancer, ovarian cancer and renal
cell carcinoma. Furthermore, B7-H4 is often detected in the tumor
microenvironment and peripheral blood (5–9). By
contrast, B7-H4 expression is rare in normal human tissues
(5,6,10–13).
The main roles of B7-H4 in tumor progression are associated with
the promotion of tumorigenicity and the regulation of the evasion
of tumor cells from immune surveillance. B7-H4 negatively regulates
T cell responses in vitro by inhibiting cell proliferation,
cell cycle progression and the cytokine production of
CD4+ and CD8+ T cells (14–16).
In addition, recent studies have demonstrated that blocking B7-H4
with human recombinant specific antibodies may prove to be a novel
antitumor therapeutic strategy (17,18).
B7-H4 has been reported to be highly expressed in
gastric cancer tissues, and to significantly correlate with tumor
invasion, lymph node metastasis, tumor progression and patient
outcome (19). In addition, B7-H4
expression in primary gastric tumors has been found to inversely
correlate with the number of tumor infiltrating T lymphocytes,
suggesting that the activation of the B7-H4 signaling pathway may
mediate tumor immune escape (20).
However, the pathobiological role of B7-H4 expression and its
effects on the tumorigenicity of gastric cancer cells have yet to
be fully established. Thus, the aims of this study were the
following: i) to evaluate the expression of B7-H4 in gastric cancer
tissues and cell lines; ii) to determine the association between
its expression and patient clinicopathological characteristics;
iii) to examine the effects of the downregulation of B7-H4 on the
proliferation, cell cycle progression and the apoptosis of the
MGC-803 human gastric cancer cell line; and iv) to investigate the
underlying mechanisms of the effects mentioned above on the MGC-803
cells. Aims 'i' and 'ii' listed above were mainly to confirm the
status of B7-H4 in gastric cancer tissues and cell lines as in a
previous report (8); the aims
'iii' and 'iv' listed above were the novelties of this study and no
related reports have been published to date associated with these
aims, at least to the best of our knowledge.
Materials and methods
Materials
The human gastric cancer cell lines used in this
study were the MGC-803, SGC-7901, AGS and KATOIII cell lines were
purchased form ATCC (Manassas, VA, USA). Fetal bovine serum (FBS),
Roswell Park Memorial Institute medium (RPMI)-1640 and Dulbecco's
modified Eagle's medium (DMEM) were obtained from PAA Laboratories
Pty Ltd. (Morningside, QLD, Australia). Oligofectamine reagent was
purchased from Invitrogen/Life Technologies (Carlsbad, CA, USA).
Small interfering RNA (siRNA) duplexes were constructed by Shanghai
GenePharma (Shanghai, China). The Cell Counting kit 8 (CCK-8) was
purchased from Dojindo Molecular Technologies (Rockville, MD, USA);
the Annexin V/propidium iodide (PI) apoptotic reagent kit was
purchased from MultiSciences Biotech (Hangzhou, China); and the In
Situ Cell Death Detection kit used for terminal deoxynucleotidyl
transferase dUTP nick-end labeling (TUNEL) assays was purchased
from Roche (Mannheim, Germany). Antibodies against the following
proteins were employed in this study: B7-H4 (Cat. no. ab209242,
Abcam, Cambridge, MA, USA). Cleaved caspase-3 (Cat. no. 9661),
cleaved caspase-9 (Cat. no. 20750), Bcl-2 (Cat. no. 15071), Bax
(Cat. no. 5023) and GAPDH (Cat. no. 5174) were purchased from Cell
Signaling Technology (Beverly, MA, USA).
Immunohistochemical (IHC) staining
IHC staining was performed in 311 gastric cancer
tissues obtained from tissue microarrays (Chaoying Biotechnology,
Shanxi, China), as previously described (21). The slides were semi-quantitatively
evaluated by calculating the product of the staining intensity
(0–3, least intense to most intense) and the percentage of stained
cells (0–4, no cells stained to >75% of cells stained), as
previously described (22). The
resulting IHC scores were classified into 4 groups as follows: -
(negative), 0 points; +, 1–4 points; ++, 5–8 points; and +++, 9–12
points. In this study, a score of <5 was considered as a low
expression, and a score of ≥5 was considered as a high expression
of B7-H4 protein. All slides were independently scored by two
independent clinical pathologists in a blinded manner.
Cell culture and siRNA transfection
The human gastric cancer cell lines, MGC-803, AGS
and SGC-7901, were cultured in RPMI-1640 medijm containing 10% FBS,
100 IU/ml penicillin and 100 μg/ml streptomycin. The KATOIII
cells were cultured in DMEM containing the same proportions of FBS,
penicillin and streptomycin. The cells were grown in a humidified
incubator at 37°C and 5% CO2.
The transfection of the MGC-803 cells with siRNA
specific for B7-H4 was performed as previously described (23). In brief, B7-H4-specific siRNA (100
nM) and scrambled siRNA (100 nM) duplexes were transfected using
oligofectamine reagent according to the manufacturer's
instructions. All siRNAs were chemically synthesized by GenePharma
(Shanghai, China). The MGC-803 cells that were transfected with
B7-H4 siRNA were classified as the B7-H4 group, those transfected
with scrambled siRNA were classified as the mock group and
untransfected cells were classified as the control group. All
experiments were carried out in triplicate (n=3).
Western blot analysis
Cell lysates were prepared using cell lysis buffer
according to the manufacturer's instructions (Cell Signaling
Technology). Equal quantities of protein from the cell lysates were
separated by 12% sodium dodecyl sulfate polyacrylamide gel
electrophoresis and transferred onto polyvinylidene difluoride
membranes. The membranes were blocked by incubation in 5% non-fat
milk for 2 h, and then incubated overnight at 4°C with primary
antibodies against B7-H4 (1:10,000), cleaved caspase-3 (1:1,000),
cleaved caspase-9 (1:1,000), Bcl-2 (1:1,000), Bax (1:1,000) and
GAPDH (1:5,000). The membranes were washed 3 times in
phosphate-buffered saline (PBS) and then incubated with anti-rabbit
IgG, horseradish peroxidase (HRP)-conjugated secondary antibodies
(Cat. no. 7074, Cell Signaling Technology). Immunoreactive proteins
were visualized with enhanced chemiluminescence (ECL) reagents
(Amersham, Piscataway, NJ, USA) and analyzed using a VersaDoc
MP5000 imaging system (Bio-Rad, Hercules, CA, USA). Relative
protein expression was quantified using Quantity One 1-D software
(Bio-Rad).
CCK-8 proliferation assay
The MGC-803 cells were plated into 96-well plates
(5×103 cells/well) and cultured in RPMI-1640 medium
containing 2% FBS. At 1–6 days after transfection, the proportions
of live cells in the B7-H4, mock and control groups were detected
using CCK-8 reagent. Following incubation for 1 h at 37°C, the
absorbance (OD) of each group was measured using a microplate
reader (iMark, Bio-Rad) at a wavelength of 450 nm. The experiment
was carried out in triplicate (n=3).
Cell cycle analysis with PI staining
The procedure for this experiment followed the
commonly used method (24). In
brief, 2×106 cells were collected and washed with PBS
and fixed with −20°C 75% ethanol; the fixed cells were then washed
with PBS and treated with 200 μg/ml DNase-free, RNaseA and
incubated at 37°C for 30 min; the cells were then washed again and
stained with 200 μg/ml propidium iodide (PI) and incubated
at room temperature for 5–10 min; the cells were analyzed on a
FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). The
experiment was carried out in triplicate (n=3).
Wound healing assay
The MGC-803 cells were seeded into 6-well plates
(5×105 cells/well) and cultured in RPMI-1640 medium
containing 2% FBS. Following transfection with the siRNA for 72 h,
scratches were created on the cell cultures using a 200 μl
micropipette tip. The cells were photographed (CKX53, Olympus,
Tokyo, Japan) at 0 and 72 h after wounding, and the wound areas
were measured and compared using Image-Pro Plus version 6.0
software. The experiment was carried out in triplicate (n=3).
Annexin V/PI staining apoptotic
assay
The MGC-803 cells were transfected with the siRNA
for 72 h and collected using trypsin. They were washed twice with
PBS and stained with Annexin V-fluorescein isothiocyanate and PI
according to the manufacturer's instructions. The percentages of
apoptotic cells were determined by fluorescence activated cell
sorting (FACS) using a FACSCalibur flow cytometer (BD Biosciences).
The experiment was carried out in triplicate (n=3).
TUNEL assay
The MGC-803 cells were seeded into 12-well plates
and transfected with siRNA for 72 h before being fixed with 4%
paraformaldehyde in PBS for 1 h. After being washed twice in PBS,
the cells were incubated with permeabilization solution (PBS; 0.1%
Triton X-100; 0.1% sodium citrate) for 2 min on ice, and then
washed in PBS. The cells were incubated with TUNEL reaction mix for
30 min at 37°C, two additional washing steps were performed and the
cells were then stained with 4′, 6-diamidino-2-phenylindole (DAPI)
for a further 7 min. The number of TUNEL-positive stained cells was
divided by the number of DAPI-stained nuclei. A total of 1,000
cells were evaluated at a magnification of ×200 (BX63,
Olympus).
Statistical analysis
The association of B7-H4 expression with various
clinicopathological parameters was assessed using the Chi-square
test and Fisher's exact test. Differences between groups were
determined by one-way ANOVA followed by a Dunnet's post hoc test. A
P-value <0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS version 16.0 software.
Results
Correlation of B7-H4 expression with
clinicopathological characteristics in gastric cancer tissues
The protein expression levels of B7-H4 were
determined in 311 gastric cancer tissue samples obtained from
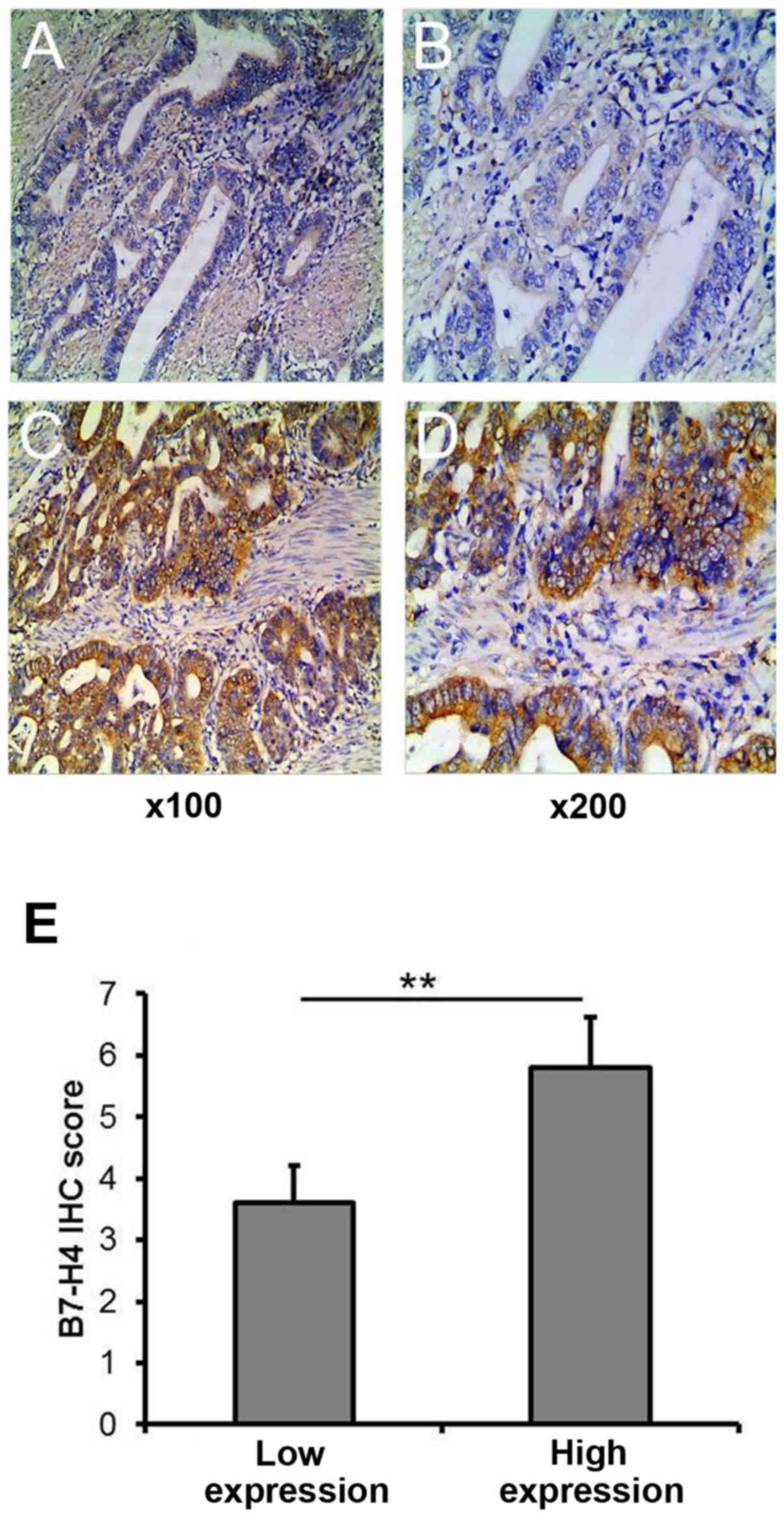
microarrays by ICH staining. We detected B7-H4 in the membrane
and/or cytoplasm of the tumor cells (Fig. 1). High expression levels of B7-H4
(scores of ≥5) were found in 130 (41.8%) cases and low expression
levels of B7-H4 (scores of <5) were found in 181 (58.2%) cases.
The associations between B7-H4 expression and the
clinicopathological characteristics of patients with gastric cancer
were determined by examining the clinicopathological information
provided by Shaanxi Chaoying Biotechnology. Sample staging was
carried out according to 6th edition of the International Union
against Cancer (UICC) tumor-node-metastasis (TNM) classification
system (25). Our results revealed
high levels of B7-H4 expression in a greater number of stage III-IV
gastric cancer tissue samples than stage I–II tissue samples (52.3
vs. 34.4%). In addition, B7-H4 expression was found to
significantly correlate with lymph node metastasis (P=0.005);
however, B7-H4 expression was not significantly associated with
patient sex, age, histological grade or the depth of invasion
(Table I).
 | Table ICorrelation of B7-H4 expression with
clinicopathological characteristics in human gastric cancer. |
Table I
Correlation of B7-H4 expression with
clinicopathological characteristics in human gastric cancer.
| Clinical
parameters | Cases | B7-H4 expression
| χ2 | P-value |
|---|
| Low (%) | High (%) |
|---|
| Sex | | | | 1.030 | 0.310 |
| Male | 225 | 127 (56.4) | 98 (43.6) | | |
| Female | 86 | 54 (62.8) | 32 (37.2) | | |
| Age (years) | | | | 0.026 | 0.873 |
| ≤50 | 78 | 46 (59.0) | 32 (41.0) | | |
| >50 | 233 | 135 (57.9) | 98 (42.1) | | |
| Histological
grade | | | | 3.913 | 0.271 |
| I | 12 | 9 (75.0) | 3 (25.0) | | |
| II | 115 | 60 (52.2) | 55 (47.8) | | |
| III | 118 | 73 (61.9) | 45 (38.1) | | |
| IVa | 66 | 40 (60.6) | 26 (39.4) | | |
| Lymph node
metastasis | | | | 7.764 | 0.005 |
| Positive | 127 | 62 (48.8) | 65 (51.2) | | |
| Negative | 184 | 119 (64.7) | 65 (35.3) | | |
| Invasion | | | | 1.115 | 0.573 |
| T1-T2 | 65 | 34 (52.3) | 31 (47.7) | | |
| T3 | 230 | 136 (59.1) | 94 (40.9) | | |
| T4 | 16 | 10 (62.5) | 6 (37.5) | | |
| TNM stage | | | | 9.939 | 0.002 |
| I–II | 183 | 120 (65.6) | 63 (34.4) | | |
| III–IV | 128 | 61 (47.7) | 67 (52.3) | | |
Expression of B7-H4 protein in human
gastric cancer cell lines
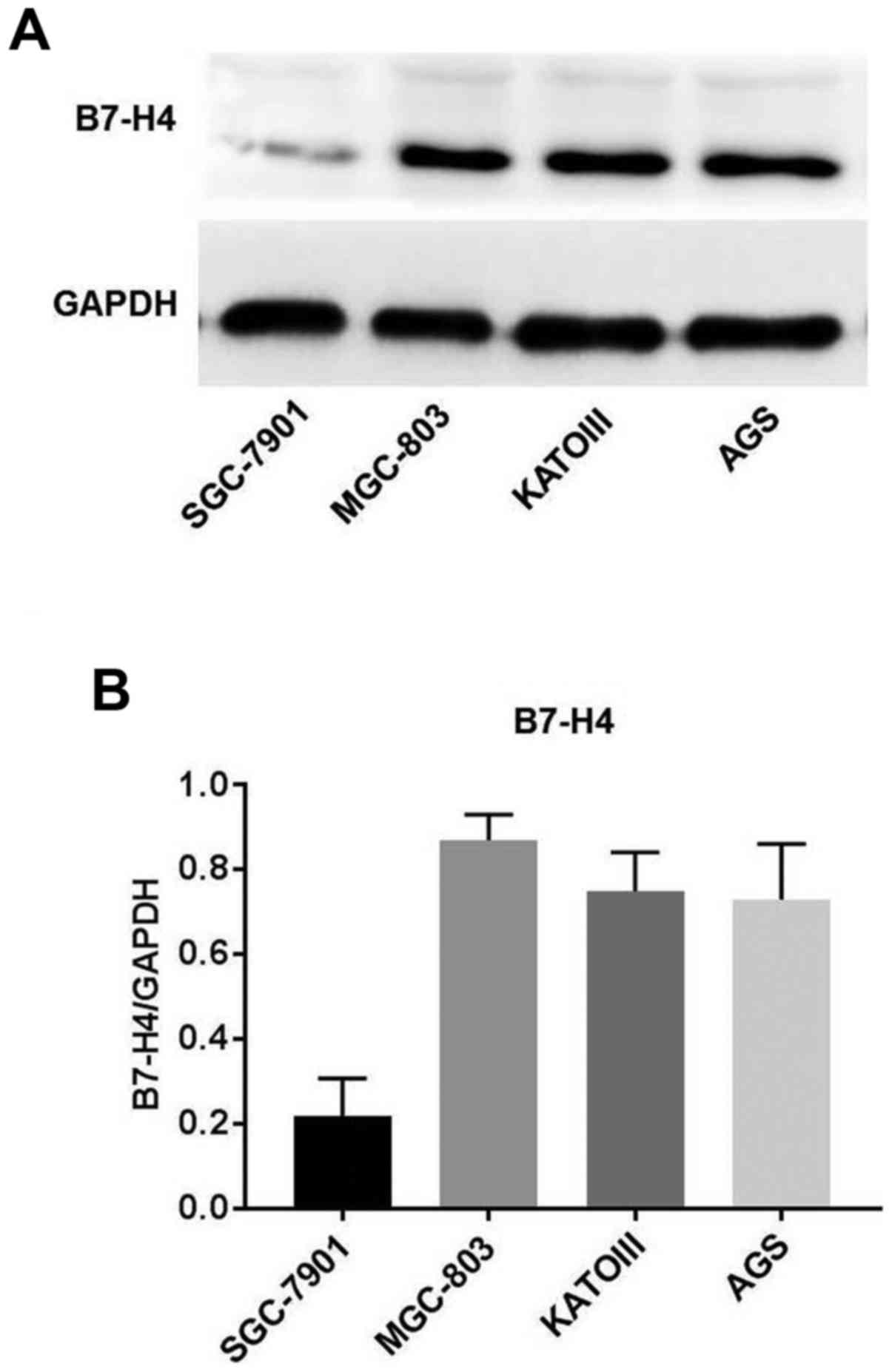
The protein expression levels of B7-H4 in the human
gastric cancer cell lines, MGC-803, SGC-7901, AGS and KATOIII, were
examined by western blot analysis. B7-H4 protein was highly
expressed in the MGC-803 cells, AGS cells and KATOIII cells,
whereas there was little or no expression of B7-H4 protein in the
SGC-7901 cells (Fig. 2). B7-H4
expression was found to be the highest in the MGC-803 cells.
Therefore, this cell line was selected for use in the subsequent
B7-H4 gene silencing experiments.
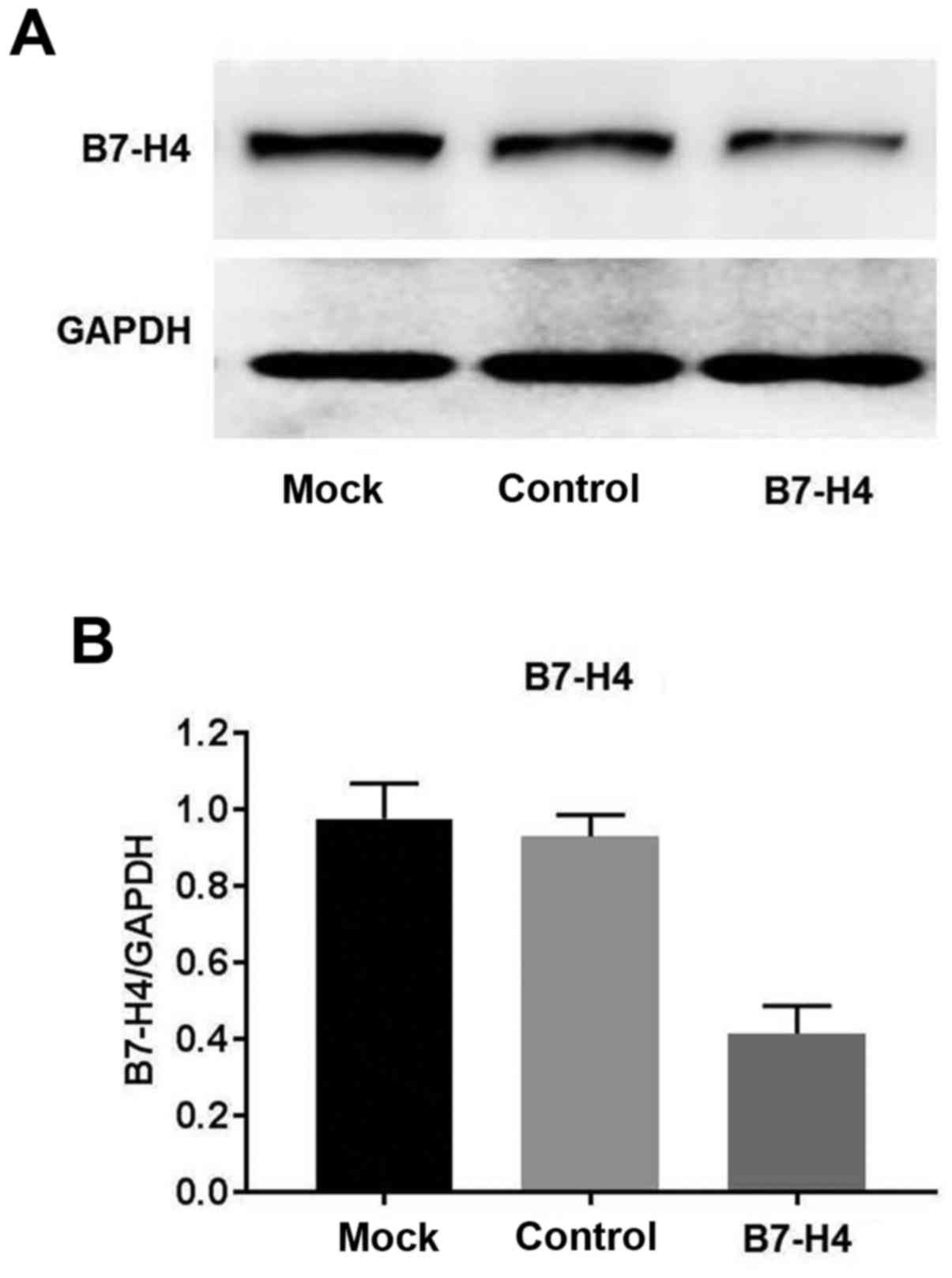
Expression of B7-H4 is effectively
knocked down in MGC-803 cells by siRNA
Western blot analysis confirmed that the expression
of B7-H4 was downregulated in the MGC-803 cells following
transfection with siRNA specific for B7-H4, compared to the cells
transfected with scrambled siRNA (mock) and the untransfected
control cells (Fig. 3).
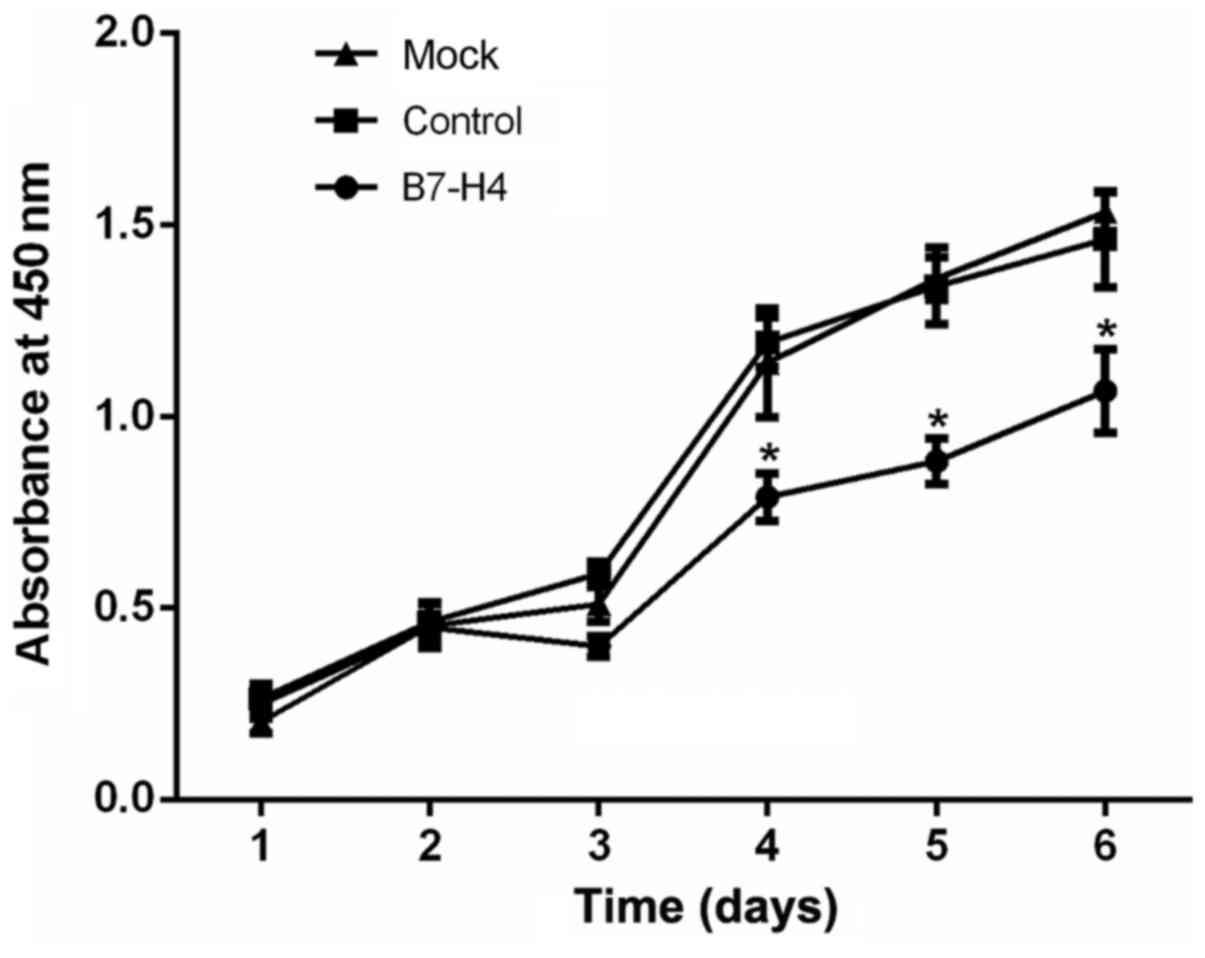
Downregulation of B7-H4 decreases the
proliferation of MGC-803 cells
The effects of B7-H4 downregulation on the
proliferative ability of the MGC-803 cells were evaluated by CCK-8
assay. The rate of proliferation following at 4 days after
transfection was significantly reduced in the B7-H4 group
(transfected with B7-H4 siRNA) compared with the mock and control
groups (P<0.05; Fig. 4). These
data indicated that the downregulation of B7-H4 suppressed the
proliferation of the MGC-803 human gastric cancer cells.
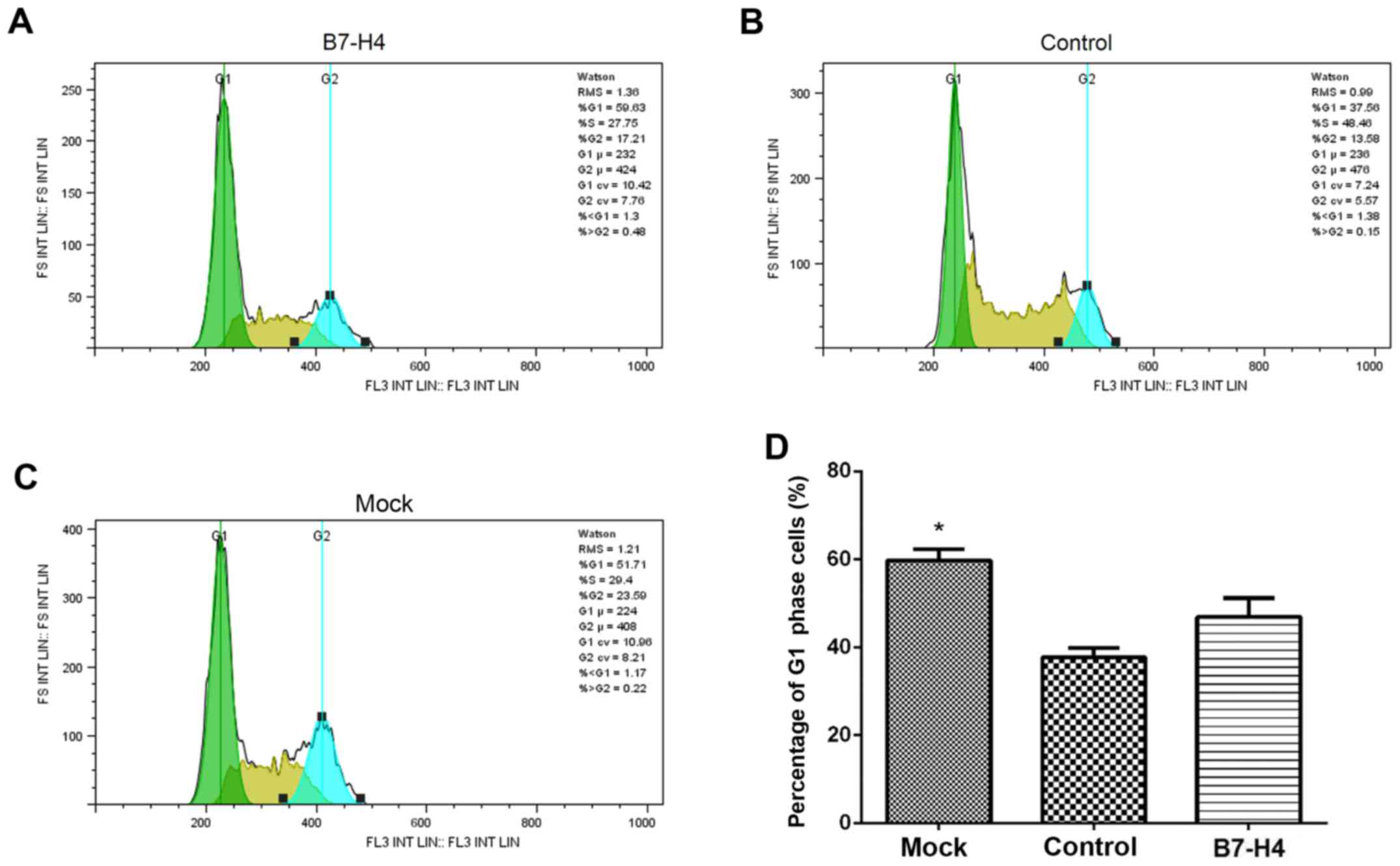
Downregulation of B7-H4 induces cell
cycle arrest in the MGC-803 cells
The effect of B7-H4 downregulation on the cell cycle
of the MGC-803 cells was evaluated by flow cytometric analysis. The
percentage of cells in the G1 phase after at 4 days after
transfection was significantly elevated in the B7-H4 group
(transfected with B7-H4 siRNA) compared to the mock and control
groups (P<0.05; Fig. 5). This
result demonstrated that the downregulation of B7-H4 induced cell
cycle arrest in the MGC-803 human gastric cancer cells.
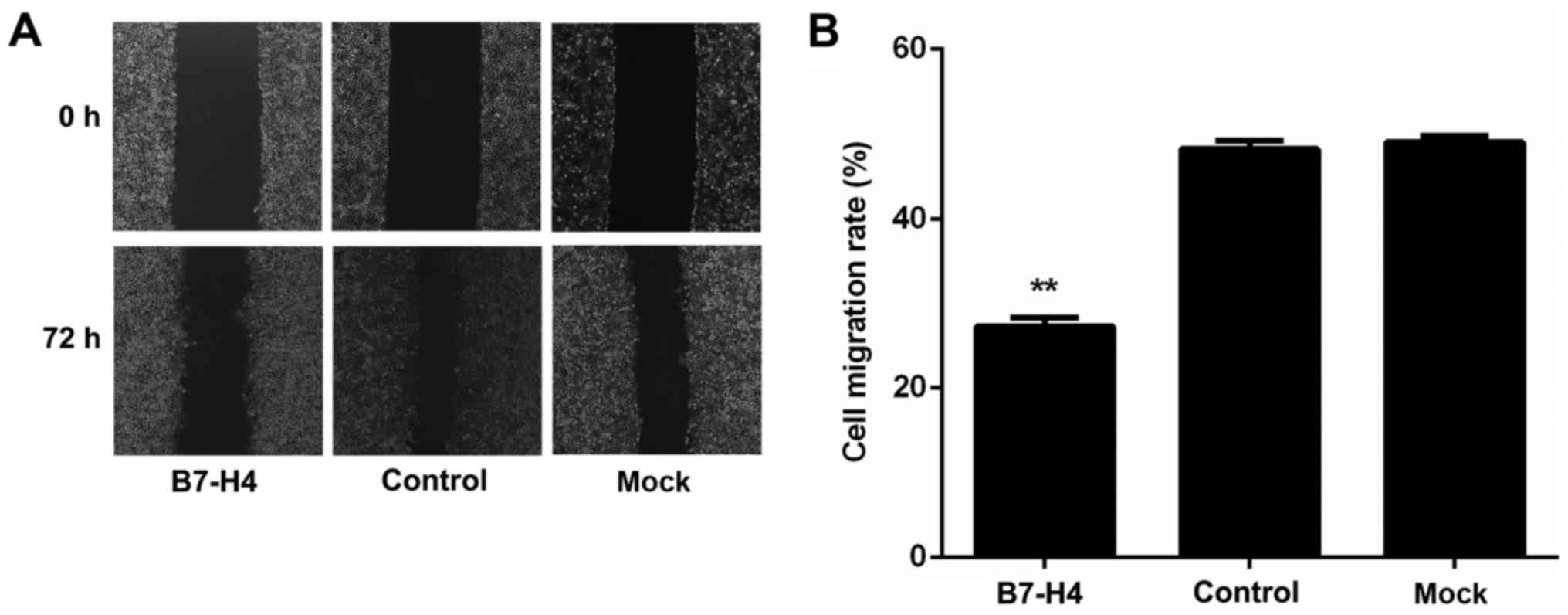
Downregulation of B7-H4 suppresses the
motility of MGC-803 cells
The effect of B7-H4 downregulation on the motility
of MGC-803 cells was evaluated by wound healing assay. The results
revealed that cell motility was significantly reduced in the B7-H4
group (transfected with B7-H4 siRNA; (27.48±1.06%) compared with
the mock group (47.68±1.24%) and the control group (49.55±1.59%);
however, there was no significant difference between the mock and
control groups (P<0.01, P<0.01 and P>0.05, respectively;
Fig. 6). These results
demonstrated that the downregulation of B7-H4 suppressed the
motility of MGC-803 human gastric cancer cells.
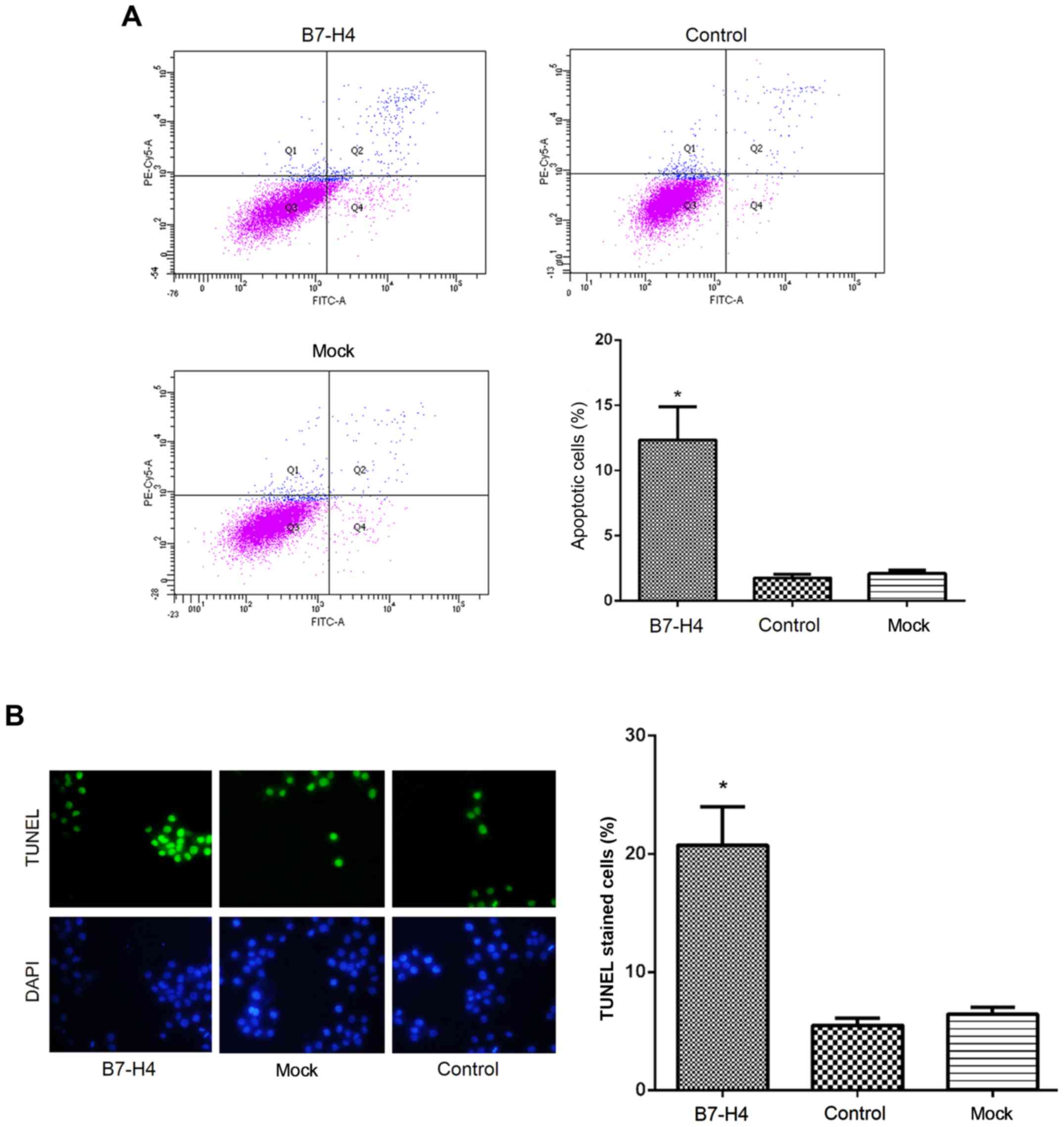
Downregulation of B7-H4 induces the
apoptosis of MGC-803 cells
The effect of B7-H4 downregulation on cell apoptosis
was evaluated by Annexin V/PI and TUNEL assays. The results of
Annexin V/PI assay revealed that the percentage of apoptotic
MGC-803 cells was significantly higher (P<0.05; Fig. 7A) in the B7-H4 group (13.45±2.12%)
than in the mock group (2.13±1.01%) and control group (1.79±0.54%).
This was consistent with the percentage of TUNEL-positive stained
cells in the B7-H4 group (21.43±5.78%), which was signifi-cantly
increased (P<0.05; Fig. 7B)
compared to the mock group (5.34±1.55%) and the control group
(6.54±1.25%). These results indicated that the downregulation of
B7-H4 induced the apoptosis of MGC-803 human gastric cancer
cells.
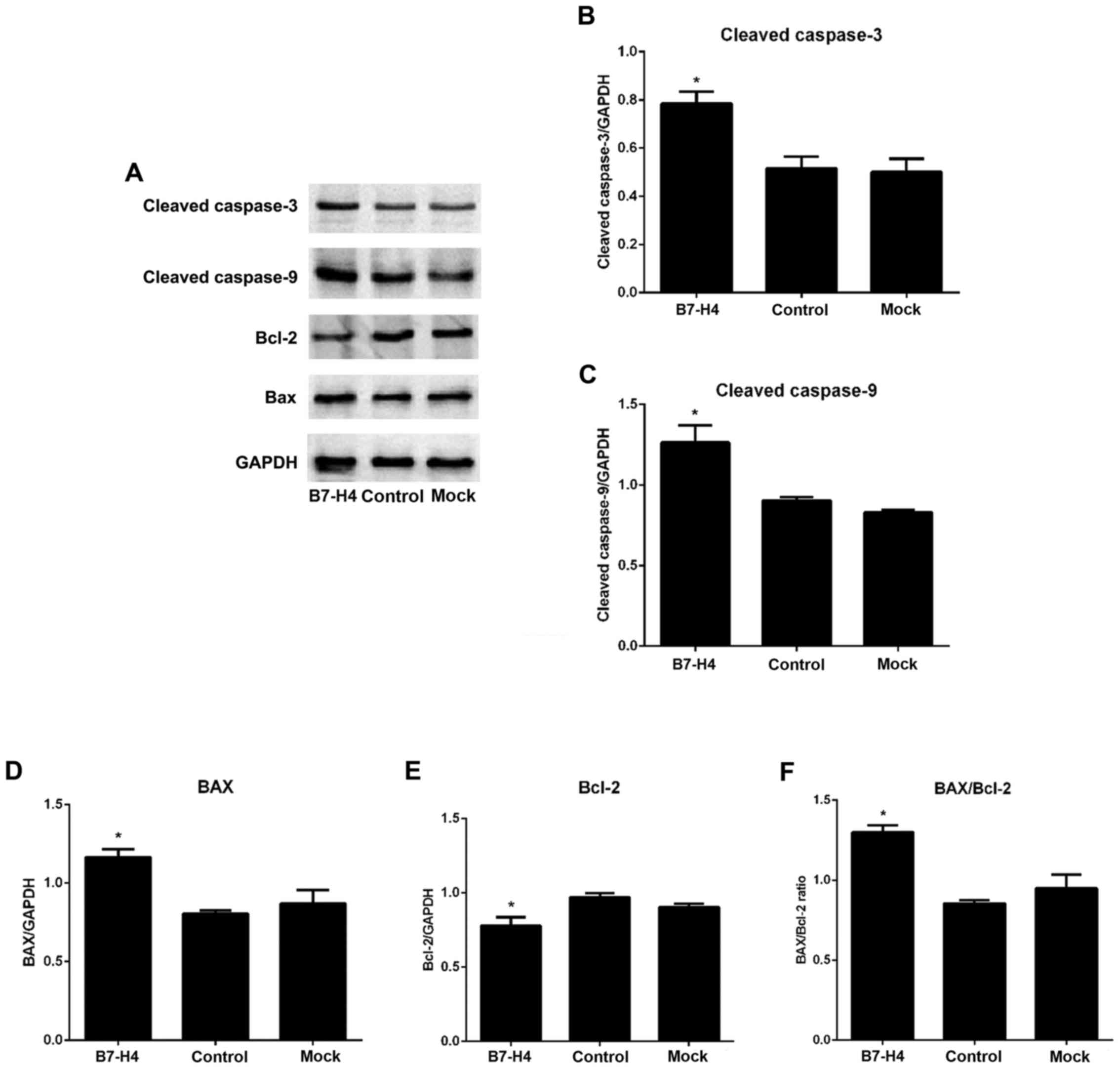
Downregulation of B7-H4 activates
caspase-3 and caspase-9, and alters the Bax/Bcl-2 ratio in the
MGC-803 cells
To investigate the effects of B7-H4 downregulation
on the apoptotic signaling pathway, we examined the expression
levels of cleaved caspase-3, cleaved caspase-9, Bax and Bcl-2 in
the MGC-830 cells. The results revealed that the expression levels
of the apoptotic markers, cleaved caspase-3 and cleaved caspase-9,
were increased in the B7-H4 group compared to the mock and control
groups. Similarly, the expression of pro-apoptotic Bax was
increased, whereas that of anti-apoptotic Bcl-2 was decreased,
thereby altering the Bax/Bcl-2 ratio (P<0.05; Fig. 8). These observations indicated that
the down-regulation of B7-H4 activated caspase-3 and caspase-9, and
altered the Bax/Bcl-2 ratio in favor of apoptosis. These results
also suggested that B7-H4 downregulation may induce the apoptosis
of MGC-803 cells via the mitochondrial pathway.
Discussion
Despite the declining incidence and mortality rates
of gastric cancer, the 5-year survival rate of patients with stage
III–IV gastric cancer is significantly lower than that of patients
with stage I–II gastric cancer (26). The expression of B7-H4 has been
found to be closely correlated with the tumor stage; furthermore,
it has been shown to be a significant independent prognostic factor
in gastric cancer (8). Consistent
with these reports, in this stuyy, we found that B7-H4 was highly
expressed in 41.8% of patients with gastric cancer, and that its
expression significantly correlated with lymph node metastasis
(P=0.005) and the TNM stage (P=0.002), with a high expression of
B7-H4 being detected in 34.4 and 52.3% of stage I–II and stage
III–IV tissues, respectively. These results confirmed those of a
previous report (8) and proved
that our investigation platform was suitable for use in further
investigations on gastric cancer materials.
B7-H4 negatively regulates T cell-mediated immune
response. Studies have shown that B7-H4 suppresses the generation
of allogeneic cytotoxic T cells by inducing cell cycle arrest in
vitro (14–16). Conversely, the blockade of
endogenous B7-H4 by specific monoclonal antibodies can promote T
cell response in vivo (14). In the tumor microenvironment, B7-H4
expression has been detected in tumor-infiltrating macrophages and
endothelial cells in small blood vessels (5,6,27).
In human ovarian cancer, tumor-associated regulatory T cells have
been found to trigger macrophages to produce the interleukin (IL)-6
and IL-10 cytokines, which in turn stimulate B7-H4 expression in
antigen-presenting cells in an autocrine/paracrine manner (27). These findings suggest that B7-H4
may contribute to the immune tolerance of tumor cells, thereby
promoting tumor progression.
Less is known about the effects of B7-H4 on gastric
cancer cell lines and the underlying mechanisms. Therefore, in this
study, we examined the expression levels of B7-H4 in human gastric
cancer cell lines, and observed the effects of the silencing of
B7-H4 by siRNA on the proliferation, motility, cell cycle arrest
and the apoptosis of the MGC-803 human gastric cancer cells. We
found that B7-H4 was highly expressed in three of the four gastric
cancer cell lines examined. B7-H4 gene silencing assays revealed
that the downregulation of B7-H4 inhibited the proliferation and
motility, and induced cell cycle arrest and the apoptosis of
MGC-803 cells, indicating that B7-H4 may play an important role in
the tumorigenesis of MGC-803 human gastric cancer cells. A previous
study demonstrated that the overexpression of B7-H4 in the SKOV3
human ovarian cancer cell line increased the rate of tumor
formation in severe combined immune deficiency mice, whereas B7-H4
knockdown increased caspase activity and the apoptosis of the SKBR3
breast cancer cell line (18).
Another study reported that B7-H4 inhibited cell apoptosis and
promoted tumor cell growth, adhesion and invasion in an
immune-defective model involving SKOV3 cells stably overexpressing
B7-H4 (28). B7-H4 has also been
shown to play an important immunity-independent role in regulating
tumorigenicity in renal cell carcinoma and pancreatic cancer cell
lines (23,29). The data of the present study on
MGC-803 cells, including the effects of B7-H4 on the cellular and
molecular levels and the underlying mechanisms were novel (at least
to the best of our knowledge), and had similarities with those of
previous reports, which expands our knowledge about the role of
B7-H4 in gastric cancer.
Balanced apoptosis is essential to maintain
homeostasis and morphogenesis in human tissues, whereas the
dysregulation of apoptosis can contribute to tumorigenesis
(30). The mitochondrial apoptotic
pathway is associated with changes in the permeability of the outer
mitochondrial membrane and the collapse of membrane potential.
Mitochondrial membrane permeability is mainly controlled by the
Bcl-2 family (31,32), which includes pro-apoptotic Bax and
anti-apoptotic Bcl-2; consequently, the Bax/Bcl-2 ratio is central
to the induction of apoptosis (31). The mitochondrial pathway has also
been implicated in the activation of caspase-9, which is an
essential initiator caspase required for apoptotic signaling
through the mitochondrial pathway and the activation of caspase-3,
which is a key executioner of apoptosis (32,33).
However, the role of B7-H4 in the mitochondrial apoptotic signaling
pathway has not been fully established in gastric cancer. In this
study, having confirmed that the downregulation of B7-H4 induced
the apoptosis of the MGC-803 cells, we examined its effect on the
expression levels of these apoptotic markers. Our results revealed
that the knockdown of B7-H4 in MGC-803 cells activated caspase-3
and caspase-9, and altered the Bax/Bcl-2 ratio in a manner that
favored apoptosis, thereby demonstrating that the downregulation of
B7-H4 induced apoptosis via the mitochondrial signaling
pathway.
In conclusion, this study demonstrated the effects
of B7-H4 on MGC-803 and clarified the underlying mechanism.
Furthermore, this study suggested that B7-H4 may be a valuable
prognostic marker and a potential target for individualized
therapies in gastric cancer.
However, this study has some limitations. For
example, the main data were from only one human gastric cancer cell
MGC-803; therefore, conclusions cannot be drawn in general on
gastric cancer. Further studies using more human gastric cancer
cell lines and even some animal models are warranted, in order to
draw more general conclusions related to gastric cancer.
Acknowledgments
Not applicable.
Notes
[1]
Funding
This study was supported by a grant from the
Zhejiang Provincial Medicine Health Science and Technology Program
(2011RCA021).
[2] Availability
of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
CL and LY performed the oncological analyses, and
the analysis and interpretation of the data; YZ and DZ designed the
study and drafted the manuscript. All authors have reviewed the
final version of the manuscript and approve it for publication.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
[7] Authors'
information
Department of Oncology, the First Affiliated
Hospital, Zhejiang University School of Medicine, Hangzhou, China
(DZ, YZ and CL); Department of Oncology, the Affiliated Dongnan
Hospital of Xiamen University, Zhangzhou, China (LY).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al ToGA Trial Investigators: Trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): A phase 3, open-label, randomised controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lennerz JK, Kwak EL, Ackerman A, Michael
M, Fox SB, Bergethon K, Lauwers GY, Christensen JG, Wilner KD,
Haber DA, et al: MET amplification identifies a small and
aggressive subgroup of esophagogastric adenocarcinoma with evidence
of responsiveness to crizotinib. J Clin Oncol. 29:4803–4810. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shah MA, Wainberg ZA, Catenacci DV,
Hochster HS, Ford J, Kunz P, Lee FC, Kallender H, Cecchi F, Rabe
DC, et al: Phase II study evaluating 2 dosing schedules of oral
foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with
metastatic gastric cancer. PLoS One. 8:e540142013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kryczek I, Zou L, Rodriguez P, Zhu G, Wei
S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al: B7-H4
expression identifies a novel suppressive macrophage population in
human ovarian carcinoma. J Exp Med. 203:871–881. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krambeck AE, Thompson RH, Dong H, Lohse
CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC and Kwon
ED: B7-H4 expression in renal cell carcinoma and tumor vasculature:
Associations with cancer progression and survival. Proc Natl Acad
Sci USA. 103:10391–10396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simon I, Zhuo S, Corral L, Diamandis EP,
Sarno MJ, Wolfert RL and Kim NW: B7-h4 is a novel membrane-bound
protein and a candidate serum and tissue biomarker for ovarian
cancer. Cancer Res. 66:1570–1575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arigami T, Uenosono Y, Hirata M, Hagihara
T, Yanagita S, Ishigami S and Natsugoe S: Expression of B7-H4 in
blood of patients with gastric cancer predicts tumor progression
and prognosis. J Surg Oncol. 102:748–752. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thompson RH, Zang X, Lohse CM, Leibovich
BC, Slovin SF, Reuter VE, Cheville JC, Blute ML, Russo P, Kwon ED,
et al: Serum-soluble B7x is elevated in renal cell carcinoma
patients and is associated with advanced stage. Cancer Res.
68:6054–6058. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Y, Wang Y, Zhao J, Gu M, Giscombe R,
Lefvert AK and Wang X: B7-H3 and B7-H4 expression in non-small-cell
lung cancer. Lung Cancer. 53:143–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Quandt D, Fiedler E, Boettcher D, Marsch
WC and Seliger B: B7-h4 expression in human melanoma: Its
association with patients' survival and antitumor immune response.
Clin Cancer Res. 17:3100–3111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tringler B, Zhuo S, Pilkington G, Torkko
KC, Singh M, Lucia MS, Heinz DE, Papkoff J and Shroyer KR: B7-h4 is
highly expressed in ductal and lobular breast cancer. Clin Cancer
Res. 11:1842–1848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi IH, Zhu G, Sica GL, Strome SE,
Cheville JC, Lau JS, Zhu Y, Flies DB, Tamada K and Chen L: Genomic
organization and expression analysis of B7-H4, an immune inhibitory
molecule of the B7 family. J Immunol. 171:4650–4654. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sica GL, Choi IH, Zhu G, Tamada K, Wang
SD, Tamura H, Chapoval AI, Flies DB, Bajorath J and Chen L: B7-H4,
a molecule of the B7 family, negatively regulates T cell immunity.
Immunity. 18:849–861. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prasad DV, Richards S, Mai XM and Dong C:
B7S1, a novel B7 family member that negatively regulates T cell
activation. Immunity. 18:863–873. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zang X, Loke P, Kim J, Murphy K, Waitz R
and Allison JP: B7x: a widely expressed B7 family member that
inhibits T cell activation. Proc Natl Acad Sci USA.
100:10388–10392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dangaj D, Lanitis E, Zhao A, Joshi S,
Cheng Y, Sandaltzopoulos R, Ra HJ, Danet-Desnoyers G, Powell DJ Jr
and Scholler N: Novel recombinant human b7-h4 antibodies overcome
tumoral immune escape to potentiate T-cell antitumor responses.
Cancer Res. 73:4820–4829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salceda S, Tang T, Kmet M, Munteanu A,
Ghosh M, Macina R, Liu W, Pilkington G and Papkoff J: The
immunomodulatory protein B7-H4 is overexpressed in breast and
ovarian cancers and promotes epithelial cell transformation. Exp
Cell Res. 306:128–141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang J, Zhu Y, Wu C, Shen Y, Wei W, Chen
L, Zheng X, Sun J, Lu B and Zhang X: Tumor expression of B7-H4
predicts poor survival of patients suffering from gastric cancer.
Cancer Immunol Immunother. 59:1707–1714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arigami T, Uenosono Y, Ishigami S,
Hagihara T, Haraguchi N and Natsugoe S: Clinical significance of
the B7-H4 coregulatory molecule as a novel prognostic marker in
gastric cancer. World J Surg. 35:2051–2057. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou D, Pan G, Zheng C, Zheng J, Yian L
and Teng X: Expression of the RON receptor tyrosine kinase and its
association with gastric carcinoma versus normal gastric tissues.
BMC Cancer. 8:3532008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hao L, Zhang C, Qiu Y, Wang L, Luo Y, Jin
M and Zhang Y, Guo TB, Matsushima K and Zhang Y: Recombination of
CXCR4, VEGF, and MMP-9 predicting lymph node metastasis in human
breast cancer. Cancer Lett. 253:34–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qian Y, Hong B, Shen L, Wu Z, Yao H and
Zhang L: B7-H4 enhances oncogenicity and inhibits apoptosis in
pancreatic cancer cells. Cell Tissue Res. 353:139–151. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walker PR, Kwast-Welfeld J, Gourdeau H,
Leblanc J, Neugebauer W and Sikorska M: Relationship between
apoptosis and the cell cycle in lymphocytes: Roles of protein
kinase C, tyrosine phosphorylation, and AP1. Exp Cell Res.
207:142–151. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
American Joint Committee on Cancer: AJCC
Cancer Staging Manual. 6th edition. Springer-Verlag; New York, NY:
2002
|
|
26
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kryczek I, Wei S, Zhu G, Myers L, Mottram
P, Cheng P, Chen L, Coukos G and Zou W: Relationship between B7-H4,
regulatory T cells, and patient outcome in human ovarian carcinoma.
Cancer Res. 67:8900–8905. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng L, Jiang J, Gao R, Wei S, Nan F, Li
S and Kong B: B7-H4 expression promotes tumorigenesis in ovarian
cancer. Int J Gynecol Cancer. 19:1481–1486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang L, Wu H, Lu D, Li G, Sun C, Song H,
Li J, Zhai T, Huang L, Hou C, et al: The costimulatory molecule
B7-H4 promote tumor progression and cell proliferation through
translocating into nucleus. Oncogene. 32:5347–5358. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schultz DR and Harrington WJ Jr:
Apoptosis: Programmed cell death at a molecular level. Semin
Arthritis Rheum. 32:345–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Korsmeyer SJ, Shutter JR, Veis DJ, Merry
DE and Oltvai ZN: Bcl-2/Bax: A rheostat that regulates an
anti-oxidant pathway and cell death. Semin Cancer Biol. 4:327–332.
1993.PubMed/NCBI
|
|
32
|
Würstle ML, Laussmann MA and Rehm M: The
central role of initiator caspase-9 in apoptosis signal
transduction and the regulation of its activation and activity on
the apoptosome. Exp Cell Res. 318:1213–1220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cohen GM: Caspases: The executioners of
apoptosis. Biochem J. 326:1–16. 1997. View Article : Google Scholar : PubMed/NCBI
|