Introduction
Odontogenic cysts of the jaws, which are
space-occupying lesions, are generally classified into two groups:
inflammatory cysts and developmental cysts (1). Inflammatory cysts mainly include
radicular cysts (RCs), while the developmental cysts include
dentigerous cysts (DCs) and odontogenic keratocysts (OKCs)
(2). RCs, which originate from
cell rests of Malassez, are the most common jaw inflammatory cysts
(52.2%) (3). DCs are associated
with the crown of an unerupted tooth (4). After being considered as benign
tumors for many years (5), OKCs
have been moved back into the cyst category by the World Health
Organization in 2017, due to their completely regress following
decompression and other inadequate evidence defining their
neoplastic nature (6).
Cell-derived microparticles (MPs) are small
(100-1,000 nm in diameter) membrane-enclosed vesicles secreted from
cells by direct budding from the plasma membrane (7). Previous studies by our group have
demonstrated that the level of circulating MPs in patients with
oral cancer are significantly increased and closely associated with
their clinical characteristics, such as blood hypercoagulation and
tumor angiogenesis (8,9). Apart from plasma, increased levels of
MPs have been also reported in various types of body fluids, such
as urine, ascites and pleural effusions in patients with malignant
tumors (10-12). MPs highly express
phosphatidylserine (PS) on their surface (13), and thus they can be easily detected
by fluorescently-labeled Annexin V (14). Additionally, subpopulations of MPs
with different cellular origins can be determined by specific
markers carried on their membrane surface (15,16).
Since odontogenic cysts are directly immersed in the
milieu of cyst fluids, the epithelial cells or surrounding cells of
odontogenic cysts may release MPs into the cavity. In the present
study, the levels of total and subtype MPs derived from the cyst
fluids (CFMPs) were examined and compared among patients with DCs,
RCs and OKCs. In addition, the potential clinical significance and
biological function of CFMPs were investigated.
Materials and methods
Study population
This study was approved by the Review Board of the
Medical Ethics Committee of the Hospital of Stomatology, Wuhan
University, Wuhan, China and conducted from April, 2016 to
February, 2017. This study included a total of 26 patients with
OKCs, 20 patients with RCs and 13 patients with DCs (Tables ITable II–III). The diagnosis of DCs, RCs and OKCs
was made according to clinical, histopathological (5) and imaging characteristics (17). All patients agreed to participate
in the study and signed informed consent forms.
 | Table ISummary of clinical characteristics
of patients with odontogenic keratocysts (OKCs). |
Table I
Summary of clinical characteristics
of patients with odontogenic keratocysts (OKCs).
| Patient No. | Sex | Age (years) | Location | Cyst fluid drained
(ml) |
|---|
| 1 | F | 35 | Left mandible | 1 |
| 2 | F | 26 | Right maxilla | 3 |
| 3 | F | 39 | Mandible | 4.5 |
| 4 | F | 47 | Left mandible | 6 |
| 5 | F | 24 | Left mandible | 2.5 |
| 6 | F | 24 | Right mandible | 2 |
| 7 | M | 32 | Left mandible | 4.5 |
| 8 | M | 52 | Left mandible | 1 |
| 9 | M | 79 | Right mandible | 5 |
| 10 | M | 24 | Right mandible | 2 |
| 11 | F | 11 | Right mandible | 5 |
| 12 | F | 52 | Right maxilla | 2 |
| 13 | F | 45 | Right mandible | 3 |
| 14 | M | 34 | Left mandible | 0.8 |
| 15 | F | 28 | Right mandible | 3 |
| 16 | M | 37 | Left maxilla | 7 |
| 17 | M | 26 | Right maxilla | 4 |
| 18 | M | 24 | Left mandible | 1 |
| 19 | F | 32 | Left mandible | 1 |
| 20 | M | 16 | Right mandible | 2 |
| 21 | F | 30 | Left maxilla | 2 |
| 22 | F | 18 | Right mandible | 1 |
| 23 | F | 27 | Maxilla | 1 |
| 24 | F | 47 | Right maxilla | 2 |
| 25 | M | 23 | Right mandible | 2 |
| 26 | M | 17 | Left maxilla | 1 |
 | Table IISummary of clinical characteristics
of patients with dentigerous cysts (DCs). |
Table II
Summary of clinical characteristics
of patients with dentigerous cysts (DCs).
| Patient No. | Sex | Age (years) | Location | Cyst fluid drained
(ml) |
|---|
| 1 | F | 30 | Left maxilla | 5 |
| 2 | F | 18 | Right mandible | 2 |
| 3 | M | 18 | Maxilla | 3 |
| 4 | F | 33 | Right maxilla | 2 |
| 5 | M | 51 | Right mandible | 9 |
| 6 | M | 42 | Left maxilla | 2 |
| 7 | F | 50 | Left mandible | 11 |
| 8 | M | 34 | Right mandible | 3.5 |
| 9 | M | 34 | Right mandible | 2 |
| 10 | M | 56 | Right mandible | 4 |
| 11 | F | 28 | Left maxilla | 5 |
| 12 | M | 36 | Right maxilla | 4 |
| 13 | F | 33 | Left mandible | 5 |
 | Table IIISummary of clinical characteristics
of patients with radicular cysts (RCs). |
Table III
Summary of clinical characteristics
of patients with radicular cysts (RCs).
| Patient No. | Sex | Age (years) | Location | Cyst fluid drained
(ml) |
|---|
| 1 | M | 14 | Left mandible | 4 |
| 2 | F | 45 | Right maxilla | 0.8 |
| 3 | F | 44 | Left mandible | 7.5 |
| 4 | M | 54 | Right maxilla | 10 |
| 5 | F | 34 | Maxilla | 2 |
| 6 | F | 63 | Right mandible | 4 |
| 7 | F | 32 | Right maxilla | 3 |
| 8 | M | 7 | Left maxilla | 2 |
| 9 | M | 43 | Left mandible | 4 |
| 10 | M | 32 | Mandible | 7 |
| 11 | F | 44 | Left mandible | 7.5 |
| 12 | M | 66 | Left maxilla | 3 |
| 13 | M | 29 | Left maxilla | 2 |
| 14 | F | 73 | Right maxilla | 5 |
| 15 | F | 51 | Right maxilla | 3 |
| 16 | M | 8 | Right maxilla | 5 |
| 17 | M | 17 | Left mandible | 3 |
| 18 | M | 24 | Left mandible | 6 |
| 19 | M | 65 | Right mandible | 5 |
| 20 | M | 41 | Right mandible | 4 |
Cyst fluid collection and CFMP
isolation
Samples of cystic fluids were obtained from the cyst
cavity by aspiration with a syringe attached to an 18G sterilized
needle prior to surgery. The cyst fluids were immediately
centrifuged at 3,000 × g for 20 min at 4°C using the Centrifuge
5810R (Eppendorf, Hamburg, Germany). Supernatants were collected
and centrifuged at 3,000 × g for a further 20 min to obtain
cell-free cyst fluids. Subsequently, an equal volume of
Ca2+/Mg2+-free highly purified phosphate-buffered saline
(PBS; Beyotime, Shanghai, China) was added to dilute the
supernatants and the dilution was centrifuged at 10,000 × g for 40
min. This step was aimed to remove apoptotic bodies and larger
vesicles. The supernatants were then centrifuged at 50,000 × g for
1 h at 4°C using the Avanti J-26 XP high-speed centrifuge (Beckman
Coulter, Irving, TX, USA) to pellet the CFMPs as previously
described (8). The CFMP pellets
were resuspended with 150 μl of PBS. Subsequently, 50
μl of the CFMP sample was prepared for flow cytometry and
the other 100 μl was immediately stored at −80°C for further
analyses.
Characterization of CFMPs by transmission
electron microscopy (TEM)
TEM was performed at the Wuhan Institute of
Virology, Chinese Academy of Sciences (Wuhan, China).
Freshly-isolated CFMPs from patients with DCs, RCs and OKCs were
placed on a copper grid. The grids were stained with 1% v/v uranyl
acetate and the samples were examined on a HT7700 transmission
electron microscope (Hitachi High-Tech; Hitachi, Tokyo, Japan) as
previously described (18).
Characterization of CFMPs by
carboxyfluorescein succinimidyl ester (CFSE) labeling
CFSE is a fluorescent dye used for the labeling of
MPs (19). It is colorless outside
of MPs; however, it can passively diffuse into MPs and become
brightly fluorescent after its cleavage of acetate groups by
esterases, which are present in MPs (19). For CFSE labeling, the CFMPs were
incubated with 10 µM CFSE (Sigma-Aldrich, St. Louis, MO,
USA) at 37°C for 30 min in the dark. Subsequently, the samples were
observed under a fluorescence microscope (Leica Microsystems,
Wetzlar, Germany) and analyzed using a BD FACSAria II flow
cytometer (BD Biosciences, San Jose, CA, USA) as previously
described (8).
Detection and quantification of CFMPs by
flow cytometry
Flow cytometric analysis was performed with a BD
FACSAria II flow cytometer (BD Biosciences) as described in our
previous studies (8,9). The size of the CFMPs was identified
with the Nile Red particles with a diameter of 0.7 to 0.9 µm
(Spherotech, Lake Forest, IL, USA). To quantify the CFMPs, the
known number of calibrator flow-count fluorosphere beads (10
µm diameter; Beckman Coulter) were added to determine the
number of CFMPs. As flow-count fluorospheres have a definite
concentration, when identical volumes of a sample and flow-count
fluorospheres are added and tested, the concentration of CFMPs can
be calculated using the following formula: (Total number of events
for the sample/total number of events for flow-count fluorospheres)
x flow-count fluorospheres assayed concentration. The
concentrations of CFMPs were then further calculated by considering
the initial volumes of cyst fluids that are listed in Tables ITable II–III. The subpopulations of CFMPs were
detected according to the expression of membrane-specific antigens
as described in our previous studies (8,9) with
minor modifications. The subsets of the CFMPs were identified as
follows: Platelet-derived MPs (PMPs,
CD31+/CD41+), endothelium-derived MPs (EMPs,
CD144+/Annexin V+), erythrocyte-derived MPs
(ErMPs, CD235a+/Annexin V+),
leukocyte-derived MPs (LMPs, CD45+/Annexin
V+) and epithelium-derived MPs (EpMPs,
EpCAM+/Annexin V+). The antibodies were added
to 10 μl of CFMPs as follows: 2 μl of
anti-CD31-APC-Cy7 (cat. no. 563653; BD Biosciences),
anti-CD41-Pacific Blue (cat. no. 305650; BioLegend, Inc., San
Diego, CA, USA), anti-CD144-PE-Cy7 (order no. 130-100-722, lot no.
5160926503; Miltenyi Biotec, Bergisch Gladbach, Germany),
anti-EpCAM-Qdot605 (cat. no. 563182); 1.5 μl of anti-Annexin
V-PerCP-Cy5.5 (cat. no. 561431), anti-CD235a-Qdot655 (cat. no.
740583), anti-CD45-Alexa Fluor 700 (cat. no. 560566) (all from BD
Biosciences), anti-RANKL-APC (cat. no. FAB6264A; R&D Systems,
Minneapolis, MN, USA) and IgG (as a control) (cat. no. 555749; BD
Biosciences). As for incubation buffers, Annexin V Binding Buffer
containing calcium (BD Biosciences) was used for anti-Annexin
V-PerCP-Cy5.5 detection, whereas PBS was used as a control for the
other antibodies. These antibodies and binding buffers were mixed
with samples for 30 min at 4°C in the dark. Subsequently, 370
μl of PBS was added to the CFMP samples and analyzed by flow
cytometry. The results were calculated using FlowJo 9.3.2
software.
Immunohistochemistry
Samples from 24 patients (2 cases were lacking the
epithelial component in the IHC test) with OKCs were fixed in 4%
paraformaldehyde and embedded in paraffin under the guidelines of
the National Institutes of Health. The tissue samples and CFMPs
used for the corollary analyses were collected from the same
patient. In brief, the sections were dewaxed, rehydrated and
antigen-retrieved by high pressure. Subsequently, the sections were
incubated with 3% hydrogen peroxide for 20 min, goat serum for 20
min under room temperature, followed by incubation with the primary
antibody [receptor activator for nuclear factor-κB ligand (RANKL),
1:200, Proteintech Group, Wuhan, China; 23408-1-AP] at 4°C
overnight. For the staining, a diaminobenzidine substrate kit, and
hematoxylin (both from Dako, Glostrup, Denmark) were used. For
RANKL evaluation, the semi-quantitative analysis of
immunohistochemical staining was performed using Image-Pro Plus 6.0
and the quantification was calculated as the mean density for each
protein (IOD/area).
DNA electrophoresis
Total RNA was isolated from the CFMPs and the
supernatants of OKCs using TRIzol reagent (Invitrogen, Carlsbad,
CA, USA) according to the manufacturer's instructions. RNA was
reverse transcribed into complementary DNA (cDNA) with Oligo(dT)
and AMV reverse transcriptase (Fermentas/Thermo Fisher Scientific,
Waltham, MA, USA). The cDNA was then used for polymerase chain
reaction (PCR) by amplifying 36 cycles for RANKL (94°C for 30 sec,
60°C for 1 min, 72°C for 1 min, and final elongation at 72°C for 10
min) on a 7900HT Real-time PCR System (Applied Biosystems, New
York, NY, USA). The primer sequences were as follows: RANKL
forward, 5′-TCAGAAGATGGCACTCACTG-3′ and reverse,
5′-AACATCTCCCACTGGCTGTA-3′. PCR products were separated in 1%
agarose gels and stained with GelRed (Biotium, Fremont, CA, USA).
The blots were imaged with a Syngene G-Box (Syngene, Cambridge,
UK).
Co-culture of CFMPs with human
immortalized oral epithelial cells (HlOECs)
HIOECs were kindly provided by Professor San-Gang He
from the School of Stomatology, Wuhan University. The HIOECs were
plated in 6-well plates and cultured in defined keratinocyte
serum-free medium (Gibco, Carlsbad, CA, USA). When the HIOECs were
grown to 80% confluence, they were co-cultured with the CFMPs (5
and 10 µg/ml; collected from 2 patients with OKCs) for 48
h.
Cellular uptake assay
This study was approved by the Medical Ethics
Committee of Hospital of Stomatology, Wuhan University. To
investigate the biological functions of CFMPs, cellular uptake
assays were performed. For this purpose, 10 six-week-old male
C57BL/6 mice were obtained from the Experimental Animal Centre of
Wuhan University. The weight of these mice ranged from 15.2 to 18.4
g. The mice were injected intraperitoneally with a lethal dose of
sodium pentobarbital (150 mg/kg) followed by cervical dislocation.
The whole body of the mice were disinfected with 70% ethanol by
soaking all the fur. The skin of the neck region was cut open, and
the muscle attachments of the mandible were removed. Subsequently,
the mandible was divided into 2 sections and pulled out. Mandibles
were kept in α-minimal essential medium (α-MEM) supplemented with
10% fetal bovine serum (FBS) (both from Gibco), 1% antibiotics (100
U/ml penicillin and 100 µg/ml streptomycin; Sigma-Aldrich).
The bone marrow was then flushed with PBS, filtered, and spun down
at 1,000 rpm for 5 min. The supernatant was discarded and the cells
were resuspended in 1 ml complete α-MEM medium. Bone marrow cells
were collected and cultured for 12 h in α-MEM containing 10% FBS in
Petri dishes. Subsequently, floating cells were collected and
plated in 96-well plates at a density of 3×104
cells/well, then cultured for 3 days in α-MEM supplemented with 10%
FBS and macrophage colony-stimulating factor (M-CSF; 20 ng/ml;
R&D Systems). Adherent cells were regarded as bone
marrow-derived macrophages (BMMs). The HIOECs were plated in 6-well
plates and cultured in defined keratinocyte serum-free medium
(Gibco). The BMMs and HIOECs were labeled with CellMask (1:2,000,
Life Technologies, Carlsbad, CA, USA) at 37°C for 30 min in the
dark and cultured on coverslips as described in our previous
studies (8,9) with some modifications. The CFMPs from
the OKCs (10 µg) were labeled with CFSE, and then added to
the CellMask-labeled BMMs and HIOECs, which were co-cultured for a
period of 2 h. The BMMs and HIOECs were then fixed in 4%
paraformaldehyde for 10 min, stained with
4′,6-diamidino-2-phenylindole (DAPI) (cat. no. ZLI-9557; Beijing
Zhongshan Golden Bridge Biotechnology, Beijing, China) for 15 min
and observed under a fluorescence microscope (Leica
Microsystems).
Cell viability assay
Cell proliferation was measured by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. The HIOECs and BMMs were seeded in a 96-well culture plate
(3×103 cells/well) and incubated overnight at 37°C with
a supply of 5% CO2. The cells were treated with or
without various concentrations of CFMPs (5 or 10 µg/ml) and
incubated for 24 or 48 h. The cells were washed with PBS, and
treated with 20 μl of MTT (5 mg/ml) and incubated for 2 h at
37°C in a CO2 incubator. The blue formazan products
formed in the cells were dissolved in 100 μl dimethyl
sulfoxide (DMSO) and measured at 450 nm using a spectrophotometer
(Power Wave XS2, serial no. 238093; BioTek Instruments, Inc.,
Winooski, VT, USA). The absorbance values were compared with those
of the control cells by GraphPad Prism 6.0 software and shown
graphically as the percentage (%) of viable cells compared to the
control.
Western blot analysis
Western blot analysis was performed using the CFMPs
and CFMPs co-cultured with the HIOECs. The CFMPs and HIOECs were
lysed in RIPA buffer, exposed to brief sonication, and proteins
were quantified using the bicinchoninic acid assay (BCA).
Subsequently, 40 µg of protein were separated by
electrophoresis on a 10% gel SDS-PAGE, and then transferred onto
polyvinylidene fluoride (PVDF) membranes. The membranes were
incubated in 5% non-fat milk solution for 1 h at room temperature
followed by overnight incubation at 4°C with the following primary
antibodies: RANKL (1:2,000, rabbit; 23408-1-AP; Proteintech Group,
Wuhan, China) and GAPDH (1:2,000, mouse, #5174; Cell Signaling
Technology, Danvers, MA, USA). The membranes were then incubated
for 1 h at room temperature with an HRP-conjugated secondary
antibody (#7074; Cell Signaling Technology). Following the
application of chemiluminescent substrates for the detection of HRP
(Life Technologies) at room temperature, the blots were developed
with ECL detection reagents (Sigma-Aldrich).
Induction of osteoclast differentiation
of BMMs
The adherent BMMs were cultured for 5 days in α-MEM
supplemented with FBS (10%), recombinant mouse M-CSF (20 ng/ml)
(R&D Systems), mouse recombinant sRANKL (50 ng/ml; PeproTech,
London, UK), anti-RANKL monoclonal antibody (1,000 ng/ml; R&D
Systems) or CFMPs (5 and 10 µg/ml), all of which were
replaced every 2 days in 96-well plates. The cells were fixed with
4% paraformaldehyde for 10 min at room temperature and stained for
tartaric-resistant acid phosphatase (TRAP) activity. For RNA
isolation, the BMMs were seeded on 6-well plates and cultured for 5
days. The BMMs were co-cultured with M-CSF (20 ng/ml) and CFMPs
isolated from 2 patients with OKCs at the concentration of 5 or 10
µg/ml. Both M-CSF (20 ng/ml)- and sRANKL (50 ng/ml)-treated
groups were selected as positive controls.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The isolation of total RNA, synthesis of cDNA and
RT-qPCR were performed. Total RNA was extracted from the BMMs using
TRIzol reagent (Invitrogen). Subsequently, 2 µg of RNA was
reverse transcribed into 20 μl of cDNA with Oligo(dT) and
AMV reverse transcriptase (Fermentas/Thermo Fisher Scientific). One
fifth of the cDNA was then used for polymerase chain reaction (PCR)
using FastStart Universal SYBR-Green Master Mix (Roche, Basel,
Switzerland) on a 7900HT Real-time PCR System (Applied Biosystems,
Waltham, MA, USA). GAPDH was used as an endogenous control. The
thermal cycling conditions comprised 94°C for 30 sec, 60°C for 1
min, 72°C for 1 min, and final elongation at 72°C for 10 min
amplifying for 36 cycles. The primer nucleotide sequences for PCR
were designed as follows: GAPDH, forward, 5′-GACGGCCGCATCTTCTTGA-3′
and reverse, 5′-CACACCGACCTTCACCATTTT-3′; nuclear factor of
activated T-cells 1 (NFATc1) forward, 5′-ACCACCTTTCCGCAACCA-3′and
reverse,5′-GGTACTGGCTTCTCTTCCGTTTC-3′; TRAP forward,
5'-CTGCTGGGCCTACAAATCATA-3′ and reverse,
5′-GGGAGTCCTCAGATCCATAGT-3′. The 2−ΔΔCq method was used
for the analysis of the data (20).
Statistical analysis
Data are expressed as the means ± SEM. Differential
levels (apart from the mean density of RANKL expression) were
investigated using the Kruskal-Wallis test followed by the Dunn's
Multiple Range test for post hoc comparisons. The mean density of
RANKL expression levels in the patient samples were analyzed by the
Student's t-test. Spearman's rank correlation analysis was used to
investigate the correlation between CFMP levels and RANKL
expression in the samples collected from the same patients. For the
comparisons, a value of P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using GraphPad Prism 6.0 software.
Results
Clinical characteristics of the patients
with odontogenic cystic lesions
Thirteen patients with DCs (7 male, 6 female), 20
patients with RCs (12 male, 8 female) and 26 patients with OKCs (11
male, 15 female) were enrolled in this study (Tables ITable II–III). The average age of the patients
with DCs, RCs and OKCs was 36±12, 39±20 and 33±15 years old,
respectively. The average diameters of the DCs, RCs and OKCs were
2.44±1.38, 2.73±1.80 and 3.66±1.53 cm, respectively. No significant
differences in age, sex and diameter distribution were found in
this study.
Characterization and quantification of
CFMPs
CFMPs from patients with DCs, RCs and OKCs were
purified by differential centrifugation. To directly visualize the
CFMPs, TEM and CFSE fluorescence labeling were performed. As shown
in Fig. 1A, the CFMPs were
100–1,000 nm membrane-bounded vesicles with a round or elliptical
structure. As shown in Fig. 1B,
the CFMPs were successfully stained with CFSE, indicating their
membrane structures. Using the Nile Red fluorescent particles with
known diameters (0.7–0.9 µm), the diameters of the CFMPs
were determined ranging from 100 to 1,000 nm (Fig. 1C). Moreover, the results of flow
cytometric analysis demonstrated that the purified CFMPs were
successfully detected by CFSE (Fig.
1D). The above-mentioned results suggest that the purified
CFMPs are in line with the basic characteristics of MPs.
Cellular origin of MPs in the cyst fluid
of DCs, RCs and OKCs
By the addition of one or more CD-specific
monoclonal antibodies, we determined the cellular origin of the MPs
present in the cyst fluid. Platelet-derived MPs
(CD31+/CD41+), endothelium-derived MPs
(CD144+/Annexin V+), erythrocyte-derived MPs
(CD235a+/Annexin V+), leukocyte-derived MPs
(CD45+/Annexin V+) and epithelium-derived MPs
(EpCAM+/Annexin V+) were identified in the
cyst fluid of DCs, RCs and OKCs. On the other hand, erythrocyte-
and epithelium-derived MPs were the leading CFMPs from the OKCs and
DCs, whereas platelet- and erythrocyte-derived MPs were the top two
CFMP subsets from the RCs (Fig.
2). These above-mentioned results showed that these CFMPs
shared similar cell origins.
Subtypes of CFMPs are significantly
increased in patients with OKCs
The results revealed that the levels of leukocyte-
and platelet-derived MPs (CD45+/Annexin V+
and CD31+/CD41+, respectively) were
significantly increased in the OKCs when compared with the DCs
(P<0.05, P<0.01 respectively), whereas no significant
differences were observed between the DCs and RCs (P>0.05), and
between the OKCs and RCs (P>0.05) (Fig. 3A and D). Significantly higher
levels of erythrocyte-, endothelium- and epithelium-derived CFMPs
were also observed in the OKCs when compared with the DCs and RCs
(P<0.05), whereas no significant differences were found between
the DCs and RCs (P>0.05) (Fig. 3B,
C and E, and Table IV).
 | Table IVCyst fluid microparticle levels in
patients with dentigerous cysts (DCs), radicular cysts (RCs) and
odontogenic keratocysts (OKCs). |
Table IV
Cyst fluid microparticle levels in
patients with dentigerous cysts (DCs), radicular cysts (RCs) and
odontogenic keratocysts (OKCs).
| Variables | OKCs | DCs | P<0.05
OKCs/DCs | RCs | P<0.05
OKCs/RCs |
|---|
| n | 26 | 13 | ND | 20 | ND |
| Agea (years) | 33±15 | 36±12 | ND | 39±20 | ND |
| Sex
(male/female) | 11/15 | 7/6 | ND | 12/8 | ND |
| Total MPsb | 6,751±5,800 | 2,755±2,471 | Y | 3,477±3,346 | Y |
|
CD31+/CD41+MPsb | 1,559±1,435 | 533±582 | Y | 891±870 | N |
| CD235a+
MPsb | 3,212±3,675 | 1,464±1,201 | Y | 2,523±2,863 | Y |
| CD45+
MPsb | 1,319±1,214 | 527±591 | Y | 639±601 | N |
| CD144+
MPsb | 996±1,144 | 357±417 | Y | 411±449 | Y |
| EpCAM+
MPsb | 1,867±2,072 | 586±534 | Y | 719±634 | Y |
The level of CFMPs is increased in
patients with OKCs
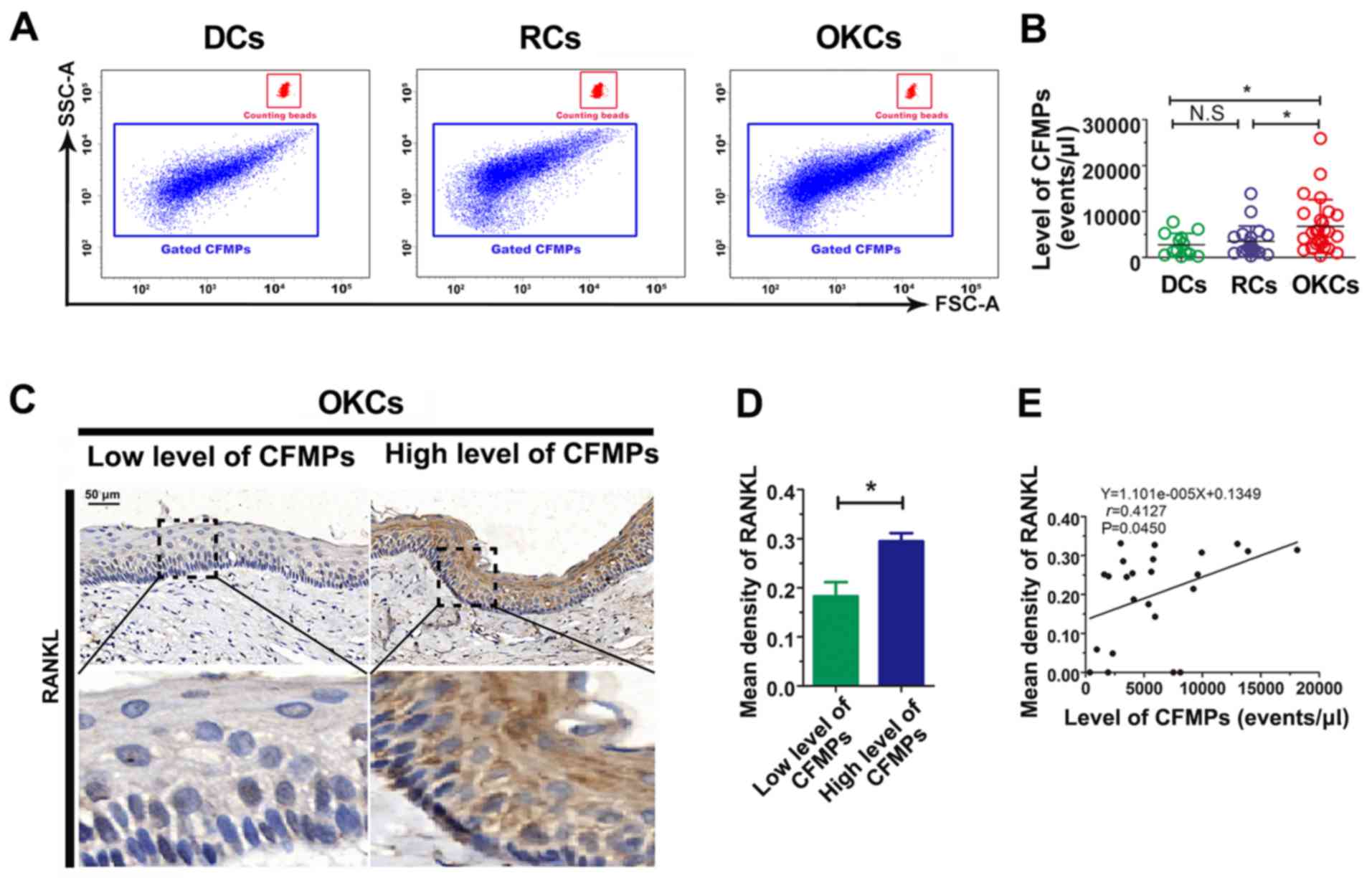
By applying flow-count beads, the concentrations of
CFMPs were determined by flow cytometry (Fig. 4A). The results revealed that the
concentration of total CFMPs in the OKCs (6,751±5,800
events/µl) was significantly higher than that in the RCs
(3,477±3,346 events/µl; P<0.05) and DCs (2,755±2,471
events/µl; P<0.05) (Fig.
4B and Table IV). However, no
significant difference was observed in the concentration of CFMPs
between the DCs and RCs (P>0.05) (Fig. 4B). The results suggest that the
level of CFMPs is elevated in OKCs, which may be associated with
the development of the lesions.
CFMPs may promote osteoclastogenesis by
upregulating the expression of RANKL in epithelial cells of
OKCs
To investigate the potential influence of CFMPs on
the osteoclastic activity of odontogenic lesions, we detected the
expression level of RANKL in the OKC samples and examined its
correlation with the level of CFMPs. According to the mean
concentration of CFMPs, the patients with OKCs were divided into 2
groups, with a high or low level of CFMPs. As shown in Fig. 4C and D, the mean density of RANKL
was markedly increased in the patients with a high level CFMPs
compared with those with a low level CFMPs (P=0.0261). The
Spearman's rank correlation test revealed that the level of total
CFMPs positively correlated with the RANKL expression level in the
samples from patients with OKCs (r=0.4127, P=0.0450) (Fig. 4E). As shown in Fig. 5A, the CFMPs were detected in the
HIOECs at 2 h. In addition, the results from DNA electrophoresis
revealed that the CFMPs from the OKCs expressed RANKL mRNA
(Fig. 5B). In addition, the HIOECs
exhibited a higher protein expression level of RANKL when compared
with the control group (Fig. 5C).
Previous studies have demonstrated that RANKL is expressed in the
components of odontogenic lesions and contributes to the bone
destruction of OKCs (21,22). Our results thus suggested that the
increased levels of CFMPs were closely associated with the
expression of RANKL in the epithelium of OKCs, indicating that the
CFMPs may be related to the osteoclastogenesis of OKCs.
CFMPs have no effects on the
proliferative ability of HIOECs and BMMs
The HIOECs and BMMs were co-cultured with or without
various concentrations of CFMPs (5 or 10 µg/ml) for 24 or 48
h. Cell viability was determined by MTT assay. The results showed
that the CFMPs (5 and 10 µg/ml) had no effect on the
viability of the HIOECs and BMMs when compared to the control group
(Fig. 6).
CFMPs isolated from OKCs promote the
osteoclastogenesis of BMMs
To further determine the biological effects of
CFMPs, a series of in vitro experiments using mouse BMMs
were carried out. The results of western blot analysis and flow
cytometry confirmed that the CFMPs from the OKCs exhibited a higher
expression of RANKL (Fig. 7).
Therefore, we examined whether the CFMPs could promote the
osteoclastogenesis of BMMs. As shown in Fig. 8A, following incubation with
CFSE-labeled CFMPs for 2 h, green fluorescence dots were detected
in the CellMask-stained BMMs, indicating that the CFMPs were
effectively taken up by the BMMs. In addition, as shown in Fig. 8B, the osteoclasts, characterized by
multinuclear giant cells and TRAP positive cells, were detected in
the M-CSF- and CFMP-treated group or the M-CSF- and sRANKL-treated
group. The quantification of TRAP staining demonstrated that the
CFMPs derived from OKCs significantly enhanced the
osteoclastogenesis of the BMMs in a concentration-dependent manner
(Fig. 8C). Additionally, the
results of RT-qPCR suggested that the CFMPs significantly promoted
the mRNA expression levels of representative markers for osteoclast
differentiation, TRAP and NFATcl (Fig.
8D and E). Moreover, the anti-RANKL monoclonal antibody (1,000
ng/ml)- and CFMP (10 µg/ml)-treated group exhibited lower
numbers of TRAP-positive cells, and lower mRNA levels of TRAP and
NFATc1. This indicated that CFMPs may promote the
osteoclastogenesis of BMMs through RANKL carried by CFMPs.
Discussion
To the best of our knowledge, this study
demonstrates for the first time, the presence of MPs shed from
platelets, endothelial cells, leukocytes, erythrocytes and
epithelial cells into the cyst fluids of patients with DCs, RCs and
OKCs, and demonstrates the elevation of CFMPs in patients with
OKCs. The levels of CFMPs are closely associated with the diameters
of lesions and RANKL expression levels in OKC tissues. In addition,
we demonstrate that CFMPs isolated from patients with OKCs
stimulate BMM differentiation and lead to the formation of
osteoclasts, suggesting that CFMPs may contribute to the
osteoclastogenesis of OKCs.
Cyst fluid is a crucial component of the
microenvironment of odontogenic cysts. The levels of proteins
and/or cytokines within the cyst fluid of OKCs have been shown to
be quite different from those in DCs or RCs. For instance, it has
been reported that the protein level of lactoferrin is
significantly higher in OKCs compared with other cysts, due to the
impermeability nature of the cyst wall of OKCs (23–25).
Epithelial cells are more frequently present in the aspirates of
OKCs when compared with those of DCs and RCs (26). Moreover, there is evidence to
indicate that the cyst fluid level of interleukin (IL)-1α in OKCs
is significantly higher than that in DC or RC fluids, associated
with a higher expression level of matrix metalloproteinase-9 (MMP9)
in the OKC epithelium (27,28).
In addition, the higher level of transforming growth factor-β
(TGF-β) within the fluids plays a significant role in inducing
RANKL expression in the stroma, which is essential for the
osteoclastogenesis of OKCs (29).
These data suggest that different components within the cyst fluids
may contribute to the development of OKCs. A number of studies have
demonstrated that MPs, which are membrane vesicles produced upon
cell apoptosis or activation (7),
are present in several human body fluids, such as plasma, saliva
and synovial fluid (30–32). These MPs naturally inherit membrane
lipids, surface antigens, cytoplasmic proteins as well as nucleic
acids from their donor cells, which could be transferred into the
target cell, and then affect various cell functions (33). However, whether MPs are present in
the cyst fluid of OKCs and their role in the development of OKCs
remains unknown.
During the release of MPs, the asymmetric
distribution of phospholipids in the two leaflets of the plasma
membrane is lost, leading to phospholipid exposure (34). Typically, MPs expose the anionic
phospholipid PS on their membrane surface, enabling their detection
by Annexin V staining (14). The
cyst fluid of OKCs usually presents as a semi-solid form, and is
characterized by a large amount of keratins (35). Due to the similar size
distributions, it is a great challenge to distinguish MPs from
protein fragments within the body fluids, which otherwise results
in a significant amount of the background noise for the
quantification of MPs (36).
Therefore, in this study, we defined CFMPs using positive staining
for Annexin V in order to distinguish MPs from cell debris or
precipitates according to a previous protocol (36). In addition, as MPs carry surface
membrane antigens from their donor cells, we tested the various
cellular origins of MPs using Annexin V and specific markers, such
as CD144 (endotheliocyte marker), CD235a (erythrocyte marker),
EpCAM (epithelium marker) or CD45 (leukocyte marker). In this
study, at least to the best of our knowledge, we demonstrate for
the first time that MPs are present in the cyst fluid of OKCs, and
have a wide origin of different cells including blood cells,
epithelial cells and inflammatory cells. Moreover, the results
demonstrated that all the subtypes of CFMPs were significantly
elevated in OKCs when compared with DCs and RCs. The majority of
previous studies have shown that the elevated level of body fluid
MPs in patients bearing tumors may be caused by the neoplastic
characteristics, such as highly cell activation, apoptosis
abnormalities or chronic inflammation (37,38).
Although the precise mechanisms behind the elevated level of CFMPs
remain to be elucidated, there are two possible reasons for the
higher level of CFMPs within OKCs. First, the aberrant
proliferation and apoptotic characteristics of the OKC epithelium
lead to the increased release of MPs, which is similar to other
tumor lesions (12,39). This could explain the elevation of
EpCAM+ MPs in OKCs compared with DCs and RCs. However,
it cannot fully account for the higher level of MPs derived from
the blood or inflammatory cells, such as CD235a+,
CD31+/CD41+ and CD45+ CFMPs.
Second, as previous studies have confirmed that the relative
impermeability of the capsule wall of OKCs could lead to specific
accumulations of proteins (23–25),
it is possible that MPs, which contain larger amounts of proteins,
are also accumulated in the cyst cavity due to the impermeability
of the capsule wall of OKCs. This may better explain the entire
elevations of various MP types in OKC cyst fluids.
For all the odontogenic cysts or tumors, bone
resorption is one of the critical events (40). It is a complex process initiated by
the proliferation of immature osteoclasts. The interaction between
RANKL and RANK promotes the osteoclast differentiation by
activating the intracellular signaling (41). In another odontogenic tumor named
ameloblastoma, it has been suggested that the co-culture of
ameloblastoma cells and rabbit bone marrow cells induces
osteoclastogenesis via the RANK/RANK/OPG signaling pathway
(22). MPs carry RANKL protein and
can be internalized and deliver their cargo into the cytosol of
recipient cells, thereby activating or inhibiting specific
signaling pathways (42). Previous
studies have demonstrated that cytokines can induce the mRNA level
of RANKL and therefore boost the protein level of RANKL in oral
epithelial cells (43). In
exploring the roles of CFMPs, we found that the level of CFMPs was
closely related with RANKL expression within the epithelium of
OKCs. Furthermore, CFMPs can promote the expression of RANKL in
HIOECs which may be closely associated with the mRNA of RANKL
contained in CFMPs. Our results indicated that CFMPs may be
representative of the tissues, and may contribute to the bone
resorption of OKCs. However, the function of CFMPs in vitro
remains to be determined.
Of note, we found that BMMs could easily uptake
CFMPs which contained RANKL mRNA and protein at 2 h, indicating
that CFMPs may exert biological effects on the recipient cells. To
investigate the biological functions of CFMPs, CFMPs were added to
the BMMs and the levels of osteoclastogenesis-related genes, such
as TRAP and NFATc1 were found to be significantly elevated in the
BMMs co-cultured with CFMPs. More importantly, we found that BMMs
could successfully differentiate into osteoclasts in the presence
of M-CSF and CFMPs, which may be a novel mechanism of
osteoclastogenesis in OKCs. The osteoclasts absorb the adjacent
bone to acquire the space of the cavity for the growth of the
lesion. This may also imply the close association between the CFMP
level and RANKL expression in the tissue. Taken together, our study
demonstrates that the level of CFMPs may be an important indicator
of the progression of OKCs.
In conclusion, the present study demonstrated that
the level of CFMPs was significantly elevated in OKCs and was
closely associated with cyst diameters. In vitro experiments
revealed that CFMPs could be internalized by BMMs, leading to
increased mRNA expression levels of NFATc1 and TRAP in the BMMs.
Further studies are warranted in order to elucidate the precise
mechanisms underlying the alternations in CFMP profiles and the
functional significance of CFMPs.
Acknowledgments
The authors would like to thank the technician, Juan
Min, from the Wuhan Institute of Virology, Chinese Academy of
Sciences for supporting our flow cytometric analysis. The authors
would also like to thank Professor San-Gang He from the School of
Stomatology, Wuhan University for providing the HIOECs.
Funding
This study was supported by grants from the National
Natural Science Foundation of China to GC (no. 81671816) and YFZ
(no. 81570994).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QWM contributed to the design of the study and wrote
the manuscript. QWM, YYZ and JYL collected the clinical samples.
QWM, JGR and WQZ performed the flow cytometry analysis and CFMP
identification. RFL and BFN performed the cell experiments. YFZ, GC
and BL performed the data analysis and revised the manuscript. All
authors have read and approved this manuscript.
Ethics approval and consent to
participate
The use of human samples was approved by the Review
Board of the Medical Ethics Committee of the Hospital of
Stomatology, Wuhan University, Wuhan, China. All patients agreed to
participate in the study and signed informed consent forms. The use
of animals was approved by the Medical Ethics Committee of Hospital
of Stomatology, Wuhan University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have not competing
interests.
References
|
1
|
Browne RM: The pathogenesis of odontogenic
cysts: A review. J Oral Pathol. 4:31–46. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kramer IR, Pindborg JJ and Shear M: The
WHO histological typing of odontogenic tumours. A commentary on the
second edition. Cancer. 70:2988–2994. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Avelar RL, Antunes AA, Carvalho RW,
Bezerra PG, Oliveira Neto PJ and Andrade ES: Odontogenic cysts: A
clinicopathological study of 507 cases. J Oral Sci. 51:581–586.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin HP, Wang YP, Chen HM, Cheng SJ, Sun A
and Chiang CP: A clinicopathological study of 338 dentigerous
cysts. J Oral Pathol Med. 42:462–467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thompson L: World Health Organization
classification of tumours: Pathology and genetics of head and neck
tumours. Ear Nose Throat J. 85:742006.PubMed/NCBI
|
|
6
|
Wright JM and Vered M: Update from the 4th
edition of the World Health Organization Classification of head and
neck tumours: Odontogenic and maxillofacial bone tumors. Head Neck
Pathol. 11:68–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
7Abels ER and Breakefield XO: Introduction
to extracellular vesicles: Biogenesis, RNA cargo selection,
content, release and uptake. Cell Mol Neurobiol. 36:301–312. 2016.
View Article : Google Scholar
|
|
8
|
Ren JG, Man QW, Zhang W, Li C, Xiong XP,
Zhu JY, Wang WM, Sun ZJ, Jia J, Zhang WF, et al: Elevated Level of
Circulating Platelet-derived Microparticles in Oral Cancer. J Dent
Res. 95:87–93. 2016. View Article : Google Scholar
|
|
9
|
Ren JG, Zhang W, Liu B, Man QW, Xiong XP,
Li C, Zhu JY, Wang WM, Jia J, Sun ZJ, et al: Clinical Significance
and Roles in Angiogenesis of Circulating Microparticles in Oral
Cancer. J Dent Res. 95:860–867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen CL, Lai YF, Tang P, Chien KY, Yu JS,
Tsai CH, Chen HW, Wu CC, Chung T, Hsu CW, et al: Comparative and
targeted proteomic analyses of urinary microparticles from bladder
cancer and hernia patients. J Proteome Res. 11:5611–5629. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Press JZ, Reyes M, Pitteri SJ, Pennil C,
Garcia R, Goff BA, Hanash SM and Swisher EM: Microparticles from
ovarian carcinomas are shed into ascites and promote cell
migration. Int J Gynecol Cancer. 22:546–552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roca E, Lacroix R, Judicone C, Laroumagne
S, Robert S, Cointe S, Muller A, Kaspi E, Roll P, Brisson AR, et
al: Detection of EpCAM-positive microparticles in pleural fluid: A
new approach to mini-invasively identify patients with malignant
pleural effusions. Oncotarget. 7:3357–3366. 2016. View Article : Google Scholar :
|
|
13
|
Tkach M and Théry C: Communication by
extracellular vesicles: Where we are and where we need to go. Cell.
164:1226–1232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mallat Z, Hugel B, Ohan J, Lesèche G,
Freyssinet JM and Tedgui A: Shed membrane microparticles with
procoagulant potential in human atherosclerotic plaques: A role for
apoptosis in plaque thrombogenicity. Circulation. 99:348–353. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burnier L, Fontana P, Kwak BR and
Angelillo-Scherrer A: Cell-derived microparticles in haemostasis
and vascular medicine. Thromb Haemost. 101:439–451. 2009.PubMed/NCBI
|
|
16
|
Nieuwland R, Berckmans RJ, McGregor S,
Boing AN, Romijn FP, Westendorp RG, Hack CE and Sturk A: Cellular
origin and procoagulant properties of microparticles in
meningococcal sepsis. Blood. 95:930–935. 2000.
|
|
17
|
Mendes RA, Carvalho JF and van der Waal I:
Characterization and management of the keratocystic odontogenic
tumor in relation to its histopathological and biological features.
Oral Oncol. 46:219–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao JY, Chen G, Gu YP, Cui R, Zhang ZL,
Yu ZL, Tang B, Zhao YF and Pang DW: Ultrasmall magnetically
engineered Ag2Se quantum dots for instant efficient labeling and
whole-body high-resolution multimodal real-time tracking of
cell-derived microvesicles. J Am Chem Soc. 138:1893–1903. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grisendi G, Finetti E, Manganaro D,
Cordova N, Montagnani G, Spano C, Prapa M, Guarneri V, Otsuru S,
Horwitz EM, et al: Detection of microparticles from human red blood
cells by multiparametric flow cytometry. Blood Transfus.
13:274–280. 2015.
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
da Silva TA, Batista AC, Mendonça EF,
Leles CR, Fukada S and Cunha FQ: Comparative expression of RANK,
RANKL, and OPG in keratocystic odontogenic tumors, ameloblastomas,
and dentigerous cysts. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 105:333–341. 2008. View Article : Google Scholar
|
|
22
|
Qian Y and Huang HZ: The role of RANKL and
MMP-9 in the bone resorption caused by ameloblastoma. J Oral Pathol
Med. 39:592–598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Douglas CW and Craig GT: Quantitation of
lactoferrin in odontogenic cyst fluids. J Clin Pathol. 42:180–183.
1989. View Article : Google Scholar
|
|
24
|
Jurisic V, Terzic T, Colic S and Jurisic
M: The concentration of TNF-alpha correlate with number of
inflammatory cells and degree of vascularization in radicular
cysts. Oral Dis. 14:600–605. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skaug N: Proteins in fluid from
non-keratinizing jaw cysts. 4. Concentrations of immunoglobulins
(IgG, IgA and IgM) and some non-immunoglobulin proteins: Relevance
to concepts of cyst wall permeability and clearance of cystic
proteins. J Oral Pathol. 3:47–61. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Patidar M, Shetty P, Patidar N, Mittal S,
Singh H and Chethna: Biochemical and cytological comparison of
keratocystic odontogenic tumours to nonkeratinising odontogenic
cysts fluid. J Clin Diagn Res. 9:ZC34–ZC38. 2015.PubMed/NCBI
|
|
27
|
Ninomiya T, Kubota Y, Koji T and Shirasuna
K: Marsupialization inhibits interleukin-1alpha expression and
epithelial cell proliferation in odontogenic keratocysts. J Oral
Pathol Med. 31:526–533. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kubota Y, Ninomiya T, Oka S, Takenoshita Y
and Shirasuna K: Interleukin-1alpha-dependent regulation of matrix
metalloproteinase-9(MMP-9) secretion and activation in the
epithelial cells of odontogenic jaw cysts. J Dent Res.
79:1423–1430. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamada C, Aikawa T, Okuno E, Miyagawa K,
Amano K, Takahata S, Kimata M, Okura M, Iida S and Kogo M: TGF-β in
jaw tumor fluids induces RANKL expression in stromal fibroblasts.
Int J Oncol. 49:499–508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Berckmans RJ, Sturk A, van Tienen LM,
Schaap MC and Nieuwland R: Cell-derived vesicles exposing coagulant
tissue factor in saliva. Blood. 117:3172–3180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Berckmans RJ, Nieuwland R, Tak PP, Boing
AN, Romijn FP, Kraan MC, Breedveld FC, Hack CE and Sturk A:
Cell-derived microparticles in synovial fluid from inflamed
arthritic joints support coagulation exclusively via a factor
VII-dependent mechanism. Arthritis Rheum. 46:2857–2866. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Berckmans RJ, Nieuwland R, Boing AN,
Romijn FP, Hack CE and Sturk A: Cell-derived microparticles
circulate in healthy humans and support low grade thrombin
generation. Thromb Haemost. 85:639–646. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van der Pol E, Boing AN, Harrison P, Sturk
A and Nieuwland R: Classification, functions, and clinical
relevance of extracellular vesicles. Pharmacol Rev. 64:676–705.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Piccin A, Murphy WG and Smith OP:
Circulating microparticles: Pathophysiology and clinical
implications. Blood Rev. 21:157–171. 2007. View Article : Google Scholar
|
|
35
|
Aragaki T, Michi Y, Katsube K, Uzawa N,
Okada N, Akashi T, Amagasa T, Yamaguchi A and Sakamoto K:
Comprehensive keratin profiling reveals different histopathogenesis
of keratocystic odontogenic tumor and orthokeratinized odontogenic
cyst. Hum Pathol. 41:1718–1725. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dey-Hazra E, Hertel B, Kirsch T, Woywodt
A, Lovric S, Haller H, Haubitz M and Erdbruegger U: Detection of
circulating microparticles by flow cytometry: Influence of
centrifugation, filtration of buffer, and freezing. Vasc Health
Risk Manag. 6:1125–1133. 2010.PubMed/NCBI
|
|
37
|
Becker A, Thakur BK, Weiss JM, Kim HS,
Peinado H and Lyden D: Extracellular vesicles in cancer:
Cell-to-cell mediators of metastasis. Cancer Cell. 30:836–848.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Todorova D, Simoncini S, Lacroix R,
Sabatier F and Dignat-George F: Extracellular vesicles in
angiogenesis. Circ Res. 120:1658–1673. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kichi E, Enokiya Y, Muramatsu T, Hashimoto
S, Inoue T, Abiko Y and Shimono M: Cell proliferation, apoptosis
and apoptosis-related factors in odontogenic keratocysts and in
dentigerous cysts. J Oral Pathol Med. 34:280–286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Main DM: The enlargement of epithelial jaw
cysts. Odontol Revy. 21:29–49. 1970.PubMed/NCBI
|
|
41
|
Wada T, Nakashima T, Hiroshi N and
Penninger JM: RANKL-RANK signaling in osteoclastogenesis and bone
disease. Trends Mol Med. 12:17–25. 2006. View Article : Google Scholar
|
|
42
|
Deng L, Wang Y, Peng Y, Wu Y, Ding Y,
Jiang Y, Shen Z and Fu Q: Osteoblast-derived microvesicles: A novel
mechanism for communication between osteoblasts and osteoclasts.
Bone. 79:37–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fujihara R, Usui M, Yamamoto G, Nishii K,
Tsukamoto Y, Okamatsu Y, Sato T, Asou Y, Nakashima K and Yamamoto
M: Tumor necrosis factor-α enhances RANKL expression in gingival
epithelial cells via protein kinase A signaling. J Periodontal Res.
49:508–517. 2014. View Article : Google Scholar
|