Introduction
Type 2 diabetes mellitus (T2DM) has been reported to
increase the risk of various types of cancer, including colorectal
cancer (CRC) (1-3). A low survival rate is evident in
diabetic patients with CRC, even in patients that have undergone
complete curative surgical treatment for CRC (4). Hyperglycemia is one of the most
direct internal environmental alterations in patients with T2DM
(5,6). It has previously been demonstrated
that hyperglycemia is a key factor in the mechanisms underlying
diabetes-associated increased cancer risk (7). Furthermore, hyperglycemia activates
numerous pathways to promote the progression of cancer; for
example, it increases the levels of insulin-like growth factor 1
(IGF1), upregulates protein kinase B (AKT)/mammalian target of
rapamycin (mTOR) signaling and enhances WNT/β-catenin signaling
(8). Therefore, identifying the
factor that affects the glycolytic pathway in CRC may help to
increase the survival rate of diabetic patients with CRC.
Our previous studies demonstrated that
arginine-specific mono-ADP-ribosyltransferase 1 (ART1) promotes
proliferation, invasion and metastasis of CRC in vitro and
in vivo (9,10). Upregulation of the expression of
ART1 activates the Ras homologue gene family mer A/ROCK1 pathway,
which affects the expression of phosphorylated-AKT (p-AKT), c-Fos
and c-Myc, and promotes the proliferation and invasion of CRC cells
(9). Furthermore, ART1 upregulates
hypoxia-inducible factor 1α (HIF-1α) via the phosphoinositide
3-kinase (PI3K)/AKT signaling pathway, in order to promote the
expression of angiogenic factors, such as vascular endothelial
growth factor and basic fibroblast growth factor, and to enhance
angiogenesis in cancer tissue (11). It has also been revealed that the
expression levels of ART1 are higher in CRC tissues from patients
with T2DM compared with in non-diabetic CRC tissues. Furthermore,
overexpression of ART1 can increase the growth of transplanted CT26
tumors in streptozocin (STZ)-induced diabetic Balb/c mice (Chen
et al, unpublished data). These findings indicated that ART1
may be associated with glycolysis in CRC; however, the mechanism
remains unclear. It is well known that tumor cells rely on
increasing aerobic glycolysis to obtain energy, in which glycolytic
enzymes serve a significant role; these enzymes include pyruvate
dehydrogenase kinase 1 (PDK1) and lactate dehydrogenase A (LDHA)
(12-14). In addition, c-Myc may regulate
aerobic glycolysis through acting on metabolic enzymes, including
hexokinase 2, LDHA and PDK1 (15).
However, to the best of our knowledge, the effects of ART1 on the
expression of glycolytic enzymes have yet to be determined. In the
present study, the expression levels of AKT/mTOR, c-Myc and the
downstream glycolytic enzymes PDK1 and LDHA were detected, and the
generation of adenosine triphosphate (ATP) and lactic acid were
measured, in order to investigate whether ART1 could regulate
glycolytic enzymes via the AKT/mTOR/c-Myc pathway and thus modulate
the generation of ATP and lactic acid.
β-caryophyllene (BCP), which is a natural
sesquiterpene obtained from spices, is present in numerous plants
worldwide, including Syzygium aromaticum and Cinnamomum
cassia. It has been reported that BCP possesses significant
anticancer activities by affecting the growth and proliferation of
numerous types of cancer cell (16). In addition, BCP may inhibit the
proliferation of HCT-116 and HT-29 CRC cells. BCP can bind to
cannabinoid receptor 2 (CB2), thus stimulating mitogen-activated
protein kinase (MAPK) and inactivating the PI3K/AKT/mTOR pathway,
in order to suppress tumor growth and promote tumor apoptosis
(17). Furthermore, BCP has a
beneficial effect on glucose homeostasis by increasing the
secretion of insulin and restoring glucose homeostasis in diabetic
rats (18); therefore, BCP exerts
both hypoglycemic and antitumor effects. The present study
hypothesized that, if ART1 could regulate the glycolytic process
via the AKT/mTOR/c-Myc pathway, BCP may inhibit ART1-regulated
glycolysis through interfering with this pathway. Therefore, the
present study assessed the effects of BCP on glycolysis and its
underlying mechanism in CRC in response to ART1 overexpression.
Materials and methods
Cells and reagents
The CT26 cell line was provided by Professor Y.Q.
Wei (Sichuan University, Chengdu, China). ART1-cDNA, untransfected,
lentivirus (LV)-control and ART1-short hairpin (sh)RNA CT26 cell
lines were established in previous experiments (19,20).
The experimental groups were as follows: ART1-cDNA group, CT26
cells in which ART1 was overexpressed; ART1-shRNA group, CT26 cells
in which ART1 was silenced; untransfected group, untransfected CT26
cells; LV-control group, CT26 cells which were transfected with an
empty LV vector. The untransfected and LV-control groups were
considered the control groups. To detect the effects of BCP
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) on ART1-induced
glycolysis, ART1-cDNA CT26 cells were incubated under high-glucose
conditions (25 mM) at 37°C for 48 h in the control group. In the
experimental groups, ART1-cDNA CT26 cells were treated with glucose
(25 mM) and 50 μM BCP [dissolved in 0.1% dimethyl sulfoxide
(DMSO) (D-5879, Sigma-Aldrich, Merck KGaA)] at 37°C for 48 h.
glucose (25 mM) and 0.1% DMSO at 37°C for 48 h, or glucose (25 mM)
and 10 μM pyrrolidinedithiocarbamic acid (PDTC) at 37°C for
48 h. Each group of CT26 cells was cultured in RPMI-1640 medium
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal bovine serum (Pan-Biotech GmbH, Aidenbach, Germany),
100 μg/ml streptomycin and 100 U/ml penicillin (Beyotime
Institute of Biotechnology, Shanghai, China) at 37°C in an
incubator containing 5% CO2. Cells were cultured in the
presence of glucose (25 mM) (21)
in complete culture medium for 48 h. The bicinchoninic acid (BCA)
protein assay kit, Hoechst staining kit, adenosine triphosphate
(ATP) assay kit and PDTC were purchased from Beyotime Institute of
Biotechnology. The lactic acid assay kit was purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). STZ and
nicotinamide (NA) were purchased from Sigma-Aldrich; Merck
KGaA.
Western blot analysis
Cells from each group were collected following
exposure to high-glucose conditions. Total protein was extracted
using whole-cell lysis buffer (Beyotime Institute of
Biotechnology). In addition, the subcutaneous tumors were cut into
small pieces, weighed, homogenized and then lysed with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology; 100 μl/10 mg) for 30 min on ice. The lysate
was transferred into a 1.5 ml centrifuge tube and centrifuged at
16,099 × g for 5 min at 4°C. Protein concentrations were evaluated
using the BCA protein assay kit (Beyotime Institute of
Biotechnology). Proteins (20 μg total protein/well) were
separated by 8-10% SDS-PAGE and were then transferred onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were then incubated at room temperature for 2 h
in a blocking solution; 5% bovine serum albumin (0332; Amresco LLC,
Solon, OH, USA) in Tris-buffered saline containing 0.1% Tween-20
(TBST) was used when phosphorylated proteins were to be detected,
whereas 5% non-fat milk in TBST was used when unphosphorylated
proteins were to be detected. Once the membranes had been washed in
TBST, they were incubated with primary antibodies overnight at 4°C.
The following primary antibodies (dilutions, 1:1,000) were used:
AKT (#4691T), phosphory-lated (p)-AKT (ser473) (#4060T), mTOR
(#2983P), p-mTOR (ser2448) (#5536T), PDK1 (#3062T), c-Myc (#5605T),
LDHA (#3558S), B-cell lymphoma 2-associated X protein (Bax)
(#14796S) and cleaved caspase-3 (#9664T) (all from Cell Signaling
Technology, Inc., Danvers, MA, USA), ART1 (A10103; ABclonal, Inc.,
Woburn, MA, USA) and β-actin (bsm-33036M; BIOSS, Beijing, China).
The membranes were washed three times with TBST, and were then
incubated with secondary antibodies [peroxidase-conjugated goat
anti-rabbit immunoglobulin (Ig)G (1:2,000, AS014; ABclonal, Inc.)
or peroxidase-conjugated goat anti-mouse IgG (1:2,000, AS003,
ABclonal, Inc.) for 2 h at room temperature. Subsequently, the
membranes were washed with TBST three times. Blots were visualized
and images were captured using a ChemiDoc XRS system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and chemiluminescence
(BeyoECL Plus; Beyotime Institute of Biotechnology). All
experiments were repeated in triplicate.
Cell survival assay
ART1-cDNA CT26 cells were seeded in 96-well plates
at a density of 1×104 cells/well. After 24 h, the cells
were exposed to increasing concentrations of BCP (0, 5, 10, 20 and
50 μM) and a high concentration of glucose (25 mM) for 24,
48 and 72 h. Cell Counting Kit-8 (CCK-8) (7sea Biotech, Shanghai,
China) reagent (10 μl) was added to each plate and the cells
were incubated for 1 h at 37°C. The absorbance was recorded using a
microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) at
an optical density (OD) of 450 nm. The percentage of inhibition was
calculated using the following formula: [1 − (mean OD of
experimental group − mean OD of blank control group) / (mean OD of
control group − mean OD of blank control group)] × 100%. The
percentage of proliferation was calculated using the formula:
[(mean OD of experimental group − mean OD of control group) / mean
OD of control group] × 100%.
ATP content assay
To measure glycolysis-mediated production of ATP,
cells were cultured in a 6-well plate and treated with a high
concentration of glucose (25 mM) and BCP (50 μM) for 48 h.
Cellular ATP levels were measured using a firefly luciferase-based
ATP assay kit (cat. no. S0026; Beyotime Institute of
Biotechnology). Briefly, protein was suspended in standard reaction
buffer containing luciferin and luciferase, and was then assessed
according to the manufacturer's protocol. Luminescence was measured
using a Glomax® Multi Detection system (Promega
Corporation, Madison, WI, USA).
Lactic acid measurement
ART1-cDNA CT26 cells in a 6-well plate were
incubated with or without BCP (50 μM) under high-glucose (25
mM) conditions at 37°C and 5% CO2 for 48 h. The
supernatant was then collected into Eppendorf tubes and centrifuged
at 111.8 × g for 5 min. The amount of lactic acid in the
supernatant was determined using a lactic acid test kit (A019-2;
Nanjing Jiancheng Bioengineering Institute), according to the
manufacturer's protocol.
Staining with Hoechst 33258
ART1-cDNA CT26 cells were cultured in 6-well plates
(1×105 cells/well) under high-glucose conditions (25 mM)
in the presence or absence of BCP (50 μM) at 37°C and 5%
CO2 for 48 h. After removal of the culture medium, the
cells were fixed 4% paraformaldehyde (0.5 ml) for 10 min and washed
twice with PBS. After treatment with Hoechst 33258 (0.5 ml) for 5
min in the dark, stained nuclei were observed under a fluorescence
microscope (ZOE™ Fluorescent Cell Imager; Bio-Rad Laboratories,
Inc.). The apoptotic ratio (AR) was calculated using the following
formula: AR = [apoptotic cells / total cell count] × l00%.
Flow cytometry
ART1-cDNA CT26 cells were seeded in 6-well plates
(1×105 cells/well) and were cultured with a high
concentration of glucose (25 mM) in the presence of absence of BCP
(50 μM) at 37°C. After being cultured for 48 h, suspended
and adherent cells were collected by centrifugation (111.8 × g for
5 min), washed twice with PBS and resuspended in binding buffer
(70% ethanol) prior to centrifugation at 111.8 × g for 5 min.
Subsequently, the collected cells were treated with RNase A (10
g/ml; cat. no. 2158; Takara Biotechnology Co., Ltd., Dalian, China)
for 1 h at 37°C and were then treated with 50 mg/ml propidium
iodide (PI; cat.no. P1304MP; Thermo Fisher Scientific, Inc.)
overnight at 4°C before flow cytometry (FACSVantage SE; BD
Biosciences, Franklin Lakes, NJ, USA). The proliferation index (PI)
was measured according to the following formula: PI = (G2 + S) /
(G1 + S + G2). Apoptosis was evaluated using the Annexin
V-fluorescein isothiocyanate Apoptosis Detection kit (C1062;
Beyotime Institute of Biotechnology), according to the
manufacturer's protocol for flow cytometry (FACSVantage SE; BD
Biosciences). The apoptotic percentage was determined as follows:
Apoptotic rate (%) = [apoptotic cells (A)/total cell count (T)] ×
100.
Establishment of the animal model
The present study was approved by the ethics
committee of Chongqing Medical University (Chongqing, China). Adult
male Balb/c mice (n=48; weight, 25±2 g; age, 6-8 weeks) were
obtained from the Experimental Animal Center of Chongqing Medical
University. They were maintained under optimal conditions (12-h
light/dark cycle; humidity, 60±5%; temperature, 22±3°C; free access
to food and water). Mice were injected intraperitoneally (i.p.)
with STZ (STZ in 0.1 M citric acid buffer, pH 4.5; 100 mg/kg on
days 1 and 3) after being fasted for 16 h (with free access to
water). NA in saline (120 mg/kg) was injected i.p. 15 min prior to
the administration of STZ (22).
The weight of each mouse and the concentration of glucose in the
blood were measured to ensure that the diabetic mouse model was
properly established. Subsequently, CT26 cells were harvested from
each of the four cell groups; a 200-μl single cell
suspension containing 2×106 cells was injected into the
right forelimb of diabetic Balb/c mice to establish the CT26
transplanted tumor model for 14 days in diabetic Balb/c mice. In
addition, BCP was dissolved in corn oil and administered
intragastrically (200 mg/kg body weight/day) (18). In the ART1-cDNA + BCP group,
diabetic Balb/c mice, which had been inoculated with ART1-cDNA CT26
cells for 10 days, were treated with BCP. The same dose of corn oil
was administered intragastrically to diabetic Balb/c mice
inoculated with ART1-cDNA CT26 cells to generate the control group.
Tumor volume was computed from diameters measured using a digital
caliper, using the following formula: V = ab2 / 2, where
a refers to the maximum diameter and b to the minimum diameter.
Following sacrifice of the mice, all tumors were obtained and used
for western blotting.
Statistical analysis
All experiments were conducted at least three times.
Data were analyzed using SPSS 18.0 software (SPSS, Chicago, IL,
USA). Data are presented as the means ± standard deviation.
Statistical analysis was performed using Student's t-test or
one-way analysis of variance followed by the least significant
difference test, which was used to analyze the ranked data and the
differences between each group. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of ART1 on the generation of ATP
and lactic acid in CT26 cells
Lactic acid and ATP concentrations were detected in
the four cell groups under high-glucose conditions, in order to
investigate whether ART1 regulates aerobic glycolysis through the
generation of ATP and lactic acid. Compared with in the
untransfected and LV-control groups, lactic acid and ATP
concentrations were significantly increased in the ART1-cDNA group,
whereas those in the ART1-shRNA group were decreased (Fig. 1A and B).
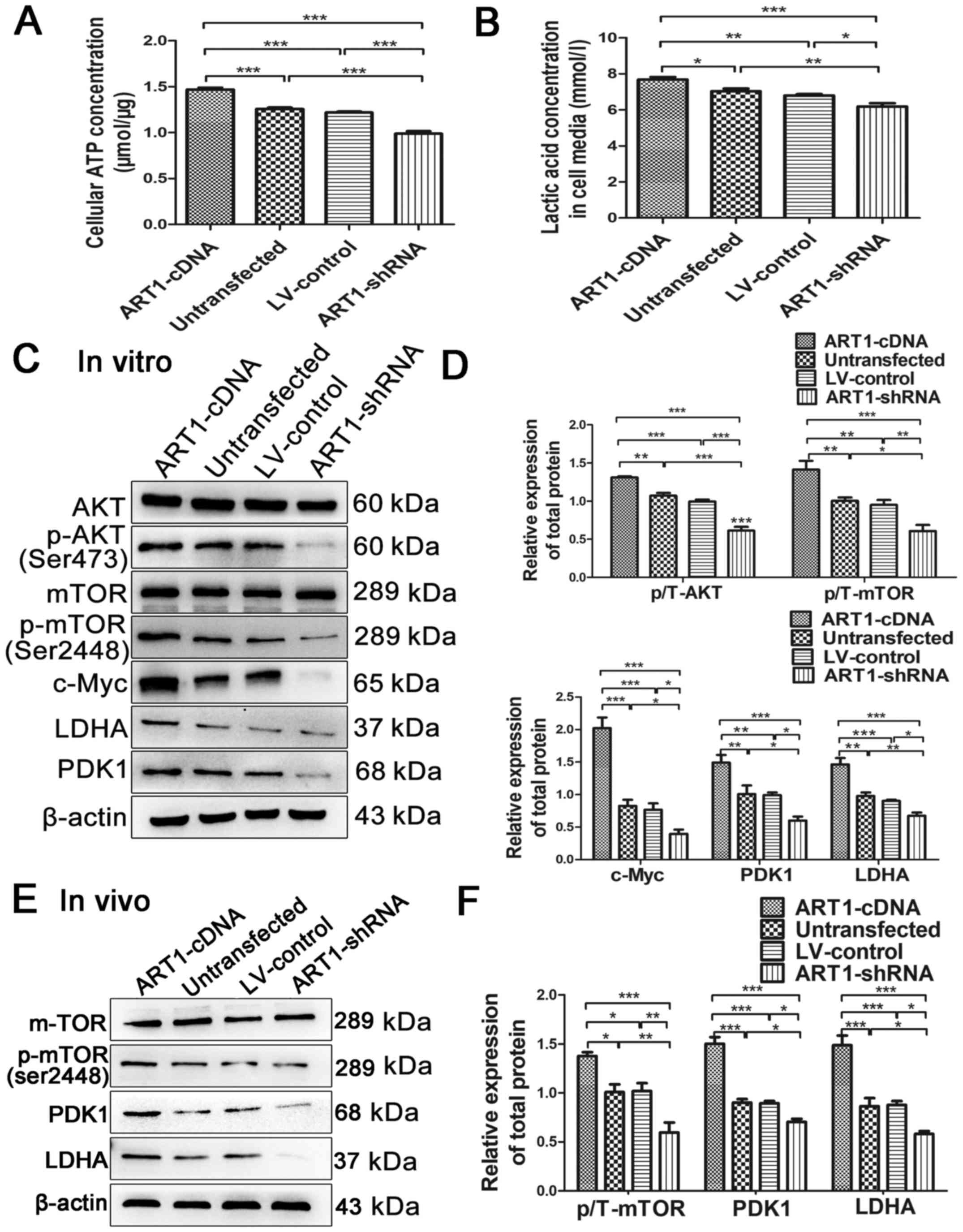 | Figure 1ART1 regulates the expression levels
of glycolytic enzymes via AKT/mTOR. (A and B) Effects of ART1 on
ATP and lactic acid concentrations were detected. (C and D) Effects
of ART1 on the expression levels of AKT, p-AKT, mTOR, p-mTOR,
c-Myc, LDHA and PDK1 in the ART1-cDNA, ART1-shRNA, LV-control and
untransfected CT26 cells treated with high glucose (25 mM) for 48
h. (E and F) Effects of ART1 on the expression levels of mTOR,
p-mTOR, LDHA and PDK1 in ART1-cDNA, ART1-shRNA, LV-control and
untransfected CT26 cell tumors from diabetic Balb/c mice.
*P<0.05, **P<0.01,
***P<0.001. AKT, protein kinase B; ART1,
arginine-specific mono-ADP-ribosyltransferase 1; ATP, adenosine
triphosphate; LDHA, lactate dehydrogenase; LV, lentivirus; mTOR,
mammalian target of rapamycin; p-, phosphorylated; PDK1, pyruvate
dehydrogenase kinase 1; shRNA, short hairpin RNA. |
Effects of ART1 on the expression of AKT,
p-AKT, mTOR, p-mTOR, c-Myc, PDK1 and LDHA in vitro and in vivo
Each group was treated with high glucose for 48 h,
after which the expression levels of AKT, p-AKT, mTOR and p-mTOR
were measured by western blotting. Compared with in the
untransfected and LV-control groups, the expression levels of
p-AKT/AKT and p-mTOR/mTOR were increased in the ART1-cDNA group;
however, they were decreased in the ART1-shRNA group (P<0.01;
Fig. 1C and D). The expression
levels of c-Myc, PDK1 and LDHA were also significantly increased in
the ART1-cDNA group and decreased in the ART1-shRNA group
(P<0.05), indicating that ART1 may induce activation of c-Myc
signaling cascades, thus affecting PDK1 and LDHA expression
(Fig. 1C and D).
To confirm whether alterations in ART1 may affect
the expression levels of mTOR, p-mTOR, PDK1 and LDHA, the
expression levels of these proteins were detected in CT26 cell
allograft transplanted tumors in diabetic Balb/c mice. The results
demonstrated that the expression levels of p-mTOR, PDK1 and LDHA in
the ART1-cDNA CT26 group were increased, whereas the expression
levels of p-mTOR, PDK1 and LDHA were decreased in the ART1-shRNA
group, as compared with in the untransfected and LV-control groups
(P<0.05; Fig. 1E and F). The
expression levels of mTOR exhibited no significant difference in
the four groups (P>0.05; Fig. 1E
and F). These findings suggested that ART1 may impact
glycolysis though regulating the AKT/mTOR molecular signal.
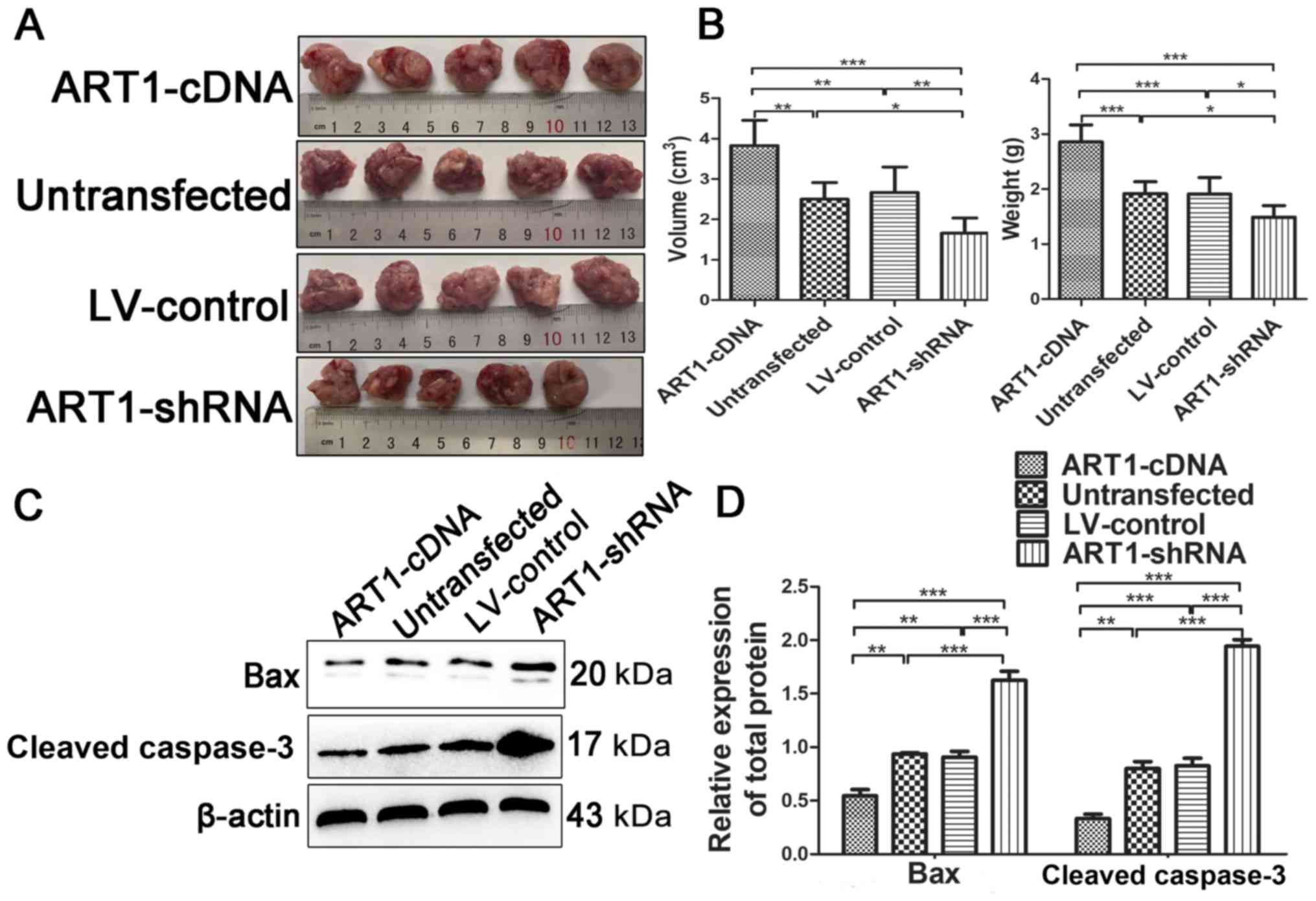
Effects of ART1 on the growth of CT26
cell allograft transplanted tumors in diabetic Balb/c mice
A total of 14 days after the CT26 cells were
inoculated into diabetic Balb/c mice, tumor weight and volume were
measured in each group. Compared with in the untransfected and
LV-control groups, tumor weight and volume were significantly
increased in the ART1-cDNA group and decreased in the ART1-shRNA
group (P<0.01; Fig. 2A and B).
Furthermore, the expression levels of Bax and cleaved caspase-3,
which are indicators of apoptosis, were decreased in the tumor
tissues from the ART1-cDNA group and increased in the ART1-shRNA
group, as compared with in the LV-control and untransfected groups
(P<0.001 Fig. 2C and D). These
findings suggested that overexpression of ART1 may upregulate the
growth and diminish cellular apoptosis of CT26 cell tumors in
diabetic mice.
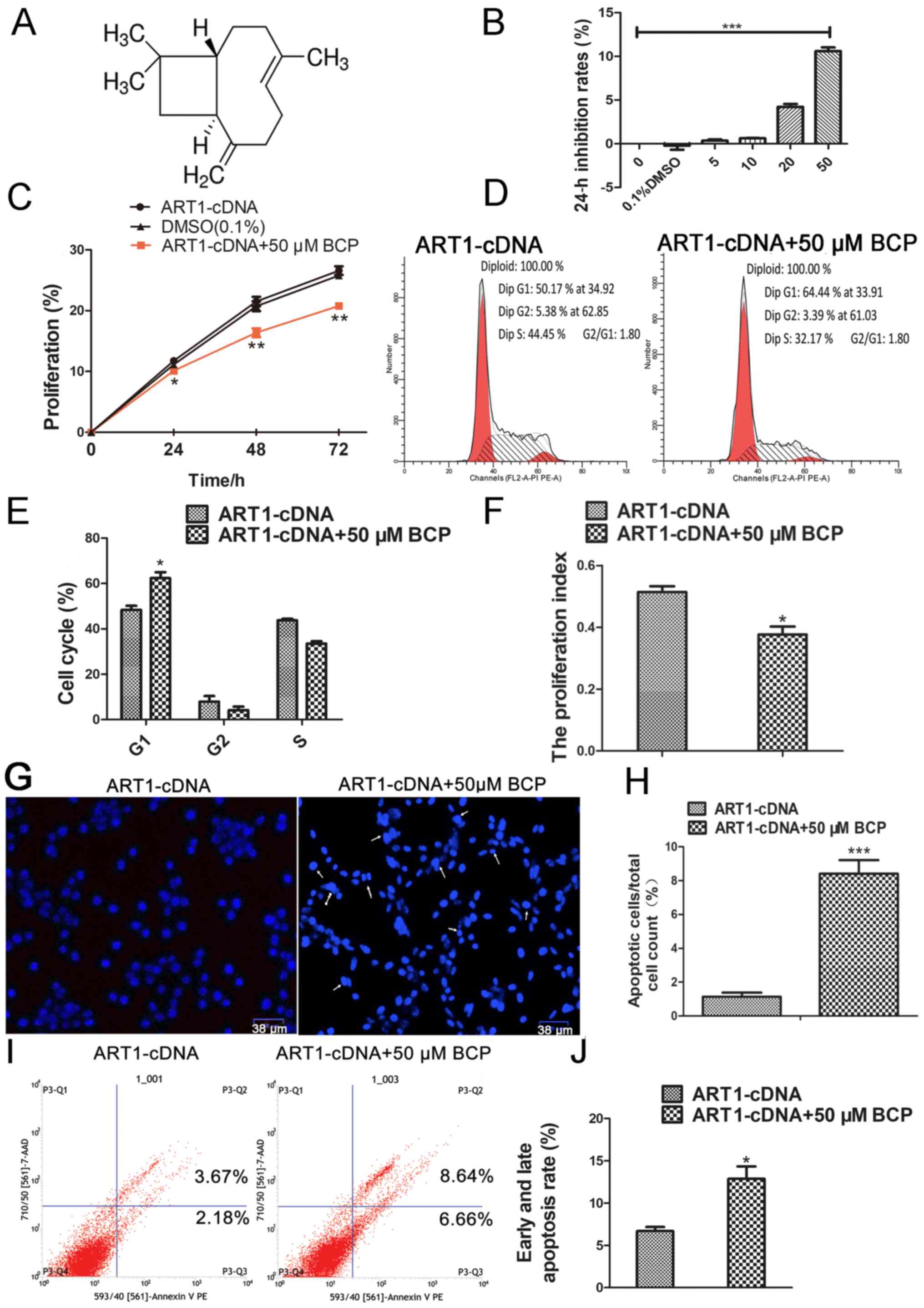
Effects of BCP on the proliferation and
apoptosis of ART1-cDNA CT26 cells
In order to investigate the effects of BCP (Fig. 3A) on the proliferation of ART1-cDNA
CT26 cells, a CCK-8 assay was used to measure cellular viability.
The results demonstrated that the inhibitory rate of ART1-cDNA CT26
cell proliferation was increased when cells were cultured with BCP
(50 μM) for 24 h (P<0.001; Fig. 3B). To further analyze this effect,
ART1-cDNA CT26 cells cultured in high-glucose (25 mM) were treated
with BCP (50 μM) for 48 h, in order to detect the effects of
BCP on cell proliferation, cell cycle progression and apoptosis.
The proliferative capacity of ART1-cDNA CT26 cells cultured with
BCP was decreased compared with the ART1-cDNA CT26 cells cultured
without BCP (P<0.05 Fig. 3C).
Flow cytometry indicated that the proportion of ART1-cDNA CT26
cells treated with BCP in G1 phase was increased and the PI was
decreased compared with the ART1-cDNA CT26 cells cultured without
BCP (P<0.05; Fig. 3D–F).
Staining with Hoechst 33342 demonstrated that the number of
apoptotic bodies was increased in ART1-cDNA CT26 cells treated with
BCP compared with in the absence of BCP (P<0.001; Fig. 3G and H). The rate of apoptosis was
further analyzed by flow cytometric analysis; the apoptotic rate in
ART1-cDNA CT26 cells treated with BCP (12.88±2.53%) was higher than
in ART1-cDNA CT26 cells not treated with BCP (6.7±0.845%)
(P<0.05; Fig. 3I and J).
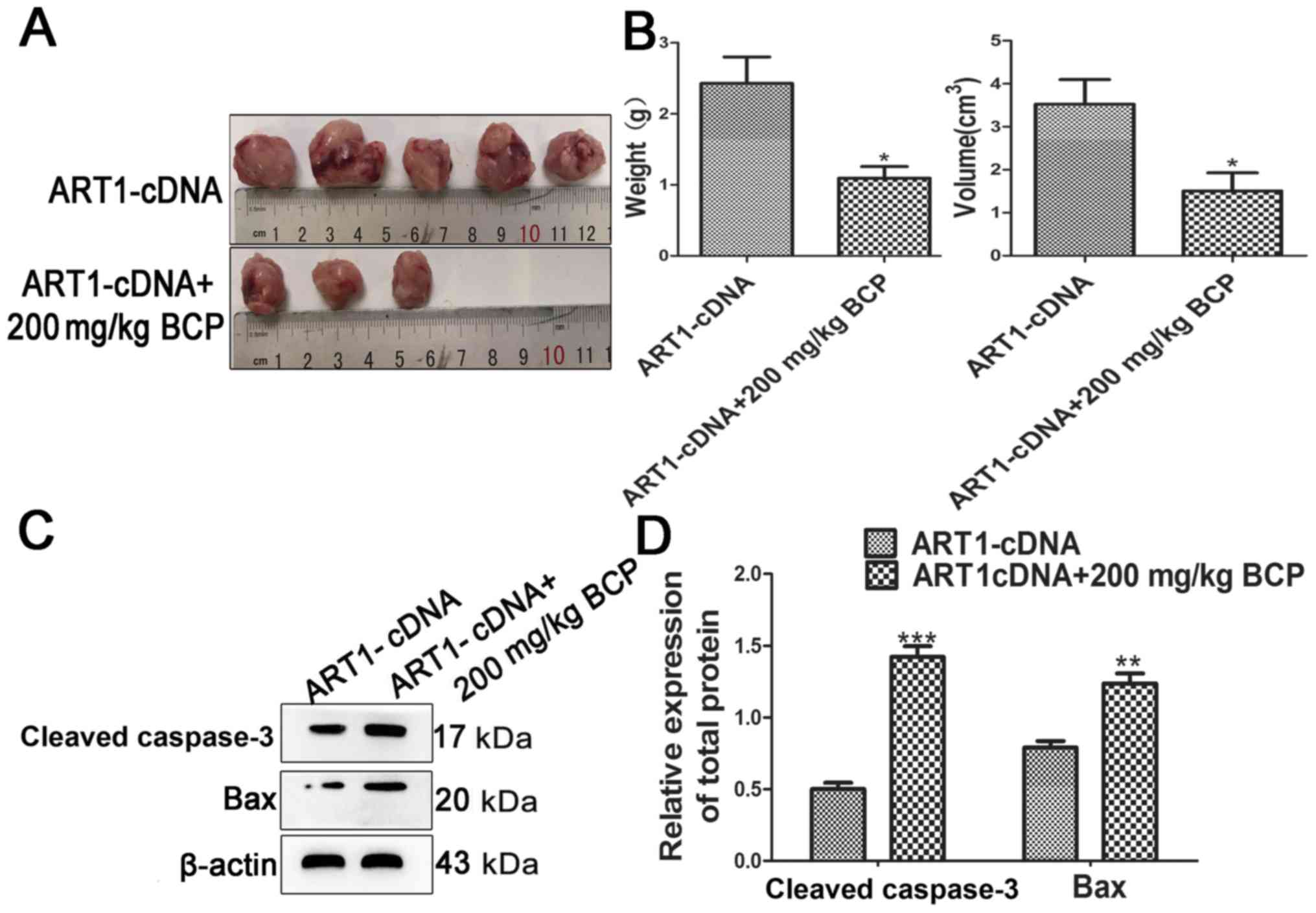
Effects of BCP on the growth of ART1-cDNA
CT26 cell allograft transplanted tumors in diabetic Balb/c
mice
BCP was dissolved in corn oil and administered
intragastrically (200 mg/kg b.w.). In the ART1-cDNA + BCP group,
diabetic Balb/c mice, which were inoculated with ART1-cDNA CT26
cells for 10 days, were treated with BCP (200 mg/kg b.w.). In the
control group, the same dose of corn oil was administered
intragastrically to diabetic Balb/c mice, which were inoculated
with ART1-cDNA CT26 cells. Tumor weight and volume were
significantly decreased in the ART1-cDNA + BCP group compared with
in the control group (P<0.05; Fig.
4A and B). Furthermore, the expression levels of
apoptosis-associated proteins (Bax and cleaved caspase-3) were
increased in the ART1-cDNA + BCP group compared with in the control
group (Fig. 4C and D). These
findings indicated that BCP may inhibit the growth and enhance the
apoptotic effects of ART1 overexpression on transplanted tumors in
diabetic mice.
Effects of BCP on the generation of ATP
and lactic acid in ART1-cDNA CT26 cells
The intracellular ATP content and lactic acid
content in the supernatant of ART1-cDNA CT26 cells treated with
high glucose and BCP were assessed, in order to investigate the
effects of BCP on aerobic glycolysis. Compared with in the
untreated ART1-cDNA CT26 cells, the concentrations of ATP and
lactic acid were decreased in the ART1-cDNA + BCP group (Fig. 5A and B).
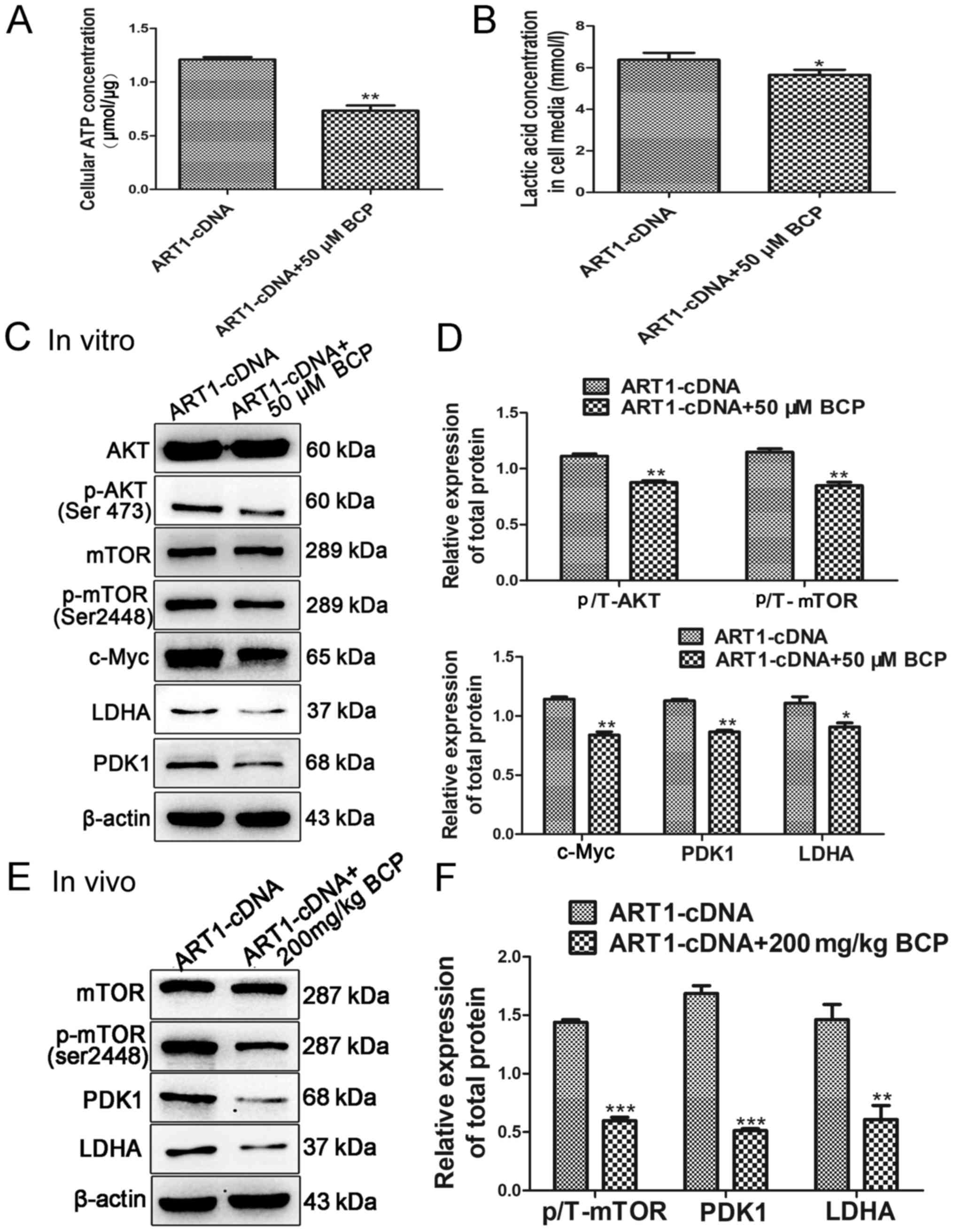 | Figure 5BCP regulates glycolysis through
inhibiting the AKT/mTOR pathway. (A and B) BCP reduced the
concentrations of ATP and lactic acid in ART1-cDNA CT26 cells. (C
and D) Western blot analysis detected the protein expression levels
of p-AKT, p-mTOR, c-Myc, PDK1 and LDHA, which were downregulated in
ART1-cDNA CT26 cells treated with BCP. The protein expression
levels of AKT and mTOR were not significantly different between the
two groups. (E and F) Protein expression levels of p-mTOR, PDK1 and
LDHA were also reduced in ART1-cDNA CT26 cell tumors from diabetic
Balb/c mice treated with BCP. The protein expression level of mTOR
exhibited no significant difference between the two groups.
*P<0.05, **P<0.01,
***P<0.001, vs. ART1-cDNA group. AKT, protein kinase
B; ART1, arginine-specific mono-ADP-ribosyltransferase 1; ATP,
adenosine triphosphate; BCP, β-caryophyllene; LDHA, lactate
dehydrogenase; mTOR, mammalian target of rapamycin; p-,
phosphorylated; PDK1, pyruvate dehydrogenase kinase 1. |
Effects of BCP on the expression levels
of AKT/mTOR, c-Myc, PDK1 and LDHA in vivo and in vitro
The expression levels of p-AKT, p-mTOR, c-Myc, PDK1
and LDHA were all decreased in ART1-cDNA CT26 cells treated with
BCP under high-glucose conditions compared with in ART1-cDNA CT26
cells cultured without BCP (P<0.05; Fig. 5C and D). Conversely, the expression
levels of AKT and mTOR exhibited no significant difference between
the groups (P>0.05; Fig. 5C and
D). Furthermore, the expression levels of p-mTOR, PDK1 and LDHA
were decreased in ART1-cDNA CT26 cell tumors obtained from mice
treated with BCP compared with in ART1-cDNA CT26 cell tumors
obtained from untreated mice (P<0.01; Fig. 5E and F). These findings indicated
that BCP may inhibit the AKT/mTOR pathway to downregulate the
levels of PDK1 and LDHA in CT26 CRC cells overexpressing ART1.
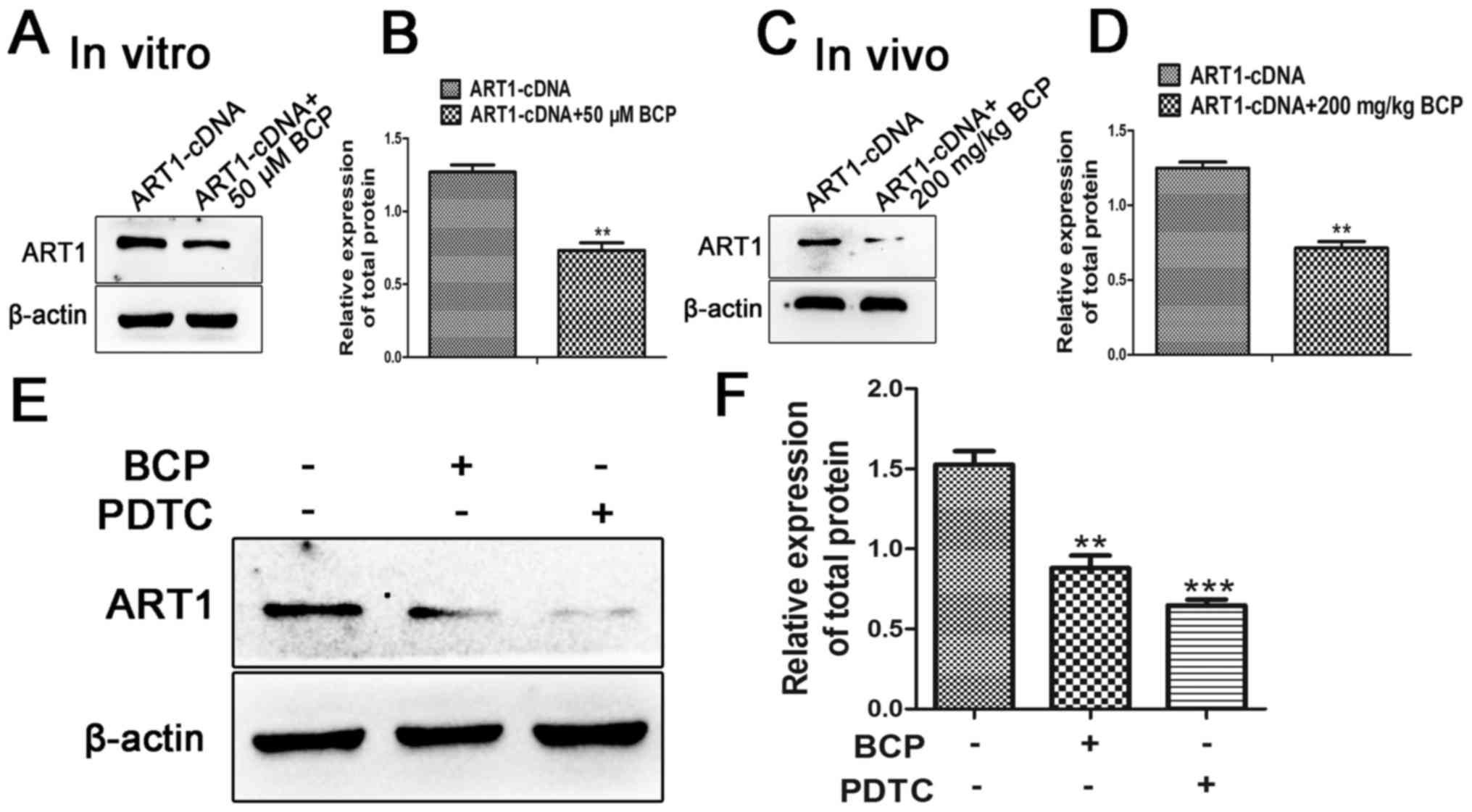
BCP and PDTC inhibit the expression of
ART1 in CT26 cells
The present study demonstrated that the expression
levels of ART1 were inhibited when ART1-cDNA CT26 cells were
treated with BCP in vitro and in vivo (P<0.01;
Fig. 6A–D); however, the
underlying mechanism was unclear. It was therefore hypothesized
that BCP may inhibit the expression of ART1 through regulating
NF-κB; however, to the best of our knowledge, there are no
indications as to whether NF-κB can control the expression of ART1.
To elucidate whether the NF-κB pathways were involved in regulating
the expression of ART1, ART1-cDNA CT26 cells were treated with the
NF-κB inhibitor, PDTC. The results demonstrated that PDTC and BCP
could downregulate the expression of ART1 (P<0.01; Fig. 6E and F).
Discussion
Previous studies have demonstrated that T2DM
increases the risk of CRC (1,23,24).
A previous meta-analysis confirmed that T2DM has a positive
correlation with the risk of CRC (1). Hyperglycemia is one of the key
mechanisms connecting the potential risk of cancer with diabetes
(25); hyperglycemia is often
associated with increases in the risk of several types of cancer
(26,27). A previous study revealed that only
~27% of patients with CRC had normal concentrations of glucose in
the blood. Furthermore, compared with in patients with normal blood
glucose levels, patients with high blood glucose levels have larger
tumor diameters, more poorly differentiated and aggressive tumors,
and present with advanced Tumor Node Metastasis stage tumors
(28). However, the mechanism
underlying how high blood glucose levels contribute to the
development of CRC is currently unclear. Our previous studies
demonstrated that ART1 may promote the proliferation, invasion and
metastasis of CRC, and inhibit the apoptosis of CRC in vitro
and in vivo (9,29). In addition, it was observed that
the expression of ART1 in human CRC tissue from patients with T2DM
is much higher than that in non-diabetic CRC tissue (Chen et
al, unpublished data). The present study demonstrated that
overexpression of ART1 may increase the growth of transplanted CT26
tumors in STZ-induced diabetic Balb/c mice. To confirm the effects
of ART1 on aerobic glycolysis in CRC cells, the study demonstrated
that ART1 could upregulate lactic acid and ATP concentrations in
CT26 cells, which were cultured in a high-glucose environment.
Lactic acid is a specific product of glycolysis. In addition,
glucose can be oxidized to produce ATP under aerobic conditions,
whereas, under anoxic conditions, ATP can be generated by
glycolysis (14); therefore, both
lactic acid and ATP may reveal the level of glycolysis (30). The present study indicated that
ART1 may be considered a regulatory factor of glycolysis in
CRC.
In our previous study, ART1 was revealed to
upregulate the expression of p-AKT, c-Myc and c-Fos, in order to
promote the proliferation and invasive potential of CT26 cells
(9); ART1 also inhibits the
apoptosis of CT26 cells via the PI3K/AKT pathway (29). A previous study demonstrated that
the PI3K/AKT pathway serves a critical role in promoting aerobic
glycolysis (31). AKT has also
been reported to protect cancer cells from bioenergetic stress by
enhancing uptake of glucose and storage of glycogen, which has a
critical role in promoting the generation of ATP (32). The PI3K/AKT/mTOR pathway is also
upregulated in human diabetic nephropathy (33). Whether or not ART1 promotes
glycolysis via the PI3K/AKT/mTOR pathway is currently unclear. The
present study indicated that the expression levels of p-AKT and
p-mTOR were increased in the ART1 overexpression group, whereas
p-AKT and p-mTOR expression levels were decreased when ART1 was
downregulated. PDK1 is a key enzyme that negatively regulates the
activity of the pyruvate dehydrogenase complex through
phosphorylation (34). PDK1 is
directly transactivated by HIF-1α, and HIF-1α is also involved in
dysregulating c-Myc to promote glycolysis (35). HIF-1α, which is a downstream target
of mTOR, is a major transcription factor that controls cellular
adaptation to hypoxia and promotes glycolytic metabolism (36). Therefore, modulation of mTOR may
regulate the expression of PDK1. LDHA catalyzes the last step of
anaerobic glycolysis, and is abnormally expressed in numerous types
of human cancer (37,38). Furthermore, c-Myc activates the
expression of LDHA and increases production of lactate (12); it also increases the uptake of
glucose by regulating LDHA and glucose transporter 1 expression
(15,39). In the present study, overexpression
of ART1 increased the expression levels of c-Myc, PDK1 and LDHA in
CT26 cells in vitro and in CT26 cell tumors in vitro,
whereas the opposite effect was detected when ART1 expression was
silenced. Above all, ART1 may regulate glycolysis in CRC through
activating the AKT/mTOR pathway to upregulate the expression of
c-Myc, PDK1 and LDHA.
The proliferation of MC38 colon cancer cells has
been reported to be elevated and their apoptosis diminished in a
T2DM environment (8). Another
study observed that local IGF-1 and IGF-1R mRNA are overexpressed
in T2DM patients with CRC. IGF-1 activates signaling via the
PI3K/AKT/mTOR and RAS/RAF/MAPK pathways that enhance the
proliferation and survival of colorectal cancer cells (40). The metabolic switch to aerobic
glycolysis in cancer cells involves mTOR-mediated expression of
glycolytic enzymes through the activation of HIF-1α, NF-κB and
c-Myc (41,42). The present study revealed that ART1
could upregulate glycolysis in CRC through activating the AKT/mTOR
pathway, thus enhancing the proliferative ability and weakening the
apoptosis of CT26 cells. Therefore, it is important to identify an
effective and accessible drug that inhibits the AKT/mTOR pathway
mediated by ART1, in order to regulate the levels of glycolysis in
CRC.
BCP is a natural bicyclic sesquiterpene, which is
found in large quantities in essential oils from cloves, cinnamon,
black pepper and cannabis (18,43).
A previous report indicated that oral administration of BCP for 45
days decreases the concentration of glucose in the blood and
increases plasma insulin; it has been reported to be more effective
than gliben-clamide (18).
Therefore, in the present study, BCP was added to CT26 cells, which
overexpressed ART1 under high glucose conditions, and to CT26 cell
tumors in diabetic Balb/c mice. The results demonstrated that the
concentrations of ATP and lactic acid were decreased following
treatment with BCP. It was therefore suggested that BCP may affect
glycolysis in ART1-overexpressing CT26 cells. Cannabinoid
receptors, CB1 and CB2, are G-protein-coupled receptors and the
main components of the endocannabinoid system (44). These receptors have important roles
in the maintenance of the balance in energy and metabolism. BCP
exclusively binds to CB2 and stimulates MAPK and PI3K signaling
pathways, which in turn can activate proapoptotic proteins and
inactivate anti-apoptotic proteins (17,45).
The present study revealed that BCP inhibited the expression of
p-AKT, p-mTOR, c-Myc, PDK1 and LDHA in ART1-overexpressing CT26
cells and tumors. In CRC and pancreatic cancer cells, BCP inhibits
their growth (16). Furthermore,
the present study revealed that following treatment of
ART1-overexpressing CT26 cells and tumors with BCP, proliferation
was reduced and apoptosis was enhanced. Therefore, it may be
considered that BCP exerts a useful action on inhibiting
ART1-induced glycolysis via the AKT/mTOR pathway, which may inhibit
the proliferation and enhance the apoptosis of CRC cells.
The present study also aimed to determine how BCP
affects ART1-induced glycolysis via the AKT/mTOR pathway. Notably,
the expression of ART1 was diminished in ART1-overexpressing CT26
cells following treatment with BCP. It is well known that AKT
regulates NF-κB-dependent gene transcription and upregulates the
activity of the cAMP response element binding protein CR1/2, which
is necessary for the transcription of anti-apoptotic genes
(45). In addition, it has been
suggested that BCP binds to CB2 to regulate the PI3K/AKT/NF-κB
pathway (44). In our previous
study, it was observed that NF-κB is inhibited by poly (ADP-ribose)
polymerase 1 (PARP1) and NF-κB was revealed to inhibit PARP1 by
negative feedback in LoVo cells (46). It has also been observed that ART1
inhibits the expression of PARP1 in CT26 cells (19). Some researchers consider
mono-ADP-ribosylation to be the basis of poly-(ADP-ribosy)lation;
the addition of more than one ADP-ribose units to a
mono-ADP-ribosylated molecule may result in the initiation of
poly-(ADP-ribosy)lated modification (47,48).
ART1 and PARP1, which are catalyzing enzymes of
mono-ADP-ribosylation and poly-ADP-ribosylation respectively, are
closely connected in CRC. Therefore, it was hypothesized that NF-κB
may also regulate the expression of ART1; to the best of our
knowledge, no previous study has reported this. To explore this,
PDTC (an inhibitor of NF-κB) was added to ART1-overexpressing CT26
cells; the expression levels of ART1 were decreased following
treatment with PDTC. Therefore, inhibition of NF-κB may
downregulate the expression of ART1. These findings suggested that
BCP may inhibit ART1-induced glycolysis via the AKT/mTOR pathway,
possibly through the downregulation of NF-κB, which may decrease
the expression of ART1. However, exactly how NF-κB regulates the
expression of ART1 requires further study.
In conclusion, the present study revealed that, in
CRC cells, ART1 may increase the generation of ATP and lactic acid
by upregulating the AKT/mTOR/c-Myc pathway, thus increasing the
expression of PKD1 and LDHA, and affecting the proliferation and
apoptosis of CT26 cells under high-glucose conditions. Conversely,
the plant product BCP inhibited ART1-induced glycolysis through the
AKT/mTOR pathway, which could suppress the proliferation and
enhance the apoptosis of CRC cells. Therefore, ART1 may have an
important role in glycolysis in CRC, and is proposed as a
therapeutic target for the treatment of diabetic patients with CRC.
Furthermore, BCP may be considered an important lead for the
identification of novel drugs for the treatment of diabetic
patients with CRC.
Acknowledgments
The authors would like to thank Professor YQ Wei
(Sichuan University, Chengdu, China) for his gift of the CT26 cell
line. Sincere thanks to Professor Michael D. Threadgill (Department
of Pharmacy and Pharmacology, University of Bath) who performed
language editing to improve language skills of the article.
Abbreviations:
|
ART1
|
arginine-specific
mono-ADP-ribosyltrans-ferase 1
|
|
ATP
|
adenosine triphosphate
|
|
BCP
|
β-caryophyllene
|
|
CB2
|
cannabinoid receptor 2
|
|
CRC
|
colorectal cancer
|
|
HIF-1α
|
hypoxia-inducible factor 1α
|
|
IGF1
|
insulin-like growth factor 1
|
|
LDHA
|
lactate dehydrogenase A
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NA
|
nicotinamide
|
|
p-AKT
|
phosphorylated-AKT
|
|
PDK1
|
pyruvate dehydrogenase kinase 1
|
|
PDTC
|
pyrrolidinedithiocarbamic acid
|
|
T2DM
|
type 2 diabetes mellitus
|
|
STZ
|
streptozocin
|
Funding
The present study was supported by the Innovation
Project of Graduate Student in Chongqing (grant no. CYS16120); the
Scientific Research Foundation of Chongqing Medical University
(grant no. 201413); the National Nature Science Foundation of China
(grant no. 30870946); the Science and Technology Plan Project of
Yuzhong District in Chongqing (grant no. 20140106); and the
National High Technology Research and Development Program of China
(grant no. 2012AA02A201).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author contributions
LZ performed experiments, analyzed the data and
wrote the manuscript. MLZ helped to construct the animal model and
participated in the experiment to detect protein expression levels
in CT26 cells. YT conducted several experiments and edited the
manuscript. YLW designed and conducted the experiments. MX designed
and modified the figures. ML analyzed and interpreted the data. QSL
performed the Hoechst 33258 staining. LY performed the flow
cytometric analysis. XL detected the concentrations of lactic acid
and ATP. WWC participated in the construction of the transplanted
tumor model in diabetic Balb/c mice.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Chongqing Medical University (Chongqing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guraya SY: Association of type 2 diabetes
mellitus and the risk of colorectal cancer: A meta-analysis and
systematic review. World J Gastroenterol. 21:6026–6031. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu F, Guo Y, Wang H, Feng J, Jin Z, Chen
Q, Liu Y and He J: Type 2 diabetes mellitus and risk of colorectal
adenoma: A meta-analysis of observational studies. BMC Cancer.
16:6422016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu HL, Fang H, Xu WH, Qin GY, Yan YJ, Yao
BD, Zhao NQ, Liu YN, Zhang F, Li WX, et al: Cancer incidence in
patients with type 2 diabetes mellitus: A population-based cohort
study in Shanghai. BMC Cancer. 15:8522015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mills KT, Bellows CF, Hoffman AE, Kelly TN
and Gagliardi G: Diabetes mellitus and colorectal cancer prognosis:
A meta-analysis. Dis Colon Rectum. 56:1304–1319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duan W, Shen X, Lei J, Xu Q, Yu Y, Li R,
Wu E and Ma Q: Hyperglycemia, a neglected factor during cancer
progression. BioMed Res Int. 2014:4619172014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masur K, Vetter C, Hinz A, Tomas N,
Henrich H, Niggemann B and Zänker KS: Diabetogenic glucose and
insulin concentrations modulate transcriptome and protein levels
involved in tumour cell migration, adhesion and proliferation. Br J
Cancer. 104:345–352. 2011. View Article : Google Scholar
|
|
7
|
Shin HY, Jung KJ, Linton JA and Jee SH:
Association between fasting serum glucose levels and incidence of
colorectal cancer in Korean men: The Korean Cancer Prevention
Study-II. Metabolism. 63:1250–1256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teng JA, Wu SG, Chen JX, Li Q, Peng F, Zhu
Z, Qin J and He ZY: The activation of ERK1/2 and JNK MAPK signaling
by insulin/IGF-1 is responsible for the development of colon cancer
with type 2 diabetes mellitus. PLoS One. 11:e01498222016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu JX, Xiong W, Zeng Z, Tang Y, Wang YL,
Xiao M, Li M, Li QS, Song GL and Kuang J: Effect of ART1 on the
proliferation and migration of mouse colon carcinoma CT26 cells in
vivo. Mol Med Rep. 15:1222–1228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song GL, Jin CC, Zhao W, Tang Y, Wang YL,
Li M, Xiao M, Li X, Li QS, Lin X, et al: Regulation of the
RhoA/ROCK/AKT/β-catenin pathway by arginine-specific
ADP-ribosytransferases 1 promotes migration and
epithelial-mesenchymal transition in colon carcinoma. Int J Oncol.
49:646–656. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang L, Xiao M, Li X, Tang Y and Wang YL:
Arginine ADP-ribosyltransferase 1 promotes angiogenesis in
colorectal cancer via the PI3K/Akt pathway. Int J Mol Med.
37:734–742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He TL, Zhang YJ, Jiang H, Li XH, Zhu H and
Zheng KL: The c-Myc-LDHA axis positively regulates aerobic
glycolysis and promotes tumor progression in pancreatic cancer. Med
Oncol. 32:1872015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeoung NH: Pyruvate dehydrogenase kinases:
Therapeutic targets for diabetes and cancers. Diabetes Metab J.
39:188–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wahlström T and Henriksson MA: Impact of
MYC in regulation of tumor cell metabolism. Biochim Biophys Acta.
1849:563–569. 2015. View Article : Google Scholar
|
|
16
|
Dahham SS, Tabana YM, Iqbal MA, Ahamed MB,
Ezzat MO, Majid AS and Majid AM: The anticancer, antioxidant and
antimicrobial properties of the sesquiterpene β-caryophyllene from
the essential oil of Aquilaria crassna. Molecules. 20:11808–11829.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Demuth DG and Molleman A: Cannabinoid
signalling. Life Sci. 78:549–563. 2006. View Article : Google Scholar
|
|
18
|
Basha RH and Sankaranarayanan C:
β-caryophyllene, a natural sesquiterpene, modulates carbohydrate
metabolism in strepto-zotocin-induced diabetic rats. Acta
Histochem. 116:1469–1479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang Y, Wang YL, Yang L, Xu JX, Xiong W,
Xiao M and Li M: Inhibition of arginine ADP-ribosyltransferase 1
reduces the expression of poly(ADP-ribose) polymerase-1 in colon
carcinoma. Int J Mol Med. 32:130–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuang J, Wang YL, Xiao M, Tang Y, Chen WW,
Song GL, Yang X and Li M: Synergistic effect of arginine-specific
ADP-ribosyltransferase 1 and poly(ADP-ribose) polymerase-1 on
apoptosis induced by cisplatin in CT26 cells. Oncol Rep.
31:2335–2343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leontieva OV, Demidenko ZN and
Blagosklonny MV: Rapamycin reverses insulin resistance (IR) in
high-glucose medium without causing IR in normoglycemic medium.
Cell Death Dis. 5:e12142014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng W, Wu P, Du Y, Wang Y, Zhou N, Ge Y
and Yang Z: Puerarin improves cardiac function through regulation
of energy metabolism in Streptozotocin-Nicotinamide induced
diabetic mice after myocardial infarction. Biochem Biophys Res
Commun. 463:1108–1114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Bruijn KMJ, Arends LR, Hansen BE,
Leeflang S, Ruiter R and van Eijck CH: Systematic review and
meta-analysis of the association between diabetes mellitus and
incidence and mortality in breast and colorectal cancer. Br J Surg.
100:1421–1429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang M, Hu RY, Wu HB, Pan J, Gong WW, Guo
LH, Zhong JM, Fei FR and Yu M: Cancer risk among patients with type
2 diabetes mellitus: A population-based prospective study in China.
Sci Rep. 5:115032015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
García-Jiménez C, García-Martínez JM,
Chocarro-Calvo A and De la Vieja A: A new link between diabetes and
cancer: Enhanced WNT/β-catenin signaling by high glucose. J Mol
Endocrinol. 52:R51–R66. 2013. View Article : Google Scholar
|
|
26
|
Lin CY, Lee CH, Huang CC, Lee ST, Guo HR
and Su SB: Impact of high glucose on metastasis of colon cancer
cells. World J Gastroenterol. 21:2047–2057. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takatani-Nakase T, Matsui C, Maeda S,
Kawahara S and Takahashi K: High glucose level promotes migration
behavior of breast cancer cells through zinc and its transporters.
PLoS One. 9:e901362014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui G, Zhang T, Ren F, Feng W-M, Yao Y,
Cui J, Zhu G-L and Shi Q-L: High blood glucose levels correlate
with tumor malignancy in colorectal cancer patients. Med Sci Monit.
21:3825–3833. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiao M, Tang Y, Wang YL, Yang L, Li X,
Kuang J and Song GL: ART1 silencing enhances apoptosis of mouse
CT26 cells via the PI3K/Akt/NF-κB pathway. Cell Physiol Biochem.
32:1587–1599. 2013. View Article : Google Scholar
|
|
30
|
Seyfried TN and Shelton LM: Cancer as a
metabolic disease. Nutr Metab (Lond). 7:72010. View Article : Google Scholar
|
|
31
|
Vazquez A, Liu J, Zhou Y and Oltvai ZN:
Catabolic efficiency of aerobic glycolysis: The Warburg effect
revisited. BMC Syst Biol. 4:582010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng KW, Agarwal R, Mitra S, Lee JS,
Carey M, Gray JW and Mills GB: Rab25 increases cellular ATP and
glycogen stores protecting cancer cells from bioenergetic stress.
EMBO Mol Med. 4:125–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kume S, Thomas MC and Koya D: Nutrient
sensing, autophagy, and diabetic nephropathy. Diabetes. 61:23–29.
2012. View Article : Google Scholar :
|
|
34
|
Sun W, Xie Z, Liu Y, Zhao D, Wu Z, Zhang
D, Lv H, Tang S, Jin N, Jiang H, et al: JX06 Selectively inhibits
pyruvate dehydro-genase kinase PDK1 by a covalent cysteine
modification. Cancer Res. 75:4923–4936. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim JW, Gao P, Liu YC, Semenza GL and Dang
CV: Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively
induce vascular endothelial growth factor and metabolic switches
hexo-kinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol.
27:7381–7393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun Q, Chen X, Ma J, Peng H, Wang F, Zha
X, Wang Y, Jing Y, Yang H, Chen R, et al: Mammalian target of
rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is
critical for aerobic glycolysis and tumor growth. Proc Natl Acad
Sci USA. 108:4129–4134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sheng SL, Liu JJ, Dai YH, Sun XG, Xiong XP
and Huang G: Knockdown of lactate dehydrogenase A suppresses tumor
growth and metastasis of human hepatocellular carcinoma. FEBS J.
279:3898–3910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi M, Cui J, Du J, Wei D, Jia Z, Zhang J,
Zhu Z, Gao Y and Xie K: A novel KLF4/LDHA signaling pathway
regulates aerobic glycolysis in and progression of pancreatic
cancer. Clin Cancer Res. 20:4370–4380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Broecker-Preuss M, Becher-Boveleth N,
Bockisch A, Dührsen U and Müller S: Regulation of glucose uptake in
lymphoma cell lines by c-MYC- and PI3K-dependent signaling pathways
and impact of glycolytic pathways on cell viability. J Transl Med.
15:1582017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu R, Hu LL, Sun A, Cao YJ, Tang T, Zhang
XP and Zhang QH: mRNA expression of IGF-1 and IGF-1R in patients
with colorectal adenocarcinoma and type 2 diabetes. Arch Med Res.
45:318–324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Csibi A and Blenis J: Appetite for
destruction: The inhibition of glycolysis as a therapy for tuberous
sclerosis complex-related tumors. BMC Biol. 9:692011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Düvel K, Yecies JL, Menon S, Raman P,
Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S,
et al: Activation of a metabolic gene regulatory network downstream
of mTOR complex 1. Mol Cell. 39:171–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yassa HD and Tohamy AF: Extract of Moringa
oleifera leaves ameliorates streptozotocin-induced Diabetes
mellitus in adult rats. Acta Histochem. 116:844–854. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brown I, Cascio MG, Rotondo D, Pertwee RG,
Heys SD and Wahle KW: Cannabinoids and omega-3/6 endocannabinoids
as cell death and anticancer modulators. Prog Lipid Res. 52:80–109.
2013. View Article : Google Scholar
|
|
45
|
Hussain AR, Ahmed SO, Ahmed M, Khan OS, Al
Abdulmohsen S, Platanias LC, Al-Kuraya KS and Uddin S: Cross-talk
between NFkB and the PI3-kinase/AKT pathway can be targeted in
primary effusion lymphoma (PEL) cell lines for efficient apoptosis.
PLoS One. 7:e399452012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pan J, Fauzee NJ, Wang YL, Sheng YT, Tang
Y, Wang JQ, Wu WQ, Yan JX and Xu J: Effect of silencing PARG in
human colon carcinoma LoVo cells on the ability of HUVEC migration
and proliferation. Cancer Gene Ther. 19:715–722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Boulikas T: At least 60 ADP-ribosylated
variant histones are present in nuclei from dimethylsulfate-treated
and untreated cells. EMBO J. 7:57–67. 1988.PubMed/NCBI
|
|
48
|
Burzio LO, Riquelme PT and Koide SS: ADP
ribosylation of rat liver nucleosomal core histones. J Biol Chem.
254:3029–3037. 1979.PubMed/NCBI
|