Introduction
With increasing problems of infertility (1,2), the
number of initiated treatments using assisted reproductive
technology (ART) is rising (3).
ART is a reproductive technology developed to achieve pregnancy as
a fertility treatment. ART contributed to ~1.6% of births in the
United States in 2013 (4). At
present, embryo cryopreservation is an essential technology used in
ART (5), and it is important in
ART as, rather than immediately transferring into the uterus,
embryo cryopreservation allows supplementary embryos to be reserved
for later pregnancy attempts (6,7).
Controlled-rate freezing and vitrification are two cryopreservation
techniques that prevail in embryo cryopreservation. Although the
former was the first to be applied and developed, the latter has
gained increased attention due to its increasingly efficacious
clinical outcomes (8).
Vitrification is a cryopreservation method whereby the embryo can
be cooled at ultra-fast rates (9).
The main advantage of vitrification is the absence of ice crystal
formation, which reduces the damage accompanying chilling.
Furthermore, it does not rely on expensive programmable freezing
equipment (10-12). Although the proportion of births
has been increased by embryo cryopreservation due to significant
improvements in technology (13),
adverse perinatal outcomes have been commonly observed in
pregnancies following ART, compared with those women who conceive
naturally and with preeclampsia (PE) (14,15).
In addition, compared with fresh embryo transplantation, increased
risk of PE in embryo cryopreservation has been reported (15).
As an obstetrical complication, PE is a condition
that emerges after the 20th week of gestation. This disorder
accounts for preterm deliveries and subsequent neonatal morbidity
(16-18). PE is a primary contributor to the
poor prognosis of the mother and the baby, and it is usually
accompanied by the occurrence of high blood pressure, an increase
in the level of urine protein and other severe organ impairments
(19,20). In addition, coagulant dysfunction
also involved in PE (21). A
previous study reported a maternal mortality rate of 50,000-60,000
caused by PE each year worldwide (22). Although extensive efforts have been
made, the exact etiology of PE remains to be elucidated. It has
been suggested that PE is a systemic maternal inflammatory response
in which oxidative stress occurs. According to previous studies
(23,24), the pathogenesis of PE can be
divided into two stages, namely abnormal placentation and
endothelial dysfunction. The implantation of placenta in early
pregnancy is mainly realized by the trophoblast infiltration of
spiral arteries, proteolytic enzymes and the activity of adhesion
molecules (24).
Multiple growth factors are involved in the progress
of PE (25). As a secreted
cytokine that serves as heparin- binding growth factor,
pleiotrophin (PTN) is associated with various cellular events via
distinct receptors (26),
including inflammatory conditions (27). The expression of PTN, receptor
syndecan-1 (SDC1) and receptor protein tyrosine phosphatase β/ζ
(RPTPβ/ζ) are found in the placenta, and are critical to the
infiltration of trophoblast cells and damage of the vascular
endothelium. PTN/RPTPβ/ζ also regulates the oxidative stress
response (26). As two of the
receptors of PTN, anaplastic lymphoma kinase (ALK) and syndecan-3
(SDC3) are essential to human placentation (26). However, to the best of our
knowledge, the effect of PTN on and its receptors in PE in
pregnancy following transplantation of embryo cryopreservation by
vitrification have not been investigated or presented.
Therefore, the objective of the present study was to
investigate the effect of embryo cryopreservation by vitrification
in terms of the risk of PE. The effect of PTN knockout on PE in
pregnancy following vitrified-warmed embryo transfer was also
examined. This may reveal a biomarker for the prediction of
pregnancy outcome following transplantation of embryo
cryopreservation.
Materials and methods
Patient samples
The 188 patients recruited were those who received
ART treatment between April, 2012 and November, 2016 at Luoyang
Central Hospital Affiliated to Zhengzhou University (Luoyang,
China). All protocols associated with humans in the present study
were approved by the Review Board of Luoyang Central Hospital
Affiliated to Zhengzhou University. All participants provided
permission to cooperate to undertake the relevant study and
provided written informed consent. The study included 188 cycles.
The inclusion criteria were as follows: Age, 20-38 years, embryo
freezing storage period between 30 and 1,080 days, vitrification
was applied as an embryo cryopreservation method. Patients with a
history of chronic hypertension (and/or used antihypertensive
medication prior to pregnancy), multifetal gestation, or metabolic
dysfunctions were excluded from the study. According to standard
criteria (26), PE was defined as
gestational hypertension with proteinuria.
Animals
The study was performed under the approval of the
Animal Ethics Committee of Luoyang Central Hospital Affiliated to
Zhengzhou University. The PTN−/− mice were generated by
deleting exons 2-4 on a background of 129/OlaxC57BL/6 J as
described in two previous studies (28,29).
The animals (614 female mice, 8-10 weeks old, weighing 18-25 g)
obtained from Nanjing Biomedical Research Institute (Nanjing,
China) were divided into [WT (304 mice) group and PTN−/−
(310 mice) group] for the preparation of false pregnancy. The
animals had free access to food and water and were housed at 22°C
(60-70% humidity, 12 h light/12 dark). The average body weight of
the mice in each group was between 20 and 25 g (8-10 weeks of age).
The genotype of PTN−/− mice was identified using a
polymerase chain reaction method as previously described (30). The primers used were as follows:
Forward, 5′-GATTGAACAAGATGGATTGC-3′ and reverse, 5′-CAT
TTAGGCAAACAGGAAGGACG-3′. The genomic DNA extracted from tails of
PTN−/− (a total of 310 mice) and WT mice (a total of 304
mice) was used as the template. The temperature protocols was set
as: 94°C, 5 min; 35 cycles of 94°C, 30 sec, 63°C, 30 sec, 72°C 1
min; final extension at 72°C, 10 min.
In vitro fertilization
An intraperitoneal injection of permanent- magnet
synchronous generator (PMSG) (5 IU/mice; Ningbo Sansheng
Pharmaceutical Co., Ltd., Ningbo, China) was administered to female
mice (75 mice, 8 weeks old, weighing 18 g). After 48 h, human
chorionic gonadotropin was then injected (5 IU/mouse; Ningbo
Sansheng Pharmaceutical Co., Ltd.). The eggs were collected 14 h
later. In brief, the female mice (weight, 18 g) were sacrificed by
cervical dislocation and following disinfection, the fallopian
tubes were rapidly isolated and washed in PBS working buffer. The
abdomen of the fallopian tube was then opened with ophthalmic
forceps under a stereomicroscope. The egg granule cell complex was
collected and washed in PBS and HTF medium (Quinn’s; SAGE-In
vitro Fertilization, Inc., Trumbull, CT, USA). The sperm from
the cauda epididymis of male mice (25 mice, 8 weeks old) were
incubated in HTF medium covered with paraffin oil (Sigma; EMD
Millipore, Billerica, MA, USA) at 37°C for 1 h (5% CO2
atmosphere). Following this treatment, the sperm (final sperm
concentration, 2.0×106/ml) were co-cultured with eggs
droplets (30-40/droplets) for 4-6 h for in vitro
fertilization (fertilization rate, 74.36%). The fertilized eggs in
cleavage medium covered with paraffin oil (Sigma; EMD Millipore)
were maintained for 36-48 h in order to acquire four-cell stage
embryos. Blastula medium (BM; SAGE-In vitro Fertilization,
Inc.) was used to culture four-cell embryos for obtaining
blastocysts.
Vitrified freezing
In brief, frozen liquid containing vitrification
solution (VS) solution and equilibration solution (ES) (both from
Kitazato, Tokyo, Japan) were prepared 30 min in advance. The
blastocysts were maintained in ES for 10 min and in VS for 30 sec.
Finally, the embryos were transferred onto the top of Cryotop
(Kitazato) with a minimal volume of embryo solution attached, which
were immediately stored in liquid nitrogen. The contact time of the
embryo with the VS liquid was between 30 and 60 sec. For thawing,
the blastocysts maintained in Cryotop were collected and directly
immersed in thawing solution for 60 sec, and then in diluent
solution for 60 sec, in washing solution (WS)1 for 3 min, and in
WS2 for 3 min. The blastocysts were then transferred into BM
covered with mineral oil for 1.5-2.5 h.
Blastocyst transfer
The male mice (8 weeks old, weighing 20-22 g,
Nanjing Biomedical Research Institute) were vascularized under the
anesthesia status. The mice were anesthetized by the intravenous
administration of sodium pentobarbital solution (2 mg/ml; Solarbio,
Beijing, China) (30-50 mg/kg body weight). In brief, following
anesthesia, the mice were disinfected with 70% ethanol. The vas
deferens of the mice was ‘picked up’ with tweezers and was ligated.
The vas deferens was then cut off in the middle of the two
ligatures. The healed vasectomized male mice were mated with
estrous female mice from the WT group and PTN−/− group
as already described. The following morning, the female mice with
copulatory plugs were selected as recipients for pseudopregnancy
for 12 h. The frozen-thawed blastocysts were then transferred into
the recipients at 2.5 days of pseudopregnancy.
Tax treatment
Following the blastocyst transfer, the pregnant WT
and PTN−/− mice were injected intraperitoneally with
either corn oil taxsolution (3 mg/40 g body weight; Sigma; EMD
Millipore) or an equal volume of corn oil vehicle solution on day
10 of pregnancy. The tax was injected for 5 days consecutively.
This dose regimen has been reported previously (31). Four groups were established in
terms of the genotype and treatment: WT corn oil (Wt) (63 mice), WT
tax (tax/Wt) (62 mice), PTN−/− corn oil
(PTN−/−) (64 mice) and the PTN−/−tax
(tax/PTN−/−) (64 mice). Following 19-21 days of
blastocyst transfer, the next-generation mice were born. According
to the occurrence PE of in mice during pregnancy, the mice in each
group were further divided into PE and non-PE mice. Thus, there
were 8 groups as follows: WT corn oil, PE/WT corn oil (PE/Wt), WT
tax (tax/Wt), PE/WT tax (PE/tax/Wt), PTN−/− corn oil
(PTN−/−), PTN−/− corn oil
(PE/PTN−/−), the PTN−/− tax
(tax/PTN−/−) and PE/PTN−/− tax
(PE/tax/PTN−/−).
Enzyme-linked immunosorbent assay
(ELISA)
The blood samples were collected via venipuncture
into tubes containing anticoagulants. The protein level of PTN in
serum was determined on covered 96-well ELISA plates, as previously
reported (32). Rabbit anti-human
PTN monoclonal antibodies (1:2,000, ab14025; Abcam, Cambridge, MA,
USA) were diluted in Tris-buffered saline (TBS) and incubated at
4°C overnight. Biotinylated affinity-purified anti-rabbit secondary
antibody (1:50,000, ab6720; Abcam) was added into the wells and
incubated at room temperature for l h. Streptavidin/alkaline
phosphatase conjugate (Roche Diagnostics GmbH, Mannheim, Germany)
was added and maintained at room temperature. The absorbance at 405
nm was measured on a plate reader. Recombinant human PTN (R&D
Systems, Inc.) was used as a standard control. The concentration of
TNF-α was also measured using an ELISA kit (ab181421; Abcam)
according to the protocols. The kit mainly consists of anti-TNF-α
antibodies and the regent for the ELISA assay. The absorbance of
TNF-α was determined at 450 nm.
Blood pressure measurement
The non-invasive tail-cuff method using the CODA™
system (Kent Scientific, Torrington, CT, USA) was applied to
monitor the blood pressure of mice following the steps described in
a previous study (33). The
measurements were all performed 5 min prior to the start of the
experiment. The assessments were repeated four times for each
measurement.
Measurement of urine protein
concentration
The urine was sampled using a bladder massage
method, as described previously (34). The quantity of urine protein was
determined using a Bradford assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Standard solutions were diluted with Bradford
regent, and the mixture of the two was incubated at room
temperature for 5 min. The absorbance was read at 595 nm and then a
standard curve was obtained. The urine protein level was assessed
according to this curve.
Immunohistohemistry (IHC) and hematoxylin
and eosin (H&E) staining
The paraffin-embedded placental and kidney tissues
from the pseudopregnant mice were dewaxed and rehydrated. For
H&E staining, the sections (4-µm-thick) were stained
with hematoxylin at room temperature for 10 min. Following
incubation with 1% hydrochloric acid ethanol for 3 sec, the section
was stained with 0.5% eosin for 30 sec. For IHC, the sections were
preheated in distilled water at 37°C. The sections were first
heated at 120°C in citric acid buffer and then maintained at room
temperature for 20 min for antigen retrieval. Fetal calf serum
(Gibco/Thermo Fisher Scientific, Waltham, MA, USA) in PBS (20%) was
incubated with the sections for 10 min. The primary antibody was
added onto the sections and maintained at 4°C overnight following
removal of the serum. The secondary antibody (biotinylated) (Cell
Signaling Technology, Inc.) was incubated for 30 min. Subsequently,
peroxidase-conjugated streptavidin biotin (Cell Signaling
Technology, Inc.) was added and maintained for 30 min at room
temperature. Diaminobenzidine was used to identify the peroxidase
activity. Finally, the sections were counterstained with Mayer’s
hematoxylin (Sangon Biotech Co., Ltd., Shanghai, China). Primary
antibodies used in IHC were as follows: Anti-PTN goat polyclonal
(1:20, cat. no. ab223674; Abcam), anti-RPTPβ/ζ mouse monoclonal
(1:200, cat. no. sc-33664; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), anti-SDC1 rabbit monoclonal (1:2,000, cat. no. ab128936;
Abcam) and anti-SDC3 rabbit monoclonal (1:20, ab36653, antibody).
The corresponding goat anti-rabbit IgG (ab64256, 1:200) or goat
anti-mouse IgG (ab64255, 1:200) secondary antibodies used were all
biotinylated and purchased from Abcam.
Western blot analysis
Lysates from the placenta were denatured in boiling
water for 5 min. The lysates (25 µg/lane) were mixed with
loading buffer and electrophoresed on a 12% SDS PAGE gel. The
proteins were then transferred onto PVDF membranes. To block the
non-specific proteins, not fatty milk was incubated with the
membrane for 2 h at room temperature. Anti-pleiotrophin (1:10,000,
cat. no. ab79411; Abcam), anti- RPTPβ/ζ (1:800, cat. no. sc33664;
Santa Cruz Biotechnology, Inc.), anti-Syndecan-1 (1:1,000, cat. no.
ab181789), anti-SDC3 (1:1,000, cat. no. ab155952) (both from
Abcam), anti-anaplastic lymphoma kinase (ALK; cat. no. sc-398791;
Santa Cruz Biotechnology, Inc.) and anti-actin antibodies (1:1,000,
cat. no. ab8226; Abcam) were used as the primary antibodies. The
primary antibodies were incubated with the membranes at 4°C
overnight. The appropriate HRP-conjugated IgG secondary antibody
(ab6721, 1:2,000; Abcam) was incubated with the membranes at room
temperature for 1 h. An ECL system (Amersham; GE Healthcare Life
Sciences, Chalfont, UK) was used to detect the bands.
Statistical analysis
Data are shown as the means ± standard deviation
(SD) and were analyzed using a two-tailed Student’s t-test or
one-way analysis of variance followed by Tukey’s post hoc test.
P<0.05 was considered to indicate a statistically significant
difference. GraphPad Prism version 6.0 (GraphPad Software, Inc., La
Jolla, CA, USA) was used to perform the statistical analyses.
Results
Clinical outcome results of
vitrified-warmed embryo transfer
PE in pregnancies following ART has gained increased
attention (35). The clinical
characteristics of the patients in the present study are summarized
in Table I. It was found that the
incidence of PE following vitrified-warmed embryo transfer was
13.5% (12/89). PE is considered to be systematical inflammatory
responses. The concentration of TNF-α was determined by ELISA,
which is closely associated with inflammation (25). Notably, as shown in Table II, increased secretion of TNF-α
was observed in patients with PE than that in normal pregnant women
(29±4 vs. 12±3 pg/ml, P<0.05). It was also found that the
activity of PTN was reduced in patients with PE (6±13 ng/ml) when
compared with the normal patients (8±17 ng/ml).
 | Table IClinical outcomes of vitrified-warmed
embryo transfer in humans. |
Table I
Clinical outcomes of vitrified-warmed
embryo transfer in humans.
| Factor | Number |
|---|
| Women | 188 |
| Embryos
vitrified | 441 |
| Embryos
recovered | 302 |
| Embryos
transferred | 132 |
| Clinical pregnancy
rate | 89 |
| Abortions | 13 |
| Stillbirth and
neonatal death | 2 |
| Preeclampsia | 12 |
 | Table IIConcentrations of TNF-α and PTN in
pregnant women. |
Table II
Concentrations of TNF-α and PTN in
pregnant women.
| Group | TNF-α (pg/ml) | PTN (ng/ml) |
|---|
| PE patients | 29±4 | 6±13 |
| Normal
patients | 12±3 | 8±17 |
| P-value | <0.05 | 0.69 |
Outcome of vitrified-warmed embryo
transfer in mice
To investigate the potential influence of PTN on the
incidence of PE following vitrified-warmed embryo transfer, the
present study evaluated the pregnancy outcome of the conditional
induced PTN-deficient mice. As shown in Table III, the knockdown of PTN did not
affect the pregnancy rate, which was ~40% among the four groups.
However, compared with the PTN−/− group (P<0.05), the
birth rate in the tax/PTN−/− group decreased by almost
half. In addition, the incidence of PE was significantly increased
by the deficiency of PTN compared with that in the
PTN−/− group (P<0.05).
 | Table IIIOutcomes of vitrified-warmed embryo
transfer in mice. |
Table III
Outcomes of vitrified-warmed embryo
transfer in mice.
| Factor | Wt | tax/Wt |
PTN−/− |
tax/PTN−/− |
|---|
| Animals (n) | 152 | 152 | 156 | 155 |
| Transferred embryos
(n) | 694 | 695 | 706 | 703 |
| Pregnancy rate, n
(%) | 63 (41.4) | 62 (40.7) | 64 (41) | 64 (41) |
| Total births, n
(%) | 211 (30.5) | 210 (30.2) | 215 (30.4) | 123 (17.5)a |
| PE (%) | 9 (14.3) | 9 (14.5) | 10 (14.7) | 24 (37.5)a |
As increased hypertension and urine protein content
are two principal features of PE, the present study monitored the
change of blood pressure and the content of urine protein in mice
prior to and following their pregnancies at certain stages. These
two parameters in control mice (non-PE mice) were maintained at a
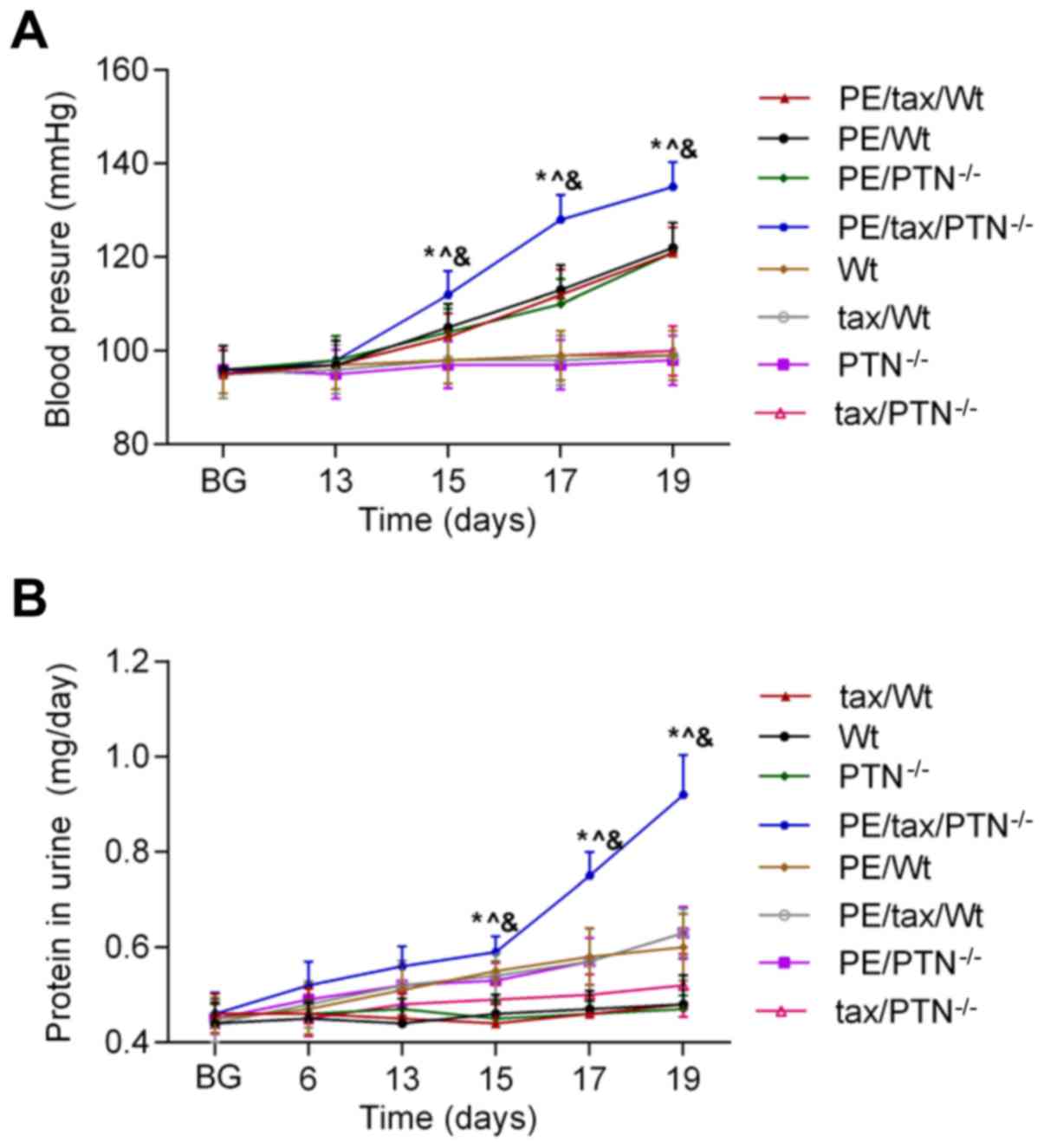
normal level throughout the study. As shown in Fig. 1, the blood pressure of the PE mice
remained stable prior to pregnancy and in early pregnancy (during
the first 13 days of pregnancy). At day 15 of pregnancy, a
significant increase in blood pressure was observed in these PE
mice. In addition, the blood pressure in those PE mice that lacked
PTN was higher than that in the other PE mice (PE/Wt. vs.
PE/tax/PTN−/− at 15 days: 105±5.0 vs. 112±4.8 mmHg,
P<0.05). Additionally, the protein content of urine in the PE
mice began to increase from day 13 of pregnancy, and was higher in
the PE mice that lacked PTN than in the other mice with PE (Wt vs.
tax/PTN−/− at 13 days: 0.51±0.05 vs. 0.565±0.038 mg/day,
P<0.05). From day 19 of pregnancy, the blood pressure and the
urine protein content reached the highest levels in the PE mice
that lacked PTN.
Concentrations of TNF-α and PTN in
mice
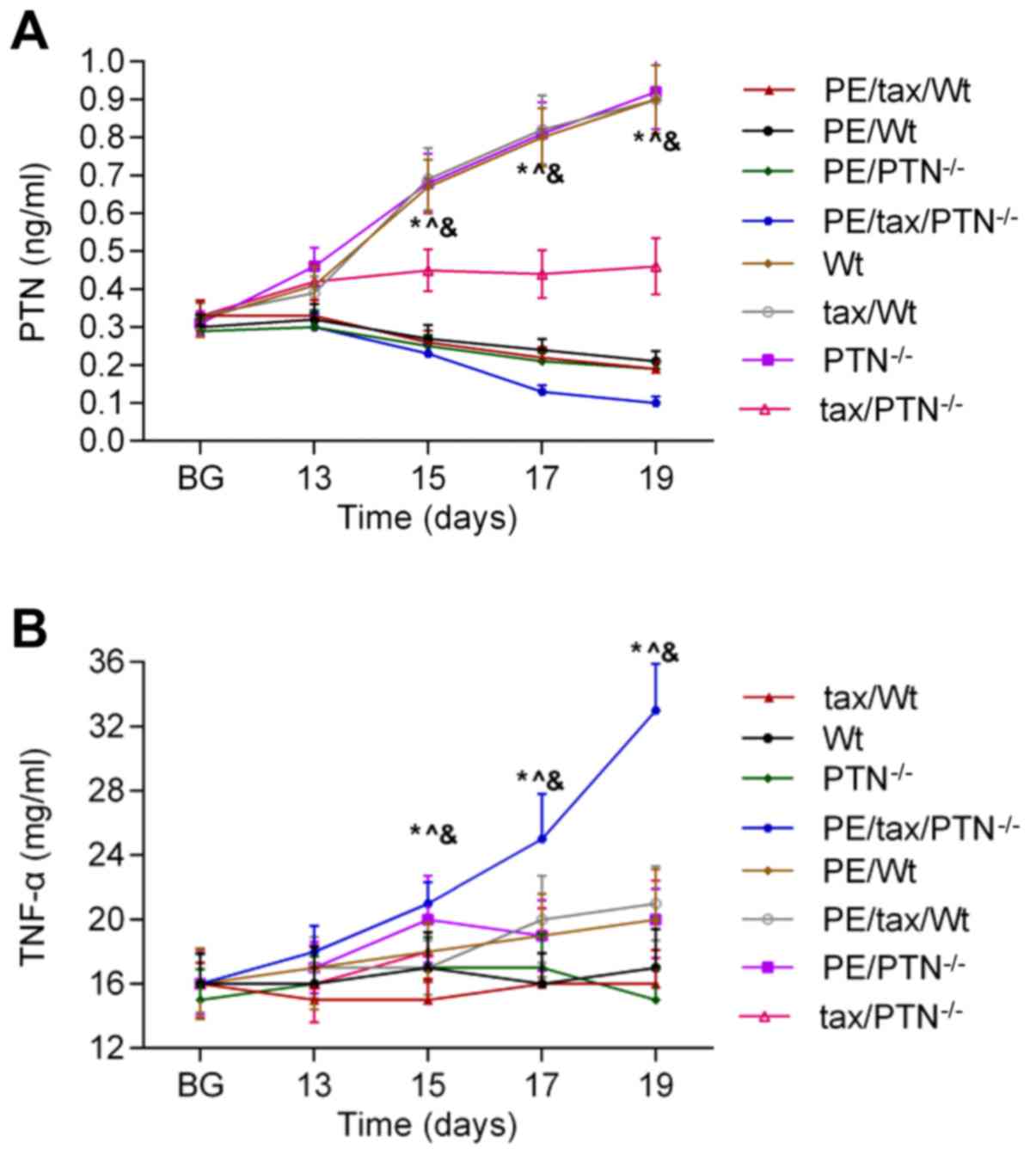
To determine the effect of PTN on PE following
vitrified-warmed embryo transfer, the activities of PTN and TNF-α
were identified in mice. As shown in Fig. 2A, the release of PTN was reduced in
the PE mice as pregnancy progressed, and the release of PTN was
higher in the PE mice that lacked PTN than in the other mice with
PE. The secretion of TNF-α showed an increasing trend in the PE
mice as pregnancy progressed. The level of PTN was low in the PE
mice that lacked PTN compared with the other mice with PE, and the
content of TNF-α was highest in the PE mice that lacked PTN
(Fig. 2B).
Expression of PTN and its receptors in
placental tissue
PTN is involved in inflammation via its distinct
receptors (36). The present study
compared the expression level of PTN and its receptors in the
placenta of PE and normal mice. As shown in Fig. 3, the expression levels of PTN,
SDC1, SDC3 and RPTPβ/ζ were reduced in the PE mice when compared
with levels in the normal mice, whereas the expression level of ALK
was similar among the groups. It appears that the expression levels
of SDC1 and SDC3 were not affected significantly by the knockdown
of PTN, whereas the expression of RPTPβ/ζ appeared to be suppressed
by the knockdown of PTN. In order to determine whether PTN and its
receptors are involved in PE following vitrified-warmed embryo
transfer, the expression pattern of PTN and its receptors in each
group were determined by IHC (n=4). Staining for the expression of
PTN was present in the cytoplasm and mesenchyme (ME) in the
placenta of control mice, whereas perinuclear and ME staining was
weaker in the placenta of PE mice (Fig. 4). The PTN staining faded out in
mice that lacked PTN, although PTN staining remained present in the
tax/ PTN−/− mice as the efficiency of the conditional
knockout was not 100% effective (negative staining for the isotype
control of PTN; data not shown). The expression of PTN was almost
absent in the tax/PTN−/−PE mice (Fig. 4D). In the placental tissues of the
control mice, strong fetal macrophage (FM) staining for SDC1 was
also observed. Fainter staining was observed in the ME and fetal
vessels (FV). In the PE mice, not all FMs were stained and other
staining had almost disappeared (Fig.
5). The expression ofSDC1 appeared to be reduced in the
PTN-knockout mice (Fig. 5D).
Strong diffuse cytoplasmic staining for RPTPβ/ζ was also noted.
Intensive staining in the ME, villous cytotrophoblasts,
syncytiotrophoblasts and FMs were observed in the control mice.
However, this staining was weaker or absent in the placenta of the
PE mice (Fig. 6).
 | Figure 3Expression of PTN and its receptors.
(A) Western blots for the expression level of PTN and its
receptors. (B) Determination of the protein level of PTN and its
receptors. *P<0.05. The experiments were repeated at
least three times independently. PE, preeclampsia; Wt, wild-type;
tax, tamoxifen; PTN, pleiotrophin; SDC1,syndecan-1;
SDC3,syndecan-3; RPTPβ, receptor protein tyrosine phosphatase β/ζ;
ALK, anaplastic lymphoma kinase; P, PE; N, normal. |
 | Figure 5Immunohistochemistry for the
expression pattern of RPTPβ/ζ in the placenta of the (A) Wt group
(arrows indicate ME, VT and ST), (B) tax/Wt group (arrows indicate
FM, VT and ST), (C) PTN−/− group (arrows indicate ST),
and (D) tax/PTN−/−group (arrows indicate the ME). The
experiments were repeated at least three times independently. PE,
preeclampsia; Wt, wild-type; tax, tamoxifen; PTN, pleiotrophin;
RPTPβ/ζ, receptor protein tyrosine phosphatase β/ζ; ME, mesenchyme;
VT, villous cytotrophoblast; ST, syncytiotrophoblast; FM, fetal
macrophage. Magnification, x100 for the upper row and x200 for the
lower row. |
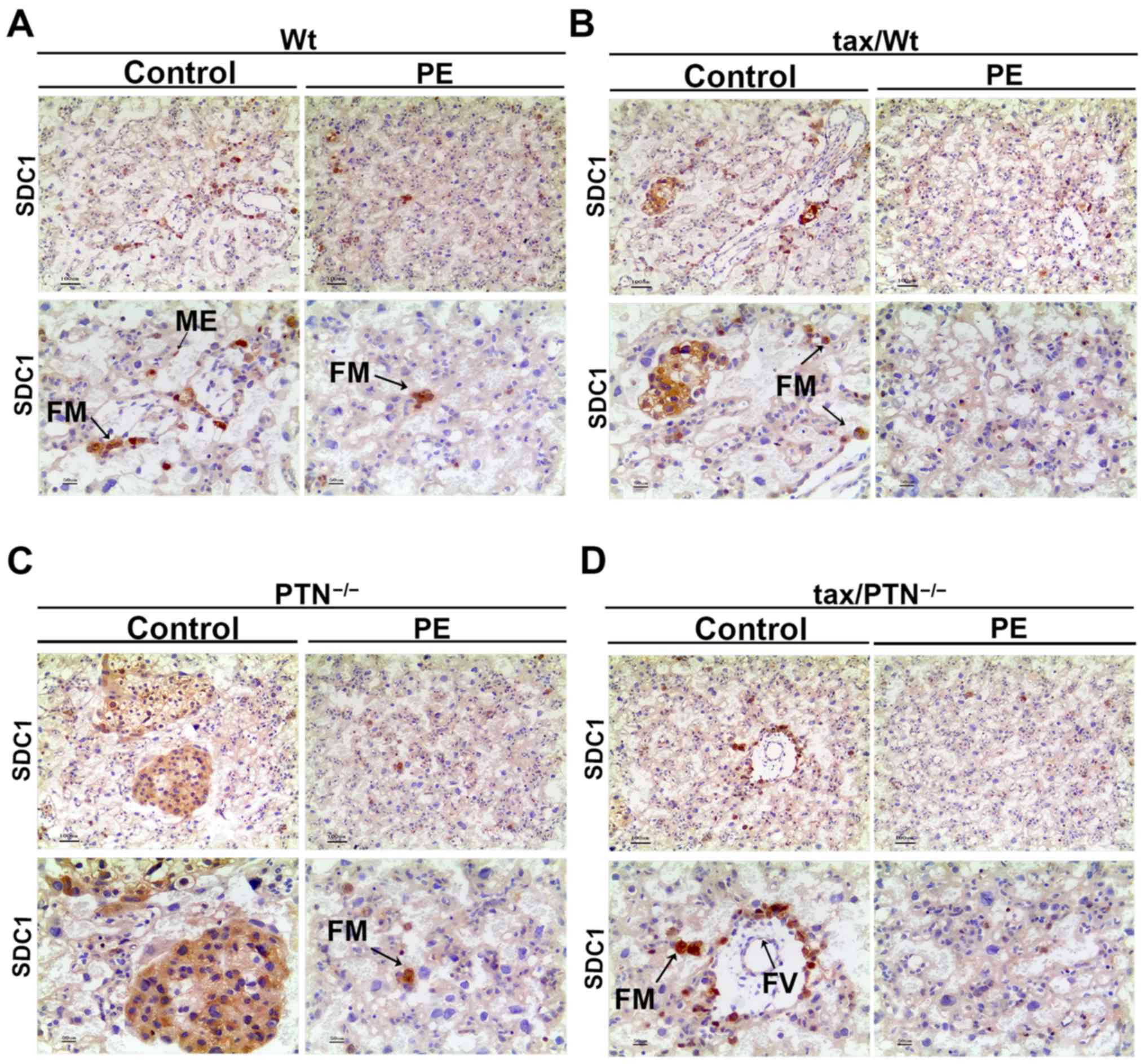 | Figure 6Immunohistochemistry for the
expression pattern of SDC1 in the placenta. Expression of SDC1 in
the (A) Wt group (arrows indicate FM and ME), (B) tax/Wt group
(arrows indicate FM), (C) PTN−/− group (arrows indicate
FM), and (D) tax/PTN−/−group (arrows indicate FM and
FV). The experiments were repeated at least three times
independently. PE, preeclampsia; Wt, wild-type; tax, tamoxifen;
PTN, pleiotrophin;SDC1, syndecan-1; ME, mesenchyme, FM, fetal
macrophage; FV, fetal vessel. Magnification, x100 for the upper row
and x200 for the lower row. |
Kidney injury in mice
Renal damage is often connected to PE (37). Therefore, the present study
examined kidney injury in the mice. As shown in Fig. 7, the glomeruli, renal capsule and
tubular structures were clear in the kidneys of the control mice.
However, the Bowman’s capsule and the opening of capillary loops
were reduced, and inflammatory cell infiltration increased in the
PE mice. In addition, kidney injury was more severe in the
PTN-knockout PE mice.
Discussion
The risk of PE in pregnancies following ART is high
(38). PE is a maternal
complication accompanied with high blood pressure and an increase
of urine protein during pregnancy. The incidence of PE is 5-8%
worldwide (39,40). The present study analyzed the
outcome of clinical pregnancy following embryo transfer. The
results showed that the incidence of PE was higher (12%) in mice
conceiving through vitrified-thawed embryo transfer. Therefore, it
is of medical importance to further investigate the molecular
mechanism of PE.
PE is considered to be a type of systemic
inflammatory responses. Poor placentation and endothelial disorder
are two critical stages of PE (24). Heparin-binding growth factors,
including PTN, have been reported to be altered in PE. PTN is
distributed in the placenta and is important to placentation and
inflammation via its receptors. The results of the present study
showed that the concentrations of TNF-α and PTN were increased and
decreased, in pregnant women with PE, compared with those in non-PE
women, respectively. The enhanced secretion of TNF-α was in
accordance with a previous study (41). In addition, to a certain extent,
the decreased PTN suggested its potential role in PE. However, less
is known regarding the role of PTN on the incidence of PE following
embryo transfer. Therefore, the present study focused on whether
and how PTN knockout affects the risk of PE following
vitrified-thawed embryo transfer.
Compared with the wild-type group, the present study
found that the risk of PE in PTN−/− mice was increased
following treatment with tax. In addition, the blood pressure and
urine protein content were increased in PE mice as time progressed,
and these reached the highest levels on day 19 of pregnancy.
Furthermore, the blood pressure and urine protein content were
higher in the PE mice treated with tax than that in the other PE
mice. In addition, the level of PTN increased as pregnancy
progressed in the non-PE mice, but declined in the PE mice. By
contrast, the level of TNF-α remained at a steady level in the
non-PE mice, but increased in the PE mice as pregnancy progressed.
Similar to blood pressure and urine protein content, the activity
of TNF-α was higher in the PE mice treated with tax than that in
the other PE mice. These results indicated that the reduction of
PTN increased the risk of PE following vitrified-thawed embryo
transfer.
The function of PTN is largely dependent on its
receptors (42). To further
examine the role of PTN in the occurrence of PE, western blot
analysis was performed to determine the expression of PTN and its
receptors, including SDC1, SDC3, RPTPβ/ζ and ALK, in the placenta.
The data showed that the expression of RPTPβ/ζ was reduced in PE
mice treated with tax, compared with that in mice treated with corn
oil, and that the expression levels of SDC1 and SDC3 were
marginally suppressed in the PE mice treated with tax. As a low
expression of SDC1 has been demonstrated to promote PE (43), IHC was used to identify the
expression pattern of PTN, RPTPβ/ζ and SDC1 in the placenta. The
results showed that the staining for PTN was perinuclear, and was
also observed in FMs and ME, which was in line with a previous
study (26). These results
confirmed the pro-angiogenic effect of PTN in these sites (44). The receptors SDC1 and RPTPβ/ζ
shared certain overlapping expression patterns as PTN. These
results were partly in line with a previous study (26). In the present study, the finding
that the staining of PTN in the syncytial microvillous membrane was
adjacent to areas of completely unstained membrane was recorded,
and this suggested its variation in the membrane (45). However, the expression of PTN and
its receptors was weaker in the PE mice. Notably, the expression
levels of SDC1 and RPTPβ/ζ were reduced in PE conditions, with no
changes in PE mice with or without tax treatment. Although this
result was not in accordance with the results from the western blot
analysis, the possibility that the receptors of PTN may be
associated with the incidence of PE following embryo transfer
cannot be excluded. Further investigations are required to examine
the role of its receptors in more detail. In addition, the present
study observed that the kidney injury caused by PE was increased by
the knockout of PTN. Therefore, these results indicated that the
reduced activity of PTN was associated with PE following embryo
transfer.
To the best of our knowledge, the present study
provides the first comprehensive investigation of the potential
role of PTN in PE following vitrified-thawed embryo transfer.
However, the underlying mechanisms remain to be fully elucidated.
The results showed that PTN may be relevant to the inflammatory
response in PE, however, as a heparin-binding factor, PTN may
affect the balance of the clotting/anticoagulant system via
heparin, affecting the homeostasis of the clotting/anticoagulant in
PE. In addition, PTN and its receptor have been reported to be
involved in the metabolism of catecholamines, and thereby regulate
oxidative stress in PE (26,46).
Therefore, further investigation of the role of PTN and its
receptors on PE following ART are required.
In conclusion, the risk of PE was shown to increase
following vitrified-thawed embryo transfer; the knockout of PTN
enhanced the incidence and symptoms of PE in pregnant mice
following embryo transfer. In addition, the inflammatory response
was more marked in PE mice that lacked PTN than that in other PE
mice. The expression of PTN and its receptors reflected their
possible role in PE. The present study suggested a potential
direction for the detailed investigation of PTN in PE following
embryo transfer.
Funding
This study was supported by the Henan Provincial
Science and Technology Project (grant no. 201602359).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
SL wrote the main manuscript and analyzed the data.
FW performed the experiments. GL conceived and designed the study.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All protocols associated with humans in the present
study were approved by the Review Board of Luoyang Central Hospital
Affiliated to Zhengzhou University. All participants provided
permission to cooperate to undertake the relevant study and
provided written informed consent. Animal experiments were
performedwith the approval of the Animal Ethics Committee of
Luoyang Central Hospital Affiliated to Zhengzhou University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Sharma A, Singh AK and Singh SK:
Increasing prevalence of male infertility and stress factors: An
overview. Int J Clin Cases Invest. 3:17–24. 2011.
|
|
2
|
Araoye MO: Epidemiology of infertility:
Social problems of the infertile couples. West Afr J Med.
22:190–196. 2003.PubMed/NCBI
|
|
3
|
Kol S: Assisted Reproductive Technology
(ART). Encyclopedia of Endocrine Diseases. Academic Press; New
York, NY: pp. 269–277. 2004, View Article : Google Scholar
|
|
4
|
Sunderam S, Kissin DM, Crawford SB, Folger
SG, Jamieson DJ, Warner L and Barfield WD: Assisted Reproductive
Technology Surveillance - United States, 2013. MMWR Surveill Summ.
64:1–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saragusty J: Arav A Current progress in
oocyte and embryo cryopreservation by slow freezing and
vitrification. Reproduction. 141:1–19. 2011. View Article : Google Scholar
|
|
6
|
Veeck LL: Does the developmental stage at
freeze impact on clinical results post-thaw? Reprod Biomed Online.
6:367–374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anderson AR, Wilkinson SS, Price S and
Crain JL: Reduction of high order multiples in frozen embryo
transfers. Reprod Biomed Online. 10:402–405. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Desai N, Blackmon H, Szeptycki J and
Goldfarb J: Cryoloop vitrification of human day 3 cleavage-stage
embryos: Post- vitrification development, pregnancy outcomes and
live births. Reprod Biomed Online. 14:208–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vajta G and Nagy ZP: Are programmable
freezers still needed in the embryo laboratory? Review on
vitrification. Reprod Biomed Online. 12:779–796. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuleshova LL and Lopata A: Vitrification
can be more favorable than slow cooling. Fertil Steril. 78:449–454.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liebermann J and Tucker MJ: Effect of
carrier system on the yield of human oocytes and embryos as
assessed by survival and developmental potential after
vitrification. Reproduction. 124:483–489. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vajta G and Kuwayama M: Improving
cryopreservation systems. Theriogenology. 65:236–244. 2006.
View Article : Google Scholar
|
|
13
|
Doody KJ: Cryopreservation and delayed
embryo transfer-assisted reproductive technology registry and
reporting implications. Fertil Steril. 102:27–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pastore LM and Williams CD: Perinatal
outcomes in singletons following in vitro fertilization: A
meta-analysis. Obstet Gynecol. 104:4122004. View Article : Google Scholar
|
|
15
|
Opdahl S, Henningsen AA, Tiitinen A, Bergh
C, Pinborg A, Romundstad PR, Wennerholm UB, Gissler M, Skjærven R
and Romundstad LB: Risk of hypertensive disorders in pregnancies
following assisted reproductive technology: A cohort study from the
CoNARTaS group. Hum Reprod. 30:1724–1731. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roberts JM: Preeclampsia: New approaches
but the same old problems. Am J Obstet Gynecol. 199:443–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roberts JM, Pearson GD, Cutler JA,
Lindheimer MD and National Heart Lung; Blood Institute: Summary of
the NHLBI Working Group on Research on Hypertension During
Pregnancy. Hypertens Pregnancy. 22:109–127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Myklestad K, Vatten LJ, Salvesen KA, Davey
Smith G and Romundstad PR: Hypertensive disorders in pregnancy and
paternal cardiovascular risk: A population-based study. Ann
Epidemiol. 21:407–412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Redman CW and Sargent IL: Latest advances
in understanding preeclampsia. Science. 308:1592–1594. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sircar M, Thadhani R and Karumanchi SA:
Pathogenesis of preeclampsia. Curr Opin Nephrol Hypertens.
24:131–138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Hua Z, Zhang K, Meng K and Hu Y:
Therapeutic effects of anticoagulant agents on preeclampsia in a
murine model induced by phosphatidylserine/phosphatidylcholine
microvesicles. Placenta. 30:1065–1070. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
American College of Obstetricians and
Gynecologists; Task Force on Hypertension in Pregnancy:
Hypertension in pregnancy. Report of the American College of
Obstetricians and Gynecologists’ Task Force on Hypertension in
Pregnancy. Obstet Gynecol. 122:1122–1131. 2013.
|
|
23
|
Redman CW and Sargent IL: Placental stress
and pre-eclampsia: a revised view. Placenta. 30(Suppl A): pp.
S38–S42. 2009, View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mohaupt M: Molecular aspects of
preeclampsia. Mol Aspects Med. 28:169–191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Das UN: Cytokines, angiogenic, and
antiangiogenic factors and bioactive lipids in preeclampsia.
Nutrition. 31:1083–1095. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ball M, Carmody M, Wynne F, Dockery P,
Aigner A, Cameron I, Higgins J, Smith SD, Aplin JD and Moore T:
Expression of pleiotrophin and its receptors in human placenta
suggests roles in trophoblast life cycle and angiogenesis.
Placenta. 30:649–653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Achour A, M’bika JP, Baudouin F, Caruelle
D and Courty J: Pleiotrophin induces expression of inflammatory
cytokines in peripheral blood mononuclear cells. Biochimie.
90:1791–1795. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amet LE, Lauri SE, Hienola A, Croll SD, Lu
Y, Levorse JM, Prabhakaran B, Taira T, Rauvala H and Vogt TF:
Enhanced hippocampal long-term potentiation in mice lacking
heparin- binding growth-associated molecule. Mol Cell Neurosci.
17:1014–1024. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
del Olmo N, Gramage E, Alguacil LF,
Pérez-Pinera P, Deuel TF and Herradón G: Pleiotrophin inhibits
hippocampal long-term potentiation: A role of pleiotrophin in
learning and memory. Growth Factors. 27:189–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gramage E, Herradón G, Martín YB,
Vicente-Rodríguez M, Rojo L, Gnekow H, Barbero A and Pérez-García
C: Differential phosphoproteome of the striatum from pleiotrophin
knockout and midkine knockout mice treated with amphetamine:
Correlations with amphetamine-induced neurotoxicity. Toxicology.
306:147–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hayashi S and McMahon AP: Efficient
recombination in diverse tissues by a tamoxifen-inducible form of
Cre: A tool for temporally regulated gene activation/inactivation
in the mouse. Dev Biol. 244:305–318. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aigner A, Brachmann P, Beyer J, Jäger R,
Raulais D, Vigny M, Neubauer A, Heidenreich A, Weinknecht S,
Czubayko F, et al: Marked increase of the growth factors
pleiotrophin and fibroblast growth factor-2 in serum of testicular
cancer patients. Ann Oncol. 14:1525–1529. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krege JH, Hodgin JB, Hagaman JR and
Smithies O: A noninvasive computerized tail-cuff system for
measuring blood pressure in mice. Hypertension. 25:1111–1115. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Monahan E and Yamazaki K: An improved
urine collection technique for laboratory mice: The bladder massage
method. Lab Animal. 22:38–39. 1993.
|
|
35
|
Martin AS, Monsour M, Kawwass JF, Boulet
SL, Kissin DM and Jamieson DJ: Risk of preeclampsia in pregnancies
after assisted reproductive technology and ovarian stimulation.
Matern Child Health J. 20:pp. 2050–2056. 2016, View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kadomatsu K: The midkine family in cancer,
inflammation and neural development. Nagoya J Med Sci. 67:71–82.
2005.
|
|
37
|
Wester-Rosenlöf L, Casslé V, Axelsson J,
Edström-Hägerwall A, Gram M, Holmqvist M, Johansson ME, Larsson I,
Ley D, Marsal K, et al: A1M/α1-microglobulin protects from heme-
induced placental and renal damage in a pregnant sheep model of
preeclampsia. PLoS One. 9:pp. e863532014, View Article : Google Scholar
|
|
38
|
Wang YA, Chughtai AA, Farquhar CM, Pollock
W, Lui K and Sullivan EA: Increased incidence of gestational
hypertension and preeclampsia after assisted reproductive
technology treatment. Fertil Steril. 105:920–926.e2. 2016.
View Article : Google Scholar
|
|
39
|
Högberg U: The World Health Report 2005:
‘Make every mother and child count’ - including Africans. Scand J
Public Health. 33:409–411. 2005. View Article : Google Scholar
|
|
40
|
Al-Jameil N, Aziz Khan F, Fareed Khan M
and Tabassum H: A brief overview of preeclampsia. J Clin Med Res.
6:1–7. 2014.PubMed/NCBI
|
|
41
|
Shaw J, Tang Z, Schneider H, Saljé K,
Hansson SR and Guller S: Inflammatory processes are specifically
enhanced in endothelial cells by placental-derived TNF-α:
Implications in preeclampsia (PE). Placenta. 43:1–8. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu C, Zhu S, Wu M, Han W and Yu Y:
Functional receptors and intracellular signal pathways of midkine
(MK) and pleiotrophin (PTN). Biol Pharm Bull. 37:511–520. 2014.
View Article : Google Scholar
|
|
43
|
Gandley RE, Althouse A, Jeyabalan A,
Bregand-White JM, McGonigal S, Myerski AC, Gallaher M, Powers RW
and Hubel CA: Low soluble syndecan-1 precedes preeclampsia. PLoS
One. 11:pp. e01576082016, View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Palmieri D, Mura M, Mambrini S and Palombo
D: Effects of Pleiotrophin on endothelial and inflammatory cells:
Pro-angiogenic and anti-inflammatory properties and potential role
for vascular bio-prosthesis endothelialization. Adv Med Sci.
60:287–293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Anjum N, Baker PN, Robinson NJ and Aplin
JD: Maternal celiac disease autoantibodies bind directly to
syncytiotrophoblast and inhibit placental tissue transglutaminase
activity. Reprod Biol Endocrinol. 7:162009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hung JH: Oxidative stress and antioxidants
in preeclampsia. J Chin Med Assoc. 70:430–432. 2007. View Article : Google Scholar : PubMed/NCBI
|