Introduction
Melanoma is a skin malignancy with a high mortality
rate (1) and an increasing
incidence worldwide (2-4). Therefore, the development of novel
antineoplastic agents and the investigation of their underlying
mechanisms are imperative in the treatment of this malignant
tumor.
Long non-coding RNAs (lncRNAs) generally consist of
>200 nucleotides and do not encode proteins (5). XIST was the first documented
functional lncRNA identified in the early 1990s (6). Initially, lncRNAs were generally
considered as transcriptional noise or useless sequences (7). However, recent studies have
demonstrated that lncRNAs play important roles in several
biological processes, including dosage compensation, regulation of
gene expression, genomic imprinting, nuclear organization and
compartmentalization. Changes in the expression of lncRNAs are
associated with the occurrence of several diseases, including
psoriasis, Alzheimer’s disease, heart disease and cancer. A growing
body of evidence indicates that lncRNAs play an important role in
the diagnosis and prognosis of various malignancies, including
clear cell renal cell carcinoma (8), glioma (9) and osteosarcoma (10). Recently, accumulating evidence
suggests that lncRNAs are also implicated in the progression and
prognosis of melanoma (11-14).
Of note, the expression and distribution of lncRNAs differ
according to the cell type to match their possible regulatory
role.
Taurine upregulated 1 (TUG1) is a novel lncRNA
located on chromosome 22q12, which is composed of 6.7-kb
nucleotides (15). Previous
reports have demonstrated that TUG1 lncRNA is largely overexpressed
and positively regulates oncogenesis in various types of cancer,
such as glioma (16), esophageal
squamous cell carcinoma (17),
endometrial and colorectal cancer (18,19),
hepatocellular carcinoma (20) and
osteosarcoma (21). However, in
certain cancers, such as non-small-cell lung cancer, TUG1 has been
reported to be expressed at a relatively low level and acts as a
tumor suppressor (22). These
reports indicate that TUG1 may play different roles in different
types of cancer cells, and TUG1 activity may change under different
tumor microenvironment conditions.
The data of the present study demonstrated that TUG1
was highly expressed in human melanoma tissues and cell lines.
Moreover, TUG1 facilitated cell proliferation and invasion and
suppressed cell apoptosis by sequestering miR-29c-3p from its
target gene regulator of G-protein signaling 1 (RGS1) in melanoma
cells. The results indicate that TUG1 may be promising as a
potential diagnostic marker and therapeutic target for
melanoma.
Materials and methods
Ethics statement
The present study was approved by the Ethics
Committee of the Xinxiang Central Hospital (Xinxiang, China). The
sample collection and surgical procedures were not harmful to the
patients. Written informed consent was obtained from all patients.
The research protocol conformed to the principles outlined in the
Declaration of Helsinki.
Clinical samples
Between March 2014 and November 2017, a total of 40
melanoma tissues, 30 benign nevi and 15 metastatic melanoma tissues
were obtained from patients who presented at the Xinxiang Central
Hospital (Xinxiang, China). The patients had never received
chemotherapy or radiotherapy prior to surgery. All the samples were
immediately snap- frozen in liquid nitrogen and stored at −80°C
prior to RNA extraction.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
TRIzol reagent (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was used to extract total RNA from the cells or
the tissue samples. The RNA purity was confirmed by the A260/A280
ratio. cDNA was synthesized by reverse transcription using the
TaqMan® MicroRNA Reverse Transcription kit (Thermo
Fisher Scientific, Inc.) in accordance with the manufacturer’s
instructions. TaqMan® Universal PCR Master Mix Kit
(Thermo Fisher Scientific, Inc.) was used to amplify the products
by PCR. The relative levels were calculated using the
2-ΔΔCq method. The primers were purchased from RiboBio
Co. Ltd. (Guangzhou, China). The primer sequences were as follows:
TUG1 forward: 5′-AACTACCTGGACCGCTTCCT-3′ and reverse: 5′-CCAC
TTGAGCTTGTTCACCA-3′. miR-29c levels were detected by TaqMan
microRNA assay (Applied Biosystems; Thermo Fisher Scientific,
Foster City, CA, USA).
Cell culture and cell transfection
The human melanoma cell lines A375 and SK-MEL-2 were
obtained from the American Type Culture Collection (Manassas, VA,
USA). The cells were routinely cultured in complete Dulbecco’s
modified Eagle’s medium (Gibco; Thermo Fisher Scientific, Grand
Island, NY, USA) with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific), 100 U/ml penicillin and 100 µg/ml
streptomycin (Sigma-Aldrich; Merck KGaA, St. Louis, MO, USA). The
cultured cells were routinely passaged every 2-3 days. All cell
lines used were between passages 3 and 8 during the
experiments.
According to the manufacturer’s instructions, the
cells were cultured in 6-well plates and transfected with 2
µg of each plasmid in each well alone in the presence of 4
µl Lipofectamine 200 (Invitrogen; Thermo Fisher Scientific,
Carlsbad, CA, USA). TUG1, control RNA sequences and TUG1 siRNA
(si-TUG1) were purchased from GenePharma (Shanghai, China).
miR-29c-3p mimics, inhibitors, and the respective negative control
(NC) were purchased from RiboBio (Guangzhou, China). The si-TUG1
sequence was GGTGGTTGAAAGGAATCCT. The TUG1 cDNA was amplified and
subcloned into a pcDNA3.1 vector (Invitrogen; Thermo Fisher
Scientific) in order to produce the TUG1 over-expression
vector.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was evaluated using CCK-8
(Dojindo, Kumamoto, Japan). After 48 h of transfection, 3,000 cells
(100 µl/well) were seeded in 96-well plates. The cells were
grown for 24, 48 and 72 h and 10 µl of CCK-8 were added to
each well. The samples were subsequently incubated at 37°C for 4 h.
A scanning multi- well spectrophotometer (Thermo Scientific, Inc.)
was used to measure the absorbance at 450 nm.
Flow cytometric analysis
After 48 h of transfection, the cells were detached
using trypsin for 2 min at 37°C, collected and rinsed twice with
phosphate-buffered saline (PBS). The cells were counted and diluted
to a density of 106 cells/ml. The apoptotic cells were verified by
an Annexin V/FITC kit (KGA108, Nanking, China) according to the
manufacturer’s instructions. The collected cells were then
centrifuged and the supernatants were discarded. The cells were
resuspended in 200 µl binding buffer. A total of 2 µl
of Annexin V-FITC solution and 5 µl of 1 µg/ml
propidium iodide were subsequently added to the cells. The cells
were incubated in the dark at 37°C for 30 min. The population of
cells was then defined. FITC−and PI− cells
were designated as viable, FITC+ and PI+
cells were designated as late apoptotic or necrotic, and
FITC+ and PI− cells were designated as
apoptotic. The results are representative of three independent
experiments and each sample was run in triplicate.
Transwell invasion assays
The invasive ability of the cells was assessed by
Transwell invasion assays (Corning Inc., Corning, NY, USA).
Briefly, 8-µm Transwell filter inserts were coated with
Matrigel (10 mg/l, BD Biosciences, San Jose, CA, USA).
Approximately 3×104 transfected cells were resuspended
in 200 µl serum-free medium and added onto 8-µm
Transwell filter inserts. A total of 500 µl medium
containing 20% fetal bovine serum were added to the lower chamber
as a chemoattractant. Following incubation for 24 h, the cells in
the upper chamber were removed with a cotton swab. The cells
invading to the lower surface of the membrane were fixed with
methanol and then stained with 0.1% crystal violet solution for
10-20 min and the number of invading cells was counted. Each
experiment was performed in triplicate.
Western blot analysis
Cells (~1×107) were collected and lysed
in RIPA buffer (Beyotime Biotech, Nantong, China). This buffer was
supplemented with protease (PMSF) and phosphatase inhibitors
(Na-ortho-vanadate, NaF). Equal amounts of total protein were
extracted from the cultured cells or tissues and separated by 8-10%
SDS-PAGE. The samples were transferred onto polyvinylidene
difluoride (PVDF) membranes. The PVDF membranes were incubated with
primary antibodies overnight at 4°C: RGS1 (1:1,000, ab117077),
Bcl-2 (1:1,000, ab196495) and MMP2 (1:1,000, ab37150). After
washing, the PVDF membrane was incubated with horseradish
peroxidase-conjugated secondary antibodies (1:5,000, ab150077) at
room temperature for 1 h. Antibodies were purchased from Abcam
(Cambridge, MA, USA). The secondary antibodies bound on the PVDF
membrane reacted with ECL substrate (Pierce Chemical Co, Rockford,
IL, USA) and the protein bands were exposed to X-ray films. The
results were normalized to the expression of the internal control
β-actin.
Luciferase reporter assay
The human melanoma cell lines A375 and SK-MEL-2 were
co-transfected with miR-29c-3p mimics or miR-control, pmiR-reporter
luciferase vector containing a specific sequence of wild-type
and/or a mutant TUG1 fragment. The transfection was achieved using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
Following transfection and incubation for 48 h, the Dual-luciferase
Reporter Assay System (Promega Corporation, Madison, WI, USA) was
employed to evaluate the luciferase activities. The relative
luciferase activity was normalized against the Renilla luciferase
activity. Each experiment was performed in triplicate.
Pull-down assay
Pull-down assay was performed according to the
manufacturer’s instructions (Thermo Fisher Scientific, Inc.).
Briefly, the biotinylated TUG1 probe or control probe were
incubated with Dynabeads M-280 Streptavidin (Thermo Fisher
Scientific, Inc.) after being dissolved in binding and washing
buffer for 10 min at 25°C, followed by generation of the
probe-coated beads. Subsequently, the probe-coated beads were
incubated with A375 or SK-MEL-2 cell lysates. Finally, the enriched
RNA complexes in the beads were purified using TRIzol
(Sigma-Aldrich; Merck KGaA) and detected using RT-qPCR. The TUG1
probe sequence was as follows: 5′-AAGA CTGAATCGGACTGCGTTAGA-3′; The
negative control sequence was as follows: 5′-AAGACTGACCCAGACTTCA
CAGCA-3′.
Immunohistochemical (IHC) staining
Standardized and automated IHC was used to detect
the expression of RGS1 in benign nevi and melanoma tissues.
Briefly, human benign nevi and melanoma tissues were fixed in 3%
formaldehyde and embedded in paraffin. 0.3%
H2O2 was applied to block endogenous
peroxidase activity. Subsequently, the sections were deparaffinized
in a series of graded alcohols and microwaved in EDTA buffer for 10
min at 450 W. Later on, the sections were washed with PBS and
incubated with anti-RGS1 primary antibodies at 37°C for 30 min.
Horseradish peroxidase and diaminobenzidine were used as substrates
to assess RGS1 expression.
Animal tumor model
BALB/c nude mice 7-8 weeks old were obtained from
the Shanghai Laboratory Animal Center (Shanghai, China) and housed
in barrier facilities with a 12-h light/dark cycle.
TUG1-transfected A375 cells were subcutaneously injected into the
right flank of the nude mice. The tumors were measured weekly and
their volumes were calculated according to the equation: V =
(length × width2)/2. All animals were euthanized using
CO2 after 4 weeks. The animal experiments were fully
approved by the Ethics Committee of Animal Experiments of the
Xinxiang Central Hospital. In addition, the present study was
strictly performed in accordance with the recommendations in the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health.
Statistical analysis
All the data are expressed as mean ± standard
deviation. The comparison between two groups was analyzed by
Student’s t-test. The comparisons among multiple groups were
performed with one-way analysis of variance followed by Dunnett’s
test. A P-value <0.05 was considered to indicate statistically
significant differences. The survival analysis was performed using
Kaplan-Meier plots and log-rank tests. A P-value <0.05 was
considered to indicate statistically significant differences.
Results
TUG1 expression in melanoma tissues and
its association with overall survival
Recently, various studies indicated that
overexpression of TUG1 may predict poor prognosis in cancer
patients (23,24). Therefore, the expression levels of
TUG1 in melanoma tissues, benign nevi, primary melanoma tissues and
metastatic melanoma tissues were detected by RT-qPCR assays. TUG1
was found to be overexpressed in melanomas compared with benign
nevi (Fig. 1A). Moreover, the
expression of TUG1 was upregulated in metastatic melanoma compared
with primary melanoma tissues. Kaplan-Meier survival analysis and
the log-rank test demonstrated that patients with high TUG1
expression exhibited significantly decreased overall survival
(Fig. 1B, 27.3 vs. 57.9%,
respectively; hazard ratio = 2.44; 95% confidence interval:
1.57-3.78; P<0.05). The data revealed that the expression levels
of TUG1 in melanoma tissues was upregulated, which was in
accordance with previous reports (25,26).
Furthermore, TUG1 expression was found to be positively associated
with patient survival and cancer metastasis.
TUG1 expression in melanoma cells
To determine the expression of TUG1 in melanoma
cells, TUG1 expression levels were initially investigated using
RT-qPCR assays. TUG1 was found to be highly expressed in melanoma
cell lines (Fig. 2A). Moreover,
TUG1 was more highly expressed in SK-MEL-2 cells compared with A375
cells. A TUG1 overexpression plasmid and siRNA sequences against
TUG1 were used and transfected to melanoma cell lines in order to
investigate the role of this protein in melanoma.
Subsequently, the transfection efficiencies of TUG1
overexpression and knockdown were validated by RT-qPCR analysis.
The transfection of TUG1 resulted in marked upregulation of TUG1
expression compared with the negative control (vector) in A375
cells (Fig. 2B). It was also
observed that the expression of TUG1 was notably downregulated in
SK-MEL-2 cells transfected with si-TUG1 in comparison with the
transfection of non-specific siRNA (NC).
TUG1 regulates the proliferation,
apoptosis and invasion of melanoma cells
It has been reported that the upregulation of TUG1
may promote the proliferation of cancer cells (27,28),
although its potential contribution to the pathogenicity of
melanoma remains elusive. Therefore, the proliferation of melanoma
cells was assessed using CCK-8 assays at 0, 24, 48 and 72 h
following transfection. The overexpression of TUG1 significantly
promoted A375 cell proliferation, while TUG1 knockdown
significantly suppressed SK-MEL-2 cell proliferation (Fig. 3A). The effect of TUG1 on melanoma
cell apoptosis was validated by flow cytometry. Overexpression of
TUG1 suppressed the induction of apoptosis in A375 cells, whereas
the depletion of TUG1 by si-TUG1 induced the apoptotic cascade in
SK-MEL-2 cells (Fig. 3B).
Similarly, the Transwell invasion assay indicated that TUG1
upregulation significantly induced the invasion of A375 cells,
whereas knockdown of TUG1 significantly reduced the invasive
capacity of SK-MEL-2 cells (Fig.
3C). As expected, the overexpression of TUG1 enhanced the
proliferation and invasive ability of A375 cells, whereas the
depletion of TUG1 suppressed the proliferation and invasive
activity of SK-MEL-2 cells.
TUG1 negatively regulates the expression
levels of miR-29c-3p and the levels of apoptosis- and
invasion-related proteins
Various reports have demonstrated that TUG1 can
recruit and modulate miRNAs that compete with the function and
expression of competitive endogenous (ce)RNAs (18,29).
To investigate the interaction between TUG1 and miR-29c-3p, RT-qPCR
assays were performed to determine the levels of the expression of
miR-29c-3p. The data indicated that overexpression of TUG1
suppressed the expression of miR-29c-3p in A375 cells, whereas the
depletion of TUG1 by si-TUG1 promoted the expression of miR-29c-3p
in SK-MEL-2 cells (Fig. 4A,
P<0.05). Moreover, the application of miR-29c-3p mimics reversed
the suppressive effect of TUG1 in A375 cells, and the miR-29c-3p
inhibitor reversed the induction of SK-MEL-2 cells by si-TUG1
(Fig. 4B, P<0.05). The
expression levels of the apoptosis-related protein Bcl-2 and the
invasion-related protein matrix metalloproteinase (MMP)2 were then
examined. The overexpression of TUG1 increased the expression of
Bcl-2 and MMP2 in A375 cells (Fig. 4C
and D). The depletion of TUG1 by si-TUG1 suppressed the
expression levels of Bcl-2 and MMP2 in SK-MEL-2 cells. Moreover,
miR-29c-3p mimics reversed the induction of A375 cells by TUG1. The
miR-29c-3p inhibitor reversed the suppressive effect of si-TUG1 on
SK-MEL-2 cells. These findings indicated that TUG1 negatively
regulated the expression of miR-29c-3p and the expression levels of
the apoptosis- and invasion-related proteins.
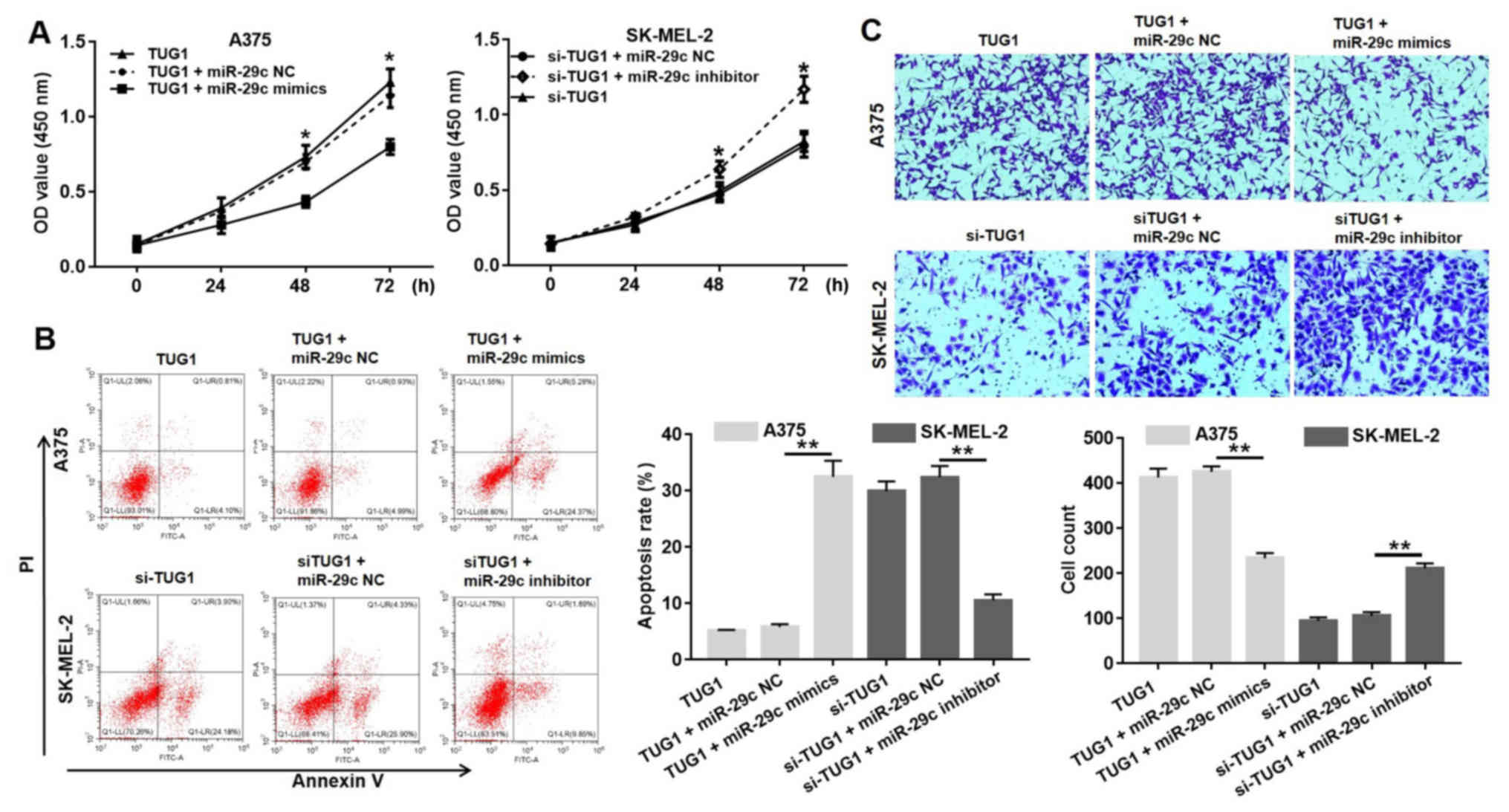
TUG1 promotes growth and invasion of
melanoma cells via targeting miR-29c-3p
To further confirm the regulatory effect of
miR-29c-3p on TUG1, CCK8 assay was performed to evaluate cell
viability. The CCK-8 assay indicated that miR-29c-3p mimics could
reverse the inductive effects of TUG1 on A375 cells, and miR-29c-3p
inhibitor could reverse the inhibitory effects of si-TUG1 on
melanoma cell proliferation (Fig.
5A). In addition, it was demonstrated that miR-29c-3p mimics
increased the apoptotic rate of A375 cells compared with the TUG1
group (Fig. 5B). The application
of the miR-29c-3p inhibitor reduced the apoptotic rate of SK-MEL-2
cells transfected with si-TUG1. Transwell assays indicated that
treatment with miR-29c-3p mimics reduced considerably the number of
A375 cells that invaded the lower chamber compared with NC cells
(transfected with TUG1 plus miR-29c NC). The application of the
miR-29c-3p inhibitor considerably increased the number of SK-MEL-2
cells that invaded the lower chamber compared with the NC cells
(transfected with si-TUG1 plus miR-29c NC). Collectively, si-TUG1
exerted its anti-proliferative, anti-invasive and pro-apoptotic
effects partly by targeting miR-29c-3p in melanoma cells.
RGS1 is a target of miR-29C-3p that is
regulated by TUG1
In recent years, the majority of studies have
demonstrated that lincRNA may modulate miRNAs as a ceRNA or
molecular sponge (30). To
determine whether TUG1 acts in a similar manner, the online
software Starbase 2.0 was used to predict the miRNA target sites.
Bioinformatic analysis indicated that miR-29c-3p had putative
binding sites with TUG1 (Fig. 6A).
Subsequently, dual luciferase reporter assays were performed in
order to explore the interaction between miR-29c-3p and TUG1. The
data indicated that TUG1 upregulated the luciferase activity of
pmirGLO-TUG1. Moreover, miR-29c-3p mimics reversed the inductive
effect of pmirGLO-TUG1, and reduced the luciferase activity of
pmirGLO-TUG1 (Fig. 6B). In
addition, western blot assays were used to identify whether TUG1
overexpression markedly improved RGS1 protein levels, while this
effect of TUG1 on RGS1 expression was markedly reduced by
miR-29c-3p mimics in A375 cells (Fig.
6C). In contrast to TUG1 overexpression, TUG1 knockdown caused
a significant reduction in RGS1 protein expression and the
miR-29c-3p inhibitor reversed the inhibitory effect of si-TUG1 on
the expression levels of RGS1 in SK-MEL-2 cells (Fig. 6C). Furthermore, the pull-down assay
indicated that miR-29c-3p was precipitated by the TUG1 probe
(Fig. 6D). These data revealed
that TUG1 acted as a ceRNA to regulate the expression of miR-29c-3p
and promote the expression of RGS1 in melanoma.
TUG1 regulates RGS1 expression via
miR-29C-3p
The crucial role of miRNAs in regulating protein
expression via post- transcriptional repression of mRNAs has been
extensively investigated (31). In
light of the above results, the TargetScan algorithm we used to
determine whether RGS1 is the target of miR-29c-3p. RGS1 was found
to be a prominent target of miR-29c-3p (Fig. 7A). To confirm that RGS1 is a direct
target of miR-29c-3p, the full length 3′-untranslated region (UTR)
fragments of RGS1 and corresponding mutant counterparts were cloned
directly downstream of the firefly luciferase gene. miR-29c-3p
transfection significantly suppressed the luciferase activity of
the reporter with RGS1 wild-type 3′-UTR (Fig. 7B). IHC staining indicated that RGS1
was highly expressed in human melanoma tissues compared with benign
nevi (Fig. 7C). Additionally,
correlation analysis revealed that the TUG1 expression level was
positively correlated to that of RGS1 (R2=0.7377, P<0.01)
(Fig. 7D).
TUG1 promotes melanoma growth in BALB/c
nude mice
To further evaluate the role of TUG1 in vivo,
a subcutaneous tumor model was established. A375 cells were
transfected with pcDNA3.1-TUG1 and injected into nude mice.
pcDNA3.1-TUG1 cell-derived xenograft tumors grew faster compared
with pcDNA3.1 cell-derived xenograft tumors (Fig. 8A). The mean tumor weight in the
pcDNA3.1-TUG1 group was also signifi-cantly higher compared with
that of the pcDNA3.1 cell-derived xenograft tumors (Fig. 8B and C). We further validated that
TUG1 expression levels in tumor tissues of the pcDNA3.1-TUG1 group
were considerably higher compared with those in the pcDNA3.1 group
(Fig. 8D). Moreover, the
expression of miR-29c was downregulated in the tumor tissues of the
pcDNA3.1-TUG1 group (Fig. 8E),
while the level of RGS1 was upregulated by pcDNA3.1-TUG1 group
(Fig. 8F and G). Additionally,
there was no statistically significant difference in the body
weight of mice between the pcDNA3.1 and pcDNA3.1-TUG1 groups
(Fig. 8H).
Discussion
Numerous studies have recently demonstrated that
lncRNAs play an important therapeutic role in a variety of human
diseases, including cancer. Moreover, a number of lncRNAs have been
found to be abnormally expressed in melanoma cell lines (11,32).
An increasing number of reports have revealed that the expression
of lncRNAs is regulated in a tissue-specific manner. lncRNAs play
specific roles in several pathological processes, including
tumorigenesis, angiogenesis, invasion and metastasis (33). These characteristics make lncRNAs
possible candidates as diagnostic or prognostic cancer biomarkers.
Over the past few years, a number of lncRNAs have been identified.
A total of 77 lncRNAs have been identified as significantly
dysregulated in melanoma cell lines and in primary melanoma samples
from patients (34).
TUG1 is a critical regulator of cell proliferation
in several cancer types, such as glioblastoma (35), osteosarcoma (26), bladder cancer (36) and colorectal cancer (37). TUG1 participates in the
proliferation, migration, invasion and apoptosis of cancer cells.
However, the role of TUG1 in melanoma cell lines and primary
melanomas remains unknown. The function of TUG1 and underlying
mechanism of action in melanoma remain largely unknown. Thus, there
is an urgent need to investigate the expression levels and
elucidate the underlying regulatory mechanism of TUG1 in
melanoma.
The data of the present study indicated that the
expression of TUG1 was upregulated in melanoma cell lines and
primary melanoma samples, and that this expression was correlated
with poor overall survival. Moreover, we determined the role of
TUG1 in melanoma progression. The findings were consistent with
those of previous reports (38)
and demonstrated that the knockdown of TUG1 suppressed cell
proliferation and invasion and induced cell apoptosis in melanoma.
The overexpression of TUG1 promoted the growth and invasion of
melanoma cells, and inhibited the induction of apoptosis. The data
indicated that TUG1 played an important role in melanoma,
suggesting that it may act as an oncogene.
Although a large number of lncRNAs were reported to
play critical roles in human malignancies, the underlying
mechanisms by which lncRNAs modulate tumor progression remain
unclear (39). Recently, lncRNAs
have been reported to act as ceRNAs that downregulate the
expression and activities of miRNAs. The downregulation of miRNAs
can subsequently adjust the suppression of miRNA targets. It was
hypothesized that TUG1 targets miRNAs in melanoma. In line with the
previous reports, our results indicated that TUG1 acted as an
endogenous sponge of miR-29c-3p, suppressing miR-29c-3p expression.
Recent reports have revealed that miR-138-5p can suppress cervical
(38) and pancreatic cancer
(40) progression by targeting
SIRT1. The TargetScan algorithm was used to identify whether RGS1
was the target gene of miR-29C-3p. It was observed that miR-29c-3p
could reverse the inhibitory effect of TUG1 on melanoma cancer cell
progression, which may be involved in the activation of RGS1. Taken
together, these data indicated that TUG1 facilitated cell
proliferation and invasion and suppressed apoptosis by regulating
the miR-29c-3p/RGS1 axis in melanoma, suggesting that lncRNA TUG1
is a promising diagnostic marker for melanoma patients. The results
further indicated that the regulation of RGS1 in melanoma by TUG1
required the activity of miR-29C-3p.
In summary, the present study verified that TUG1
plays a key role in melanoma progression as an oncogene by
promoting proliferation and invasion of melanoma cells.
Mechanistically, the results of the present study revealed that
TUG1 facilitated proliferation and invasion and suppressed the
induction of apoptosis by regulating the miR-29c-3p/RGS1 axis in
melanoma. The results suggest that the lncRNA TUG1 may be a useful
prognostic biomarker in melanoma patients, as well as a novel
potential therapeutic target for melanoma, which may be further
investigated in future experiments.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
YW, GL and AL are responsible for sample collection,
experiment design and execution. LR and KW analyzed and interpreted
the experimental data. AL drafted the manuscript, reviewed and
approved the final draft of this manuscript prior to submission.
All the authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The research protocol was approved by the Ethics
Committee of Xinxiang Central Hospital (approval no. 2017068801).
The sample collection and surgical procedures were not harmful to
the patients. Written informed consent was obtained from all
patients. The research protocol conformed to the principles
outlined in the Declaration of Helsinki. The animal experiments
were fully approved by the Ethics Committee of Animal Experiments
of the Xinxiang Central Hospital. In addition, the present study
was strictly performed in accordance with the recommendations in
the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests to disclose.
Acknowledgments
The authors gratefully acknowledge support from the
Ke Wu (Xinxiang Central Hospital) for the valuable suggestions.
Abbreviations:
|
TUG1
|
taurine upregulated gene 1
|
|
RGS1
|
regulator of G-protein signaling 1
|
|
WT
|
wild-type
|
|
MT
|
mutant
|
References
|
1
|
Maddodi N and Setaluri V: Role of UV in
cutaneous melanoma. Photochem Photobiol. 84:528–536. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torre LA, Sauer AM, Chen MS Jr,
Kagawa-Singer M, Jemal A and Siegel RL: Cancer statistics for Asian
Americans, Native Hawaiians, and Pacific Islanders, 2016:
Converging incidence in males and females. CA Cancer J Clin.
66:182–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu T and Du Y: lncRNAs: From basic
research to medical application. Int J Biol Sci. 13:295–307. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown CJ, Hendrich BD, Rupert JL,
Lafrenière RG, Xing Y, Lawrence J and Willard HF: The human XIST
gene: Analysis of a 17 kb inactive X-specific RNA that contains
conserved repeats and is highly localized within the nucleus. Cell.
71:527–542. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clark MB, Amaral PP, Schlesinger FJ,
Dinger ME, Taft RJ, Rinn JL, Ponting CP, Stadler PF, Morris KV,
Morillon A, et al: The reality of pervasive transcription. PLoS
Biol. 9:e1000625discussion e1001102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang HM, Yang FQ, Chen SJ, Che J and
Zheng JH: Upregulation of long non-coding RNA MALAT1 correlates
with tumor progression and poor prognosis in clear cell renal cell
carcinoma. Tumour Biol. 36:2947–2955. 2015. View Article : Google Scholar
|
|
9
|
Gao K, Ji Z, She K, Yang Q and Shao L:
Long non-coding RNA ZFAS1 is an unfavourable prognostic factor and
promotes glioma cell progression by activation of the Notch
signaling pathway. Biomed Pharmacother. 87:555–560. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Zhao L and Wang Q: Overexpression of
long non-coding RNA HOTTIP increases chemoresistance of
osteosarcoma cell by activating the Wnt/β-catenin pathway. Am J
Transl Res. 8:2385–2393. 2016.
|
|
11
|
Khaitan D, Dinger ME, Mazar J, Crawford J,
Smith MA, Mattick JS and Perera RJ: The melanoma-upregulated long
noncoding RNA SPRY4-IT1 modulates apoptosis and invasion. Cancer
Res. 71:3852–3862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Flockhart RJ, Webster DE, Qu K,
Mascarenhas N, Kovalski J, Kretz M and Khavari PA: BRAFV600E
remodels the melanocyte transcriptome and induces BANCR to regulate
melanoma cell migration. Genome Res. 22:1006–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang L, Zhang W, Su B and Yu B: Long
noncoding RNA HOTAIR is associated with motility, invasion, and
metastatic potential of metastatic melanoma. BioMed Res Int.
2013:2510982013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pasmant E, Sabbagh A, Vidaud M and Bièche
I: ANRIL, a long, noncoding RNA, is an unexpected major hotspot in
GWAS. FASEB J. 25:444–448. 2011. View Article : Google Scholar
|
|
15
|
Rapicavoli NA and Blackshaw S: New meaning
in the message: Noncoding RNAs and their role in retinal
development. Dev Dyn. 238:2103–2114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Katsushima K, Natsume A, Ohka F, Shinjo K,
Hatanaka A, Ichimura N, Sato S, Takahashi S, Kimura H, Totoki Y, et
al: Targeting the Notch-regulated non-coding RNA TUG1 for glioma
treatment. Nat Commun. 7:136162016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang L, Wang W, Li G, Sun C, Ren Z, Sheng
H, Gao H, Wang C and Yu H: High TUG1 expression is associated with
chemotherapy resistance and poor prognosis in esophageal squamous
cell carcinoma. Cancer Chemother Pharmacol. 78:333–339. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu L, Chen X, Zhang Y, Hu Y, Shen X and
Zhu W: Long non-coding RNA TUG1 promotes endometrial cancer
development via inhibiting miR-299 and miR-34a-5p. Oncotarget.
8:31386–31394. 2017.PubMed/NCBI
|
|
19
|
Sun J, Ding C, Yang Z, Liu T, Zhang X,
Zhao C and Wang J: The long non-coding RNA TUG1 indicates a poor
prognosis for colorectal cancer and promotes metastasis by
affecting epithelial-mesenchymal transition. J Transl Med.
14:422016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang MD, Chen WM, Qi FZ, Sun M, Xu TP, Ma
P and Shu YQ: Long non-coding RNA TUG1 is up-regulated in
hepatocellular carcinoma and promotes cell growth and apoptosis by
epigenetically silencing of KLF2. Mol Cancer. 14:1652015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma B, Li M, Zhang L, Huang M, Lei JB, Fu
GH, Liu CX, Lai QW, Chen QQ and Wang YL: Upregulation of long
non-coding RNA TUG1 correlates with poor prognosis and disease
status in osteosarcoma. Tumour Biol. 37:4445–4455. 2016. View Article : Google Scholar
|
|
22
|
Zhang EB, Yin DD, Sun M, Kong R, Liu XH,
You LH, Han L, Xia R, Wang KM, Yang JS, et al: P53-regulated long
non-coding RNA TUG1 affects cell proliferation in human non-small
cell lung cancer, partly through epigenetically regulating HOXB7
expression. Cell Death Dis. 5:e12432014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iliev R, Kleinova R, Juracek J, Dolezel J,
Ozanova Z, Fedorko M, Pacik D, Svoboda M, Stanik M and Slaby O:
Overexpression of long non-coding RNA TUG1 predicts poor prognosis
and promotes cancer cell proliferation and migration in high-grade
muscle-invasive bladder cancer. Tumour Biol. 37:13385–13390. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang PQ, Wu YX, Zhong XD, Liu B and Qiao
G: Prognostic significance of overexpressed long non-coding RNA
TUG1 in patients with clear cell renal cell carcinoma. Eur Rev Med
Pharmacol Sci. 21:82–86. 2017.PubMed/NCBI
|
|
25
|
Zhang Q, Geng PL, Yin P, Wang XL, Jia JP
and Yao J: Downregulation of long non-coding RNA TUG1 inhibits
osteosarcoma cell proliferation and promotes apoptosis. Asian Pac J
Cancer Prev. 14:2311–2315. 2013. View Article : Google Scholar
|
|
26
|
Yun-Bo F, Xiao-Po L, Xiao-Li L, Guo-Long
C, Pei Z and Fa-Ming T: lncRNA TUG1 is upregulated and promotes
cell proliferation in osteosarcoma. Open Med (Wars). 11:163–167.
2016.
|
|
27
|
Li G, Liu K and Du X: Long Non-Coding RNA
TUG1 Promotes proliferation and inhibits apoptosis of osteosarcoma
cells by sponging miR-132-3p and upregulating SOX4 expression.
Yonsei Med J. 59:226–235. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu H, Zhou G, Fu X, Cui H, Pu G, Xiao Y,
Sun W, Dong X, Zhang L, Cao S, et al: Long noncoding RNA TUG1 is a
diagnostic factor in lung adenocarcinoma and suppresses apoptosis
via epigenetic silencing of BAX. Oncotarget. 8:101899–101910.
2017.PubMed/NCBI
|
|
29
|
Li J, An G, Zhang M and Ma Q: Long
non-coding RNA TUG1 acts as a miR-26a sponge in human glioma cells.
Biochem Biophys Res Commun. 477:743–748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mazar J, Sinha S, Dinger ME, Mattick JS
and Perera RJ: Protein-coding and non-coding gene expression
analysis in differentiating human keratinocytes using a
three-dimensional epidermal equivalent. Mol Genet Genomics.
284:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View Article : Google Scholar
|
|
34
|
Xie HW, Wu QQ, Zhu B, Chen FJ, Ji L, Li
SQ, Wang CM, Tong YS, Tuo L, Wu M, et al: Long noncoding RNA
SPRY4-IT1 is upregulated in esophageal squamous cell carcinoma and
associated with poor prognosis. Tumour Biol. 35:7743–7754. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao Z, Wang B, Hao J, Man W, Chang Y, Ma
S, Hu Y, Liu F and Yang J: Downregulation of the long non-coding
RNA taurine- upregulated gene 1 inhibits glioma cell proliferation
and invasion and promotes apoptosis. Oncol Lett. 15:4026–4032.
2018.PubMed/NCBI
|
|
36
|
Jiang H, Hu X, Zhang H and Li W:
Down-regulation of lncRNA TUG1 enhances radiosensitivity in bladder
cancer via suppressing HMGB1 expression. Radiat Oncol. 12:652017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cao J, Han X, Qi X, Jin X and Li X: TUG1
promotes osteosarcoma tumorigenesis by upregulating EZH2 expression
via miR-144-3p. Int J Oncol. 51:1115–1123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu J, Shi H, Liu H, Wang X and Li F: Long
non-coding RNA TUG1 promotes cervical cancer progression by
regulating the miR-138-5p-SIRT1 axis. Oncotarget. 8:65253–65264.
2017.PubMed/NCBI
|
|
39
|
Cheetham SW, Gruhl F, Mattick JS and
Dinger ME: Long noncoding RNAs and the genetics of cancer. Br J
Cancer. 108:2419–2425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tian S, Guo X, Yu C, Sun C and Jiang J:
miR-138-5p suppresses autophagy in pancreatic cancer by targeting
SIRT1. Oncotarget. 8:11071–11082. 2017.PubMed/NCBI
|