Introduction
Immunotoxins are fusion proteins consisting of an
Ig-derived targeting domain and a highly toxic protein or peptide.
These have been under intense study over the past few decades, and
several immunotoxins are under pre-clinical and clinical evaluation
(www.clinicaltrials.gov) (1,2). In
2018, the first immunotoxin was approved by the US Food and Drug
Administration (FDA), namely moxetumomab pasudotox for the
treatment of hairy cell leukemia (3). Fusion proteins, consisting of a toxic
protein or peptide coupled to a non-Ig-derived affinity protein are
also under evaluation and are denoted fusion toxins (4). One fusion toxin has been approved by
the FDA for therapy, namely denileukin diftitox for the treatment
of cutaneous T-cell lymphoma (5).
The toxin part of immunotoxins and fusion toxins is
often of bacterial or plant origin. A well-studied toxin is PE38,
which is a truncated version of Pseudomonas exotoxin A
(ETA), where domain I, responsible for target cell interaction has
been removed. When PE38-containing constructs are internalized, a
cleavage by furin occurs in domain II, followed by release into the
cytosol. PE38 then exerts its toxic effect through ribosylation of
elongation factor 2, effectively preventing further protein
synthesis, which leads to cell death. A drawback with PE38-based
constructs is the relatively high immunogenicity, limiting the
number of consecutive injections possible in animals and humans
before anti-drug antibodies are formed (6). Several efforts to decrease the
immunogenicity have been undertaken, where B- and T-cell epitopes
reactive in humans and mice have been identified and removed
(7-10). One of these variants is PE38X8
(10). It has 8 mutations to
remove mouse B-cell epitopes and has previously been found suitable
for fusion with different targeting domains (10-12).
Efforts to minimize the size of the deimmunized versions of PE38
have also been undertaken, where a large portion of the
translocation domain II has been replaced by a furin cleavage site
and a spacer with the amino acid sequence GGS, leading to PE25, a
deimmunized and size-decreased variant with a toxicity that is
reported to be similar as PE38 (13,14).
PE25 has 10 mutations in the catalytic domain III to suppress B-
and T-cell epitopes.
In recent years, alternatives to Ig-derived
targeting domains have begun to emerge, consisting of small
engineered affinity proteins, so-called alternative scaffold
proteins (15). They are often
based on naturally occurring proteins or protein-domains, and their
development has been driven by the notion that antibodies have
limitations in different applications. A few fusion toxins,
including alternative scaffold proteins have been described in the
literature (11,12,16-19),
although their properties are relatively unexplored.
Albumin binding domain (ABD)-derived affinity
proteins (ADAPTs) are a novel group of alternative scaffold
proteins originating from one of the albumin-binding domains
(GA148-GA3) of streptococcal protein G (20). These non-immunoglobulin-based
ligands fold into a three-helical bundle motif, have no naturally
occurring cysteines and usually have high thermal and proteolytic
stability. ADAPTs consist of only 46 amino acids, which renders
them considerably smaller than the majority of other folded
alternative scaffold proteins. Furthermore, in contrast to several
other alternative scaffold proteins, the ADAPTs have been designed
to allow for bi-specificity by preserving the surface residues
interacting with albumin when selecting for novel binders (21). However, for some ADAPTs, the
interaction with albumin is not desired and has been removed. Among
others, ADAPTs binding to the tumor cell markers, human epidermal
growth factor receptor 2 (HER2) and human epidermal growth factor
receptor 3 (HER3), have been described (22,23).
HER2 (also known as ErbB2) is a cell surface bound
tyrosine kinase receptor that belongs to the human epidermal growth
factor receptor family with important roles in cancer development
(24). HER2 is a clinically
validated target and is often overexpressed in breast, ovarian and
gastric cancer, while its expression in normal cells is limited
(25). Several HER2 targeted drugs
have been approved for clinical use by the FDA e.g., Herceptin
(trastuzumab) (26), Perjeta
(pertuzumab) (27) and Kadcyla
(trastuzumab emtansine) (28).
A group of ADAPTs binding to HER2 has been generated
and has been found to interact with the receptor with different
affinities (23). The most studied
variant, ADAPT6, is a ligand targeting domain IV of HER2
with an equilibrium dissociation constant (KD) of 0.5
nM. The natural affinity for serum albumin in this variant has been
removed.
The small sizes of ADAPTs that do not have affinity
for albumin, such as ADAPT6, are associated with short
in vivo half-lives due to rapid renal clearance. When used
for in vivo molecular imaging, where rapid target
accumulation in combination with rapid clearance of unbound
molecules is desirable, this feature has proven to be highly
beneficial. ADAPT6 has been investigated as a
radionuclide molecular imaging agent and appears to be a promising
imaging agent for visualization of HER2 expression in human tumors
implanted in mice (29,30). However, for therapeutic
applications, a short residence time in circulation often
correlates with the need for multiple injections, which limits the
applicability of small proteins. Hence, strategies to extend the
in vivo half-life of small protein therapeutics have been
developed. One strategy is to fuse the targeting molecule to an
albumin-binding moiety to increase the size of the construct in
vivo by non-covalent association with albumin. Thereby the
cut-off size of glomerular filtration in the kidneys can be
exceeded. Association with serum albumin also takes advantage of
the long serum half-life of albumin, mediated by the interaction
with the neonatal Fc receptor (FcRn). The same albumin-binding
domain used for development of ADAPTs has been reported as fusion
partner of several protein-therapeutics, for half-life extension
(31-33). Furthermore, affinity-improved
variants of this domain have been developed, where
ABD035 with its femtomolar affinity for human serum
albumin (HSA) has shown to further prolong the serum half-life
(20).
In this study, fusion toxins were created,
consisting of ADAPT6 coupled to truncated versions of
ETA (PE38X8 or PE25), with or without ABD035.
Biochemical characterization, including toxicity to cell lines with
differential HER2 expression levels, was performed and revealed
highly potent fusion toxins with exquisite specificity for cell
lines expressing the HER2 receptor. The biodistribution in mice was
also investigated.
Materials and methods
General
All chemicals were from Sigma-Aldrich or Merck
unless otherwise stated. Restriction enzymes were from New England
Biolabs.
Gene construction
The gene encoding ADAPT6-ABD-PE25 with
the N-terminal amino acid sequence MHEHEHEDANS was synthesized by
Thermo Fisher Scientific and delivered in the pMK-RQ vector
(Novagen). The gene was sub-cloned into the expression vector,
pET-26b(+) (Novagen), with NdeI and XhoI restriction
enzymes surrounding the gene, resulting in the vector,
pET26-ADAPT6-ABD-PE25. The construct also included a
BamHI restriction site between the gene fragments encoding
ADAPT6 and the ABD, as well as two NcoI
restriction sites surrounding the gene fragment encoding the ABD
domain. The ABD used in this study was ABD035, an
engineered version with improved affinity for HSA (34). The gene fragment encoding PE25 was
derived from a deimmunized version of PE38 with the following amino
acid alterations: R427A, F443A, D463A, R467A, L477H, R490A, R494A,
R505A, R538A and L552E, and the deletion of the majority of domain
II (∆251-273 and ∆285-394) (10).
A furin cleavage site was placed at the N-terminus of PE25 and it
was connected to domain III of PE25 with the amino acids GGS. The
expression vector for ADAPT6-PE25 was created by the
digestion of pET26-ADAPT6-ABD-PE25 with NcoI
followed by re-ligation of the vector. The expression vector for
ADAPT6-ABD-PE38X8 was created by isolation of the gene
fragment encoding ADAPT6 by restriction digestion of
pET26-ADAPT6-ABD-PE25 with NdeI and BamHI,
followed by ligation with the expression vector encoding
ZHER2:2891-ABD-PE38X8, which had been cut with the same
enzymes, replacing ZHER2:2891 (11). The expression vector for
ZTaq-ABD-PE25 was created by replacing the gene fragment
encoding ADAPT6 in pET26-ADAPT6-ABD-PE25 with
the gene encoding ZTaq (35), using the NdeI and
BamHI restriction sites. All connections between all domains
in the constructs included linkers with the amino acids sequence
(S4G)3. All constructs were verified by DNA
sequencing. The vector containing the gene for expression of free
HEHEHE-DANS-ADAPT6 (36), not fused to any peptides, was
included for blocking experiments.
Protein expression and purification
Escherichia coli (E. coli) [BL21 Star
(DE3)] (Thermo Fisher Scientific) was used for expression of the
fusion toxins and free ADAPT6. Cells harboring the
expression plasmids were grown in 500 ml cultures in tryptic soy
broth supplemented with 5 g/l yeast extract at 37°C until
OD600 reached 1.5, after which protein expression was
induced by isopropyl β-D-1-thiogalactopyranoside (IPTG; Appolo
Scientific) at a concentration of 1 mM. Protein expression was
carried out for 2.5 h after which the cells were harvested by
centrifugation (4°C, 7,000 × g, 10 min). E. coli cells
expressing ADAPT6-PE25 were resuspended in 20 ml loading
buffer (300 mM NaCl, 50 mM Na-phosphate, pH 7.0) supplemented with
Complete EDTA-free protease inhibitor cocktail (Roche Diagnostics)
and lysed by sonication (Sonics VCX-750; Sonics & Material).
ADAPT6-PE25 was purified from the supernatant by
immobilized metal-ion affinity chromatography (IMAC) on a
Ni-Sepharose 6 Fast Flow resin (GE Healthcare) under native
conditions according to the manufacturer's protocol with imidazole
elution. The eluted material was pooled and diluted 5 times with
deionized water. Subsequently, the material was loaded on an anion
exchange HiTrap Q HP column (1 ml; GE Healthcare). The running
buffer had the following composition: 60 mM NaCl, 10 mM
Na-phosphate, pH 7.0. Bound material was eluted by a NaCl-gradient
from 0.06 to 1 M. The fractions containing ADAPT6-PE25
were pooled and buffer was exchanged to PBS (10 mM Na-phosphate,
2.7 mM KCl, 137 mM NaCl, pH 7.4) by passage through a PD-10
desalting column (GE Healthcare).
Cell pellets containing fusion toxins, including the
ABD were resuspended in TST-buffer [25 mM tris(hydroxymethyl)
aminomethane, 1 mM EDTA, 200 mM NaCl, 0.05% Tween-20, pH 8.0]
supplemented with Complete EDTA-free protease inhibitor cocktail
and lysed by sonication. The proteins were purified by affinity
chromatography on a HiTrap NHS sepharose column (GE Healthcare)
with immobilized HSA. The supernatant following sonication was
loaded on the column after it had been equilibrated with TST, with
subsequent washing of the column with TST. The column was further
washed with 5 mM ammonium acetate (pH 5.5) followed by elution with
0.5 M acetic acid. Fractions containing protein were pooled
followed by buffer exchange to PBS by passage over a PD-10 column.
Protein concentrations were determined by the BCA protein assay kit
(Thermo Fisher Scientific). The molecular mass of the fusion toxins
was determined by liquid chromatography electrospray ionization
mass spectrometry (Agilent Technologies). The samples were diluted
to a final concentration of 100 ng/μl in 1X PBS prior to
analysis on a Bruker impact II time of flight instrument equipped
with an ESI source. The samples were injected via an online
connected Dionex UltiMate 3000 ultra-high performance liquid
chromatography (UHPLC) system (Thermo Fisher Scientific). The UHPLC
was equipped with a ProSwift RP-4H column (1×50 mm, product no.
069477, Thermo Fisher Scientific). The chromatography used for the
analysis utilized two solvents: Solvent A (3% acetonitrile, 0.1%
formic acid) and solvent B (95% acetonitrile, 0.1% formic acid) and
was conducted using a flow rate of 200 μl/min. The gradient
used was as follows: 4% solvent B for 2 min, 4-90% solvent B within
6 min, 90% solvent B for 2 min, 90-4% solvent B within 1 min
followed by 4% solvent B for 4 min. In order to achieve spray, a
capillary voltage of 4.5 kV was applied. The mass spectrometer was
run in a positive mode with a mass range from 300 to 3.000 m/z and
a scan rate of 1 Hz. Spectra were created by averaging every 2
scans. SDS-PAGE was performed by loading approximately 10 μg
sample in each lane of a 4-12% gel, followed by electrophoretic
separation. The gel was stained with gelcode blue safe protein
stain (Thermo Fisher Scientific). The purities were determined by
densitometric analysis of the lanes on the SDS-PAGE gel shown in
Fig. 1B. The lanes were visualized
using a Chemidoc XRS+ and were analyzed by Image Lab 4.1 software
(Bio-Rad laboratories). Free ADAPT6 was purified by heat
treatment (90°C, 10 min) followed by IMAC purification as
previously described (36).
Biosensor analysis
A Biacore 3000 and a Biacore T200 instrument (GE
Healthcare) were used for biosensor analysis. The extracellular
domain of HER2 (Sino Biological) was immobilized on a CM5-chip by
amine coupling in sodium acetate buffer at pH 4.5. On a second
CM5-chip, HSA (Novozymes) and MSA (Sigma-Aldrich) were immobilized
in the same manner. The final immobilization level of the
extracellular domain of HER2 was 320 RU. The final immobilization
level of HSA and MSA was 265 and 207 RU respectively. Reference
flow cells were created on both chips by activation and
deactivation. HBS-EP [10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 150 mM
NaCl, 3 mM EDTA, 0.05% Tween-20, pH 7.4] was used as running buffer
and for dilution of the analytes. All experiments were performed at
25°C with a flow-rate of 50 μl/min. The surfaces were
regenerated after each injection by 10 mM HCl. The kinetic
parameters for the interaction between fusion toxins and HER2 was
derived from Fig. S1 using
Biacore T200 evaluation software (version 2.0) by simultaneously
considering single injections of each dilution of the analyte in
the dilution series. Each dilution series was injected twice. The
kinetic parameters for the interaction between fusion toxins and
HSA and MSA was derived from Fig.
S2 using Biaevaluation (version 4.1) by simultaneously
considering all sensorgrams recorded for single injections of each
dilution of the analyte in the dilution series. Each dilution
series was injected twice.
A Biacore 8K (GE Healthcare) was used for the
biosensor analysis presented in Fig.
S3. The extracellular domains of HER2, HSA and MSA were
immobilized on flow cell 2 in different channels on a CM5-chip by
amine coupling in sodium acetate buffer at pH 4.5. Flow cell 1 in
each channel was activated/deactivated and used as reference. The
final immobilization levels were as follows: 721 RU (HER2), 945 RU
(HSA) and 437 RU (MSA). PBS supplemented with 0.05% Tween-20 was
used for the dilution of the samples and as running buffer.
Cells and cell culture
SKBR3 (breast cancer), AU565 (breast cancer), SKOV3
(ovarian cancer) and A549 (lung cancer) cell lines were obtained
from ATCC and were grown in the media recommended: McCoy's 5a or
Dulbecco's modified Eagle's medium (Sigma-Aldrich) in a humidified
incubator at 37°C in 5% CO2 atmosphere. The cell lines
were grown for a maximum of 3 months after resuscitation and were
routinely tested for mycoplasma infection.
Cytotoxicity of the fusion toxins
To evaluate the cytotoxicity of the fusion toxins on
the SKBR3, AU565, SKOV3 and A549 cell lines, approximately 5,000
cells/well were seeded in 96-well plates and were allowed to attach
for 4 h. Subsequently, the medium was replaced with fresh medium
containing the fusion toxins and the cells were incubated for 72 h
at 37°C, followed by the assessment of cell viability by the
measurement of intracellular dehydrogenase activity using a CCK-8
kit according to the manufacturer's protocol (Sigma-Aldrich). The
obtained absorbance values were analyzed by Graphpad Prism
(Graphpad) to derive IC50 values. The data points were
fitted with a log (inhibitor) vs. response (4 parameters)
function.
Radiolabeling
Purified fusion toxins were conjugated with a
benzylisothiocyanate derivative of the CHX-A''-DTPA chelator
(Macrocyclics) as previously described (12). In brief, the conjugates were mixed
with 111InCl3 (8-10 MBq in 20-30 μl
0.05 M HCl) and incubated for 60 min at room temperature. For
purification of the radiolabeled conjugates, the mixture was passed
through a NAP-5 column pre-equilibrated and eluted with PBS. The
radiochemical yield and purity of the conjugates were determined
using silica-impregnated ITLC (Instant Thin Layer Chromatography)
strips (150-771 DARK GREEN Tec-Control Chromatography strips,
Biodex Medical Systems) eluted with 0.2 M citric acid and measured
using the Cyclone Storage Phosphor System (PerkinElmer). To
evaluate the stability of the labeling, the radiolabeled conjugates
were incubated with a 500-fold molar excess of EDTA at room
temperature for 4 h, and the percentage of protein-bound
radioactivity was determined using radio-ITLC as mentioned
above.
Biodistribution
Comparative biodistribution studies of
111In-labeled fusion toxins were performed in 32 female
NMRI mice (Taconic M&B Denmark, 8-10 weeks old at arrival).
Mice were housed with free access to food and water in rooms with
controlled temperature and humidity in an animal facility at
Uppsala University. The mice (weighing 25.0±0.7 g) were randomly
divided into 8 groups with 4 mice in each group. The animals were
injected intravenously with 1 μg (20 kBq) radiolabeled
fusion toxin per animal in 100 μl PBS containing 2% BSA. At
4 and 24 h after the injection, the mice were sacrificed by
injection of a lethal dose of anesthesia (20 μl of
Ketalar-Rompun solution per gram body weight; Ketalar, 200 mg/kg
body weight; Rompun, 20 mg/kg body weight, i.p.) followed by heart
puncture and exsanguination with a heparinized syringe. Organs and
tissue samples were collected, weighed and the radioactivity was
measured using an automated gamma-spectrometer. The animal
experiments were planned and performed in accordance with Sweden's
national legislation on laboratory animals' protection. The animal
studies were approved by the local ethics committee for animal
research in Uppsala, Sweden.
Flow cytometry
To investigate the HER2 expression level in the
SKOV3, SKBR3, AU565, and A549 cell lines, 2×105 cells
were incubated with trastuzumab obtained in the form of Herceptin
(cat. no. 115140, Apoteket) (5 μg/ml) as primary antibody
for 30 min, followed by Alexa Fluor 647 conjugated goat anti-human
IgG (H+L) (Thermo Fisher Scientific) (cat. no. 1A-21445) (5
μg/ml) as secondary antibody for 30 min. The cells were
analyzed on a Gallios flow cytometer (Beckman Coulter). A total of
10,000 events were recorded for each sample and plotted using
Kaluza software (Beckman Coulter).
Statistical analysis
Statistical analysis was performed using Prism 8 for
macOS (version 8.0.2) (Graphpad). An unpaired two-tailed Student's
t-test was used when comparing 2 groups of values. One-way ANOVA
with Tukey's (Fig. 2) or
Bonferroni's (Table IV) post hoc
multiple comparisons tests were used when comparing more than 2
groups. The cut-off value for significance was P<0.05. The
number of repeats/measurements in each group were at least 4. The
data are presented as the means ± 1 SD.
 | Table IVComparative biodistribution of the
111In-labeled fusion toxins in mice 4 and 24 h following
intravenous injection.a |
Table IV
Comparative biodistribution of the
111In-labeled fusion toxins in mice 4 and 24 h following
intravenous injection.a
|
ZHER2:2891-ABD-PE38X8 |
ADAPT6-ABD-PE38X8 |
ADAPT6-ABD-PE25 |
ADAPT6-PE25 |
|---|
| At 4 h
post-injection |
| Blood | 6.0±0.7c,d,e | 9.0±0.7d,e | 3.2±0.1e | 0.2±0.1 |
| Heart | 2.8±0.3d,e | 3.0±0.8 | 1.1±0.2 | 0.7±0.1 |
| Lung | 2.4±1.3 | 3.3±0.4d | 1.3±0.1e | 3.1±0.4 |
| Sal. gland | 1.0±0.2e | 1.5±0.2d,e | 0.7±0.2 | 0.2±0.1 |
| Liver | 23.0±2.0c | 9.0±0.4d,e | 43.0±7.0 | 33.0±6.0 |
| Spleen | 9.0±1.0c | 3.3±0.3d | 19.0±4.0 | 7.0±3.0 |
| Pancreas | 0.6±0.1 | 0.9±0.2 | 0.4±0.1 | 0.3±0.2 |
| Stomach | 1.1±0.4 | 1.0±0.1e | 0.5±0.2 | 0.2±0.1 |
| Kidney | 8.0±4.0c | 58.0±3.0d,e | 13.0±1.0e | 28.0±4.0 |
| Colon | 0.9±0.1c,e | 1.1±0.1e | 0.6±0.3 | 0.3±0.1 |
| Skin | 1.0±0.1d,e | 1.8±0.3d,e | 0.6±0.1e | 0.2±0.1 |
| Muscle | 0.6±0.1d | 0.7±0.1d,e | 0.3±0.1 | 0.3±0.1 |
| Bone | 1.9±0.2 | 1.3±0.1 | 1.1±0.3 | 1.6±0.4 |
| GI tractb | 4.2±0.5 | 5.2±0.3 | 6.9±4.4e | 2.6±0.5 |
| Carcassb | 16.0±1.0d,e | 22.0±1.8b,c | 4.0±3.0 | 7.0±1.0 |
| At 24 h
post-injection |
| Blood | 2.0±0.1e | 1.8±0.1d,e | 1.3±0.2e | 0.1±0.1 |
| Heart | 1.8±0.2c,d,e | 1.0±0.1e | 0.7±0.2 | 0.5±0.1 |
| Lung | 1.7±0.2c | 1.0±0.2 | 0.8±0.1 | 2.6±0.8 |
| Sal. gland | 1.2±0.1c,d,e | 0.7±0.1e | 0.6±0.1e | 0.2±0.1 |
| Liver | 22.0±2.0c | 6.0±1.0d,e | 32.0±5.0 | 27.0±3.0 |
| Spleen | 9.0±2.0c,e | 2.2±0.4d | 11.4±1.3e | 4.0 ±1.0 |
| Pancreas | 0.6±0.1e | 0.4±0.1e | 0.4±0.2 | 0.1±0.1 |
| Stomach | 0.7±0.1d,e | 0.5±0.1e | 0.4±0.2 | 0.2±0.1 |
| Kidney | 9.0±1.0c,e | 46.0±2.0d,e | 11.0±1.0e | 19.0±1.0 |
| Colon | 0.9±0.1c,d | 0.3±0.1 | 0.3±0.1 | 0.3±0.2 |
| Skin | 1.2±0.1e | 1.3±0.3e | 0.7±0.2e | 0.1±0.1 |
| Muscle | 0.5±0.1d,e | 0.4±0.1 | 0.3±0.1 | 0.2±0.1 |
| Bone | 1.8±0.3c | 0.7±0.2e | 0.8±0.2 | 1.6±0.2 |
| GI tracta | 2.7±0.7 | 1.1±0.2 | 1.0±0.3 | 0.6±0.2 |
| Carcassb | 13.3±1.4d,e | 11.5±1.6d,e | 7.0±1.0 | 5.4±0.5 |
Results
Construction, purification and initial
biochemical characterization
To investigate the feasibility of utilizing ADAPTs
for specific delivery of toxins to cancer cells, two constructs
consisting of ADAPT6, specifically targeting the HER2
receptor, coupled to PE25 were produced, with or without an ABD. To
investigate the differences between PE25 and PE38X8 as part of a
protein fusion with ADAPT6, a third construct consisting
of ADAPT6 coupled to ABD and PE38X8 was also produced.
As negative control in the in vitro experiments, a protein
with similar size and fold, ZTaq, was used in lieu of
ADAPT6 fused to the ABD and PE25. ZTaq
interacts specifically with DNA polymerase from Thermus aquaticus
and was not expected to interact with any protein of human origin.
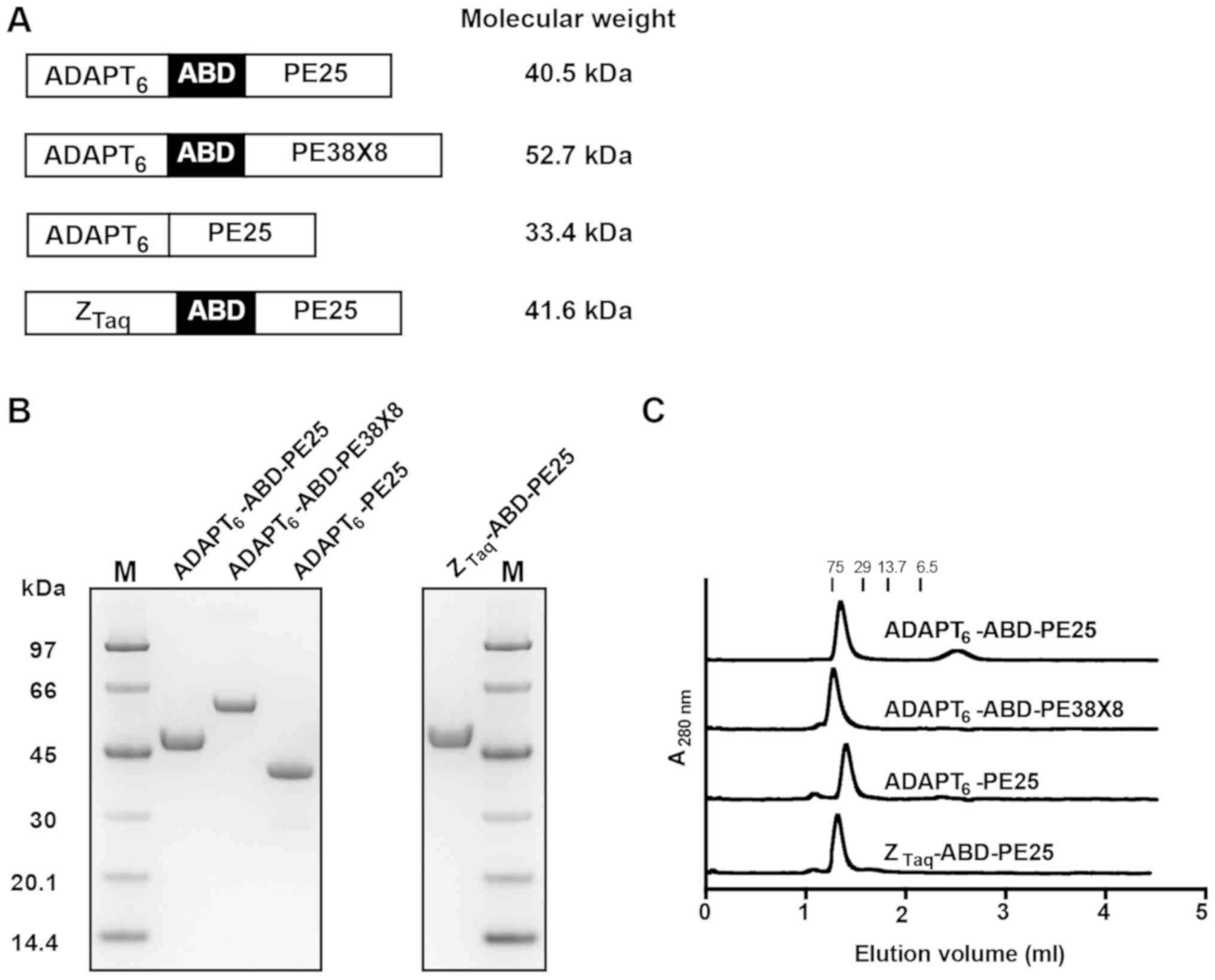
A schematic description of all constructs is presented in Fig. 1A.
The fusion toxins were expressed in the cytoplasm of
E. coli. ADAPT6-PE25 was successfully
purified by IMAC followed by anion exchange chromatography. The
other constructs, including the ABD, were purified by HSA-based
affinity chromatography. Proteins recovered following purification
were analyzed by SDS-PAGE and the gel revealed homogenous proteins
of the expected molecular weight with a purity between 95.1 and
99.8% (Fig. 1B and Table I).
 | Table IBiochemical characterization of the
fusion toxins. |
Table I
Biochemical characterization of the
fusion toxins.
| Fusion toxin | Purity (%)a | Calc. Mw (Da) | Found Mw
(Da)b |
|---|
|
ADAPT6-ABD-PE25 | 99.8 | 40,549 | 40,547 |
|
ADAPT6-ABD-PE38X8 | 97.0 | 52,673 | 52,669 |
|
ADAPT6-PE25 | 95.1 | 33,364 | 33,362 |
|
ZTaq-ABD-PE25 | 99.0 | 41,554 | 41,553 |
To investigate the possible formation of aggregates,
the purified proteins were analyzed by size-exclusion
chromatography under native conditions (Fig. 1C). The elution profiles revealed
mono-disperse proteins with a molecular weight of a monomer. Mass
spectrometry confirmed the correct molecular masses of the fusion
toxins (Table I).
Surface plasmon resonance analysis
The ability of the fusion toxins to interact with
HER2 was investigated by biosensor analysis. The dilution series of
the fusion toxins were sequentially injected over a surface with
immobilized extracellular domain of HER2, to derive the kinetic
constants and affinity of the interaction (Table II and Fig. S1). The KD was similar
for ADAPT6-ABD-PE25, ADAPT6-ABD-PE38X8 and
ADAPT6-PE25 and ranged from 11 to 26 nM.
ZTaq-ABD-PE25, lacking a HER2-binding domain, did not
interact with the surface with immobilized HER2 (Fig. S3).
 | Table IIAffinity constants for fusion toxins
interacting with HER2.a |
Table II
Affinity constants for fusion toxins
interacting with HER2.a
| Fusion toxin |
ka/104
(1/Ms)b |
kd/10−4
(1/s)b |
KD/10−8 (M)b |
|---|
|
ADAPT6-ABD-PE25 | 2.46±0.01 | 3.68±0.01 | 1.50±0.01 |
|
ADAPT6-ABD-PE38X8 | 1.06±0.01 | 2.76±0.03 | 2.60±0.03 |
|
ADAPT6-PE25 | 4.05±0.01 | 4.24±0.01 | 1.05±0.01 |
The ability of the ABD-containing fusion toxins to
interact with serum albumins was investigated by injection of
dilution series over flow cells with immobilized HSA or MSA. The
kinetic constants and affinity were derived from the sensorgrams
(Table III and Fig. S2). The affinities (KD)
for HSA ranged from 1.1 to 2.2 nM, for the different constructs.
The affinities for MSA were slightly weaker and KD
ranged from 3.6 to 6.5 nM. ADAPT6-PE25, lacking an
albumin binding domain, did not interact with the surfaces with
immo-bilized HSA or MSA (Fig.
S3).
 | Table IIIAffinity constants for fusion toxins
interacting with human and mouse serum albumin.a |
Table III
Affinity constants for fusion toxins
interacting with human and mouse serum albumin.a
| Human serum albumin
| Mouse serum albumin
|
|---|
| Fusion toxin |
ka/105
(1/Ms)b |
kd/10−4
(1/s)b |
KD/10−9 (M)b |
ka/105
(1/Ms)b |
kd/10−3
(1/s)b |
KD/10−9v (M)b |
|---|
|
ADAPT6-ABD-PE25 | 1.86±0.01 | 2.01±0.01 | 1.08±0.01 | 2.91±0.06 | 1.90±0.01 | 6.53±0.08 |
|
ADAPT6-ABD-PE38X8 | 1.72±0.05 | 3.69±0.23 | 2.15±0.20 | 3.95±0.20 | 2.23±0.15 | 5.63±0.09 |
|
ZTaq-ABD-PE25 | 1.82±0.03 | 3.2±0.17 | 1.76±0.07 | 4.21±0.35 | 1.49±0.01 | 3.55±0.33 |
In vitro cytotoxicity
The in vitro cytotoxicity was measured by
treating SKBR3, SKOV3 and AU565 cells, all with a high HER2
expression (Fig. S4), as well as
A549 cells with moderate HER2 expression (Fig. S4), with serial dilutions of the
fusion toxins followed by measurement of cell viability. The fusion
toxins specifically interacting with HER2 demonstrated picomolar
IC50 values on SKBR3, SKOV3 and AU565 cells (Fig. 2A). For SKBR3 cells, the
IC50 values were 84 pM with a 95% confidence interval
between 69 and 100 pM (ADAPT6-ABD-PE25), 35 pM with a
95% confidence interval between 30 and 40 pM
(ADAPT6-ABD-PE38X8), and 46 pM with a 95% confidence
interval between 38 and 56 pM (ADAPT6-PE25). For the
AU565 cells the IC50 values were 130 pM with a 95%
confidence interval between 96 and 170 pM
(ADAPT6-ABD-PE25), 130 pM with a 95% confidence interval
between 100 and 160 pM (ADAPT6-ABD-PE38X8), and 46 pM
with a 95% confidence interval between 31 and 68 pM
(ADAPT6-PE25). For SKOV3 cells, the IC50
values were 310 pM with a 95% confidence interval between 260 and
370 pM (ADAPT6-ABD-PE25), 180 pM with a 95% confidence
interval between 150 and 210 pM (ADAPT6-ABD-PE38X8), and
140 pM with a 95% confidence interval between 120 and 160 pM
(ADAPT6-PE25). A comparison of the
ADAPT6-ABD-PE25 and ADAPT6-ABD-PE38X8 thus
revealed that the fusion toxin, including PE38X8 was slightly more
cytotoxic. A comparison of ADAPT6-PE25 with
ADAPT6-ABD-PE25, revealed that the inclusion of the ABD
lowered the cytotoxicity slightly. The IC50 values of
the non-target control, ZTaq-ABD-PE25 were found to be
5,700 pM with a 95% confidence interval between 4,100 and 9,100 pM
for SKBR3 cells. The relative viability of AU565 and SKOV3 cells at
the concentrations used were not affected to such an extent by
ZTaq-ABD-PE25 that the IC50 values could be
measured. The difference in cytotoxicity between the HER2-specific
fusion toxins and the ZTaq-control was 2 to 3 orders of
magnitude for the 3 cell lines with a high HER2 expression.
All fusion toxins demonstrated substantially weaker
cytotoxic potential for A549 cells with a moderate HER2 expression
(Fig. 2A). The IC50
values were 15,000 pM with a 95% confidence interval between 13,000
and 19,000 pM (ADAPT6-ABD-PE25) and 5,600 pM with a 95%
confidence interval between 4,500 and 7,300 pM
(ADAPT6-ABD-PE38X8). The relative viability of A549
cells at the concentrations used was not affected to such an extent
by ZTaq-ABD-PE25 that the IC50 values could
be measured.
Rescue of SKOV3 viability
One of the ADAPT6-containing variants,
ADAPT6-ABD-PE25, was investigated further. Its
dependence on HER2 availability for activity was determined by
treating SKOV3 cells with a constant concentration of
ADAPT6-ABD-PE25 in the presence of increasing
concentrations of free ADAPT6 to potentially block
receptor binding sites on HER2, preventing interaction with the
fusion toxin. As shown in Fig. 2B,
SKOV3 cell viability was gradually increased by an increase in the
concentration of free ADAPT6 and full viability was
found at 1,000-fold excess, strongly indicating that
ADAPT6-ABD-PE25 is dependent on specific interaction
with HER2 for intoxication. Transferrin, which does not interact
with HER2, was used as a control. It was added in the same molar
excess (1,000-fold) as free ADAPT6 and was found to not
affect the efficiency of ADAPT6-ABD-PE25 (Fig. 2C). Free ADAPT6 and
transferrin did not affect cell viability when used alone.
Influence of contact time
The capability of fusion toxins to deliver their
cytotoxic effect is dependent on their ability to be internalized
by the target cell, which in turn is dependent on the contact time.
To examine the relationship between fusion toxin contact time and
cell viability, SKOV3 cells were incubated with a constant
concentration of ADAPT6-ABD-PE25 for different times
after which the toxin was removed. The viability decreased with
increasing contact time (Fig. 2D)
for the duration of the experiment (24 h). The half-maximum effect
was reached after approximately 30 min.
Biodistribution in mice
To investigate biodistribution, the fusion toxins
were radiolabeled with 111In, resulting in conjugates
with high radiochemical purity (>95%) and high stability during
EDTA challenge (Table SI). The
conjugates were subsequently injected into NMRI mice followed by
measurement of radioactive uptake in different organs (Table IV). As a positive control, the
HER2-specific fusion toxin ZHER2:2891-ABD-PE38X8 was
included. This fusion toxin has previously been thoroughly
characterized (11,12), where the targeting domain
ZHER2:2891 is a size-matched control with a fold similar
to ADAPT6. The comparison of the values at 4 and 24 h
revealed that the uptake in the different organs of all conjugates
followed a similar pattern, but with lower values at 24 h. The
comparison of ADAPT6-ABD-PE25 and ADAPT6-ABD-PE38X8 at
24 h post-injection (p.i.) revealed a 5-fold higher uptake in liver
(32.0±5.0 vs. 6.0±1.0 %ID/g) and a 4-fold lower in kidney (11.0±1.0
vs. 46.0±2.0 %ID/g) for the toxin containing PE25.
ADAPT6-ABD-PE25 also had a significantly lower uptake in
blood at both time points compared to ADAPT6-ABD-PE38X8.
By comparing ADAPT6-ABD-PE25 with
ADAPT6-PE25, a profound impact of the addition of ABD on
half-life in circulation was observed. At 4 h, the uptake in blood
was significantly higher (3.2±0.1 %ID/g) for the compound with an
ABD compared to 0.2±0.1 %ID/g for the variant lacking ABD. The
uptake in kidney of ADAPT6-ABD-PE25 was also decreased
compared to ADAPT6-PE25 (11.0±1.0 vs. 19.0±1.0 %ID/g at
24 h p.i.). However, the uptake in liver remained similar for both
constructs (27.0±3.0 vs. 32.0±5.0 %ID/g at 24 h p.i).
Discussion
In this study, we demonstrated that an ADAPT,
specifically interacting with HER2, can be expressed as a fusion to
different truncated variants of ETA, leading to potent fusion
toxins with high specificity for HER2 expressing cells. The fusion
toxins could be expressed in a soluble form in E. coli and
easily purified to homogeneity. By contrast, PE38-derived
immunotoxins, where immunoglobulin-based targeting domains are
utilized, may require more advanced host cells or a refolding step
during purification (37). Soluble
expression in a simple prokaryotic host cell for ADAPT-based fusion
toxins may be an important cost-of-goods advantage in a potential
future commercial manufacturing process, when large amounts of
GMP-grade (good manufacturing practice-grade) material is
needed.
ADAPTs comprise a class of novel engineered affinity
proteins and they are some of the smallest folded affinity proteins
(Mw of 5 kDa). The size is one important parameter to consider
during development of protein therapeutics for intended cancer
therapy, and it has previously been shown that a smaller size leads
to more efficient accumulation in solid tumors (38). This is a consequence of the
typically inefficient lymphatic drainage and an increased
interstitial pressure in solid tumors, leading to that the rate of
penetration is mostly dependent on diffusivity, and hence the size,
of the protein therapeutic (39,40).
Inefficient penetration and distribution in the tumor may lead to
untargeted portions of the tumor and portions where the
concentration of the therapeutic molecule is low. This may in turn
lead to selection for resistant clones. One of the fusion toxins
investigated in this study, ADAPT6-PE25 (Mw of 33 kDa),
is one of the smallest fusion toxins or immunotoxins created.
However, since ADAPT6-PE25 is also
considerably smaller than the cut-off of the glomerular filter in
the kidneys (ca 60 kDa), its blood retention was low, likely a
consequence of quick excretion to urine. A slightly larger version,
ADAPT6-ABD-PE25, including an ABD for in vivo
half-life extension was therefore also evaluated (Mw of 41 kDa).
This version was found to interact strongly with MSA and HSA, and
should thus form a 108 kDa complex with serum albumin in
vivo, well above the cut-off of the glomerular filtration.
Indeed, the blood retention at both 4 and 24 h p.i. was
significantly higher for the fusion toxin including the ABD. In
addition to an increase in size, the interaction with albumin
extends serum half-life by 'piggybacking' on the FcRn-dependent
intracellular rescue system for serum albumin (41).
The internalization of free ADAPT6 has
previously been investigated in SKOV3 cells, and it was found that
it is indeed internalized (36).
In this study, the cells were efficiently killed by the fusion
toxins including ADAPT6, which act on cytosolic
elongation factor 2, suggesting that also the fusion toxins are
efficiently internalized.
Previously, fusion toxins utilizing an affibody
molecule (ZHER2:2891) with specific affinity for HER2
have been evaluated (11,12). This targeting domain is slightly
larger than ADAPT6 (58 compared to 46 amino acids), but
with a similar, anti-parallel 3-helix fold. ZHER2:2891
has a higher affinity for HER2, which was reflected in the
affinities of the fusion toxins for HER2,
ZHER2:2891-ABD-PE38X8 had an equilibrium dissociation
constant of 5 nM (11) compared to
ADAPT6-ABD-PE38X8 which had an equilibrium dissociation
constant of 26 nM. In vitro, ADAPT6-ABD-PE38X8
was 6- to 7-fold less potent compared to
ZHER2:2891-ABD-PE38X8. The contact time needed to reach
50% viability (Fig. 2D) was also
longer for ADAPT6-ABD-PE38X8 (30 min) compared to
ZHER2:2891-ABD-PE38X8 (15 min). Both of the above could
be a consequence of the weaker affinity of
ADAPT6-ABD-PE38X8 for HER2. Compared to the
non-targeting control, ZTaq-ABD-PE25, the cytotoxic
potential of the 3 ADAPT6 containing fusion toxins had 2
to 3 orders of magnitude lower IC50 values on the cell
lines with a high HER2 expression, which translates into a large
therapeutic window. A strong affinity is desired for efficient
receptor binding, but too strong affinity may hamper tumor
penetration (42). The balance
between binding-strength and tumor accumulation is more important
for constructs smaller than the pores of the glomerular filter in
the kidneys and is thus more important for ADAPT6-PE25
than for the constructs including the ABD.
Two different truncated versions of ETA were
investigated in this study, PE38X8 and PE25, which have both been
found to retain a similar cytotoxic potential as the parental PE38
toxin (10,14). In the context of a C-terminal
fusion to ADAPT6-ABD, the cytotoxicity was found to be
slightly weaker for the PE25 version on the cell lines with a high
HER2 expression: SKBR3, AU565 and SKOV3 cells. For the
moderate-expressing cell line, A549, the PE38X8-containing fusion
toxin was 4-fold more potent than the PE25-containing fusion toxin
(IC50 3.4 nM versus 15 nM). However, the slight loss of
cytotoxic potential of ADAPT6-ABD-PE25 on A549 cells may
be compensated by other favorable properties; its smaller size may
lead to increased tumor accumulation and its deimmunizing mutations
should decrease formation of neutralizing antibodies in vivo
(14).
The biodistribution of the ABD-containing fusion
toxins revealed a significantly higher accumulation in blood at
both 4 h and 24 h post injection compared to
ADAPT6-PE25, clearly demonstrating the circulation
half-life extension effect of the ABD. Surprisingly,
ADAPT6-ABD-PE25 was found to have a lower blood
retention than ADAPT6-ABD-PE38X8, indicating a possible
hampering of the serum albumin interaction, even though the
affinity for mouse serum albumin was similar in the biosensor
experiment (Table III). It has
to be noted that the biosensor is an artificial system, and
interaction conditions with immobilized albumin may not exactly
mimic the in vivo conditions. This indicates that animal
studies are required for a proper evaluation. Another factor
leading to the reduced blood retention of
ADAPT6-ABD-PE25 may be an elevated sequestering by the
liver. The hepatic uptake of both ADAPT6-ABD-PE25 and
ADAPT6-PE25 was several-fold higher than the uptake of
ADAPT6-ABD-PE38X8. A lower liver uptake of
ADAPT6-ABD-PE38X8 may also be associated with a lower
hepatic toxicity, although animal studies have demonstrated that an
uptake of ETA-derivatives in liver was well tolerated (17). All 4 constructs accumulated in the
kidneys, although to varying extents. Since a radionuclide with
residualizing properties is used, the accumulation measured in the
kidneys is proportional to the sum of the uptake and lysosomal
degradation during the course of the whole experiment. Generally,
the uptake of proteins from primary urine in the kidneys is mainly
carried out by the megalin/cubulin complex of receptors (43). This complex recognizes a variety of
different ligands, including vitamins and proteins. The
accumulation of radioactivity in the kidneys of the 4 constructs is
dependent on several parameters, including the affinity between the
construct and the megalin/cubulin complex outside of the cells, the
potential triggering of endocytosis and the release inside the
endosomes of the kidney cells. In addition, the rate at which the
peptide linker between ADAPT6 and the toxin is cleaved
may also influence transport to the lysosomes for degradation. The
comparison of ADAPT6 and ZHER2:2891 as
targeting domains in the context of an N-terminal fusion to
ABD-PE38X8 (Table IV), revealed
that blood retention was increased, accumulation in liver and
spleen was decreased, and accumulation in kidney was increased when
employing ADAPT6. Apparently, the targeting part of a
fusion toxin may have a substantial influence on
biodistribution.
In conclusion, it was found that fusion toxins
consisting of a HER2-targeting ADAPT coupled to ETA-derived
cytotoxic domains, are highly potent agents for specific killing of
HER2-expressing cells. Inclusion of an ABD was found to prolong the
residence time in blood, which increases the bioavailability. On
the whole, the findings motivate further pre-clinical and clinical
evaluations.
Supplementary Materials
Funding
This study was financially supported by a grant
from the Swedish cancer foundation (Cancerfonden; CAN 2015/746) and
Vinnova (grant no. 2016-04060). The funding bodies did not
participate in the design of the study, collection, analysis or
interpretation of data or writing of the manuscript.
Availability of data and materials
All data and materials included in this article are
available from the corresponding author upon reasonable
request.
Authors' contributions
HL, SL, MA, AO, VT, SH and TG designed the study
and experiments. HL, HD, MA, JG, AO and VT performed the
experiments. HL, HD, MA, JG, AO, VT, SH and TG analyzed and
interpreted the data. SL, MA, AO, VT, SH and TG wrote the
manuscript. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The animal experiments were planned and performed
in accordance with Sweden's national legislation on laboratory
animals' protection. The animal studies were approved by the local
ethics committee for animal research in Uppsala, Sweden.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
ABD
|
albumin binding domain
|
|
ADAPT
|
ABD-derived affinity protein
|
|
E. coli
|
Escherichia coli
|
|
ETA
|
Pseudomonas exotoxin A
|
|
FcRn
|
neonatal Fc receptor
|
|
GMP
|
good manufacturing practice
|
|
HER2
|
human epidermal growth factor
receptor 2
|
|
IMAC
|
immobilized metal-ion affinity
chromatography
|
|
IPTG
|
isopropyl
β-D-1-thiogalactopyranoside
|
|
ITLC
|
instant thin layer chromatography
|
|
KD
|
equilibrium dissociation constant
|
|
MSA
|
mouse serum albumin
|
Acknowledgments
The authors are grateful to Mr. Andreas Hober
(Department of protein science, KTH Royal institute of technology,
Stockholm, Sweden) for providing expert technical assistance.
References
|
1
|
Alewine C, Hassan R and Pastan I: Advances
in anticancer immunotoxin therapy. Oncologist. 20:176–185. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akbari B, Farajnia S, Ahdi Khosroshahi S,
Safari F, Yousefi M, Dariushnejad H and Rahbarnia L: Immunotoxins
in cancer therapy: Review and update. Int Rev Immunol. 36:207–219.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kreitman RJ, Dearden C, Zinzani PL,
Delgado J, Karlin L, Robak T, Gladstone DE, le Coutre P, Dietrich
S, Gotic M, et al: Moxetumomab pasudotox in relapsed/refractory
hairy cell leukemia. Leukemia. 32:1768–1777. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin-Killias P, Stefan N, Rothschild S,
Plückthun A and Zangemeister-Wittke U: A novel fusion toxin derived
from an EpCAM-specific designed ankyrin repeat protein has potent
antitumor activity. Clin Cancer Res. 17:100–110. 2011. View Article : Google Scholar
|
|
5
|
Prince HM, Duvic M, Martin A, Sterry W,
Assaf C, Sun Y, Straus D, Acosta M and Negro-Vilar A: Phase III
placebo-controlled trial of denileukin diftitox for patients with
cutaneous T-cell lymphoma. J Clin Oncol. 28:1870–1877. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Onda M, Nagata S, FitzGerald DJ, Beers R,
Fisher RJ, Vincent JJ, Lee B, Nakamura M, Hwang J, Kreitman RJ, et
al: Characterization of the B cell epitopes associated with a
truncated form of Pseudomonas exotoxin (PE38) used to make
immunotoxins for the treatment of cancer patients. J Immunol.
177:8822–8834. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu W, Onda M, Lee B, Kreitman RJ, Hassan
R, Xiang L and Pastan I: Recombinant immunotoxin engineered for low
immunogenicity and antigenicity by identifying and silencing human
B-cell epitopes. Proc Natl Acad Sci USA. 109:11782–11787. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Onda M, Beers R, Xiang L, Lee B, Weldon
JE, Kreitman RJ and Pastan I: Recombinant immunotoxin against
B-cell malignancies with no immunogenicity in mice by removal of
B-cell epitopes. Proc Natl Acad Sci USA. 108:5742–5747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mazor R, Vassall AN, Eberle JA, Beers R,
Weldon JE, Venzon DJ, Tsang KY, Benhar I and Pastan I:
Identification and elimination of an immunodominant T-cell epitope
in recombinant immunotoxins based on Pseudomonas exotoxin A. Proc
Natl Acad Sci USA. 109:E3597–E3603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Onda M, Beers R, Xiang L, Nagata S, Wang
Q-C and Pastan I: An immunotoxin with greatly reduced
immunogenicity by identification and removal of B cell epitopes.
Proc Natl Acad Sci USA. 105:11311–11316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu H, Seijsing J, Frejd FY, Tolmachev V
and Gräslund T: Target-specific cytotoxic effects on
HER2-expressing cells by the tripartite fusion toxin
ZHER2:2891-ABD-PE38X8, including a targeting affibody
molecule and a half-life extension domain. Int J Oncol. 47:601–609.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Altai M, Liu H, Orlova A, Tolmachev V and
Gräslund T: Influence of molecular design on biodistribution and
targeting properties of an Affibody-fused HER2-recognising
anticancer toxin. Int J Oncol. 49:1185–1194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weldon JE, Skarzynski M, Therres JA,
Ostovitz JR, Zhou H, Kreitman RJ and Pastan I: Designing the
furin-cleavable linker in recombinant immunotoxins based on
Pseudomonas exotoxin A. Bioconjug Chem. 26:1120–1128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mazor R, Onda M, Park D, Addissie S, Xiang
L, Zhang J, Hassan R and Pastan I: Dual B- and T-cell
de-immunization of recombinant immunotoxin targeting mesothelin
with high cytotoxic activity. Oncotarget. 7:29916–29926. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wurch T, Pierré A and Depil S: Novel
protein scaffolds as emerging therapeutic proteins: From discovery
to clinical proof-of-concept. Trends Biotechnol. 30:575–582. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zielinski R, Lyakhov I, Jacobs A, Chertov
O, Kramer-Marek G, Francella N, Stephen A, Fisher R, Blumenthal R
and Capala J: Affitoxin - a novel recombinant, HER2-specific,
anticancer agent for targeted therapy of HER2-positive tumors. J
Immunother. 32:817–825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zielinski R, Lyakhov I, Hassan M, Kuban M,
Shafer-Weaver K, Gandjbakhche A and Capala J: HER2-affitoxin: A
potent therapeutic agent for the treatment of HER2-overexpressing
tumors. Clin Cancer Res. 17:5071–5081. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Simon M, Stefan N, Borsig L, Plückthun A
and Zangemeister-Wittke U: Increasing the antitumor effect of an
EpCAM-targeting fusion toxin by facile click PEGylation. Mol Cancer
Ther. 13:375–385. 2014. View Article : Google Scholar
|
|
19
|
Ham S, Min KA, Yang JW and Shin MC: Fusion
of gelonin and anti-insulin-like growth factor-1 receptor (IGF-1R)
affibody for enhanced brain cancer therapy. Arch Pharm Res.
40:1094–1104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nilvebrant J and Hober S: The
albumin-binding domain as a scaffold for protein engineering.
Comput Struct Biotechnol J. 6:e2013030092013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alm T, Yderland L, Nilvebrant J, Halldin A
and Hober S: A small bispecific protein selected for orthogonal
affinity purification. Biotechnol J. 5:605–617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nilvebrant J, Åstrand M, Löfblom J and
Hober S: Development and characterization of small bispecific
albumin-binding domains with high affinity for ErbB3. Cell Mol Life
Sci. 70:3973–3985. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nilvebrant J, Åstrand M, Georgieva-Kotseva
M, Björnmalm M, Löfblom J and Hober S: Engineering of bispecific
affinity proteins with high affinity for ERBB2 and adaptable
binding to albumin. PLoS One. 9:e1030942014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hynes NE and MacDonald G: ErbB receptors
and signaling pathways in cancer. Curr Opin Cell Biol. 21:177–184.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tai W, Mahato R and Cheng K: The role of
HER2 in cancer therapy and targeted drug delivery. J Control
Release. 146:264–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
von Minckwitz G, Procter M, de Azambuja E,
Zardavas D, Benyunes M, Viale G, Suter T, Arahmani A, Rouchet N,
Clark E, et al: APHINITY Steering Committee and Investigators:
Adjuvant pertuzumab and trastuzumab in early HER2-positive breast
cancer. N Engl J Med. 377:122–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Verma S, Miles D, Gianni L, Krop IE,
Welslau M, Baselga J, Pegram M, Oh DY, Diéras V, Guardino E, et al
EMILIA Study Group: Trastuzumab emtansine for HER2-positive
advanced breast cancer. N Engl J Med. 367:1783–1791. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lindbo S, Garousi J, Åstrand M, Honarvar
H, Orlova A, Hober S and Tolmachev V: Influence of
histidine-containing tags on the biodistribution of ADAPT scaffold
proteins. Bioconjug Chem. 27:716–726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Garousi J, Lindbo S, Nilvebrant J, Åstrand
M, Buijs J, Sandström M, Honarvar H, Orlova A, Tolmachev V and
Hober S: ADAPT, a novel scaffold protein-based probe for
radionuclide imaging of molecular targets that are expressed in
disseminated cancers. Cancer Res. 75:4364–4371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hopp J, Hornig N, Zettlitz KA, Schwarz A,
Fuss N, Müller D and Kontermann RE: The effects of affinity and
valency of an albumin-binding domain (ABD) on the half-life of a
single-chain diabody-ABD fusion protein. Protein Eng Des Sel.
23:827–834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Makrides SC, Nygren PÅ, Andrews B, Ford
PJ, Evans KS, Hayman EG, Adari H, Uhlén M and Toth CA: Extended in
vivo half-life of human soluble complement receptor type 1 fused to
a serum albumin-binding receptor. J Pharmacol Exp Ther.
277:534–542. 1996.PubMed/NCBI
|
|
33
|
Orlova A, Jonsson A, Rosik D, Lundqvist H,
Lindborg M, Abrahmsen L, Ekblad C, Frejd FY and Tolmachev V:
Site-specific radiometal labeling and improved biodistribution
using ABY-027, a novel HER2-targeting affibody
molecule-albumin-binding domain fusion protein. J Nucl Med.
54:961–968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jonsson A, Dogan J, Herne N, Abrahmsén L
and Nygren PÅ: Engineering of a femtomolar affinity binding protein
to human serum albumin. Protein Eng Des Sel. 21:515–527. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gunneriusson E, Nord K, Uhlén M and Nygren
P: Affinity maturation of a Taq DNA polymerase specific affibody by
helix shuffling. Protein Eng. 12:873–878. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Garousi J, Lindbo S, Honarvar H, Velletta
J, Mitran B, Altai M, Orlova A, Tolmachev V and Hober S: Influence
of the N-terminal composition on targeting properties of
radiometal-labeled anti-HER2 scaffold protein ADAPT6.
Bioconjug Chem. 27:2678–2688. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pastan I, Beers R and Bera TK: Recombinant
immunotoxins in the treatment of cancer. Methods Mol Biol.
248:503–518. 2004.PubMed/NCBI
|
|
38
|
Zahnd C, Kawe M, Stumpp MT, de Pasquale C,
Tamaskovic R, Nagy-Davidescu G, Dreier B, Schibli R, Binz HK,
Waibel R, et al: Efficient tumor targeting with high-affinity
designed ankyrin repeat proteins: Effects of affinity and molecular
size. Cancer Res. 70:1595–1605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thurber GM, Schmidt MM and Wittrup KD:
Antibody tumor penetration: Transport opposed by systemic and
antigen-mediated clearance. Adv Drug Deliv Rev. 60:1421–1434. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heldin CH, Rubin K, Pietras K and Östman
A: High interstitial fluid pressure - an obstacle in cancer
therapy. Nat Rev Cancer. 4:806–813. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Roopenian DC and Akilesh S: FcRn: The
neonatal Fc receptor comes of age. Nat Rev Immunol. 7:715–725.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schmidt MM and Wittrup KD: A modeling
analysis of the effects of molecular size and binding affinity on
tumor targeting. Mol Cancer Ther. 8:2861–2871. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nielsen R, Christensen EI and Birn H:
Megalin and cubilin in proximal tubule protein reabsorption: From
experimental models to human disease. Kidney Int. 89:58–67. 2016.
View Article : Google Scholar : PubMed/NCBI
|