According to the 2018 cancer statistics, it was
estimated that 234,030 cases of lung and bronchus cancer were newly
diagnosed in the United States (1). Lung cancer is the primary cause of
cancer-related deaths worldwide and results in >1.3 million
deaths per year (2). Lung cancer
mainly includes non-small cell lung cancer (NSCLC) and small cell
lung cancer (SCLC). NSCLC constitutes 85% of all lung cancer cases,
including lung adenocarcinoma, squamous cell carcinoma and large
cell lung cancer (3-5). The lung cancer incidence rate is
increasing worldwide, especially female morbidity (6). Despite the discovery of multiple
mutations and targeted drugs, such as for the genes epidermal
growth factor receptor (EGFR), KRAS and MET, the prognosis of
advanced lung cancer patients remains poor, with a 5-year survival
rate stagnant at ~5% (7). Known
risk factors, such as smoking habits, air pollution and genetic
variations, have an important impact on lung cancer development and
clinical outcomes (8).
Long non-coding RNAs (lncRNAs) are ~200 nt in
length, lack the protein coding potential, and constitute ~70% of
the non-coding RNAs (9,10). Except for their role as competing
endogenous RNA (ceRNA) to sponge microRNAs (miRNAs), lncRNAs have
also been shown to interact with DNA, RNA and various proteins,
thereby having crucial roles in diverse physiological and
pathological functions (11).
Appropriate lncRNA expression is essential for normal cell function
and is precisely regulated by epigenetic mechanisms and various
other molecules. Recent reports have found that dysregulation of
lncRNA expression induces tumorigenesis, invasiveness and drug
resistance through diverse mechanisms in multiple types of cancer
(12,13). lncRNAs are also important, complex
controlling factors in the pathogenesis of lung cancer (14-17).
In the present review, the behavior and environment-induced
dysregulation of lncRNA expression was summarized in regards to
lung cancer, their functions and molecular mechanisms were
examined, and their potential as biomarkers for the diagnosis and
prognosis of lung cancer was explored.
Many large-scale investigations, including
microarray profiling and deep sequencing data, have revealed that
the derangement of lncRNA expression is a primary feature in lung
cancer initiation and progression (18,19).
The lncRNA expression levels are precisely regulated in the
physiological state and are potentially disturbed in the
pathological state by diverse mechanisms. The influence of chemical
compounds and the local tumor microenvironments responsible for the
regulation of lncRNA expression should not be ignored.
Additionally, the function of epigenetic modification in tumor
progression is likely involved. Abnormal epigenetic regulation can
lead to aberrant activation of lncRNAs without involving any
changes in the DNA sequences. Various transcription factors can
bind within the promoter regions of lncRNAs to activate or inhibit
their transcription. These regulation patterns of dysregulated
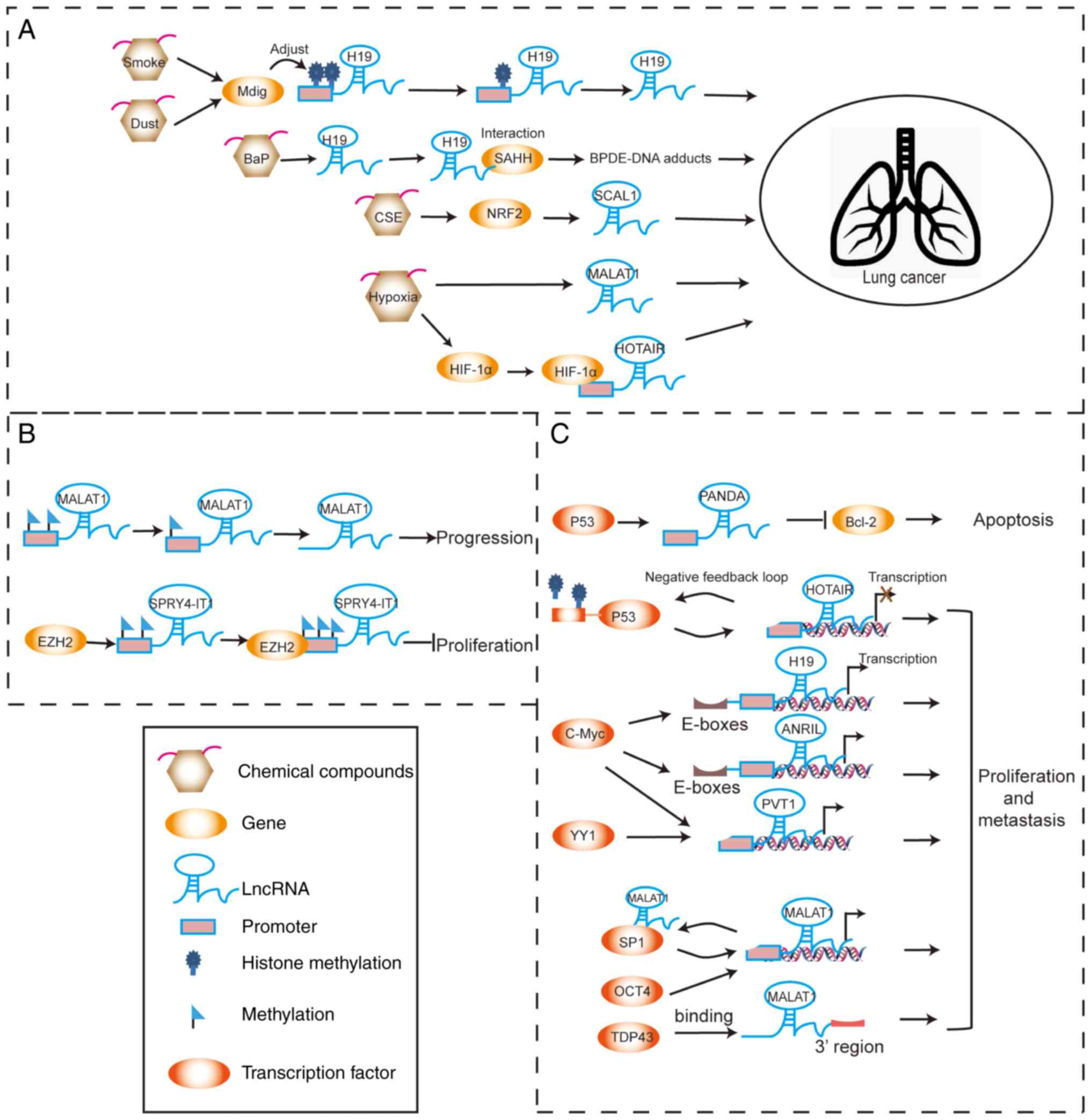
lncRNAs in lung cancer are summarized in Fig. 1 and Table I.
It has been reported that H19 is significantly
elevated in the airway epithelium of healthy 20 pack-year smokers
compared with non-smokers (20).
Mineral dust-induced gene (Mdig) is associated with environmental
exposure to smoke and dust, which influences the progression of
lung cancer. Mdig regulates the expression of H19 by regulating the
levels of trimethylated histone 3 lysine 9 (H3K9me3) at the
promoter region of H19 (21).
Benzo(a)pyrene (BaP) increases H19 expression and its interaction
with the S-adenosylhomocysteine hydrolase protein. By contrast, H19
knockdown suppresses the formation of
benzo(a)pyrene-7,8-dihydrodiol-9,10-epoxide (BPDE)-DNA adducts,
which decreases the risk for lung cancer (22). Smoke-associated and
cancer-associated lncRNA-1 (SCAL1) is located on the chromosome
5q14.3 locus. High expression of SCAL1 in lung cancer cells is
induced by cigarette smoke extract. SCAL1 is upregulated by nuclear
factor erythroid 2-related factor 2 (NRF2) and serves a functional
role in cytoprotection against cigarette smoke-induced toxicity.
These findings suggest that SCAL1 has an important role in the
antioxidant pathway (23).
Hypoxia induces upregulation of the lncRNA
metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) in
lung cancer (24).
Hypoxia-inducible factor 1α (HIF-1α) can bind to the
hypoxia-sensitive elements on the promoter region of HOX transcript
antisense RNA (HOTAIR) and activate the transcription of HOTAIR, as
well as promote NSCLC proliferation and metastasis under hypoxia
conditions (25).
The methylated levels of MALAT1 promoter are low in
lung cancer cells or tissues. Treatment with the methyl donor,
S-adenosylmethionine, suppresses MALAT1 expression in lung cancer
cells (26). In such cases, the
lncRNA sprouty RTK signaling antagonist 4 intronic transcript 1
(SPRY4-IT1), located at chromosome 5q31.3, is upregulated and
promotes proliferation and metastasis of cancer cells (27). However, SPRY4-IT1 is expressed at
low levels in NSCLC tissues and inhibits the proliferation and
epithelial-mesenchymal transition (EMT) of NSCLC cancer cells.
Enhancer of zeste homolog 2 (EZH2) can directly bind to SPRY4-IT1
and silence its transcription in NSCLCs (28,29).
p53 has been shown to bind the promoter region of
HOTAIR and suppress its transcription. By contrast, HOTAIR enhances
H3K27me3 modification within the p53 promoter and inhibits p53
expression in the lung cancer cell line A549. This negative
feedback loop of HOTAIR-p53 promotes the progression of lung cancer
(30). On the contrary, p53
increases expression of p21-associated non-coding RNA DNA
damage-activated (PANDAR), which is a tumor suppressor gene that is
downregulated in human NSCLC tissues (31). PANDAR can interact with nuclear
transcription factor Y subunit α (NF-YA) and low expression of
PANDAR increases NF-YA binding to the promoter of B cell lymphoma-2
(Bcl-2); this leads to an increase in Bcl-2 expression, thereby
inhibiting NSCLC cell apoptosis (32). Binding of c-Myc to the E-boxes near
the H19 imprinting control region activates the transcription of
H19 in lung cancer (33). Notably,
c-Myc also binds to the E-box element upstream of antisense ncRNA
in INK4 locus (ANRIL) and induces its expression in NSCLC cells
(34). The transcription factors,
c-Myc and Yin Yang 1 (YY1), can activate transcription of the
lncRNA plasmacytoma variant translocation 1 (PVT1), by binding to
its promoter region in lung cancer (35,36).
The transcription factor, specificity protein 1 (SP1), promotes
MALAT1 transcription and MALAT1 directly binds to SP1 protein to
enhances its stability. This MALAT1-SP1 positive feedback loop has
been demonstrated to promote the progression of lung cancer
(37). Octamer binding
transcription factor 4 (OCT4) has been reported to increase MALAT1
transcription by binding to its promoter enhancer region, thereby
inducing upregulation of MALAT1 expression in lung cancer (38). MALAT1 expression has also been
shown to be regulated by TAR DNA-binding protein 43 (TDP43) in lung
cancer (39).
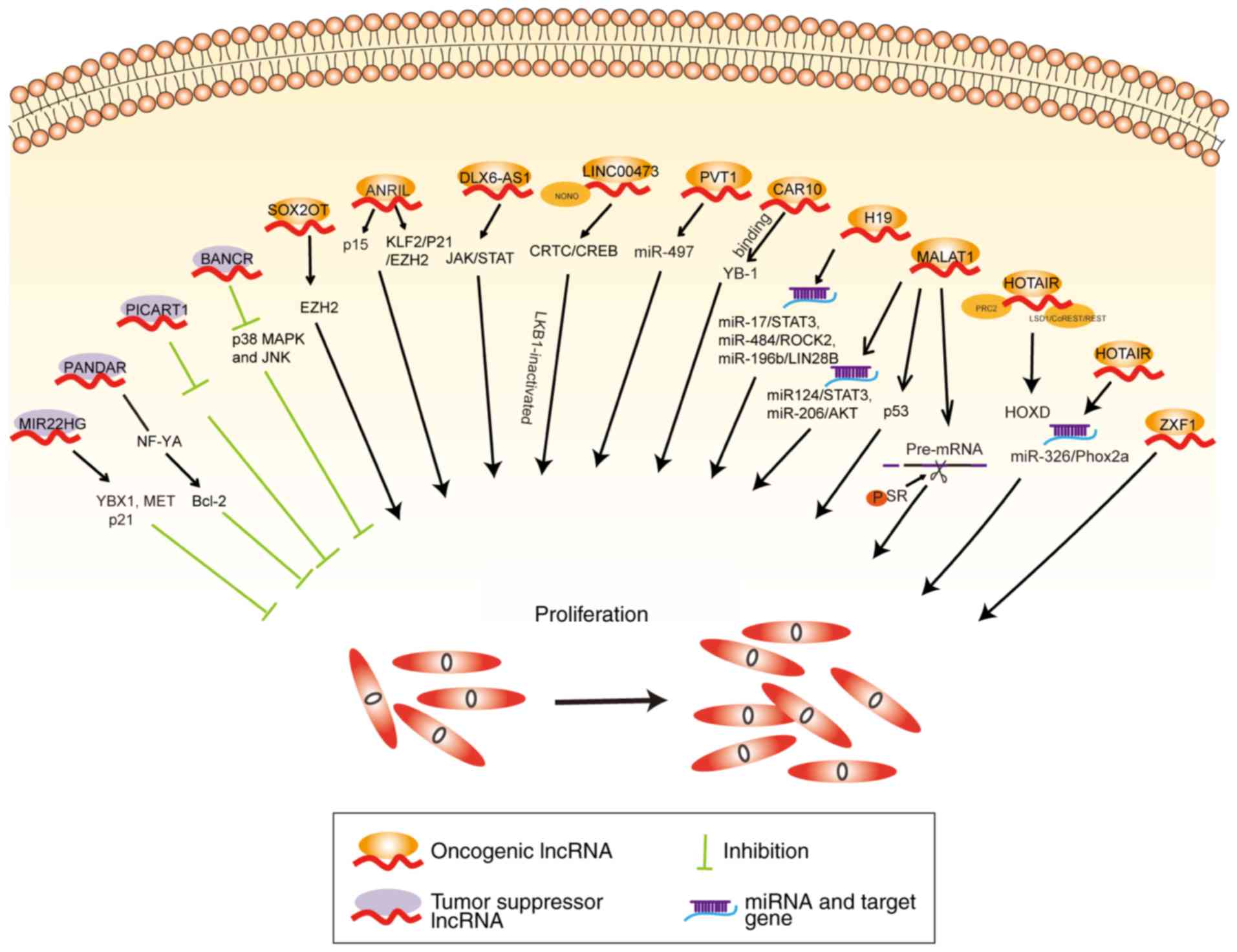
In lung cancer progression, abnormally regulated
lncRNAs act as vital factors to regulate the gene signaling network
at the transcriptional, post-transcriptional and post-translational
level, and thus, alter various malignant behaviors and treatment
responses of lung cancer (Table
II).
MALAT1 can act as a ceRNA to regulate miR-124/STAT3
and miR-206/AKT expression to promote NSCLC progression (40,41).
MALAT1 binds with serine/arginine splicing factor (SR) in the
nuclear speckle domains and increases SR phosphorylation followed
by regulation of the alternative splicing of pre-mRNA (42). MALAT1 suppresses p53 activity by
binding to a minimal region of p53 promoter that regulates
downstream genes influencing the cell cycle progression of lung
cancer cells (43). Downregulation
of MALAT1 has been shown to inhibit NSCLC progression by inhibiting
autophagy (44). The 5'end of
HOTAIR binds with the polycomb repressive complex 2 (PRC2)
resulting in histone H3 being trimethylated at lysine 27, while the
3'domain binds to the histone demethylase complexes (lysine
demethylase 1A/REST corepressor 1/RE1 silencing transcription
factor) facilitating histone H3 lysine 4 demethylation, which
causes homeobox D cluster (HOXD) gene silencing (45). Silencing of HOTAIR decreases
miR-326 expression, which regulates paired like homeobox 2A
(Phox2a) and inhibits tumor cell proliferation and migration in
lung cancer (46). H19 knockdown
evidently restrains NSCLC cell proliferation (47-49).
Notably, H19 functions as a ceRNA sponge for miR-17 to modulate
signal transducer and activator of transcription 3 (STAT3)
expression (50), and as a ceRNA
sponge for miR-484 to regulate the expression of Rho associated
coiled-coil containing protein kinase 2 (ROCK2) (51), thereby promoting lung cancer
development. Finally, H19 sponges miR-196b to elevate LIN28B
expression, which accelerates the proliferation of lung cancer
cells (52).
Another intergenic non-coding RNA, LINC00473, has
been demonstrated to be the most upregulated lncRNA in liver kinase
B (LKB1)-inactivated NSCLC tissues. LINC00473 interacts with
non-POU domain-containing octamer-binding protein (NONO) and
subsequently facilitates NONO/CREB regulated transcription
coactivator 1 (CRTC1) interaction and CREB-mediated transcription,
to promote the proliferation of LKB1-inactivated NSCLC cells
(53). Another lncRNA, DLX6-AS1,
is located on the chromosome 7q21.3 and has been found to be
upregulated in lung adenocarcinoma tissues comparted with adjacent
normal tissues (54). DLX6-AS1
alters JAK/STAT signaling to promote proliferation of lung
adenocarcinoma cells (54).
Another study demonstrated that the knockdown of ANRIL induced cell
cycle arrest at the G1/G0 phase and promoted cell cycle apoptosis
(34). In addition, depletion of
ANRIL increased p15 expression and induced cell-cycle arrest at the
G2/M phase of lung cancer cells (55). Knockdown of ANRIL has been found to
reduce EZH2 binding with Krüppel-like factor 2 (KLF2) and p21
promoter, and to also inhibit the proliferation of PC9 NSCLC cells
(56). SOX2 overlapping transcript
(SOX2OT) is encoded on chromosome 3q26.3 locus, and has been found
to be upregulated in 53.01% of NSCLCs and significantly associated
with poor survival in patients lung cancer. Thus, silencing of
SOX2OT can suppress cell proliferation by causing G2/M arrest via
regulation of EZH2 expression (57).
Similarly, BRAF-activated non-protein coding RNA
(BANCR) is an antitumor lncRNA of 693 bp, located on the chromosome
9q21.11 (58). Knockdown of BANCR
induces p38 mitogen-activated protein kinase (MAPK) and JNK
activation, which promotes lung cancer cell proliferation and
migration (59). By contrast,
other lncRNAs, such as p53 inducible cancer associated RNA
transcript 1 (PICART1), can inhibit JAK2/STAT3 signaling to
suppress lung cancer proliferation and induce apoptosis (60). Another lncRNA, MIR22 host gene
(MIR22HG), also has a tumor suppressive role in lung cancer, by
inhibiting oncogenes Y-box binding protein 1 (YBX1) and MET, while
increasing p21 expression (61).
The lncRNA chromatin-associated RNA 10 (CAR10) can regulate the
expression of neighboring genes, which was first confirmed in human
fibroblasts (62). Previous
studies have shown that CAR10 can act as an oncogene by binding to
the transcription factor YBX1 and subsequently increase the
proliferation of lung cancer cells (63). A schematic illustrating the
aforementioned lncRNAs and their roles in proliferation of lung
cancer cells is shown in Fig.
2.
HOTAIR also promotes the invasion and metastasis of
lung cancer cells by regulating homeobox A5 (HOXA5), miR-613 and
14-3-3σ expression (67-69). Ono et al (70) found that patients with elevated
expression of HOTAIR were more prone to lymph node metastasis and
recurrence. HOTAIR interacts with lymphoid-specific helicase
(HELLS) and affects the forkhead box A (FOXA) 2/FOXA1 expression
ratio, thereby promoting invasion and migration of lung
adenocarcinoma cells (71). PVT1
has been shown to regulate miR-497 expression and to competitively
bind with miR-200a and miR-200b, to upregulate matrix
metalloproteinase 9 (MMP9) expression and promote the metastasis of
NSCLC (72,73). ANRIL suppression has been shown to
inhibit the invasion and migration of lung tumor cells (74,75).
LINC00963 is highly expressed in NSCLC tissues and interacts with
phosphoglycerate kinase (PGK1) to prevent its ubiquitination,
leading to activation of the AKT/mTOR oncogenic signaling pathway.
In addition, LINC00963 interacts with NONO to activate
CRTC/CREB-mediated transcription promoting the metastasis of lung
cancer cells (76). Knockdown of
the lncRNA ACTA2 antisense RNA 1 (ACTA2-AS1, also known as ZXF1)
inhibits the invasion and migration of lung cancer cells (77). Finally, Ge et al found that
CAR10 acted as a ceRNA for miR-30 and miR-203 and induced EMT by
regulating Snail family transcriptional repressor 1 (SNAI1) and
SNAI2 expression (78). A
schematic illustrating the aforementioned lncRNAs and their roles
in invasion and metastasis of lung cancer cells is shown in
Fig. 3.
Medical treatment for lung cancer mainly includes
platinum-based chemotherapy and molecular-targeted drugs, such as
epidermal growth factor receptor tyrosine kinase inhibitors
(EGFR-TKIs) (79,80). However, drug resistance at many
instances leads to failure of treatment (81,82).
Previous studies have shown that multidrug resistant (MDR) A549/DDP
cells were primarily caused by changes to the cell membrane
transporters, abnormal target enzymes and irregular apoptosis
pathway (83-85). In recent years, there has been
evidence that some lncRNAs are also involved in the drug resistance
mechanism of lung cancer (Fig.
4).
The levels of several lncRNAs, including MALAT1, H19
and HOTAIR, have been demonstrated to be upregulated in
cisplatin-resistant lung cancer (86-88),
whereas maternally expressed 3 (MEG3) and AK126698 are
downregulated in drug-resistant A549/DDP lung cancer cells
(89,90). MALAT1 acts as a ceRNA to sponge
miR-101 and then regulates SRY-box transcription factor 9 (SOX9)
and MCL1 to enhance cisplatin resistance (91,92).
Furthermore, MALAT1 induces cisplatin resistance via STAT3
activation, and upregulation of multidrug resistance-associated
protein 1 (MRP1) and multidrug resistance 1 (MDR1) expression
(86). HOTAIR increases cisplatin
resistance in A549 cells by decreasing p21 expression and
activating the Wnt signaling pathway (93). HOTAIR upregulates HOXA1 by
decreasing the expression of DNA methyltransferase (DNMT) 1 and
DNMT3b, resulting in chemoresistant SCLC (94,95).
By contrast, MEG3 expression is decreased in cisplatin-resistant
A549/DDP lung cancer cells and cisplatin-insensitive lung
adenocarcinoma tissues (89).
Overexpression of MEG3 has been reported to mediate
re-sensitization to cisplatin in drug resistant A549/DDP cells and
animal models (89). MEG3 affects
cisplatin sensitivity partially via regulation of the p53 and
WNT/β-catenin signaling pathways (89). AK126698 is also found at high
expression levels in DDP-sensitive A549 cells compared with the
drug resistant A549/DDP cells. As a result, AK126698 knockdown has
been demonstrated to decrease the apoptosis of A549 cells following
cisplatin treatment via activation of Wnt signaling (90).
In addition, HOTAIR increases the radiation
resistance in lung cancer via downregulation of Wnt inhibitory
factor 1 (WIF-1) and activation of the Wnt signaling pathway
(104). Similarly, PVT1 also
decreases the radiosensitivity of NSCLC cells via sponging of
miR-195 (105). BANCR was
demonstrated to be highly expressed in Lewis lung tumor-bearing
mice after radiation therapy (106). Knockdown of BANCR expression
promoted cancer cell viability after radiation therapy, and mice
with lower BANCR expression had larger tumor sizes (106). These studies could help predict
which patients may best respond to radiotherapy.
lncRNAs have complex roles in the initiation and
progression of lung cancer, thereby affecting the prognosis of
patients. lncRNAs are prevailing in the plasma with relative
stability, which is suitable for early diagnosis of lung cancer.
Recently, abundant lncRNAs have also been detected in serum
exosomes with specific and characteristic expression markers in
patients with lung cancer, suggesting that they could be utilized
as potential clinical biomarkers.
Several reports have found that increased HOTAIR
levels in patients with lung cancer and upregulation of HOTAIR
expression correlates with the pathological staging and poor
prognosis of lung cancer (107,108). Plasma HOTAIR expression levels
could be a biomarker for the diagnosis and monitoring of NSCLC
patients (109). Similarly, H19
is upregulated in NSCLC tissues and negatively correlated with the
survival of lung cancer patients (21,49).
PVT1 has been shown to be overexpressed in NSCLC tissues, and
elevated PVT1 expression levels have been demonstrated as an
independent prognostic factor for NSCLC (110-112). Wu et al (110) reported that PVT1 was also
overexpressed in lung squamous cell carcinoma. Notably,
overexpression of the lncRNA ZXF1, positioned at chromosome
10q23.31 with a length of 3,985 bp, was found to be significantly
related
to lymph node metastasis and poor prognosis in
patients with lung adenocarcinoma (77). ANRIL is overexpressed in NSCLC
tissues and cell lines and elevated ANRIL levels are correlated
with poor prognosis in NSCLC patients (74). ANRIL can be found in the plasma of
NSCLC patients and acts as an extremely sensitive diagnostic tool
with an area under ROC curve (AUC) value of 0.798 (113). Circulating ANRIL expression may
be used as a predictor in the early diagnosis of NSCLC (113). Similarly, SOX2OT is upregulated
in serum samples of NSCLC and its expression is significantly
associated with the overall survival (OS) rate of lung cancer
patients (114). Several studies
have reported overexpression of MALAT1 in tumor tissue as well as
peripheral blood of NSCLC patients (115-118). Weber et al (119) found that MALAT1 expression in the
peripheral blood of NSCLC patients was higher compared with healthy
controls and was characterized by high specificity and sufficient
sensitivity (AUC=0.79). Similarly, Zhang et al (120) indicated that abundant expression
of serum exosomal MALAT1 in NSCLC patients was positively
associated with tumor stage and lymph node metastasis, suggesting
that MALAT1 can act as a tumor biomarker for prognosis and
diagnosis in NSCLC.
Emerging substantial research has confirmed that
abnormally regulated lncRNAs have crucial roles in the malignant
biology of lung cancer. However, available information about lncRNA
dysregulation mechanisms in lung cancer remain limited. Further
research into the mechanisms by which smoking and air pollution
regulate lncRNA expression and by which lncRNAs affect lung cancer
initiation and progression will provide valuable information to
improve our understanding of lung cancer.
lncRNAs demonstrate diverse and dynamic functions
depending on their subcellular localization and interacting
molecules. At present, lncRNA remains a poorly understood genomic
product; especially their functions in the nucleus as chromatin
architecture regulators are unclear. In the future, the
construction of a lncRNA-mediated gene expression network and
associated signaling pathway network will further reveal the
function and molecular mechanisms of lncRNA in proliferation,
metastasis, and therapeutic response of lung cancer.
lncRNA-specific expression patterns in cancer
subtypes and their stability in body fluid provides a valuable
choice as biomarkers for lung cancer. Existing studies of lncRNAs
as biomarkers in lung cancer have laid the foundation for clinical
application, but require further wider screening and validation in
large cohorts. Such studies will further elucidate the potential of
lncRNAs as diagnostic markers and treatment targets for lung
cancer.
This study was supported by grants from the Overseas
Expertise Introduction Project for Discipline Innovation (111
project, grant no. 111-2-12), the National Natural Science
Foundation of China (grant no. 81602030) and the Hunan Province
Natural Sciences Foundation of China (grant nos. 2019JJ40395,
2019JJ40436 and 2019JJ40161).
All data generated or analyzed during this study are
included in this published article.
ZL designed and revised the manuscript. LJ wrote the
manuscript and drew figures. RW collected the related literature
and created the tables. All the authors read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng TY, Cramb SM, Baade PD, Youlden DR,
Nwogu C and Reid ME: The International epidemiology of lung cancer:
Latest trends, disparities, and tumor characteristics. J Thorac
Oncol. 11:1653–1671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Behera M, Owonikoko TK, Gal AA, Steuer CE,
Kim S, Pillai RN, Khuri FR, Ramalingam SS and Sica GL: Lung
adenocarcinoma staging using the 2011 IASLC/ATS/ERS classification:
A pooled analysis of adenocarcinoma in situ and minimally invasive
adenocarcinoma. Clin Lung Cancer. 17:e57–e64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xue K, Li FF, Chen YW, Zhou YH and He J:
Body mass index and the risk of cancer in women compared with men:
A meta-analysis of prospective cohort studies. Eur J Cancer Prev.
26:94–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Groot PM, Wu CC, Carter BW and Munden
RF: The epidemiology of lung cancer. Transl Luna Cancer Res.
7:220–233. 2018. View Article : Google Scholar
|
|
7
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Malhotra J, Malvezzi M, Negri E, La
Vecchia C and Boffetta P: Risk factors for lung cancer worldwide.
Eur Respir J. 48:889–902. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cabili MN, Trapnell C, Goff L, Koziol M,
Tazon-Vega B, Regev A and Rinn JL: Integrative annotation of human
large intergenic noncoding RNAs reveals global properties and
specific subclasses. Genes Dev. 25:1915–1927. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marchese FP, Raimondi I and Huarte M: The
multidimensional mechanisms of long noncoding RNA function. Genome
Biol. 18:2062017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun Q, Hao Q and Prasanth KV: Nuclear long
noncoding RNAs: Key regulators of gene expression. Trends Genet.
34:142–157. 2018. View Article : Google Scholar :
|
|
12
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin C and Yang L: Long noncoding RNA in
cancer: Wiring signaling circuitry. Trends Cell Biol. 28:287–301.
2018. View Article : Google Scholar :
|
|
14
|
Ding X, Zhang S, Li X, Feng C, Huang Q,
Wang S, Wang S, Xia W, Yang F, Yin R, et al: Profiling expression
of coding genes, long noncoding RNA, and circular RNA in lung
adenocarcinoma by ribosomal RNA-depleted RNA sequencing. FEBS Open
Bio. 8:544–555. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang RX, Chen WJ, He RQ, Zeng JH, Liang L,
Li SK, Ma J, Luo DZ and Chen G: Identification of a RNA-Seq based
prognostic signature with five lncRNAs for lung squamous cell
carcinoma. Oncotarget. 8:50761–50773. 2017.PubMed/NCBI
|
|
16
|
Tian X, Zhang H, Zhang B, Zhao J, Li T and
Zhao Y: Microarray expression profile of long non-coding RNAs in
paclitaxel-resistant human lung adenocarcinoma cells. Oncol Rep.
38:293–300. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang J, Lin J, Liu T, Chen T, Pan S, Huang
W and Li S: Analysis of lncRNA expression profiles in non-small
cell lung cancers (NSCLC) and their clinical subtypes. Lung Cancer.
85:110–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng F, Wang R, Zhang Y, Zhao Z, Zhou W,
Chang Z, Liang H, Zhao W, Qi L, Guo Z and Gu Y: Differential
expression analysis at the individual level reveals a lncRNA
prognostic signature for lung adenocarcinoma. Mol Cancer.
16:982017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gibb EA, Warren RL, Wilson GW, Brown SD,
Robertson GA, Morin GB and Holt RA: Activation of an endogenous
retrovirus-associated long non-coding RNA in human adenocarcinoma.
Genome Med. 7:222015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaplan R, Luettich K, Heguy A, Hackett NR,
Harvey BG and Crystal RG: Monoallelic up-regulation of the
imprinted H19 gene in airway epithelium of phenotypically normal
cigarette smokers. Cancer Res. 63:1475–1482. 2003.PubMed/NCBI
|
|
21
|
Chen B, Yu M, Chang Q, Lu Y, Thakur C, Ma
D, Yi Z and Chen F: Mdig de-represses H19 large intergenic
non-coding RNA (lincRNA) by down-regulating H3K9me3 and
heterochromatin. Oncotarget. 4:1427–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu Y, Wang W, Li X, Liu Y, Niu Y, Zhang B,
Nie J, Pan B, Wang R and Yang J: lncRNA H19 interacts with
S-adenosylhomocysteine hydrolase to regulate LINE-1 Methylation in
human lung-derived cells exposed to Benzo[a]pyrene. Chemosphere.
207:84–90. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thai P, Statt S, Chen CH, Liang E,
Campbell C and Wu R: Characterization of a novel long noncoding
RNA, SCAL1, induced by cigarette smoke and elevated in lung cancer
cell lines. Am J Respir Cell Mol Biol. 49:204–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu L, Tang J, Huang X, Zhang T and Feng X:
Hypoxia exposure upregulates MALAT-1 and regulates the
transcriptional activity of PTB-associated splicing factor in A549
lung adenocarcinoma cells. Oncol Lett. 16:294–300. 2018.PubMed/NCBI
|
|
25
|
Zhou C, Ye L, Jiang C, Bai J, Chi Y and
Zhang H: Long noncoding RNA HOTAIR, a hypoxia-inducible factor-1α
activated driver of malignancy, enhances hypoxic cancer cell
proliferation, migration, and invasion in non-small cell lung
cancer. Tumour Biol. 36:9179–9188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo F, Guo L, Li Y, Zhou Q and Li Z:
MALAT1 is an oncogenic long non-coding RNA associated with tumor
invasion in non-small cell lung cancer regulated by DNA
methylation. Int J Clin Exp Pathol. 8:15903–15910. 2015.
|
|
27
|
Khaitan D, Dinger ME, Mazar J, Crawford J,
Smith MA, Mattick JS and Perera RJ: The melanoma-upregulated long
noncoding RNA SPRY4-IT1 modulates apoptosis and invasion. Cancer
Res. 71:3852–3862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun M, Liu XH, Lu KH, Nie FQ, Xia R, Kong
R, Yang JS, Xu TP, Liu YW, Zou YF, et al: EZH2-mediated epigenetic
suppression of long noncoding RNA SPRY4-IT1 promotes NSCLC cell
proliferation and metastasis by affecting the
epithelial-mesenchymal transition. Cell Death Dis. 5:e12982014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wen X, Han XR, Wang YJ, Fan SH, Zhuang J,
Zhang ZF, Shan Q, Li MQ, Hu B, Sun CH, et al: Effects of long
noncoding RNA SPRY4-IT1-mediated EZH2 on the invasion and migration
of lung adenocarcinoma. J Cell Biochem. 119:1827–1840. 2018.
View Article : Google Scholar
|
|
30
|
Zhai N, Xia Y, Yin R, Liu J and Gao F: A
negative regulation loop of long noncoding RNA HOTAIR and p53 in
non-small-cell lung cancer. Onco Targets Ther. 9:5713–5720. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hung T, Wang Y, Lin MF, Koegel AK, Kotake
Y, Grant GD, Horlings HM, Shah N, Umbricht C, Wang P, et al:
Extensive and coordinated transcription of noncoding RNAs within
cell-cycle promoters. Nat Genet. 43:621–629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han L, Zhang EB, Yin DD, Kong R, Xu TP,
Chen WM, Xia R, Shu YQ and De W: Low expression of long noncoding
RNA PANDAR predicts a poor prognosis of non-small cell lung cancer
and affects cell apoptosis by regulating Bcl-2. Cell Death Dis.
6:e16652015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barsyte-Lovejoy D, Lau SK, Boutros PC,
Khosravi F, Jurisica I, Andrulis IL, Tsao MS and Penn LZ: The c-Myc
oncogene directly induces the H19 noncoding RNA by allele-specific
binding to potentiate tumorigenesis. Cancer Res. 66:5330–5337.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu Y, Zhou X, Xu L, Rong C, Shen C and
Bian W: Long noncoding RNA ANRIL could be transactivated by c-Myc
and promote tumor progression of non-small-cell lung cancer. Onco
Targets Ther. 9:3077–3084. 2016.PubMed/NCBI
|
|
35
|
Huang T, Wang G, Yang L, Peng B, Wen Y,
Ding G and Wang Z: Transcription Factor YY1 modulates lung cancer
progression by activating lncRNA-PVT1. DNA Cell Biol. 36:947–958.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wan L, Sun M, Liu GJ, Wei CC, Zhang EB,
Kong R, Xu TP, Huang MD and Wang ZX: Long noncoding RNA PVT-1
promotes non-small cell lung cancer cell proliferation through
epigenetically regulating LATS2 expression. Mol Cancer Ther.
15:1082–1094. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li S, Ma F, Jiang K, Shan H, Shi M and
Chen B: Long non-coding RNA metastasis-associated lung
adenocarcinoma transcript 1 promotes lung adenocarcinoma by
directly interacting with specificity protein 1. Cancer Sci.
109:1346–1356. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jen J, Tang YA, Lu YH, Lin CC, Lai WW and
Wang YC: Oct4 transcriptionally regulates the expression of long
non-coding RNAs NEAT1 and MALAT1 to promote lung cancer
progression. Mol Cancer. 16:1042017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo F, Jiao F, Song Z, Li S, Liu B, Yang
H, Zhou Q and Li Z: Regulation of MALAT1 expression by TDP43
controls the migration and invasion of non-small cell lung cancer
cells in vitro. Biochem Biophys Res Commun. 465:293–298. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li S, Mei Z, Hu HB and Zhang X: The lncRNA
MALAT1 contributes to non-small cell lung cancer development via
modulating miR-124/STAT3 axis. J Cell Physiol. 233:6679–6688. 2018.
View Article : Google Scholar
|
|
41
|
Tang Y, Xiao G, Chen Y and Deng Y: lncRNA
MALAT 1 p romotes migration and invasion of non-small-cell lung
cancer by targeting miR-206 and activating Akt/mTOR signaling.
Anticancer Drugs. 29:725–735. 2018.PubMed/NCBI
|
|
42
|
Tripathi V, Ellis JD, Shen Z, Song DY, Pan
Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, et al: The
nuclear-retained noncoding RNA MALAT1 regulates alternative
splicing by modulating SR splicing factor phosphorylation. Mol
Cell. 39:925–938. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tano K, Onoguchi-Mizutani R, Yeasmin F,
Uchiumi F, Suzuki Y, Yada T and Akimitsu N: Identification of
minimal p53 promoter region regulated by MALAT1 in human lung
adenocarcinoma Cells. Front Genet. 8:2082018. View Article : Google Scholar :
|
|
44
|
Ma J, Wu K, Liu K and Miao R: Effects of
MALAT1 on proliferation and apoptosis of human non-small cell lung
cancer A549 cells in vitro and tumor xenograft growth in vivo by
modulating autophagy. Cancer Biomark. 22:63–72. 2018. View Article : Google Scholar
|
|
45
|
Tsai MC, Manor O, Wan Y, Mosammaparast N,
Wang JK, Lan F, Shi Y, Segal E and Chang HY: Long noncoding RNA as
modular scaffold of histone modification complexes. Science.
329:689–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang R, Chen X, Xu T, Xia R, Han L, Chen
W, De W and Shu Y: miR-326 regulates cell proliferation and
migration in lung cancer by targeting phox2a and is regulated by
HOTAIR. Am J Cancer Res. 6:173–186. 2016.PubMed/NCBI
|
|
47
|
Feng L, Xie Y, Zhang H and Wu Y: miR-107
targets cyclin-dependent kinase 6 expression, induces cell cycle G1
arrest and inhibits invasion in gastric cancer cells. Med Oncol.
29:856–863. 2012. View Article : Google Scholar
|
|
48
|
Cui J, Mo J, Luo M, Yu Q, Zhou S, Li T,
Zhang Y and Luo W: c-Myc-activated long non-coding RNA H19
downregulates miR-107 and promotes cell cycle progression of
non-small cell lung cancer. Int J Clin Exp Pathol. 8:12400–12409.
2015.
|
|
49
|
Zhang E, Li W, Yin D, De W, Zhu L, Sun S
and Han L: c-Myc-regulated long non-coding RNA H19 indicates a poor
prognosis and affects cell proliferation in non-small-cell lung
cancer. Tumour Biol. 37:4007–4015. 2016. View Article : Google Scholar
|
|
50
|
Huang Z, Lei W, Hu HB, Zhang H and Zhu Y:
H19 promotes non-small-cell lung cancer (NSCLC) development through
STAT3 signaling via sponging miR-17. J Cell Physiol. 233:6768–6776.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang Q, Li X, Li X, Li X and Chen Z:
lncRNA H19 promotes epithelial-mesenchymal transition (EMT) by
targeting miR-484 in human lung cancer cells. J Cell Biochem.
119:4447–4457. 2018. View Article : Google Scholar
|
|
52
|
Ren J, Fu J, Ma T, Yan B, Gao R, An Z and
Wang D: lncRNA H19-elevated LIN28B promotes lung cancer progression
through sequestering miR-196b. Cell Cycle. 17:1372–1380. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen Z, Li JL, Lin S, Cao C, Gimbrone NT,
Yang R, Fu DA, Carper MB, Haura EB, Schabath MB, et al:
cAMP/CREB-regulated LINC00473 marks LKB1-inactivated lung cancer
and mediates tumor growth. J Clin Invest. 126:2267–2279. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li J, Li P, Zhao W, Yang R, Chen S, Bai Y,
Dun S, Chen X, Du Y, Wang Y, et al: Expression of long non-coding
RNA DLX6-AS1 in lung adenocarcinoma. Cancer Cell Int. 15:482015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Naemura M, Murasaki C, Inoue Y, Okamoto H
and Kotake Y: Long noncoding RNA ANRIL regulates proliferation of
non-small cell lung cancer and cervical cancer cells. Anticancer
Res. 35:5377–5382. 2015.PubMed/NCBI
|
|
56
|
Nie FQ, Sun M, Yang JS, Xie M, Xu TP, Xia
R, Liu YW, Liu XH, Zhang EB, Lu KH and Shu YQ: Long noncoding RNA
ANRIL promotes non-small cell lung cancer cell proliferation and
inhibits apoptosis by silencing KLF2 and P21 expression. Mol Cancer
Ther. 14:268–277. 2015. View Article : Google Scholar
|
|
57
|
Hou Z, Zhao W, Zhou J, Shen L, Zhan P, Xu
C, Chang C, Bi H, Zou J, Yao X, et al: A long noncoding RNA Sox2ot
regulates lung cancer cell proliferation and is a prognostic
indicator of poor survival. Int J Biochem Cell Biol. 53:380–388.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Flockhart RJ, Webster DE, Qu K,
Mascarenhas N, Kovalski J, Kretz M and Khavari PA: BRAFV600E
remodels the melanocyte transcriptome and induces BANCR to regulate
melanoma cell migration. Genome Res. 22:1006–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jiang W, Zhang D, Xu B, Wu Z, Liu S, Zhang
L, Tian Y, Han X and Tian D: Long non-coding RNA BANCR promotes
proliferation and migration of lung carcinoma via MAPK pathways.
Biomed Pharmacother. 69:90–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhao JM, Cheng W, He XG, Liu YL, Wang FF
and Gao YF: Long non-coding RNA PICART1 suppresses proliferation
and promotes apoptosis in lung cancer cells by inhibiting
JAK2/STAT3 signaling. Neoplasma. 65:779–789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Su W, Feng S, Chen X, Yang X, Mao R, Guo
C, Wang Z, Thomas DG, Lin J, Reddy RM, et al: Silencing of long
noncoding RNA MIR22HG triggers cell Survival/Death signaling via
oncogenes YBX1, MET, and p21 in lung cancer. Cancer Res.
78:3207–3219. 2018.PubMed/NCBI
|
|
62
|
Mondal T, Rasmussen M, Pandey GK, Isaksson
A and Kanduri C: Characterization of the RNA content of chromatin.
Genome Res. 20:899–907. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wei MM, Zhou YC, Wen ZS, Zhou B, Huang YC,
Wang GZ, Zhao XC, Pan HL, Qu LW, Zhang J, et al: Long non-coding
RNA stabilizes the Y-box-binding protein 1 and regulates the
epidermal growth factor receptor to promote lung carcinogenesis.
Oncotarget. 7:59556–59571. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu M, Sun W, Liu Y and Dong X: The role
of lncRNA MALAT1 in bone metastasis in patients with non-small cell
lung cancer. Oncol Rep. 36:1679–1685. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gutschner T, Hammerle M, Eissmann M, Hsu
J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, et al:
The noncoding RNA MALAT1 is a critical regulator of the metastasis
phenotype of lung cancer cells. Cancer Res. 73:1180–1189. 2013.
View Article : Google Scholar
|
|
66
|
Li J, Wang J, Chen Y, Li S, Jin M, Wang H,
Chen Z and Yu W: lncRNA MALAT1 exerts oncogenic functions in lung
adenocarcinoma by targeting miR-204. Am J Cancer Res. 6:1099–1107.
2016.PubMed/NCBI
|
|
67
|
Jiang C, Yang Y, Yang Y, Guo L, Huang J,
Liu X, Wu C and Zou J: Long noncoding RNA (lncRNA) HOTAIR affects
tumorigenesis and metastasis of non-small cell lung cancer by
upregulating miR-613. Oncol Res. 26:725–734. 2018. View Article : Google Scholar
|
|
68
|
Wang R, Yan B, Li Z, Jiang Y, Mao C, Wang
X and Zhou X: Long non-coding RNA HOX transcript antisense RNA
promotes expression of 14-3-3σ in non-small cell lung cancer. Exp
Ther Med. 14:4503–4508. 2017.PubMed/NCBI
|
|
69
|
Liu XH, Liu ZL, Sun M, Liu J, Wang ZX and
De W: The long non-coding RNA HOTAIR indicates a poor prognosis and
promotes metastasis in non-small cell lung cancer. BMC cancer.
13:4642013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ono H, Motoi N, Nagano H, Miyauchi E,
Ushijima M, Matsuura M, Okumura S, Nishio M, Hirose T, Inase N and
Ishikawa Y: Long noncoding RNA HOTAIR is relevant to cellular
proliferation, invasiveness, and clinical relapse in small-cell
lung cancer. Cancer Med. 3:632–642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang R, Shi Y, Chen L, Jiang Y, Mao C, Yan
B, Liu S, Shan B, Tao Y and Wang X: Theratio of FoxA1 to FoxA2 in
lung adenocarcinoma is regulated by lncRNA HOTAIR and chromatin
remodeling factor LSH. Sci Rep. 5:178262015. View Article : Google Scholar
|
|
72
|
Guo D, Wang Y, Ren K and Han X: Knockdown
of lncRNA PVT1 inhibits tumorigenesis in non-small-cell lung cancer
by regulating miR-497 expression. Exp Cell Res. 362:172–179. 2018.
View Article : Google Scholar
|
|
73
|
Chen W, Zhu H, Yin L, Wang T, Wu J, Xu J,
Tao H, Liu J and He X: lncRNA-PVT1 facilitates invasion through
upregulation of MMP9 in nonsmall cell lung cancer cell. DNA Cell
Biol. 36:787–793. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lin L, Gu ZT, Chen WH and Cao KJ:
Increased expression of the long non-coding RNA ANRIL promotes lung
cancer cell metastasis and correlates with poor prognosis. Diagn
Pathol. 10:142015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li Z, Yu X and Shen J: ANRIL: A pivotal
tumor suppressor long non-coding RNA in human cancers. Tumour Biol.
37:5657–5661. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yu T, Zhao Y, Hu Z, Li J, Chu D, Zhang J,
Li Z, Chen B, Zhang X, Pan H, et al: Metalnc9 facilitates lung
cancer metastasis via a PGK1-activated AKT/mTOR pathway. Cancer
Res. 77:5782–5794. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang L, Zhou XF, Pan GF and Zhao JP:
Enhanced expression of long non-coding RNA ZXF-1 promoted the
invasion and metastasis in lung adenocarcinoma. Biomed
Pharmacother. 68:401–407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ge X, Li GY, Jiang L, Jia L, Zhang Z, Li
X, Wang R, Zhou M, Zhou Y, Zeng Z, et al: Long noncoding RNA CAR10
promotes lung adenocarcinoma metastasis via miR-203/30/SNAI axis.
Oncogene. 38:3061–3076. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Falzone L, Salomone S and Libra M:
Evolution of cancer pharmacological treatments at the turn of the
third millennium. Front Pharmacol. 9:13002018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sun YW, Xu J, Zhou J and Liu WJ: Targeted
drugs for systemic therapy of lung cancer with brain metastases.
Oncotarget. 9:5459–5472. 2017.
|
|
81
|
Yang CJ, Hung JY, Tsai MJ, Wu KL, Liu TC,
Chou SH, Lee JY, Hsu JS, Huang MS and Chong IW: The salvage therapy
in lung adenocarcinoma initially harbored susceptible EGFR mutation
and acquired resistance occurred to the first-line gefitinib and
second-line cytotoxic chemotherapy. BMC Pharmacol Toxicol.
18:212017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mitsudomi T: Advances in target therapy
for lung cancer. Jpn J Clin Oncol. 40:101–106. 2010. View Article : Google Scholar
|
|
83
|
Konieczna A, Novakova V, Medalova J, Erceg
S and Klabusay M: Thiazolidinediones regulate the level of ABC
transporters expression on lung cancer cells. Klin Onkol.
28:431–438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gao Y, Li W, Liu X, Gao F and Zhao X:
Reversing effect and mechanism of soluble resistance-related
calcium-binding protein on multidrug resistance in human lung
cancer A549/DDP cells. Mol Med Rep. 11:2118–2124. 2015. View Article : Google Scholar
|
|
85
|
Patel NR, Pattni BS, Abouzeid AH and
Torchilin VP: Nanopreparations to overcome multidrug resistance in
cancer. Adv Drug Deliv Rev. 65:1748–1762. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fang Z, Chen W, Yuan Z, Liu X and Jiang H:
lncRNA-MALAT1 contributes to the cisplatin-resistance of lung
cancer by upregulating MRP1 and MDR1 via STAT3 activation. Biomed
Pharmacother. 101:536–542. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu MY, Li XQ, Gao TH, Cui Y, Ma N, Zhou Y
and Zhang GJ: Elevated HOTAIR expression associated with cisplatin
resistance in non-small cell lung cancer patients. J Thorac Dis.
8:3314–3322. 2016. View Article : Google Scholar
|
|
88
|
Wang Q, Cheng N, Li X, Pan H, Li C, Ren S,
Su C, Cai W, Zhao C, Zhang L and Zhou C: Correlation of long
non-coding RNA H19 expression with cisplatin-resistance and
clinical outcome in lung adenocarcinoma. Oncotarget. 8:2558–2567.
2017.
|
|
89
|
Liu J, Wan L, Lu K, Sun M, Pan X, Zhang P,
Lu B, Liu G and Wang Z: The long noncoding RNA MEG3 contributes to
cispl-atin resistance of human lung adenocarcinoma. PLoS One.
10:e01145862015. View Article : Google Scholar
|
|
90
|
Yang Y, Li H, Hou S, Hu B, Liu J and Wang
J: The noncoding RNA expression profile and the effect of lncRNA
AK126698 on cisplatin resistance in non-small-cell lung cancer
cell. PLoS One. 8:e653092013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chen W, Zhao W, Zhang L, Wang L, Wang J,
Wan Z, Hong Y and Yu L: MALAT1-miR-101 SOX9 feedback loop modulates
the chemo-resistance of lung cancer cell to DDP via Wnt signaling
pathway. Oncotarget. 8:94317–94329. 2017.PubMed/NCBI
|
|
92
|
Wang H, Wang L, Zhang G, Lu C, Chu H, Yang
R and Zhao G: MALAT1/miR-101 3p/MCL1 axis mediates cisplatin
resistance in lung cancer. Oncotarget. 9:7501–7512. 2017.
|
|
93
|
Liu Z, Sun M, Lu K, Liu J, Zhang M, Wu W,
De W, Wang Z and Wang R: The long noncoding RNA HOTAIR contributes
to cisplatin resistance of human lung adenocarcinoma cells via
downregualtion of p21(WAF1/CIP1) expression. PLoS One.
8:e772932013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Rhee I, Bachman KE, Park BH, Jair KW, Yen
RW, Schuebel KE, Cui H, Feinberg AP, Lengauer C, Kinzler KW, et al:
DNMT1 and DNMT3b cooperate to silence genes in human cancer cells.
Nature. 416:552–556. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Fang S, Gao H, Tong Y, Yang J, Tang R, Niu
Y, Li M and Guo L: Long noncoding RNA-HOTAIR affects
chemoresistance by regulating HOXA1 methylation in small cell lung
cancer cells. Lab Invest. 96:60–68. 2016. View Article : Google Scholar
|
|
96
|
Kris MG, Natale RB, Herbst RS, Lynch TJ
Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H,
Sandler A, et al: Efficacy of gefitinib, an inhibitor of the
epidermal growth factor receptor tyrosine kinase, in symptomatic
patients with non-small cell lung cancer: A randomized trial. JAMA.
290:2149–2158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wheler J, Falchook G, Tsimberidou AM, Hong
D, Naing A, Piha-Paul S, Chen SS, Heymach J, Fu S, Stephen B, et
al: Revisiting clinical trials using EGFR inhibitor-based regimens
in patients with advanced non-small cell lung cancer: A
retrospective analysis of an MD Anderson Cancer Center phase I
population. Oncotarget. 4:772–784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chang YS, Choi CM and Lee JC: Mechanisms
of epidermal growth factor receptor tyrosine kinase inhibitor
resistance and strategies to overcome resistance in lung
adenocarcinoma. Tuberc Respir Dis (Seoul). 79:248–256. 2016.
View Article : Google Scholar
|
|
99
|
Cheng N, Li X, Zhao C, Ren S, Chen X, Cai
W, Zhao M, Zhang Y, Li J, Wang Q and Zhou C: Microarray expression
profile of long non-coding RNAs in EGFR-TKIs resistance of human
non-small cell lung cancer. Oncol Rep. 33:833–839. 2015. View Article : Google Scholar
|
|
100
|
Liu Y, Jiang H, Zhou H, Ying X, Wang Z,
Yang Y, Xu W, He X and Li Y: Lentivirus-mediated silencing of
HOTAIR lncRNA restores gefitinib sensitivity by activating
Bax/caspase-3 and suppressing TGF-α/EGFR signaling in lung
adenocarcinoma. Oncol Lett. 15:2829–2838. 2018.PubMed/NCBI
|
|
101
|
Cheng N, Cai W, Ren S, Li X, Wang Q, Pan
H, Zhao M, Li J, Zhang Y, Zhao C, et al: Long non-coding RNA UCA1
induces non-T790M acquired resistance to EGFR-TKIs by activating
the AKT/mTOR pathway in EGFR-mutant non-small cell lung cancer.
Oncotarget. 6:23582–23593. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Dong S, Qu X, Li W, Zhong X, Li P, Yang S,
Chen X, Shao M and Zhang L: The long non-coding RNA, GAS5, enhances
gefitinib-induced cell death in innate EGFR tyrosine kinase
inhibitor-resistant lung adenocarcinoma cells with wide-type EGFR
via downregulation of the IGF-1R expression. J Hematol Oncol.
8:432015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yang Y, Jiang C, Yang Y, Guo L, Huang J,
Liu X, Wu C and Zou J: Silencing of lncRNA-HOTAIR decreases drug
resistance of Non-small cell lung cancer cells by inactivating
autophagy via suppressing the phosphorylation of ULK1. Biochem
Biophys Res Commun. 497:1003–1010. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Chen J, Shen Z, Zheng Y, Wang S and Mao W:
Radiotherapy induced Lewis lung cancer cell apoptosis via
inactivating β-catenin mediated by upregulated HOTAIR. Int J Clin
Exp Pathol. 8:7878–7886. 2015.
|
|
105
|
Wu D, Li Y, Zhang H and Hu X: Knockdown of
lncrna PVT1 enhances radiosensitivity in Non-small cell lung cancer
by sponging Mir-195. Cell Physiol Biochem. 42:2453–2466. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Chen JX, Chen M, Zheng YD, Wang SY and
Shen ZP: Up-regulation of BRAF activated non-coding RNA is
associated with radiation therapy for lung cancer. Biomed
Pharmacother. 71:79–83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Loewen G, Jayawickramarajah J, Zhuo Y and
Shan B: Functions of lncRNA HOTAIR in lung cancer. J Hematol Oncol.
7:902014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Nakagawa T, Endo H, Yokoyama M, Abe J,
Tamai K, Tanaka N, Sato I, Takahashi S, Kondo T and Satoh K: Large
noncoding RNA HOTAIR enhances aggressive biological behavior and is
associated with short disease-free survival in human non-small cell
lung cancer. Biochem Biophys Res Commun. 436:319–324. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Li N, Wang Y, Liu X, Luo P, Jing W, Zhu M
and Tu J: Identification of Circulating long noncoding RNA HOTAIR
as a novel biomarker for diagnosis and monitoring of Non-small cell
lung cancer. Technol Cancer Res Treat. Jan 1–2017. View Article : Google Scholar : Epub ahead of
print.
|
|
110
|
Wu X, Ruan L, Yang Y and Mei Q:
Identification of crucial regulatory relationships between long
non-coding RNAs and protein-coding genes in lung squamous cell
carcinoma. Mol Cell Probes. 30:146–152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Cui D, Yu CH, Liu M, Xia QQ, Zhang YF and
Jiang WL: Long non-coding RNA PVT1 as a novel biomarker for
diagnosis and prognosis of non-small cell lung cancer. Tumour Biol.
37:4127–4134. 2016. View Article : Google Scholar
|
|
112
|
Yang YR, Zang SZ, Zhong CL, Li YX, Zhao SS
and Feng XJ: Increased expression of the lncRNA PVT1 promotes
tumori-genesis in non-small cell lung cancer. Int J Clin Exp
Pathol. 7:6929–6935. 2014.
|
|
113
|
Hu X, Bao J, Wang Z, Zhang Z, Gu P, Tao F,
Cui D and Jiang W: The plasma lncRNA acting as fingerprint in
non-small-cell lung cancer. Tumour Biol. 37:3497–3504. 2016.
View Article : Google Scholar
|
|
114
|
Xie Y, Zhang Y, Du L, Jiang X, Yan S, Duan
W, Li J, Zhan Y, Wang L, Zhang S, et al: Circulating long noncoding
RNA act as potential novel biomarkers for diagnosis and prognosis
of non-small cell lung cancer. Mol Oncol. 12:648–658. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Schmidt LH, Spieker T, Koschmieder S,
Schaffers S, Humberg J, Jungen D, Bulk E, Hascher A, Wittmer D,
Marra A, et al: The long noncoding MALAT-1 RNA indicates a poor
prognosis in non-small cell lung cancer and induces migration and
tumor growth. J Thorac Oncol. 6:1984–1992. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ji P, Diederichs S, Wang W, Böing S,
Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et
al: MALAT-1, a novel noncoding RNA, and thymosin beta4 predict
metastasis and survival in early-stage non-small cell lung cancer.
Oncogene. 22:8031–8041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Lin L, Li H, Zhu Y, He S and Ge H:
Expression of metastasis-associated lung adenocarcinoma transcript
1 long non-coding RNA in vitro and in patients with non-small cell
lung cancer. Oncol Lett. 15:9443–9449. 2018.PubMed/NCBI
|
|
118
|
Tian X and Xu G: Clinical value of lncRNA
MALAT1 as a prognostic marker in human cancer: Systematic review
and meta-analysis. BMJ Open. 5:e0086532015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Weber DG, Johnen G, Casjens S, Bryk O,
Pesch B, Jöckel KH, Kollmeier J and Brüning T: Evaluation of long
noncoding RNA MALAT1 as a candidate blood-based biomarker for the
diagnosis of non-small cell lung cancer. BMC Res Notes. 6:5182013.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhang R, Xia Y, Wang Z, Zheng J, Chen Y,
Li X, Wang Y and Ming H: Serum long non coding RNA MALAT-1
protected by exosomes is up-regulated and promotes cell
proliferation and migration in non-small cell lung cancer. Biochem
Biophys Res Commun. 490:406–414. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Sun M, Liu XH, Wang KM, Nie FQ, Kong R,
Yang JS, Xia R, Xu TP, Jin FY, Liu ZJ, et al: Downregulation of
BRAF activated non-coding RNA is associated with poor prognosis for
non-small cell lung cancer and promotes metastasis by affecting
epithelial-mesenchymal transition. Mol Cancer. 13:682014.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Liang W, Lv T, Shi X, Liu H, Zhu Q, Zeng
J, Yang W, Yin J and Song Y: Circulating long noncoding RNA GAS5 is
a novel biomarker for the diagnosis of nonsmall cell lung cancer.
Medicine (Baltimore). 95:e46082016. View Article : Google Scholar
|
|
123
|
Tantai J, Hu D, Yang Y and Geng J:
Combined identification of long non-coding RNA XIST and HIF1A-AS1
in serum as an effective screening for non-small cell lung cancer.
Int J Clin Exp Pathol. 8:7887–7895. 2015.PubMed/NCBI
|