Introduction
Pancreatic cancer (PC) is a malignant tumor that is
difficult to diagnose and treat (1). The incidence of PC and its mortality
rate have increased significantly in recent years, and with a
5-year survival rate of <1%, it has one of the poorest prognoses
of malignant tumors (2). PC
treatment is mainly based on comprehensive surgical intervention.
However, the surgical resection success rate is low and the
postoperative recurrence rate is high (3). Chemotherapy is the main form of
treatment for unresectable PC and is one of the main strategies to
prevent its postoperative recurrence. Gemcitabine is considered the
'gold standard' chemotherapy regimen for advanced PC carcinoma and
the postoperative recurrence of PC (4). However, the effective rate of
gemcitabine chemotherapy is <10%, and its long-term use leads to
cancer resistance to chemotherapy (5). Therefore, the development of novel
and effective chemotherapy drugs is necessary. Studies have shown
that curcumin, thymol and other similar compounds significantly
inhibit PC cell proliferation (6,7).
Evodiamine (EVO) is a chemical substance extract of
plant origin, it is usually extracted from the genus
Tetradium, trees of the family Rutaceae (8). EVO has been shown to reduce fat
uptake in studies in mice (9). The
mechanism underlying the effect of EVO has not been established,
however, it is expected that EVO acts primarily as a thermogenic
substance (10). It has also
reported that EVO can influence serotonin reuptake in the brain
(11). In previous years, there
have been multiple publications focusing on various aspects of EVO,
in terms of its effects on proliferation, apoptosis and autophagy
(12-14). As the main component of the Chinese
medicine formulation Evodia (15),
EVO has inhibitory effects on cervical, lung, colon and
nasopharyngeal cancer and other tumor types, likely mediated by the
inhibition of tumor proliferation, metastasis and angiogenesis and
the promotion of tumor cell apoptosis and autophagy (16). The present study was conducted to
determine the effects of EVO on the proliferation, apoptosis and
autophagy of human PC cell lines and an in situ xenograft
model of PC in nude mice, and to investigate its possible mechanism
of action.
Materials and methods
Cell culture, treatment and
chemicals
The human SW1990 and PANC-1 PC cell lines were
obtained from the Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences (Shanghai, China) and were routinely
cultured in Roswell Park Memorial Institute (RPMI)-1640 medium and
Dulbecco's modified Eagle's medium (DMEM; both from Gibco; Thermo
Fisher Scientific, Inc.). All cell lines were supplemented with 10%
fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) and
1% penicillin/streptomycin solution at 37°C in a humidified
atmosphere of 5% CO2. The nutrient medium was replaced
every 2-3 days and the cells were subcultured when they reached
70-80% adherence to the bottom of the culture plate, followed by
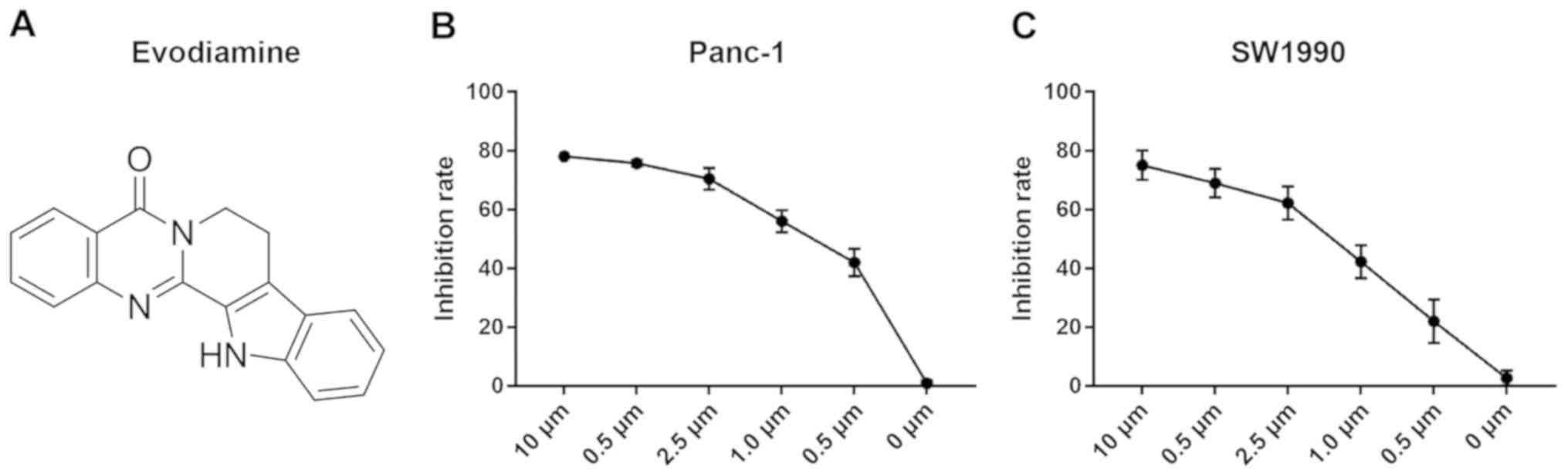
digestion with tryptase. EVO (purity >99%; Fig. 1A) was purchased from Sigma; Merck
KGaA and dissolved in dimethyl sulfoxide (DMSO; Nacalai Tesque,
Kyoto, Japan) at 0.2 mol/l to produce the stock solution. The final
DMSO concentration in the media did not exceed 0.1%. LY294002 (Akt
inhibitor), U0126 [extracellular signal-regulated kinase (ERK)1/2
inhibitor] and SB203580 (p38 inhibitor) were obtained from Merck
KGaA. Fluorine-18-labeled fluorodeoxyglucose (18F-FDG) was provided
by Zhejiang University (Hangzhou, China).
Antibodies
Rabbit monoclonal antibodies against phosphory-lated
(p)-Akt (Ser473) (D9E) (cat. no. CST 4060), Akt (C67E7; cat. no.
CST 4691), p-ERK (Thr202/Tyr204) (D13.14.4E) (cat. no. 4370), ERK
(137 F5) (cat. no. 4695), p-p38 (Thr180/Tyr182) (D3F9) (cat. no.
4551), p38 (D13E1) (cat. no. 8690), phosphorylated signal
transducer and activator of transcription activator 3 (p-STAT3;
Tyr705) (D3A7) (cat. no. 9145), STAT3 (79D7) (cat. no. 4904), P62
(D5E2) (cat. no. 8025) and LC3 (D3U4C) (cat. no. 12741) were
purchased from Cell Signaling Technology, Inc. Glyceraldehyde
3-phosphate dehydrogenase (GAPDH; cat. no. sc-47724) and HRP
AffiniPure Goat Anti-Rabbit IgG (H+L, cat. no. A32731) were
obtained from Santa Cruz Biotechnology, Inc.
Cell survival rate detection using Cell
Counting Kit (CCK)-8
The cells were seeded into 96-well plates at a
density of 5×10³ cells per well in 100 µl of the respective
medium overnight and were then divided into five groups, each
comprising five wells. The cells were treated with different
concentrations (0, 0.5, 1.0, 2.5, 5.0 and 10.0 µM) of the
EVO at 4°C, and then cultured the cells at 37°C for 48 h. Wells
treated with 0.1% mass fraction dimethyl sulfoxide (DMSO) without
the drug were used as the control. The blank retainer group was set
at the same time. At 1 h prior to the end of incubation, 10
µl CCK-8 (Beyotime Institute of Biotechnology) was added to
each well. The plates were mixed for 10 min on a gyratory shaker,
and absorbance at the 450 nm wavelength was measured with an ELISA
reader (BioTek ELx800, BioTek Instruments, Inc.).
Western blot analysis of autophagy- and
apoptosis-related protein expression in PC cells
Following treatment, the cells exposed to
phosphatase inhibitors were collected, total protein was extracted,
the concentration was measured in all samples using the Bradford
Protein Assay kit, and the protein samples (30-100 µg) were
separated using 10-15% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis. The separated proteins were then transferred onto
a polyvinylidene fluoride membrane, which was blocked for 1.5 h in
blocking buffer consisting of 5% milk in Tris-buffered saline (TBS)
containing 0.05% Tween-20, and then incubated with the different
primary antibodies (1:400) overnight at 4°C. The membrane was
washed with TBS containing 0.1% Tween-20, incubated with a
horseradish peroxidase (HRP)-conjugated secondary antibody
(1:1,000) for 1 h at 25°C. Primary and secondary antibodies were
diluted in Tris-buffered saline (TBS) containing 0.05% Tween-20.
The immunoreactive bands were then visualized using enhanced
chemiluminescence kits. The density of the immunoreactive bands was
analyzed using the Image J software (v1.8.0; National Institute of
Health).
Scratch wound model
The PC cells (5×105 cells/well) were
seeded in 6-well plates and allowed to adhere overnight without
serum-starvation. At 80-90% confluence, a 'reference line' scratch
was made at the bottom of the plate using a sterile 100-µl
pipette tip. Following washing three times with phosphate-buffered
saline (PBS), the cells were incubated with EVO (1, 5 and 10
µM) or the vehicle (DMSO) medium to detect cells in the
absence of cell growth migration. Following processing for 0 and 24
h, a digital camera following analysis with an MX4R Inspection
microscope (Canon, Inc., magnification, ×100) was used to capture
micrographs of the cells that had migrated across the reference
line in different fields. Compared with the control, the rate of
movement was quantified according to the migrated distance of the
cells from the reference line to the center. All experiments were
repeated three times.
Cell colony-forming assay
To determine the cell colony-forming units, 500
cells per well were seeded into 6-well plates with 2 ml of culture
medium overnight. The cells were pretreated with EVO, mixed with
DMSO for 8-12 h, washed twice with PBS, and then transferred to
fresh medium to allow the cells to grow for 7 days. The colonies
were washed with PBS and then fixed with 4% methanol for 15 min at
room temperature. The cells were washed then twice with PBS and
stained with 1% crystal violet (25% methanol) for 10 min at room
temperature. An image of the stained cell colonies was captured
using a digital camera following analysis with an MX4R Inspection
microscope. The cell size in the field of view is magnified 10
times. Three independent experiments were conducted for each
assay.
Assessment of apoptosis using flow
cytometry
Apoptosis was assessed using an Annexin V/propidium
iodide (PI) kit (BD Biosciences Pharmingen). Exponentially growing
cells were seeded in 6-well plates and then treated with EVO (1,5
and 10 µM) or DMSO (vehicle control, final concentration of
0.1%) for 48 h. Treated cells were harvested at room temperature
and stained with Annexin V and PI for 15 min and analyzed using
flow cytometry with a FACSCalibur instrument (BD Biosciences). The
exported data was processed and analyzed using FlowJo Software
version 7.6.1 (FlowJo LLC).
Immunofluorescence analysis of LC3
expression in PC cells
The PC cells were cultured in a nutrient-deficient
medium for 24 h (17). The
well-attached cells (5×105 cells/well) were placed
directly onto coverslips to allow them to grow. The coverslips
containing logarithmic growth-phase cells were placed in a 6-well
plate, fixed in 4% paraformaldehyde (1 ml per well) for 30 min and
washed with PBS three times for 5 min each. The cells were then
permeabilized with PBS containing 1% Triton X-100 (PBST) for 15
min, washed three times with PBS for 5 min each, and blocked with
blocking solution (normal donkey serum; Gibco; Thermo Fisher
Scientific, Inc.) for 30 min in 4°C. Non-specific staining was
assessed by replacing the LC3 antibody (D3U4C, cat. no. 12741) with
PBS. In the control group, only DMSO was added. The coverslips were
subsequently incubated with anti-LC3 (1:400) overnight at 4°C and
rinsed with PBST three times (flushing for 5 min each time). For
indirect immunofluorescence, the coverslips were further incubated
with HRP AffiniPure Goat Anti-Rabbit IgG (H+L, cat. no. A32731;
1:50) in the dark at room temperature for 1 h and then rinsed with
PBST three times. Primary and secondary antibodies were diluted in
Tris-buffered saline (TBS) containing 0.05% Tween-20. Following
each 5-min rinse, the coverslips were rinsed again with distilled
water, one drop of the encapsulated tablet was dropped, the
cover-slips were mounted and then examined using fluorescence
microscopy.
4′,6-diamidino-2-phenylindole (DAPI)
staining
Following the indicated treatments, the cells were
fixed with 4% parafor-maldehyde for 20 min and were washed
thoroughly with PBS between each reaction to remove any residual
solvent. The cells were stained with DAPI for 15 min in 4°C and
viewed under a fluorescence microscope equipped with an ultraviolet
(UV) filter.
Orthotopic PC xenograft nude mouse model
establishment
Female BALB/c nude mice, (age, 4-6 weeks; weight,
weight 20-25 g) were obtained from Shanghai SLAC Laboratory Animal
Co., Ltd. for tumor implantation. All mice were maintained in a
sterile environment under constant temperature (25°C) and humidity,
with a 12-h light/dark cycle, and free access to food and water.
Their care adhered to the laboratory animal regulations of the
Ministry of Science and Technology of the People's Republic of
China (http://www.most.gov.cn/kytj/kytjzcwj/200411/t20041108_32465.hgrowthtm).
The feed and drinking water of nude mice were purchased from
Beijing Keao Xieli Feed Co., Ltd.
A total of 40 mice were used, and the food, water
and bedding of the immunocompromised mice were sterilized and
replaced at least once per week. PANC-1 PC cells (5×106)
in log phase were collected in 50 µl serum-free DMEM per
mouse and orthotopically implanted into anesthetized athymic nude
mice. In brief, a small laparotomy was performed on the left upper
quadrant of each mouse. Anesthesia of the mice was induced with 3%
isoflurane and maintained with 2% isoflurane. The PANC-1 cells were
injected subcapsularly in a region of the pancreas just beneath the
spleen. Successful subcapsular intra-pancreatic injection of the
cancer cells was identified by the appearance of a fluid bleb
without intraperito-neal (i.p) leakage (18). All surgical procedures were
performed under sterile conditions using a 10X microscope. At 1
week post-implantation, the mice were medially randomized into four
groups to receive i.p. injections of the vehicle (0.9% sodium
chloride) or different concentrations of EVO (10, 20 and 30 mg/kg)
(19) three times per week for 2
weeks.
Micro positron emission tomography (PET)
imaging
PET imaging was performed 4 weeks after the nude
mice were treated. The mice were fasted 1 day before imaging and
injected via the tail vein with 0.1 mCi 18F-FDG/mouse. Anesthesia
of the mice was induced using 3% isoflurane and maintained with 2%
isoflurane, placed in the prone position, and scanned along the
long axis of the Micro PET scanner. Data were collected following
the administration of 18F-FDG-PET for 10 min and the ingestion time
was 1 h after tracer injection. Static acquisition in 3D was
performed using a micro PET imaging system (R4, Concorde
Microsystems, Inc.). For quantitation, the regions of interest
(ROIs) method and standard uptake values (SUVs) were used and
statistically analyzed using micro PET ASIPro6.0.5.0 (Concorde
Microsystems, Inc.). The ROI perimeters were drawn around the tumor
and the area around the coronal section showing the best tumor
depiction. All images were displayed using the same color scale.
The highest and average intakes (percentage injected doses) in the
tumor and surrounding ROIs, respectively were recorded and
calculated (20). The
tumor/non-tumor (T/N) ratio, which indicates tumor growth
metabolism, of the semi-quantitative analysis was calculated using
the following formula: T/N ratio = maximum pixel uptake in the
tumor ROI / average pixel uptake in the surrounding ROI. The SUVs
for ROIs of different groups were calculated as follows: SUV =
radioactivity concentration in ROI (mCi/mL) x body weight (g) /
injection dose (mCi), and compared. At the end of the experiment,
the mice were sacrificed by cervical dislocation 1 week after the
final PET imaging. The tumor samples were weighed and fresh frozen
in liquid nitrogen and 10% formalin prior to analysis. The mice
were sacrificed 8 weeks after implantation.
Immunohistochemical analysis of related
proteins p-AKT, p-ERK and p-P38
The formalin-fixed paraffin-embedded tumor tissue
samples were sectioned, deparaffinized, rehydrated, blocked with
10% goat serum (Gibco; Thermo Fisher Scientific, Inc.) in PBS for 1
h (4°C), and then immunostained. The sections (25×75 mm) were
incubated with p-Akt (Ser473, D9E, cat. no. CST 4060), p-ERK
(Thr202/Tyr204) (D13.14.4E, cat. no. 4370) and p-p38
(Thr180/Tyr182, D3F9, cat. no. 4551) in a moist chamber overnight
at 4°C. Following washing with PBS, the sections were incubated
with an HRP-conjugated secondary antibody for an additional 30 min
at room temperature. Following staining with hematoxylin, the
sections were evaluated using a microscope (Olympus BX51, Olympus
Corporation). Non-specific primary antibody staining was assessed
by substituting PBS for the primary antibody, and at least 10
fields were randomly selected from each section for evaluation. The
images were analyzed using Image-Pro Plus 6.0 software (Media
Cybernetics, Inc.).
Statistical analysis
The data are presented as the mean ± standard error
of the mean and the experiments were repeated three times (n=3).
The statistical package for the social sciences (SPSS) 13.0
software (SPSS, Inc.) was used for statistical analysis.
Differences in mean values between the groups were initially
analyzed using one-way analysis of variance.
Results
Cell growth effects of EVO in PC
cells
The inhibitory effects of EVO on PANC-1 and SW1990
PC cell lines were evaluated following incubation with different
concentrations for 48 h. As shown in Fig. 1B and C, EVO dose-dependently
reduced the viability of SW1990 and PANC-1 cells, indicating that
EVO markedly inhibited the growth of PC cells.
EVO inhibits colony formation in PC
cells
The colony-forming ability characterizes the
independent survival and environmental adaptation of cancer cells.
Therefore, the effect of EVO on the clonogenic survival of PC cells
was evaluated using a colony formation assay in plates. As shown in
Fig. 2A and B, EVO
dose-dependently inhibited the clonogenic survival of PANC-1and
SW1990 cells after 7 days of treatment. These findings indicated
that EVO potently inhibited PC cell growth in vitro and may
be useful for the treatment of PC.
EVO induces apoptosis of PC cells
The role of apoptosis in EVO-induced cell death was
then evaluated using Annexin V-fluorescein isothiocyanate and PI
staining. The PANC-1 and SW1990 cells were treated with EVO (1.0,
5.0 and 10 µM) for 48 h and then stained with Annexin V and
PI for the analysis of apoptosis using flow cytometry. As shown in
Fig. 3A and B, the exposure of
cells to EVO at various concentrations for 48 h led to a
dose-dependent increase in apoptosis. To determine whether
EVO-induced cell death was related to apoptosis, DAPI staining was
performed to analyze the changes in nuclear morphology. The results
revealed condensed and fragmented nuclei in both cell types
following 24 h of EVO (10 µM) treatment (Fig. 3C).
EVO inhibits the expression of
apoptosis-related proteins in PC cells
Previous data showed that the AKT/PI3K signaling
pathway serves an important role in the occurrence and development
of various tumor cells (19).
Therefore, the present study performed western blot analysis of PC
cells to examine whether EVO inhibits the expression of AKT, ERK1/2
and P38. As shown in Fig. 4A and
B, EVO inhibited the phosphorylation of AKT, ERK1/2 and P38 in
a dose-dependent manner. The role of p38, AkT and erk1/2 in
EVO-induced cell viability was also investigated. PANC-1 and SW1990
cells pretreated with LY294002, SB203580 or U0126 exhibited
decreased cell viability compared with those treated only with EVO
(Fig. 4C).
 | Figure 4EVO inhibits anti-apoptotic signal
proteins through inhibition of Akt, ERK1/2, and p38 in PANC-1 and
SW1990 cells. (A) PANC-1 and SW1990 cells were treated with
different concentrations of EVO (0-10 µM) for 24 h and then
subjected to western blotting. The levels of EVO of Akt, ERK1/2 and
p38 were investigated by western blotting with GAPDH as an internal
control. (B) Quantitative results of the protein levels of Akt,
ERK1/2 and p38, which were adjusted with the GAPDH protein level.
(C) PANC-1 and SW1990 cells were pretreated with LY294002 (20
µM), SB203580 (20 µM) or U0126 (10 µM) for 1 h
followed by treatment with EVO (2 µM) for 24 h and analyzed
using a Cell Counting Kit-8 assay. Results are shown as the mean ±
SD. *P<0.05, **P<0.01 and
***P<0.001 vs. control group; #P<0.05
vs. evodiamine alone group. EVO, evodiamine; ERK, extracellular
signal-regulated kinase; P-, phosphorylated; GAPDH, glyceraldehyde
3-phosphate dehydrogenase; CON, control; LY, LY294002; U, U0126;
SB, SB203580. |
EVO inhibits the expression of
autophagy-related LC3 and regulates apoptosis-related protein
expression in PC cells
Treatment with EVO significantly reduced the levels
of apoptotic marker STAT3 in PC cells (Fig. 5A and B). In addition, EVO reduced
the expression of LC3-II and enhanced that of P62, autophagy
markers in both cell lines, indicating that autophagy was inhibited
(Fig. 5A and B). The distribution
of LC3 was observed using cellular immunostaining (Fig. 5C). Treatment with EVO decreased the
number of LC3 puncta, also indicating that autophagy was inhibited
(Fig. 5C and D).
 | Figure 5EVO inhibits the expression of
autophagy-related LC3 and regulates apoptosis-related protein
expression in PC cells. (A) Treatment with EVO induced progressive
inhibition of autophagy in PC cell lines. Following culture in a
nutrient-deficient medium for 24 h, PANC-1 and SW1990 cells were
treated with different concentration of EVO (0, 1.0, 5.0 and 10
µm) and harvested at the indicated incubation times.
Expression levels of LC3, STAT3 and GAPDH were determined by
western blot analysis. (B) Densitometry analysis of the western
blotting results. (C) PANC-1 and SW1990 cells were immunostained
with anti-LC3 (green). Insets indicate magnified images of the
boxed area. Magnification, ×100. (D) At least 200 cells on each
slide were counted and the percentages of LC3 puncta-containing
cells were calculated. Data shown are presented as the mean ± SD of
three individual experiments. *P<0.05,
**P<0.01 and ***P<0.001 vs. CON. PC,
pancreatic cancer; EVO, evodiamine; STAT3, signal transducer and
activator of transcription activator 3; P-, phosphorylated; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; CON, control. |
EVO decreases PC cell migration
To evaluate the inhibitory effects of EVO on cell
migration, a scratch wound model was used. The wound areas were
marked and images were captured at 0 and 24 h. Compared with the
control PANC-1 and SW1990 cells, those treated with EVO at
different concentrations (1.0, 5.0 and 10 µM) migrated into
the wound with a significantly decreased distance between the edges
after 24 h, as shown in Fig. 6A and
B.
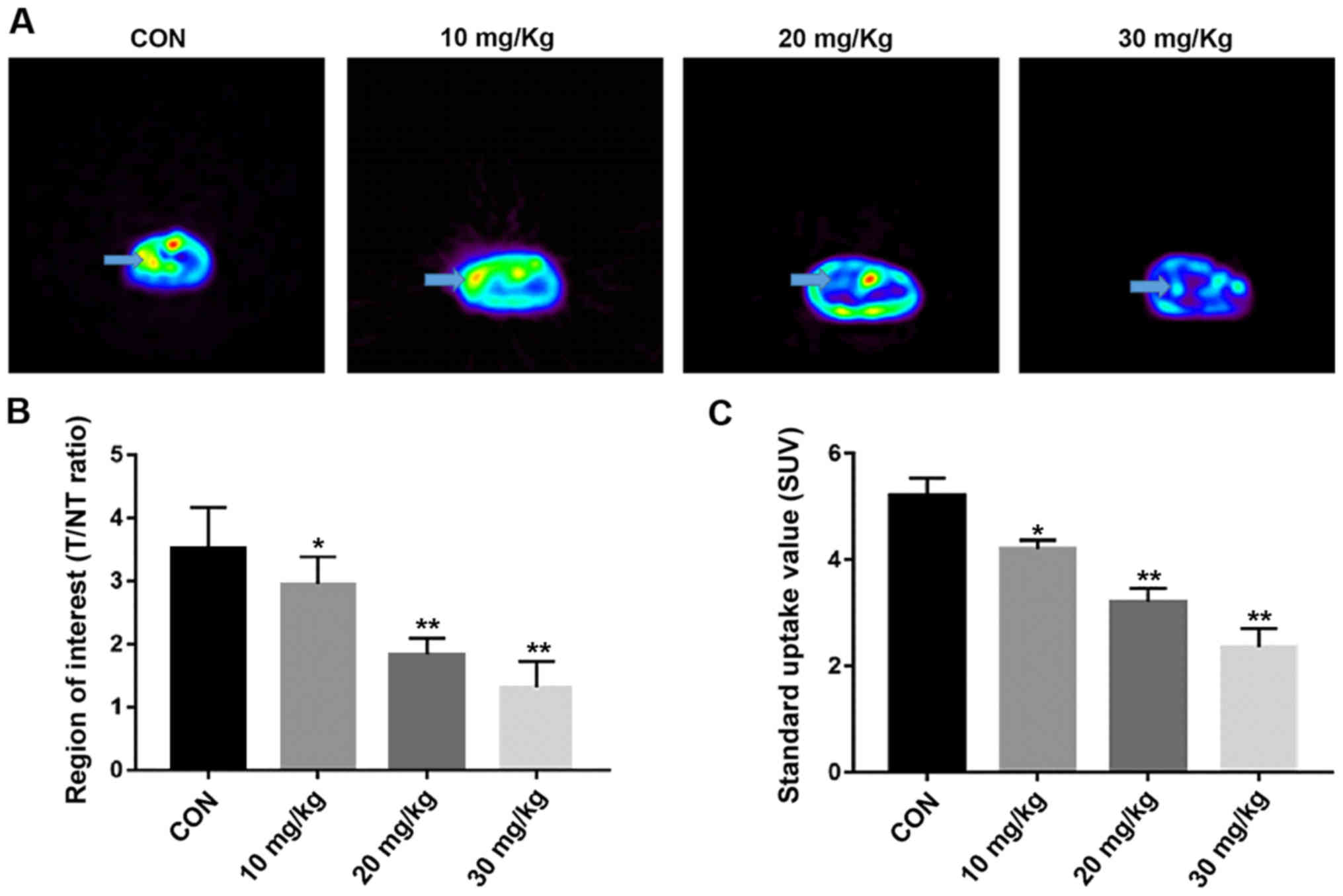
Micro PET imaging
The survival rate was of the mice was 100% and all
tumors were observed in the coronal plane of the micro PET. The
arrows in Fig. 7A show the tumor
localization. It was found that the drug decreased the T/NT ratio
in a concentration-dependent manner. The T/NT ratios of the EVO 10,
20 and 30 mg/kg groups (2.95±0.090, 1.83±0.260 and 1.31±0.142,
respectively) were lower than those of the control group (0.9%
sodium chloride, 3.52±0.095, P<0.05, Fig. 7B). The SUVs of the control and the
10, 20 and 30 mg/kg EVO groups were 5.21±0.33, 4.21±0.16, 3.11±0.26
and 2.36±0.35, respectively (Fig.
7C). The regional average intake of the tracer was
downregulated in an EVO concentration-dependent manner compared
with that of the in vivo control group (P<0.05).
EVO inhibits orthotopic xenograft growth
in nude mice
The effects of EVO on orthotopic xenografts in nude
mice were investigated (Fig. 8A).
The tumor weights (Fig. 8B and C)
of the EVO 10, 20 and 30 mg/kg groups, were 0.82±0.13, 0.67±0.18
and 0.23±0.17 g, respectively, compared with that of the control
group (1.58±0.27 g). As the concentration of EVO increased, the
body weight of nude mice also increased. In addition, the volume of
tumors in the nude mice decreased with increasing drug
concentration (Fig. 8D). These
results showed that EVO inhibited tumor growth in the nude mice in
a concentration-dependent manner.
Immunohistochemistry of the expression of
p-AKT, p-ERK and p-P38 in tumor tissues
The detection of p-AKT, p-ERK and p-P38 indicated
the inhibition of PC cell proliferation in the treatment group
(Fig. 9A and B). The expression
levels of p-AKT, p-ERK, and p-P38 were microscopically examined at
×400 magnification. Compared with those in the control group, the
expression levels of p-AKT, p-ERK and p-P38 in the tumor tissues
decreased significantly in a concentration-dependent manner
(Fig. 9B).
Discussion
PC, which is one of the most malignant types of
cancer worldwide (5), has a high
mortality rate and usually progresses to an untreatable state
(21). Gemcitabine is used to
treat patients with PC, but its effectiveness is <12% and it is
associated with considerable toxicity (2). Therefore, novel therapeutic
strategies require investigation to improve treatment outcomes.
EVO, a quinolone alkaloid, is traditionally used for stomachache
and headache in China. The present study investigated the effects
and precise mechanism of action of EVO in PC and found that EVO
significantly increased apoptosis in a concentration-dependent
manner by increasing apoptotic bodies and inducing chromosome
condensation, which serves an effective role in PC inhibition.
Early reports indicate that the treatment of A375-S2
human melanoma cells with EVO negatively affected the PI3K/AKT
signaling pathway (22,23), Studies have shown that EVO
signifi-cantly inhibits the proliferation of SGC-7901 cells and
induces cell cycle arrest in the G2/M phase, indicating that
autophagy and apoptosis were activated during EVO-induced SGC-7901
cell death (24). EVO also
affected the cell cycle of U87-MG astrocytes (25). In the present study, different
concentrations (0, 1, 5 and 10 µm) of EVO were used to treat
PANC-1 and SW1990 PC cell lines in vitro. It was found that
PC cell viability and apoptosis were increased by inhibiting the
PI3K/AKT pathway (26). The
results showed that increasing the EVO concentration between 1 and
10 µM inhibited the growth of tumor cells significantly and
their apoptosis became more obvious.
In the present study, EVO exhibited dose-dependent
cytotoxicity with a half-maximal inhibitory concentration of ~2
µM, and the results were similar in the two cell lines.
Combined with the results of the CCK assay and the pre-experiment
results, it was found that the effect of EVO on PC was
concentration-dependent in the 0-10-µm range, but the change
was not obvious >10 µm, thus the experiment was suspended
at these concentrations. It was found that, in 72 h, PC cell death
occurred instead of apoptosis, therefore the duration of 48 h was
used. The experiments demonstrated that AKT was expressed in PC
cells. It is reported that PC growth can be suppressed by
inhibiting the expression of AKT (19). The present study analyzed protein
expression using western blotting, which indicated that EVO
downregulated the expression of p-AKT, p-ERK and, p-P38 in PC cells
in a concentration-dependent manner. Multiple protein kinases, such
as the PI3K/AKT and mitogen-activated protein kinase (MAPK)/ERK
pathways, in cells are involved in cell survival and proliferation
(27). MAPKs have been shown to be
involved in the regulation of apoptosis and cell cycle-related
signaling pathways in a variety of cell models. MAPKs consists of
three major members, ERK, c-Jun N-terminal kinase and P38 MAPK
(26). The phosphorylation of P38
increased the apoptosis of PANC-1 and SW1990 PC cells. P38 is
mainly activated by a variety of stimuli, such as oxidative stress,
UV irradiation and osmotic pressure changes, mainly to induce
apoptosis (28). In addition to
MAPKs, another protein kinase, AKT, activates a range of downstream
substrates through phosphorylation, including the B-cell lymphoma
2-associated death promoter, glycogen synthase kinase 3β and
forkhead box O transcription factors, which mediate cell
proliferation (29). The present
study observed that EVO downregulated p-ERK in a dose-dependent
manner. ERK serves an important role in cell proliferation and
differentiation and is generally considered to be a regulator of
cell survival (30). The results
of the present study indicated that AKT and ERK were constitutively
activated in PC cells and that the levels of p-AKT and p-ERK were
significantly downregulated following EVO treatment in a
dose-dependent manner. Previously reported data show that PC cells
are protected by autophagy under extreme conditions (31). In the present study, it was
hypothesized that EVO mediates autophagy in human PC cells. STAT3
is constitutively activated and its prognostic value has been
associated with p-STAT3 signatures in PC (32). Therefore, targeting STAT3 signaling
with small molecule inhibitors is an emerging therapeutic strategy
for PC (33). Mechanistic studies
have indicated that EVO inhibits the formation of p-STAT3, leading
to decreased autophagy. Under normal physiological conditions,
STAT3 has low expression levels (34). Abnormal levels of p-STAT3 transform
normal fibrotic cells, showing the role of protooncogenes, which
are involved in tumor cell proliferation, differentiation,
invasion, metastasis and angiogenesis (35). Published studies show that
silencing STAT3 genetically or pharmacologically reduced the
expression of LC3 in Capan-2 cells, suggesting that STAT3
transcriptionally regulates LC3 (36). Furthermore, silencing STAT3
increased p62 levels, suggesting that STAT3 positively regulates
the activation of autophagy (36).
In addition, the present study found that EVO inhibited the
phosphorylation of STAT3 in PC cells, whereas expression of the
autophagy-related protein LC3 was downregulated and that of p62 was
upregulated. It was found that EVO promoted the apoptosis of PC
cells, whereas autophagy was suppressed. In a nutrient-poor
environment, PC cell lines have been reported to exhibit autophagy,
which supports cell survival (37). The analysis of human surgical
specimens suggested higher autophagy activity in cancer tissues
than in non-cancerous tissues (38). PC cell resistance has often been
associated with self-autophagy (31). Therefore, autophagy is a mechanism
that not only enables cells to adapt to hypoxic environments but
also supports the survival of the entire microenvironment of the PC
tissue. Therefore, autophagy is a favorable therapeutic target
(39). Cells prevent apoptosis
through mitochondrial autophagy, which is a selective autophagic
mechanism that maintains the intracellular redox state (40). Under hypoxic conditions, PC cells
can evade death through autophagy (31). In the present study, autophagy
formation was induced in PC cells through a nutrient-deficient
medium (17). The results revealed
that EVO inhibited autophagy in PC cells and thus reduced the
self-protection of PC cells. An orthotopic pancreas tumor model was
established in nude mice, and the growth of pancreatic tumors was
close to that in the human physiological environment. 18F-FDG-PET
has high sensitivity and specificity in the detection of PC in
patients with a suspected pancreatic mass. After 4 weeks of EVO
treatment of the nude mice, micro-PET imaging revealed that the
tumor mass was significantly lower than that of the untreated
control group. Regional uptake of the tracer was assessed using the
ROI method and SUVs. We found that as the concentration of EVO
increased, the body weight of nude mice increased correspondingly
compared with the control group, which also indicated that EVO also
serves a role in the health effects of nude mice while inhibiting
tumor growth. It was found that, with increasing drug
concentration, the size and volume of the tumors were increasingly
smaller, which was consistent with the in vitro experimental
results. In addition, the immunohistochemistry revealed that the
expression of P-AKT in the tumor tissues was decreased
concentration-dependently, which was also consistent with the in
vitro experiments. EVO inhibited cell migration similarly in
the two cell lines, which is similar to a previous study by
Ogasawara et al showing that EVO inhibited lung cancer
invasion and cell migration (41).
In conclusion, the present study demonstrated that
EVO exhibited potent antitumor effects on human PC cells and
promoted their apoptosis by inhibiting the phosphorylation of
PI3K/AKT and MEK/ERK pathway proteins. Furthermore, EVO promoted PC
cell growth retardation by inhibiting the phosphorylation of STAT3,
reducing phagocytosis. In the absence of nutrient deficiency, PC
cells survive by autophagy. For the first time, to the best of our
knowledge, EVO was found to inhibit the formation of autophagy in
PC cells, thereby destroying the self-protection mechanism of PC
cells. Based on these results, EVO has potential therapeutic
effects that warrant further investigation for development and
possible clinical trials for PC.
Funding
This study was supported by funding from Zhejiang
Provincial Administration of Traditional Chinese Medicine Youth
Fund Project (grant no. 2014ZQ020) and the Natural Science
Foundation of Zhejiang Project (grant nos. LQ18H290003 and
Y190029H46). Funding was also received from Wenzhou Science and
Technology Project (grant no. Y20160052).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZH and PZ contributed to the conception of the
study; HC, ZW and YL contributed significantly to analysis and
manuscript preparation; BZ, JW and MBJ performed the data analyses
and wrote the manuscript; QZ and HT helped perform the analysis
with constructive discussions.
Ethics approval and consent to
participate
This study was approved by the Laboratory Animal
Ethics Committee of Wenzhou Medical University and Laboratory
Animal Centre of Wenzhou Medical University (Wenzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: Current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Teague A, Lim KH and Wang-Gillam A:
Advanced pancreatic adenocarcinoma: A review of current treatment
strategies and developing therapies. Ther Adv Med Oncol. 7:68–84.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhan HX, Xu JW, Wu D, Wu ZY, Wang L, Hu SY
and Zhang GY: Neoadjuvant therapy in pancreatic cancer: A
systematic review and meta-analysis of prospective studies. Cancer
Med. 6:1201–1219. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Long J, Zhang Y, Yu X, Yang J, LeBrun DG,
Chen C, Yao Q and Li M: Overcoming drug resistance in pancreatic
cancer. Expert Opin Ther Targets. 15:817–828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yusufi M, Banerjee S, Mohammad M, Khatal
S, Venkateswara Swamy K, Khan EM, Aboukameel A, Sarkar FH and
Padhye S: Synthesis, characterization and anti-tumor activity of
novel thymoquinone analogs against pancreatic cancer. Bioorg Med
Chem Lett. 23:3101–3104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su J, Zhou X, Wang L, Yin X and Wang Z:
Curcumin inhibits cell growth and invasion and induces apoptosis
through down-regulation of Skp2 in pancreatic cancer cells. Am J
Cancer Res. 6:1949–1962. 2016.PubMed/NCBI
|
|
8
|
Liu AJ, Wang SH, Chen KC, Kuei HP, Shih
YL, Hou SY, Chiu WT, Hsiao SH and Shih CM: Evodiamine, a plant
alkaloid, induces calcium/JNK-mediated autophagy and
calcium/mitochondria-mediated apoptosis in human glioblastoma
cells. Chem Biol Interact. 205:20–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou X, Ren F, Wei H, Liu L, Shen T, Xu S,
Wei J, Ren J and Ni H: Combination of berberine and evodiamine
inhibits intestinal cholesterol absorption in high fat diet induced
hyperlipidemic rats. Lipids Health Dis. 16:2392017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schwarz NA, Spillane M, La Bounty P,
Grandjean PW, Leutholtz B and Willoughby DS: Capsaicin and
evodiamine ingestion does not augment energy expenditure and fat
oxidation at rest or after moderately-intense exercise. Nutr Res.
33:1034–1042. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang YN, Yang YF and Yang XW: Blood-brain
barrier permeability and neuroprotective effects of three main
alkaloids from the fruits of Euodia rutaecarpa with MDCK-pHaMDR
cell monolayer and PC12 cell line. Biomed Pharmacother. 98:82–87.
2018. View Article : Google Scholar
|
|
12
|
Kim SH, Kang JG, Kim CS, Ihm SH, Choi MG
and Lee SJ: Evodiamine Suppresses Survival, Proliferation,
Migration and Epithelial-Mesenchymal Transition of Thyroid
Carcinoma Cells. Anticancer Res. 38:6339–6352. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo XX, Li XP, Zhou P, Li DY, Lyu XT, Chen
Y, Lyu YW, Tian K, Yuan DZ, Ran JH, et al: Evodiamine Induces
Apoptosis in SMMC-7721 and HepG2 Cells by Suppressing NOD1 Signal
Pathway. Int J Mol Sci. 19:192018. View Article : Google Scholar
|
|
14
|
Tu YJ, Fan X, Yang X, Zhang C and Liang
HP: Evodiamine activates autophagy as a cytoprotective response in
murine Lewis lung carcinoma cells. Oncol Rep. 29:481–490. 2013.
View Article : Google Scholar
|
|
15
|
Han S, Woo JK, Jung Y, Jeong D, Kang M,
Yoo YJ, Lee H, Oh SH, Ryu JH and Kim WY: Evodiamine selectively
targets cancer stem-like cells through the p53-p21-Rb pathway.
Biochem Biophys Res Commun. 469:1153–1158. 2016. View Article : Google Scholar
|
|
16
|
Jiang J and Hu C: Evodiamine: A novel
anti-cancer alkaloid from Evodia rutaecarpa. Molecules.
14:1852–1859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SE, Park HJ, Jeong HK, Kim MJ, Kim M,
Bae ON and Baek SH: Autophagy sustains the survival of human
pancreatic cancer PANC-1 cells under extreme nutrient deprivation
conditions. Biochem Biophys Res Commun. 463:205–210. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pratesi G, Petrangolini G, Tortoreto M,
Addis A, Belluco S, Rossini A, Selleri S, Rumio C, Menard S and
Balsari A: Therapeutic synergism of gemcitabine and
CpG-oligodeoxynucleotides in an orthotopic human pancreatic
carcinoma xenograft. Cancer Res. 65:6388–6393. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei WT, Chen H, Wang ZH, Ni ZL, Liu HB,
Tong HF, Guo HC, Liu DL and Lin SZ: Enhanced antitumor efficacy of
gemcitabine by evodiamine on pancreatic cancer via regulating
PI3K/Akt pathway. Int J Biol Sci. 8:1–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang C, Li S and Wang MW:
Evodiamine-induced human melanoma A375-S2 cell death was mediated
by PI3K/Akt/caspase and Fas-L/NF-kappaB signaling pathways and
augmented by ubiquitin-proteasome inhibition. Toxicol In Vitro.
24:898–904. 2010. View Article : Google Scholar
|
|
23
|
Yotsumoto F, Fukami T, Yagi H, Funakoshi
A, Yoshizato T, Kuroki M and Miyamoto S: Amphiregulin regulates the
activation of ERK and Akt through epidermal growth factor receptor
and HER3 signals involved in the progression of pancreatic cancer.
Cancer Sci. 101:2351–2360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rasul A, Yu B, Zhong L, Khan M, Yang H and
Ma T: Cytotoxic effect of evodiamine in SGC-7901 human gastric
adenocarcinoma cells via simultaneous induction of apoptosis and
autophagy. Oncol Rep. 27:1481–1487. 2012.PubMed/NCBI
|
|
25
|
Liu AJ, Wang SH, Hou SY, Lin CJ, Chiu WT,
Hsiao SH, Chen TH and Shih CM: Evodiamine Induces Transient
Receptor Potential Vanilloid-1-Mediated Protective Autophagy in
U87-MG Astrocytes. Evid Based Complement Alternat Med.
2013:3548402013. View Article : Google Scholar
|
|
26
|
Wang R, Deng D, Shao N, Xu Y, Xue L, Peng
Y, Liu Y and Zhi F: Evodiamine activates cellular apoptosis through
suppressing PI3K/AKT and activating MAPK in glioma. Onco Targets
Ther. 11:1183–1192. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carnero A and Paramio JM: The
PTEN/PI3K/AKT Pathway in vivo, Cancer Mouse Models. Front Oncol.
4:2522014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhuang S and Schnellmann RG: A
death-promoting role for extracellular signal-regulated kinase. J
Pharmacol Exp Ther. 319:991–997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin S, Yang S, Pan X, Ma A, Ma J, Pei H,
Dong Y, Li S, Li W and Bi X: MicroRNA 155 promotes ox LDL induced
autophagy in human umbilical vein endothelial cells by targeting
the PI3K/Akt/mTOR pathway. Mol Med Rep. 18:2798–2806.
2018.PubMed/NCBI
|
|
30
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bellot G, Garcia-Medina R, Gounon P,
Chiche J, Roux D, Pouysségur J and Mazure NM: Hypoxia-induced
autophagy is mediated through hypoxia-inducible factor induction of
BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol.
29:2570–2581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mace TA, Ameen Z, Collins A, Wojcik S,
Mair M, Young GS, Fuchs JR, Eubank TD, Frankel WL, Bekaii-Saab T,
et al: Pancreatic cancer-associated stellate cells promote
differentiation of myeloid-derived suppressor cells in a
STAT3-dependent manner. Cancer Res. 73:3007–3018. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu Y, Zhao C, Zheng H, Lu K, Shi D, Liu Z,
Dai X, Zhang Y, Zhang X, Hu W, et al: A novel STAT3 inhibitor
HO-3867 induces cell apoptosis by reactive oxygen species-dependent
endoplasmic reticulum stress in human pancreatic cancer cells.
Anticancer Drugs. 28:392–400. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Szczepanek K, Lesnefsky EJ and Larner AC:
Multi-tasking: Nuclear transcription factors with novel roles in
the mitochondria. Trends Cell Biol. 22:429–437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aigner P, Just V and Stoiber D: STAT3
isoforms: Alternative fates in cancer? Cytokine. 118:27–34. 2019.
View Article : Google Scholar
|
|
36
|
Gong J, Muñoz AR, Chan D, Ghosh R and
Kumar AP: STAT3 down regulates LC3 to inhibit autophagy and
pancreatic cancer cell growth. Oncotarget. 5:2529–2541. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Azad MB, Chen Y, Henson ES, Cizeau J,
McMillan-Ward E, Israels SJ and Gibson SB: Hypoxia induces
autophagic cell death in apoptosis-competent cells through a
mechanism involving BNIP3. Autophagy. 4:195–204. 2008. View Article : Google Scholar
|
|
38
|
Sullivan R, Paré GC, Frederiksen LJ,
Semenza GL and Graham CH: Hypoxia-induced resistance to anticancer
drugs is associated with decreased senescence and requires
hypoxia-inducible factor-1 activity. Mol Cancer Ther. 7:1961–1973.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Greene LM, Nolan DP, Regan-Komito D,
Campiani G, Williams DC and Zisterer DM: Inhibition of late-stage
autophagy synergistically enhances
pyrrolo-1,5-benzoxazepine-6-induced apoptotic cell death in human
colon cancer cells. Int J Oncol. 43:927–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Blommaart EF, Luiken JJ, Blommaart PJ, van
Woerkom GM and Meijer AJ: Phosphorylation of ribosomal protein S6
is inhibitory for autophagy in isolated rat hepatocytes. J Biol
Chem. 270:2320–2326. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ogasawara M, Matsubara T and Suzuki H:
Inhibitory effects of evodiamine on in vitro invasion and
experimental lung metastasis of murine colon cancer cells. Biol
Pharm Bull. 24:917–920. 2001. View Article : Google Scholar : PubMed/NCBI
|