Introduction
Oral squamous cell carcinoma (OSCC) is the most
common malignant tumor of the head and neck region. It is
associated with rapid growth, strong invasiveness, early cervical
lymph node metastasis and a high rate of metastasis. Approximately
90% of oral cancers are squamous cell carcinoma or one of its
variants (1,2). It is currently one of the leading
causes of cancer-related mortality. Despite recent advances in
research and therapies, such as chemotherapy, radiotherapy and
immunotherapy in particular, the overall mortality rate of patients
with OSCC has remained constant over the past few decades, at
approximately 50% (3,4).
Tumor immune escape and chronic inflammation in the
tumor microenvironment are two important features necessary for
tumorigenesis and cancer progression. Programmed death ligand-1
(PD-L1), a ligand for the programmed cell death protein 1 (PD-1)
immunosuppressive checkpoint, can be induced in tumors by their
exposure to inflammatory factors in the tumor microenvironment,
leading to immune escape. PD-L1 protein expression in tumor cells
is upregulated upon their stimulation with interleukin (IL)-1,
IL-6, tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ),
which are located in the tumor microenvironment (5). Of these effectors, IFN-γ is the most
effective inducer of PD-L1 expression (6). Recent studies have suggested that
signaling molecules affecting the cell cycle, proliferation,
apoptosis and survival [including mitogen-activated protein kinase
(MAPK), nuclear factor-κB (NF-κB), phosphatidylinositol 3-kinase
(PI3K) and Janus kinase (JAK)/signal transducer and activator of
transcription (STAT)] are involved in the regulation of PD-L1
expression (6-9). Notably, OSCC usually exhibits host
immunosuppression and cytogenetic alterations in tumor cells. The
detailed understanding of the mechanisms through which PD-L1
expression is regulated will facilitate the identification of
pathways that inhibit PD-L1 function and modulate cancer
cell-responsive immune responses.
The protein kinase D (PKD) family consists of 3
serine/threonine kinases (termed PKCμ/PKD1, PKD2 and PKCν/PKD3).
They are extremely important regulators of diverse biological
processes involved in cell proliferation, cell migration,
differentiation, apoptosis, cardiac contraction, cardiac
hypertrophy, angiogenesis, tumorigenesis, epithelial-to-mesenchymal
transition and immune regulation (10-16).
The PKD subtypes can be localized to the plasma membrane and the
Golgi complex, and it has also been reported that they can shuttle
to the nucleus, as in the case of PKD3 (17). In recent decades, studies on the
functions and mechanisms of PKD have mainly focused on PKD1 and
PKD2. However, little is known about the function of PKD3,
particularly its mechanisms of action. There is increasing evidence
to suggest that PKD3 is connected to multiple pathways involved in
oncogenic signaling, such as protein kinase B (AKT), extracellular
signal-regulated kinase 1/2 (ERK1/2), NF-κB, STAT1 and STAT3
(16,18,19).
These signals can also trigger the expression of PD-L1 in tumor
cells. Previously, the authors' research group found that PKD2
exerted a certain regulatory effect on the expression of PD-L1
(11). However, the mechanisms
through which PKD3, as an oncogene, regulates PD-L1 expression in
OSCC cells remain unknown.
In this study, the role of PKD3 in the tumorigenesis
and progression of OSCC was examined. The results suggest that PKD3
expression is elevated in OSCC and that PKD3 regulates PD-L1
expression via STAT1 and STAT3. The findings of this study suggest
that PKD may be a promising therapeutic target for OSCC and broaden
the current understanding of the molecular mechanisms and function
of PKD3 in cancer progression.
Materials and methods
Isolation of PDLCs and cell culture
Human oral normal periodontal ligament cells (PDLCs)
were obtained from premolar teeth without inflammation and caries,
which were extracted for orthodontic treatment at the West China
Hospital of Stomatology of Sichuan University. All donors were
healthy and written informed consent was obtained from each donor
prior to tooth extraction. The extracted teeth were rinsed and
placed in phosphate-buffered saline (PBS) supplemented with 1,000
IU/ml penicillin and 1,000 μg/ml streptomycin (HyClone). The
remaining procedures were performed according to a previously
described protocol (20).
Periodontal tissues were from the middle third of the root, were
cut into 1-2 mm2 sections and placed in culture flasks
for cell culture in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific) supplemented with 10% fetal bovine serum (FBS;
Sigma-Aldrich; Merck KGaA), 100 IU/ml penicillin and 100 μg/ml
streptomycin. The present study was approved by the West China
Hospital of Stomatology Institutional Review Board.
The dysplastic oral keratinocyte (DOK) cell line and
4 OSCC cell lines (Cal-27, HSC-4, HSC-3 and SCC25) were purchased
from the American Type Culture Collection (ATCC). The SCC25 cells
were cultured in Dulbecco's modified Eagle's medium/nutrient
Mixture F12 (DMEM/F12; Gibco; Thermo Fisher Scientific) with 400
ng/ml hydrocortisone and 10% FBS (Sigma-Aldrich; Merck KGaA). The
other cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific) supplemented with 10% FBS,
100 IU/ml penicillin and 100 μg/ml streptomycin. The human normal
oral epithelial keratinocytes (HOK) were purchased from ScienCell
Research Laboratories, Inc. and were cultured in keratinocyte
serum-free medium (KSFM) supplemented with recombinant human
epidermal growth factor (5 ng/ml) and bovine pituitary extract (50
μg/ml) (Gibco; Thermo Fisher Scientific). The cells were maintained
in a humidified 5% CO2 atmosphere at 37̊C.
Patients and clinical samples
The present study included OSCC tissues specimens
from 34 patients with OSCC who underwent partial or total surgical
resection at West China Hospital of Stomatology from 2014 to 2016.
The 34 patients with OSCC enrolled in this study had not received
radiotherapy or chemotherapy prior to surgical resection. The
clinical information of the patients is presented in Table I. Clinically normal oral mucosa
specimens (>2 cm at a distance from the edge of the tumor mass)
and primary cancer tissues were collected by surgical resection.
The clinical samples were confirmed by two experienced
pathologists. This study was approved by the West China Hospital of
Stomatology Institutional Review Board and written informed consent
was obtained from each patient.
 | Table IClinical characteristics of the 34
patients with OSCC. |
Table I
Clinical characteristics of the 34
patients with OSCC.
| Patient
characteristics (n=34) | No. of
patients | Percentage (%) |
|---|
| Age (years) | | |
| <57 | 16 | 47.1 |
| >57 | 18 | 52.9 |
| Sex | | |
| Male | 26 | 76.5 |
| Female | 8 | 23.5 |
| N-regional lymph
node | | |
| Negative | 20 | 58.8 |
| Positive | 14 | 41.2 |
| Histological
grade | | |
| Grade 1 | 12 | 35.3 |
| Grade 2 | 18 | 52.9 |
| Grade 3 | 4 | 11.8 |
| TNM stage | | |
| I-II | 15 | 44.1 |
| III-IV | 19 | 55.9 |
Plasmids and transfection
The Hu-shRNA PKD3 and control shRNA plasmids were
obtained from GeneCopoeia. The Hu-shRNA construct contains the
human PKD3 gene-specific sequence (GCT CCT ACT TTC TGT GAT TAC)
shRNA expression vector psi-LVRU6GP. The plasmid containing GCT TCG
CGC CGT AGT CTT A (scrambled) was used as a control. A PKD3
overexpression plasmid and the corresponding control plasmid were
also purchased from GeneCopoeia. All plasmids were transfected into
the cells using Lipofectamine 2000 reagent according to the
manufacturer's instructions. The DOK, Cal-27 and HSC-4 cells were
seeded into 6 well plates, and transfection was carried out at
60-80% confluency. Hu-shRNA PKD3 and control shRNA plasmids were
transfected into Cal-27 and HSC-4. DOK stably expressing PKD3
protein was established by transfecting the PKD3 overexpression
plasmid. After 24 h, the cells were cultured in 0.2 μg/ml
puromycin, and PKD3 expression levels were detected by western blot
analysis.
Transient gene knockdown with siRNA
STAT1 siRNA (5′-CAC GAG ACC AAU GGU GUG GdT dT-3′;
5′-CCA CAC CAU UGG UCU CGU GdT dT-3′) was used to knockdown STAT1
expression, as previously described (21). In addition, STAT3 siRNA (5′-AAC AUC
UGC CUA GAU CGG CUA dTdT-3′; 3′-dTd TGU AGA CGG AUC UAG CCG AU-5′)
was synthesized by Dharmacon Research (22). A scrambled sequence (5′-UUC UCC GAA
CGU GUC ACG UTT-3′; 5′-ACG UGA CAC GUU CGG AGA ATT-3′) was used as
a negative control. STAT1/3 siRNA was transfected into the Cal-27
and HSC-4 cells using Lipofectamine 2000 reagent according to the
manufacturer's instructions. The knockdown efficiency was assessed
at 72 h following transfection by western blot analysis.
Cell lysates and western blot
analysis
The cells were seeded at a density of
2×105 cells per well in 6-well plates. Following
overnight incubation, the medium was replaced with maintenance
medium containing 20 ng/ml of cytokines, such as IL-1 and IL-6,
TNF-α and recombinant human IFN-γ (R&D Systems). The 'wild'
group represented untreated cells. The cells in the MIX group were
treated with 20 ng/ml of IL-1β, IL-6, TNF-α and IFN-γ.
Subsequently, the cells were washed 3 times with ice-cold PBS and
lysed with cell lysis buffer (50 mmol/l Tris-HCl at pH 7.4, 5
mmol/l EDTA, 150 mmol/l NaCl, 0.5% Nonidet P-40, 0.5 mmol/l PMSF,
0.5 mmol/l DTT and protease inhibitor cocktail) for 30 min at 4°C.
The supernatant was collected by centrifugation at 13,000 × g for
10 min. Lysates were used for western blot analysis as previously
described (11). The protein
concentration was measured with BCA protein assay reagent (Beyotime
Institute of Biotechnology). Approximately 20 μg of protein was
separated by 8% SDS-PAGE and blotted onto PVDF membranes (Bio-Rad
Laboratories, Inc.). After blocking in 5% non-fat milk for 1 h at
room temperature, the membranes were incubated with primary
antibodies on a shaker at 4̊C overnight, followed by incubation
with horseradish peroxidase (HRP) conjugated anti-mouse or
anti-rabbit immunoglobulin G (IgG; cat. no. 7076 or 7074; 1:2,000;
Cell Signaling Technology, Inc.) for 1.5 h at 37°C. The protein
bands were visualized using an enhanced chemiluminescence (ECL)
substrate kit (Millipore, Inc.) with the ECL western blotting
system (Bio-Rad Laboratories, Inc.). The primary antibodies used
for western blot were as follows: PD-L1 (cat. no. 13684; 1:1,000)
and PKD3 (cat. no. 5655; 1:1,000) from Cell Signaling Technology;
and STAT1 (cat. no. ab109320; 1:10,000), STAT3 (cat. no. ab68153;
1:2,000), phospho-STAT1(S727) (cat. no. ab109461; 1:5,000),
phospho-STAT1(Y701) (cat. no. ab29045; 1:1,000),
phospho-STAT3(S727) (cat. no. ab32143; 1:5,000),
phospho-STAT3(Y705) (cat. no. ab76315; 1:10,000) and anti-GAPDH
(cat. no. ab128915; 1:20,000) from Abcam. Phostag SDS-PAGE (Wako)
was performed according to a previously described protocol
(23). Semi-quantitative analysis
was performed by densitometry using Gel-Pro 32 software (version
3.1, Media Cybernetics, Bethesda).
Immunofluorescence and flow
cytometry
The cells were seeded on coverslips and allowed to
attach overnight. They were then washed twice with PBS for 5 min at
room temperature, fixed with 4% paraformaldehyde for 15 min at room
temperature, and then blocked in blocking buffer (1X PBS, 5% normal
goat serum, 0.3% Triton X-100™) for 60 min. Primary antibodies were
prepared by their dilution according to the datasheet guidelines in
antibody dilution buffer (1X PBS, 1% BSA, 0.3% Triton X-100™). The
primary antibodies included a polyclonal anti-PKD3 antibody (cat.
no. ab252982; 1:40; Abcam) for indirect immunofluorescence and a
PE-conjugated antibody for human PD-L1 (MIH1)(cat. no. 12-5983-42;
1:20; eBioscience) for direct immunofluorescence. Following the
removal of the blocking solution, the cells were incubated
overnight at 4̊C and then washed 3 times in PBS for 5 min each. The
cells were then incubated with fluorochrome-conjugated secondary
antibody (FITC-conjugated goat anti-rabbit IgG, cat. no. F-2765;
1:100; Invitrogen; Thermo Fisher Scientific) diluted in antibody
dilution buffer for 1 h at room temperature in the dark. The slides
were then rinsed in PBS and coverslipped with Prolong®
Gold Anti-Fade Reagent with DAPI. Imaging was performed using a
fluorescence microscope. Flow cytometry was performed according to
a previously described (5) using a
PE-conjugated antibody for human PD-L1 (MIH1) (cat. no. 12-5983-42;
1:20; eBioscience).
Reverse transcription-quantitative PCR
(RT-qPCR)
For RT-qPCR, total RNA was collected from the cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific), and
reverse transcription reactions were performed using PrimeScript RT
Master Mix (Takara Biotechnology Co., Ltd.). The resulting cDNA was
then subjected to qPCR analysis using SYBR Premix Ex Taq II (Takara
Biotechnology Co., Ltd.) and the ABI 7500 real-time PCR machine
(Applied Biosystems). The qPCR conditions were as follows: Initial
denaturation at 95̊C for 30 sec, followed by 40 cycles of 95̊C for
5 sec and 60̊C for 30 sec. The relative expression values of the
targeted genes were calculated using the comparative Cq
(2−ΔΔCq) method. The primers (forward and reverse,
respectively) used for RT-qPCR included PD-L1 (forward, 5′-CAA TGT
GAC CAG CAC ACT GAG AA-3′ and reverse, 5′-GGC ATA ATA AGA TGG CTC
CCA GAA-3′) and GAPDH (forward, 5′-ACA ACT TTG GTA TCG TGG AAG G-3′
and reverse, 5′-GCC ATC ACG CCA CAG TT TC-3′). The relative mRNA
expression of PD-L1 was normal-ized to that of GAPDH.
Immunohistochemical staining and
analysis
The OSCC tissues were fixed with 10% neutral
formalin and embedded in paraffin. A series of 5-μm-thick slices
was cut, dewaxed with xylene, and rehydrated with a series of
graded ethanol solutions. The tissue slices were boiled for 20 min
in citrate solution (10 mmol/l, pH 6.0). After cooling, the slices
were immersed in 0.3% hydrogen peroxide solution for 15 min to
block endogenous peroxidase activity. The slices were rinsed in PBS
for 5 min and blocked with 5% BSA solution at room temperature for
20 min. Subsequently, the sections were incubated overnight with
rabbit anti-human PD-L1 polyclonal antibody (cat. no. 13684; 1:200;
Cell Signaling Technology) or rabbit anti-human PKD3 polyclonal
antibody (cat. no. ab252982; 1:40; Abcam) at 4°C. The following
day, according to the manufacturer's instructions of ChemMate™
EnVision™ Detection kit (Genetech), the sections were incubated
with HRP-labeled goat anti-mouse or -rabbit secondary antibodies.
Diaminobenzidine (DAB) was used for color development, and
hematoxylin was used to stain the nuclei for 2 min at room
temperature. Finally, the slices were dehydrated, cleaned and
mounted.
ImageJ 1.48v software was used for analysis. The
staining intensity was evaluated according to the following
ratings: 0, no staining; 1, weak staining; 2, medium staining; 3,
strong staining. The H-score was calculated as follows: H-score = 0
× (% negative tumor cells) + 1 × (% weak staining) + 2 × (% medium
staining) + 3 × (% strong staining). The H-scores ranged from 0
(100% negative tumor cells) to 300 (100% strong staining of tumor
cells).
The Cancer Genome Atlas (TCGA) data
retrieval and analysis
UCSC Xena (http://xena.ucsc.edu/welcome-to-ucsc-xena/) was used
to download RNAseq data for queried genes. The gene expression data
of 564 head and neck squamous cell carcinoma (HNSCC) samples were
obtained. Linear regression curve fitting analysis was performed
using GraphPad Prism software. TCGA data were correlated using the
Spearman's r test.
Statistical analysis
All data were statistically analyzed using GraphPad
Prism software (version 6; GraphPad Software, Inc.) using one-way
analysis of variance followed by the post hoc Tukey's multiple
comparisons test. The correlation between PKD3 and PD-L1 expression
was analyzed using Spearman's correlation analysis. All statistical
results with a P-value <0.05 were considered to be
significant.
Results
Expression and localization of PD-L1 and
PKD3 in non-tumor and OSCC cell lines
The expression levels of PKD3 and PD-L1 in non-tumor
and OSCC cell lines were first measured by western blot analysis
and immunofluorescence. As shown in Fig. 1A and C, the expression levels of
PKD3 and PD-L1 were higher in the cancer cells than in the
non-cancer cells. In addition, the expression pattern of the
immunosuppressive protein, PD-L1, correlated well with the
expression pattern of PKD3 (Fig.
1B). Subsequently, the localization of PKD3 and PD-L1
expression was analyzed by double fluorescence staining. From
non-tumor cells to tumor cells, PKD3 exhibited a progressively
increasing nuclear distribution that ranged from only in the
cytoplasm, to evenly in the cytoplasm and nucleus, to mainly in the
nucleus. However, PD-L1 exhibited an intracellular distribution
that contrasted with that of PKD3. PD-L1 exhibited progressively
enhanced plasma membrane distribution ranging from evenly in the
cytoplasm and nucleus to mainly in the plasma membrane. In addition
to the increased accumulation of PKD3 in the nucleus, the staining
of PD-L1 at the cell membrane gradually increased. Taken together,
the data indicated that PKD3 and PD-L1 are upregulated and highly
positively correlated in OSCC, suggesting that PKD3 plays an
important role in the regulation of the expression of the
immunosuppressive protein, PD-L1.
Expression and localization of PD-L1 and
PKD3 in OSCC tissues
The expression and localization of PD-L1 and PKD3 in
were then analyzed 26 normal tissue and 34 tumor specimens. More
frequent and intense PKD3 and PD-L1 staining was observed in OSCC
tissues (Fig. 1D). In addition, it
was found that the nuclear localization of PKD3 was significantly
associated with tumor grade. PKD3 nuclear staining was not observed
in the normal tissues, whereas the grade I tumors exhibited 3.23%
PKD3-positive nuclear staining, grade II tumors exhibited 23.14%
positive staining, and grade III tumors exhibited 72.65% positive
staining (P<0.01; Fig. 1F). The
immunohistochemical score (H-score) was then calculated. To
determine whether PKD3 affects the level of the immunosuppressive
protein, PD-L1, linear trend and Spearman's rank correlation
coefficient tests were performed on the H-score. It was found that
a significant positive correlation existed between PKD3 and PD-L1
(P<0.0001, r=0.87; Fig. 1E).
The expression of PKD3 and PD-L1 was also analyzed in a large
number of HNSCC samples using the TCGA gene expression database. It
was found that the expression levels of PKD3 and PD-L1 were
significantly higher in the OSCC than in the normal tissues. In
addition, the expression level of PKD3 gradually increased with an
increase in the tumor grade (Fig.
S1). Indeed, it was found that PKD3 significantly and
positively correlated with PD-L1 (P<0.0001, r=0.21; Fig. 1G). These results indicate that PKD3
may be involved in the regulation of PD-L1 expression and that the
nuclear accumulation of PKD3 may play a role in the pathogenesis of
OSCC.
PD-L1 expression is induced by
inflammatory factors
To examine the effects of inflammatory factors on
the expression of PD-L1, the DOK and Cal-27 cell lines were treated
with 20 ng/ml of IL-1β, IL-6, TNF-α and IFN-γ for 24 h. The
expression of PD-L1 was determined by western blot analysis and
flow cytometry. It was found that PD-L1 protein expression was
upregulated upon the stimulation of the cells with IL-1β, IL-6,
TNF-α and IFN-γ, with IFN-γ exhibiting the most potent effect
(Fig. 2A and B).
PKD3 is required for IFN-γ-mediated PD-L1
expression
To determine whether PKD3 is involved in the
IFN-γ-induced upregulation of PD-L1 expression in OSCC, a
loss-of-function method was used to investigate the biological
relevance of PKD3 in the IFN-γ-induced expression of PD-L1. First,
shRNA was used to silence the expression of PKD3. The silencing
efficiency was confirmed at the protein level by western blot
analysis (Fig. 2C). Additional
results revealed that PKD3 knockdown decreased the expression of
PD-L1. Even with exposure to IFN-γ, PD-L1 expression was only
slightly elevated (Fig. 2D).
Moreover, the overexpression of PKD3 in the DOK cells significantly
increased the expression of PD-L1 induced by IFN-γ (P<0.001,
Fig. 3C and D). However, the
expression level of PD-L1 was only slightly elevated without IFN-γ
treatment (Fig. 3A and B). These
data thus suggest that PKD3 is responsible for IFN-γ-mediated PD-L1
expression.
IFN-γ activates PKD3, STAT1 and STAT3 in
OSCC cell lines
The findings of this study have thus far
demonstrated that PKD3 is involved in the expression of PD-L1
induced by IFN-γ. To investigate the IFN-γ-mediated signal
transduction events, the activation status of PKD3 was first
examined by using phostag SDS-PAGE immunoblot analysis of cells
exposed to IFN-γ (Fig. 4A). IFN-γ
induced a marked increase in PKD3 phosphorylation as early as 1 h.
The TCGA gene expression database was then used to analyze the
major signaling pathway members involved in the regulation of PD-L1
expression in a large number of HNSCC samples, such as MAPK, NF-κB,
PI3K and STAT1/3. It was found that only the expression of STAT1/3
was significantly associated with that of PD-L1 and PKD3 (Fig. S2). Moreover, numerous studies have
indicated that STAT1 and STAT3 play a key role in regulating the
expression of PD-L1 in HNSCC (6,9,24-26).
As expected, IFN-γ stimulation activated STAT1 and STAT3, with
their phosphorylation apparent after 1 h in all 3 cell lines
(Fig. 4A and B). In addition, the
PD-L1 mRNA levels were measured by RT-qPCR. As shown in Fig. 4C, the PD-L1 mRNA level peaked at 2
h. Moreover, the level of PD-L1 protein was gradually increased
after 6 h in the presence of IFN-γ (Fig. 4A). Thus, these results indicate
that IFN-γ induces the activation of PKD3, STAT1 and STAT3 in OSCC,
and that the activation of PKD3, STAT1 and STAT3 chronologically
follows the increase in the mRNA and protein levels of PD-L1.
PKD3 is a key kinase for the STAT1 and
STAT3 regulation of PD-L1 expression
The results presented above provide evidence of an
association between the expression of PKD3 and the signaling of
IFN-γ-induced PD-L1 expression in OSCC. To further elucidate the
role of PKD3 in the IFN-γ-mediated induction of PD-L1 expression,
it was then determined whether the IFN-γ-induced activation of
STAT1 and STAT3 in OSCC cell lines following exposure to IFN-γ was
dependent on PKD3. For this purpose, the phosphorylation levels of
STAT1 and STAT3 were examined after 1 h of treatment with or
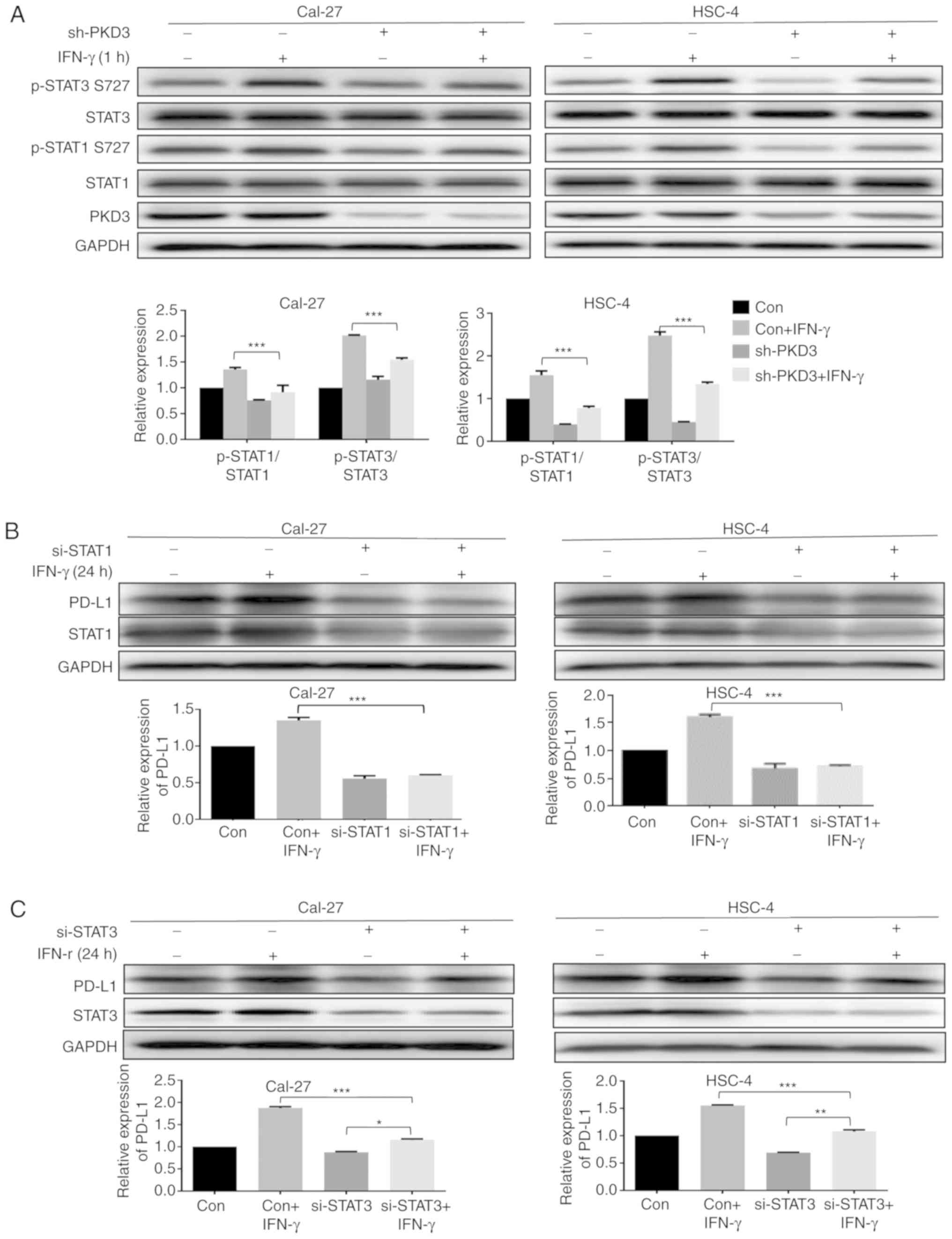
without IFN-γ. It was found that the knockdown of PKD3
significantly decreased the phosphorylation levels of STAT1 and
STAT3 at Ser727 in OSCC cell lines (P<0.001, Fig. 5A). However, their phosphorylation
levels at tyrosine residues were not markedly altered (Fig. S3). Subsequently, the relative
contribution of STAT1 and STAT3 to IFN-γ-mediated PD-L1 expression
was examined. The corresponding siRNAs effectively knocked down the
expression of STAT1 and STAT3 in OSCC cell lines. The knockdown of
STAT1 significantly abrogated the IFN-γ-induced PD-L1 upregulation
at the protein level (P<0.001, Fig.
5B). However, IFN-γ was still able to induce an increase in
PD-L1 expression after STAT3 silencing, although the elevated level
was decreased (Fig. 5C). Taken
together, these results indicate that activation of STAT1 and STAT3
is required for IFN-γ-mediated PD-L1 expression and that PKD3 is a
key kinase regulating the activation of STAT1 and STAT3, as shown
in Fig. 6.
Discussion
The PKD family, as a class of evolutionarily
conserved serine/threonine kinases, has been implicated in diverse
biological processes, such as cell proliferation, cell migration,
differentiation, apoptosis, tumorigenesis,
epithelial-to-mesenchymal transition and immune regulation
(10-15,17,18).
In this study, the role of a lesser-known member of the PKD family,
PKD3, in the IFN-γ-induced expression of the immunosuppressive
protein, PD-L1, in OSCC was investigated.
Based on the data from western blot analysis and
immunofluorescence, PKD3 expression was found to be significantly
upregulated in malignant cell lines relative to its expression in
non-tumor cells. The increase in the nuclear distribution of PKD3
in malignant tumor cells was evident. However, the specific
mechanisms underlying the nuclear accumulation of PKD3 have not
been fully elucidated. Moreover, a statistically significant
correlation was also found between PKD3 and PD-L1. The
immunohistochemical findings were also consistent with the cell
experiments. The results of immunohistochemistry and TCGA analysis
indicated that the expression level of PKD3 and the frequency and
intensity of nuclear staining gradually increased with the increase
in the tumor grade. These findings suggest a potential role of PKD3
in the progression of OSCC. These results are consistent with those
of previous studies in several types of cancer, which supports the
conclusion obtained herein that abnormal PKD3 expression and
localization promote cancer progression (18,27,28).
The expression of PD-L1 in tumor cells is mainly
regulated by two mechanisms. The external mechanism, such as IFN-γ
secreted by natural killer cells and tumor-infiltrating lymphocytes
in the tumor microenvironment (7,29-31),
can strongly induce the expression of PD-L1 in tumor cells. The
intrinsic mechanism may be present in the constitutive carcinogenic
signaling pathway of tumor cells. It is now becoming clear that the
tumor microenvironment is critical for cancer progression. In
addition, the pathogenesis of cancer is largely dependent on its
interaction with microenvironmental components. There is increasing
evidence to indicate that the induction of PD-L1 expression by
inflammatory factors (such as IL-1, IL-6, TNF-α and IFN-γ) in the
tumor microenvironment may be one of the most important factors
affecting the efficacy of tumor immunotherapy (32). Of these, IFN-γ has the most potent
inducing effect on PD-L1 expression. IFN-γ is a multifunctional
cytokine produced by natural killer cells, T cells and macrophages.
The infiltration of these cells can upregulate the expression of
PD-L1 in tumor cells and can protect tumors from immune attack
(31,33-35).
Previous studies have indicated that IFN-γ can significantly induce
tumor progression in some cases (36-38).
In some clinical trials, IFN-γ treatment has been found to exert a
negative effect on the prognosis of certain patients (39). Taken together, IFN-γ is deemed to
have a double-edged sword effect in anti-tumor immunity,
upregulating antigen processing machinery (APM) components and
human leukocyte antigen (HLA) class I expression in tumor cells.
However, it is also one of the most effective inducers of PD-L1.
Therefore, it is crucial to elucidate the molecular mechanism
underlying the induction of PD-L1 expression by IFN-γ. In HNSCC,
the expression of PKD3 is significantly increased and gradually
increases with an increase in the tumor grade. PKD3 may be a
constitutive oncogenic signal in tumor cells, and internal and
external mechanisms may co-regulate the expression of PD-L1.
One of the main findings of this study is the
observation that PKD3 is involved in the IFN-γ-mediated
upregulation of PD-L1 expression in OSCC. It was found that PKD3
knockdown in tumor cells reduced the level of PD-L1 induced by
IFN-γ and that the overexpression of PKD3 in non-tumor cells
significantly increased the level of PD-L1 induced by IFN-γ.
Previous studies had demonstrated that PD-L1 expression in HNSCC is
mainly affected by the activation of the STAT1/STAT3 pathway
(9,24,25).
To determine whether STAT1/STAT3 is involved in the regulation of
PD-L1 expression by PKD3, in this study, the TCGA gene expression
database was used to analyze the main signaling pathway members
involved in the regulation of PD-L1 expression, such as NF-κB,
MAPK, PI3K and STAT1/3 (6-9,24-26,29,40).
It was found that only the expression of STAT1/3 was significantly
associated with that of PD-L1 and PKD3. This provides direction for
future studies. Indeed, it was found that IFN-γ induced a
significant increase in the STAT1 and STAT3 phosphorylation levels.
However, a significant decrease was only observed in the
phosphorylation level of STAT1 and STAT3 at the Ser727 site after
PKD3 silencing, and there was no significant change in the
phosphorylation level at tyrosine. The activation of STATs involves
dimerization, nuclear trans-location, DNA binding and
transcriptional activation, which requires the phosphorylation of
the Ser727 site and tyrosine. The phosphorylation of tyrosine
regulates the dimerization and nuclear translocation of STATs,
which is essential for the activation of the JAK-STAT signaling
pathway. However, the phosphorylation of STAT1/3 at Ser727 is
essential for the optimal DNA binding and transcriptional activity
of STAT1/3 (41,42). Previous studies have demonstrated
that the inhibition of AKT activation can lead to the
downregulation of the IFN-γ-mediated phosphorylation of STAT1 at
Ser727, which not only downregulates the expression of the STAT1
target genes, CXCL9 and CXCL10, but also downregulates the
expression of PD-L1 (7). In
addition, ERK can directly interact with STAT1/STAT3 to
phosphorylate Ser727, increasing the viability and growth rate of
cells (41-50). AKT and ERK, as downstream targets
of PKD3, regulate the growth and survival of cancer cells (18). Taken together, these findings
suggest that the activation of STAT1/STAT3 at the Ser727 site
contributes to tumor development. These results indicate that PKD3
regulates IFN-γ-induced PD-L1 expression by regulating the
phosphorylation of STAT1/STAT3 (Fig.
6).
In conclusion, PKD3, as a serine/threonine kinase,
is involved in the regulation of STAT1/STAT3 activation. The
findings of this study shed light on the function of PKD3 in the
development of OSCC. It was demonstrated that in human OSCC, PKD3
participates in the regulation of PD-L1 expression by modulating
the activation of STAT1/STAT3, thereby providing a theoretical
basis for the combination of PKD inhibition and immunotherapy of
human OSCC.
Supplementary Data
Funding
This study was supported by National Natural Science
Foundations of China (grant nos. 81802717 and 81372892).
Availability of data and materials
All data are included in this article and its
supplementary information files.
Authors' contributions
PZ, YF and XZ designed the outline of the study. BC
and JC designed the study, conducted experiments and wrote the
manuscript. JC and ML supervised the study, contributed to data
acquisition and revised the manuscript. LW, HC, YK and JW collected
and analyzed the data. All authors have read and approved the final
version of this manuscript.
Ethics approval and consent to
participate
This study was approved by the West China Hospital
of Stomatology Institutional Review Board (approval no.
WCHSIRB-D-2013-039). Written informed consent was obtained from
each donor and patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of
interest.
Abbreviations:
|
PKD3
|
protein kinase D3
|
|
OSCC
|
oral squamous cell carcinoma
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
PD-L1
|
programmed death ligand-1
|
|
PD-1
|
programmed cell death protein 1
|
|
IFN-γ
|
interferon-γ
|
|
STAT
|
signal transducer and activator of
transcription
|
|
TCGA
|
The Cancer Genome Atlas
|
|
IL
|
interleukin
|
|
TNF
|
tumor necrosis factor
|
|
NF-κB
|
nuclear factor-κB
|
|
MAPK
|
mitogen-activated protein kinase
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
AKT
|
protein kinase B
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
APM
|
antigen processing machinery
|
|
HLA
|
human leukocyte antigen
|
Acknowledgments
The authors would like to thank the patients,
researchers, and staff involved in this study.
References
|
1
|
Sathiyasekar AC, Chandrasekar P, Pakash A,
Kumar KUG and Jaishlal MS: Overview of immunology of oral squamous
cell carcinoma. J Pharm Bioallied Sci. 8(Suppl 1): S8–S12.
2016.PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin YM, Sung WW, Hsieh MJ, Tsai SC, Lai
HW, Yang SM, Shen KH, Chen MK, Lee H, Yeh KT and Chen CJ: High
PD-L1 expression correlates with metastasis and poor prognosis in
oral squamous cell carcinoma. PLoS One. 10:e01426562015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferris RL: Immunology and immunotherapy of
head and neck cancer. J Clin Oncol. 33:3293–3304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu W, Lu L, Feng Y, Chen J, Li Y, Kong X,
Chen S, Li X, Chen Q and Zhang P: Inflammation promotes oral
squamous carcinoma immune evasion via induced programmed death
ligand-1 surface expression. Oncol Lett. 5:1519–1526. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ritprajak P and Azuma M: Intrinsic and
extrinsic control of expression of the immunoregulatory molecule
PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncol.
51:221–228. 2015. View Article : Google Scholar
|
|
7
|
Gao Y, Yang J, Cai Y, Fu S, Zhang N, Fu X
and Li L: IFN-γ-mediated inhibition of lung cancer correlates with
PD-L1 expression and is regulated by PI3K-AKT signaling. Int J
Cancer. 143:931–943. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moon JW, Kong SK, Kim BS, Kim HJ, Lim H,
Noh K, Kim Y, Choi JW, Lee JH and Kim YS: IFNγ induces PD-L1
overexpression by JAK2/STAT1/IRF-1 signaling in EBV-positive
gastric carcinoma. Sci Rep. 7:178102017. View Article : Google Scholar
|
|
9
|
Bu L, Yu G, Wu L, Mao L, Deng WW, Liu JF,
Kulkarni AB, Zhang WF, Zhang L and Sun ZJ: STAT3 induces
immunosuppression by upregulating PD-1/PD-L1 in HNSCC. J Dent Res.
96:1027–1034. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roy A, Ye J, Deng F and Wang QJ: Protein
kinase D signaling in cancer: A friend or foe? Biochim Biophys Acta
Rev Cancer. 1868:283–294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen J, Feng Y, Lu L, Wang H, Dai L, Li Y
and Zhang P: Interferon-γ-induced PD-L1 surface expression on human
oral squamous carcinoma via PKD2 signal pathway. Immunobiology.
217:385–393. 2012. View Article : Google Scholar
|
|
12
|
LaValle CR, George KM, Sharlow ER, Lazo
JS, Wipf P and Wang QJ: Protein kinase D as a potential new target
for cancer therapy. Biochim Biophys Acta. 1806:183–192.
2010.PubMed/NCBI
|
|
13
|
Evans IM and Zachary IC: Protein kinase D
in vascular biology and angiogenesis. IUBMB Life. 63:258–263. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang T, Braun U and Leitges M: PKD3
deficiency causes alterations in microtubule dynamics during the
cell cycle. Cell Cycle. 15:1844–1854. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Durand N, Borges S and Storz P: Protein
kinase D enzymes as regulators of EMT and cancer cell invasion. J
Clin Med. 5:E202016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baker J, Falconer AM, Wilkinson DJ,
Europe-Finner GN, Litherland GJ and Rowan AD: Protein kinase D3
modulates MMP1 and MMP13 expression in human chondrocytes. PLoS
One. 13:pp. e01958642018, View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rozengurt E, Rey O and Waldron RT: Protein
kinase D signaling. J Biol Chem. 280:pp. 13205–13208. 2005,
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Deng F, Singh SV and Wang QJ:
Protein kinase D3 (PKD3) contributes to prostate cancer cell growth
and survival through a PKCε/PKD3 pathway downstream of Akt and ERK
1/2. Cancer Res. 68:3844–3853. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zou Z, Zeng F, Xu W, et al: PKD2 and PKD3
Promote Prostate Cancer Cell Invasion by Modulating NF-κB- And
HDAC1-mediated Expression and Activation of uPA. J Cell Sci.
125:4800–4811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Wang CM, Zhang P, Wang X, Chen J,
Yang J, Lu W, Zhou W, Yuan W and Feng Y: Expression of programmed
death 1 ligand 1 on periodontal tissue cells as a possible
protective feedback mechanism against periodontal tissue
destruction. Mol Med Rep. 13:2423–2430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin W, Choe WH, Hiasa Y, Kamegaya Y,
Blackard JT, Schmidt EV and Chung RT: Hepatitis C virus expression
suppresses interferon signaling by degrading STAT1.
Gastroenterology. 128:1034–1041. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Konnikova L, Kotecki M, Kruger MM and
Cochran BH: Knockdown of STAT3 expression by RNAi induces apoptosis
in astrocytoma cells. BMC Cancer. 3:232003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiu W and Steinberg SF: Phos-tag SDS-PAGE
resolves agonist-and isoform-specific activation patterns for PKD2
and PKD3 in cardiomyocytes and cardiac fibroblasts. J Mol Cell
Cardiol. 99:14–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin C, Cao W, Ren Z, Tang Y, Zhang C, Yang
R, Chen Y, Liu Z, Peng C, Wang L, et al: GDNF secreted by nerves
enhances PD-L1 expression via JAK2-STAT1 signaling activation in
HNSCC. Oncoimmunology. 6:e13538602017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Concha-Benavente F, Srivastava RM, Trivedi
S, Lei Y, Chandran U, Seethala RR, Freeman GJ and Ferris RL:
Identification of the cell-intrinsic and-extrinsic pathways
downstream of EGFR and IFNγ that induce PD-L1 expression in head
and neck cancer. Cancer Res. 76:1031–1043. 2016. View Article : Google Scholar
|
|
26
|
Mann J, Hoesli R, Michmerhuizen N,
Devenport SN, Ludwig ML, Vandenberg TR, Matovina C, Jawad N,
Mierzwa M, Shuman AG, et al: Surveilling the potential for
precision medicine-driven PD-1/PD-L1-targeted therapy in HNSCC. J
Cancer. 8:332–344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang H, Xu M, Chi X, Yan Q, Wang Y, Xu W,
Zhuang K, Li A and Liu S: Higher PKD3 expression in hepatocellular
carcinoma (HCC) tissues predicts poorer prognosis for HCC patients.
Clin Res Hepatol Gastroenterol. 41:554–563. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huck B, Duss S, Hausser A and Olayioye MA:
Elevated protein kinase D3 (PKD3) expression supports proliferation
of triple-negative breast cancer cells and contributes to
mTORC1-S6K1 pathway activation. J Biol Chem. 289:3138–3147. 2014.
View Article : Google Scholar :
|
|
29
|
Mimura K, The JL, Okayama H, Shiraishi K,
Kua LF, Koh V, Smoot DT, Ashktorab H, Oike T, Suzuki Y, et al:
PD-L1 expression is mainly regulated by interferon gamma associated
with JAK-STAT pathway in gastric cancer. Cancer Sci. 109:43–53.
2018. View Article : Google Scholar
|
|
30
|
Vassilakopoulou M, Avgeris M, Velcheti V,
Kotoula V, Rampias T, Chatzopoulos K, Perisanidis C, Kontos CK,
Giotakis AI, Scorilas A, et al: Evaluation of PD-L1 expression and
associated tumor-infiltrating lymphocytes in laryngeal squamous
cell carcinoma. Clin Cancer Res. 22:704–713. 2016. View Article : Google Scholar
|
|
31
|
Taube JM, Anders RA, Young GD, Xu H,
Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL
and Chen L: Colocalization of inflammatory response with B7-h1
expression in human melanocytic lesions supports an adaptive
resistance mechanism of immune escape. Sci Transl Med. 4:pp.
127ra1372012, View Article : Google Scholar
|
|
32
|
Jiang X, Wang J, Deng X, Xiong F, Ge J,
Xiang B, Wu X, Ma J, Zhou M, Li X, et al: Role of the tumor
microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol
Cancer. 18:102019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
LaCasse CJ, Janikashvili N, Larmonier CB,
Alizadeh D, Hanke N, Kartchner J, Situ E, Centuori S, Har-Noy M,
Bonnotte B, et al: Th-1 lymphocytes induce dendritic cell tumor
killing activity by an IFN-γ-dependent mechanism. J Immunol.
187:6310–6317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maraskovsky E, Chen W and Shortman K: IL-2
and IFN-gamma are two necessary lymphokines in the development of
cytolytic T cells. J Immunol. 143:1210–1214. 1989.PubMed/NCBI
|
|
35
|
Chen L, Tourvieille B, Burns GF, Bach FH,
Mathieu-Mahul D, Sasportes M and Bensussan A: Interferon: A
cytotoxic T lymphocyte differentiation signal. Eur J Immunol.
16:767–770. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiao M, Wang C, Zhang J, Li Z, Zhao X and
Qin Z: IFNgamma promotes papilloma development by up-regulating
Th17-associated inflammation. Cancer Res. 69:2010–2017. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matsuda M, Nakamoto Y, Suzuki S, Kurata T
and Kaneko SJ: Interferon-gamma-mediated hepatocarcinogenesis in
mice treated with diethylnitrosamine. Lab Invest. 85:655–663. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hanada T, Kobayashi T, Chinen T, Saeki K,
Takaki H, Koga K, Minoda Y, Sanada T, Yoshioka T, Mimata H, et al:
IFNgamma-dependent, spontaneous development of colorectal
carcinomas in SOCS1-deficient mice. J Exp Med. 203:1391–1397. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alberts DS, Marth C, Alvarez RD, Johnson
G, Bidzinski M, Kardatzke DR, Bradford WZ, Loutit J, Kirn DH,
Clouser MC, et al: Randomized phase 3 trial of interferon gamma-1b
plus standard carboplatin/paclitaxel versus carboplatin/paclitaxel
alone for first-line treatment of advanced ovarian and primary
peritoneal carcinomas: Results from a prospectively designed
analysis of progression-free survival. Gynecol Oncol. 109:174–181.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang W, Zhang J, Zhang Z, Guo Y, Wu Y,
Wang R, Wang L, Mao S and Yao X: Overexpression of indoleamine
2,3-dioxygenase 1 promotes epithelial-mesenchymal transition by
activation of the IL-6/STAT3/PD-L1 pathway in bladder cancer.
Transl Oncol. 12:485–492. 2019. View Article : Google Scholar
|
|
41
|
Decker T and Kovarik P: Serine
phosphorylation of STATs. Oncogene. 19:2628–2637. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aziz MH, Manoharan HT, Church DR,
Dreckschmidt NE, Zhong W, Oberley TD, Wilding G and Verma AK:
Protein kinase Cepsilon interacts with signal transducers and
activators of transcription 3 (Stat3), phosphorylates Stat3Ser727,
and regulates its constitutive activation in prostate cancer.
Cancer Res. 67:8828–8838. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sakaguchi M, Oka M, Iwasaki T, Fukami Y
and Nishigori C: Role and regulation of STAT3 phosphorylation at
Ser727 in melanocytes and melanoma cells. J Invest Dermatol.
132:1877–1885. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gough DJ, Koetz L and Levy DE: The MEK-ERK
pathway is necessary for serine phosphorylation of mitochondrial
STAT3 and Ras-mediated transformation. PLoS One. 8:e833952013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Decker T and Kovarik P: Transcription
factor activity of STAT proteins: Structural requirements and
regulation by phosphorylation and interacting proteins. Cell Mol
Life Sci. 55:1535–1546. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fang Y, Zhong L, Lin M, Zhou X, Jing H,
Ying M, Luo P, Yang B and He Q: MEK/ERK dependent activation of
STAT1 mediates dasatinib-induced differentiation of acute myeloid
leukemia. PLoS One. 8:pp. e669152013, View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nitulescu II, Meyer SC, Wen QJ, Crispino
JD, Lemieux ME, Levine RL, Pelish HE and Shair MD: Mediator kinase
phosphorylation of STAT1 S727 promotes growth of neoplasms with
JAK-STAT activation. EBioMedicine. 26:112–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Goren I, Pfeilschifter J and Frank S:
Determination of leptin signaling pathways in human and murine
keratinocytes. Biochem Bioph Res Commun. 303:1080–1085. 2003.
View Article : Google Scholar
|
|
49
|
Kondo K, Shaim H, Thompson PA, Burger JA,
Keating M, Estrov Z, Harris D, Kim E, Ferrajoli A, Daher M, et al:
Ibrutinib modulates the immunosuppressive CLL microenvironment
through STAT3-mediated suppression of regulatory B-cell function
and inhibition of the PD-1/PD-L1 pathway. Leukemia. 32:960–970.
2018. View Article : Google Scholar
|
|
50
|
Zhou L, Schandené L, Mordvinov VA,
Chatelain P, Pradier O, Goldman M and Stordeur P: Trapidil inhibits
monocyte CD40 expression by preventing IFN-γ-induced STAT1 S727
phosphorylation. Int Immunopharmacol. 4:863–871. 2004. View Article : Google Scholar : PubMed/NCBI
|