Introduction
Dendritic cells (DCs) serve a critical role in the
immune system, and initiate and control the immune response. The
main function of DCs is to detect foreign antigens and present them
to T cells. After being activated by injury or inflammatory
stimuli, DCs migrate to draining lymph nodes and activate T cells
to differentiate, thus initiating the immune response (1,2).
Therefore, the migration of DCs to draining lymph nodes is
essential in mediating anti-tumor immunity. According to previous
reports, the number of CD83-positive DCs, which present foreign
antigens, is increased in cervical intraepithelial neoplasia
(3,4) and significantly decreased in draining
lymph nodes (5). These changes in
DCs may contribute to the immune evasion of tumor cells. Therefore,
it is important to elucidate the mechanisms underlying this
phenomenon.
As a cyclooxygenase (COX)-induced product of
arachidonic acid released from membrane phospholipids,
prostaglandin E2 (PGE2) modulates various pathological
and physiological processes. Numerous studies have demonstrated
that PGE2 is closely associated with the development of
various malignant lesions (6-8).
Furthermore, in cervical lesions, PGE2 expression is
higher compared with in normal tissues (9-11).
Additionally, certain studies have revealed that PGE2
modulates the migration of DCs (12,13).
Therefore, PGE2 may be associated with changes in DCs in
cervical lesions.
The development of cervical lesions has been linked
to infection with certain high-risk types of human papilloma-virus
(HPV) (14). The most prevalent
type is HPV16, which accounts for >50% of cervical malignancy
cases. The constitutive expression of E6 oncoprotein is one of the
major risk factors for the development of high-grade lesions, which
is required for the onset and maintenance of the malignant
phenotype (15). Notably, E6 has
been reported to induce decreased migration of Langerhans cells and
their precursor-like cells, one type of which is DCs (16,17).
Furthermore, the transcription of COX-2 has been reported to be
regulated by E6 (18); the COX
pathway is associated with the production of bioactive prostanoids,
including PGE2. Therefore, the production of
PGE2, which affects the migration of DCs in cervical
lesions, may be regulated by E6.
The present study used murine bone marrow DCs to
determine the effects of PGE2 on the migration of DCs,
which may be associated with changes to DCs in cervical lesions.
Furthermore, the association between E6 oncoprotein and the
upregulated expression of PGE2 in HPV16-positive
cervical lesions was investigated. The results obtained in the
present study may improve the understanding of immune evasion in
cervical lesions. Notably, the biological implications of these
findings may provide a novel perspective in the immunological
surveillance of various malignant lesions.
Materials and methods
Samples
Cervical biopsy specimens (age range, 20-80 years)
were collected between January 2012 and December 2017 from the
Department of Obstetrics and Gynecology, Daping Hospital, Army
Medical University (Third Military Medical University; Chongqing,
China). Only HPV16-positive samples were selected from samples
obtained for clinical HPV typing for subsequent experiments.
According to the cytological and histological evaluation of fresh
specimens, cervical disease status was categorized as normal
squamous epithelium (n=27), low-grade squamous intraepithelial
lesion (LSIL; n=25), high-grade squamous intraepithelial lesion
(HSIL; n=21) and squamous carcinoma (SCC; n=15). The present study
was approved by the Ethics Committee of Daping Hospital, Army
Medical University (Third Military Medical University). The
participants provided written informed consent, and this study was
conducted in accordance with the Declaration of Helsinki.
Cell culture
All animal studies were approved by the Laboratory
Animal Welfare and Ethics Committee of the Third Military Medical
University. DCs were isolated from mouse bone marrow as previously
described with slight modifications (19,20).
Balb/C mice (age, 6-8 weeks; weight, 20-25 g; n=80) were provided
by the Experimental Animal Center of Daping Hospital. Mice were
maintained in a specific pathogen-free environment at 22±2°C with
55±5% humidity under a 12-h light/dark cycle. Food and water were
provided ad libitum. Briefly, the mice were sacrificed by
cervical dislocation, the femurs and tibias were collected, bone
marrow cells were flushed out with a 1-ml syringe and the red cells
were removed using erythrocyte lysis fluid (Beyotime Institute of
Biotechnology). The remaining cells were cultured with RPMI-1640
medium supplemented with 10% FBS (both from Gibco; Thermo Fisher
Scientific, Inc.), 20 ng/ml recombinant granulocyte-macrophage
colony-stimulating factor (rGM-CSF; Peprotech, Inc.) and IL-4
(Peprotech, Inc.). On day 3, non-adherent granulocytes, as well as
B and T lymphocytes, were gently removed and fresh media were
added. The immature DCs were collected on day 6. On day 7, the DCs
were stimulated with 1 μg/ml lipopolysaccharide (LPS;
Sigma-Aldrich; Merck KGaA) for 24 h at 37°C in an atmosphere
containing 5% CO2. Mature DCs were collected on day 8.
Morphological and phenotypic tests were performed using microscopy
and flow cytometry to confirm the successful isolation of DCs.
Based on Baratelli et al (21), PGE2 (Sigma-Aldrich;
Merck KGaA) was added to the culture media of DCs at a final
concentration of 0, 5 or 10 µg/ml for 24 h.
Immunohistochemistry
Cervical biopsy specimens were sequentially fixed in
4% paraformaldehyde for ≥24 h at room temperature, dehydrated and
embedded in paraffin, then cut into 7-µm sections. After
dewaxing and rehydration, heat-mediated antigen retrieval was
performed with 1 mM EDTA (pH 9.0) in a pressure boiler for 10 min.
Microsomal PGE synthase (mPGES)-1 (1:100; cat. no. 160140; Cayman
Chemical Company), mPGES-2 (1:100; cat. no. 160145; Cayman Chemical
Company) and cytosolic PGE synthase (cPGES) (1:100; cat. no. 18219;
Cayman Chemical Company), CD83 (1:50; cat. no. bs-4826R; BIOSS) and
CD1a (prediluted; 1 drop; cat. no. ZA-0544; OriGene Technologies,
Inc.) were incubated with the sections overnight at 4°C. After
washing with PBS (pH 7.4), sections were incubated with appropriate
secondary antibodies (cat. no. PV-9000; OriGene Technologies, Inc.)
for 20 min at room temperature, according to the manufacturer's
protocol. Color reaction was developed by incubation with the DAB
detection system (cat. no. ZLI-9019; OriGene Technologies, Inc.)
for 10 sec at room temperature, and the sections were
counterstained with hematoxylin for min at room temperature. After
sequential dehydration using graded ethanol and xylene, the
sections were mounted and covered with a coverslip. The sections
were observed under a light microscope (CTR6000; Leica
Microsystems, Inc.). The images were analyzed using Image-Pro Plus
6.0 software (Media Cybernetics, Inc.).
Western blot analysis
Western blotting was performed to determine protein
expression levels. The lysates of DCs and tissue samples preserved
in liquid nitrogen were prepared using lysis buffer (cat. no.
P0013; Beyotime Institute of Biotechnology). The concentration in
all samples was measured using the BCA Protein Assay kit (cat. no.
23227; Thermo Fisher Scientific, Inc.), and the protein samples
(30-50 µg) were separated by SDS-PAGE on 10% gels. Proteins
were then transferred to nitrocellulose membranes and blocked in
PBS (containing 5% BSA and 0.1% Tween-20; both from BBI Life
Sciences Corporation). Subsequently, blots were incubated with
antibodies against mPGES-1 (1:200; cat. no. 160140; Cayman Chemical
Company), mPGES-2 (1:200; cat. no. 160145; Cayman Chemical Company)
and cPGES (1:1,000; cat. no. 18219; Cayman Chemical Company), E6
(1:200; cat. no. sc-1584; Santa Cruz Biotechnology, Inc.), tubulin
(1:1,000; cat. no. 11224-1-AP; ProteinTech Group, Inc.), or GAPDH
(1:2,000; cat. no. 60004-1-Ig; ProteinTech Group, Inc.) overnight
at 4°C. Following incubation with horseradish peroxidase-conjugated
goat anti-mouse (1:1,000; cat. no. SA00001-1; ProteinTech Group,
Inc.), goat anti-rabbit (1:1,000; cat. no. A0208; Beyotime
Institute of Biotechnology) or donkey anti-goat (1:1,000; cat. no.
A0181; Beyotime Institute of Biotechnology) secondary antibody at
room temperature for 1 h, immunoreactivities were detected using
enhanced chemiluminescence substrate (cat. no. 34577; Thermo Fisher
Scientific, Inc.). Densitometric analysis was performed using
ImageJ software (Image J bundled with 64-bit Java 1.6.0_20;
National Institutes of Health) with protein expression levels
normalized to tubulin or GAPDH.
Reverse transcription-PCR analysis
Total RNA was extracted from tissue samples
preserved in liquid nitrogen and DCs using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. cDNA was synthesized using
HiScript II Q Select RT SuperMix for qPCR (+gDNA wiper) (cat. no.
R233-01; Vazyme), following the manufacturer's protocol. PCR was
conducted using 2X Taq Plus Master Mix II (Dye Plus) (cat. no.
P213-01, Vazyme). Semi-quantitative PCR was conducted as follows:
Initial denaturation at 95°C for 3 min, followed by 26 cycles at
95°C for 15 sec, 59°C for 30 sec and 72°C for 30 sec. The primers
used for PCR are listed in Table
I. Electrophoresis was performed using 1% agarose gel and 4S
GelRed (1:10,000; BBI Life Sciences Corporation), and the images
were captured using ChemiDOC XRS system (Bio-Rad Laboratories,
Inc.). Densitometric analysis was performed using ImageJ software
(Image J bundled with 64-bit Java 1.6.0_20; National Institutes of
Health) with mRNA expression levels normalized to GAPDH.
 | Table INucleotide sequences of the primers
used for reverse transcription-PCR. |
Table I
Nucleotide sequences of the primers
used for reverse transcription-PCR.
| Gene | Sense primer | Antisense
primer |
|---|
| HPV16 E6 |
5′-AATGTTTCAGGACCCACAGG-3′ |
5′-ACTGTTGCTTGCAGTACACACAT-3′ |
| GAPDH |
5′-ATCAAGAAGGTGGTGAAGCAG-3′ |
5′-GCCAAATTCGTTGTCATACC-3′ |
Cell transfection
The double-stranded E6-specific small interfering
RNA (siRNA) sequences and control siRNA were prepared and annealed
by Neuron Biotech Co. [OBiO Technology (Shanghai) Corp., Ltd.]. Two
sequences were used for further experiments: siRNA-1,
5′-GCAATGTTTCAGGAC CCACAG-3′; siRNA-2, 5′-GCACAGAGCTGCAAACAA
CTA-3′. SiHa (5×105 cells/well; Cell Bank of the Chinese
Academy of Sciences) were seeded in 6-well plates and transfected
with 100 nmol/l siRNA using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 24 h.
Subsequently, the medium was replaced with DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS for a further 24
h, and cells were subsequently used for other experiments.
Transwell migration assay
The lower chambers of 24-well Transwell plates
(Merck KGaA) were filled with 600 µl serum-free medium
containing C-C motif chemokine ligand 19 (CCL19; 100 ng/ml;
Peprotech, Inc.). Following treatment with different concentrations
of PGE2 (0, 5 and 10 µg/ml), cultured
supernatants of SiHa cells (positive control) or cultured
super-natants of siRNA-transfected SiHa cells at 37°C for 24 h, DCs
(1×105 cells/well) resuspended in 100 μl serum-free
medium were deposited in the upper chambers of the Transwell plates
and allowed to migrate for 3 h at 37°C in an atmosphere containing
5% CO2. Since there were almost no residual cells on the
chamber membrane, the method of counting cells in the lower
compartment was used, instead of image acquisition. The number of
migrated DCs harvested from the lower chambers was counted using a
hemocytometer.
3D migration assay
DCs (45 µl; 0.45×106 cells/ml)
were embedded into a collagen matrix (final concentration of
collagen matrix, 1.7 mg/ml; BD Biosciences) in migration chambers
(Electron Microscopy Sciences). The remaining space of the chambers
was filled with medium containing 200 ng/ml CCL19. Migration of DCs
was recorded by bright-field time-lapse video microscopy at 37°C
using an inverted microscope (Carl Zeiss AG) fitted with ×10
objectives and Axiocam cameras; recording started 10 min after
injection. Cells were imaged at a frame rate of 2 min up to 61
frames. Computer-assisted cell tracking was performed with
custom-written software (ImageJ bundled with 64-bit Java 1.6.0_20;
National Institutes of Health). The average speed was calculated as
step length (μm) per minute for each cell. A total of 30 randomly
selected cells were included in one experiment.
Flow cytometry
Cultured DCs were harvested and their phenotypes
evaluated by fluorescence-activated cell sorting (FACS) analysis.
Cells were blocked for 15 min at 4°C with PBS containing 0.5% BSA,
and were then incubated with respective antibodies for 30 min at
4°C. After being washed twice with PBS, the cells were resuspended
in 200 µl PBS. The antibodies included
phycoerythrin-conjugated anti-mouse CD40 (1:100; cat. no. 12-0401;
eBioscience; Thermo Fisher Scientific, Inc.), CD80 (1:100; cat. no.
12-0801; eBioscience; Thermo Fisher Scientific, Inc.), CD86 (1:100;
cat. no. 12-0862; eBioscience; Thermo Fisher Scientific, Inc.),
major histocompatibility complex II (MHCII; 1:100; cat. no.
12-5321; eBioscience; Thermo Fisher Scientific, Inc.) and C-C
chemokine receptor type 7 (CCR7; 1:100; cat. no. 12-1971;
eBioscience; Thermo Fisher Scientific, Inc.). Same-species and
same-isotype IgG (1:100, cat. no. 12-4031; 1:100, cat. no. 12-4888;
1:100, cat. no. 12-4321; eBioscience; Thermo Fisher Scientific,
Inc.) was used as an isotype control, which was used as the
template to conduct gating. FACS analysis was performed on a
FACSCalibur flow cytometer using CellQuest Pro software (version
6.0; BD Bioscience).
PGE2 measurement
PGE2 concentration in cervical biopsy
specimens and animal tumor tissues was determined using the
PGE2-enzyme immunoassay kit (cat. no. 500141; Cayman
Chemical Company), according to the manufacturer's protocol.
In vivo migration assay
Female Balb/C-nu mice (n=8/group; age, 4-6 weeks;
weight, 20-25 g; Experimental Animal Center of Daping Hospital)
were housed in exhaust-ventilated closed system cages in a specific
pathogen-free environment. The animals were maintained at 22±2°C
with 55±5% humidity under a 12-h light/dark cycle. Food and water
were provided ad libitum. SiHa and E6-siRNA transfected SiHa
cells were cultured with DMEM supplemented with 10% FBS at 37°C in
an 5% incubator containing CO2. CaSki cells (Cell Bank
of the Chinese Academy of Sciences) were cultured with RPMI-1640
medium supplemented with 10% FBS at 37°C in a 5% CO2
incubator. Each mouse was injected with 2.5×105 cells
into the left footpad to create tumors. After ~3 weeks, when the
tumor size reached ~10 mm2, 2×106 treated DCs
were injected intratumorally. The injected DCs were labeled with
Qtracker™ 705 Cell Labeling kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions. Animals injected with
PBS were used as controls. After 48 h, the mice were sacrificed and
the popliteal lymph nodes were made into single cell suspensions
using nylon mesh and a 1-ml syringe. Subsequently, 20,000 cells
were counted by flow cytometry, the labeled DCs were detected and
the positive rate was calculated. In addition, for the detection of
labeled DCs, the popliteal lymph nodes of mice were dissected 48 h
post-DC injection, then embedded in Tissue-Tek OCT compound (Sakura
FineTek Japan) and frozen in liquid nitrogen. Cryosections (8
µm) were cut using a cryostatic microtome (Leica
Microsystems GmbH). Sections were mounted onto slides, dried and
frozen at −20°C before use. The slides were then fixed with acetone
(15 min, 4°C) and counterstained with DAPI. After washing, the
slides were mounted in 50% glycerol (in PBS) and examined using
confocal microscopy. All animal studies were approved by the
Laboratory Animal Welfare and Ethics Committee of the Third
Military Medical University. All efforts were made to minimize
animal suffering and reduce the number of animals used.
Statistical analysis
The experiments were repeated three times. Histogram
and scatter graphs were generated using GraphPad Prism 5 software
(GraphPad Software, Inc.) and were presented as the mean ± standard
deviation. Differences between multiple groups were analyzed by
one-way ANOVA followed by Dunnett's post hoc test using IBM SPSS
Statistics 19 software (IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Status of DCs in HPV16-positive cervical
lesions
The distribution of DCs in cervical biopsy specimens
was investigated. Immature DCs were characterized by CD1a
expression. Mature DCs, which capture foreign antigens to initiate
the immune response, expressed CD83. According to Fig. 1A, all HPV16-positive samples
demonstrated reduced expression of CD1a and increased expression of
CD83, compared with normal tissues. CD1a expression was
significantly reduced in the HSIL and SCC groups (P<0.05),
whereas CD83 expression was significantly upregulated in the LSIL
and HSIL groups (P<0.05). CCR7 expression was also detected;
however, not only DCs, but also cervical cells expressed CCR7 and
it was difficult to detect the expression of CCR7 on DCs only (data
not shown). These results indicated that, after capturing foreign
antigens, the majority of DCs may be trapped in the lesion site and
unable to migrate to the draining lymph nodes. The most likely
explanation of this phenomenon was that the migration ability of
DCs was suppressed. Therefore, an in vivo experiment was
performed to investigate this possibility. To mimic the
microenvironment of HPV16-positive lesions, immunodeficient mice
were injected with two HPV16-positive cell lines: SiHa and CaSki.
Labeled DCs were subsequently injected intratumorally. Control mice
not bearing tumors were directly injected with DCs into the footpad
and served as the control group. To investigate the migration of
DCs, the labeled cells in the draining lymph nodes were collected
and counted. The cell numbers from the two tumor-bearing groups
were reduced compared with the control group (Fig. 1B). These findings indicated that
the migration of DCs was inhibited in HPV16-positive cervical
lesions. However, the mechanism underlying the decreased migration
of DCs requires further investigation.
Expression of PGE2 in
HPV16-positive cervical lesions
The expression of PGE2 in cervical biopsy
specimens was investigated. As depicted in Fig. 2A, the expression of PGE2
was gradually upregulated in LSIL, HSIL and SCC samples, which was
accompanied by the development of disease. All HPV16-positive
lesions demonstrated significant upregulation compared with normal
tissues (P<0.05). Furthermore, the expression levels of
PGE2 synthases were detected, which are required for
PGE2 production. Western blotting and
immunohistochemistry were used to detect the expression levels of
three isoforms of PGE2 synthases: mPGES-1, cPGES and
mPGES-2. As demonstrated in Fig.
2B-E, the expression levels of all three PGE2
synthases were increased in HPV16-positive lesions compared with
normal tissue. Particularly, the SCC group exhibited the strongest
expression (P<0.05). These findings suggested that
PGE2 and PGE2 synthases may be upregulated in
HPV16-positive cervical lesions.
 | Figure 2Expression of PGE2 in
human papillomavirus 16-positive cervical lesions. (A) Detection of
PGE2 expression in cervical biopsy specimens by ELISA.
Evaluation of PGE2 expression was performed in normal
squamous epithelium (n=20), LSIL (n=20), HSIL (n=16) and SCC
(n=10). (B) Detection of mPGES-1, mPGES-2 and cPGES expression in
cervical biopsy specimens by western blotting. (C)
Semi-quantitative evaluation of mPGES-1, mPGES-2 and cPGES
expression. (D) mPGES-1, mPGES-2 and cPGES immunostaining in
cervical biopsy specimens. Original magnification, ×200. (E)
Semi-quantitative evaluation of mPGES-1, mPGES-2 and cPGES
expression was performed in normal squamous epithelium (n=5), LSIL
(n=5), HSIL (n=5) and SCC (n=5). *P<0.05 vs. Normal.
cPGES, cytosolic PGE synthase; HSIL, high-grade squamous
intraepithelial lesion; IOD, integrated optical density; LSIL,
low-grade squamous intraepithelial lesion; mPGES, microsomal PGE
synthase; PGE2, prostaglandin E2; SCC, squamous
carcinoma. |
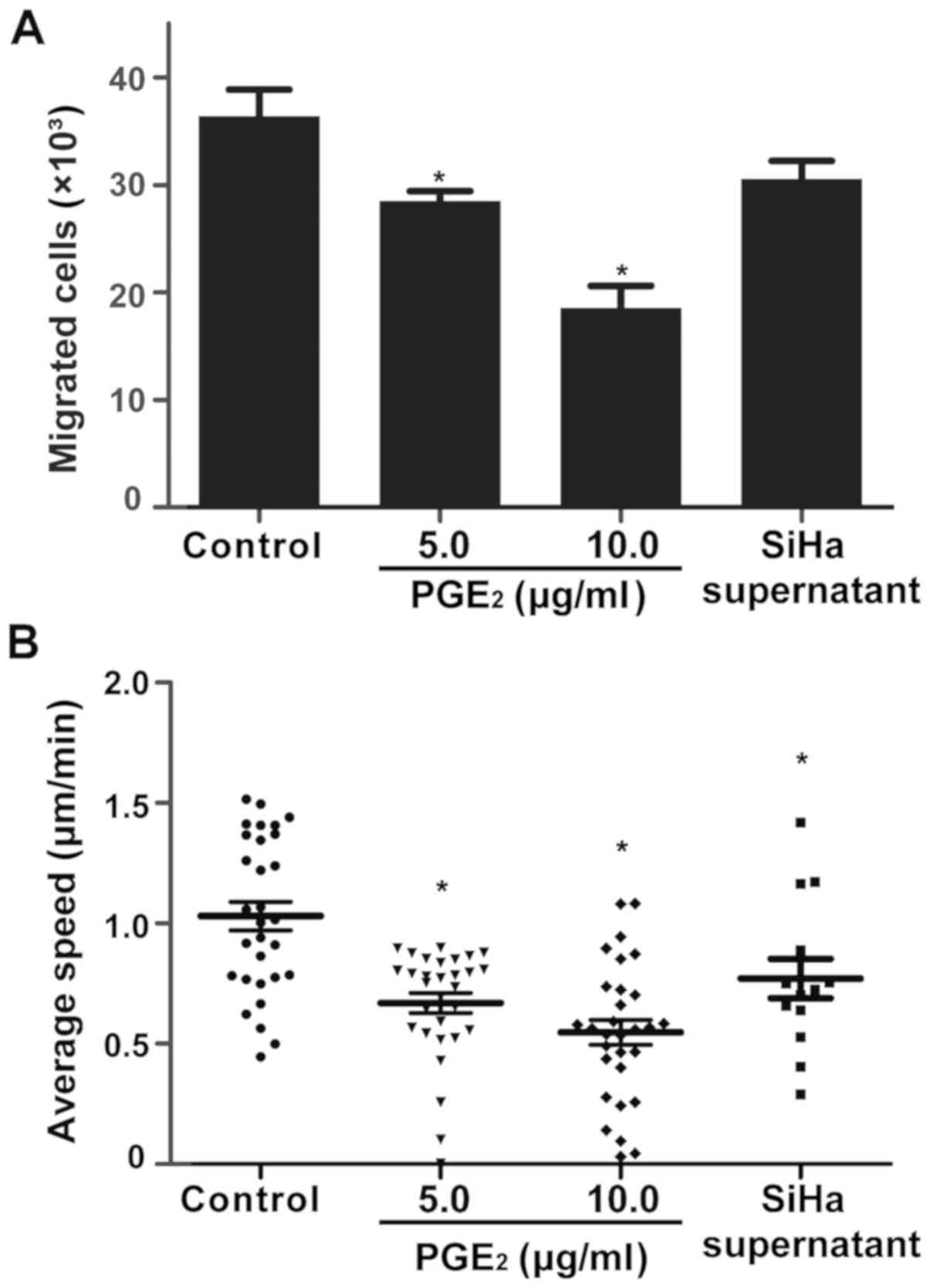
Effect of PGE2 on the
migration of DCs
The effect of PGE2 on the migration
ability of DCs was investigated. PGE2 was added to the
culture media of DCs at a final concentration of 0, 5 or 10
µg/ml. The cultured supernatant of SiHa cells was applied to
treat the DCs. The migration of DCs was determined using a
Transwell migration assay. As depicted in Fig. 3A, the migration of DCs was
significantly decreased in response to PGE2 in a
dose-dependent manner (P<0.05). Furthermore, after being treated
with SiHa supernatant, DCs exhibited declined migration ability as
well. A 3D migration assay was subsequently performed to validate
these results (Fig. 3B); the
results were in line with the Transwell migration assay. Therefore,
PGE2 may inhibit the migration of DCs in cervical
lesions.
Expression of CCR7 on DCs is affected by
PGE2
As shown in Fig. 4,
DCs exhibited marked upregulation of all co-stimulatory molecules
after being stimulated with LPS. However, the phenotypic features
of immature and mature DCs remained unchanged, following
PGE2 exposure. Since CCR7 is required for the migration
of DCs, the surface expression of CCR7 was also detected. CCR7
expression was markedly downregulated on immature and mature DCs
following PGE2 exposure. This result indicated that
PGE2 affected the migration ability of DCs.
E6 regulates PGE2 expression
in HPV16-positive cervical lesions
Due to its central role in the development of
HPV16-positive cervical lesions, the effect of the E6 oncoprotein
on PGE2 expression was investigated. Firstly, E6
expression was observed in cervical biopsy specimens. As
demonstrated in Fig. 5A, E6 was
undetectable in normal samples. Conversely, the expression of E6
gradually increased from low to high-grade lesions, which was
parallel to disease development and progression. The highest
expression was observed in SCC samples. E6-specific siRNA was
subsequently used to knock down E6 expression in SiHa cells. As
depicted in Fig. 5B, two siRNAs
were used to knock down E6 expression, with siRNA-1 exhibiting a
stronger effect. The cultured supernatant of siRNA-transfected SiHa
cells was collected and used to treat DCs in a Transwell migration
assay. As shown in Fig. 5C,
following treatment with siRNA-transfected supernatant, DCs
exhibited improved migration (P<0.05). Furthermore,
siRNA-transfected SiHa cells were used to perform an in vivo
migration assay on immunodeficient mice. The E6-knockdown SiHa
cells and control SiHa cells were applied to create tumors. The
labeled DCs were injected in the same way as aforementioned. The
labeled cells in the draining lymph nodes were collected and
counted to investigate the migration ability of DCs. As shown in
Fig. 6A and B, the numbers of DCs
were significantly increased in both siRNA-transfected groups
compared with the control group (P<0.05). This indicated that
the migration ability of DCs was improved following E6 knockdown.
Furthermore, the concentration of PGE2 in tumors was
measured. As shown in Fig. 6C,
tumors created by the E6 knockdown SiHa cells produced
significantly less PGE2 compared with normal cells
(P<0.05). These results indicated that E6 regulated the
production of PGE2 in HPV16-positive cervical lesions,
which affected the migration ability of DCs.
 | Figure 6E6 regulates PGE2
expression and affects the migration of DCs in vivo. (A and
B) Qtracker™ 705-labeled DCs were injected into groups of mice
(n=8/group), which were treated with different SiHa cells to create
tumors. After 48 h, three mice in each group were sacrificed, and
the popliteal lymph nodes were collected, snap frozen and
cryosectioned. The number of positive DCs was detected by confocal
microscopy; original magnification, ×400. Red staining indicated
Qtracker™ 705-labeled DCs; blue staining indicated nuclei. The
remaining mice were sacrificed, the popliteal lymph nodes were made
into single cell suspensions and the number of positive DCs was
detected by flow cytometry. Data are shown as means ± standard
deviation. (C) Tumor tissues were collected from each group
(control group, n=9; control siRNA group, n=6; E6 siRNA-1 group,
n=9; E6 siRNA-2 group, n=9) and detection of PGE2 concentration was
performed by ELISA. Data are shown as means ± standard deviation.
*P<0.05 vs. Control. DCs, dendritic cells;
PGE2, prostaglandin E2; siRNA, small interfering
RNA. |
Discussion
E6 is broadly expressed in different stages of
cervical lesions, and is responsible for hosT cell transformation
and disease development (22,23).
The present study revealed that E6 expression was markedly higher
in late cervical lesions than in early cervical lesions; this
result was in line with existing reports (24,25).
Following infection with HPV, DCs undergo a phenotypic conversion
from a tissue resident, antigen-capturing cell to a highly
migratory antigen-presenting cell, in a process known as
maturation. Previous studies have demonstrated that HPV regulates
the differentiation, function and distribution of DCs in infected
sites of cervical epithelial lesions, which allows for evasion of
immune surveillance (26,27). HPV may therefore promote tumor
progression by reducing the number of DCs and attenuating immune
surveillance. The present study revealed that the number of
CD1a-positive DCs was negatively associated with the degree of
cervical lesion, which was consistent with the findings of a
previous study (3). However, it
also revealed that the number of CD83-positive DCs was
significantly increased in early lesions, but decreased in late
lesions. It was speculated that in early lesions, DCs matured by
capturing antigens and accumulated at the lesion sites. In late
lesions, DCs that captured antigens may not migrate in time.
Therefore, the migration of DCs to the lymph nodes after capturing
antigens in early lesions may be a critical step in the immune
response. Khaiboullina et al (28) observed the variation and expression
of HPV-related proteins in DCs by transfecting the HPV18 gene. The
results revealed that the mRNA expression levels of E6 and E7 were
upregulated in transfected cells. In addition, the migration
ability of transfected DCs was reduced, which was accompanied with
a decreased ability to produce cytokines, which may inhibit and
delay the immune response of viral antigens. Notably, knockdown of
E6 expression restored the migration ability of DCs in the current
study; however, the mechanism by which E6 inhibited the migration
of DCs was not clarified.
As an inflammatory factor, PGE2 has been
related to carcinogenesis in several types of cancer, including
cervical cancer (29).
PGE2 stimulates carcinogenesis by promoting angiogenesis
(30), increasing proliferation of
cancer cells (31), suppressing
apoptosis of cancer cells (32)
and inducing immune tolerance (33). The activities of phospholipase A2,
COX and PGE2 synthases are essential to the biosynthesis
of PGE2. A previous study demonstrated that COX-2 is
rapidly induced by oncogenes, growth factors, cytokines and tumor
promoters, and serves an important role in the development of
cancer (34). Furthermore, COX-2
regulates tumor growth by binding to its corresponding receptors
through various downstream prostate products, instead of acting as
a signal transduction kinase. It has been reported that HPV16 and
COX-2 serve a synergistic role in cervical carcinogenesis. In
addition, certain studies have demonstrated that the expression of
COX-2 is significantly upregulated in HPV16E6/E7-positive cervical
cancer tissues and cell lines (18,35).
Prostaglandin levels in cervical cancer tissue have been reported
to be significantly higher than those in adjacent tissues, and high
expression of COX-2 is positively correlated with prostaglandin
levels (11). E6 may promote the
synthesis of COX-2 by activating the epidermal growth factor
receptor/→RAS/→MAPK/→activator protein 1 signaling pathway
(18). The present study revealed
that with the development of cervical lesions, the concentration of
PGE2 also increased. The in vivo experiments
using SiHa cells revealed that the concentration of PGE2
in tumors was significantly downregulated following E6 knockdown
compared with in the control group. This indicated that E6 may
exert a positive regulatory effect on the synthesis of
PGE2.
As an inducible synthase, mPGES-1 has been
identified to mainly interact with COX-2, whereas cPGES is not
inducible and mainly interacts with COX-1. Conversely, the role of
mPGES-2 is not clear (36). It has
been confirmed that COX-2/mPGES-1 expression is upregulated in
cervical pre-cancerous and invasive cancer tissues (37). According to Mattila et al
(38), with the disease
development of human glioma, the expression of these three
synthases is gradually increased. The results obtained in the
present study demonstrated that cPGES and mPGES-2 were also
expressed during the development of cervical lesions, and were
significantly upregulated in invasive cancer tissues. However, more
samples and further investigation is required to identify the
specific synthase that serves the predominant role in the
pathogenesis of cervical lesions.
PGE2 acts on neighboring tissues to
maintain the micro-environment in a steady state. PGE2
exerts its functions by activating four receptors: EP1-EP4.
Notably, EP2 and EP4 are expressed during the entire life cycle of
myeloid DCs (39). PGE2
regulates the functions of DCs by: i) Regulating the expression of
CCR7 on the surface of DCs to affect chemo-taxis (40,41);
ii) regulating the intracellular calcium flux and migration-related
signaling pathways (42,43); iii) regulating the expression of
MHCs, co-stimulatory molecules and markers of maturation in DCs, as
well as the ability of DCs to activate T-cell immune responses
(44); and iv) regulating the
expression of matrix metalloproteinase and tissue inhibitor of
matrix metalloproteinase in DCs, which affect the basement membrane
and allow the migration of DCs (21). Due to the important role of DCs in
specific cellular immunity, the impact and mechanism of
PGE2 are attracting increased attention. However, there
remain controversial conclusions. Some studies have revealed that
PGE2 enhances the migration and antigen-presenting
ability of DCs (12,13); therefore, PGE2 is often
used as an important component of cytokine cocktails that stimulate
the maturation of DCs in vitro. Conversely, other studies,
including the present study, have demonstrated that PGE2
inhibits the migration of DCs (21,45,46).
These differences may be explained by the fact that the
concentration and state of PGE2 used by the researchers
differed, which may cause inconsistent results.
Notably, the role of PGE2 requires
further investigation. The present results are unable to fully
elucidate the specific mechanism underlying regulation of DC
migration. This is one of the limitations of the present study. In
addition, the receptors that mediate the effect of PGE2
induced by cervical lesions are unclear and the specific signaling
pathway associated with the effects of PGE2 on migration
of DCs remains unclear. Therefore, we are currently collecting more
clinical samples to validate the results obtained in this study. In
addition, the expression of key molecules in the signaling pathway
induced by PGE2 will also be verified in these samples.
In the current study, the expression of CCR7 was slightly decreased
when the migration of DCs was inhibited by PGE2. This
finding indicated that there may be other mechanisms underlying the
regulatory effects of PGE2 on the migration of DCs, such
as those affecting formation of the cytoskeleton and focal
adhesion. Furthermore, immune evasion of cervical lesions is a
complex process; inhibited migration of mature DCs may be one
effect of immune evasion. It may regulate immature DCs to keep
dormant and remain in a steady state. Furthermore, it may induce
the production of regulatory DCs, thereby inhibiting T-cell
proliferation and keeping T cells less reactive, which eventually
induces immune tolerance. All of these are issues we aim to focus
on in our future study.
Similar to what was reported in previous studies,
the present study revealed that, in HPV16-positive cervical
lesions, the expression of PGE synthase was significantly
upregulated, resulting in the overproduction of PGE2
(18). Therefore, the use of drugs
to suppress PGE2 synthesis may reduce the risk of
cervical lesions. In the treatment of cervical cancer, patients
with upregulated expression of COX-2 have a worse prognosis than
patients who do not, irrespective of whether radiotherapy or
chemotherapy is administered (47,48).
Therefore, COX-2 inhibitors, such as ibuprofen and aspirin, may
reduce the risk of cervical cancer recurrence (29). However, COX-2 is the main
rate-limiting enzyme, and not a terminal enzyme, in the
PGE2 synthesis pathway. COX-2 affects a number of
downstream signaling pathways and is likely to have a double
effect. Therefore, the traditional treatment strategy using COX-2
as a therapeutic target is not ideal. The abnormal balance of the
PGE synthase-PGE2-EPs signaling pathway as downstream
target of COX-2 may inhibit the migration of DCs and lead to immune
evasion of cervical cancer cells. Restoring the balance of the PGE
synthase-PGE2-EPs signaling pathway may serve as a novel
therapeutic strategy for cancer.
In conclusion, the present study revealed that a
high concentration of PGE2 inhibited the migration of
DCs in HPV16-positive cervical lesions. Furthermore, the production
of PGE2 was mediated by the oncoprotein E6. Notably,
these findings may improve the understanding of immune evasion in
cervical lesions, and contribute to the treatment of cervical
cancer and other types of cancer.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81272864) and the Natural
Science Foundation of Chongqing (grant nos. CSTC2011BA5008 and
cstc2017shms-zdyfX0043).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JG and JHan designed this study. JHuang and GD
performed the majority of the experiments and were major
contributors in writing the manuscript. QZ and YC assisted with the
experiments and data analysis. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Daping Hospital, Army Medical University (Third Military Medical
University). All participants signed informed consent forms.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Professor Jiongyu Hu
and Professor Yizhi Peng (Institute of Burn Research, Southwest
Hospital, State Key Laboratory of Trauma, Burns and Combined
Injury, Third Military Medical University) for technical assistance
in preparing the manuscript.
References
|
1
|
Martin-Gayo E and Yu XG: Role of Dendritic
Cells in Natural Immune Control of HIV-1 Infection. Front Immunol.
10:13062019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carenza C, Calcaterra F, Oriolo F, Di Vito
C, Ubezio M, Della Porta MG, Mavilio D and Della Bella S:
Costimulatory molecules and immune checkpoints are differentially
expressed on different subsets of dendritic cells. Front Immunol.
10:13252019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hayati AR and Zulkarnaen M: An
immunohistochemical study of CD1a and CD83-positive infiltrating
dendritic cell density in cervical neoplasia. Int J Gynecol Pathol.
26:83–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poindexter NJ, Sahin A, Hunt KK and Grimm
EA: Analysis of dendritic cells in tumor-free and tumor-containing
sentinel lymph nodes from patients with breast cancer. Breast
Cancer Res. 6:R408–R415. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishigami S, Natsugoe S, Uenosono Y, Hata
Y, Nakajo A, Miyazono F, Matsumoto M, Hokita S and Aikou T:
Infiltration of antitumor immunocytes into the sentinel node in
gastric cancer. J Gastrointest Surg. 7:735–739. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greenhough A, Smartt HJ, Moore AE, Roberts
HR, Williams AC, Paraskeva C and Kaidi A: The COX-2/PGE2 pathway:
Key roles in the hallmarks of cancer and adaptation to the tumour
micro-environment. Carcinogenesis. 30:377–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang MT, Honn KV and Nie D:
Cyclooxygenases, prostanoids, and tumor progression. Cancer
Metastasis Rev. 26:525–534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee EJ, Kim SJ, Hahn YI, Yoon HJ, Han B,
Kim K, Lee S, Kim KP, Suh YG, Na HK, et al: 15-Keto prostaglandin
E2 suppresses STAT3 signaling and inhibits breast cancer cell
growth and progression. Redox Biol. 23:1011752019. View Article : Google Scholar :
|
|
9
|
Uefuji K, Ichikura T and Mochizuki H:
Cyclooxygenase-2 expression is related to prostaglandin
biosynthesis and angio-genesis in human gastric cancer. Clin Cancer
Res. 6:135–138. 2000.PubMed/NCBI
|
|
10
|
Watson J and Chuah SY: Prostaglandins,
steroids and human mammary cancer. Eur J Cancer Clin Oncol.
21:1051–1055. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sales KJ, Katz AA, Davis M, Hinz S,
Soeters RP, Hofmeyr MD, Millar RP and Jabbour HN: Cyclooxygenase-2
expression and prostaglandin E(2) synthesis are up-regulated in
carcinomas of the cervix: A possible autocrine/paracrine regulation
of neoplastic cell function via EP2/EP4 receptors. J Clin
Endocrinol Metab. 86:2243–2249. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saalbach A, Janik T, Busch M, Herbert D,
Anderegg U and Simon JC: Fibroblasts support migration of
monocyte-derived dendritic cells by secretion of PGE2 and MMP-1.
Exp Dermatol. 24:598–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yen JH, Khayrullina T and Ganea D:
PGE2-induced metallo-proteinase-9 is essential for dendritic cell
migration. Blood. 111:260–270. 2008. View Article : Google Scholar
|
|
14
|
Li Y, Huang K, Ji PL, Song L and Liu HT:
Cervical Infection of Oncogenic Human Papillomavirus (HPV) Types in
Beijing, China. Biomed Environ Sci. 29:734–741. 2016.PubMed/NCBI
|
|
15
|
Song D, Li H, Li H and Dai J: Effect of
human papillomavirus infection on the immune system and its role in
the course of cervical cancer. Oncol Lett. 10:600–606. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guess JC and McCance DJ: Decreased
migration of Langerhans precursor-like cells in response to human
keratinocytes expressing human papillomavirus type 16 E6/E7 is
related to reduced macrophage inflammatory protein-3alpha
production. J Virol. 79:14852–14862. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caberg JH, Hubert P, Herman L, Herfs M,
Roncarati P, Boniver J and Delvenne P: Increased migration of
Langerhans cells in response to HPV16 E6 and E7 oncogene silencing:
Role of CCL20. Cancer Immunol Immunother. 58:39–47. 2009.
View Article : Google Scholar
|
|
18
|
Subbaramaiah K and Dannenberg AJ:
Cyclooxygenase-2 transcription is regulated by human papillomavirus
16 E6 and E7 oncoproteins: Evidence of a corepressor/coactivator
exchange. Cancer Res. 67:3976–3985. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lutz MB, Kukutsch N, Ogilvie AL, Rössner
S, Koch F, Romani N and Schuler G: An advanced culture method for
generating large quantities of highly pure dendritic cells from
mouse bone marrow. J Immunol Methods. 223:77–92. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han P, Hanlon D, Sobolev O, Chaudhury R
and Edelson RL: Ex vivo dendritic cell generation-A critical
comparison of current approaches. Int Rev Cell Mol Biol.
349:251–307. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baratelli FE, Heuzé-Vourc'h N, Krysan K,
Dohadwala M, Riedl K, Sharma S and Dubinett SM: Prostaglandin
E2-dependent enhancement of tissue inhibitors of
metalloproteinases-1 production limits dendritic cell migration
through extracellular matrix. J Immunol. 173:5458–5466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghittoni R, Accardi R, Hasan U, Gheit T,
Sylla B and Tommasino M: The biological properties of E6 and E7
oncoproteins from human papillomaviruses. Virus Genes. 40:1–13.
2010. View Article : Google Scholar
|
|
23
|
Narisawa-Saito M and Kiyono T: Basic
mechanisms of high-risk human papillomavirus-induced
carcinogenesis: Roles of E6 and E7 proteins. Cancer Sci.
98:1505–1511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boldrini NT, Freitas LB, Coutinho AR,
Loureiro FZ, Spano LC and Miranda AE: High-grade cervical lesions
among women attending a reference clinic in Brazil: Associated
factors and comparison among screening methods. PLoS One.
9:e1021692014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mazumder Indra D, Singh RK, Mitra S, Dutta
S, Chakraborty C, Basu PS, Mondal RK, Roychoudhury S and Panda CK:
Genetic and epigenetic changes of HPV16 in cervical cancer
differentially regulate E6/E7 expression and associate with disease
progression. Gynecol Oncol. 123:597–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leong CM, Doorbar J, Nindl I, Yoon HS and
Hibma MH: Loss of epidermal Langerhans cells occurs in human
papillomavirus alpha, gamma, and mu but not beta genus infections.
J Invest Dermatol. 130:472–480. 2010. View Article : Google Scholar
|
|
27
|
Tran Janco JM, Lamichhane P, Karyampudi L
and Knutson KL: Tumor-infiltrating dendritic cells in cancer
pathogenesis. J Immunol. 194:2985–2991. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Khaiboullina SF, Morzunov SP, Hall MR, De
Meirleir KL, Rizvanov AA and Lombardi VC: Human dendritic cells
transfected with a human papilloma virus-18 construct display
decreased mobility and upregulated cytokine production. Int J
Oncol. 43:1701–1709. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fitzgerald DW, Bezak K, Ocheretina O,
Riviere C, Wright TC, Milne GL, Zhou XK, Du B, Subbaramaiah K, Byrt
E, et al: The effect of HIV and HPV coinfection on cervical COX-2
expression and systemic prostaglandin E2 levels. Cancer Prev Res
(Phila). 5:34–40. 2012. View Article : Google Scholar
|
|
30
|
Tsujii M, Kawano S, Tsuji S, Sawaoka H,
Hori M and DuBois RN: Cyclooxygenase regulates angiogenesis induced
by colon cancer cells. Cell. 93:705–716. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karpisheh V, Nikkhoo A, Hojjat-Farsangi M,
Namdar A, Azizi G, Ghalamfarsa G, Sabz G, Yousefi M, Yousefi B and
Jadidi-Niaragh F: Prostaglandin E2 as a potent therapeutic target
for treatment of colon cancer. Prostaglandins Other Lipid Mediat.
144:1063382019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsujii M and DuBois RN: Alterations in
cellular adhesion and apoptosis in epithelial cells overexpressing
prostaglandin endo-peroxide synthase 2. Cell. 83:493–501. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sharma S, Yang SC, Zhu L, Reckamp K,
Gardner B, Baratelli F, Huang M, Batra RK and Dubinett SM: Tumor
cyclooxy-genase-2/prostaglandin E2-dependent promotion of FOXP3
expression and CD4+ CD25+ T regulatory cell activities in lung
cancer. Cancer Res. 65:5211–5220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang F, Zhang R and Wang J:
Cyclooxygenase-2-Mediated Up-Regulation of Mitochondrial
Transcription Factor A Mitigates the Radio-Sensitivity of Cancer
Cells. Int J Mol Sci. 20:E12182019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Balan R, Amălinei C, Giuşcă SE, Ditescu D,
Gheorghiţă V, Crauciuc E and Căruntu ID: Immunohistochemical
evaluation of COX-2 expression in HPV-positive cervical squamous
intraepithelial lesions. Rom J Morphol Embryol. 52:39–43.
2011.PubMed/NCBI
|
|
36
|
Hara S, Kamei D, Sasaki Y, Tanemoto A,
Nakatani Y and Murakami M: Prostaglandin E synthases: Understanding
their pathophysiological roles through mouse genetic models.
Biochimie. 92:651–659. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Herfs M, Herman L, Hubert P, Minner F,
Arafa M, Roncarati P, Henrotin Y, Boniver J and Delvenne P: High
expression of PGE2 enzymatic pathways in cervical (pre)neoplastic
lesions and functional consequences for antigen-presenting cells.
Cancer Immunol Immunother. 58:603–614. 2009. View Article : Google Scholar
|
|
38
|
Mattila S, Tuominen H, Koivukangas J and
Stenbäck F: The terminal prostaglandin synthases mPGES-1, mPGES-2,
and cPGES are all overexpressed in human gliomas. Neuropathology.
29:156–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Scandella E, Men Y, Gillessen S, Förster R
and Groettrup M: Prostaglandin E2 is a key factor for CCR7 surface
expression and migration of monocyte-derived dendritic cells.
Blood. 100:1354–1361. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bruckner M, Dickel D, Singer E and Legler
DF: Converse regulation of CCR7-driven human dendritic cell
migration by prostaglandin E2 and liver X receptor
activation. Eur J Immunol. 42:2949–2958. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Legler DF, Krause P, Scandella E, Singer E
and Groettrup M: Prostaglandin E2 is generally required for human
dendritic cell migration and exerts its effect via EP2 and EP4
receptors. J Immunol. 176:966–973. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Scandella E, Men Y, Legler DF, Gillessen
S, Prikler L, Ludewig B and Groettrup M: CCL19/CCL21-triggered
signal transduction and migration of dendritic cells requires
prostaglandin E2. Blood. 103:1595–1601. 2004. View Article : Google Scholar
|
|
43
|
Shimabukuro-Vornhagen A, Liebig TM,
Koslowsky T, Theurich S and von Bergwelt-Baildon MS: The ratio
between dendritic cells and T cells determines whether
prostaglandin E2 has a stimulatory or inhibitory effect. Cell
Immunol. 281:62–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rubio MT, Means TK, Chakraverty R, Shaffer
J, Fudaba Y, Chittenden M, Luster AD and Sykes M: Maturation of
human monocyte-derived dendritic cells (MoDCs) in the presence of
prostaglandin E2 optimizes CD4 and CD8 T cell-mediated responses to
protein antigens: Role of PGE2 in chemokine and cytokine expression
by MoDCs. Int Immunol. 17:1561–1572. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Spaggiari GM, Abdelrazik H, Becchetti F
and Moretta L: MSCs inhibit monocyte-derived DC maturation and
function by selectively interfering with the generation of immature
DCs: Central role of MSC-derived prostaglandin E2. Blood.
113:6576–6583. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sá-Nunes A, Bafica A, Lucas DA, Conrads
TP, Veenstra TD, Andersen JF, Mather TN, Ribeiro JM and
Francischetti IM: Prostaglandin E2 is a major inhibitor of
dendritic cell maturation and function in Ixodes scapularis saliva.
J Immunol. 179:1497–1505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim YB, Kim GE, Cho NH, Pyo HR, Shim SJ,
Chang SK, Park HC, Suh CO, Park TK and Kim BS: Overexpression of
cyclooxygenase-2 is associated with a poor prognosis in patients
with squamous cell carcinoma of the uterine cervix treated with
radiation and concurrent chemotherapy. Cancer. 95:531–539. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hugo de Almeida V, Guimarães IDS, Almendra
LR, Rondon AMR, Tilli TM, de Melo AC, Sternberg C and Monteiro RQ:
Positive crosstalk between EGFR and the TF-PAR2 pathway mediates
resistance to cisplatin and poor survival in cervical cancer.
Oncotarget. 9:30594–30609. 2018.PubMed/NCBI
|