Introduction
Prostate cancer (PCa) is one of the most frequently
occurring malignant tumors among males, and is associated with high
mortality and morbidity rates (1,2). To
date, no standard regimen for PCa treatment has been established.
Chemotherapy, immu-notherapy and radiotherapy are the primary
cancer treatments used following surgery (3). For patients with PCa, one goal of
treatment is to initiate the apoptosis of cancer cells (4,5).
Tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL), a member of the TNF protein
family, is known to trigger the death of various cancer cells, but
not normal cells (6,7), by binding the death receptors, DR-4
and DR-5, and by recruiting FADD and caspase-8 to create a
death-inducing complex (8). TRAIL
has been used as an apoptosis-inducing factor in various human
cancers, and is thus a good candidate for use in novel cancer
therapies (9). However, numerous
cancer cells acquire resistance to TRAIL-induced apoptosis
(5). Thus, for the treatment of
PCa, considerable attention has recently been focused on overcoming
the resistance of cancer cells to TRAIL.
Neferine is a major bisbenzylisoquinoline alkaloid
present in Nelumbo nucifera Gaertn. green seed embryos
(10). Recently, neferine has been
demonstrated to exhibit efficient antitumor activities in HepG2
cells and human lung cancer cells (11,12),
and to suppress the propagation of osteosarcoma cells (13). Further, neferine treatment has been
shown to induce the release of reactive oxygen species (ROS) and
trigger the mitochondrial apoptosis of liver and lung cancer cells
(11,12).
The autophagic flux, which involves the degradation
and recycling of damaged and harmful cellular components, is an
important process for maintaining metabolism and energy homeostasis
(14). Apoptosis leads to
programmed cell death, whereas the autophagic flux can lead either
to survival or death (15). During
the induction of the autophagic flux, beclin-1 triggers the
transformation of cytosolic microtubule-associated protein
1A/1B-light chain 3 (LC3-I) into LC3-phosphatidylethanolamine
conjugate (LC3-II). The conversion of LC3-I to LC3-II and the
recruitment of p62/SQTMI to the autophagosomal membrane are
considered to be key features of the autophagic flux and are
indicators that this process has been induced and activated
(16-18), although the specific molecular
pathways for this process in cancer cells remain unclear. c-Jun
N-terminal kinase (JNK) is a stress-induced member of the
mitogen-activated protein kinase (MAPK) family. JNK plays
fundamental roles in cell growth, differentiation, attenuation and
apoptosis (19). In the present
study, the ability of neferine treatment to enhance the
TRAIL-initiated apoptosis of PCa cells was assessed. The results
indicate that combined treatment of PCa cells with and neferine and
TRAIL is more effective than treatment with either substance
alone.
Materials and methods
Cells and cell culture
Human PCa cells (DU145 and LNCaP; Korean Cell Line
Bank) were maintained in RPMI-1640 medium containing 10% fetal
bovine serum (FBS). During experimentation, cells were grown in
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) containing 1%
FBS. Cells were grown at 37˚C and 5% CO2 in a humidified
incubator.
Reagents
Neferine was acquired from Sigma-Aldrich; Merck
KGaA, and TRAIL was acquired from Abfrontier.
Determination of cell cytotoxicity
Cytotoxicity is the amount of toxiciticy affecting
cells. A cytotoxic agent can lead to a decrease in cell viability,
the activation of apoptosis and the alteration of autophagy
(20-22). Several methods for cytotoxicity
assay, such as trypan blue stain, lactate dehydrogenase (LDH)
assay, 3-(4,5-dimethyl-2-thiazoly)-2,5-dephenyl-2H-tetrazo-lium
bromide (MTT) assay. In the present study, cell viability was
assessed, and crystal violet and trypan blue staining was used to
examine cells treated with a combination of neferine and TRAIL.
Cell viability assay
The DU145 and LNCaP cells seeded in 12-well plates
were treated with 0, 5, 10, or 20 µM neferine for 18 h, cells were
subseqently treated with 200 ng/ml TRAIL for 2 h and chloroquine
(CQ; cat. no. c6628; Sigma-Aldrich; Merck KGaA) was treated 10 µM 1
h prior neferine or TRAIL treatment. In addition, the JNK
inhibitor, SP600125 (cat. no. s5567; Sigma-Aldrich; Merck KGaA),
was used to treat the cells at 1 µM for 1 h prior to neferine or
TRAIL treatment. Cell morphology was evaluated using a light
microscope (Nikon Corp.), and cell viability was evaluated using a
crystal violet assay as previously described (23).
Trypan blue exclusion assay
Cell viability was evaluated using a trypan blue
exclusion assay. The DU145 and LNCap cells were seeded in a 24-well
plate and following treatment, the cells were dissociated from
plate with trypsin-EDTA and were then suspensed in 1 ml PBS per
well followed by the addition of 1 ml trypan blue solution
(Sigma-Aldrich; Merck KGaA). Follwing incubation for 5 min at 20˚C,
the cells were counted using a hemocytometer (Marienfeld Corp.) and
examined using a light microscope (Nikon Corp.). Each treatment was
performed in triplicate, and the results are expressed as a
percentage relative to the untreated controls.
Immunofluorescence staining
To determine the effects of neferine on
TRAIL-induced apoptosis, the levels of cleaved casapase-8 (Ac-cas8)
and caspase-3 (Ac-cas3) were detected. In addition, Also, changes
in the levels of p62 and LC-3 Ⅱ proteins were detected to monitor
the autophagic flux. DU145 cells were cultured on
poly-L-lysine-coated coverslips. Differentiated cells that had been
treated were fixed with 4% paraformaldehyde and permeabilized with
0.1% Triton X-100. The cells were then incubated in blocking
solution overnight at 4˚C and bathed in a solution containing
anti-p62 (1:250; cat. no. PAS-20839; Invitrogen; Thermo Fisher
Scientific, Inc.) and anti-p-JNK (1:500; cat. no. c.s 9255s; Cell
Signaling Technology; Inc.) antibodies for 2-3 h at room
temperature. After washing with PBS, the cells were incubated in
the dark at 20˚C with secondary antibodies (Alexa Fluor®
488-conjugated donkey polyclonal anti-rabbit; 1:500; cat. no.
A-21206; Thermo Fisher Scientific, Inc. and Alexa FluorTM 546 goat
anti-mouse IgG (1:500; cat. no. A-21206; Thermo Fisher Scientific,
Inc.) for 2 h. A fluorescence microscope (Nikon Corp.) was used to
visualize immunostaining.
Transmission electron microscopy
(TEM)
Cells were bathed in a solution containing 2%
glutaraldehyde, 2% paraformal-dehyde and 0.05 M sodium cacodylate
(pH 7.2) (all from Electron Microscopy Sciences) for 2 h at 4˚C.
The cells were then fixed by incubation with 1% osmium tetroxide
(Electron Microscopy Sciences) for 1 h at 4˚C, dehydrated with
increasing concentrations of ethanol (25, 50, 70, 90 and 100%) for
5 min at each concentration, and embedded in epoxy resin (Embed
812; Electron Microscopy Sciences) for 48 h at 60˚C following the
manufacturers' instructions. Ultrathin sections (60-nm-thick) were
sliced using an LKB-III ultratome (Leica Microsystems GmbH). The
slices were stained with 0.5% uranyl acetate (Electron Microscopy
Sciences) for 20 min and with 0.1% lead citrate (Electron
Microscopy Sciences) for 7 min at room temperature. Fluorescent
images were recorded using the Hitachi H7650 electron microscope
(Hitachi, Ltd.; magnification, ×10,000) located at the Center for
University-Wide Research Facilities (CURF) at Jeonbuk National
University.
Western blot analysis
Western blot analysis was performed as previously
described (3). Total protein
extraction was using immunoprecipitation assay buffer (Qiagen,
Inc.). The supernatant was collected by centrifugation (11,200 ×
g); 4˚C; 10 min; the protein concentration was determined using the
BCA protein assay kit (Thermo Fisher Scientific; Inc.). Proteins
(30 µg) were separated on 10% SDS-PAGE gels and blotted onto
polyvinylidene fluoride membranes. The membranes were blocked with
5% non-fat dried milk at 25˚C for 1 h antibodies against the
flowing proteins were used; β-actin (1:10,000; cat. no. A5441;
Sigma-Aldrich; Merck KGaA), LC3A/B (1:1,000; cat. no. c.s 4108s;
Cell Signaling Technology; Inc.), p62 (1:250; cat. no. PAS-20839;
Invitrogen; Thermo Fisher Scientific, Inc.), ATG5 (1:1,000; cat.
no. c.s 12994s; Cell Signaling Technology; Inc.), cleaved caspase-3
(1:1,000; cat. no. c.s 9661; Cell Signaling Technology; Inc.)
(activation of caspase-3 requires proteolytic processing of its
inactive zymogen into activated p17 and p12 fragments, cleavage of
caspase-3 requires aspartic acid at thep1 position), cleaved
caspase-8 (1:1,000; cat. no. 551242; BD Pharmingen) (caspase-8 is
produced as a proenzyme (55/50 kDa, doublet) it is cleaved into
smaller subunits (40/36 kDa, doublet). and p-JNK (1:500; cat. no.
c.s 9255s; Cell Signaling Technology; Inc.). The membranes were
then incubated with horseradish peroxidase-conjugated secondary
antibodies (cat. nos. ADI-SAB-100 and ADI-SAB-300; 1:1,000; Enzo
Life Sciences, Inc.) at 25˚C for 1 h. The immune reactive protein
bands were visualized using enhanced chemiluminescence detection
system (GE Healthcare Life Sciences) and detected with
chemiluminescence imaging system (Fusion FX7; Viber Lourmat). The
intensities of the protein bands were determined using Image J Java
1.8.0 software.xs.
Small interfering RNA transfection
Media RPMI-1640 (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% FBS (Atlas Biologicals) was seeded with DU145
cells, and 24 h later, the cells were transfected with
silencer-select small interfering RNA (ATG5 siRNA; oligo ID
HSS114103; Sequence GGU UUG GAC GAA UUC CAA CUU GUU U; Invitrogen;
Thermo Fisher Scientific, Inc.) using Lipofectamine 2000 as per the
manufacturer's recommendations. Simultaneously, a negative control
(Invitrogen; Thermo Fisher Scientific, Inc.) was trans-fected with
a non-targeting siRNA. The cells were incubated with ATG5 siRNA or
negative control siRNA for 6 h and the medium was then changed to
RPMI-1640 with 10% FBS for 24 h. The cells were then treated with
neferine or neferine in combination with TRAIL.
Statistical analysis
All experiments were performed in triplicate, and
the data are reported as the means ± standard error. One-way
factorial analysis of variance (ANOVA), followed by Duncan's
post-hoc test, was performed to evaluate the statistical
significance of the differences between the treatment and control
groups.
Results
Effects of neferine treatment on the
TRAIL-induced apoptosis of PCa cells
In the present study, in order to investigate the
synergistic effects of combination treatment with neferine and
TRAIL on PCa cells, the viability of neferine only-treated cells,
TRAIL only-treated cells, and neferine and TRAIL combine-treated
cells was compared. No significants changes were observed in the
viability of the cells treated with neferine or TRAIL only;
however, a significant decrease was detected in the viability of
the cells treated with the combination of neferine and TRAIL
(Fig. 1). To evaluate the effects
of neferine treatment on PCa cell apoptosis, the DU145 and LNCaP
cells were treated with various concentrations of neferine for 18 h
and then exposed to TRAIL for a further 2 h. As shown in Fig. 1, treatment with TRAIL or neferine
alone resulted in only marginal cell death and did not cause any
novel morphological changes. By contrast, when cells were treated
with both neferine and TRAIL, cell viability decreased
significantly (Fig. 1). These data
indicate that neferine treatment sensitizes human PCa cells to
TRAIL-induced apoptosis.
Neferine treatment induces an autophagic
flux and promotes TRAIL-mediated apoptosis
In response to neferine treatment, the expression of
LC3-II increased markedly in the DU145 cells, whereas p62
expression decreased significantly (Fig. 2A). The results obtained for p62
protein levels measured by immunofluorescence staining were
consistent with those obtained by western blot analysis (Fig. 2B). TEM indicated that autophagic
vacuoles were secreted by the neferine-treated cells (Fig. 2C). In addition, the cells treated
with a combination of neferine and TRAIL exhibited higher
expression levels of Ac-cas3 and Ac-cas8 (Fig. 2D). Caspase-8 activation also was
detected in the cells treated with a combination of neferine and
TRAIL, but not in the cells treated with neferine or TRAIL alone
(Fig. 2D). Certain studies have
compared the combination of two agents§ to either agent alone to
investigate the synergistic effects of the two agents (24-26).
These results suggest that neferine treatment can initiate
autophagy in DU145 cells.
Inhibition of autophagy attenuates the
neferine-mediated sensitization of TRAIL-induced apoptosis
Cell morphological analysis revealed that treatment
with chloroquine (CQ), an autophagy inhibitor, attenuated cellular
apoptosis mediated by combined treatment with neferine and TRAIL
(Fig. 3A). Co-treatment with
neferine, TRAIL and CQ resulted in a distinct improvement in the
viability of the DU145 cells (Fig.
3B-D). The autophagic flux activation by neferine was confirmed
by the inspection of the autophagic flux following treatment with
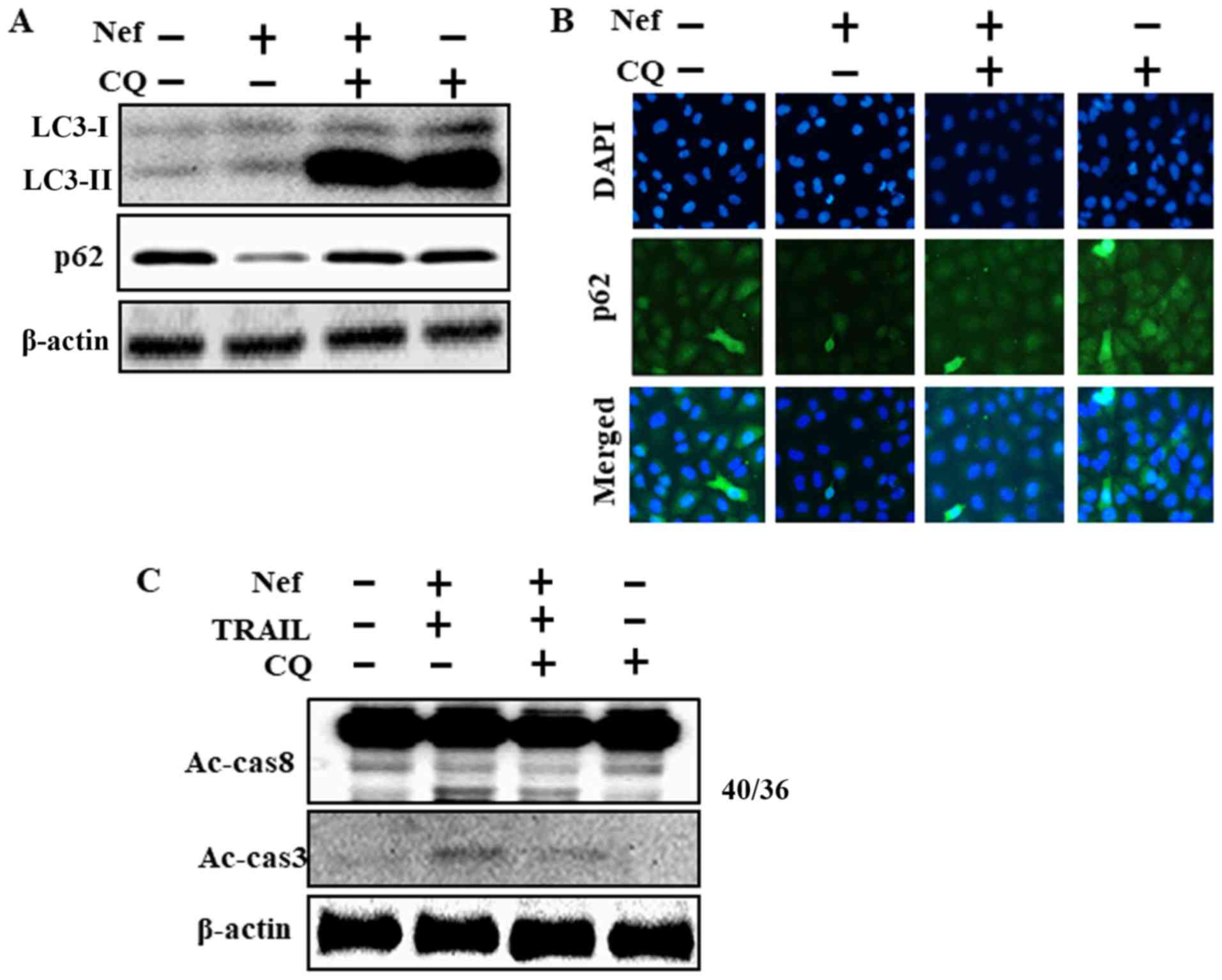
CQ as an autophagy inhibitor. CQ treatment led to the accumulation
of membrane-bound LC3-II and an increase in p62 levels, these
results indicated that CQ blocked neferine-induced autophagy
(Fig. 4A). Immunofluorescence
staining indicated that CQ treatment resulted in an increase in p62
protein levels (Fig. 4B). CQ also
attenuated the upregulation of Ac-cas8 and Ac-cas3 that was
observed following treatment with neferine and TRAIL (Fig. 4C). These results indicate that CQ
modulates neferine-mediated, TRAIL-triggered apoptosis by
inhibiting the autophagic flux.
Synergistic apoptosis mediated by
neferine and TRAIL is blocked by the genetic inhibition of the
autophagic flux
Cell morphological analysis indicated that
transfection with ATG5 siRNA (Fig.
5A). Co-treatment with neferine, TRAIL and ATG5 siRNA
significantly attenuated cell death and markedly increased the
viability of the DU145 cells (Fig.
5B-D). In the cells in which ATG5 was knocked down, the LC3-II
protein levels were markedly decreased and p62 protein levels were
markedly increased (Fig. 6A). The
protein levels of p62 measured by immunofluorescence staining were
similar to the levels measured by western blot analysis (Fig. 6B). Co-treatment of the cells with
neferine, ATG5 siRNA and TRAIL resulted in a decrease in Ac-cas3
and Ac-cas8 expression (Fig. 6C).
These results suggested that ATG5 siRNA blocked the synergistic
cell death induced by neferine and TRAIL treatment.
Neferine treatment induces JNK
activation
As revealed by western blot analysis and
immunofluorescence staining, neferine treatment of the DU145 cells
resulted in a dose-dependent increase in the p-JNK protein
expression levels (Fig. 7A and B).
In the cells that were treated with 1 µM SP600125 for 1 h prior to
neferine treatment, a decrease in neferine-induced p-JNK expression
was observed. SP600125 treatment also increased the viability of
the cells that had been treated with neferine and TRAIL (Fig. 7D and E). These results indicate
that neferine treatment causes an increase in JNK expression that
triggers TRAIL-mediated death of DU145 cells.
Discussion
TRAIL has been demonstrated to stimulate the
apoptosis of cancer cells without harming normal cells; therefore,
TRAIL administration is regarded as a prospective treatment
strategy against cancer (27-29).
However, TRAIL resistance has been observed in a number of
different types of cancer (30).
Neferine is a bisbenzyl isoquinoline alkaloid that has been shown
to exert a number of biological effects, such as the inhibition of
cancer cell proliferation (31).
The autophagic flux, a process of lysosomal degradation of
misfolded and unneeded proteins, plays a crucial role in
maintaining homeostasis in healthy cells, and can also lead to the
destruction of damaged or cancerous cells (32-34).
JNK plays a critical role in inducing autophagy and triggering
cellular apoptosis (35). Cell
viability is the ratio of the initial cell number minus the dead
cell number to the initial cell number. In the present study, cell
viability assay was used to assess apoptotic cell death. Cleaved
caspase-3 and caspase-8 were also detected for the assessment of
apoptosis (Figs. 2D, 4C and 6C).
TRAIL has received considerable attention as a novel
anticancer agent. Although various types of tumor cells are
sensitive to TRAIL-initiated apoptosis, other cells, including PCa
cells, are TRAIL-resistant (36).
The data of the present study demonstrated that while TRAIL
treatment alone did not trigger the apoptosis of DU145 cells,
treatment of the cells with neferine prior to TRAIL treatment
resulted in increased cell death (Fig.
1). Recent research has revealed that neferine attenuates
cancer cell proliferation and induces autophagy (12,31,37).
The present study demonstrated that neferine initiated autophagy in
PCa cells through the formation of autophagosomes and the
conversion of LC3-I to LC3-II (Fig.
2), and that pharmacological (Figs. 3 and 4) and genetic (Figs. 5 and 6) autophagy inhibitors attenuated
neferine-mediated, TRAIL-triggered apoptosis.
Autophagy and apoptosis are self-destructive
processes in response to cell stress. These two processes are
activated by different signaling pathway, but also interact to each
other. They finally lead to cell death and decrease cell viability,
particularly that of cancer cells; however, these two processes
have different biomarkers for identification. Apoptosis is
programmed cell death. There are certain biomarkers for monitoring
apoptosis, such as caspase, mitochondrial potential, the sub G1
population, DNA fragmentation and nuclear condensation (38). Western blot analysis is a powerful
method for the detection of apoptosis. During apoptosis the levels
of a number of proteins are altered. The levels of caspases, such
as caspase-3, caspase-8 and caspase-9 are significantly increased
during apoptosis (39). In
addition, the levels of Bcl family proteins are altered during
apoptosis (40). The
depolarization of mitochondrial potential is the central mechanism
of apoptosis (41). The induction
of apoptosis induces DNA fragmentation and nuclear condensation,
leading to nuclear morphological changes, and this increases the
Sub G1 cell population (42).
These methods can identify apoptotic cell death. Autophagy selects
and tags cytoplasmic components and organelles into the
autophago-some and which are then degraded by the lysosome. There
are some available methods with which to detect autophagy
structures and monitor the autophagic flux (43). In the present study, LC-3 and p62
protein expression was examined by western blot analysis and
immunocytochemistry. LC-3 and p62 are typical protein makers of the
autophagic flux (43). LC-3 as an
autophagosome marker, is presented in Figs. 2A, 4A and 6A. LC-3 is a microtubule-associated
protein 1A/1B-light chain, and during autophagy the cytosolic form
of LC-3 (LC-3I) is conjugated to phosphatidyl-ethanolamine (PE) to
form LC-3-PE (LC-3II). Subsequently, LC-3II contributes to
autophagosome formation. LC-3 has been used as a maker of
autophagosome formation (44,45).
The present study detected p62 as a maker of the autophagic flux
(Figs. 2A and B, 4A and B, and 6A and B). p62 is sequestosome-1, and is
an ubiquitin-binding protein. It delivers cytosolic protein to the
autophagosome and directly binds to LC-3II. p62 allows the
cytosolic protein to locate into the autophagosome and be degraded.
A decrease in p62 expression can represent an increase in the
autophagic flux. In several studies, the expression of p62 has been
monitored for the investigation of the autophagic flux (46,47).
The present study also detected autophagosome structure using TEM
(Fig. 2C). The autophagosome is a
double membrane vesicle, substrates some cellular organelles and
aggregated proteins. TEM is a conventional method used to identify
the autophagosome and monitor the morphology of the autophagosome
(48).
The present study also demonstrated that neferine
treatment increased p-JNK protein expression in a dose-dependent
manner (Fig. 7A and B). The
effects of neferine treatment were suppressed in the presence of
SP600125, which inhibits p-JNK activity (Fig. 7C and D). In conclusion, the
findings of the present study demonstrate that neferine treatment
mediates TRAIL-the induced death of DU145 tumor cells via the
autophagic flux and JNK pathway. These results indicate that
combined treatment with neferine and TRAIL may be effective against
TRAIL-resistant cancers.
Funding
The present study was supported by the National
Research Foundation of the Korea Grant (NRF) funded by the Ministry
of Education (2019R1A6A1A03033084).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
UMDN, HY and SYP designed, executed the study and
analyzed data. UMDN and HY wrote the manuscript. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hao L, Zhao Y, Li ZG, He HG, Liang Q,
Zhang ZG, Shi ZD, Zhang PY and Han CH: Tumor necrosis
factor-related apop-tosis-inducing ligand inhibits proliferation
and induces apoptosis of prostate and bladder cancer cells. Oncol
Lett. 13:3638–3640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nazim UM, Jeong JK and Park SY:
Ophiopogonin B sensitizes TRAIL-induced apoptosis through
activation of autophagy flux and downregulates cellular FLICE-like
inhibitory protein. Oncotarget. 9:4161–4172. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang S, Wang Y, Chen Z, Kim S, Iqbal S,
Chi A, Ritenour C, Wang YA, Kucuk O and Wu D: Genistein enhances
the efficacy of cabazitaxel chemotherapy in metastatic
castration-resistant prostate cancer cells. Prostate. 73:1681–1689.
2013.PubMed/NCBI
|
|
5
|
Klosek M, Mertas A, Krol W, Jaworska D,
Szymszal J and Szliszka E: Tumor necrosis factor-related
apoptosis-inducing ligand-induced apoptosis in prostate cancer
cells after treatment with xanthohumol-a natural compound present
in humulus lupulus L. Int J Mol Sci. 17:pii: E837. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wiley SR, Schooley K, Smolak PJ, Din WS,
Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA,
et al: Identification and characterization of a new member of the
TNF family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang S and El-Deiry WS: TRAIL and
apoptosis induction by TNF-family death receptors. Oncogene.
22:8628–8633. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kischkel FC, Lawrence DA, Chuntharapai A,
Schow P, Kim KJ and Ashkenazi A: Apo2L/TRAIL-dependent recruitment
of endogenous FADD and caspase-8 to death receptors 4 and 5.
Immunity. 12:611–620. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei RJ, Zhang XS and He DL:
Andrographolide sensitizes prostate cancer cells to TRAIL-induced
apoptosis. Asian J Androl. 20:200–204. 2018. View Article : Google Scholar :
|
|
10
|
Sivalingam KS, Paramasivan P, Weng CF and
Viswanadha VP: Neferine potentiates the antitumor effect of
cisplatin in human lung adenocarcinoma cells via a
mitochondria-mediated apoptosis pathway. J Cell Biochem.
118:2865–2876. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Poornima P, Quency RS and Padma VV:
Neferine induces reactive oxygen species mediated intrinsic pathway
of apoptosis in HepG2 cells. Food Chem. 136:659–667. 2013.
View Article : Google Scholar
|
|
12
|
Poornima P, Weng CF and Padma VV:
Neferine, an alkaloid from lotus seed embryo, inhibits human lung
cancer cell growth by MAPK activation and cell cycle arrest.
Biofactors. 40:121–131. 2014. View Article : Google Scholar
|
|
13
|
Zhang X, Liu Z, Xu B, Sun Z, Gong Y and
Shao C: Neferine, an alkaloid ingredient in lotus seed embryo,
inhibits proliferation of human osteosarcoma cells by promoting p38
MAPK-mediated p21 stabilization. Eur J Pharmacol. 677:47–54. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li T, Su L, Zhong N, Hao X, Zhong D,
Singhal S and Liu X: Salinomycin induces cell death with autophagy
through activation of endoplasmic reticulum stress in human cancer
cells. Autophagy. 9:1057–1068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu YP, Li L, Xu L, Dai EN and Chen WD:
Cantharidin suppresses cell growth and migration, and activates
autophagy in human non-small cell lung cancer cells. Oncol Lett.
15:6527–6532. 2018.PubMed/NCBI
|
|
17
|
White E: The role for autophagy in cancer.
J Clin Invest. 125:42–46. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Parzych KR and Klionsky DJ: An overview of
autophagy: morphology, mechanism, and regulation. Antioxid Redox
Signal. 20:460–473. 2014. View Article : Google Scholar :
|
|
19
|
Fan S, Qi M, Yu Y, Li L, Yao G, Tashiro S,
Onodera S and Ikejima T: P53 activation plays a crucial role in
silibinin induced ROS generation via PUMA and JNK. Free Radic Res.
46:310–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuntz S, Wenzel U and Daniel H:
Comparative analysis of the effects of flavonoids on proliferation,
cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J
Nutr. 38:133–142. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bonavida B, Ng C, Jazirehi A, Schiller G
and Mizutani Y: Selectivity of TRAIL-mediated apoptosis of cancer
cells and synergy with drugs: The trail to non-toxic cancer
therapeutics. Int J Oncol. 15:793–1595. 1999.PubMed/NCBI
|
|
22
|
Ji D, Zhang Z, Cheng L, Chang J, Wang S,
Zheng B, Zheng R, Sun Z, Wang C, Zhang Z, et al: The combination of
RAD001 and MK-2206 exerts synergistic cytotoxic effects against
PTEN mutant gastric cancer cells: Involvement of MAPK-dependent
autophagic, but not apoptotic cell death pathway. PLoS One.
9:e851162014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nazim UM, Moon JH, Lee JH, Lee YJ, Seol
JW, Eo SK, Lee JH and Park SY: Activation of autophagy flux by
metformin down-regulates cellular FLICE-like inhibitory protein and
enhances TRAIL-induced apoptosis. Oncotarget. 7:23468–23481. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Labsch S, Liu L, Bauer N, Zhang Y,
Aleksandrowicz E, Gladkich J, Schönsiegel F and Herr I:
Sulforaphane and TRAIL induce a synergistic elimination of advanced
prostate cancer stem-like cells. Int J Oncol. 44:1470–1480. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lagadec C, Adriaenssens E, Toillon R,
Chopin V, Romon R, Van Coppenolle F, Hondermarck H and Le Bourhis
X: Tamoxifen and TRAIL synergistically induce apoptosis in breast
cancer cells. Oncogene. 27:1472–1477. 2008. View Article : Google Scholar
|
|
26
|
Zhu H, Ding WJ, Wu R, Weng QJ, Lou JS, Jin
RJ, Lu W, Yang B and He QJ: Synergistic anti-cancer activity by the
combination of TRAIL/APO-2L and celastrol. Cancer Invest. 28:23–32.
2010. View Article : Google Scholar
|
|
27
|
Oh YT, Yue P, Wang D, Tong JS, Chen ZG,
Khuri FR and Sun SY: Suppression of death receptor 5 enhances
cancer cell invasion and metastasis through activation of
caspase-8/TRAF2-mediated signaling. Oncotarget. 6:41324–41338.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han B, Yao W, Oh YT, Tong JS, Li S, Deng
J, Yue P, Khuri FR and Sun SY: The novel proteasome inhibitor
carfilzomib activates and enhances extrinsic apoptosis involving
stabilization of death receptor 5. Oncotarget. 6:17532–17542. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
von Karstedt S, Montinaro A and Walczak H:
Exploring the TRAILs less travelled: TRAIL in cancer biology and
therapy. Nature reviews. Cancer. 17:352–366. 2017.
|
|
30
|
Selvarajoo K: A systems biology approach
to overcome TRAIL resistance in cancer treatment. Prog Biophys Mol
Biol. 128:142–154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Poornima P, Weng CF and Padma VV: Neferine
from Nelumbo nucifera induces autophagy through the inhibition of
PI3K/Akt/mTOR pathway and ROS hyper generation in A549 cells. Food
Chem. 141:3598–3605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Petersen A, Larsen KE, Behr GG, Romero N,
Przedborski S, Brundin P and Sulzer D: Expanded CAG repeats in exon
1 of the Huntington's disease gene stimulate dopamine-mediated
striatal neuron autophagy and degeneration. Hum Mol Genet.
10:1243–1254. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim SW, Lee JH, Moon JH, Nazim UM, Lee YJ,
Seol JW, Hur J, Eo SK, Lee JH and Park SY: Niacin alleviates
TRAIL-mediated colon cancer cell death via autophagy flux
activation. Oncotarget. 7:4356–4368. 2016.
|
|
35
|
Zhang Y, Wu Y, Cheng Y, Zhao Z, Tashiro S,
Onodera S and Ikejima T: Fas-mediated autophagy requires JNK
activation in HeLa cells. Biochem Biophys Res Commun.
377:1205–1210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stuckey DW and Shah K: TRAIL on trial:
Preclinical advances in cancer therapy. Trends Mol Med. 19:685–694.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu L, Zhang X, Li Y, Lu S, Lu S, Li J,
Wang Y, Tian X, Wei JJ, Shao C and Liu Z: Neferine induces
autophagy of human ovarian cancer cells via p38 MAPK/JNK
activation. Tumour Biol. 37:8721–8729. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Musumeci G, Castrogiovanni P, Trovato FM,
Weinberg AM, Al-Wasiyah MK, Alqahtani MH and Mobasheri A:
Biomarkers of chondrocyte apoptosis and autophagy in
osteoarthritis. Int J Mol Sci. 16:20560–20575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fiandalo MV and Kyprianou N: Caspase
control: Protagonists of cancer cell apoptosis. Exp Oncol.
34:165–175. 2012.PubMed/NCBI
|
|
40
|
Huang K: Mechanism of Bax/Bak Activation
in Apoptotic Signaling (unpublished PhD thesis). University of
Nebraska Medical Center; 2019
|
|
41
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tounekti O, Belehradek J Jr and Mir L:
Relationships between DNA fragmentation, chromatin condensation,
and changes in flow cytometry profiles detected during apoptosis.
Exp Cell Res. 217:506–516. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoshii SR and Mizushima N: Monitoring and
measuring autophagy. Int J Mol Sci. 18:18652017. View Article : Google Scholar :
|
|
44
|
Tanida I, Ueno T and Kominami E: LC3 and
autophagy. Methods Mol Biol. 445:77–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ganesher A, Chaturvedi P, Sahai R, Meena
S, Mitra K, Datta D and Panda G: New spisulosine derivative
promotes robust autophagic response to cancer cells. Eur J Med
Chem. 188:1120112020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Han X, Guo L, Jiang X, Wang Y, Wang Z and
Li D: Curcumin inhibits cell viability by inducing apoptosis and
autophagy in human colon cancer cells. Proceed Anticancer Res.
3:21–25. 2019.
|
|
47
|
Chiou JT, Huang CH, Lee YC, Wang LJ, Shi
YJ, Chen YJ and Chang LS: Compound C induces autophagy and
apoptosis in parental and hydroquinone-selected malignant leukemia
cells through the ROS/p38 MAPK/AMPK/TET2/FOXP3 axis. Cell Biol
Toxicol. Jan 3–2020.Epub ahead of prin. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Opipari AW Jr, Tan L, Boitano AE, Sorenson
DR, Aurora A and Liu JR: Resveratrol-induced autophagocytosis in
ovarian cancer cells. Cancer Res. 64:696–703. 2004. View Article : Google Scholar : PubMed/NCBI
|