Introduction
Breast cancer is the most common malignant tumor
affecting women worldwide and its incidence is increasing. However,
the overall mortality rate has been reduced by early diagnostic
programs and optimized treatment (1). Radiation has been adopted as a
standard therapy for breast cancer and external beam radiotherapy
is generally used for chest wall and total breast irradiation
(2). However, breast cancer is a
heterogeneous disease and differs greatly among different patients,
and even within each individual tumor (3); thus, radiotherapy sometimes results
in unsatisfactory effects in local and regional control for certain
subtypes of breast cancer and treatment failure seems to be due to
radiation resistance (4).
Radiation is not tumor-specific but can directly
kill cancer cells and has thus been used in the treatment of cancer
patients (5). However, major
drawbacks of radiation therapy are the undesirable effects on
normal tissue and the radiation resistance of cancer cells. In
particular, radiation can induce lymphocyte damage as conventional
radiation fields frequently include hematopoietic bone marrow or
large blood volumes. Derangements in lymphocyte function creates an
immunosuppressive condition in cancer patients, as radiation
increases transforming growth factor (TGF)-β secretion and the
infiltration of regulatory T cells (Tregs) into the tumor
microenvironment (6,7). These results suggest that radiation
may perversely, generate an anticancer immunity that promotes tumor
recurrence.
The status of a host's immune response affects both
the development and progression of cancer. Cancer cells often
acquire immunotolerance during malignancy and contribute to the
infiltration of immunosuppressive cells in the tumor
micro-environment (8,9). Tumor-associated macrophages (TAMs)
and Tregs are both well-known immunosuppressive cells in the tumor
microenvironment and these cells inhibit the development of an
efficient antitumor response by secretion of interleukin (IL)-10 or
TGF-β (10,11). Previous studies have reported that
infiltrating TAMs and Tregs are directly related to the progression
of breast cancer (12,13) and TAM or Treg depletion increases
effector T cell activation (14,15).
Cancer cells can release molecules that induce TAM differentiation
into immunosuppressive forms, such as the M2 type, or recruit Tregs
in the tumor microenvironment (10,11,16).
Furthermore, ionizing radiation induces the expression of
immunosuppressive cytokines, such as TGF-β and IL-10, and is
associated with the immunotolerance of cancer cells (17,18).
Therefore, there is a critical need for the development of novel
approaches that can be used clinically to overcome immune
suppression within the tumor microenvironment and to enhance the
radiation sensitivity of tumor cells.
Resveratrol is found in red wine and is contained in
various food components that have known biological activities
(19). A number of studies have
indicated that resveratrol suppresses the initiation, promotion and
progression of a variety of solid tumors (20-22),
and increases immune responses in mice by enhancing lymphocyte
proliferation and suppressing the population of Tregs (23). It has also been shown to enhance
radiation-induced cell death in various cancer cell lines, as well
as the radiation sensitivity of tumor cells (24). However, resveratrol has several
disadvantages, including its photosensitivity, its metabolic
instability and the high doses required for biological activity.
Therefore, in a previous study, the authors synthesized new
derivatives, including HS-1793, that overcomes some of the
drawbacks associated with resveratrol (25). Previous studies by the authors
demonstrated that HS-1793 exerted anticancer effects on various
cancer cell lines (24-26), enhanced the radiosensitivity of
FM3A cells under hypoxic conditions (27) and exhibited radioprotective
activity in CHO cells (28). In
particular, HS-1793 has been shown to enhance antitumor immunity by
reducing the number of Tregs and M-2 phenotype TAMs, in mammary
tumors of mice (26,29).
The present study demonstrates that HS-1793
increases lymphocyte proliferation and inhibits the DNA damage of
lymphocytes in irradiated tumor-bearing mice. In addition, is shown
to HS-1793 inhibit the infiltration of Tregs and TAMs, and to
decrease the secretion of immunosuppressive cytokines, such as
IL-10 and TGF-β. Thus, HS-1793 may increase the effectiveness of
radiation through the enhancement of antitumor immunity in a murine
breast tumor model. These results may be useful for the
understanding of the pharmacological action of HS-1793 in designing
a combination therapy utilizing both pharmacology and
radiation.
Materials and methods
Preparation of the resveratrol analogue,
HS-1793
To obtain HS-1793, the stilbene double bond present
in resveratrol was substituted with a naphthalene ring as described
in a previous study (25). The
structure of HS-1793 is illustrated in Fig. 1A. A stock solution was made in
absolute ethanol at 125 g/l, and working dilutions were made
directly in saline. The control vehicle was saline containing
equivalent amounts of ethanol.
Cells and cell culture
The FM3A murine breast cancer cell line originating
from the mammary gland of the C3H/He mouse was provided by
Professor C.D. Kang, Pusan National University College of Medicine.
The cells were cultured in RPMI-1640 (Welgene) supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 U/ml streptomycin (complete medium) under
humidified conditions at 37°C and 5% CO2 in an
incubator.
In vitro cell growth assay
FM3A cells (5×103 cells per well) were
seeded in 96-well plates in RPMI-1640 supplemented with 10% FBS.
The cells were exposed to HS-1793 treatment (0-10 µg/ml) or
irradiation (0-16 Gy) with a BioBeam 8000 (Gamma-Service Medical
GmbH) irradiator at a dose rate of 1.88 Gy/min and were cultured
for 24 or 48 h. In addition, some cells were treated with 0.25,
0.5, or 1 µg/ml of HS-1793 for 24 h prior to 4 Gy
irradiation. To determine cell growth,
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)
was added to each well (25 µl/well) after 24 or 48 h and
incubated at 37°C for 4 h. The blue dye absorbed by the cells was
dissolved in dimethyl sulfoxide (100 µl/well), and the
absorbance was measured with a spectrophotometer (Bio-Rad
Laboratories, Inc.) at 490 nm. The IC50 values were
calculated using GraphPad Prism (Ver. 8; GraphPad Software
Inc).
Clonogenic survival assay
The cell survival assay is based on the
clonogenicity of cells to divide indefinitely and form colonies
(28,30). In brief, the FM3A cells were
trypsinized, counted and plated in triplicate per data point into
100 mm dishes. The cells were treated with HS-1793 (0.25, 0.5, 1
µg/ml) and incubated at 37°C for 24 h prior to irradiation
(2, 4, 6, 8 and 10 Gy). After approximately 14 days, the colonies
were fixed with 95% methanol at room temperature for 30 min,
stained with 1% crystal violet for 10 min and counted. The plating
efficiency (PE) was based on a previously described method
(28).
Animal experiments
The animal experiments were ethically approved by
the Committee on the Use and Care of Animals of Dong-A University
Hospital (DIACUC-10-14) and the Dong Nam Institute of Radiological
and Medical Sciences (DIRAMS AEC-2011-003). Female C3H/He mice, 6
weeks of age (20-25 g), were obtained from Central Lab. Animal Inc.
Mice were housed under standard laboratory conditions (22±2°C, 60%
relative humidity, 12/12 h light/dark cycle, and provided with food
and water ad libitum), and animal health and behavior were
daily monitored. A total of 75 mice were used in 15 experimental
groups with 5 mice per group; 4 groups to determine the radiation
dose, 6 groups to analyze radiation-induced DNA damage in
lymphocytes, and 5 groups to measure tumor growth and lymphocyte
function. FM3A cells (2×106 cells/50 µl) were
inoculated subcutaneously into the right flanks of female C3H/He
mice to induce tumors. When the tumors grew to a size of
approximately 80-125 mm3, the mice were irradiated with
a linear accelerator (Elekta Ltd.) at a dose rate of 5 Gy/min. In
the case of HS-1793 treatment (0.5 or l mg/kg), the mice were
intraperitoneally injected 24 h prior to irradiation and twice a
week for 3 weeks after irradiation. The tumor growth of 70
tumor-bearing mice was observed for 30 days after the first
treatment and the mice were euthanized by using the CO2
method (20% of the chamber volume was displaced per min by the flow
rate of CO2). Tumor volume was calculated using the
following equation: Volume=Width2 × length × 0.52.
Additional humane endpoints included a tumor burden that exceeded
20 mm in any one dimension; tumors that became ulcerated, necrotic
or infected; and tumors that interfered with eating or impaired
ambulation. The volume of the largest tumor was 3,935
mm3 and the largest diameter of a single tumor was 19.8
mm. None of the animals developed multiple tumors and none were
found dead. After sacrificing the tumor-bearing mice, the
splenocytes were prepared from an aseptically removed spleen, and
paraffin-embedded sections (4-µm-thick) were prepared from
tumor tissue.
Lymphocyte proliferation assay
Lymphocyte proliferation was determined by
5-bromo-2-deoxyuridine (BrdU) incorporation assay using a cell
suspension at 5×105 cells/well in flat-bottom 96-well
microculture plates. The isolated lymphocytes from tumor-bearing
mice were cultured for 48 h with concanavalin A (Con A; 5
µg/m; Sigma-Aldrich; Merck KGaA) and further incubated for
24 h in the presence of 10 µl of the BrdU solution in RPMI
medium (1:100 diluent). BrdU incorporation was measured using a
Cell Proliferation ELISA BrdU kit (Roche Diagnosis GmbH) following
the supplier's specifications.
Comet assay
The mouse spleens were aseptically removed, and a
single-cell suspension was prepared by gently teasing the cells
through a sterile stainless-steel screen. After removing red blood
cells, the splenocytes were suspended in PBS. The dye exclusion
test was used to determine the number of viable cells present in a
cell suspension. Equal parts of 0.4% Trypan blue dye and the cell
suspension was mixed, loaded to a hemocytometer, and examined
immediately under a optical microscope (Olympus Corp.). The viable
cell density was adjusted to 1-2×105/ml and stored at
4°C. The lymphocytes were mixed with low melting point agarose at
37°C. The comet assay was performed as previously described
(28). In brief, this mixture was
placed on top of a layer of 0.5% normal melting point agarose on a
slide covered with a coverslip, and then incubated at 4°C until
solid. The slide was placed in chilled lysis buffer (100 mM EDTA,
2.5 M sodium chloride, 10 mM Trizma base and 1%
N-lauroylsarcosinate, adjusted to pH 10.0, with 1% Triton X-100)
and unwinding buffer (1 mM EDTA and 300 mM sodium hydroxide,
pH>13), respectively, and subjected to electrophoresis. All
slides were gently washed with 0.4 M Tris buffer, stained with Gel
green DNA dye (Biotium, Inc.) and analyzed under a fluorescence
microscope (Carl Zeiss Microscopy GmbH). The images were captured,
and a minimum of 100 comets per slide, in triplicate for a group,
were analyzed using Metafer 4 software (Carl Zeiss GmbH), which
yields % DNA in the tail, tail length, tail moment (TM) and olive
moment (OM) directly. The parameter TM is the product of tail
length and % DNA in the tail, and the olive moment is the product
of the distance between the center of the head and the center of
the tail and % DNA in the tail (30).
Flow cytometric analysis
Lymphocyte subpopulations among splenocytes were
analyzed with a flow cytometer (Beckman Coulter, Inc.) following
standard surface staining procedures using the appropriately
diluted antibodies as follows: PE-conjugated anti-mouse CD4 (1:100,
cat. no. 553730, BD Biosciences), FITC-conjugated anti-mouse CD8a
(1:100, cat. no. 553031, BD Biosciences), PE/Cy7-conjugated
anti-mouse CD25 (1:100, cat. no. 60-0251, Tonbo Biosciences) and
isotype control antibodies (1:100, cat. no. 553989, cat. no.
553929, BD Biosciences; cat. no. 60-430, cat. no. 35-4714, Tonbo
Biosciences) (the cells were incubated with the antibodies at 4°C
for 30 min). For the confirmation of FoxP3-expressing Tregs and
interferon (IFN)-γ-expressing CD8+ T cells, standard
surface staining procedures were combined with an intracellular
staining method using FITC-conjugated anti-mouse FoxP3 (1:100, cat.
no. 35-5773, Tonbo Biosciences) and PE/Cy7-conjugated anti-mouse
IFN-γ (1:100, cat. no. 561040, BD Biosciences) antibodies,
respectively. For the intracellular FoxP3 and IFN-γ titration, the
cells were pre-incubated with Protein Transport Inhibitor
containing Monensin (BD Biosciences) at 37°C for 6 h prior to
harvesting the cells. Quantification was performed by FACS analysis
using a Beckman Coulter FC500 (Beckman Coulter, Inc.).
Enzyme-linked immune absorbent spot
(ELISpot) assay
ImmunoSpot plates (Merck Millipore) were washed with
35% ethanol at room temperature for 1 min, coated with anti-mouse
IFN-γ antibody (1:100, cat. no. 551881, BD Biosciences) dissolved
in PBS at 4°C overnight, and blocked with BSA (10 g/l in PBS) for 1
h. Splenocytes (5×104 cells/well) from each mouse were
seeded and incubated with stimulant cocktail and HS-1793 at 37°C
for 24 h. The plate was treated with biotinylated detection
antibody at room temperature for 2 h and streptavidin-HRP (100
µl/well) at room temperature for 2 h. After washing the
plate, a chromogenic substrate and H2O2 were
added to each well to produce spots. The numbers of spots were
analyzed using the AID ELISpot Reader System (Autoimmun Diagnostika
GmbH).
Cytotoxicity assay
Splenocytes (3×107 cells/5 ml) were
re-stimulated with mitomycin C (10 µg/ml) for 20 min and
washed with PBS. The re-stimulated splenocytes (effector cells)
were incubated with target cells (2×104 FM3A cells) at
various effector/target ratios (10:1, 25:1 and 50:1) in 96-well
round-bottom microplates (200 µl) for 3 days. Following
centrifugation, at room temperature for 10 min, 100 µl of
supernatants were collected, and the lactate dehydrogenase (LDH)
released from the target cells was measured with an LDH release
assaying kit (Roche Applied Science) according to the
manufacturer's instructions. Percentage cytotoxicity was calculated
by the following formula: % Cytotoxicity=
(ODexperiment-ODeffector
spontaneous-ODtarget spontaneous)/ODtarget
maximum- ODtarget spontaneous ×100.
The ODspontaneous amount of effector and
target cells was controlled by separate incubations of the
respective populations. ODmaximum was measured after
lysis of the target cells with 2% Triton X-100.
Histopathological analysis
Tumor-infiltrating TAMs and Tregs were evaluated by
immunofluorescent staining of deparaffinized sections
(5-µm-thick) and the tissue sections were incubated at 4°C
with PE-conjugated anti-mouse CD25 (1:100, cat. no. 553866; BD
Biosciences) or CD206 (1:100, cat. no. 141706; BioLegend),
FITC-conjugated anti-mouse IFN-γ antibodies (1:100, cat. no.
ab21039; Abcam) for over-night. Counterstaining was performed with
VECTASHIELD Antifade Mounting Medium with DAPI (Vector
Laboratories). The slides were observed and photographed on a Carl
Zeiss LSM 700 confocal microscope and processed by ZEN 2009 (Carl
Zeiss Microscopy GmbH).
Cytokine production assay
Splenocytes were cultured with Con A (5
µg/ml) for 24 h at 107 cells/ml in serum-free
RPMI medium containing 200 µg/ml BSA. The IL-4, IL-10, TGF-β
and IFN-γ concentrations in the culture supernatants of the
splenocytes were determined with ELISA kits (BD Biosciences)
according to the manufacturer's instructions.
Statistical analysis
Results are expressed as the means ± standard
deviation (SD). Statistical significance was tested using the
Statistical Package for the Social Sciences statistical software
for Windows, Ver. 18.0 (SPSS Inc.). Significant differences were
evaluated by one-way analysis of variance for multiple comparisons.
Tukey's post hoc test was used to compare several groups with
different controls, and Dunnett's post hoc test was used to compare
all other samples with a single control. P<0.05 was considered
to indicate a statistically significant difference.
Results
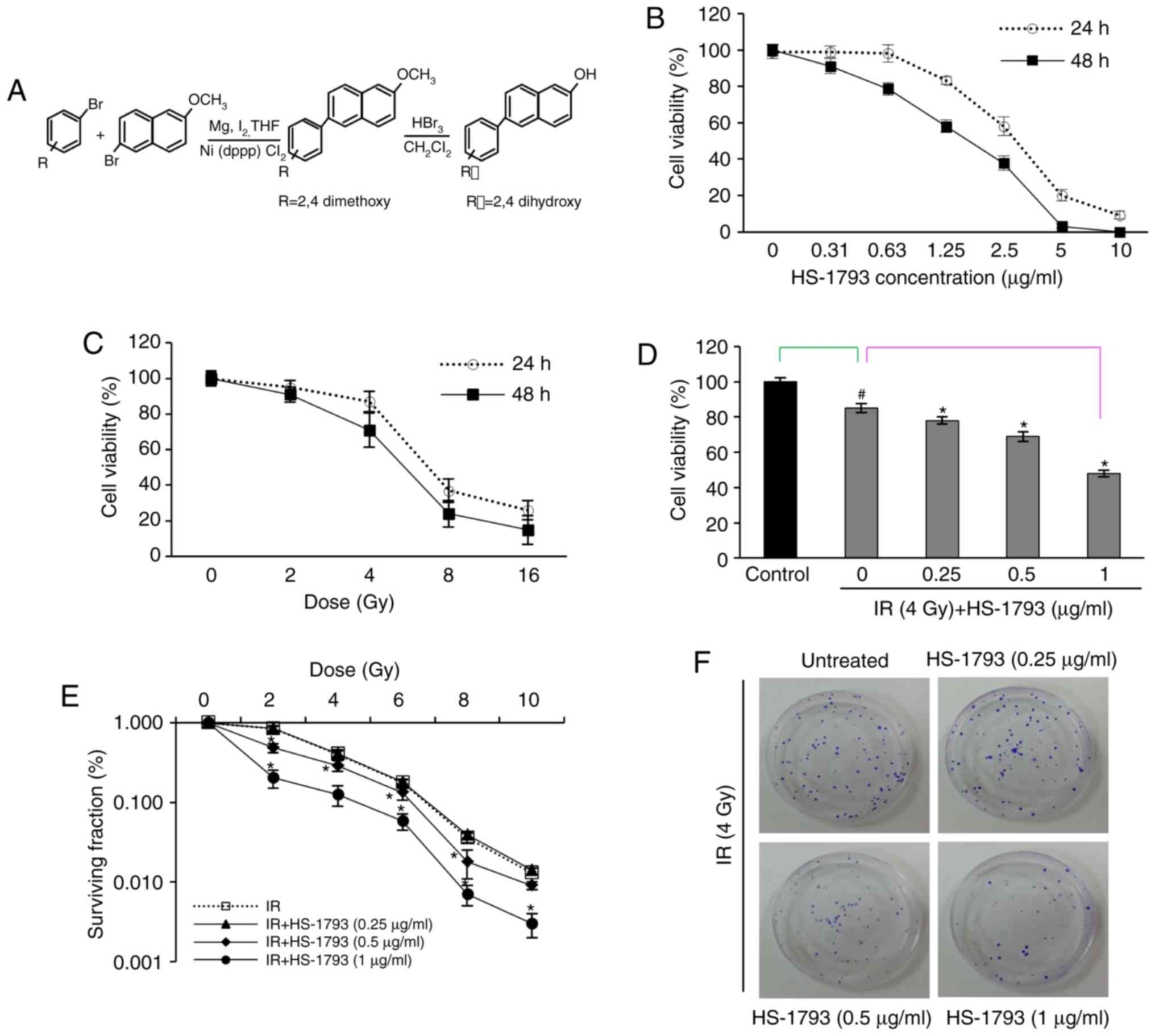
HS-1793 enhances the radiation-induced
death pf FM3A cells
The effect of HS-1793 on radiation-induced death of
murine breast cancer cells was investigated and the viability of
FM3A cells was measured by MTT assay at various HS-1793
concentrations and/or radiation doses. HS-1793 inhibited cell
growth in a dose-dependent manner and the IC50 value was
1.9 µg/ml at 48 h (Fig.
1B). The survival curve of gamma-radiation was generated for
the FM3A cells (Fig. 1C) and a
gamma-radiation dose of 4 Gy, which yielded 29% growth inhibition,
was selected for use in combination with HS-1793. To determine
whether HS-1793 can enhance the sensitivity of the FM3A cells to
radiation-induced cell death, the cells were plated for MTT assay
and clonogenic cell survival assay. As shown Fig. 1D, HS-1793 treatment exerted a
significant growth inhibitory effect on the 4 Gy-exposed FM3A cells
in a dose-dependent manner. In addition, the colony formation
assays with HS-1793 reduced the plating efficiency of the FM3A
cells and sensitized the FM3A cells to radiation treatment at 0.5
and 1 µg/ml (Fig. 1E and
F). These results suggest that HS-1793 can enhance radiation
induced cell death of murine breast cancer cells.
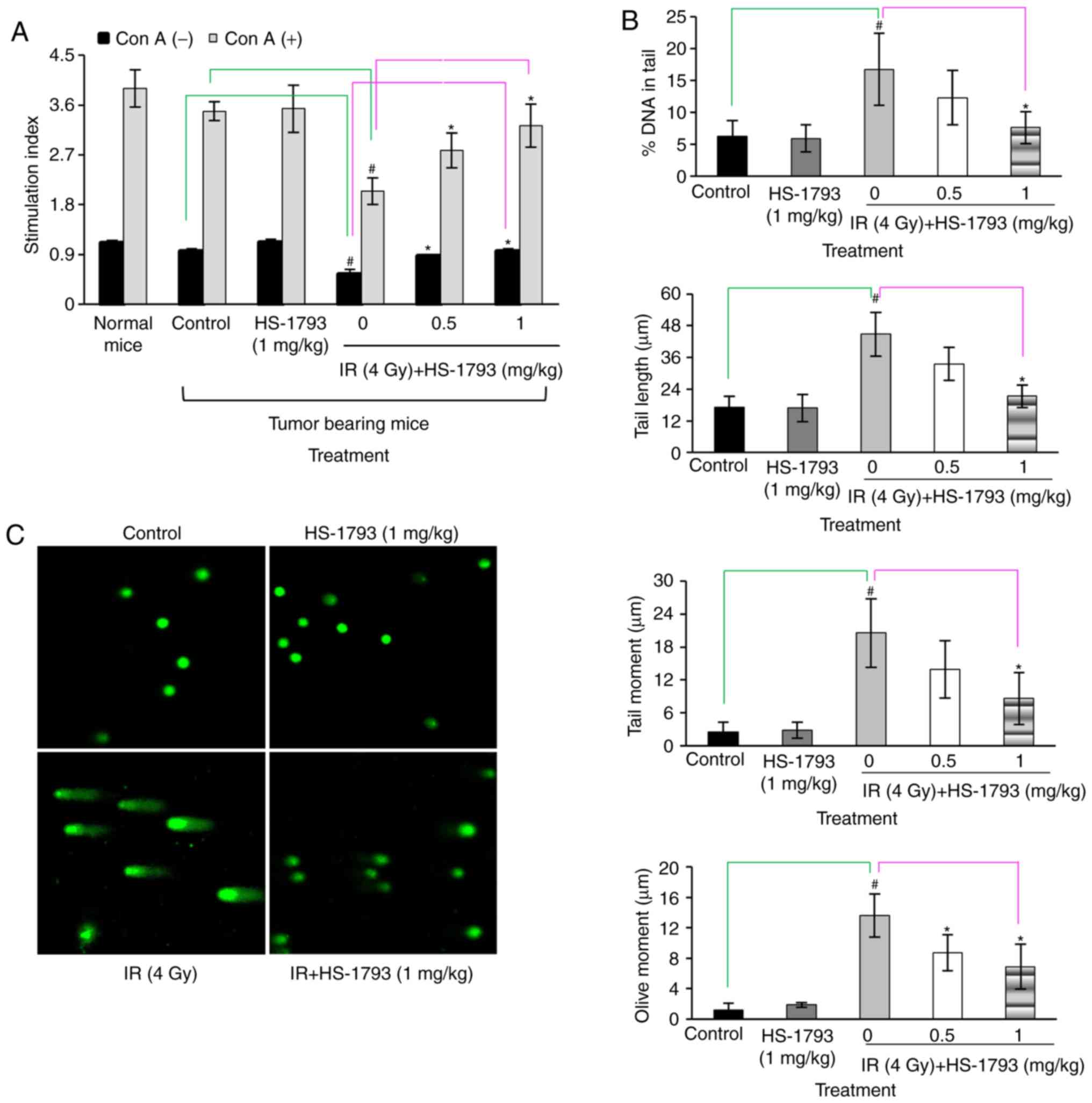
HS-1793 induces lymphocyte proliferation
by decreasing radiation-induced DNA damage in irradiated
tumor-bearing mice
Lymphocytes are known to be very radiosensitive as
radiation causes DNA cross-linkage and cells undergo cell death
rapidly, with some cells being affected within 24 h of irradiation
(31). A previous study by the
authors demonstrated that HS-1793 induced radioprotective effects
through free radical scavenging and that this activity inhibited
cellular DNA damage (28). The
present study therefore examined whether a similar pattern of
results could be obtained with the FM3A cell line, which can be
used for animal experiments. A gamma-radiation dose of 4 Gy,
exhibited an 18% growth inhibition, and was selected as the dose
for use in combination with HS-1793 (Fig. 2B). The protective effects of
HS-1793 on lymphocyte proliferation in the 4 Gy-irradiated
tumor-bearing mice were then investigated. The results revealed
that HS-1793 did not directly induce lymphocyte proliferation in
tumor-bearing mice with or without Con A stimulation. Gamma
radiation induced a reduction in lymphocyte proliferation, whereas
combination treatment with HS-1793 prevented the decrease in
radiation-induced lymphocyte proliferation in the presence or
absence of Con A in tumor-bearing mice (Fig. 3A). Subsequently, whether HS-1793
treatment inhibits radiation-induced DNA damage in lymphocytes of
tumor-bearing mice was examined by comet assays to measure DNA
breakage. The lymphocytes were isolated from the spleens of
tumor-bearing mice following irradiation at 24 h and the DNA damage
of lymphocytes was analyzed by comet assay. No significant
differences were observed between the control and HS-1793 alone
groups, whereas the 4 Gy-irradiated lymphocytes exhibited an
increased DNA damage in comet parameters, such as % DNA in tail,
tail length, tail moment and olive moment. However, HS-1793
treatment inhibited the radiation-induced DNA damage of lymphocytes
from the tumor-bearing mice (Fig. 3B
and C). These data suggest that HS-1793 treatment increases
lymphocyte proliferation by inhibiting DNA damage.
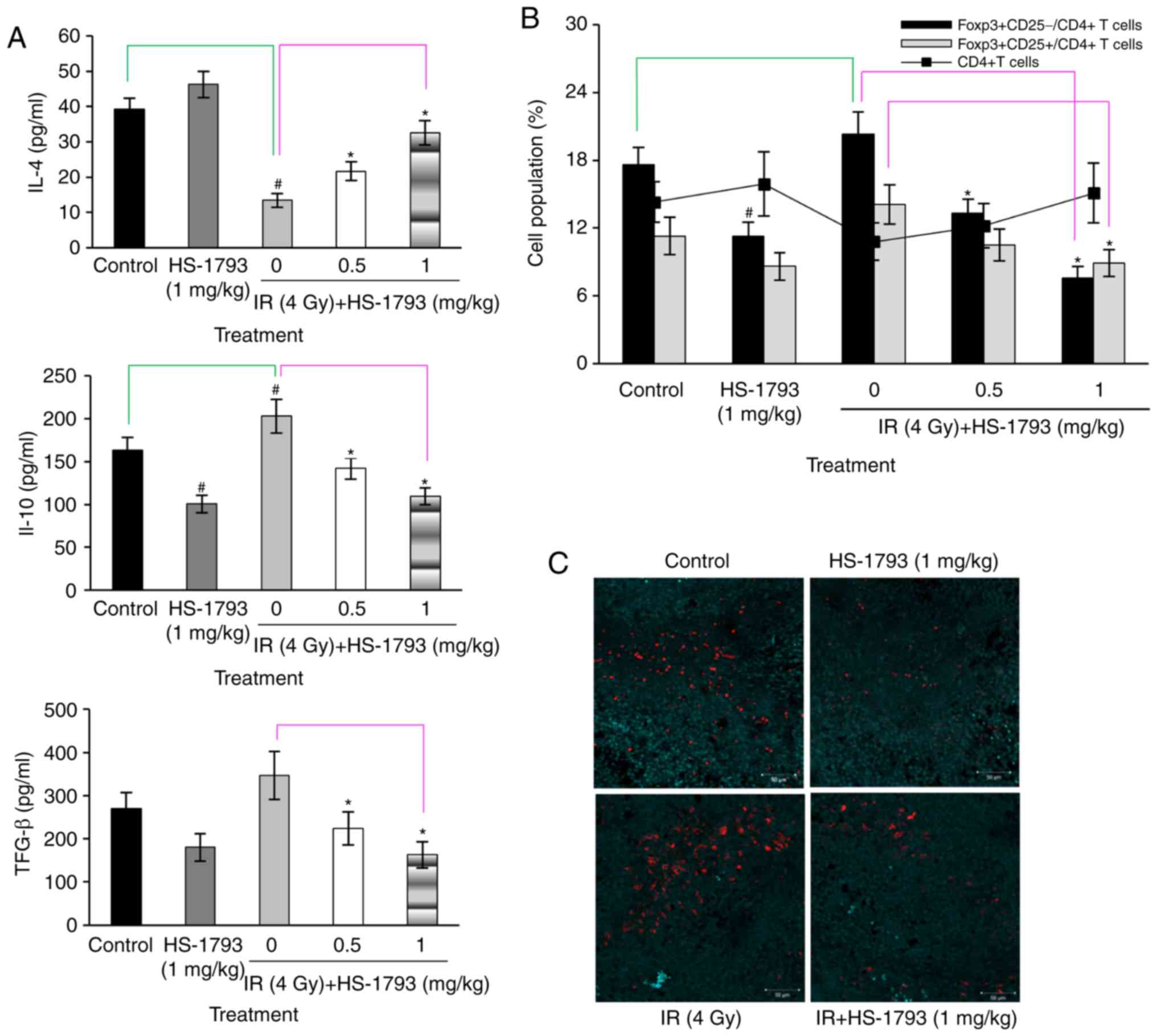
HS-1793 inhibits the induction of Tregs
in irradiated tumor-bearing mice
To examine whether HS-1793 can modulate the
production of IL-4 for T cell development and immune suppressive
cytokines, such as IL-10 and TGF-β by radiation treatment, the
levels of cytokines in the cultured supernatants of Con
A-stimulated lymphocytes were measured. As shown Fig. 4A, HS-1793 treatment increased IL-4
production; however, this increase was reduced by radiation
treatment in the tumor-bearing mice. The administration of HS-1793
significantly enhanced IL-4 secretion in the irradiated
tumor-bearing mice. IL-10 and TGF-β are important factors involved
in Treg induction (11,32). Therefore, whether HS-1793 modulates
the induction of Tregs by the regulation of IL-10 and TGF-β
production in irradiated tumor-bearing mice was investigated.
HS-1793 treatment inhibited the secretion of IL-10 and TGF-β in
both the control and irradiated tumor-bearing mice, whereas
irradiation efficiently increased the secretion of both IL-10 and
TGF-β in the tumor-bearing mice not exposed to the compound
(Fig. 4A). It is possible that
radiation induced Tregs, resulting in the enhanced production of
IL-10 and TGF-β. The proportion of Treg markers
(FoxP3+CD25+) among CD4+ T cells
was determined by flow cytometric analysis in all mice. The cell
ratio of FoxP3+CD25+ to CD4+ cells
exhibited some differences between the control and irradiated
tumor-bearing mice groups, and irradiation increased the proportion
of Tregs in the tumor-bearing mice. By contrast, HS-1793 treatment
significantly decreased the population of
FoxP3+CD25+ cells among CD4+ cells
in irradiated tumor-bearing mice (Fig.
4B). This confirmed that HS-1793 treatment reduced the
infiltration of Tregs in tumor tissue following irradiation
(Fig. 4C).
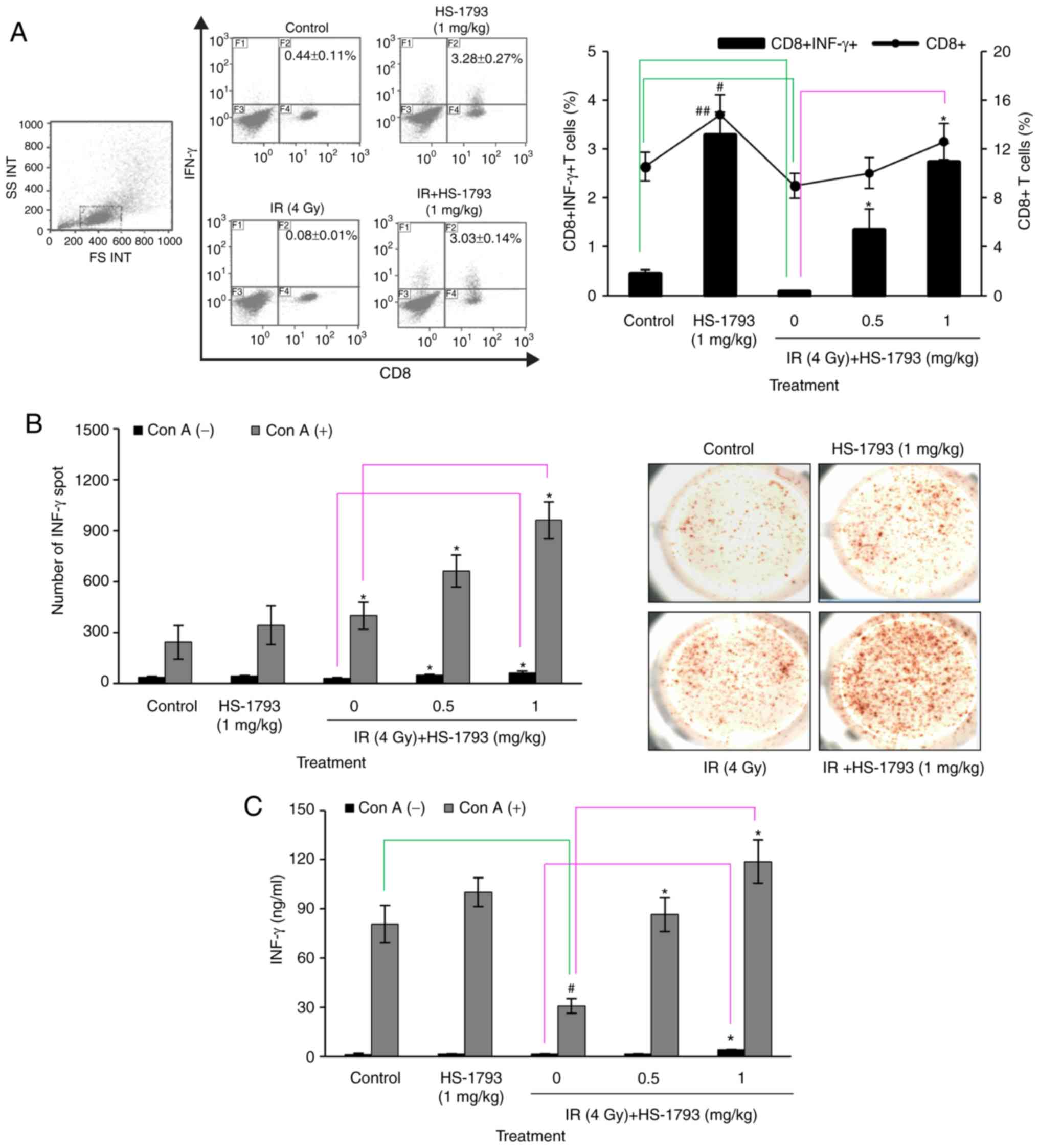
HS-1793 increases the amount of
IFN-γ-expressing CD8+ T cells in irradiated
tumor-bearing mice
The authors have previously demonstrated that
HS-1793 significantly increased IFN-γ secretion in the cultured
supernatants of splenocytes and tumor tissue of tumor-bearing mice
(26). Therefore, the present
study investigated whether HS-1793 treatment can enhance effector T
cells involved in the antitumor immunity of irradiated
tumor-bearing mice. The frequency of CD8+ T cells was
reduced in the irradiated tumor-bearing mice, but was prominently
recovered in tne HS-1793-treated mice in comparison with the
control tumor-bearing mice (Fig.
5A). In addition, the results from assays of IFN-γ secretion
evaluated by ELISpot and ELISA were consistent with the frequency
of CD8+ T cells observed (Fig. 5B and C).
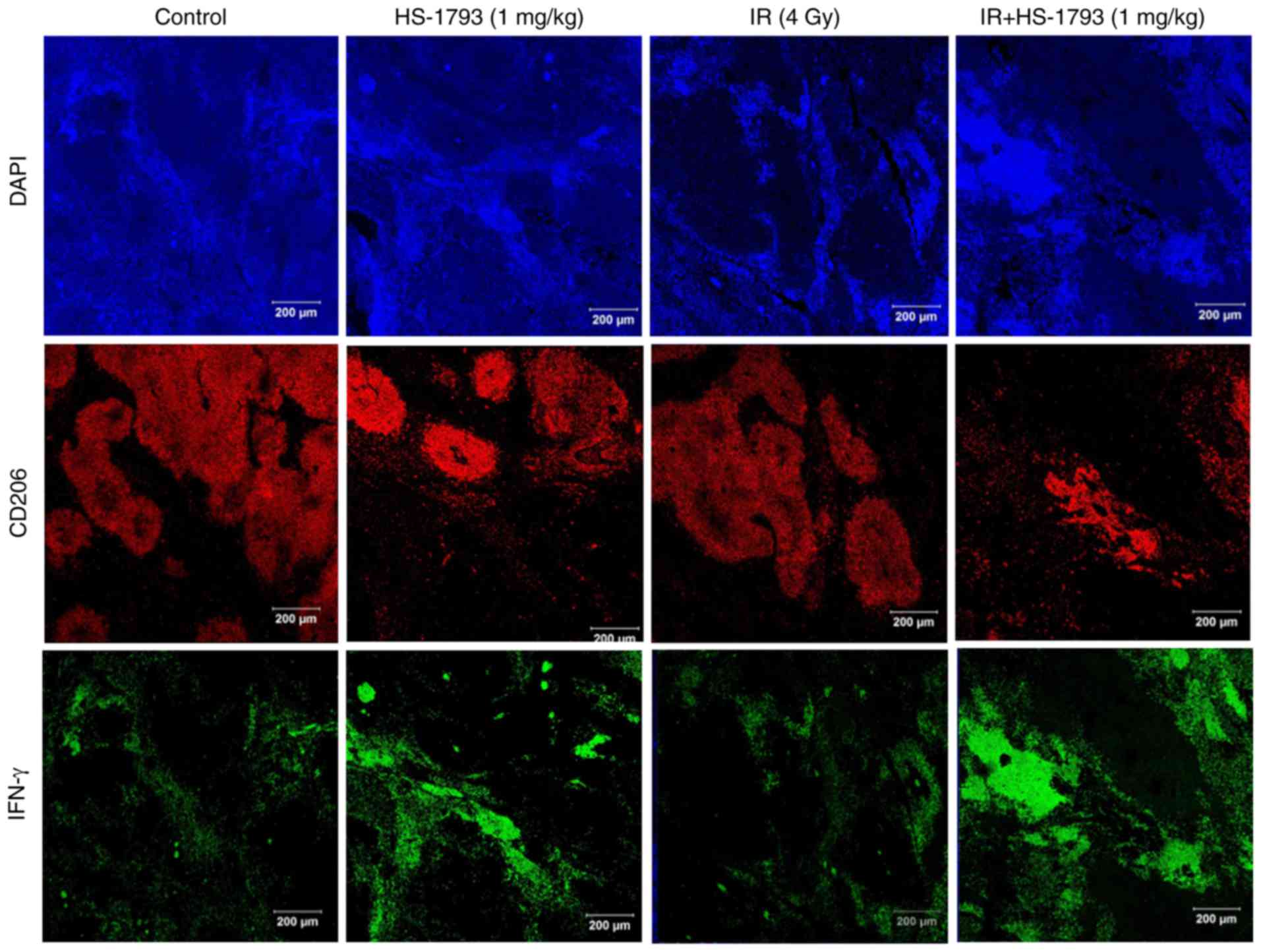
HS-1793 inhibits the infiltration of TAMs
in tumor tissue of irradiated tumor-bearing mice via upregulation
of IFN-γ
It has been previously reported that HS-1793
treatment increases IFN-γ expression and suppresses TAM
infiltration in tumor tissues compared to control mice (29). Therefore, the present study whether
the HS-1793-induced IFN-γ secretion inhibited TAM infiltration in
tumor tissue following radiation treatment. The distribution of
CD206+ TAMs in irradiated tumor tissue was similar
compared to that of the control tumor tissue. By contrast,
treatment with HS-1793 suppressed the infiltration of
CD206+ TAMs both in the control tumor-bearing mice and
in the irradiated tumor-bearing mice. These results are consistent
with the IFN-γ expression observed in the tumor tissue (Fig. 6). Taken together, these results
suggest that HS-1793 may overcome TAM-induced immunosuppression by
modulating IFN-γ secretion, although radiation elicits a number of
changes in the tumor microenvironment that are unfavorable for the
induction of antitumor immunity.
HS-1793 significantly enhances the effect
of radiotherapy on tumor growth via the induction of antitumor
immunity
Although the HS-1793 injections did not completely
remove the established tumors and protect against tumor formation,
HS-1793 did reduce tumor growth and attenuated tumor formation in a
previous study by the authors (26). Therefore, in the present study,
whether HS-1793 and radiation can enhance the antitumor effects in
tumor-bearing mice compared to radiation alone was investigated.
Cytotoxicity assays were performed for the tumor-specific lysis
activity of effector T cells following combination treatment. As
shown in Fig. 7A, HS-1793 induced
an increase in cytotoxicity compared with the control or
irradiation alone at all E:T ratios, and also enhanced the
cytotoxicity of target FM3A cells in irradiated tumor-bearing mice.
Furthermore, the therapeutic efficacy of HS-1793 in reducing tumor
size following radiotherapy was observed. The C3H/He mice were
intraperitoneally injected with HS-1793 (0.5 and 1 mg/kg) twice a
week for 3 weeks in the control or irradiated tumor-bearing mice,
for the evaluation of the therapeutic antitumor effects (Fig. 2A). Irradiation inhibited the tumor
growth of the FM3A cells in a dose-dependent manner and the dose
for treatment was 4 Gy, which exerted a 23% inhibition (Fig. 7B). In addition, treatment with
HS-1793 (1 mg/kg) alone produced a 44% inhibition of tumor growth
in FM3A cell allografts, and combination treatment with HS-1793 and
4 Gy exerted a significant inhibitory effect on tumor growth
(Fig. 7B and C). In particular,
HS-1793 (1 mg/kg) treatment exerted an 89% inhibition of tumor
growth in the irradiated tumor bearing mice. These results
demonstrate that combining HS-1793 with radiotherapy is effective
at inducing antitumor immunity.
Discussion
An important goal of novel agents acting on novel
targets in cancer treatment is the induction of apoptosis or the
debilitation of cancer cells without excessive damage to normal
cells. Interest in bioactive compounds on cancer treatments and
prevention has steadily increased over the past two decades and the
majority of these compounds that exhibit chemopreventive activity
have been identified in vegetables, fruits, barks, leaves, spices
and grains (33). Naturally
occurring bioactive compounds can potentially be useful in
complementary therapy for breast cancer patients (34). Recently, the combination of natural
products with either chemotherapy or radiotherapy has been shown to
improve outcomes for breast cancer and it is a useful strategy for
patients with late-stage breast cancer who cannot be treated with
surgery (35,36). Radiotherapy has often been
associated with the recurrence or metastasis of cancer as radiation
induces the resistance of tumor cells and in addition, damages
healthy cells (28,37). Thus, a number of combination
therapies have been developed to overcome radiation-induced
resistance, and a large number of clinical trials investigating
novel drugs derived from natural products and radiotherapy
combinations have been performed (38,39).
HS-1793 is a resveratrol derivative and several
studies have demonstrate that it has more potent anti cancer
activity than its parent compound. It is also not any highly
cytotoxic than resveratrol (25).
Evidence from in vitro and in vivo studies has
indicated that the antitumor effects of HS-1793 are mediated
through apoptosis in a number of different cancer cell lines
(25,26,40)
and through the induction of antitumor immune responses by
modifying the balance between effector T cells and immune
suppressive cells in a mouse breast tumor model (26,29).
In addition, in a previous study by the authors, it was
demonstrated that HS-1793 enhanced the radiosensitivity of mouse
breast cancer cells under hypoxic conditions (27). In the present study, the results
demonstrated that HS-1793 not only enhanced the radiation-induced
death of FM3A cells, but also inhibited tumor growth in FM3A cell
allografts when compared to radiation or treatment with the
compound alone. Thus, these data suggest that HS-1793 may be a
potential candidate for use in combination with radiotherapy.
The exposure of cells to ionizing radiation results
in a range of DNA damage, including strand breaks, base damage and
crosslinking, which in turn induces apoptosis in
radiation-sensitive tissues, including lymphocytes (41,42).
The reduction in the number of lymphocytes is associated with
immunosuppressive functions in cancer patients treated with
radiotherapy. The induction of a systemic antitumor immune response
involves the coordinated participation of several different T
lymphocytes. In particular, cytotoxic CD8+ T lymphocytes
are usually responsible for the direct killing of tumor cells and
the CD4+ T lymphocyte subpopulation is also required for
the induction of an efficient antitumor immune response (43,44).
A small fraction of CD4+ cells known as Tregs, express
CD25 and Foxp3 on the cell surface, and are considered to be
suppressive elements of the immune system. They have been shown to
be involved directly or indirectly in tumor progression by
modulating certain constituents of the tumor microenvironment
(11,45). Ionizing radiation is associated
with immunosuppressive functions as Tregs are more resistant to
radiation than other lymphocytes (46,47).
A previous study demonstrated that HS-1793 exerted radio-protective
effects through the inhibition of cellular DNA damage (28). In this light, it is critical to
investigate whether HS-1793 can provide protection against the
radiation-induced damage of lymphocytes and overall immune
suppression in irradiated tumor-bearing mice.
In the present study, it was demonstrated that
HS-1793 increased lymphocyte proliferation with or without Con A
stimulation in irradiated tumor-bearing mice via the inhibition of
DNA damage to lymphocytes Moreover, HS-1793 treatment decreased the
proportion of Tregs in irradiated tumor-bearing mice when compared
to the controls or irradiation alone. Radiation affects the
function of Tregs by altering the expression of cellular activation
markers and by regulating cytokine expression within the tumor
microenvironment (18). Indeed,
IL-4 is essential for naturally occurring Treg homeostasis or
activation, and Tregs are refractory to TCR (T cell
receptor)-induced proliferation (48). In addition, IL-4 inhibits
TGF-β-induced Foxp3 expression and thus suppresses the generation
of new Foxp3+ Tregs (49). Importantly, TGF-β is strongly
induced by radiation as an immunosuppressive factor and is known to
activate Tregs rather than effector T cells (46,50).
IL-10 also potentiates the differentiation of human induced Treg
cells via STAT3 and Foxo1 (51).
In a previous study, it was demonstrated that HS-1793 promoted IL-4
secretion in tumor-derived T lymphocytes and immunosuppressive
cytokines, such as TGF-β (26,29).
In the present study, it was found that the administration of
HS-1793 increased IL-4 production, and decreased TGF-β and IL-10
production in irradiated tumor-bearing mice. These data suggest
that HS-1793 plays an important role in the downregulation of
activated Tregs by radiotherapy.
Cancer cells promote the recruitment of Tregs,
specific subsets of TAMs and myeloid-derived suppressor cells
(MDSCs), via the secretion of TGF-β or IL-10 (10,52).
TAMs have been shown to inhibit the development of efficient
anti-tumor responses, and in doing so they promote tumor growth,
invasion, metastasis and activate tumor-promoting genes in cancer
cells. In particular, TAMs of the M2 phenotype exhibit a high
expression of CD68, CD204, CD206, vascular endothelial growth
factor (VEGF) and matrix metalloproteinase (MMP)-9 (52). In addition, TAMs effectively
inhibit the induction of proper antitumor T cell responses through
the production of immunosuppressive cytokines, such as TGF-β and
IL-10, which promote the induction and infiltration of
CD4+CD25+FoxP3+ T cells (Tregs) at
the tumor site (33,52). The authors previously reported that
HS-1793 administration significantly increased IFN-γ-expressing
CD8+ T cells in splenocytes, as well as in tumor tissue
and CD206+ macrophage infiltration was decreased at the
tumor site with a higher expression of IFN-γ. In addition, IFN-γ
led to the switch of the established human TAMs from the M-2 to M-1
phenotype. IFN-γ re-treated TAMs secreted lower levels of mediators
with immunosuppressive and/or pro-tumoral properties, such as
IL-10, TGF-β, VEGF and MMP-9 than untreated M-2 phenotype TAMs
(29). In the present study, it
was demonstrated that HS-1793 in combination with radiation
decreased CD206+ macrophage infiltration in the tumor
site when compared to the control or radiation alone. However,
there was no difference in CD206+ macrophage
infiltration in the tumor site between the unirradiated and
irradiated controls. These results were also supported by the
increased IFN-γ-expressing CD8+ T cells in splenocytes
of tumor-bearing mice and in tumor tissue following the combination
of HS-1793 and radiation. IFN-γ plays a key role in tumor
surveillance and immunoediting and blocks TGF-β-mediated Treg cell
differentiation (29). In
addition, IFN-γ producing CD8+ T cells (cytotoxic T
lymphocytes; CTL) which kill tumor cells and impede tumor growth
are observed as a reflection of a tumor-related immune responses
and are recognized as the principal effectors of a local anti-tumor
immune response (29). As
demonstrated in the present study, the combination of HS-1793 and
radiation significantly increased IFN-γ-expressing CD8+
T cells in splenocytes and enhanced IFN-γ secretion and
cytotoxicity against tumor cells, which may be related to
HS-1793-induced Treg depletion. More importantly, HS-1793
administration markedly improved radiation-induced tumor growth
inhibition in the murine breast cancer model. These results
indicate that there seems to be an association between the presence
of immunosuppressive cells, such as Tregs or TAMs and the capacity
to develop an anti-tumor immune response by HS-1793 supplementation
in the radiation-induced tumor microenvironment.
Tumor heterogeneity is one of the hallmarks of
malignancy. Although the present study was limited as it evaluated
only FM3A murine breast cancer cells, HS-1793 was shown to be a
promising candidate when used in combination with radiotherapy to
modulate the tumor microenvironment and promote immunotherapy in
breast cancer. Given the inter-tumor heterogeneity and intratumoral
heterogeneity that are considered to account for the differences in
clinical behavior and treatment responses in breast cancer, a
further potential strategy to overcome treatment resistance such as
targeting mutations would be required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Dong-A
University research fund and the Dongnam Institute of Radiological
and Medical Sciences (DIRAMS) grant funded by the Korea government
(MSIT) (nos. 50493-2014 and 50496-2019).
Availability of data and materials
All data generated during this research are included
in this article or are available on proper request from the
corresponding authors.
Authors' contributions
JSK, WSJ and MHJ were involved in the
conceptualization of the study and in the methodology. CGL, YRK,
SKJ and SJO performed the experiments. WSJ and MHJ were involved in
data analysis, and in the writing and preparation of the original
draft. JSK, WSJ and MHJ were involved in the writing, reviewing and
editing of the manuscript. WSJ and MHJ supervised the study. MHJ
was involved in funding acquisition. All the above-mentioned
authors participated in the conception and design of the study. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were ethically approved by
the Committee on Use and Care of Animals of Dong-A University
Hospital (DIACUC-10-14) and Dong Nam Institute of Radiological and
Medical Sciences (DIRAMS AEC-2011-003).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Basu P and Maier C: Phytoestrogens and
breast cancer: In vitro anticancer activities of isoflavones,
lignans, coumestans, stilbenes and their analogs and derivatives.
Biomed Pharmacother. 107:1648–1666. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clarke M, Collins R, Darby S, Davies C,
Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al:
Effects of radio-therapy and of differences in the extent of
surgery for early breast cancer on local recurrence and 15-year
survival: An overview of the randomised trials. Lancet.
366:2087–2106. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Turashvili G and Brog E: Tumor
heterogeneity in breast cancer. Front Med (Lausanne). 4:2272017.
View Article : Google Scholar
|
|
4
|
Grantzau T and Overgaard J: Risk of second
non-breast cancer after radiotherapy for breast cancer: A
systematic review and meta-analysis of 762,468 patients. Radiother
Oncol. 114:56–65. 2015. View Article : Google Scholar
|
|
5
|
Bartelink H, Horiot JC, Poortmans PM,
Struikmans H, Van den Bogaert W, Fourquet A, Jager JJ, Hoogenraad
WJ, Oei SB, Wárlám-Rodenhuis CC, et al: Impact of a higher
radiation dose on local control and survival in breast-conserving
therapy of early breast cancer: 10-Year results of the randomized
boost versus no boost EORTC 22881-10882 trial. J Clin Oncol.
25:3259–3265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balogh A, Persa E, Bogdándi EN, Benedek A,
Hegyesi H, Sáfrány G and Lumniczky K: The effect of ionizing
radiation on the homeostasis and functional integrity of murine
splenic regulatory T cells. Inflamm Res. 62:201–212. 2013.
View Article : Google Scholar
|
|
7
|
Deng G: Tumor-infiltrating regulatory T
cells: Origins and features. Am J Clin Exp Immunol. 7:81–87.
2018.PubMed/NCBI
|
|
8
|
Stanton SE and Disis ML: Clinical
significance of tumor-infiltrating lymphocytes in breast cancer. J
Immunother Cancer. 4:592016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Loi S, Sirtaine N, Piette F, Salgado R,
Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, et al:
Prognostic and predictive value of tumor-infiltrating lymphocytes
in a phase III randomized adjuvant breast cancer trial in
node-positive breast cancer comparing the addition of docetaxel to
doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin
Oncol. 31:860–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takeya M and Komohara Y: Role of
tumor-associated macro-phages in human malignancies: Friend or foe?
Pathol Int. 66:491–505. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Du Y, Lin X, Qian Y, Zhou T and
Huang Z: CD4+CD25+ regulatory T cells in tumor immunity. Int
Immunopharmacol. 34:244–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cassetta L and Pollard JW: Repolarizing
macrophages improves breast cancer therapy. Cell Res. 27:963–964.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shou J, Zhang Z, Lai Y, Chen Z and Huang
J: Worse outcome in breast cancer with higher tumor-infiltrating
FOXP3+ Tregs : A systematic review and meta-analysis. BMC Cancer.
16:6872016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peranzoni E, Lemoine J, Vimeux L, Feuillet
V, Barrin S, Kantari-Mimoun C, Bercovici N, Guérin M, Biton J,
Ouakrim H, et al: Macrophages impede CD8 T cells from reaching
tumor cells and limit the efficacy of anti-PD-1 treatment. Proc
Natl Acad Sci USA. 115:E4041–E4050. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Buchan SL, Dou L, Remer M, Booth SG, Dunn
SN, Lai C, Semmrich M, Teige I, Mårtensson L, Penfold CA, et al:
Antibodies to costimulatory receptor 4-1BB enhance anti-tumor
immunity via T regulatory cell depletion and promotion of CD8 T
cell effector function. Immunity. 49:958–970 e957. 2018. View Article : Google Scholar
|
|
16
|
Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR
and Yang SM: Macrophages in tumor microenvironments and the
progression of tumors. Clin Dev Immunol. 2012:9480982012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahmed MM, Guha C, Hodge JW and Jaffee E:
Immunobiology of radiotherapy: New paradigms. Radiat Res.
182:123–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lumniczky K and Safrany G: The impact of
radiation therapy on the antitumor immunity: Local effects and
systemic consequences. Cancer Lett. 356:114–125. 2015. View Article : Google Scholar
|
|
19
|
Kulkarni SS and Canto C: The molecular
targets of resveratrol. Biochim Biophys Acta. 1852:1114–1123. 2015.
View Article : Google Scholar
|
|
20
|
Zhao Y, Tang H, Zeng X, Ye D and Liu J:
Resveratrol inhibits proliferation, migration and invasion via Akt
and ERK1/2 signaling pathways in renal cell carcinoma cells. Biomed
Pharmacother. 98:36–44. 2018. View Article : Google Scholar
|
|
21
|
Elshaer M, Chen Y, Wang XJ and Tang X:
Resveratrol: An over-view of its anti-cancer mechanisms. Life Sci.
207:340–349. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scarlatti F, Sala G, Ricci C, Maioli C,
Milani F, Minella M, Botturi M and Ghidoni R: Resveratrol
sensitization of DU145 prostate cancer cells to ionizing radiation
is associated to ceramide increase. Cancer Lett. 253:124–130. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Y, Paik JH, Cho D, Cho JA and Kim CW:
Resveratrol induces the suppression of tumor-derived CD4+CD25+
regulatory T cells. Int Immunopharmacol. 8:542–547. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baatout S, Derradji H, Jacquet P, Ooms D,
Michaux A and Mergeay M: Enhanced radiation-induced apoptosis of
cancer cell lines after treatment with resveratrol. Int J Mol Med.
13:895–902. 2004.PubMed/NCBI
|
|
25
|
Jeong SH, Jo WS, Song S, Suh H, Seol SY,
Leem SH, Kwon TK and Yoo YH: A novel resveratrol derivative,
HS1793, overcomes the resistance conferred by Bcl-2 in human
leukemic U937 cells. Biochem Pharmacol. 77:1337–1347. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeong MH, Yang KM, Choi YJ, Kim SD, Yoo
YH, Seo SY, Lee SH, Ryu SR, Lee CM, Suh Hs and Jo WS: Resveratrol
analog, HS-1793 enhance anti-tumor immunity by reducing the
CD4+CD25+ regulatory T cells in FM3A tumor bearing mice. Int
Immunopharmacol. 14:328–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi YJ, Heo K, Park HS, Yang KM and Jeong
MH: The resveratrol analog HS-1793 enhances radiosensitivity of
mouse-derived breast cancer cells under hypoxic conditions. Int J
Oncol. 49:1479–1488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jeong MH, Yang KM, Jeong DH, Lee CG, Oh
SJ, Jeong SK, Lee KW, Jo YR and Jo WS: Protective activity of a
novel resveratrol analogue, HS-1793, against DNA damage in
137Cs-irradiated CHO-K1 cells. J Radiat Res. 55:464–475.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jeong SK, Yang K, Park YS, Choi YJ, Oh SJ,
Lee CW, Lee KY, Jeong MH and Jo WS: Interferon gamma induced by
resveratrol analog, HS-1793, reverses the properties of tumor
associated macrophages. Int Immunopharmacol. 22:303–310. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang WJ, Long LM, Yang N, Zhang QQ, Ji WJ,
Zhao JH, Qin ZH, Wang Z, Chen G and Liang ZQ: NVP-BEZ235, a novel
dual PI3K/mTOR inhibitor, enhances the radiosensitivity of human
glioma stem cells in vitro. Acta Pharmacol Sin. 34:681–690. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eberlein U, Scherthan H, Bluemel C, Peper
M, Lapa C, Buck AK, Port M and Lassmann M: DNA damage in peripheral
blood lymphocytes of thyroid cancer patients after radioiodine
therapy. J Nucl Med. 57:173–179. 2016. View Article : Google Scholar
|
|
32
|
Zhu Q, Wu X, Wu Y and Wang X: Interaction
between Treg cells and tumor-associated macrophages in the tumor
microenvironment of epithelial ovarian cancer. Oncol Rep.
36:3472–3478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu DP, Li Y, Meng X, Zhou T, Zhou Y, Zheng
J, Zhang JJ and Li HB: Natural antioxidants in foods and medicinal
plants: Extraction, assessment and resources. Int J Mol Sci.
18:E962017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sinha D, Sarkar N, Biswas J and Bishayee
A: Resveratrol for breast cancer prevention and therapy:
Preclinical evidence and molecular mechanisms. Semin Cancer Biol.
40-41:209–232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
da Costa Araldi IC, Bordin FPR, Cadona FC,
Barbisan F, Azzolin VF, Teixeira CF, Baumhardt T, da Cruz IBM,
Duarte MMMF and Bauermann LF: The in vitro radiosensitizer
potential of resveratrol on MCF-7 breast cancer cells. Chem Biol
Interact. 282:85–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dewangan J, Tandon D, Srivastava S, Verma
AK, Yapuri A and Rath SK: Novel combination of salinomycin and
resveratrol synergistically enhances the anti-proliferative and
pro-apoptotic effects on human breast cancer cells. Apoptosis.
22:1246–1259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barker HE, Paget JT, Khan AA and
Harrington KJ: The tumour microenvironment after radiotherapy:
Mechanisms of resistance and recurrence. Nat Rev Cancer.
15:409–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sharma RA, Plummer R, Stock JK, Greenhalgh
TA, Ataman O, Kelly S, Clay R, Adams RA, Baird RD, Billingham L, et
al: Clinical development of new drug-radiotherapy combinations. Nat
Rev Clin Oncol. 13:627–642. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Malik A, Sultana M, Qazi A, Qazi MH,
Parveen G, Waquar S, Ashraf AB and Rasool M: Role of natural
radiosensitizers and cancer cell radioresistance: An update. Anal
Cell Pathol (Amst). 2016:61465952016.
|
|
40
|
Jeong SH, Lee JS, Jeong NY, Kim TH, Yoo
KS, Song S, Suh H, Kwon TK, Park BS and Yoo YH: A novel resveratrol
analogue HS-1793 treatment overcomes the resistance conferred by
Bcl-2 and is associated with the formation of mature PML nuclear
bodies in renal clear cell carcinoma Caki-1 cells. Int J Oncol.
35:1353–1360. 2009.PubMed/NCBI
|
|
41
|
Cerda H, Delincee H, Haine H and Rupp H:
The DNA 'comet assay' as a rapid screening technique to control
irradiated food. Mutat Res. 375:167–181. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bausinger J and Speit G: The impact of
lymphocyte isolation on induced DNA damage in human blood samples
measured by the comet assay. Mutagenesis. 31:567–572. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Makkouk A and Weiner GJ: Cancer
immunotherapy and breaking immune tolerance: New approaches to an
old challenge. Cancer Res. 75:5–10. 2015. View Article : Google Scholar
|
|
44
|
Shiku H: Importance of CD4+ helper T-cells
in antitumor immunity. Int J Hematol. 77:435–438. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shen Y, Wei Y, Wang Z, Jing Y, He H, Yuan
J, Li R, Zhao Q, Wei L, Yang T and Lu J: TGF-β regulates
hepatocellular carcinoma progression by inducing Treg cell
polarization. Cell Physiol Biochem. 35:1623–1632. 2015. View Article : Google Scholar
|
|
46
|
Son CH, Bae JH, Shin DY, Lee HR, Jo WS,
Yang K and Park YS: Combination effect of regulatory T-cell
depletion and ionizing radiation in mouse models of lung and colon
cancer. Int J Radiat Oncol Biol Phys. 92:390–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kachikwu EL, Iwamoto KS, Liao YP, DeMarco
JJ, Agazaryan N, Economou JS, McBride WH and Schaue D: Radiation
enhances regulatory T cell representation. Int J Radiat Oncol Biol
Phys. 81:1128–1135. 2011. View Article : Google Scholar :
|
|
48
|
Yang WC, Hwang YS, Chen YY, Liu CL, Shen
CN, Hong WH, Lo SM and Shen CR: Interleukin-4 supports the
suppressive immune responses elicited by regulatory T cells. Front
Immunol. 8:15082017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dardalhon V, Awasthi A, Kwon H, Galileos
G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et
al: IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together
with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells.
Nat Immunol. 9:1347–1355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Martin M, Lefaix J and Delanian S:
TGF-beta1 and radiation fibrosis: A master switch and a specific
therapeutic target? Int J Radiat Oncol Biol Phys. 47:277–290. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hsu P, Santner-Nanan B, Hu M, Skarratt K,
Lee CH, Stormon M, Wong M, Fuller SJ and Nanan R: IL-10 Potentiates
Differentiation of Human Induced Regulatory T Cells via STAT3 and
Foxo1. J Immunol. 195:3665–3674. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Solinas G, Germano G, Mantovani A and
Allavena P: Tumor-associated macrophages (TAM) as major players of
the cancer-related inflammation. J Leukoc Biol. 86:1065–1073. 2009.
View Article : Google Scholar : PubMed/NCBI
|