Introduction
Ovarian cancer is the most lethal gynecological
malignancy in the United States; in 2017, the estimated number of
new cases for ovarian cancer was 22,440, and the estimated number
of deaths was 14,080 (1). In
China, the estimated number of new cases of ovarian cancer was
52,100, and the estimated number of deaths was 22,500 in 2015
(2). The high mortality of ovarian
cancer is mainly due to its diagnosis at an advanced stage
(3). Despite the progress in
chemotherapy after surgery, most patients develop chemoresistance
(4). Thus, 5-year survival is ~40%
for patients with ovarian cancer (5). An improved understanding of the
genetic and molecular pathogenesis of this disease and targeted
therapies are urgently needed to improve patient prognosis.
The human microRNA (miRNA/miR)-508-3p gene is
located at Xq27.3, and alterations in miR-508-3p expression have
been reported in several types of cancer (6-8). A
previous study reported that low miR-508-3p expression levels are
associated with an advanced stage of serous ovarian cancer
(7), suggesting that deregulation
of miR-508-3p in ovarian cancer may be associated with the
tumorigenesis and progression of this disease. Zhai et al
(6) reported that miR-508-3p
suppressed cell proliferation and migration in renal cell
carcinoma. miR-508-3p also exerts a tumor suppressor function by
directly targeting nuclear factor-κB subunit 1 and RELA
proto-oncogene in gastric cancer (9). However, the role of miR-508-3p in
ovarian cancer and the potential underlying molecular mechanisms
remain largely unknown.
Cyclin A2 (CCNA2) regulates the cell cycle by
binding and activating cyclin-dependent kinase 2 (CDK2) and thus
promotes transition through the G1/S and G2/M phases. CCNA2 is
overexpressed in various types of cancer, including ovarian cancer
(10,11). Matrix metalloproteinase 7 (MMP7) is
a member of the MMP family and is also upregulated in multiple
types of cancer, such as ovarian and colorectal cancer (12,13).
However, the molecular mechanisms leading to the upregulation of
CCNA2 and MMP7 in ovarian cancer remain unclear. The present study
aimed to examine the potential role of miR-508-3p in human ovarian
cancer progression and to determine whether miR-508-3p inhibits
cell proliferation, migration and invasion by directly targeting
CCNA2 and MMP7 in ovarian cancer.
Materials and methods
Patient samples
A total of 130 patients who were diagnosed with
serous ovarian cancer at the Department of Gynecology and
Obstetrics in Tianjin Medical University General Hospital (Tianjin,
China) according to a pathological report between January 2009 and
December 2016 were selected for the current study. The mean age of
the patients was 57 years (range, 35-76 years). This study was
approved by the Ethics Committee of Tianjin Medical University
General Hospital. All participants signed consent forms prior to
the surgical procedure. Pathological specimens were collected
during the primary surgery. The ovaries of patients were resected
during the surgery, and the adjacent non-tumor tissues were
collected 1.0 cm away from the tumor. The specimens were fixed in
formalin in room temperature for 24 h and embedded in paraffin.
Then, 5-μm continuous sections were prepared for HE
staining, in situ hybridization of miR-508-3p and
immunohistochemical staining of CCNA2, MMP7 and proliferation
marker protein Ki67. Each slide was re-evaluated by an expert
pathologist without knowledge of patient clinical data before the
experiments were performed. Only specimens containing >70% of
tumor tissue were used for the study. The cases were classified
according to the FIGO stage system (2013) (14). The clinicopathological data were
collected, including age, FIGO stage and grade (Table SI). In this cohort, the median
follow-up duration was 36.8 months (range, 1.2-94 months). A total
of 78% of patients were alive at the last follow-up. Overall
survival (OS) times were calculated from the initial cytoreductive
surgery and considered censored for patients who were alive at or
died after the last follow-up.
To further evaluate the results of the survival data
from Tianjin Medical University General Hospital, the ovarian
carcinoma cohort was obtained from The Cancer Genome Atlas (TCGA;
https://portal.gdc.cancer.gov/). The
association between miR-508-3p expression and survival of 452
patients with ovarian cancer was analyzed using TCGA data.
Methods and cell culture
The ovarian cancer cell lines SKOV3, HeyA8 and
A2780, and the cervical cancer cell line HeLa were obtained from
the American Type Culture Collection and maintained in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) without any
antibiotics. The cells were incubated at 37°C in an atmosphere
containing 5% CO2. Human miR-508-3p mimic
(hsa-miR-508-3p) and scrambled negative control (miR-ctrl) were
obtained from GE Healthcare Dharmacon, Inc. Small interfering RNA
(siRNA) targeting CCNA2 or MMP7 and scrambled
negative siRNA control were purchased from Sigma-Aldrich; Merck
KGaA. The pcDNA3.1 plasmids (Promega Corporation) expressing CCNA2
or MMP7 and empty vectors (EV) were constructed by Shanghai
GenePharma Co., Ltd.
miRNA, siRNA and plasmid
transfection
The ovarian cancer cell lines SKOV3, HeyA8 and A2780
(2×105 per well) were seeded in 6-well plates and
allowed to attach for at least 16 h. miR-508-3p mimic (miR-508-3p),
miR-ctrl or siRNA were transfected into cells using
Lipofectamine® RNAiMAX (Invitrogen; Thermo Fisher
Scientific, Inc.) at a final concentration of 50 nM. Plasmids (500
ng) were transfected into cells using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). The transfection
reagents were used according to the manufacturer's instructions,
and the transfections were performed at 37°C for 48 h. Total RNA
and protein were collected 48 h post-transfection. Cell
supernatants were collected at 24 h for ELISA.
Reverse transcription-quantitative (RT-q)
PCR
Total RNA were isolated from the ovarian cancer cell
lines SKOV3, HeyA8 and A2780 using the mirVana™ miRNA Isolation kit
(Ambion; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. A total of 50 ng RNA was reverse
transcribed to cDNA using a Taqman MicroRNA Reverse Transcription
kit (cat. no. 4366596; Applied Biosystems; Thermo Fisher
Scientific, Inc.) for the reverse transcription of miRNA and U6
(16°C for 30 min, 42°C for 30 min, 85°C for 5 min and store at 4°C)
or Taqman Reverse Transcription reagents (cat. no. 8080234; Applied
Biosystems; Thermo Fisher Scientific, Inc.) for the reverse
transcription of CCNA2 and MMP7 mRNA (25°C for 10 min, 42°C for 50
min, 70°C for 15 min and store at 4°C). TaqMan RT-PCR assay probes
for CCNA2 (cat. nos. A15629 and A15630) and MMP7 (cat. nos. A15629
and A15630) were purchased from Thermo Fisher Scientific, Inc.
TaqMan miRNA assay kits were purchased from Applied Biosystems;
Thermo Fisher Scientific, Inc (cat. no. 4427975).
The probes of PCR for miR-508-3p (cat. no. AP01571)
and U6 (cat. no. AP01501) were purchased from HaiGene Co., Ltd. The
qPCR instrument used was Applied Biosystems Real-Time PCR System
(BioRad Laboratories, Inc.) with the following thermocycling
conditions: 95°C for 10 min, followed by 50 cycles of 95°C for 15
sec and 60°C for 1 min. U6 and GAPDH were used as normalization
controls. Data were analyzed by the 2−ΔΔCq method
(15).
MTT assay
The
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphen-yltetrazolium bromide (MTT)
substrate (Sigma-Aldrich; Merck KGaA) was used to assay cell
proliferation according to the manufacturer's instructions.
Briefly, SKOV3, HeyA8 and A2780 cells transfected with miR-508-3p,
miR-ctrl, siRNA-ctrl, siRNA-CCNA2 or siRNA-MMP7 were seeded into
96-well plates at a density of 1×103 cells/well with 200
μl RPMI-1640 medium supplemented with 10% FBS. Then, 5 mg/ml
MTT reagent was added to the medium (without FBS) at a ratio of 1:9
and was used to replace the medium in each well (100
μl/well) at different time points (1, 2, 3 and 4 days),
followed by incubation at 37°C for 4 h. After removal of the
medium, 150 μl dimethylsulfoxide was added to each well, and
the cells were gently agitated for 5 min at 37°C. Absorbance values
were determined using a microplate reader at 590 nm. Each
experiment contained three replicates.
Colony formation assay
The ovarian cancer cell lines SKOV3, HeyA8 and A2780
were harvested 24 h after transfection with miR-508-3p or miR-ctrl,
seeded in a fresh 6-well plate (1,000 cells/well) and incubated for
14 days. Colony formation was analyzed by staining cells with 0.1%
crystal violet at room temperature for 30 min. The colonies were
observed under an inverted phase contrast microscope (Olympus
corporation), and a cluster containing >50 cells was considered
a colony. The efficiency of colony formation was calculated as
follows: Colony formation rate = (No. of colonies / No. of seeded
cells) x 100%.
Cell cycle analysis
SKOV3, HeyA8 and A2780 cells were transfected with
miR-508-3p, miR-ctrl, siRNA-ctrl or siRNA-CCNA2. At 48 h
post-transfection, the cells were harvested by trypsinization,
washed with PBS and fixed in ice-cold 70% ethanol at 4°C overnight.
The cells were then washed in PBS and incubated with 50 μl
100 g/ml ribo-nuclease (Thermo Fisher Scientific, Inc.) for 20 min
at room temperature to ensure that only the DNA was stained. Then,
the cells were incubated in 200 μl 50 g/ml propidium iodide
for 30 min at 37°C in the dark. Cell cycle distribution was assayed
using a FACSCalibur™ Flow Cytometer (BD Biosciences). The data were
analyzed with the ModFit LT v3.3 software (BD Biosciences).
Transwell assays
For Transwell experiments, 24 h post-transfection,
SKOV3 and HeyA8 cells (5×104 cells/well) were seeded
into the upper wells of the Transwell chamber in RPMI-1640 medium
without serum. The Transwell chamber was pre-coated with Matrigel
(BD Biosciences) for 30 min at 37°C for the invasion assay or left
uncoated for the migration assay. The cells were allowed to migrate
or invade toward RPMI-1640 medium containing 10% FBS for 24 h at
37°C. Then, the cells on the lower surface were fixed with 4%
paraformaldehyde at room temperature for 30 min and stained with
0.1% crystal violet at room temperature for 15 min. The cells on
the upper surface were removed using a cotton swab. The migrated or
invaded cells were counted in six randomly selected fields (×400
magnification) under a positive phase contrast microscope (Olympus
Corporation).
Western blot analysis
SKOV3 or HeyA8 cells were solubilized in RIPA Lysis
and Extraction Buffer (Thermo Fisher Scientific, Inc.) supplemented
with Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific,
Inc.). Protein quantification was performed using BCA Protein Assay
kit (Abcam). Equal amounts (40 μg) of protein from each
sample was subjected to SDS-PAGE on a 10% SDS-acrylamide gel and
loaded onto a PVDF membrane. The membrane was blocked by 5% skimmed
milk TBS + Tween-20 (1,000:1) buffer at room temperature for 1 h
and probed with the following primary antibodies: CCNA2 (1:200;
cat. no. sc-596; Santa Cruz Biotechnology, Inc.), MMP7 (1:200; cat.
no. sc-80205; Santa Cruz Biotechnology, Inc.), β-actin (1:1,000;
cat. no. sc-8432; Santa Cruz Biotechnology, Inc.), MMP2 (1:1,000;
cat. no. ab37150; Abcam), pRb (1:1,000; cat. no. 9307; Cell
signaling Technology, Inc.) CDK4 (1:1,000; cat. no. sc-53636; Santa
Cruz Biotechnology, Inc.), CDK6 (1:1,000; cat. no. sc-53638; Santa
Cruz Biotechnology, Inc.) and Ki67 (1:1,000; cat. no. AF0198; Cell
Signaling Technology, Inc.) overnight at 4°C. The membrane was
washed three times by TBS + Tween-20 (1000:1) buffer at room
temperature for 10 min. The secondary antibodies included
horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG
(1:4,000; cat. no. ab205718; Abcam) and HRP-conjugated horse
anti-mouse IgG (1:6,000; cat. no. 7076; Cell Signaling
Technologies, Inc.). The secondary antibody incubation was
performed at room temperature for 1 h. The proteins were visualized
using the SuperSignal West Pico Chemiluminescent Substrate (Pierce;
Thermo Fisher Scientific, Inc.).
ELISA
The concentration of MMP7 in the conditioned media
from cultured cells was determined by a MMP7 ELISA kit (cat. no.
PDMP700; R&D Systems, Inc.) according to the manufacturer's
instructions. Briefly, the collected conditioned media from SKOV3
or HeyA8 cells were added to a well coated with an MMP7 polyclonal
antibody and immunosorbed by a biotinylated monoclonal anti-human
MMP7 antibody at 37°C for 1 h. The color development catalyzed by
horseradish peroxidase was terminated with 2.5 mol/l sulfuric acid.
The protein concentration was determined by a microplate reader at
450 nm.
Luciferase reporter assay
The 3′-untranslated regions (UTRs) of the CCNA2 and
MMP7 genes, each of which contained one putative miR-508-3p binding
site identified by TargetScan (http://www.targetscan.org/vert_71/) and miRBase
(http://www.mirbase.org/) databases, were
amplified by PCR from cDNA derived from HeyA8 cells and inserted
into the multiple cloning sites of the pmirGLO vector (Promega
Corporation). The primers used were as follows: CCNA2
forward,
5′-AAGTATATGGTGTACAGTTTTTAACTTAGGTTTTAATTTTTTCTGAATACAGAAGTTGTG-3′
and reverse,
5′-CACAACTTCTGTATTCAGAAAAAATTAAAACCTAAGTTAAAAACTGTACACCATATACTT-3′;
MMP7 forward, 5′-CGGCTAGCTCAGGCAGAACATCCATT CATTC-3′ and
reverse, 5′-GCTCTAGACATTTATTGACATCTACCCACTGCA-3′. Two mutant (mt)
CCNA2 and MMP7 3′-UTR reporter vectors that lacked
the binding sites for miR-508-3p were created through site-directed
mutagenesis using a QuikChange kit (Stratagene; Agilent
Technologies, Inc.). The primers used for site-directed mutagenesis
were as follows: CCNA2 3′-UTR-mt forward,
5′-TACTTGTCAATATTTAAACATGGTTTGCTGAAAATGGTATTTTCCCCC-3′ and reverse,
5′-GGGGAAAATACCATTTTCAGCAAACCATGTTTAAATATTGACAAGTA-3′; MMP7
3′-UTR-mt forward, 5′-GGATTGTATATCATTGTTGCGAATTGATAAGCACTGTTCC-3′
and reverse, 5′-GGAACAGTGCTTAT CAATTCGCAACAATGATATACAATCC-3′. All
constructed clones were verified by DNA sequencing performed by
Beijing Genomics Institute (Shenzhen, China).
For the luciferase reporter assay, 0.5 μg
pmirGLO, pmirGLO-3′-UTR-wild-type or pmirGLO-3′-UTR-M was
transfected into HeLa cells that were cultured in 24-well plates
together with 50 nM miR-508-3p or miR-ctrl using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). At 48 h post-transfection, the cells were
subjected to lysis, and firefly and Renilla luciferase
activities were determined using a Dual-Luciferase Reporter Assay
system (Promega Corporation) as previously described (16). Firefly luciferase activity was
normalized to Renilla luciferase activity for each construct
and compared with that of the miR-ctrl group.
miRNA in situ hybridization (ISH) and
immunohistochemistry (IHC)
miRNA ISH was performed as previously described
(15). Briefly, the patient tissue
sections were deparaffinized and incubated for 30 min at 37°C with
20 μg/ml proteinase K. Then, the slides were dehydrated,
followed by hybridization with 100 nM DIG-labeled miRCURY LNA™
detection probe hsa-miR-508-3p (cat. no. 611776-350; Qiagen GmbH)
for 2 h at 55°C. Next, anti-DIG reagent (sheep anti-DIG-AP at 1:500
in antibody diluent; cat. no. 11093274910; Roche Applied Science)
was added, and the sections were incubated for 60 min at room
temperature. Then, freshly prepared alkaline phosphatase (AP)
substrate [nitro blue tetrazolium chloride
(NBT)/5-bromo-4-chloro-3-indolyl-phosphate, toluidine salt (BCIP);
cat. no. 11697471001; Roche Applied Science) was applied to the
sections, and the sections were incubated for 2 h at 30°C away from
light. The U6 probe was used as a positive control for each
section. The stained sections were reviewed by two independent
pathologists under an inverted phase contrast microscope (Olympus
Corporation) at ×200 magnification. Signals in tumor cells were
visually quantified using a scoring system between 0 and 9; the
scores were assigned based on the intensity of the signal and the
percentage of positive cells as follows: i) Signal: 0, no signal;
1, weak signal; 2, intermediate signal; and 3, strong signal; and
ii) percentage: 0, 0%; 1, <25%; 2, 25-50%; and 3, >50%. The
scores were multiplied to achieve a final score. The low and high
miR-508-3p expression groups were based on the mean of miR-508-3p
ISH score and, the low and high CCNA2 or MMP7 expression groups
were based on the mean of the IHC scores.
IHC staining was performed on the tumor tissues from
130 patients with serous ovarian cancer. The sections were immersed
in eBioscience™ IHC Antigen Retrieval solution (cat. no.
00-4956-58; Thermo Fisher Scientific, Inc.) and heated in a
microwave for 15 min. Subsequently, the sections were blocked with
goat serum (cat. no. 16210064; Thermo Fisher Scientific, Inc.) at
room temperature for 1 h and incubated with rabbit anti-human Ki-67
(1:200; cat. no. AP101M; EMD Millipore), mouse anti-human CCNA2
(1:200; cat. no. sc-596; Santa Cruz Biotechnology, Inc.) and mouse
anti-human MMP7 (1:100, cat. no. sc-80205; Santa Cruz
Biotechnology, Inc.) primary antibodies overnight at 4°C. The
secondary antibody (anti-rabbit IgG; cat. no. SPN-9001; OriGene
Technologies, Inc.) was then added at room temperature for 1 h.
Finally, the sections were incubated with diaminobenzidine (30 mg
dissolved in 100 ml Tris buffer) for 5 min, rinsed with running
water and counterstained with hematoxylin for 1 min at room
temperature.
Ki-67-positive cells were defined as those with
brown staining in the nucleus, whereas MMP7-positive cells were
defined as those with immunoreactivity in the cytoplasm.
CCNA2-positive cells were considered as those with immunoreactivity
in the nucleus and cytoplasm. The expression of these proteins was
evaluated using the percentage of positive tumor cells in 1,000
tumor cells. The results were analyzed by two independent
pathologists, and scores were assigned based on the intensity and
the percentage of the stained tissues described above.
Statistical analysis
The results are presented as the mean ± standard
deviation. The cell culture experiments were performed at least
three times and in triplicate. Wilcoxon test was performed using
GraphPad Prism version 6 software (GraphPad Software, Inc.).
Pearson's χ2 test, Kruskal-Wallis test with Dunn's post
hoc test, Kaplan-Meier analysis, log-rank test and Cox's
proportional hazards regression model were performed using SPSS
21.0 software (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-508-3p suppresses the proliferation,
migration and invasion and increases the G1 phase distribution of
ovarian cancer cells
miR-508-3p mimics were transfected into HeyA8, SKOV3
and A2780 cells. The results of the RT-qPCR analysis demonstrated
increased expression of miR-508-3 compared with the miR-ctrl group
in all the three cell lines (P<0.01; Fig. S1), and the MTT assay revealed that
miR-508-3p overexpression resulted in the suppression of cell
proliferation at all time points compared with the miR-ctrl group
(Fig. 1A). At 96 h
post-transfection, miR-508-3p inhibited the viability of HeyA8
cells by 35.3% (P<0.05), SKOV3 cells by 34.8% (P<0.05) and
A2780 cells by 33.4% (P<0.05) compared with miR-ctrl (Fig. 1A). In addition, miR-508-3p impaired
the colony formation efficiency of ovarian cancer cells; cells
transfected with miR-508-3p displayed lower colony formation
capacity compared with those transfected with miR-ctrl (P<0.05),
further confirming the long-term anti-proliferative effect of
miR-508-3p (Fig. 1B).
When miR-508-3p was transfected into HeyA8 and SKOV3
cells, the proportion of cells in the S phase decreased compared
with the corresponding miR-ctrl-transfected cells (P<0.05), and
concomitantly, the proportion of cells in G1 phase significantly
increased (P<0.05; Fig. 1C). To
examine whether miR-508-3p was involved in apoptosis, Annexin V
apoptosis assay was performed after transfection of miR-508-3p or
miR-ctrl. SKOV3 and HeyA8 cells exhibited a mild non-significant
increase in apoptotic rates at 48 h post-transfection with
miR-508-3p compared with those in the miR-ctrl groups (data not
shown). These results suggested that the suppressive effect of
miR-508-3p on ovarian cancer cell proliferation was mediated by
cell cycle arrest.
To examine the suppressive effects of miR-508-p on
ovarian cancer cell invasion and migration, transient mimic
transfections were performed, and the results demonstrated that
miR-508-3p suppressed the migration and invasion of SKOV3 and HeyA8
cells (P<0.05; Fig. 1D). These
results provided evidence that miR-508-3p may be an important
inhibitor of the viability, migration and invasion of ovarian
cancer cells.
miR-508-3p directly targets the 3′-UTRs
of CCNA2 and MMP7
To identify genes potentially regulated by
miR-508-3p, the TargetScan and miRBase databases were searched, and
CCNA2 and MMP7 were identified as potential target
genes of miR-508-3p. TargetScan analysis predicted one miR-508-3p
binding site in the 3′-UTR of the CCNA2 gene and one in the
3′-UTR of the MMP7 gene (Fig.
2E), suggesting that miR-508-3p may directly target
CCNA2 and MMP7. To confirm this, SKOV3 and HeyA8
cells were transfected with miR-508-3p or miR-ctrl followed by
measurement of CCNA2 and MMP7 mRNA and protein levels
48 h post-transfection. The results demonstrated that CCNA2
and MMP7 mRNA (Fig. 2A and
C) and protein (Fig. 2B and D)
levels were significantly downregulated following miR-508-3p
transfection compared with those in the miR-ctrl groups in SKOV3
and HeyA8 cells.
Luciferase reporter assays were performed to examine
whether miR-508-3p directly targeted CCNA2 and MMP7.
The 3′-UTRs of CCNA2 or MMP7 were cloned into the
pmirGLO vector to generate pmirGLO-CCNA2 and
pmirGLO-MMP7 constructs. Co-transfection of
pmirGLO-CCNA2 with miR-508-3p into HeLa cells resulted in
65.7% lower level of luciferase activity compared with that
following co-transfection with miR-ctrl, suggesting that miR-508-3p
directly targeted CCNA2 (Fig. 2F).
Similarly, co-transfection of pmirGLO-MMP7 with miR-508-3p
resulted in 37.3% less luciferase activity compared with
co-transfection with miR-ctrl, suggesting that miR-508-3p directly
targeted MMP7 (Fig.
2F).
To confirm that miR-508-3p specifically regulated
CCNA2 and MMP7 through the predicted binding sites,
the constructs pmirGLO-CCNA2-mt and pmirGLO-MMP7-mt, in which the
miR-508-3p binding site sequences on the 3′-UTRs of the two genes
were deleted, were generated and co-transfected with miR-508-3p or
miR-ctrl into HeLa cells. Deletion of the miR-508-3p binding sites
from the 3′-UTR of CCNA2 or MMP7 abolished the
effects of miR-508-3p on the luciferase activity (Fig. 2F). These results indicated that the
CCNA2 and MMP7 genes were direct targets of
miR-508-3p.
miR-508-3p inhibits CCNA2/pRb/CDK4/6
signaling and decreases MMP7 levels in cells and culture
supernatants
The possible mechanism of the suppressive effects of
miR-508-3p on cancer cell proliferation may be induced by
CCNA2/pRb/CDK4/6 (17); thus, the
expression levels of pRb, CDK4 and CDK6 were determined in the
present study. At 48 h post-transfection of miR-508-3p into SKOV3
and HeyA8 cells, the protein levels of pRb and CDK6 were decreased
compared with those in cells transfected with miR-ctrl, but CDK4
levels did not change (Fig. 3A).
These results indicated that miR-508-3p may suppress the
proliferation of ovarian cancer cells by decreasing CCNA2, pRb and
CDK6 levels.
 | Figure 3miR-508-3p suppresses
CCNA2/pRb/CDK4/6 signaling and MMP7 levels, and CCNA2 is involved
in miR-508-3p-induced inhibition of the cell cycle. (A) Western
blot assay results demonstrated that miR-508-3p attenuated pRb,
CDK6 and MMP2 protein levels. (B) Detection of MMP7 levels in the
supernatant of ovarian cancer cells transfected with miR-508-3p or
miR-ctrl by ELISA. (C-E) SKOV3 cells were transfected with
siRNA-ctrl or siRNA-CCNA2, and the (C) protein level, (D) cell
proliferation and (E) percentage of cells in each phase of the cell
cycle were assessed by western blot, MTT and flow cytometry assays,
respectively. (F-H) SKOV3 cells were co-transfected with CCNA2
vector or EV with miR-508-3p or miR-ctrl, and the (F) protein
level, (G) cell proliferation and (E) percentage of cells in the G1
phase were assessed by western blot, MTT and flow cytometry assays,
respectively. Kruskal-Wallis test was used to analyze the data.
*P<0.05, **P<0.01,
***P<0.001. miR, microRNA; ctrl, control; CCNA2,
cyclin A2; MMP7, matrix metalloproteinase 7; siRNA, small
interfering RNA; EV, empty vector. |
Cell mobility may be induced by MMPs, which are
known as direct regulators of tumor metastasis (18). In addition to MMP7, miR-508-3p also
downregulated MMP2 protein levels (Fig. 3A). MMP7 and MMP2 levels in the
culture supernatant were also examined, and the results
demonstrated that the level of MMP7 was decreased after
transfection of miR-508-3p compared with that in cells transfected
with miR-ctrl (Fig. 3B),
indicating that miR-508-3p suppressed ovarian cancer cell migration
and invasion by decreasing MMP7 levels.
CCNA2 is involved in the
miR-508-3p-induced proliferation suppression and cell cycle
arrest
To investigate whether the proliferation suppressive
effect of miR-508-3p on ovarian cancer cells was mediated by
repression of CCNA2, SKOV3 cells were transfected with siRNA-ctrl
or siRNA-CCNA2. At 48 h post-transfection, knockdown of CCNA2 led
to decreased expression of pRb, mimicking the suppressive effect of
miR-508-3p (Fig. 3C). At 4 days
post-transfection, knockdown of CCNA2 led to a significant
suppression of cell proliferation (P<0.05; Fig. 3D). Cell proliferation was also
suppressed at 2 and 3 days post-transfection, but no significant
differences were observed between siRNA-CCNA2 and siRNA-ctrl when
analyzed by the Kruskal-Wallis test (P>0.05; Fig. 3D). Knockdown of CCNA2 also led to
cell cycle arrest and increased G1 phase distribution, which was
similar to the results of miR-508-3p overexpression; however, no
statistical differences of G1 phase distribution were observed
between siRNA-CCNA2 and siRNA-ctrl when analyzed by the
Kruskal-Wallis test (P>0.05; Fig.
3E). These findings indicated that suppression of CCNA2 may be
required for proliferation inhibition and cell cycle arrest.
Based on the above results, SKOV3 cells were
co-transfected with miR-508-3p mimics and a plasmid overexpressing
CCNA2 to further study whether ectopic expression of CCNA2 may
rescue the inhibitory effects of miR-508-3p. As presented in
Fig. 3F, overexpression of CCNA2
partially rescued the decreased expression of pRb. At 48 h
post-transfection, co-transfection of miR-508-3p and an empty
vector led to significant suppression of cell proliferation
compared with that of miR-ctrl and empty vector, which was
attenuated by CCNA2 plasmid (P<0.05; Fig. 3G). Thus, ectopic expression of
CCNA2 partially rescued cell proliferation suppression mediated by
miR-508-3p. In addition, co-transfection of miR-508-3p and an empty
vector induced G1 phase arrest compared with that of miR-Ctrl and
empty vector, but the difference was not statistically significant
(P=0.05; Fig. 3H); this was also
attenuated by CCNA2 plasmid. Taken together, these findings
indicated that miR-508-3p may induce the proliferation suppression
and cell cycle arrest of ovarian cancer cells by targeting
CCNA2.
MMP7 is involved in the
miR-508-3p-induced suppression of migration and invasion
To elucidate the possible mechanism by which
miR-508-3p suppressed ovarian cancer cell migration and invasion,
SKOV3 cells were transfected with siRNA-ctrl or siRNA-MMP7. At 48 h
post-transfection with siRNA-MMP7, the MMP7 levels in the whole
cell lysate and cultured supernatant were decreased compared with
those in the siRNA-ctrl transfected cells (P<0.05; Fig. 4A and B). In addition, SKOV3 and
HeyA8 cells transfected with siRNA-MMP7_1 or siRNA-MMP7_2 exhibited
significantly decreased migratory capacity compared with those
transfected with siRNA-ctrl, which was in accordance with the
effect of miR-508-3p (P<0.05; Fig.
4C and F).
 | Figure 4MMP7 is involved in the
miR-508-3p-induced inhibition of ovarian cancer cell migration and
invasion. (A-C) SKOV3 cells were transfected with siRNA-ctrl or
siRNA-MMP7, and the (A) protein level of MMP7, (B) supernatant
level of MMP7 and (C) migratory capacity were analyzed by western
blot, ELISA and Transwell assays, respectively. (D-E) SKOV3 cells
were co-transfected with the MMP7 vector or EV and miR-508-3p or
miR-ctrl, and the (D) protein level and (E) invasive capacity were
assessed by western blot and Transwell assays, respectively.
Quantification results of (C and E) are presented in (F and G),
respectively. Kruskal-Wallis test was used in this figure.
*P<0.05, **P<0.01 vs. control. miR,
microRNA; ctrl, control; CCNA2, cyclin A2; MMP7, matrix
metalloproteinase 7; siRNA, small interfering RNA; EV, empty
vector. |
To study whether ectopic expression of MMP7 may
rescue the inhibition of migration and invasion, cells were
co-transfected SKOV3 with miR-508-3p mimics and the plasmid
expressing MMP7. As demonstrated in Fig. 4D and E, the suppressive effects of
miR-508-3p were partially reversed by ectopic expression of MMP7.
At 48 h post-transfection, miR-508-3p and empty vector led to a
significant suppression of migration (P<0.05; Fig. 4E and G) and invasion (P<0.05;
Fig. 4E and G) compared with that
in the miR-Ctrl and empty vector group, which was attenuated by the
MMP7 plasmid. These results demonstrated that the effect of the
miR-508-3p mimics was consistent with that of siRNA-MMP7 on SKOV3
cells, suggesting that miR-508-3p may inhibit the migration and
invasion of ovarian cancer cells by directly targeting MMP7.
High miR-508-3p expression is associated
with low CCNA2 and MMP7 expression and improved OS
Considering that miR-508-3p expression impaired
tumor aggressiveness in the cellular models, its expression was
evaluated in a cohort of 130 clinically annotated serous tumor
samples. Expression of miR-508-3p and the control U6 small RNA was
evaluated by ISH, whereas CCNA2 and MMP7 were evaluated by IHC in
the same tissue samples.
To compare the levels of miR-508-3p, as well as
CCNA2 and MMP7 in ovarian cancer with normal ovarian tissue, their
expression was also evaluated in adjacent non-tumor tissue. The
results demonstrated that miR-508-3p expression in ovarian cancer
was significantly lower compared with that in adjacent non-tumor
tissue (P<0.001; Fig. 5A). By
contrast, CCNA2 expression in cancer tissue was significantly
higher compared with that in adjacent non-tumor tissue (P<0.001;
Fig. 5B). The results of MMP7
analysis were similar to those of CCNA2 (P<0.05; Fig. 5C).
 | Figure 5miR-508-3p expression in ovarian
cancer is lower compared with that in the adjacent non-tumor
tissue, and is associated with low CCNA2 and MMP7 expression
levels. (A-C) Expression of (A) miR-508-3p, (B) CCNA2 and (C) MMP7
in ovarian cancer and adjacent non-tumor tissue. (D) Representative
images of ISH of miR-508-3p and IHC for CCNA2, MMP7 and Ki67 in
cases expressing low or high levels of miR-508-3p. (E) Bar charts
demonstrating the association between the expression of miR-508-3p
and CCNA2, MMP7 and Ki-67 determined by IHC. Student's t-test and
log-rank test was used to analyze the data. Patients were
stratified into high and low expression subgroups using the mean
miR-508-3p, CCNA2 or MMP7 expression scores.
**P<0.01, ***P<0.001. miR, microRNA;
CCNA2, cyclin A2; MMP7, matrix metalloproteinase 7; ISH, in situ
hybridization; IHC, immunohistochemistry. |
A significant decrease in miR-508-3p expression was
observed with disease progression from stages I-II to III-IV
(P=0.028; Table SI). No
significant differences in clinicopathologic characteristics were
observed between the CCNA2 low and high groups (Table SII) or between the MMP7 low and
high groups (Table SIII). In
addition, both CCNA2 and MMP7 IHC scores in the tissues from the
miR-508-3p low group were significantly higher compared with those
from the miR-508-3p high group (P<0.001; Fig. 5D and 5E). The Ki67 expression score in the
miR-508-3p low group was also significantly higher compared with
that in the miR-508-3p high group (P<0.001). Univariate log-rank
analysis demonstrated that older age, advanced FIGO stage, residual
disease >1 cm, high CCNA2 and MMP7 and low miR-508-3p expression
were significantly associated with a shorter OS time (Table I). A multivariate model revealed
that age, residual disease size, miR-508-3p and CCNA2 were
independent predictors for OS in ovarian cancer (Table II). Kaplan-Meier analysis
demonstrated that patients with high expression levels of
miR-508-3p exhibited longer OS times compared with those with low
levels of miR-508-3p (median OS 43.91 vs. 27.90 months,
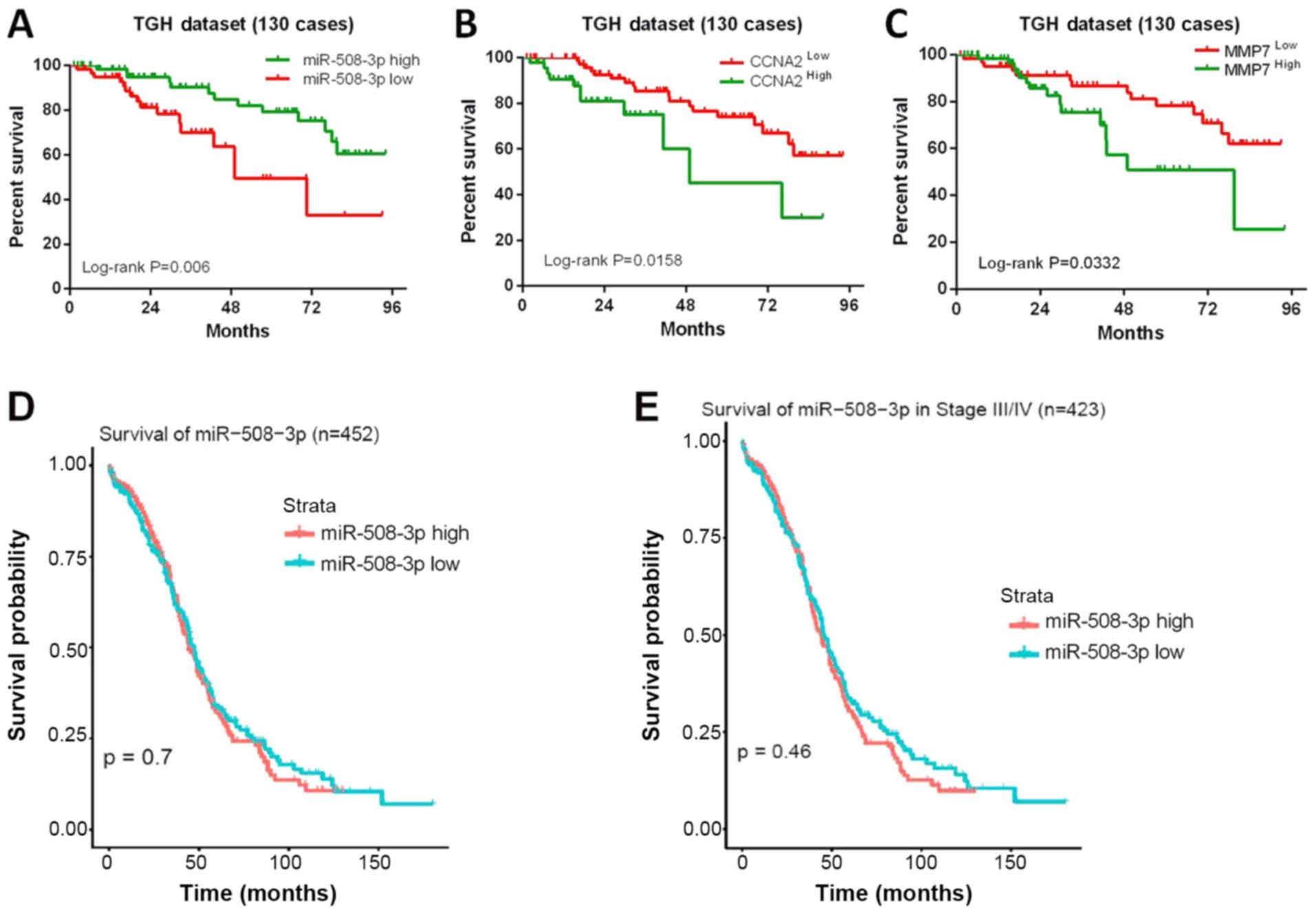
respectively; log-rank P=0.006; Fig.
6A). Accordingly, high levels of CCNA2 or MMP7 predicted a
shorter OS time compared with low expression levels in a univariate
analysis of patients with ovarian cancer (median OS 45.36 vs. 22.61
months, respectively; log-rank P=0.0158 for CCNA2; median OS 45.15
vs. 28.16 months, respectively; log-rank P=0.0332 for MMP7;
Fig. 6B and C).
 | Table IUnivariate log-rank analysis of
prognostic factors in ovarian cancer. |
Table I
Univariate log-rank analysis of
prognostic factors in ovarian cancer.
| Factors | No. (130) | No. dead | Overall survival
|
|---|
| HR | 95% CI | P-value |
|---|
| Age, years | | | | | |
| <59 | 65 | 12 | 2.265 | 1.069-4.797 | 0.033a |
| ≥59 | 65 | 17 | | | |
| Gradeb | | | | | |
| Low | 33 | 5 | 1.411 | 0.5936-3.354 | 0.436 |
| High | 97 | 24 | | | |
| FIGO stageb | | | | | |
| I+II | 40 | 6 | 2.194 | 1.025-4.696 | 0.043a |
| III+IV | 90 | 23 | | | |
| Residual disease,
cm | | | | | |
| ≤1 | 95 | 13 | 4.294 | 2.792-15.16 | <0.001a |
| >1 | 35 | 16 | | | |
| Ascites
cytology | | | | | |
| Negative | 84 | 17 | 1.988 | 0.8722-4.530 | 0.102 |
| Positive | 46 | 12 | | | |
| miR-508-3p | | | | | |
| Low | 61 | 16 | 0.3224 | 0.1437-0.7234 | 0.006a |
| High | 69 | 13 | | | |
| CCNA2 | | | | | |
| Low | 81 | 18 | 2.390 | 1.269-7.739 | 0.016a |
| High | 49 | 11 | | | |
| MMP7 | | | | | |
| Low | 66 | 14 | 2.086 | 1.117-5.196 | 0.033a |
| High | 64 | 15 | | | |
 | Table IIMultivariate Cox regression analysis
for overall survival in ovarian cancer. |
Table II
Multivariate Cox regression analysis
for overall survival in ovarian cancer.
| Factors | Overall survival
|
|---|
| HR | 95% CI | P-value |
|---|
| Age (<59 vs. ≥59
years) | 2.410 | 1.104-5.263 | 0.027a |
| FIGO stageb (I+II vs. III+IV) | 1.468 | 0.532-4.049 | 0.458 |
| Residual disease
(≤1 vs. >1 cm) | 3.342 | 1.474-7.576 | 0.004a |
| miR-508-3p (low vs.
high) | 0.412 | 0.183-0.928 | 0.032a |
| CCNA2 (low vs.
high) | 2.429 | 1.025-5.756 | 0.044a |
| MMP7 (low vs.
high) | 2.084 | 0.881-4.929 | 0.094 |
To evaluate these results, ovarian carcinoma data
from TCGA were analyzed. Among 452 patients with ovarian cancer,
Kaplan-Meier analysis demonstrated that compared with patients with
low miR-508-3p expression, those with high expression levels of
miR-508-3p did not exhibit a longer OS time (log-rank P=0.07;
Fig. 6D). The same conclusion was
reached for the 423 patients with stage III-IV ovarian cancer
(log-rank P=0.46; Fig. 6E).
Discussion
Emerging evidence from previous studies has
demonstrated that miRNAs regulate carcinogenesis and cancer
development (19-21). Several studies have reported that
miR-508-3p may function as a tumor suppressor or promoter in
different tumors, such as renal cell carcinoma and gastric cancer
(6,9,22).
But its exact roles and clinical relevance in ovarian cancer remain
unclear. The results of the present study demonstrated that
miR-508-3p may act as a tumor suppressor to inhibit cell
proliferation, migration and invasion in ovarian cancer. To the
best of our knowledge, the present study was the first to identify
that CCNA2 and MMP7 were direct targets of miR-508-3p
in ovarian cancer. In addition, the results demonstrated that the
expression levels of miR-508-3p and CCNA2 were negatively
associated, and that they were independent predictors for the OS
time of patients with ovarian cancer.
The results of the present study demonstrated that
miR-508-3p regulated the proliferation and cell cycle by directly
binding to the 3′-UTR of CCNA2 in ovarian cancer cells. In
these experiments, HeLa cells were used for the Luciferase assays,
as HeLa cells exhibit higher transfection efficiency compared with
ovarian cancer cells. The results identified a direct binding site
between miR-508-3p and the 3′-UTRs of the target genes. These
direct targeted relationship is also likely to exist in ovarian
cancer cells. CCNA2 is a main mitotic cyclin that controls the cell
cycle by binding CDK1 and CDK2 (23). CCNA2 contributes to controlling the
S phase by initiating DNA synthesis and, subsequently, mitotic
entry (24). Abnormal expression
of CCNA2 has been detected in various types of cancer, such as
non-small cell lung cancer and colorectal carcinoma (25-27),
and deregulation of CCNA2 is associated with chromosomal
instability and tumor proliferation (28). CCNA2/CDK complexes phosphorylate
proteins such as Rb (28), and
phosphorylation of RB results in its inactivation and upregulation
of CDK4/6, which allows progression through G1 into the S phase
(29). The present results
revealed that miR-508-3p induced proliferation suppression and G1
phase arrest in ovarian cancer cells, which were mediated by
targeting the CCNA2/pRb/CDK4/6 signaling pathway. Thus, repressing
CCNA2/pRb/CDK4/6 signaling may provide a rationale for the
treatment of ovarian cancer.
To investigate the possible mechanisms that underlie
the regulation of ovarian cancer cell migration and invasion
potential by miR-508-3p, MMPs, which are known to be direct
regulators of tumor metastasis, were examined in the present study.
The results demonstrated that MMP2 and MMP7 were downregulated by
miR-508-3p. MMP2 may not be directly regulated by miR-508-3p, as no
miR-508-3p targeting sequence was identified in the 3′-UTR of MMP.
Upregulation of MMP7, including in serum, has been reported in
various types of cancer (30-32),
including ovarian (12) and
gastric cancer (30). In addition,
high serum MMP7 levels alone (33)
or in combinations with chromogranin A are independently associated
with disease-specific survival in prostate cancer (34). MMP7 induces
epithelial-to-mesenchymal transformation (EMT) in cancer by
disrupting the E-cadherin/β-catenin complex and upregulating EMT
transcription factors (35), thus
facilitating migration and invasion. The results of the present
study demonstrated that MMP7 was a direct functional target of
miR-508-3p via the analysis of the luciferase reporter assay.
miR-508-3p downregulated the mRNA and protein levels of MMP7 in
ovarian cancer cells. The results also confirmed that the
miR-508-3p-induced attenuation of MMP7 levels, including those in
the supernatant, significantly decreased the migration and invasion
of ovarian cancer cells. These results were further validated by
the rescue experiments.
The results of the present study indicated that
miR-508-3p expression was decreased in serous ovarian cancer
compared with that in normal ovarian tissue. A previous study has
demonstrated that the methylation of miRNA promoters may explain
the deregulation of miRNAs in cancer (15). However, the molecular mechanism of
miR-508-3p downregulation remains unclear in ovarian cancer. Zhao
et al (36) have reported
that miR-508-3p downregulation in ovarian cancer is due to promoter
hypermethylation, and that miR-508-3p may be a potential new
biomarker for ovarian cancer. Further studies are required to
investigate the deregulation mechanism. The results of the present
study confirmed that miR-508-3p expression was associated with
improved prognosis and was an independent predictor for OS in
ovarian cancer by multivariate analysis, highlighting its potential
value as a prognostic biomarker in ovarian cancer.
It has been reported that CCNA2 expression in tumors
may also have prognostic value (11,37).
In the present study, high CCNA2 expression was associated with
poor survival. Multivariate analysis revealed that CCNA2 was an
independent predictor of OS in ovarian cancer. A previous study has
reported that upregulation of MMP7 in serum was associated with a
significantly impaired OS in cancers (31). The present data demonstrated that
MMP7 expression in tumor tissues was associated with a poor
survival of patients with ovarian cancer, but MMP7 was not an
independent predictor for OS. The results of the present study also
demonstrated that CCNA2 and MMP7 expression was negatively
associated with miR-508-3p expression in tumor tissues, which
suggested that miR-508-3p downregulation may contribute to the
increased expression of CCNA2 and MMP7 in ovarian cancer. Thus,
CCNA2 and MMP7 may be promising therapeutic candidates for ovarian
cancer.
Taken together, the results of the present study
demonstrated that miR-508-3p suppressed the proliferation,
migration and invasion of ovarian cancer cells by directly
regulating CCNA2 and MMP7 and may serve as an independent
prognostic factor for the OS of patients with ovarian cancer. Thus,
these results are encouraging and suggest that miR-508-3p and its
downstream molecules CCNA2 and MMP7 are potential targets for the
treatment of ovarian cancer.
Supplementary Data
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81772790 to FXX, 81602292
to FG and 81472761 to GYL), the Science & Technology Foundation
for Selected Overseas Chinese Scholars, Bureau of Personnel of
China, Tianjin (grant no. 2016017 to FG), Tianjin Medical
University General Hospital Grant (grant no. ZYYFY2014004 to FG)
and the Postgraduate Innovation Fund of 13th Five-year
comprehensive investment, Tianjin Medical University (grant no.
YJSCX201812 to KZ).
Availability of data and materials
All data generated or analyzed during this study are
available from the corresponding author on reasonable request.
Authors' contributions
FG and FXX designed the study. FG, KZ, MYL and LC
performed the experiments. GYL, YY, WYT and FT performed the
statistical analysis. YFZ, CG, JPG and YMW analyzed the clinical
specimens. FG, YMW and FXX wrote and revised the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Tianjin Medical University General Hospital (TGH) and all
procedures followed the principles of the Declaration of Helsinki.
All subjects provided written informed consent prior to
participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Professor Shizhu Yu
and Professor Chunsheng Kang (Department of Neuropathology, Tianjin
Neurological Institute, Tianjin Medical University General
Hospital) for their discussion and revision of this manuscript.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nasioudis D, Kahn R, Chapman-Davis E, Frey
MK, Caputo TA, Witkin SS and Holcomb K: Impact of hospital surgical
volume on complete gross resection (CGR) rates following primary
debulking surgery for advanced stage epithelial ovarian carcinoma.
Gynecol Oncol. 154:401–404. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rojas V, Hirshfield KM, Ganesan S and
Rodriguez-Rodriguez L: Molecular Characterization of Epithelial
Ovarian Cancer: Implications for Diagnosis and Treatment. Int J Mol
Sci. 17:172016. View Article : Google Scholar
|
|
5
|
Allemani C, Weir HK, Carreira H, Harewood
R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A,
et al CONCORD Working Group: Global surveillance of cancer survival
1995-2009: Analysis of individual data for 25,676,887 patients from
279 population-based registries in 67 countries (CONCORD-2).
Lancet. 385:977–1010. 2015. View Article : Google Scholar
|
|
6
|
Zhai Q, Zhou L, Zhao C, Wan J, Yu Z, Guo
X, Qin J, Chen J and Lu R: Identification of miR-508-3p and
miR-509-3p that are associated with cell invasion and migration and
involved in the apoptosis of renal cell carcinoma. Biochem Biophys
Res Commun. 419:621–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu X, Zhang X, Bi T, Ding Y, Zhao J, Wang
C, Jia T, Han D, Guo G, Wang B, et al: MiRNA expression signature
for potentially predicting the prognosis of ovarian serous
carcinoma. Tumour Biol. 34:3501–3508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hidaka H, Seki N, Yoshino H, Yamasaki T,
Yamada Y, Nohata N, Fuse M, Nakagawa M and Enokida H: Tumor
suppressive microRNA-1285 regulates novel molecular targets:
Aberrant expression and functional significance in renal cell
carcinoma. Oncotarget. 3:44–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang T, Kang W, Zhang B, Wu F, Dong Y,
Tong JH, Yang W, Zhou Y, Zhang L, Cheng AS, et al: miR-508-3p
concordantly silences NFKB1 and RELA to inactivate canonical NF-κB
signaling in gastric carcinogenesis. Mol Cancer. 15:92016.
View Article : Google Scholar
|
|
10
|
Yamamoto S, Tsuda H, Miyai K, Takano M,
Tamai S and Matsubara O: Cumulative alterations of p27-related
cell-cycle regulators in the development of
endometriosis-associated ovarian clear cell adenocarcinoma.
Histopathology. 56:740–749. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee YH, Heo JH, Kim TH, Kang H, Kim G, Kim
J, Cho SH and An HJ: Significance of cell cycle regulatory proteins
as malignant and prognostic biomarkers in ovarian epithelial
tumors. Int J Gynecol Pathol. 30:205–217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song N, Liu H, Ma X and Zhang S: Placental
growth factor promotes metastases of ovarian cancer through
miR-543-regulated MMP7. Cell Physiol Biochem. 37:1104–1112. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu B, Liu X and Chang H: MicroRNA-143
inhibits colorectal cancer cell proliferation by targeting MMP7.
Minerva Med. 108:13–19. 2017.
|
|
14
|
Berek JS, Crum C and Friedlander M: Cancer
of the ovary, fallopian tube, and peritoneum. Int J Gynaecol
Obstet. 119(Suppl 2): S118–S129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang D, Sun Y, Hu L, Zheng H, Ji P, Pecot
CV, Zhao Y, Reynolds S, Cheng H, Rupaimoole R, et al: Integrated
analyses identify a master microRNA regulatory network for the
mesenchymal subtype in serous ovarian cancer. Cancer Cell.
23:186–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Parker BC, Annala MJ, Cogdell DE, Granberg
KJ, Sun Y, Ji P, Li X, Gumin J, Zheng H, Hu L, et al: The
tumorigenic FGFR3-TACC3 gene fusion escapes miR-99a regulation in
glioblastoma. J Clin Invest. 123:855–865. 2013.PubMed/NCBI
|
|
17
|
Zarkowska T, U S, Harlow E and Mittnacht
S: Monoclonal antibodies specific for underphosphorylated
retinoblastoma protein identify a cell cycle regulated
phosphorylation site targeted by CDKs. Oncogene. 14:249–254. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Hu Y, Cui J, Zhou Y and Chen L:
Coordinated targeting of MMP-2/MMP-9 by miR-296-3p/FOXCUT exerts
tumor-suppressing effects in choroidal malignant melanoma. Mol Cell
Biochem. 445:25–33. 2018. View Article : Google Scholar
|
|
19
|
Sun Y, Hu L, Zheng H, Bagnoli M, Guo Y,
Rupaimoole R, Rodriguez-Aguayo C, Lopez-Berestein G, Ji P, Chen K,
et al: miR-506 inhibits multiple targets in the
epithelial-to-mesenchymal transition network and is associated with
good prognosis in epithelial ovarian cancer. J Pathol. 235:25–36.
2015. View Article : Google Scholar
|
|
20
|
Liu G, Sun Y, Ji P, Li X, Cogdell D, Yang
D, Parker Kerrigan BC, Shmulevich I, Chen K, Sood AK, et al:
miR-506 suppresses proliferation and induces senescence by directly
targeting the CDK4/6-FOXM1 axis in ovarian cancer. J Pathol.
233:308–318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Y, Guo F, Bagnoli M, Xue FX, Sun BC,
Shmulevich I, Mezzanzanica D, Chen KX, Sood AK, Yang D, et al: Key
nodes of a microRNA network associated with the integrated
mesen-chymal subtype of high-grade serous ovarian cancer. Chin J
Cancer. 34:28–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin C, Liu A, Zhu J, Zhang X, Wu G, Ren P,
Wu J, Li M, Li J and Song L: miR-508 sustains phosphoinositide
signalling and promotes aggressive phenotype of oesophageal
squamous cell carcinoma. Nat Commun. 5:46202014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cribier A, Descours B, Valadão AL,
Laguette N and Benkirane M: Phosphorylation of SAMHD1 by cyclin
A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep.
3:1036–1043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Das E, Jana NR and Bhattacharyya NP:
MicroRNA-124 targets CCNA2 and regulates cell cycle in
STHdh(Q111)/Hdh(Q111) cells. Biochem Biophys Res Commun.
437:217–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van Olphen SH, Ten Kate FJ, Doukas M,
Kastelein F, Steyerberg EW, Stoop HA, Spaander MC, Looijenga LH,
Bruno MJ, Biermann K and ProBar-Study Group: value of cyclin A
immunohistochemistry for cancer risk stratification in Barrett
esophagus surveillance: A multicenter case-control study. Medicine
(Baltimore). 95:e54022016. View Article : Google Scholar
|
|
26
|
Cooper WA, Kohonen-Corish MR, McCaughan B,
Kennedy C, Sutherland RL and Lee CS: Expression and prognostic
significance of cyclin B1 and cyclin A in non-small cell lung
cancer. Histopathology. 55:28–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nozoe T, Inutsuka S, Honda M, Ezaki T and
Korenaga D: Clinicopathologic significance of cyclin A expression
in colorectal carcinoma. J Exp Clin Cancer Res. 23:127–133.
2004.PubMed/NCBI
|
|
28
|
Gopinathan L, Tan SL, Padmakumar VC,
Coppola V, Tessarollo L and Kaldis P: Loss of Cdk2 and cyclin A2
impairs cell proliferation and tumorigenesis. Cancer Res.
74:3870–3879. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Srethapakdi M, Liu F, Tavorath R and Rosen
N: Inhibition of Hsp90 function by ansamycins causes retinoblastoma
gene product-dependent G1 arrest. Cancer Res. 60:3940–3946.
2000.PubMed/NCBI
|
|
30
|
Xu J, e C, Yao Y, Ren S, Wang G and Jin H:
Matrix metalloproteinase expression and molecular interaction
network analysis in gastric cancer. Oncol Lett. 12:2403–2408. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Klupp F, Neumann L, Kahlert C, Diers J,
Halama N, Franz C, Schmidt T, Koch M, Weitz J, Schneider M, et al:
Serum MMP7, MMP10 and MMP12 level as negative prognostic markers in
colon cancer patients. BMC Cancer. 16:4942016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu R and Tian Y: Astrocyte elevated
gene-1 increases invasiveness of NSCLC through up-regulating MMP7.
Cell Physiol Biochem. 37:1187–1195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maurel J, Nadal C, Garcia-Albeniz X,
Gallego R, Carcereny E, Almendro V, Mármol M, Gallardo E, Maria
Augé J, Longarón R, et al: Serum matrix metalloproteinase 7 levels
identifies poor prognosis advanced colorectal cancer patients. Int
J Cancer. 121:1066–1071. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Niedworok C, Tschirdewahn S, Reis H,
Lehmann N, Szucs M, Nyirady P, Romics I, Rubben H and Szarvas T:
serum chromogranin a as a complementary marker for the prediction
of prostate cancer-specific survival. Pathol Oncol Res. 23:643–650.
2017. View Article : Google Scholar
|
|
35
|
Zhang Q, Liu S, Parajuli KR, Zhang W,
Zhang K, Mo Z, Liu J, Chen Z, Yang S, Wang AR, et al:
Interleukin-17 promotes prostate cancer via MMP7-induced
epithelial-to-mesenchymal transition. Oncogene. 36:687–699. 2017.
View Article : Google Scholar :
|
|
36
|
Zhao L, Wang W, Xu L, Yi T, Zhao X, Wei Y,
Vermeulen L, Goel A, Zhou S and Wang X: Integrative network biology
analysis identifies miR-508-3p as the determinant for the
mesenchymal identity and a strong prognostic biomarker of ovarian
cancer. Oncogene. 38:2305–2319. 2019. View Article : Google Scholar :
|
|
37
|
Li HP, Ji JF, Hou KY, Lei YT, Zhao HM,
Wang J, Zheng J, Liu JY, Wang MP, Xiao Y, et al: Prediction of
recurrence risk in early breast cancer using human epidermal growth
factor 2 and cyclin A2. Chin Med J (Engl). 123:431–437. 2010.
|