Introduction
Cancer cells have the ability to evade or escape
immune monitor and destruction, which leads to carcinogenesis and
cancer progression (1).
Immunosuppression and evasion can be mediated via a variety of
mechanisms, such as interleukin (IL)-10 or transforming growth
factor (TGF)-β induced Th2 polarization (2-5), the
overexpression of Fas ligand/TRAIL (6,7), the
overexpression of complement inhibitors (DAF and CD55) (8), the loss of MHC class I molecules or
tumor antigens, as well as the overexpression of indoleamine
2,3-dioxygenase (IDO), a tryptophan catabolizing enzyme (9-13).
It has been demonstrated that tryptophan catabolism by IDO1
mediates a mechanism that suppresses T-cells, providing balance or
feedback control in immune reactions (14,15).
Over the past decade, a novel immunosuppression factor, namely the
tryptophan catabolizing enzyme, IDO2, has attracted attention and
has stimulated research in tumor and autoimmune diseases (16). IDO2 and IDO1 are homologous
proteins and they are arranged in tandem on the same chromosome.
Similar to the classical immunosuppression enzyme IDO1, IDO2 can
degrade tryptophan, although it's the efficiency is less than that
of IDO1 (17-19). Previous studies have demonstrated
their similar functions in the immune system. It has been reported
that IDO2 is expressed in dendritic cells (DCs) (17,20)
and that IDO2 is expressed in peripheral blood DCs by a
steady-state model and may contribute to the homeostatic
tolerogenic capacity of DCs under healthy conditions (21). IDO2 gene-transfected 293 cells have
been shown to inhibit CD4+ and CD8+ T-cell
proliferation in a co-culture system (18). Additionally, IDO2 is critical for
IDO1-mediated T-cell regulation and performs a non-redundant
function in inflammation (22).
DCs are antigen-presenting cells, forms key link
between the innate and adaptive immune responses and play a pivotal
role in the initiation of the immune response. Due to their unique
ability, DCs attract interest in tumor immunotherapy and vaccine
development. The activation of DCs is essential for the stimulation
of immunity. It has been demonstrated in vitro and in
vivo that DCs loaded with tumor antigens (ex vivo)
successfully induce the activity of cytotoxic T-cells against tumor
cells in animal models or clinical trials (23-25).
In previous studies, the authors successfully developed IDO-siRNA
based antitumor therapeutics through the direct knockdown of IDO in
mice with tumors (26-28) or using IDO-silenced DCs as
antitumor vaccines (29,30). However, role of IDO2 in DC-mediated
antitumor immunity has not yet been studied, at least to the best
of our knowledge. In view of the role of IDO2 in immunosuppression,
it was hypothesized that the silencing of IDO2 in DCs would
activate the DCs to enhance the antitumor response and furthermore,
to suppress tumor progression.
In the present study, the potent gene silencing
method was applied to knock down IDO2 expression in DCs. In ex vivo
experiments, it was demonstrated that the silencing of IDO2
promoted DCs maturation, which elicited strong T-cell responses.
Using a murine lung cancer model, it was found that the
IDO2-silenced DC-based cancer vaccine effectively suppressed tumor
growth and enhanced the antitumor immune response in vivo. To the
best of our knowledge, this is the first study to report the role
of IDO2 in DCs in a murine lung cancer model. The IDO2-silenced
DC-based cancer vaccine may thus prove to be a novel potent cancer
therapy through the depletion of immune suppression and the
reinstallation of anticancer immunity.
Materials and methods
Animals and cancer cell lines
A total of 80 female C57/BL6 mice and 20 female
BABL/C mice (6 to 8 weeks old, weighing 18-22 g) were purchased
from Changsha Laboratory Animal Co. Ltd. All the mice were kept in
a specific pathogen-free grade environment, without dietary
restrictions, at a temperature of 25±2°C and 60±5% air relative
humidity. All animal experiments complied with the Regulations for
the Administration of Affairs Concerning Experimental Animals of
China and ethics approval was obtained from the Institutional
Animal Care and Use Committee of Nanchang University. The LLC cell
line was purchased from the China Center for Type Culture
Collection (CCTCC) and cultured in DMEM (Invitrogen, Life
Technologies; Thermo Fisher Scientific, Inc.) containing 10% FBS,
L-glutamine, penicillin and streptomycin in 5% CO2 and
at 37°C.
Generation of C57BL/6 bone marrow-derived
DCs
DCs were generated from C57BL/6 bone marrow
progenitor cells as previously described (31). Briefly, the femurs of mice were
removed and the marrow cavity was flushed with RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) to obtain bone marrow
progenitor cells. After washing with RPMI-1640 medium twice, the
obtained cells were plated in 6-well tissue culture plates. Each
well was supplemented with 4 ml DC induction medium which contained
RPMI-1640 medium, 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
10 ng/ml recombinant murine IL-4 and 10 ng/ml
granulocyte-macrophage colony-stimulating factor (GM-CSF;
PeproTech, Inc.). The induced cells were maintained at 37°C in 5%
humidified CO2 and the medium was replaced with new DC
induction medium described above every 2 days.
Synthesis of IDO2 siRNA and gene
silencing
The siRNA targeting murine IDO2 mRNA was designed in
accordance with the target sequence selection method and
synthesized by the manufacturer (Sigma-Aldrich; Merck KGaA). GL2
siRNA targeting the luciferase gene was used as the control siRNA.
IDO2 (5'-GUCAUGUCCUGCACCCUAA-3') and GL2
(5'-GCAUGCGCCUUAUGAAGCU-3') siRNAs were transfected into the DCs
using Lipofectamine2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Briefly, the cells were collected, centrifuged
(1,000 × g) for 5 min, resuspended in Opti MEM®
serum-reduced medium (Invitrogen, Life Technologies; Thermo Fisher
Scientific, Inc.) and then plated in 12-well plates
(2×106 cells/well). A total of 1 µg IDO2- or GL2-siRNA
was incubated with 2 µl Lipofectamine 2000 reagent in 200 µl of
optimal serum-reduced medium at room temperature for 20 min and the
mixture was then gently added to the cells in each group.
IDO2 mRNA quantification by RT-qPCR
DCs were collected, lysed and total RNA was
extracted according to the manual of manufacturer (TRIzol reagent;
Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (1 µg) was
used as a template to synthesize cDNA by reverse transcriptase
(MMLV-RT, Invitrogen; Thermo Fisher Scientific, Inc.). qPCR were
conducted using a Stratagene Mx3000P QPCR System (Agilent
Technologies, Inc.) and 2X SYBR-Green PCR Master Mix (Life
Technologies; Thermo Fisher Scientific, Inc.) was used to perform
the reactions according to manufacturer's protocol. The PCR
thermocycling conditions were as follows: Denaturation for 2 min at
95°C, followed by 44 cycles of denaturation for 10 sec at 95°C, and
extension for 20 sec at 58°C. The following primer sequences were
used for target gene amplifications: IDO2 forward,
5'-GTGGGGCTGGTCTATGAAGGTG-3' and reverse,
5'-TGGTGGCAGCGGAGATAATGTA-3'; IDO1 forward,
5'-GGGCTTTGCTCTACCACATCCACT-3' and reverse,
5'-ACATCGTCATCCCCTCGGTTCC-3'; and GAPDH forward,
5'-TGATGACATCAAGAAGGTGGTGAA-3' and reverse, 5'-TCCTTG
GAGGCCATGTAGGCCAT-3'. Differences in gene expression were
calculated using the ∆∆Cq method (32).
Western blot analysis
The cells were collected, washed twice with PBS, and
re-suspended in iced RIPA buffer (Cell Signaling Technology, Inc.)
for 30 min. Lysates were centrifuged (20,000 × g) for 30 min at 4°C
to obtain the total protein. A total of 50 µg of total protein was
then separated on a 12% SDS-PAGE and transferred to a
nitrocellulose membrane. To reduce background intensity, 5%
fat-free milk in TBS-T (0.25% Tween-20) was used to block the
membrane. To probe the target protein, the blocked membranes were
incubated with a rabbit anti-mouse IDO2 monoclonal antibody (cat.
no. sc-374159, 1:500, clone C-9, Santa Cruz Biotechnology, Inc.) or
β-actin (cat. no. sc-58673, 1:5,000, clone 2Q1055, Santa Cruz
Biotechnology, Inc.) overnight at 4°C. Following incubation, the
membrane was washed with PBST for 3 times and then incubated with a
mouse anti-rabbit IgG-HRP antibody (sc-2357, 1:5,000, Santa Cruz
Biotechnology, Inc.) for 1.5 h at room temperature. The ECL system
was used for membrane color rendering (GE Healthcare).
Flow cytometry
Flow cytometry was used to analyze the
characterization of DCs and the induction of regulatory T-cells
(Tregs) in vitro. DCs were harvested and stained with
FITC-CD11c mAb (cat. no. MA5-16877, clone N418), PE-CD80 mAb (cat.
no. 12-0801-82, clone 16-10A1), PE-CD86 mAb (cat. no. 12-0862-82,
clone GL1) and Pe-Cy5-CD40 mAb (cat. no. 15-0401-82, clone 1C10)
(eBioscience; Thermo Fisher Scientific, Inc.) for 30 min at 4°C.
For Treg subsets analyzing, the cells were stained with FITC-Foxp3
mAb (cat. no. 11-5773-82, clone FJK-16s), PE-CD4 mAb (cat. no.
12-0041-82, clone GK1.5) and PeCy5-CD25 mAb (cat. no. 15-0251-82,
clone PC61.5) (eBioscience; Thermo Fisher Scientific, Inc.) for 30
min at 4°C. Flow cytometry was also used to determine the apoptosis
of T-cells and subsets of regulatory T-cells from the
tumor-draining lymph nodes or spleen from the tumor-bearing mice
in vivo. To explore cell apoptosis of T cells, PE-CD4 mAb or
PeCy5-CD8 mAb (cat. no. 15-0081-82, clone 53-6.7) (eBioscience;
Thermo Fisher Scientific, Inc.) were used to pre-stained the
T-cells for 30 min at 4°C and then stained with FITC-Annexin V (BD
Pharmingen) for 5 min at 4°C. All cells were examined using a BD
FACSCalibur flow cytometer (BD Biosciences) and the results were
analyzed using FlowJo software (Tree Star, Inc.).
Mixed lymphocyte reaction (MLR) and Treg
induction
T-cells from the lymph nodes of naïve BALB/c mice
were used to perform the allogeneic MLR and Treg induction.
Briefly, the lymph nodes of mice were collected and paced on 6-well
plate which contains a 200 mesh screen strainer and 2 ml RPMI-1640
medium and then grinded, purified and enriched using nylon wool
columns. T-cells were labeled with 5(6)-carboxyfluorescein diacetate
N-succinimidyl ester (CFSE) (eBioscience; Thermo Fisher Scientific,
Inc.) for mixed lymphocyte reaction, while the unlabeled cells were
used for Treg induction. IDO2-siRNA- or GL2-siRNA-transfected DCs
(1×105) and T-cells (1×106) were co-cultured
in 24-well plates. The mixed lymphatic reaction lasted for 3 days
and Treg induction lasted for 5 days. The proliferation of T-cells
(using CFSE) and CD4+CD25+Foxp3+
Tregs was analyzed by flow cytometry using a BD FACSCalibur flow
cytometer (BD Biosciences).
Preparation of IDO2-silenced DC
vaccine
The in vitro induced DCs were transfected with IDO2-
or GL2 (control)-siRNA on day 5 of culture. To prepare tumor
antigen, the mouse LLC lung cancer cells were dissolved using a
6-cycle freeze-thaw method and then centrifuged (20,000 × g) for 30
min at 4°C to obtain the total tumor antigen. The LLC lysate (50
ng/ml) was added to the culture medium following transfection and
kept for 24 h to allow the loading of lung cancer antigen by DCs.
Antigen-pulsed DCs were stimulated to mature using tumor necrosis
factor (TNF)-α (20 ng/ml, PeproTech, Inc.) overnight. The
IDO2-silenced or control, antigen-loaded mature DCs were collected
for used in the following in vivo experiments.
Treatment with IDO2-silenced DC
vaccine
In order to examine the antitumor effect of the
vaccine in vivo, C57/BL6 mice were used to establish a lung
cancer model, and were grouped as follows: Healthy mice, tumor mice
with sham treatment, tumor mice untreated with control DCs and
IDO2-silenced DCs. Each group contained 4 mice and the experiment
was repeated 3 times. LLC cells (5×105 cells in 100 µl
cell suspension) were subcutaneously injected into the upper hind
leg of each mouse. The tumor-bearing mice were treated with
1×106 of the IDO2-silenced or control DC vaccine from
day 3 of LLC cell inoculation, once every 5 days for 4 times. The
tumor diameter was measured using a caliper every other day when
tumors appeared. The tumor volumes were calculated using the
following formula: Tumor volume (mm3) = width
(mm)2 × length (mm) × 0.5.
Cytotoxic T lymphocyte (CTL)-mediated
tumor cell lysis assay
The cytotoxicity of CD8+ T-cells from
DC-treated tumor bearing mice was analyzed using a nonradioactive
cytotoxicity assay kit (Promega Corp.), in order to determine the
tumor-specific lysis against lung cancer cells. Briefly,
CD8+ T-cells were isolated from the draining lymph nodes
of tumor-bearing mice using immunomagnetic beads (Miltenyi Biotec).
The target tumor cells (105 LLCs) were incubated with the
CD8+ T-cells at a ratio of 1:25, 1:50 and 1:100 for 4 h
and the supernatant was then collected for the measurement of
lactate dehydrogenase (LDH) using a coupled enzymatic assay. The
intensity of the color indicated the number of lysed cells. The
cytotoxic activity of CTL (%) = [(absorbance OD) - (spontaneous
effector cell LDH release OD) - (spontaneous target cell LDH
release OD)]/[(maximal LDH release OD) - (spontaneous target cell
LDH release OD)] x100%.
Cytokine secretion assay
The tumor-bearing mice treated with the different DC
vaccines were anesthetized by an intraperitoneal injection of
chloral hydrate (400 mg/kg) and then blood was obtained by cardiac
puncture. The death of the mice was judged by the absence of
corneal reflex, heartbeat and respiration for >5 min. The blood
was placed in a micro-centrifuge tube at room temperature for half
an hour and then centrifuged (3,000 × g) for 20 min at room
temperature. Serum was collected from the upper layer of the blood
using a pipette, removed to another clean micro-centrifuge tube at
and stored in a storage freezer (-80°C) for use in following
experiments. ELLSA kits (eBioscience; Thermo Fisher Scientific,
Inc.) were used to detect the levels of interferon (IFN)-γ, TNF-α,
IL-10 and TGF-β in serum according to the manufacturer
instructions.
Statistical analysis
Data are presented as the means ± SD. The Student's
t-test (2-tailed) was used to determine differences between 2
means. Differences between multiple groups were analyzed by one-way
ANOVA, followed, if necessary, by Tukey's test (≥3 groups). For all
statistical analyses, P-values <0.05 were considered to indicate
statistically significant differences.
Results
expression of IDO2 in DCs
The expression of IDO1 in DCs has a potent
immunosuppressive effect and can induce Tregs in the
microenvironment (14). IDO2, a
homologous protein of IDO1, is expressed in human myeloid and
plasma-like DCs, which can induce Treg cell production in
vitro (21). However, the
tendency of IDO2 expression during the maturation of DCs has not
yet been reported, at least to the best of our knowledge.
Therefore, the present study first investigated the changes
occurring in IDO2 expression during DC maturation. To determine the
IDO2 expression level, bone marrow-derived DCs were induced and
total RNA or protein was collected every other day. As shown in
Fig. 1A, the transcriptional level
of IDO2 increased gradually during DC culture, reaching peak levels
on the 7th day, and then decreasing. At the same time, the change
in the IDO2 protein level was also detected (Fig. 1B). The results revealed that the
change in protein expression was consistent with that observed for
mRNA expression.
In order to examine the role of IDO2 in bone
marrow-derived DCs, siRNA technology was used for gene silencing.
To determine the siRNA efficacy, the DCs were transfected with
Lipofectamine 2000 coated with IDO2-siRNA or GL2-siRNA (control
siRNA). Following 48 h of transfection, the expression of IDO2 mRNA
decreased significantly and the silencing efficiency was >70%
(Fig. 1C). The silencing effect
was further determined by western blot analysis to determine
protein level of IDO2 (Fig. 1D).
The results of both RT-qPCR and western blot analysis revealed that
IDO2-siRNA was effective in knocking down the expression of IDO2 in
bone marrow-derived DCs.
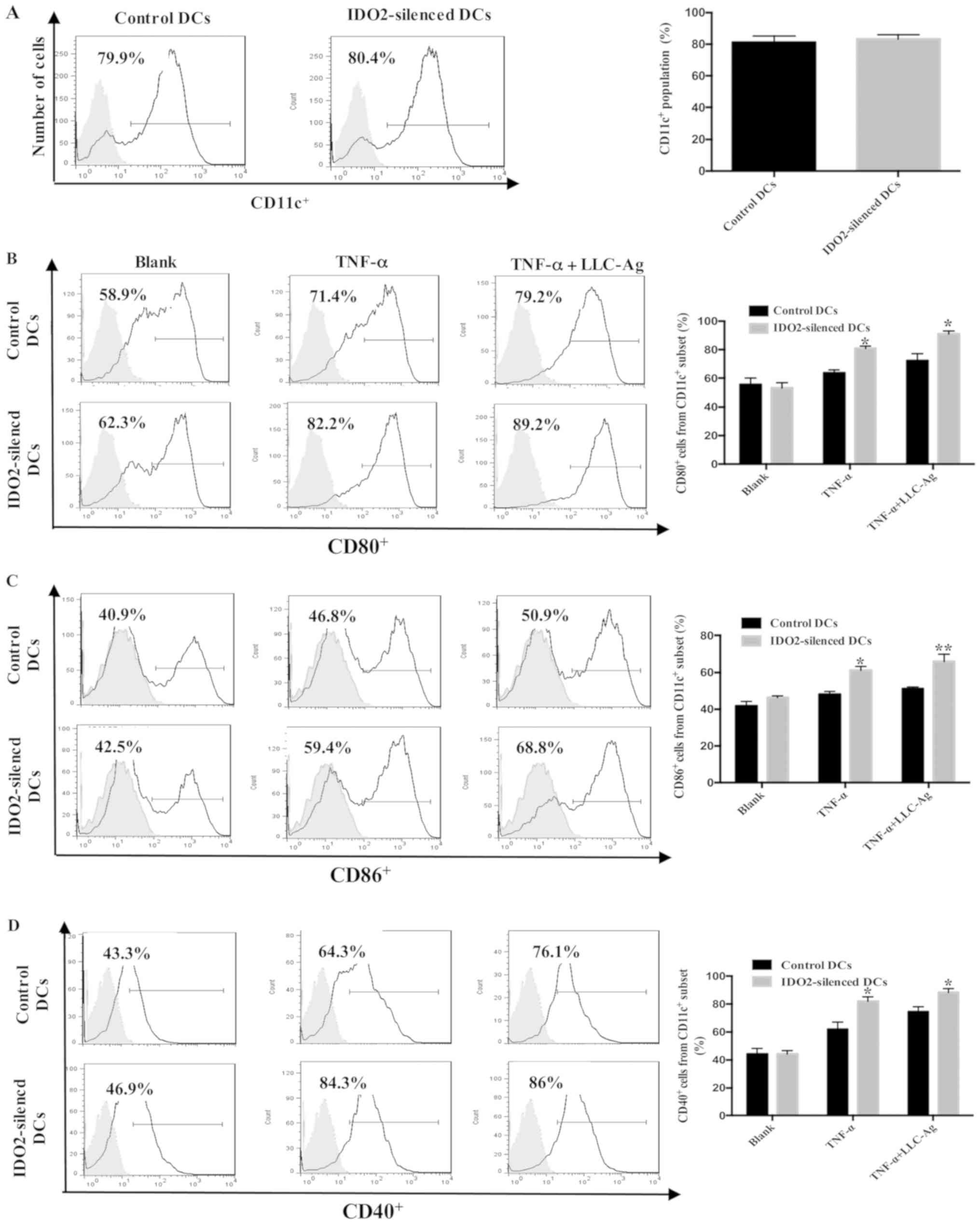
Effect of IDO2 gene silencing on DC
phenotype and maturation
Immunologically competent mature DCs are the most
efficient antigen-presenting cells (APCs). Upon stimulation with
antigen, the phenotype of DCs changes from an immature status to
mature APCs and activates T-cell proliferation (33). In the present study, to investigate
the role of IDO2 in the maturation of DCs, bone marrow-derived DCs
were transfected with IDO2-siRNA or GL2 (control)-siRNA as
presented in Fig. 1, followed by
the assessment of DC maturation markers. As shown in Fig. 2, although IDO2 gene silencing did
not affect the expression of CD11c, CD80, CD86 and CD40 on the
surface of DCs, it significantly increased the percentage of mature
DCs following treatment with TNF-α or TNF-α plus tumor antigen.
These data suggest that IDO2 gene silencing can enhance the
sensitivity of DCs to TNF-α and tumor antigen, which can promote DC
maturation.
IDO2 gene silencing in DCs enhances the
T-cell response and reduces Treg induction
As previously demonstrated, IDO2 gene transfected
into 293 cells can inhibit the CD4+ and CD8+
T-cell proliferation in a co-culture system (18). Therefore, the present study
investigated whether IDO2-silenced DCs may affect the stimulation
of T-cell proliferation. To evaluate the capacity of DCs to
stimulate T-cell responses following the gene silencing of IDO2,
mixed leukocyte reaction was performed. DCs cultured from C57BL/6
mice were transfected with IDO2-siRNA, or GL2-siRNA as the control.
The results revealed that compared to transfection with the control
siRNA, IDO2 gene silencing in the DCs initiated a potent T-cell
response (Fig. 3A). The potential
for gene silencing of IDO2 to alter the Treg induction by DCs was
then determined. The IDO2-silenced DCs were co-cultured with naïve
allogeneic T-cells for 5 days. The percentage of
CD4+CD25+Foxp3+ Tregs was
significantly decreased when the T-cells incubated with
IDO2-silenced DCs, as compared with that of the cells incubated
with control DCs (Fig. 3B).
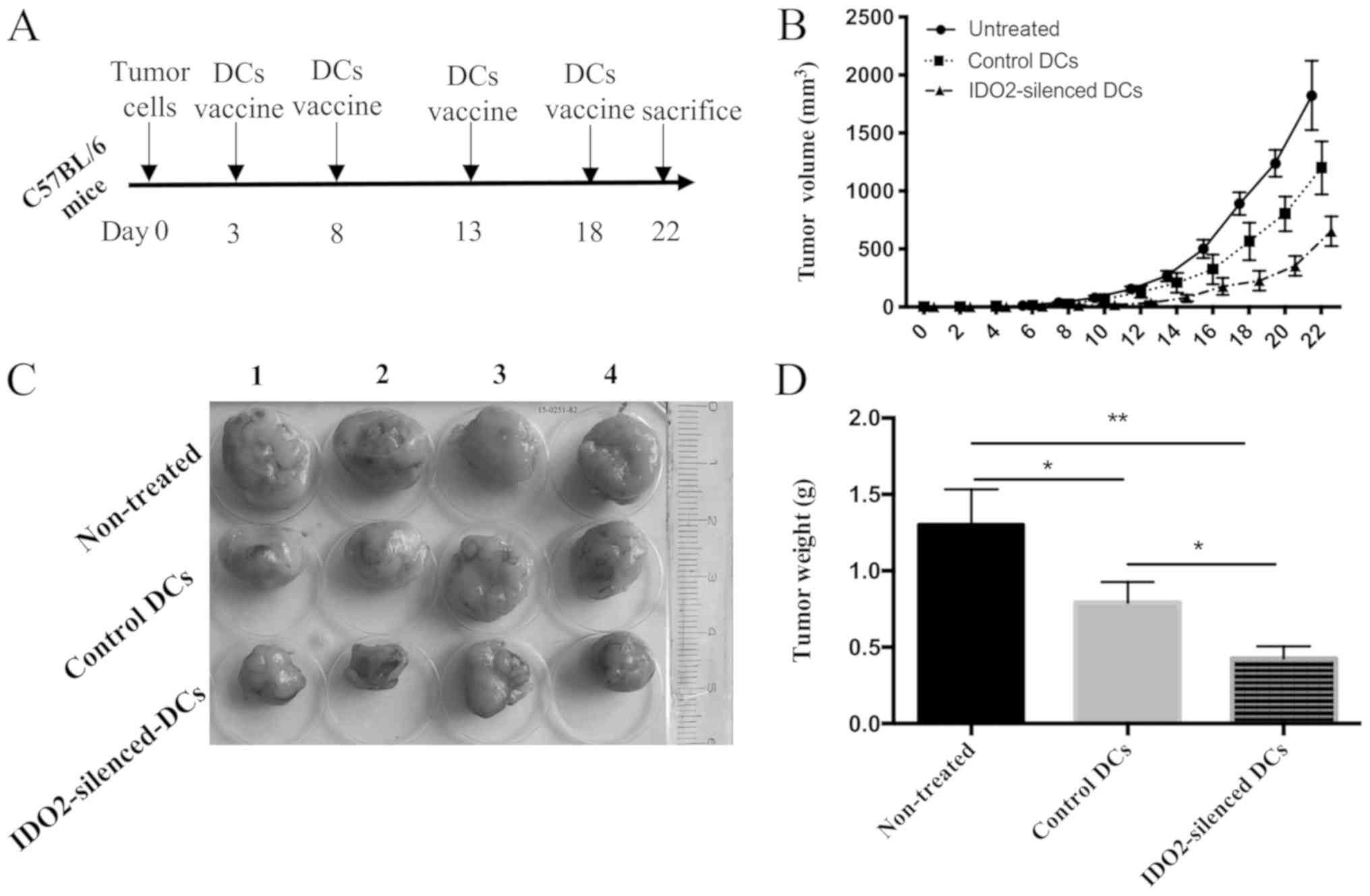
Administration of IDO2-silenced DCs
suppresses tumor progression
To evaluate the therapeutic efficacy of
IDO2-silenced DCs in lung cancer, IDO2-siRNA- or
GL2-siRNA-transfected DCs were loaded with lysates of LLC cells,
and subsequently used to treat mice inoculated with LLC tumors.
According to the tumor growth curve and tumor images, the tumors of
untreated mice were larger and the length of largest tumor was
approximately 19 mm and the diameter was approximately 15 mm.
(volume, 19 × 15 × 15 × 0.5=2,137.5 mm3). Compared to
the untreated mice, the two DC vaccines inhibited tumor growth,
although the inhibitory effects of the IDO2-silenced DC vaccine on
tumor growth were more evident (Fig.
4B and C). At end point of observation (death or time elapse),
excised tumor weight was measured. Tumor weight derived from the
mice treated with IDO2 silenced DC was significantly lighter than
the tumor from control DC-treated mice and non-treated mice
(Fig. 4D).
These results suggested that the IDO2-silenced DCs
were a superior tumor vaccine, which displayed robust antitumor
efficacy in suppressing LLC tumor growth.
IDO2-silenced DC-based tumor vaccine
enhances the antitumor immune response
To characterize the mechanisms responsible for the
enhanced antitumor activity mediated by the IDO2-silenced DCs, the
apoptosis of T-cells, and Tregs from the tumor-draining lymph nodes
or spleen from the tumor-bearing mice were analyzed. The results
revealed that the IDO2-silenced DCs significantly decreased the
number of apoptotic CD8+ T-cells and apoptotic
CD4+ T-cells (Fig. 5A and
B). To examine the changes in the percentage of Tregs,
FITC-labeled Foxp3, PE-labeled CD25 and Pecy5-labeled CD4
antibodies were used to stain the T-cells (Fig. 5C and D). Compared to the control
DC-treated mice and untreated mice, the percentage of Tregs in the
both spleen and lymph nodes from IDO2-silenced DC-treated mice
exhibited a significant decrease which was close to that of the
healthy mice. Furthermore, we examined the cytotoxic activity of
CD8+ T-cells by a CTL mediated tumor cell lysis assay.
As shown in Fig. 5E,
CD8+ T-cells from the mice which were treated with the
IDO2-silenced DC vaccine exhibited a higher lysis capacity than the
CD8+ T-cells from the conventional DC vaccine-treated
mice, suggesting that the IDO2-silenced DC vaccine induced stronger
activities of CTLs against LLC cells. Taken together, these data
demonstrated that the IDO2-silenced DC-based tumor vaccine enhanced
the antitumor immune responses in the murine lung cancer model.
Treatment with IDO2-silenced DC-based
tumor vaccine affects cytokine secretion profiles
To further explore the mechanisms responsible for
the enhanced antitumor activity mediated by the IDO2-silenced DCs,
at the end of the in vivo animal experiments, the
tumor-bearing mice were sacrificed and serum was collected to
determine the cytokine secretion profiles. Cytokines associated
tumor progression (4 types) were selected and the levels of these
were measured by ELISA. Compared with the control DC-treated mice
and the untreated mice, the levels of IFN-γ (Fig. 6A) and TNF-α (Fig. 6B) in the IDO2-silenced DC-treated
mice were significantly increased by >50%, while the
concentrations of TGF-β (Fig. 6C)
and IL-10 (Fig. 6D) were
decreased. These data suggested that the IDO2-silenced DC-based
tumor vaccine altered the secretion profiles that favor immune
reaction against tumors.
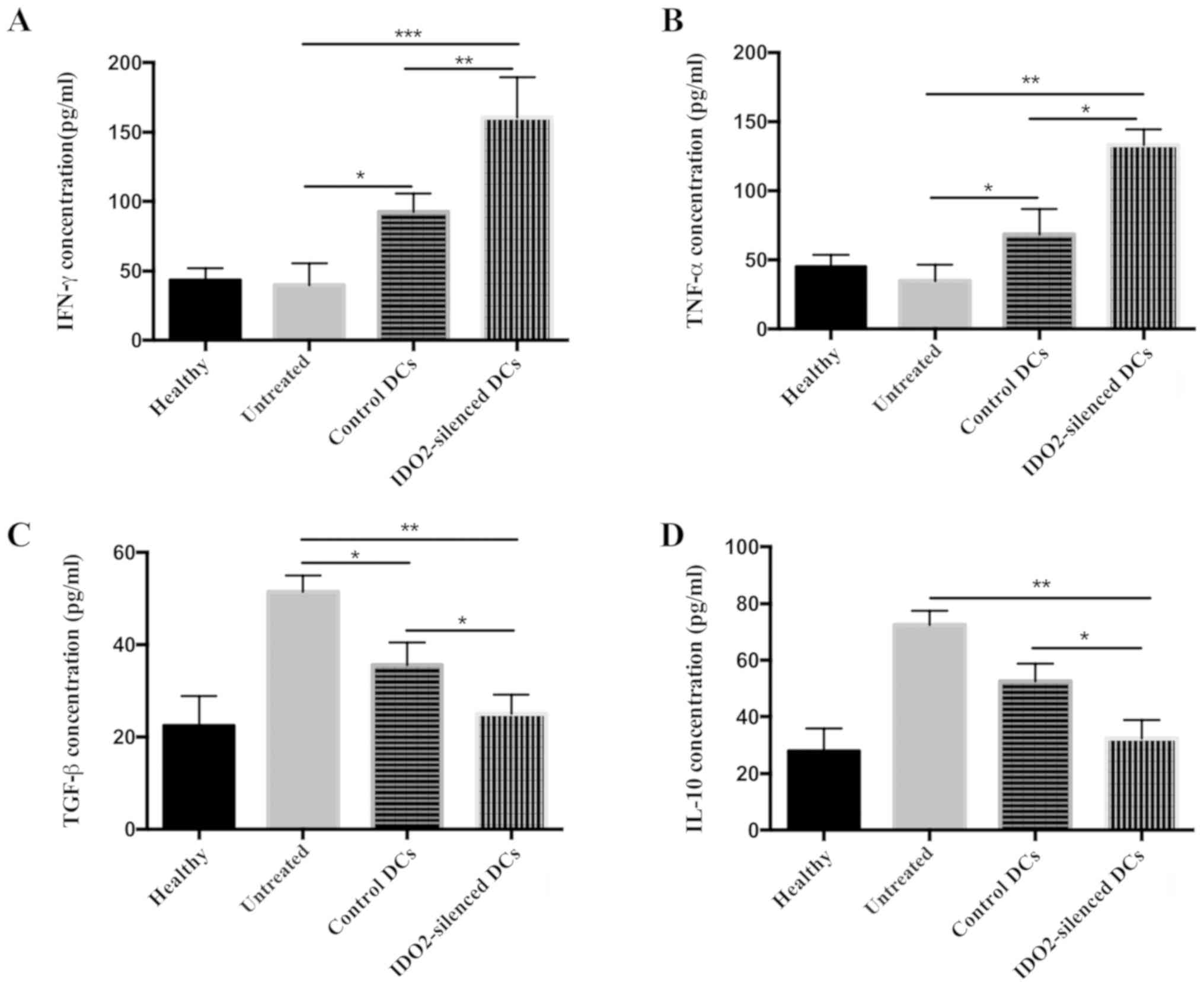 | Figure 6Treatment with IDO2-silenced DC-based
tumor vaccine alters cytokine secretion profiles. Blood was
collected from the differently treated mice at the end of the
experiment and centrifuged to obtain serum. The concentration of
(A) IFN-γ, (B) TNF-α, (C) TGF-β and (D) IL-10 in serum was
determined by ELISA. *P<0.05, **P<0.01
and ***P<0.001. IDO2, indoleamine 2,3-dioxygenase 2;
DCs, dendritic cells; IFN-γ, interferon γ; TNF-α, tumor necrosis
factor α; TGF-β, transforming growth factor β; IL-10, interleukin
10. |
Discussion
DCs are the most potent antigen presenting cells in
the immune system. They interact with a number of immune cells in
the immune response, whereas mature DCs can activate T-cells
effectively. Theoretically speaking, as a therapeutic vaccine, DCs
can produce more effective immune protection for cancer patients
than other immune methods. However, the results of clinical studies
have demonstrated that the effect of currently used DC tumor
vaccines is not as potent as was originally expected; DC-based
vaccines have produced disappointing results in clinical trials,
despite being safe for clinical use (34,35).
Negating the function of immunosuppressive molecules may become an
effective strategy with which to enhance the effectiveness of
cancer vaccines (29). A novel
mechanism of immunosuppression through IDO2 has recently attracted
attention and has stimulated research in immunology (18,20-22,36-38).
In the present study, it was demonstrated that the silencing of
IDO2 gene in DCs increased DC-stimulated T-cell proliferation and
reduced the induction of Tregs in vitro. Further in
vivo experiments revealed that the IDO2-silenced DC-based tumor
vaccine inhibited tumor growth and enhanced the antitumor immunity
ability in a mouse model of lung cancer.
It has been reported that IDO2 is stably expressed
in peripheral DCs and may contribute to the homeostatic
tolero-genic capacity of DCs in healthy conditions (21). However, the expression of IDO2 in
cultured DCs, as the initial step to establish the DC tumor
vaccine, remains unclear. Therefore, the present study first
determined the expression of IDO2 in the process of culture at both
the mRNA and protein level. The results revealed that the
expression of IDO2 increased gradually, peaking on the 7th day
(Fig. 1A and B). DC-based vaccines
for anticancer are usually utilized between days 5 to 7 (39,40).
Thus, the high expression of IDO2 may have a negative impact on the
therapeutic efficacy. To further clarify the effects of IDO2 on
DCs, gene interference was utilized to silence IDO2 gene expression
in DCs and subsequently to implement a series of experiments. As
was expected, the knockdown of IDO resulted in an enhanced immune
response and antitumor effects.
DCs play a pivotal role in cancer immune responses.
They induce adaptive immune responses by taking up, processing and
presenting antigens to T-cells, and can be used as the basis of
cancer vaccines (41). Indeed, the
number of DCs in cancer patients decreases (42,43)
and this is usually a disorder of differentiation and maturation
(44). It is well known that
CD8+ T-cells play a particularly important role in
mediating the antitumor immune response and effector CTLs can
directly kill cancer cells when recognizing tumor antigen, which is
presented by DCs (45). Naturally,
Foxp3+ Treg cells exist at a very low frequency in the
tumor condition; however, an increase in the Foxp3+ Treg
population has been reported and has been hypothesized to promote
tumor tolerance (46). Thus, the
ideal DC tumor vaccine should have the following characteristics:
Efficient tumor antigen loading, a high expression of the
costimulatory molecule, stimulating robust T lymphocyte
proliferation, particularly CD8+ T-cells and inducing
fewer inhibitory T-cells. In the present study, to improve the
effi-ciency of the DC-based cancer vaccine, IDO2 was silenced in
DCs. For tumor antigen loading, the IDO2-silenced DCs were then
challenged by LLC lysis for 24 h and then stimulated for maturation
with TNF-α. Notably, although IDO2 silencing did not alter the
differentiation and maturity of DCs, the levels of the surface
costimulatory molecules, CD40, CD80 and CD86, were upregulated in
varying degrees when stimulated by tumor antigen and TNF-α
(Fig. 2). In a mixed lymphocyte
reaction, the IDO2-silenced DCs significantly increased T
lymphocyte proliferation and reduced Treg cell induction (Fig. 3). Based on the above-mentioned ex
vivo results, it was postulate that the IDO2-silenced DC-based
tumor vaccine would possess a more potent antitumor capacity. To
determine this, LLC lung cancer-bearing mice were treated with the
IDO2-silenced DC vaccine. As was expected, compared to the
untreated and control DC-treated group, the IDO2-silenced DCs
suppressed tumor growth (Fig. 4)
and induced a more potent antitumor immune response in the mouse
model of lung cancer (Figs. 5 and
6).
IDO2 is expressed in primary cancer cells, as well
as in DCs (47). It has been
reported that the decomposition of tryptophan by IDO2 can induce
the phosphorylation of eIF2A, a transcription initiation factor, to
regulate cell proliferation and immune response. Although the
enzymatic activities of IDO1 and IDO2 can both promote the
upregulation of liver-enriched inhibitory protein (LIP), of note,
the upregulation of LIP induced by IDO1 is reversed following
tryptophan supplementation, while the upregulation of LIP induced
by IDO2 can not be reversed even after tryptophan supplementation.
It has been speculated that IDO2 is closely related to tumor
growth, invasion and distant metastasis (17). Another study found that in the
co-culture system of T-cells and IDO2-overexpressing tumor cells,
IDO2 expression inhibited the proliferation of CD4+
T-cells and CD8+ T-cells, and the inhibition of
CD4+ T lymphocyte proliferation could not be reversed
after increasing the tryptophan content (18). In human peripheral DCs, IDO2 is
stably expressed in medullary and plasma DCs, and can induce Treg
production (21). In IDO2 knockout
mice, the number of Tregs induced by IDOl decreased significantly,
indicating that IDO2 and IDO1 are interrelated in regulating immune
homeostasis and IDO2 plays an immunosuppressive role in immune
regulation (22). In the present
study, it was demonstrated that the silencing of IDO2 expression in
DCs more potently induced allogeneic T-cell proliferation in vitro.
In addition, the utilization of the IDO2-silenced DC-based vaccine
to challenge lung cancer-bearing mice decreased apoptotic
CD4+T-cells and CD8+T-cells in vivo.
Furthermore, the knockdown of IDO2 in DCs decreased Treg generation
and evoked enhanced antitumor effects.
In conclusion, the present study demonstrates that
silencing the immunosuppressive gene, IDO2, in DCs ex vivo using
siRNA may be an effective treatment strategy for lung cancer. The
targeted silencing of IDO2 in DCs may prove to be a useful strategy
with which to enhance antitumor immune responses and to improve the
potential of immunotherapy to suppress tumor growth in cancer
patients.
Funding
The present study was partially supported by grants
from the Natural Science Foundation of China (grant nos. 81673009
and 81960113), the Jiangxi Natural Science Foundation (grant no.
20171BAB215082), the Science and Technology Project of Jiangxi
Education Department (grant no. GJJ180966), the Science and
Technology Plan of Jiangxi Health and Family Planning Commission
(grant no. 20195664) and by the Canadian Institutes of Health
Research (CIHR).
Availability of data and materials
All the datasets generated and analyzed in the
present study are included in this published article.
Authors' contributions
YL and WM designed the study. HG, XC, HL, PX and CF
performed the experiments. MT, YZ, YL and WM analyzed the data. YL
and YZ wrote the manuscript, WM revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments complied with the Regulations
for the Administration of Affairs Concerning Experimental Animals
of China and were approved by the Institutional Animal Care and Use
Committee of Nanchang University, China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have not competing
interests.
Abbreviations:
|
IDO2
|
indoleamine 2,3-dioxygenase 2
|
|
DCs
|
dendritic cells
|
|
siRNA
|
small interfering RNA
|
|
GL2
|
luciferase gene duplex
|
|
CFSE
|
5(6)-carboxyfluorescein diacetate
N-succinimidyl ester
|
|
LLC
|
Lewis lung cancer
|
|
CTL
|
cytotoxic T lymphocyte
|
Acknowledgements
The authors are very grateful to Dr Hongmei Wang,
Department of Immunology, Nanchang University, for providing
assistance with performing the flow cytometry and analyzing the
results.
References
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frumento G, Rotondo R, Tonetti M, Damonte
G, Benatti U and Ferrara GB: Tryptophan-derived catabolites are
responsible for inhibition of T and natural killer cell
proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med.
196:459–468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Vita F, Orditura M, Galizia G, Romano
C, Lieto E, Iodice P, Tuccillo C and Catalano G: Serum
interleukin-10 is an independent prognostic factor in advanced
solid tumors. Oncol Rep. 7:357–361. 2000.PubMed/NCBI
|
|
4
|
Berghella AM, Pellegrini P, Del Beato T,
Adorno D and Casciani CU: IL-10 and sIL-2R serum levels as possible
peripheral blood prognostic markers in the passage from adenoma to
colorectal cancer. Cancer Biother Radiopharm. 12:265–272. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Orditura M, Romano C, De Vita F, Galizia
G, Lieto E, Infusino S, De Cataldis G and Catalano G: Behaviour of
interleukin-2 serum levels in advanced non-small-cell lung cancer
patients: Relationship with response to therapy and survival.
Cancer Immunol Immunother. 49:530–536. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kase H, Aoki Y and Tanaka K: Fas ligand
expression in cervical adenocarcinoma: Relevance to lymph node
metastasis and tumor progression. Gynecol Oncol. 90:70–74. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sheehan KM, O'Donovan DG, Fitzmaurice G,
O'Grady A, O'Donoghue DP, Sheahan K, Byrne MF, Conroy RM, Kay EW
and Murray FE: Prognostic relevance of Fas (APO-1/CD95) ligand in
human colorectal cancer. Eur J Gastroenterol Hepatol. 15:375–380.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gorter A and Meri S: Immune evasion of
tumor cells using membrane-bound complement regulatory proteins.
Immunol Today. 20:576–582. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uyttenhove C, Pilotte L, Théate I,
Stroobant V, Colau D, Parmentier N, Boon T and Van den Eynde BJ:
Evidence for a tumoral immune resistance mechanism based on
tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med.
9:1269–1274. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muller AJ, DuHadaway JB, Donover PS,
Sutanto-Ward E and Prendergast GC: Inhibition of indoleamine
2,3-dioxygenase, an immunoregulatory target of the cancer
suppression gene Bin1, potentiates cancer chemotherapy. Nat Med.
11:312–319. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Friberg M, Jennings R, Alsarraj M,
Dessureault S, Cantor A, Extermann M, Mellor AL, Munn DH and
Antonia SJ: Indoleamine 2,3-dioxygenase contributes to tumor cell
evasion of T cell-mediated rejection. Int J Cancer. 101:151–155.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muller AJ and Prendergast GC: Marrying
immunotherapy with chemotherapy: Why say IDO? Cancer Res.
65:8065–8068. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Munn DH and Mellor AL: IDO and tolerance
to tumors. Trends Mol Med. 10:15–18. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mellor AL and Munn DH: IDO expression by
dendritic cells: Tolerance and tryptophan catabolism. Nat Rev
Immunol. 4:762–774. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Puccetti P: On watching the watchers: IDO
and type I/II IFN. Eur J Immunol. 37:876–879. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ball HJ, Sanchez-Perez A, Weiser S, Austin
CJ, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS and
Hunt NH: Characterization of an indoleamine 2,3-dioxygenase-like
protein found in humans and mice. Gene. 396:203–213. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Metz R, Duhadaway JB, Kamasani U,
Laury-Kleintop L, Muller AJ and Prendergast GC: Novel tryptophan
catabolic enzyme IDO2 is the preferred biochemical target of the
antitumor indoleamine 2,3-dioxygenase inhibitory compound
D-1-methyl-tryptophan. Cancer Res. 67:7082–7087. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qian F, Liao J, Villella J, Edwards R,
Kalinski P, Lele S, Shrikant P and Odunsi K: Effects of
1-methyltryptophan stereo-isomers on IDO2 enzyme activity and
IDO2-mediated arrest of human T cell proliferation. Cancer Immunol
Immunother. 61:2013–2020. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuasa HJ, Takubo M, Takahashi A, Hasegawa
T, Noma H and Suzuki T: Evolution of vertebrate indoleamine
2,3-dioxygenases. J Mol Evol. 65:705–714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun T, Chen XH, Tang ZD, Cai J, Wang XY,
Wang SC and Li ZL: Novel 1-alkyl-tryptophan derivatives
downregulate IDO1 and IDO2 mRNA expression induced by
interferon-gamma in dendritic cells. Mol Cell Biochem. 342:29–34.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trabanelli S, Očadlíková D, Ciciarello M,
Salvestrini V, Lecciso M, Jandus C, Metz R, Evangelisti C,
Laury-Kleintop L, Romero P, et al: The SOCS3-independent expression
of IDO2 supports the homeostatic generation of T regulatory cells
by human dendritic cells. J Immunol. 192:1231–1240. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Metz R, Smith C, DuHadaway JB, Chandler P,
Baban B, Merlo LM, Pigott E, Keough MP, Rust S, Mellor AL, et al:
IDO2 is critical for IDO1-mediated T-cell regulation and exerts a
non-redundant function in inflammation. Int Immunol. 26:357–367.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Neill DW, Adams S and Bhardwaj N:
Manipulating dendritic cell biology for the active immunotherapy of
cancer. Blood. 104:2235–2246. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iwashita Y, Goto S, Tominaga M, Sasaki A,
Ohmori N, Goto T, Sato S, Ohta M and Kitano S: Dendritic cell
immunotherapy with poly(D, L-2,4-diaminobutyric acid)-mediated
intratumoral delivery of the interleukin-12 gene suppresses tumor
growth significantly. Cancer Sci. 96:303–307. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nestle FO, Alijagic S, Gilliet M, Sun Y,
Grabbe S, Dummer R, Burg G and Schadendorf D: Vaccination of
melanoma patients with peptide- or tumor lysate-pulsed dendritic
cells. Nat Med. 4:328–332. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Y, Zhang Y, Zheng X, Zhang X, Wang H,
Li Q, Yuan K, Zhou N, Yu Y, Song N, et al: Gene silencing of
indoleamine 2,3-dioxygenase 2 in melanoma cells induces apoptosis
through the suppression of NAD+ and inhibits in vivo tumor growth.
Oncotarget. 7:32329–32340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng X, Koropatnick J, Li M, Zhang X,
Ling F, Ren X, Hao X, Sun H, Vladau C, Franek JA, et al:
Reinstalling antitumor immunity by inhibiting tumor-derived
immunosuppressive molecule IDO through RNA interference. J Immunol.
177:5639–5646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yujuan Z, Na S, Jiamin F, Yanling L,
Xuelin Z, Shanshan P, Zhi Y, Xianfang Z, Yiguo C, et al: Synergic
therapy of melanoma using GNRs-MUA- PEI/siIDO2-FA through targeted
gene silencing and plasmonic photothermia. RSC. 6:79236–79237.
2016.
|
|
29
|
Zheng X, Koropatnick J, Chen D, Velenosi
T, Ling H, Zhang X, Jiang N, Navarro B, Ichim TE, et al: Silencing
IDO in dendritic cells: A novel approach to enhance cancer
immunotherapy in a murine breast cancer model. Int J Cancer.
132:967–977. 2013. View Article : Google Scholar
|
|
30
|
Zhang Y, Fu J, Shi Y, Peng S, Cai Y, Zhan
X, Song N, Liu Y, Wang Z, et al: A new cancer immunotherapy via
simultaneous DC mobilization and DC-targeted IDO gene silencing
using an immune-stimulatory nanosystem. Int J Cancer.
143:2039–2052. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng X, Vladau C, Zhang X, Suzuki M,
Ichim TE, Zhang ZX, Li M, Carrier E, Garcia B, Jevnikar AM, et al:
A novel in vivo siRNA delivery system specifically targeting
dendritic cells and silencing CD40 genes for immunomodulation.
Blood. 113:2646–2654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vacchelli E, Vitale I, Eggermont A,
Fridman WH, Fučíková J, Cremer I, Galon J, Tartour E, Zitvogel L,
Kroemer G, et al: Trial watch: Dendritic cell-based interventions
for cancer therapy. OncoImmunology. 2:e257712013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Galluzzi L, Senovilla L, Vacchelli E,
Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E,
Zitvogel L and Kroemer G: Trial watch: Dendritic cell-based
interventions for cancer therapy. OncoImmunology. 1:1111–1134.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sørensen RB, Køllgaard T, Andersen RS, van
den Berg JH, Svane IM, Straten P and Andersen MH: Spontaneous
cytotoxic T-Cell reactivity against indoleamine 2,3-dioxygenase-2.
Cancer Res. 71:2038–2044. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Køllgaard T, Klausen TW, Idorn M,
Holmgaard RB, Straten PT and Andersen MH: Association of a
functional Indoleamine 2,3-dioxygenase 2 genotype with specific
immune responses. OncoImmunology. 1:441–447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Merlo LMF, Pigott E, DuHadaway JB, Grabler
S, Metz R, Prendergast GC and Mandik-Nayak L: IDO2 is a critical
mediator of autoantibody production and inflammatory pathogenesis
in a mouse model of autoimmune arthritis. J Immunol. 192:2082–2090.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baek S, Lee SJ, Kim MJ and Lee H:
Dendritic cell (DC) vaccine in mouse lung cancer minimal residual
model; Comparison of monocyte-derived DC vs. hematopoietic stem
cell derived-DC. Immune Netw. 12:pp. 269–276. 2012, View Article : Google Scholar
|
|
40
|
Hsu YL, Huang MS, Cheng DE, Hung JY, Yang
CJ, Chou SH and Kuo PL: Lung tumor-associated dendritic
cell-derived amphiregulin increased cancer progression. J Immunol.
187:1733–1744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gardner A and Ruffell B: Dendritic cells
and cancer immunity. Trends Immunol. 37:855–865. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ghirelli C, Reyal F, Jeanmougin M,
Zollinger R, Sirven P, Michea P, Caux C, Bendriss-Vermare N,
Donnadieu MH, Caly M, et al: Breast cancer cell-derived GM-CSF
licenses regulatory Th2 induction by plasmacytoid predendritic
cells in aggressive disease subtypes. Cancer Res. 75:2775–2787.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xi HB, Wang GX, Fu B, Liu WP and Li Y:
Survivin and PSMA loaded dendritic cell vaccine for the treatment
of prostate cancer. Biol Pharm Bull. 38:827–835. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hossain DM, Dos Santos C, Zhang Q,
Kozlowska A, Liu H, Gao C, Moreira D, Swiderski P, Jozwiak A, Kline
J, et al: Leukemia cell-targeted STAT3 silencing and TLR9
triggering generate systemic antitumor immunity. Blood. 123:15–25.
2014. View Article : Google Scholar :
|
|
45
|
Fu C and Jiang A: Dendritic cells and CD8
T cell immunity in tumor microenvironment. Front Immunol.
9:30592018. View Article : Google Scholar
|
|
46
|
Koos D, Josephs SF, Alexandrescu DT, Chan
RC, Ramos F, Bogin V, Gammill V, Dasanu CA, De Necochea-Campion R,
Riordan NH, et al: Tumor vaccines in 2010: Need for integration.
Cell Immunol. 263:138–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lob S, Konigsrainer A, Schafer R,
Rammensee HG, Opelz G and Terness P: Levo- but not dextro-1-methyl
tryptophan abrogates the IDO activity of human dendritic cells.
Blood. 111:2152–2154. 2008. View Article : Google Scholar
|