Introduction
Cancer is the leading cause of death worldwide, and
almost 50% of all new cancer cases globally were diagnosed in the
elderly (1). Despite recent
advances in multimodal treatment including surgery, chemotherapy,
and radiotherapy, the outcomes of treatment for gastroenterological
(GI) cancers remain poor (2).
The impacts of post-operative abdominal infectious
complications such as anastomotic leakage and bile leakage increase
distant hematogenous metastasis and result in poor long-term
survival after curative resection for GI cancers such as
colorectal, esophageal, and hepatobiliary cancer (3-7).
Traditional ideas propose that these potential mechanisms are
involved in the systemic immunosuppressive effects of sepsis and
inflammation (3). High levels of
interleukin (IL)-1, IL-6, IL-8, and tumor necrosis factor (TNF)-α
can decrease the number and function of cytotoxic lymphocytes,
natural killer (NK) cells, and dendritic antigens (8-10).
These changes promote a direct oncogenic effect, stimulating
circulating tumor cell (CTC) adhesion and the development of
distant hematogenous metastasis (11,12).
CTCs are actively released by the tumor and disseminate through the
bloodstream, but the molecular mechanisms of distant hematogenous
metastasis have not been fully investigated.
The preferential organ of metastasis is the liver
for various GI cancers (13-15).
However, the liver is an important organ in immune surveillance
(16-18). The immune compartment of the liver
harbors diverse innate cell populations such as Kupffer, NK, and
gamma delta (γδ) T cells (19,20).
These cells attack bacteria, microbial products, and CTCs, which
generally causes distant metastasis failure (21).
Activated platelet-mediated neutrophil extracellular
traps (NETs) have gained attention as a host defense response to
bacterial infection (22,23). Normally, the liver has no capillary
structure, so when bacteria reach the liver, they spread throughout
the body. Released extracellularly from activated neutrophils in
response to both infection and the sterile inflammatory process,
NETs form a three-dimensional meshwork that traps and kills cancer
cells as well as bacteria within their matrices as part of the
host's defense mechanism (22-24).
However, excessive NET formation releases anti-microbial granule
proteins such as damage-associated molecular patterns (DAMPs)
including nuclear protein high mobility group box 1 (HMGB1),
histones, and myeloperoxidase, which can lead to tissue and
endothelial damage (23,25). NET formation occurs in the sinusoid
of the liver and in the alveolar wall of the lung (26,27).
We previously demonstrated that progression from sepsis to liver
dysfunction, characterized by NETs and activated-platelet
aggregation, cause portal hypertension and liver fibrosis, and
progress to veno-occlusive disease (VOD) (26). Furthermore, we also reported that
these changes in the lung cause pulmonary hyper-tension, acute lung
injury (ALI), and acute respiratory distress syndrome (ARDS) by
severe sepsis (27).
Platelets, anucleate hematopoietic cells, cannot be
detected by traditional hematoxylin and eosin staining. Therefore,
the presence of platelets around primary tumor cells is difficult
to recognize. However, activated platelets surrounding CTCs in the
cancer microenvironment and immune system have recently gained
attention. Placke et al reported that MHC class I present on
the cell surface of platelets in blood vessels are transferred to
cancer cells, where they directly adhere to CTCs in a cell-cell
adhesion manner and surround the CTCs completely, which results in
escape from innate immune surveillance (28). Furthermore, we also demonstrated
that cancer cells are already surrounded by activated platelets at
the primary site before entering blood vessels (29,30).
Our study showed that platelet adhesion around cancer cells can be
detected both in blood vessels and at the primary site, where they
exhibit characteristics of epithelial-mesenchymal transition (EMT)
in pancreatic cancer (29).
Furthermore, we also reported that cancer cells surrounded by
platelets could be associated with EMT and chemoresistance in
patients with breast cancer (30).
From these findings, we hypothesized that
post-operative abdominal infectious complications could induced NET
formation and activated platelet aggression in the liver, which
traps CTCs, with CTCs with EMT characteristics escaping from innate
immune surveillance through platelet adhesion eventually promoting
metastasis. Furthermore, we hypothesized that the switching of CD44
isoforms, which has an important role in EMT, was involved in this
process. In the present study, we investigated the association
between cancer cells with platelet affinity, EMT, and distant
hematogenous metastasis in abdominal infectious complications.
Materials and methods
Reagents
Transforming growth factor (TGF)-β and
lipopolysaccharide (LPS) were purchased from Sigma-Aldrich; Merck
KGa.
Cell lines and cell culture
The human pancreatic ductal adenocarcinoma (PDAC)
cell lines used in this study included Capan-1, BxPC-3, Panc-1, and
MiaPaCa-2, which were purchased from American Type Culture
Collection (ATCC). A previous study reported that Capan-1 and
BxPC-3 have an epithelial phenotype, while Panc-1 and MiaPaCa-2
have a mesenchymal phenotype (31). We also demonstrated that Capan-1
and BxPC-3 cells can form liver metastases in immunodeficient mice
(32). Capan-1 and BxPC-3 were
maintained in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS; Iwaki, Japan), 2 mM glutamine (Nissui Pharmaceutical,
Tokyo, Japan), and 10 U/ml penicillin-streptomycin (Gibco-BRL;
Thermo Fisher Scientific, Inc.). Panc-1 and MIA PaCa-2 cells were
maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS,
glutamine, and penicillin-streptomycin. The cells were seeded in
gelatin-coated 75-cm2 flasks (BD BioCoat) and cultured
in 10 ml medium at 37°C in a humidified atmosphere of 5%
CO2. To induce a change from the epithelial to the
mesenchymal phenotype, BxPC-3 cells were treated with TGF-β (5
ng/ml) for 28 days and were passaged every 3 days.
Platelet preparation
Blood was obtained from healthy donors who for at
least 10 days had not taken medication known to affect platelet
function. All healthy donors gave their written informed consent
before providing blood samples. Blood was collected by venipuncture
through a 19-gauge butterfly needle without a tourniquet to avoid
platelet activation. For the preparation of platelet-rich plasma
(PRP), blood was collected into a syringe containing 3.2% trisodium
citrate as anticoagulant, then centrifuged at 170 × g for 10 min at
room temperature. For the preparation of washed platelets, blood
was collected into acid-citrate-dextrose (ACD: 38 mM citric acid,
75 mM sodium citrate, 124 mM D-glucose) as anticoagulant. Blood was
centrifuged at 170 × g for 10 min at room temperature. PRP was
acidified to pH 6.5 with ACD, and PGE1 (1 mM) was added to avoid
platelet activation during centrifugation. Platelets were pelleted
by centrifugation at 720 × g for 10 min. The supernatant was
removed and the platelet pellet was resuspended in JNL buffer (130
mM NaCl, 10 mM sodium citrate, 9 mM NaHCO3, 6 mM
D-glucose, and 0.9 mM MgCl2, 0.81 mM
KH2PO4, and 10 mM Tris, pH 7.4) and
supplemented with 1.8 mM CaCl2.
Co-culture with cell lines and PRP
A total of 5.0×104 pancreatic cancer
cells were grown on two-well Lab-Tek chamber slides (Thermo Fisher
Scientific, Inc.) for 24 h. Then, they were co-cultured with
5.0×106 PRP for 24 h. For visualization of activated
platelets, cancer cells were fixed in a mixture of methanol and
acetone (1:1) for 10 min. Following treatment with protein block
serum (Dako Cytomation) for 10 min, the sections were incubated
with anti-CD42b antibody (dilution 1:100; cat. no. ab134087; Abcam)
at 4°C overnight. The Envision-polymer solution (horseradish
peroxidase, HRP; Dako Cytomation) was then applied for 1 h. Signals
were developed in 0.02% 3,3'-diaminobenzidinetetrahydrochloride
(DAB) solution containing 0.1% H2O2. Sections
were then lightly counterstained with hematoxylin and examined
using a fluorescence microscope (original magnification ×400,
Olympus, Tokyo, Japan).
Western blotting
Approximately 5×106 cells were lysed in
RIPA buffer [50 mmol/l Tris-HCl (pH 8.0), 150 mmol/l sodium
chloride, 0.5 w/v% sodium deoxycholate, 0.1 w/v% sodium dodecyl
sulfate, and 1.0 w/v% NP-40 substitute (Wako)] containing 1%
protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA). The
protein concentration of each lysate was measured using a BCA
protein assay kit (Pierce Biotechnology; Thermo Fisher Scientific,
Inc.). Protein from each sample was loaded onto 12.5% SDS-PAGE gels
(Bio-Rad, USA) and subjected to electrophoresis. An amount of 20
µg of proteins were transferred to a PVDF membrane (Bio-Rad,
USA) and blocked with blocking solution (0.1% Tween-20; EZ Block
ATTO Corp.) at room temperature for 30 min. Blots were incubated
overnight at 4°C with each primary antibody (see below). The
membranes were incubated with IRDye 800CW-conjugated goat
anti-mouse IgG (dilution 1:10,000; cat. no. 926-32212; LICOR
Bioscience) or IRDye 680CW-conjugated goat anti-rabbit IgG
(dilution 1:10,000; cat. no. 926-32213; LICOR Bioscience) for 1 h
at room temperature. The immunoblots were visualized using an ECL
Plus western blotting detection system (GE Healthcare Japan Ltd.,
Japan) and the Light-Capture system (ATTO). To ensure equal protein
loading, β-actin levels were measured using an anti-β-actin
monoclonal antibody (dilution 1:3,000; cat. no. A2228;
Sigma-Aldrich; Merck KGa), anti-CD44 variant 6 monoclonal antibody
(dilution 1:500; cat. no. ab78960; Abcam), anti-CD44 standard
monoclonal antibody (dilution 1:250; cat. no. BBA10; R&D
Systems), anti-ZEB1 monoclonal antibody (dilution 1:500; cat. no.
ab203829; Abcam). anti-ESRP-1 poly-clonal antibody (RBM35A,
dilution 1:500; cat. no. ab107278; Abcam), anti-E-cadherin
monoclonal antibody (dilution 1:1,000; cat. no. sc7870; Santa Cruz
Biotechnology, Inc.), and anti-vimentin monoclonal antibody
(dilution 1:1,000; cat. no. sc6260; Santa Cruz Biotechnology,
Inc.).
CD44 knockdown
Panc-1 cells were seeded into 75-cm2
flasks at a density of 1×106 cells/ml. The next day, the
cells were transiently transfected with a short interfering RNA
(siRNA) targeting CD44 (30 nM) or negative control siRNA (30 nM;
Silencer® Select Negative Control No. 1 siRNA; catalog
no. 4390843; Ambion; Thermo Fisher Scientific, Inc.) using
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.). The
CD44 siRNA sequence was forward, 5′UAU UCC ACG UGG AGA AAA
ATT3′ and reverse, 3′UUU UUC UCC ACG UGG AAU ACA5′. After 72 h, the
cells were collected. Fluorescence microscopy was performed to
examine cells stained with CD42b, and the expression of CD44s and
vimentin was also assessed by western blotting.
Experimental animals
All animal experiments were performed according to
the standard guidelines of Kanazawa University. All procedures were
in accordance with the Fundamental Guidelines for Proper Conduct of
Animal Experiments and Related Activities in Academic Research
Institutions, under the jurisdiction of the Ministry of Education,
Culture, Sports, Science, and Technology of Japan and with the
Helsinki Declaration of 1964 and later versions (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/).
This study was approved by the Research Ethics Committee of
Kanazawa University (AP-163774; Kanazawa, Japan). We used male
BALB/c mice (aged 6-10 weeks and weighing 20-22 g; Charles River
Laboratories, Kanagawa, Japan) as a sepsis model to observe the
aggregation of platelets and metastasis formation in infectious
complications. All the animals were housed under specific
pathogen-free conditions with a 12-h dark/light cycle at 25°C, and
fed standard food and aseptic water.
Abdominal infectious model
An abdominal infectious model was prepared as
previously described (26).
Lipopolysaccharide (LPS) was dissolved in saline and diluted to
0.0003 g/ml. The solution was administered at a concentration of 1
mg/kg and injected intraperitoneally into mice. After 24 h,
5×106 cells/100 µl BxPC-3, TGF-β-treated BxPC-3,
and Panc-1 cells were injected into the spleen and the animals were
carefully monitored. Intrasplenic injection of cancer cells
reproduces circulating tumor cells (CTCs), and a previous study was
similarly performed to generate a liver metastasis model (33). At 1 h following intrasplenic
injection of cancer cells, mice were euthanized and analyzed. Mice
injected with Panc-1 cells were also euthanized and analyzed at 3
and 6 h following intrasplenic injection. For the control group,
mice were administered 500 µl saline intraperitoneally,
which represented the non-infectious model.
Immunohistochemistry
Excised organs were fixed in 10% neutral buffered
formalin and embedded in paraffin. The specimens were sliced into
3-mm sections and embedded in paraffin. Each paraffin block was
further sliced into 0.5-l-µm-thick sections and mounted on
slides. The expression levels of cytokeratin (mouse monoclonal IgG,
dilution 1:50; Abcam) to detect the presence of human cytoplasm,
p-selectin (mouse monoclonal IgG, dilution 1:100; Abcam) to examine
the presence of activated platelets and hepatic sinusoid
endothelial cells, and mab4383 (mouse monoclonal IgG, dilution
1:200; EMD Millipore) to examine the presence of nuclei of human
cell types were assessed by immunohistochemistry. Deparaffinized
sections were pretreated by autoclaving in 10% citric acid buffer
(pH 8.0) at 120°C for 15 min. Following treatment with protein
block serum (Dako Cytomation) for 10 min and incubation with 2%
skim milk for 30 min to block non-specific reactions, the sections
were incubated with primary antibody at 4°C overnight.
Envision-polymer solution (horseradish peroxidase, HRP; Dako
Cytomation) was then applied for 1 h. Signals were developed in
0.02% 3,3'-diamino-benzidine tetrahydrochloride (DAB) solution
containing 0.1% H2O2. Sections were then
lightly counterstained with hematoxylin and examined using a
fluorescence microscope (original magnification ×400, Olympus,
Tokyo, Japan). The number of Panc-1 cells positively stained by
mab4383 was calculated by counting within the original field of
magnification at ×40 at 1, 3, and 6 h following intrasplenic
injection.
Statistical analysis
Values are expressed as mean ± SD. Comparisons were
made using one-way analysis of variance or Student's t-test
followed by Tukey's post hoc test using SPSS statistical software,
version 11.0 (IBM, Corp.). P-values <0.05 indicated a
statistically significant difference.
Results
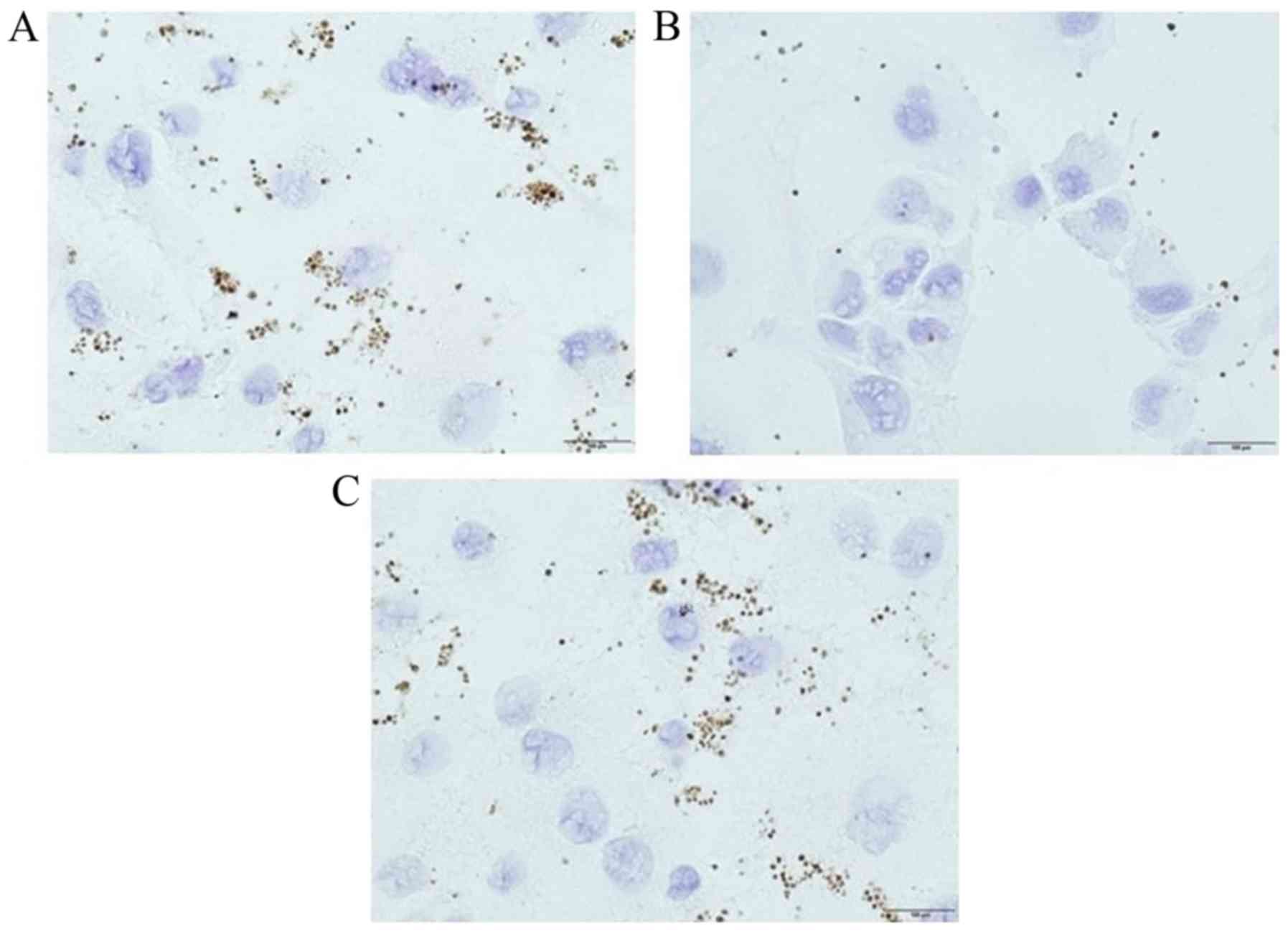
Platelet adhesion to pancreatic cancer
cells
Capan-1 and BxPC-3 cells showed slight platelet
adhesion and cell-to-cell adhesion (Fig. 1A and B), while Panc-1 and MiaPaca-2
cells showed loss of cell-to-cell adhesion and were completely
surrounded by many activated platelets, which were positive for
CD42b staining (Fig. 1C and D).
Based on these results, we decided to use BxPc-3 cells as the
epithelial cancer cell line, and Panc-1 cells as the mesenchymal
cancer cell line in subsequent experiments.
Adhesion molecules influenced by EMT
A panel of PDAC cell lines was examined by western
blotting for the expression of CD44 isoforms, transcriptional
factors, and EMT markers. BxPC-3 cells showed a reduction in
vimentin and upregulation of E-cadherin expression, and Panc-1
cells showed reduction in E-cadherin and upregulation of vimentin
expression. TGF-β-mediated BxPC-3 showed the same findings as
Panc-1 and demonstrated a change from an epithelial phenotype to a
mesenchymal phenotype. Furthermore, western blotting identified a
reduction in CD44-standard and zinc finger E-box-binding homeobox 1
(ZEB-1) and upregulation of CD44-variant6 and epithelial splicing
regulatory protein 1 (ESRP-1) expression in BxPC-3 cells. However,
Panc-1 and TGF-β-treated BxPC-3 cells were identified as having
reduced CD44-variant 6 and ESRP-1, and upregulation of
CD44-standard and ZEB-1 expression (Fig. 2). Blots were re-probed for β-actin
to ensure equal protein loading in each lane. Results are
representative data from three separate experiments.
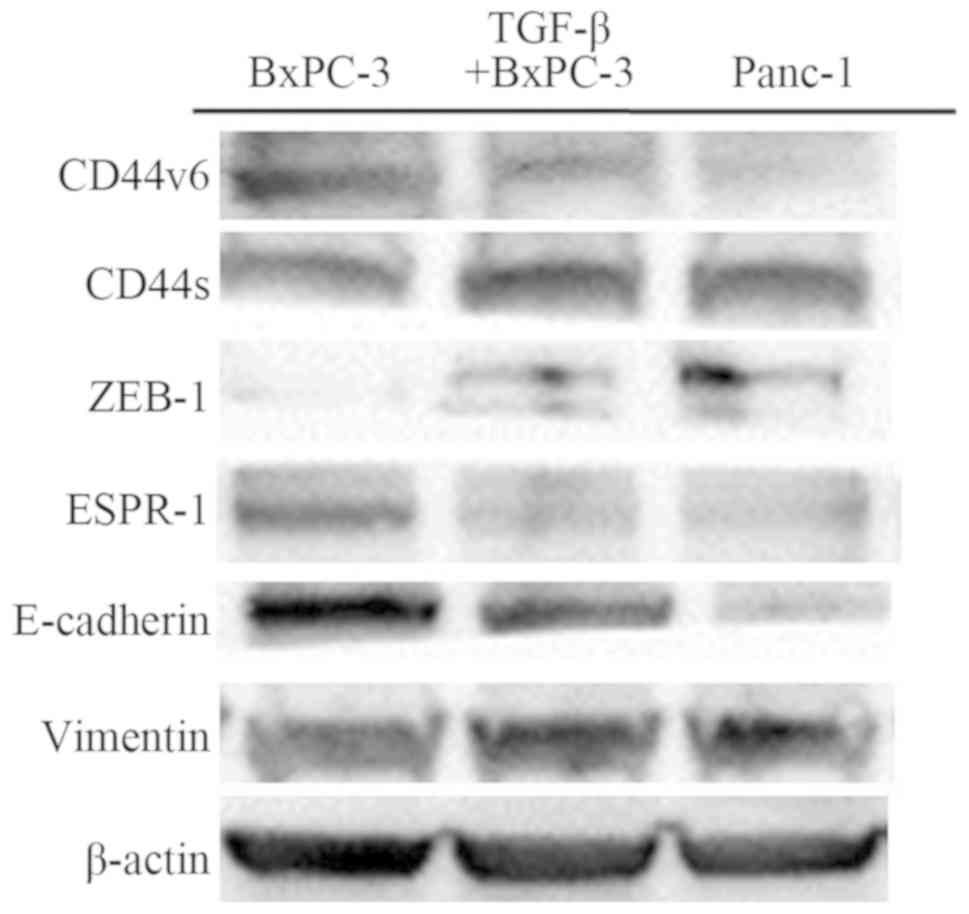 | Figure 2Western blot analysis of CD44v6,
CD44s, ZEB-1, ESRP-1, E-cadherin, vimentin, and β-actin in BxPC-3,
TGF-β-treated BxPC-3, and Panc-1 cells. The average signal
intensity was standardized to that of β-actin. CD44v6, CD44-version
6; CD44s, CD44-standard; ZEB-1, zinc finger E-box-binding homeobox
1; ESRP-1, epithelial splicing regulatory protein 1. |
Platelet adhesion to TGF-β-treated
BxPC-3
BxPC-3 cells showed slight platelet adhesion and
cell-to-cell adhesion (Fig. 3A),
while TGF-β-treated BxPC-3 cells were demonstrated to show more
preferential platelet adhesion than BxPC-3 cells and loss of
cell-to-cell adhesion (Fig. 3B).
In other words, switching CD44 from the variant isoform to the
standard isoform through EMT changed the affinity for
platelets.
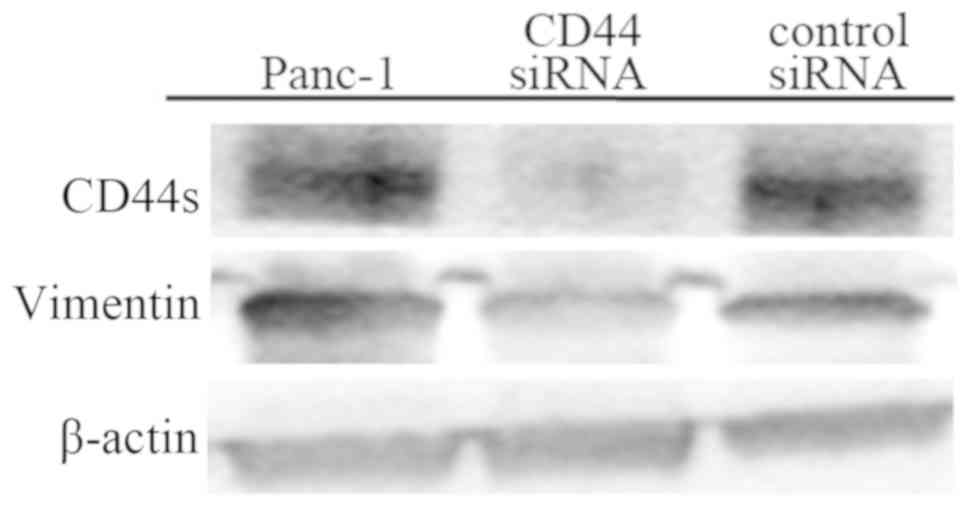
Effect of CD44 knockdown by CD44
siRNA
Panc-1 and control siRNA-treated Panc-1 cells
preferentially adhered to platelets, which were positive for CD42b
staining (Fig. 4A and C), while
CD44 siRNA-treated Panc-1 cells showed slight platelet
adhesion (Fig. 4B). In western
blotting, Panc-1 cells showed upregulated CD44s and vimentin
expression, while CD44 siRNA-treated Panc-1 cells
demonstrated a reduction in CD44s and vimentin expression (Fig. 5). These findings suggested that
CD44 knockdown by CD44 siRNA could change the
mesenchymal phenotype and reduce the affinity of cancer cells for
platelets.
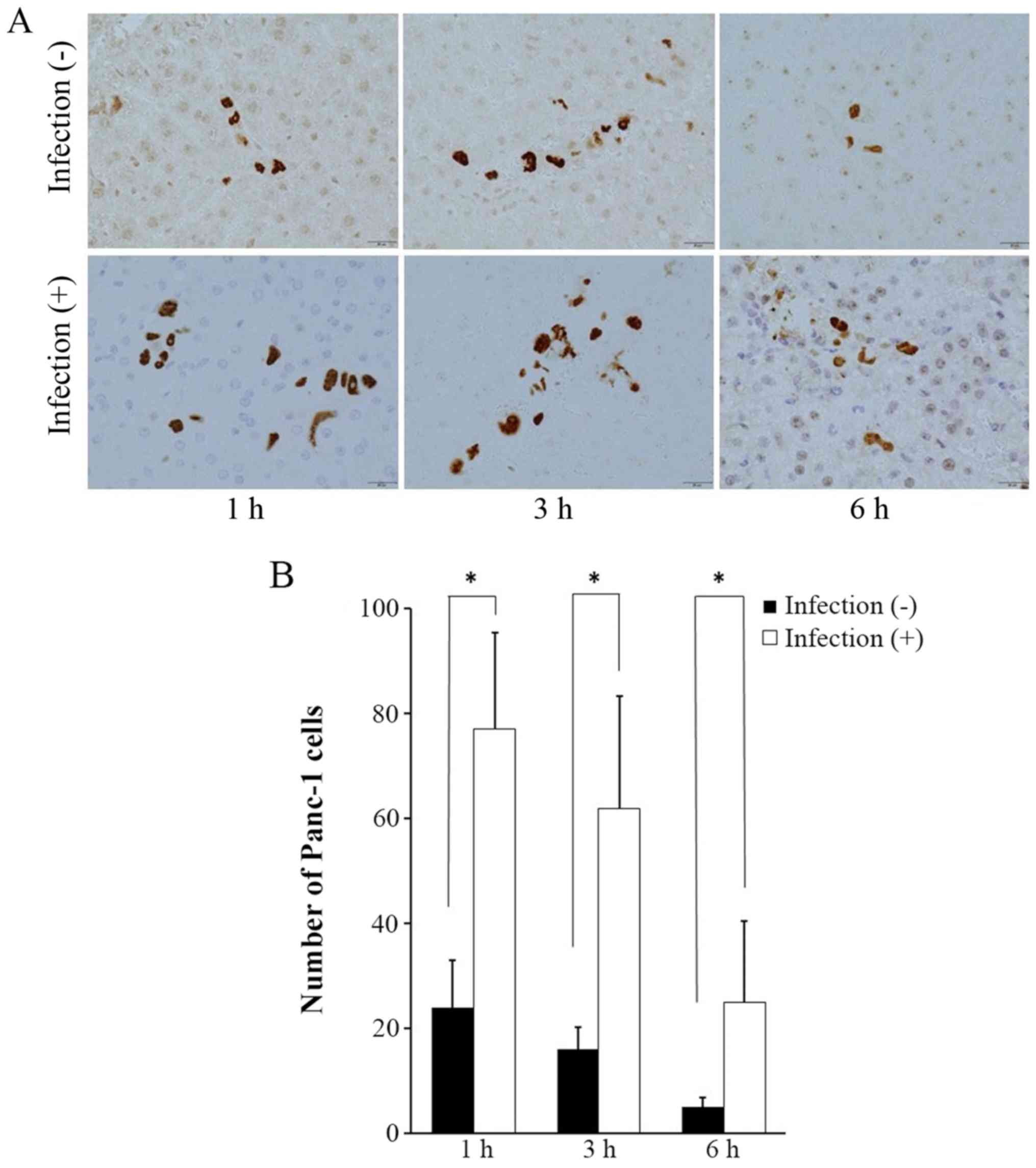
Mab4383 expression for examination of
cancer cell viability
In the liver of the non-infectious model, a small
number of Panc-1 cells was identified. In the liver of the
abdominal infectious model, positive staining for mab4383 was
higher than that in the non-infectious model. In both models,
Panc-1 cells showed a gradual decrease in number (Fig. 6A), while there were significantly
more Panc-1 cells in the infectious models than in the
non-infectious models at all times (Fig. 6B).
Cytokeratin expression for examination of
cancer cell viability
In the liver of the non-infectious model, cancer
cells were not found regardless of the cancer cell line type (data
not shown). In the liver of the abdominal infectious model,
positive staining for cytokeratin was weak in the specimens
regardless of the cancer cell line type (Fig. 7). There were very few viable BxPC-3
cells, epithelial phenotype cancer cells, because the nuclei were
deformed and there were few circular cells, with most cells in an
apoptotic state (Fig. 7A and D).
Conversely, there were some viable TGF-β-treated BePC-3 (Fig. 7B and E) and Panc-1 cells (Fig. 7C and F) and mesenchymal phenotype
cells, as shown by cytokeratin staining, which formed a focal
cluster within the liver sinusoid.
P-selectin expression for examination of
platelet adhesion
In the liver of the non-infectious model, activated
platelets were not observed, while a few activated hepatic sinusoid
endothelial cells were observed (data not shown). In the liver of
the abdominal infectious model, activated sinusoidal endothelial
cells stained with p-selectin were observed more frequently than in
the non-infectious model (Fig. 8).
In cancer cells with the epithelial phenotype (BxPC-3 cells), very
few viable cells were surrounded by activated platelets stained
with p-selectin, while apoptotic cancer cells were not surrounded
by activated platelets (Fig. 8A and
D). Conversely, some viable mesenchymal phenotype cancer cells
[TGF-β-treated BePC-3 (Fig. 8B and
E) and Panc-1 cells (Fig. 8C and
F)] were completely surrounded by adherent platelets.
Discussion
Human cancer cells are generally recognized as
xenogeneic and exogenous material and cannot be engrafted in
immunocompetent mice because of innate immune surveillance.
However, our study demonstrated that cancer cells which adhered to
activated platelets were viable in the abdominal infectious model
at 1 h following intrasplenic injection of cancer cells.
Additionally, cells with the mesenchymal phenotype, Panc-1 and
TGF-β-treated BxPC-3 cells, which express the CD44s isoform,
clearly showed these changes. Therefore, our study confirmed that
escape from innate immune surveillance was induced by the enhanced
adhesion of activated platelets by epithelial-mesenchymal
transition (EMT) and switching of CD44 from the variant to the
standard isoform.
Circulating tumor cells (CTCs) are present in
advanced GI cancer patients including those presenting with
esophageal (34), colorectal
(35-38), and pancreatic cancer (39,40).
Cancer metastasis formation in animal studies is promoted by
injecting cancer cells into blood vessels (33). Tien et al reported that CTCs
in the portal vein were detected in 58.3% of patients with
periampullary or pancreatic carcinoma undergoing
pancreaticoduodenectomy. Furthermore, 28.3% of patients had liver
metastases within 6 months after surgery, and liver metastases
developed soon after surgery in patients with a high portal venous
CTC count (41). Most CTCs are
eliminated by immune cells such as natural killer (NK) cells, but
pathophysiologically, cancer metastasis formation from CTCs has not
been fully elucidated.
Previous studies reported that neutrophil
extracellular traps (NETs) have strong adhesive properties, which
enable them to bind to pathogens including CTCs in blood vessels,
and that they have important roles in tumor progression and
metastasis (24,33,42).
We previously demonstrated that aggregated platelets are surrounded
by NETs in hepatic sinusoids in the abdominal infectious model
using mice intraperitoneally injected with LPS (26). In other words, NETs interact with
platelets and leukocytes and are pro-thrombotic, acting as
three-dimensional frameworks for both fibrin deposition and later
stabilization of thrombi; thus, we named them 'immune-thrombosis'
(43-45). Immune-thrombosis comprising NETs
and aggregated platelets can trap CTCs, but have some disadvantages
including not only endothelial cell damage and detachment through
alarmins, including HMGB1 and histones, but also the release of
various cytokines and chemokines including TGF-β, vascular
endothelial growth factor-A (VEGF-A) and plasminogen activator
inhibitor-1 (PAI-1), which affect cancer progression (23,25).
Based on these mechanisms, we postulated that the hepatic sinusoids
had already undergone pre-metastatic niche formation in abdominal
infectious complications, where they contribute to binding CTCs,
which likely results in metastasis.
Activated platelets express p-selectin on their cell
surface and can adhere to CD44, a ligand of p-selectin (28). CD44 is also an adhesion molecule
and is involved in cell-to-cell and cell-to-matrix adhesion, cell
proliferation, differentiation and trafficking, and is highly
expressed on stem cells (46,47).
CD44 is encoded by 20 exons and undergoes extensive
alternative splicing to generate CD44 standard (CD44s) and CD44
variant (CD44v) forms. Zhao et al reported that cells
expressing high levels of CD44 predominantly showed the CD44s
isoform, displayed a mesenchymal phenotype, and were highly
invasive, while epithelial phenotype cell lines expressed CD44v
(31). Our findings are also in
agreement with this previous report and demonstrate that epithelial
phenotype cell lines express CD44v and ESRP-1, the latter of which
is suppressed by ZEB-1, and mesenchymal phenotype cell lines
express CD44s and ZEB-1, which is an EMT-associated transcription
factor. EMT is involved in invasion and tumor metastasis is a
property of stem cells, whereas, mesenchymal-to-epithelial (MET)
switch may favor tumor growth (48). Cancer cells have phenotypic
plasticity, which confers survival capabilities through phenotypic
changes induced by EMT and MET in response to various environmental
factors. CD44 may have a role in regulating these processes.
In the present study, cells with a mesenchymal
phenotype and expressing CD44s were more preferentially adherent to
activated platelets than cells with an epithelial phenotype that
expressed CD44v. We also found that BxPC-3 cells could switch CD44
isoforms from the variant to the standard isoform through EMT via
TGF-β and a reduction in ESRP-1 and that TGF-β-treated BxPC-3 cells
were preferentially adherent to activated platelets. Furthermore,
we demonstrated that CD44 knockdown by CD44 siRNA
resulted in a reduction in platelet adhesion. From these findings,
cancer cells are suggested to have phenotypic plasticity. In
particular, we hypothesized that CD44s is involved in cluster
formation via platelet adhesion and enhanced platelet adhesion
contributes to escape from innate immune surveillance. A previous
study reported that EMT indeed provides epithelial tumor cells with
enhanced migratory, invasive, and survival abilities that enable
them to participate in the liberation of CTCs into blood vessels
(49-51). CTCs may be able to travel in the
blood vessels as clusters or microemboli to escape innate immune
surveillance, and therefore have higher metastatic potential.
Our study demonstrated the escape from innate immune
surveillance through activated platelet adhesion using an
infectious mouse model by injecting LPS intraperitoneally into
mice. In the non-infectious model, which has no NET formation in
the liver, we did not identify viable cancer cells in the liver
sinusoid, regardless of the epithelial or mesenchymal phenotype. In
the infectious model, which has NET formation in the liver,
epithelial cancer cells such as BePC-3 had slight platelet adhesion
and were mostly in an apoptotic state. However, mesenchymal cancer
cells such as Panc-1 and TGF-β-treated BxPC-3 cells had abundant
platelet adhesion and formed clusters and were in a viable state at
1 h following intrasplenic injection of cancer cells despite the
xenogeneic and exogenous material in immunocompetent mice. To
summarize, when cancer cells express CD44v and have an epithelial
phenotype, they are not trapped by immune thrombosis because of the
reduced platelet adhesion, and are recognized as non-self by
immunocompetent cells, which results in an apoptotic state induced
by the innate immune surveillance. However, when cancer cells
express CD44s and have a mesenchymal phenotype, they are trapped by
immune thrombosis and form clusters due to the upregulated platelet
adhesion and are recognized as self by MHC class I present on the
cell surface of platelets. Consequently, these cells can escape
from innate immune surveillance of immunocompetent cells and liver
metastasis develops (28). From
these findings, we suspect that this process is the initial
invasion step. Literature reporting xenografts in animal models has
suggested that CTC clusters have higher metastatic potential than
single CTCs (52). Furthermore, Yu
et al reported that CTCs of breast cancer expressing a
mesenchymal phenotype predominantly formed multicellular clusters
surrounded by platelets (53).
Though innate immune surveillance was not examined in this study,
these findings agree with our results and support the idea that
platelet adhesion could also have a role in the survival of
CTCs.
Our study results suggest that NET formation and
platelet adhesion may be potential therapeutic targets not only in
sepsis but also in cancer progression (41). In previous studies, Yu et al
reported that aspirin therapy, which is an antiplatelet agent in
patients with acute respiratory distress syndrome (ARDS)/acute lung
injury (ALI), was associated with reduced incidence rate (54), while Boyle et al reported
that aspirin is associated with reduced intensive care unit
mortality (55). Furthermore,
recent reports suggest that aspirin prevents distant metastasis;
this could account for the reduction in cancer deaths (56-58).
These studies showed that platelets have an important function in
the progression of ARDS/ALI and cancer, but there is no detailed
mechanism of platelet association such as escape from innate immune
surveillance. We previously reported that the antiplatelet agent
cilostazol could inhibit NET formation in a monocrotaline-induced
model (59). Our data in the
present study suggested a potential role for antiplatelet agents in
suppressing NET formation and immune surveillance in the tumor
microenvironment.
In conclusion, abdominal infectious complications
induced NET formation and immune-thrombosis in the liver sinusoids.
Cancer cells have phenotypic plasticity that is adapted for various
environments by switching CD44 isoform and changing the affinity
for platelets. CTC clusters surrounded by activated platelets,
especially those more preferentially adherent to CD44s, as well as
mesenchymal phenotype cancer cells, could escape from immune
surveillance, which results in the first step of cancer metastasis
formation. In the progression of distant hematogenous metastasis,
it is important that not just NETs but also activated platelet
aggregates escape from innate immune surveillance. We are now
conducting research to elucidate the mechanisms of MHC class I
molecules in NETs and activated platelet aggregates. We believe
that it may be necessary to undertake treatment management
including administration of antiplatelet agents to prevent distant
hematogenous metastasis in GI cancer patients with post-operative
abdominal infectious complications.
Acknowledgments
We are grateful to members of the Department of
Gastroenterological Surgery of Kanazawa University for their
helpful suggestions.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
MO, TY and TO contributed to the conception and
design of the study, as well as data acquisition and
interpretation. MO and TY drafted the manuscript. HT and SF
analyzed and interpret the data, and critically reviewed the
manuscript. TO supervised and also conceived and designed the
study. All authors contributed to the interpretation of the
findings, and reviewed, edited, and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Research Ethics
Committee of Kanazawa University (AP-163774; Kanazawa, Japan). All
procedures followed were in accordance with the Fundamental
Guidelines for Proper Conduct of Animal Experiments and Related
Activities in Academic Research Institutions (http://www.clar.med.tohoku.ac.jp/data/kitei/10th/hoi1-10th-E-monkashou.pdf#search='Fundamental+Guidelines+for+Proper+Conduct+of+Animal+Experiments+and+Related+Activities+in+Academic+Research+Institutions').
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pilleron S, Sarfati D, Janssen-Heijnen M,
Vignat J, Ferlay J, Bray F and Soerjomataram I: Global cancer
incidence in older adults, 2012 and 2035: A population-based study.
Int J Cancer. 144:49–58. 2019. View Article : Google Scholar
|
|
2
|
Carioli G, Malvezzi M, Bertuccio P, Hashim
D, Waxman S, Negri E, Boffetta P and La Vecchia C: Cancer mortality
in the elderly in 11 countries worldwide, 1970-2015. Ann Oncol.
30:1344–1355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farid SG, Aldouri A, Morris-Stiff G, Khan
AZ, Toogood GJ, Lodge JP and Prasad KR: Correlation between
postoperative infective complications and long-term outcomes after
hepatic resection for colorectal liver metastasis. Ann Surg.
251:91–100. 2010. View Article : Google Scholar
|
|
4
|
Ito H, Are C, Gonen M, D'Angelica M,
Dematteo RP, Kemeny NE, Fong Y, Blumgart LH and Jarnagin WR: Effect
of postoperative morbidity on long-term survival after hepatic
resection for meta-static colorectal cancer. Ann Surg.
247:994–1002. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kataoka K, Takeuchi H, Mizusawa J, Igaki
H, Ozawa S, Abe T, Nakamura K, Kato K, Ando N and Kitagawa Y:
Prognostic impact of postoperative morbidity after esophagectomy
for esophageal cancer: Exploratory analysis of JCOG9907. Ann Surg.
265:1152–1157. 2017. View Article : Google Scholar
|
|
6
|
Lerut T, Moons J, Coosemans W, Van
Raemdonck D, De Leyn P, Decaluwé H, Decker G and Nafteux P:
Postoperative complications after transthoracic esophagectomy for
cancer of the esophagus and gastroesophageal junction are
correlated with early cancer recurrence: Role of systematic grading
of complications using the modified Clavien classification. Ann
Surg. 250:798–807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Braunwarth E, Primavesi F, Göbel G,
Cardini B, Oberhuber R, Margreiter C, Maglione M, Schneeberger S,
Öfner D and Stättner S: Is bile leakage after hepatic resection
associated with impaired long-term survival? Eur J Surg Oncol.
45:1077–1083. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dranoff G: Cytokines in cancer
pathogenesis and cancer therapy. Nat Rev Cancer. 4:11–22. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ogura M, Takeuchi H, Kawakubo H, Nishi T,
Fukuda K, Nakamura R, Takahashi T, Wada N, Saikawa Y, Omori T, et
al: Clinical significance of CXCL-8/CXCR-2 network in esophageal
squamous cell carcinoma. Surgery. 154:512–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okamura A, Takeuchi H, Matsuda S, Ogura M,
Miyasho T, Nakamura R, Takahashi T, Wada N, Kawakubo H, Saikawa Y,
et al: Factors affecting cytokine change after esopha-gectomy for
esophageal cancer. Ann Surg Oncol. 22:3130–3135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pachot A, Cazalis MA, Venet F, Turrel F,
Faudot C, Voirin N, Diasparra J, Bourgoin N, Poitevin F, Mougin B,
et al: Decreased expression of the fractalkine receptor CX3CR1 on
circulating monocytes as new feature of sepsis-induced
immunosuppression. J Immunol. 180:6421–6429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Panis Y, Ribeiro J, Chrétien Y and
Nordlinger B: Dormant liver metastases: An experimental study. Br J
Surg. 79:221–223. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hackl C, Neumann P, Gerken M, Loss M,
Klinkhammer-Schalke M and Schlitt HJ: Treatment of colorectal liver
metastases in Germany: A ten-year population-based analysis of 5772
cases of primary colorectal adenocarcinoma. BMC Cancer. 14:8102014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J and Chen L: Current status and
progress in gastric cancer with liver metastasis. Chin Med J
(Engl). 124:445–456. 2011.
|
|
15
|
Murakami Y, Satoi S, Sho M, Motoi F,
Matsumoto I, Kawai M, Honda G, Uemura K, Yanagimoto H, Shinzeki M,
et al: National comprehensive cancer network resectability status
for pancreatic carcinoma predicts overall survival. World J Surg.
39:2306–2314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krenkel O and Tacke F: Liver macrophages
in tissue homeostasis and disease. Nat Rev Immunol. 17:306–321.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guilliams M, Dutertre CA, Scott CL,
McGovern N, Sichien D, Chakarov S, Van Gassen S, Chen J, Poidinger
M, De Prijck S, et al: Unsupervised high-dimensional analysis
aligns dendritic cells across tissues and species. Immunity.
45:669–684. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nolan JP: The role of intestinal endotoxin
in liver injury: A long and evolving history. Hepatology.
52:1829–1835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crispe IN: The liver as a lymphoid organ.
Annu Rev Immunol. 27:147–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jenne CN and Kubes P: Immune surveillance
by the liver. Nat Immunol. 14:996–1006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davies LC, Jenkins SJ, Allen JE and Taylor
PR: Tissue-resident macrophages. Nat Immunol. 14:986–995. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brinkmann V, Reichard U, Goosmann C,
Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y and Zychlinsky A:
Neutrophil extracellular traps kill bacteria. Science.
303:1532–1535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brinkmann V and Zychlinsky A: Neutrophil
extracellular traps: Is immunity the second function of chromatin?
J Cell Biol. 198:773–783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Erpenbeck L and Schön MP: Neutrophil
extracellular traps: Protagonists of cancer progression? Oncogene.
36:2483–2490. 2017. View Article : Google Scholar
|
|
25
|
Chen R, Kang R, Fan XG and Tang D: Release
and activity of histone in diseases. Cell Death Dis. 5:e13702014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sakurai K, Miyashita T, Okazaki M,
Yamaguchi T, Ohbatake Y, Nakanuma S, Okamoto K, Sakai S, Kinoshita
J, Makino I, et al: Role for neutrophil extracellular traps (NETs)
and platelet aggregation in early sepsis-induced hepatic
dysfunction. In Vivo. 31:1051–1058. 2017.PubMed/NCBI
|
|
27
|
Miyashita T, Ahmed AK, Nakanuma S, Okamoto
K, Sakai S, Kinoshita J, Makino I, Nakamura K, Hayashi H, Oyama K,
et al: A three-phase approach for the early identification of acute
lung injury induced by severe sepsis. In Vivo. 30:341–349.
2016.PubMed/NCBI
|
|
28
|
Placke T, Örgel M, Schaller M, Jung G,
Rammensee HG, Kopp HG and Salih HR: Platelet-derived MHC class I
confers a pseudonormal phenotype to cancer cells that subverts the
anti-tumor reactivity of natural killer immune cells. Cancer Res.
72:440–448. 2012. View Article : Google Scholar
|
|
29
|
Miyashita T, Tajima H, Makino I,
Nakagawara H, Kitagawa H, Fushida S, Harmon JW and Ohta T:
Metastasis-promoting role of extravasated platelet activation in
tumor. J Surg Res. 193:289–94. 2015. View Article : Google Scholar
|
|
30
|
Ishikawa S, Miyashita T, Inokuchi M,
Hayashi H, Oyama K, Tajima H, Takamura H, Ninomiya I, Ahmed AK,
Harman JW, et al: Platelets surrounding primary tumor cells are
related to chemoresistance. Oncol Rep. 36:787–774. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao S, Chen C, Chang K, Karnad A,
Jagirdar J, Kumar AP and Freeman JW: CD44 expression level and
isoform contributes to pancreatic cancer cell plasticity,
invasiveness, and response to therapy. Clin Cancer Res.
15:5592–5604. 2016. View Article : Google Scholar
|
|
32
|
Ohta T, Nakagawara H, Arakawa H, Futagami
F, Tsukioka Y, Kitagawa H, Kayahara M, Nagakawa T and Miyazaki I: A
new strategy for the therapy of pancreatic cancer invasion and
metastasis by protease inhibitor and protein pump inhibitor agents.
Jpn J Gastroenterol Surg. 29:888–892. 1996. View Article : Google Scholar
|
|
33
|
Cools-Lartigue J, Spicer J, McDonald B,
Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P and Ferri L:
Neutrophil extracellular traps sequester circulating tumor cells
and promote metastasis. J Clin Invest. 123:3446–3458. 2013.
View Article : Google Scholar
|
|
34
|
Qiao Y, Li J, Shi C, Wang W, Qu X, Xiong
M, Sun Y, Li D, Zhao X and Zhang D: Prognostic value of circulating
tumor cells in the peripheral blood of patients with esophageal
squamous cell carcinoma. Onco Targets Ther. 10:1363–1373. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cohen SJ, Punt CJ, Iannotti N, Saidman BH,
Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et
al: Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Groot Koerkamp B, Rahbari NN, Büchler MW,
Koch M and Weitz J: Circulating tumor cells and prognosis of
patients with resectable colorectal liver metastases or widespread
meta-static colorectal cancer: A meta-analysis. Ann Surg Oncol.
20:2156–2165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rahbari NN, Aigner M, Thorlund K, Mollberg
N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M and Weitz
J: Meta-analysis shows that detection of circulating tumor cells
indicates poor prognosis in patients with colorectal cancer.
Gastroenterology. 138:1714–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Negin BP and Cohen SJ: Circulating tumor
cells in colorectal cancer: Past, present, and future challenges.
Curr Treat Options Oncol. 11:1–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dotan E, Alpaugh RK, Ruth K, Negin BP,
Denlinger CS, Hall MJ, Astsaturov I, McAleer C, Fittipaldi P,
Thrash-Bingham C, et al: Prognostic significance of MUC-1 in
circulating tumor cells in patients with metastatic pancreatic
adenocarcinoma. Pancreas. 45:1131–1135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kurihara T, Itoi T, Sofuni A, Itokawa F,
Tsuchiya T, Tsuji S, Ishii K, Ikeuchi N, Tsuchida A, Kasuya K, et
al: Detection of circulating tumor cells in patients with
pancreatic cancer: A preliminary result. J Hepatobiliary Pancreat
Surg. 15:189–195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tien YW, Kuo HC, Ho BI, Chang MC, Chang
YT, Cheng MF, Chen HL, Liang TY, Wang CF, Huang CY, et al: A high
circulating tumor cell count in portal vein predicts liver
metastasis from periampullary or pancreatic cancer: A high portal
venous CTC count predicts liver metastases. Medicine (Baltimore).
95:e34072016. View Article : Google Scholar
|
|
42
|
Richardson JJR, Hendrickse C, Gao-Smith F
and Thickett DR: Neutrophil extracellular trap production in
patients with colorectal cancer in vitro. Int J Inflam.
2017:49150622017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Engelmann B and Massberg S: Thrombosis as
an intravascular effector of innate immunity. Nat Rev Immunol.
13:34–45. 2013. View Article : Google Scholar
|
|
44
|
Nakanuma S, Miyashita T, Hayashi H, Tajima
H, Takamura H, Tsukada T, Okamoto K, Sakai S, Makino I, Kinoshita
J, et al: Extravasated platelet aggregation in liver zone 3 may
correlate with the progression of sinusoidal obstruction syndrome
following living donor liver transplantation: A case report. Exp
Ther Med. 9:1119–1124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Miyashita T, Nakanuma S, Ahmed AK, Makino
I, Hayashi H, Oyama K, Nakagawara H, Tajima H, Takamura H, Ninomiya
I, et al: Ischemia reperfusion-facilitated sinusoidal endothelial
cell injury in liver transplantation and the resulting impact of
extravasated platelet aggregation. Eur Surg. 48:92–98. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Keysar SB and Jimeno A: More than markers:
Biological significance of cancer stem cell-defining molecules. Mol
Cancer Ther. 9:2450–2457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zoller M: CD44: Can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Brabletz T: EMT and MET in metastasis:
Where are the cancer stem cells? Cancer Cell. 22:699–701. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Alix-Panabières C, Mader S and Pantel K:
Epithelial-mesenchymal plasticity in circulating tumor cells. J Mol
Med (Berl). 95:133–142. 2017. View Article : Google Scholar
|
|
50
|
Bourcy M, Suarez-Carmona M, Lambert J,
Francart ME, Schroeder H, Delierneux C, Skrypek N, Thompson EW,
Jérusalem G, Berx G, et al: Tissue factor induced by
epithelial-mesenchymal transition triggers a procoagulant state
that drives metastasis of circulating tumor cells. Cancer Res.
76:4270–4282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cortés-Hernández LE, Eslami-S Z and
Alix-Panabières C: Circulating tumor cells as the functional aspect
of liquid biopsy to understand the metastatic cascade in solid
cancer. Mol Aspects Med. 72:1008162020. View Article : Google Scholar
|
|
52
|
Aceto N, Toner M, Maheswaran S and Haber
DA: En route to metastasis: Circulating tumor cell clusters and
epithelial-to-mesenchymal transition. Trends Cancer. 1:44–52. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yu H, Ni YN, Liang ZA, Liang BM and Wang
Y: The effect of aspirin in preventing the acute respiratory
distress syndrome/acute lung injury: A meta-analysis. Am J Emerg
Med. 36:1486–1491. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Boyle AJ, Di Gangi S, Hamid UI, Mottram
LJ, McNamee L, White G, Cross LJ, McNamee JJ, O'Kane CM and McAuley
DF: Aspirin therapy in patients with acute respiratory distress
syndrome (ARDS) is associated with reduced intensive care unit
mortality: A prospective analysis. Crit Care. 19:1092015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rothwell PM, Wilson M, Price JF, Belch JF,
Meade TW and Mehta Z: Effect of daily aspirin on risk of cancer
metastasis: A study of incident cancers during randomised
controlled trials. Lancet. 379:1591–1601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liao X, Lochhead P, Nishihara R, Morikawa
T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K, et
al: Aspirin use, tumor PIK3CA mutation, and colorectal-cancer
survival. N Engl J Med. 367:1596–1606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sahasrabuddhe VV, Gunja MZ, Graubard BI,
Trabert B, Schwartz LM, Park Y, Hollenbeck AR, Freedman ND and
McGlynn KA: Nonsteroidal anti-inflammatory drug use, chronic liver
disease, and hepatocellular carcinoma. J Natl Cancer Inst.
104:1808–1814. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Takada S, Miyashita T, Yamamoto Y, Kanou
S, Munesue S, Ohbatake Y, Nakanuma S, Okamoto K, Sakai S, Kinoshita
J, et al: Soluble thrombomodulin attenuates endothelial cell damage
in hepatic sinusoidal obstruction syndrome. In Vivo. 32:1409–1417.
2018. View Article : Google Scholar : PubMed/NCBI
|