Introduction
Breast cancer (BC) is a common malignancy that
threatens the health of women globally, and it has been predicted
that the incidence of BC will reach ~3.2 million new cases per year
by 2050 globally (1). The
heterogeneity of BC in genetic and epigenetic alterations limits
its progression of treatment development (2,3).
Deep understanding of the molecular mechanisms behind BC
progression is pivotal for identifying novel targets for diagnosis,
therapy and prognosis of BC.
Circular RNAs (circRNAs) form covalently continuous
circular structures without 3′- or 5′-ends (4,5).
CircRNAs regulate cellular physiological (6,7)
and pathological (8,9) processes by acting as transcription
modulators and microRNA (miRNA/miR) sponges (10). There are increasing studies
focusing on the miRNA sponge role of circRNAs in cancer (11-13). Through sponging and sequestering
miRNAs, the downstream mRNAs are released from miRNA inhibition,
causing the upregulation of mRNAs (13). For instance, Ding et al
(14) revealed that circ-ATP8A2
accelerated the progression of cervical cancer via sponging miR-433
to upregulate EGFR expression. Sun et al (15) found that circ-SFMBT2 facilitated
gastric cancer progression via acting as a sponge for miR-182-5p to
elevate the abundance of cAMP responsive element binding protein 1.
In the present study, the expression pattern and function of
circ_0000511 in BC development was investigated.
miRNAs exert pro-tumor or antitumor roles in cancer
through modulating the expression levels of mRNAs via degrading
them or blocking their translation (16,17). miR-326 has been reported to
suppress the progression of BC via regulating ErbB/PI3K signaling
(18). In the present study, the
working mechanism of miR-326 in BC progression was
investigated.
Transcriptional co-activator with PDZ-binding motif
(TAZ) was identified for the first time due to its interaction with
14-3-3 proteins (19). In
addition to the interaction with 14-3-3, TAZ was found to interact
with SLC9A3 regulator 2, translation elongation factor EF-1a and
paired box 3 (19-21). Chan et al (22) have identified the promoting roles
of TAZ in the migration and invasion of BC cells. However, the
molecular mechanism behind TAZ in the progression of BC remains to
be elucidated.
The present study analyzed the influences of
circ_0000511 on the malignant phenotypes of BC cells, and the
circ_0000511-miRNA-mRNA axis in BC cells was explored to illustrate
its working mechanism.
Materials and methods
Tissue samples
BC tissue specimens and adjacent non-tumor tissue
specimens (5 cm from tumor tissues) were collected from 50 female
patients (median age, 53 years; age range, 29-86 years) with BC at
The First Hospital of Jilin University (Changchun, China) between
March 2016 and November 2017. The exclusion criteria were as
follows: i) Patients who received preoperative treatment, including
chemotherapy or radiotherapy; and ii) patients who had other types
of tumor in other organs. The inclusion criteria were as follows:
i) All clinicopathological diagnoses were confirmed by two
pathologists; ii) none of the patients received any treatments
before surgery; iii) no history of other synchronous malignancies;
and iv) no death in the perioperative period. All samples were
immediately frozen in liquid nitrogen. All participants provided
written informed consent before surgical resection. The present
study was approved by the Ethics Committee of The First Hospital of
Jilin University.
Cell culture
A panel of human BC cell lines (MCF-7, SK-BR-3,
MDA-MB-231 and MDA-MB-468) and a human breast epithelial cell line
(MCF-10A) were purchased from BeNa Culture Collection. BC cell
lines and MCF-10A cell line were cultured with RPMI-1640 (MCF-10A
cell line) or DMEM (BC cell lines) (both from Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin (100 units/ml)/streptomycin
(100 μg/ml) mixture (Beijing Solarbio Science &
Technology Co., Ltd.) at 37°C with 5% CO2.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA samples were isolated from tissues and cells
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. RT was conducted
using the Bio-Rad iScript kit (Bio-Rad Laboratories, Inc.) and the
TaqMan RT kit (Applied Biosystems; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol, to obtain cDNA, which was
later used as the template for qPCR. The amplification of
circ_0000511, ribonuclease P RNA component H1 (RPPH1) and TAZ was
performed using iQSYBR-Green SuperMix (Bio-Rad Laboratories, Inc.),
while the PCR reaction of miR-326 was conducted using the TaqMan
MicroRNA assay kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). RT-qPCR started at 95°C for 5 min, followed by 40 cycles at
95°C for 30 sec, 60°C for 40 sec and 72°C for 30 sec. GAPDH (for
circ_0000511, RPPH1 and TAZ) and U6 (for miR-326) served as the
internal references. The quantification was performed with the
2−ΔΔCq method (23).
The specific primers are displayed in Table I.
 | Table IPrimers used for reverse
transcription-quantitative PCR. |
Table I
Primers used for reverse
transcription-quantitative PCR.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| circ_0000511 |
CCTCCTTTGCCGGAGCTT |
GGTCCACGGCATCTCCTG |
| RPPH1 |
GAGCTGAGTGCGTCCTGTC |
TCAGGGAGAGCCCTGTTAGG |
| miR-326 |
CCTCTGGGCCCTTCCTCCAG | Universal
primer |
| TAZ |
AGTACCCTGAGCCAGCAGAA |
GATTCTCTGAAGCCGCAGTT |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
| GAPDH |
GTGAAGGTCGGAGTCAACGG |
GAGGTCAATGAAGGGGTCATTG |
RNase R treatment
RNase R digestion was used to test the stability and
circular structure of circ_0000511 and RPPH1 mRNA. RNA was isolated
from MCF-7 and MDA-MB-468 cells using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.), and 10 μg RNA
was digested with 40 U RNase R (Epicentre; Illumina, Inc.). The
enrichment of circ_0000511 and RPPH1 mRNA was detected by RTq-PCR,
as aforementioned.
Cell transfection
Small interfering (si)RNA of circ_0000511
(si-circ_0000511), scrambled siRNA negative control (si-NC), short
hairpin (sh)RNA of circ_0000511 using pGIPZ vector
(sh-circ_0000511), pGIPZ empty vector (sh-NC), miR-326 mimics
(miR-326), miR-NC, miR-326 inhibitor (anti-miR-326), anti-miR-NC,
TAZ overexpression plasmid (TAZ) and pcDNA empty vector (pcDNA)
were purchased from Shanghai GenePharma Co., Ltd. The sequences
were as follows: si-circ_0000511 sense, 5′-AAU GGC UGA GGU GAG UUC
CCA-3′ and antisense, 5′-GGA ACU CAC CUC AGC CAU UGG-3′; si-NC
sense, 5′-AAC AGG CAC ACG UCC CAG CGU-3′ and antisense, 5′-ACG CUG
GGA CGU GUG CCU GUU-3′; miR-326, 5′-AGG UCU GGA CCG UAA GUC AG-3′;
miR-NC, 5′-UGG ACG GAC CAC UGA CAU G-3′; anti-miR-326, 5′-CGU GGA
UCG AAC GUU GGA C-3′; and anti-miR-NC, 5′-ACG GAC GUU GAG ACA CGG
G-3′. A total of 1 μg plasmid or 0.5 μM
oligonucleotides was transfected into MCF-7 or MDA-MB-468 cells
using Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After transfection for 7 h at 37°C, the culture
medium was replaced by fresh complete culture medium. BC cells were
utilized for further analysis after 24 h of transfection.
Colony formation assay
A total of 150 MCF-7 or MDA-MB-468 cells in the
control group and experimental groups were seeded into 6-well
plates and allowed to grow for 2 weeks at 37°C. The culture medium
was replenished every 6 days. The visible colonies were immobilized
using 4% poly methanol for 15 min at room temperature, and stained
using 0.5% crystal violet for 10 min at room temperature. Colonies
containing >50 cells were counted under a light microscope.
MTT assay
MCF-7 and MDA-MB-468 cells were seeded into 96-well
plates to settle down at 37°C overnight. The next day, BC cells
were transfected with plasmids or RNAs, as aforementioned. After
transfection for specific intervals (0, 1, 2 or 3 days), BC cells
were incubated with 10 μl MTT (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 4 h. A total of 100 μl DMSO
(Sangon Biotech Co., Ltd.) was added to the wells to dissolve the
formazan product. The absorbance was detected at 570 nm.
Flow cytometry
The apoptotic rate of transfected MCF-7 and
MDA-MB-468 cells was assessed by flow cytometry. BC cells in the
early and late stage of apoptosis were differentiated from normal
or necrotic BC cells through double staining with Annexin V-FITC
and PI at 37°C for 15 min in the dark (both Beijing Solarbio
Science & Technology Co., Ltd.). The apoptotic rate was
evaluated using FlowJo software (v7.6; FlowJo LLC) on a FC-500 flow
cytometer (Beckman Coulter, Inc.).
Western blot assay
After plasmid or RNA transfection, MCF-7 and
MDA-MB-468 cells were lysed using cell lysis buffer (Abcam). The
BCA method was used to examine the concentrations of different
protein samples. Protein samples (25 μg/lane) were separated
via 10% SDS-PAGE and transferred to PVDF membranes (Bio-Rad
Laboratories, Inc.). After incubating with 5% skimmed milk for 1 h
at room temperature, the PVDF membranes were incubated overnight at
4°C with specific primary antibodies, including anti-B cell
leukemia/lymphoma 2 (anti-Bcl-2; 1:5,000; cat. no. ab185002;
Abcam), anti-Bcl-2 associated X apoptosis regulator (anti-Bax;
1:8,000; cat. no. ab32503; Abcam), anti-cleaved caspase-3
(1:10,000; cat. no. ab2302; Abcam), anti-TAZ (1:5,000; cat. no.
ab242313; Abcam) and anti-GAPDH (1:20,000; cat. no. ab181602;
Abcam). The next day, an HRP-conjugated secondary antibody
(1:5,000; cat. no. ab205718; Abcam) was incubated with the PVDF
membranes for 2 h at room temperature. The immunoreactive protein
bands were measured using an enhanced chemiluminescent
visualization (ECL) kit (Pierce; Thermo Fisher Scientific, Inc.).
Image Lab analysis software (v4.0; Bio-Rad Laboratories, Inc.) was
used to analyze the intensities of protein bands.
Transwell assays
Transwell chambers (Costar; Corning, Inc.) were used
in the present study. For Transwell migration assays, 200 μl
cell suspension (1×104 MCF-7 and MDA-MB-468 cells in
serum-free DMEM) was added to the upper chambers. The lower
chambers contained culture medium with 10% FBS. After incubation at
37°C for 24 h, BC cells on the upper surface of the membrane were
discarded using a cotton swab, and the migrated BC cells on the
lower surface of the membrane were stained using 0.5% crystal
violet for 10 min at room temperature. Five random fields at a
magnification of ×100 were selected to count the number of
migrating cells under a light microscope.
For Transwell invasion assays, the upper chambers
were pre-coated with 40 μl Matrigel (BD Biosciences) at the
dilution of 1:8 at 37°C for 30 min. The remaining steps were
similar as for the Transwell migration assays.
Bioinformatic prediction
The circ_0000511-miRNAs interactions and
miR-326-mRNAs interactions were explored using the StarBase
database (http://starbase.sysu.edu.cn).
Dual-luciferase reporter assay
To test the direct interaction between miR-326 and
circ_0000511 or TAZ mRNA, the wild-type (wt) partial sequences in
circ_0000511 and TAZ mRNA 3′-untranslated region (UTR), containing
the putative binding sequence with miR-326, were amplified and
constructed in the psiCHECK-2 luciferase vector (Promega
Corporation), generating circ_0000511-wt and TAZ-wt. Meanwhile,
circ_0000511-mutant (mut) and TAZ-mut luciferase plasmids
containing the mutant binding sites with miR-326 were generated
using GeneArtä Site-Directed Mutagenesis System (Invitrogen; Thermo
Fisher Scientific, Inc.). Subsequently, the constructed luciferase
plasmids were co-transfected into BC cells with miR-NC or miR-326
using Lipofectamine 3000, as aforementioned. Luciferase activities
were measured after 24 h of transfection using the Dual Luciferase
Reporter Assay kit (Promega Corporation). The firefly luciferase
activity was normalized to Renilla luciferase activity.
Xenografts
A total of 10 female 4-week-old BALB/c nude mice
(weight, 16-20 g) from Orient Bio, Inc., were randomly divided into
two groups with 5 mice in each group. Mice were maintained under
pathogen-free conditions at 22±2°C and 60% humidity with a 12-h
light/dark cycle, and were supplied with food and water ad
libitum. The weight of mice was measured twice a week to
monitor the health of mice. Mice weight in the two groups exhibited
no significant difference during the experiment. The maximum volume
of xenograft tumor was <1,000 mm3. The mice were
euthanized by carbon dioxide suffocation. The CO2
displacement rate used for mouse euthanasia was 30% chamber
volume/min. The MCF-7 cell line stably transfected with sh-NC or
sh-circ_0000511 was collected and re-suspended in PBS buffer. A
total of 200 μl cell suspension containing 2×106
MCF-7 cells was subcutaneously injected into the right flank of
nude mice. The length and width of tumor masses were detected every
week until 4 weeks after injection when the mice were euthanized.
The tumor volume was analyzed with the following formula: Volume =
width2 × length × 0.5. The tumors were weighed after
removing the tumors from the mice. The abundance of circ_0000511,
miR-326 and TAZ mRNA and protein was examined by RT-qPCR and
western blot assay, respectively. Animal manipulation was approved
by the Research Ethics Committee of The First Hospital of Jilin
University.
Statistical analysis
The results were analyzed using GraphPad Prism
software (v7.0; GraphPad Software, Inc.) and the data are displayed
as the mean ± SD. All in vitro experiments were repeated
three times. Differences between BC tissues and adjacent normal
tissues were analyzed using paired Student's t-test, while unpaired
Student's t-test was utilized to assess the differences between two
groups of BC cells. One-way ANOVA followed by Tukey's test was used
to analyze the differences in multiple groups. Linear correlation
analysis was performed using Spearman's correlation coefficient.
P<0.05 was considered to indicate a statistically significant
difference.
Results
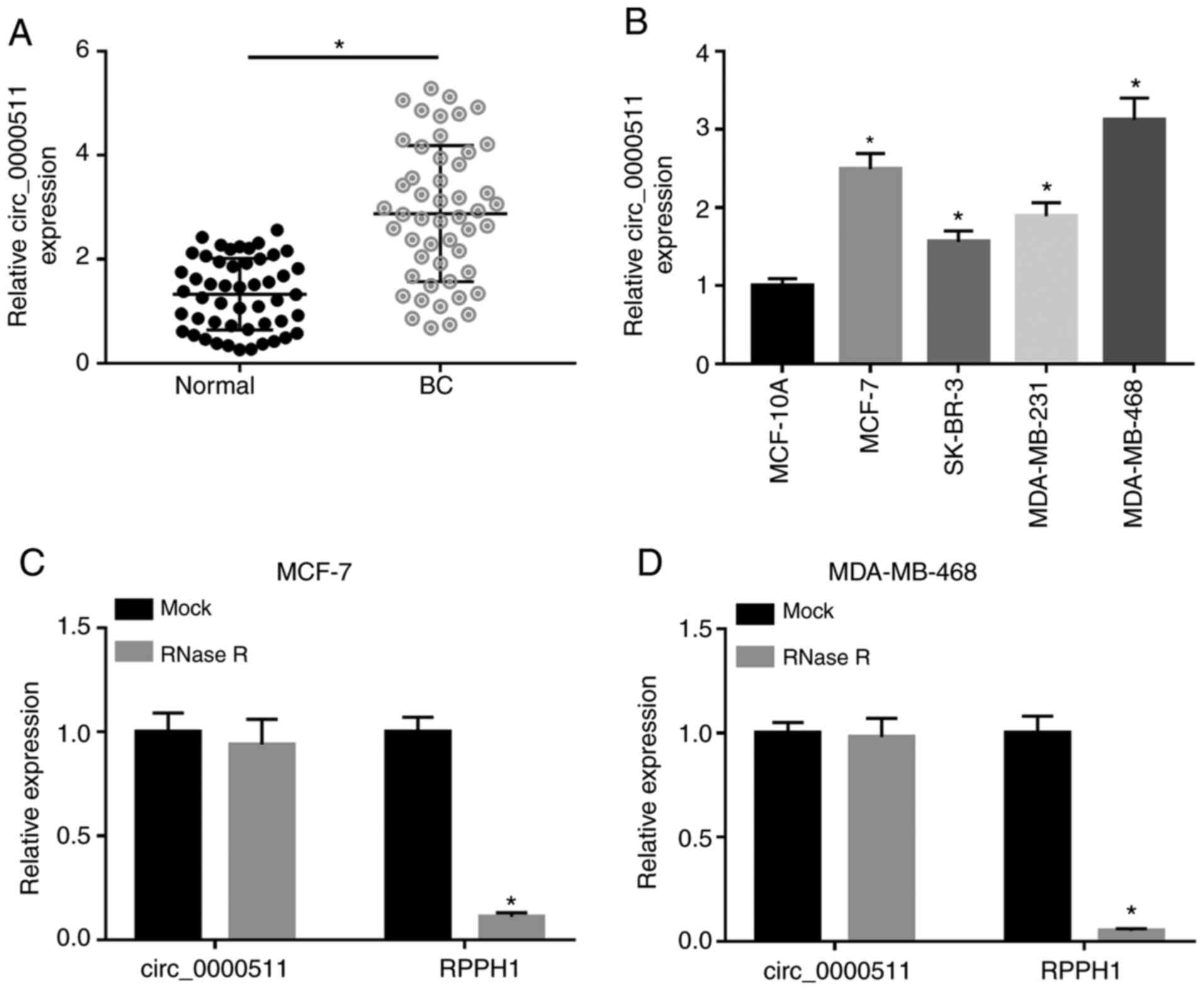
Circ_0000511 expression is upregulated in
BC
Circ_0000511 expression was significantly elevated
in BC tissues compared with in matching adjacent normal tissues
(Fig. 1A). The expression levels
of circ_0000511 in the normal breast epithelial MCF-10A cell line
and four BC cell lines (MCF-7, SK-BR-3, MDA-MB-231 and MDA-MB-468)
were detected by RT-qPCR. As shown in Fig. 1B, circ_0000511 expression was
significantly enhanced in BC cell lines compared with in the
MCF-10A cell line. Moreover, to test the stability and loop
structure of circ_0000511, exonuclease RNase R was used in
subsequent experiments to digest linear RNA but not circular RNA,
and the linear form of circ_0000511 (RPPH1 mRNA) was used as the
control. RNA samples isolated from BC cells were equally divided
into two parts, and these two parts were exposed to RNase R or not.
As shown in Fig. 1C and D, RNase
R addition significantly downregulated the expression levels of
RPPH1, while the abundance of circ_0000511 remained almost
unchanged in the RNase R treatment and no treatment groups,
suggesting the loop structure of circ_0000511. Overall,
circ_0000511 was identified as a circular RNA, and its expression
was upregulated in BC tissues and cell lines.
Circ_0000511 accelerates the
proliferation, migration and invasion, and impedes the apoptosis of
BC cells
si-circ_0000511 was designed to silence circ_0000511
in BC cell lines to test the biological effects of circ_0000511.
Circ_0000511 abundance was significantly downregulated in MCF-7 and
MDA-MB-468 cells transfected with si-circ_0000511 compared with the
si-NC-transfected group (Fig.
2A). MCF-7 and MDA-MB-468 cell lines were chosen for
loss-of-function experiments due to their higher expression of
circ_0000511 compared with the other two BC cell lines (SK-BR-3 and
MDA-MB-231). The proliferation of BC cells was analyzed using
colony formation and MTT assays. As shown in Fig. 2B, circ_0000511 silencing
significantly decreased the number of colonies, suggesting that
circ_0000511 interference suppressed the proliferative ability of
BC cells. Additionally, the results of MTT assay revealed that
circ_0000511-knockdown impaired the proliferation of BC cells
(Fig. 2C and D). Furthermore,
circ_0000511 interference triggered the apoptosis of BC cells
(Fig. 2E). To further clarify the
influence of circ_0000511 interference on the apoptosis of BC
cells, western blot assay was conducted to detect the protein
expression levels of Bcl-2, Bax and Cleaved caspase-3 in BC cells.
As shown in Fig. 2F and G, the
expression levels of the pro-apoptotic proteins (Bax and Cleaved
caspase-3) were significantly upregulated in BC cells with the
silencing of circ_0000511, while circ_0000511 interference
significantly downregulated the expression levels of the
anti-apoptotic protein Bcl-2, demonstrating that
circ_0000511-knockdown promoted the apoptosis of BC cells. The
migratory and invasive abilities were both significantly suppressed
with the silencing of circ_0000511 compared with in the si-NC group
(Fig. 2H and I), suggesting that
circ_0000511-knockdown restrained the migration and invasion of BC
cells. Overall, high circ_0000511 expression increased the
malignant behaviors of BC cells.
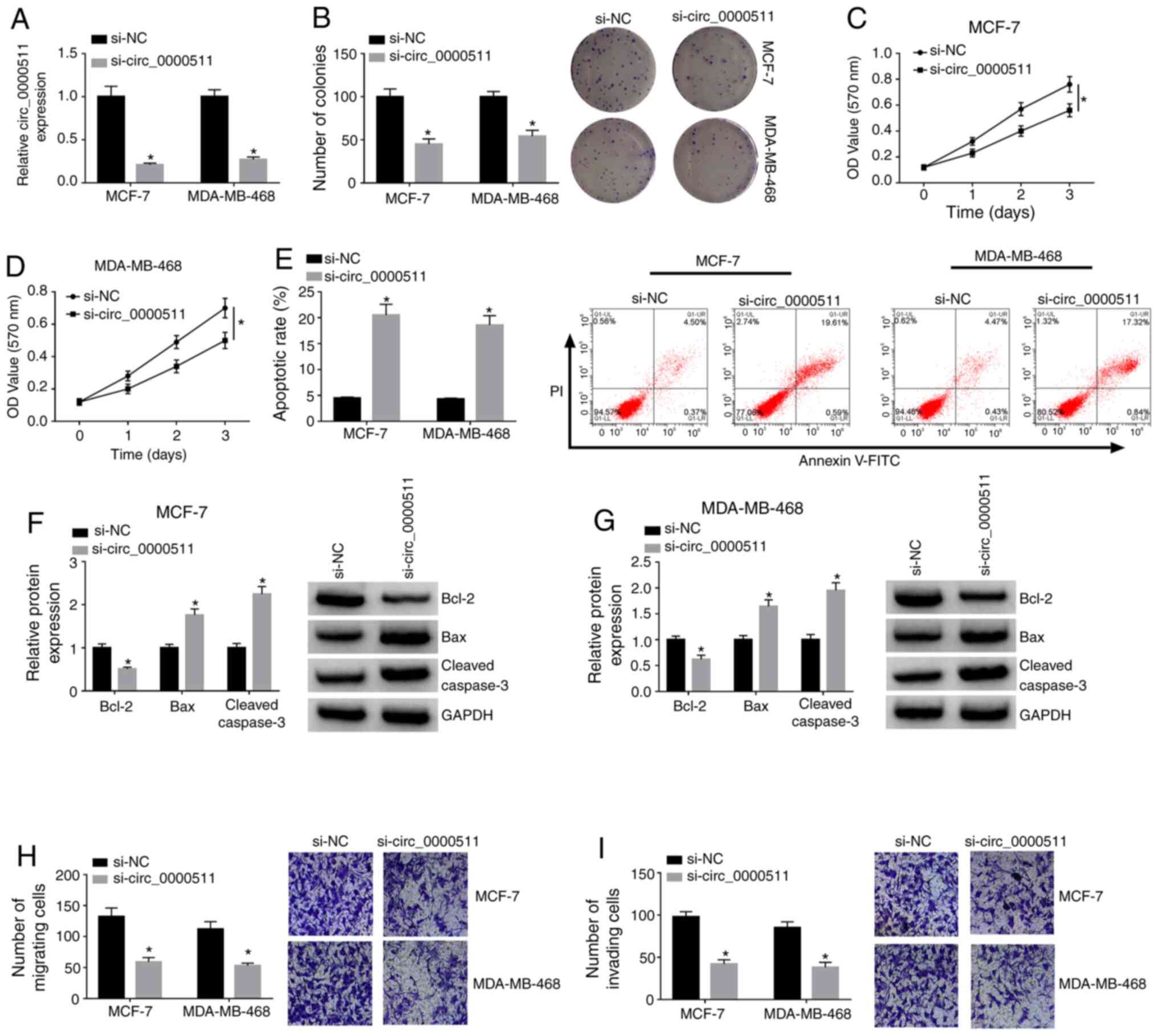 | Figure 2Circ_0000511 accelerates the
proliferation, migration and invasion, and impedes the apoptosis of
BC cells. MCF-7 and MDA-MB-468 cells were transfected with si-NC or
si-circ_0000511. (A) Circ_0000511 expression was detected in BC
cells by reverse transcription-quantitative PCR. (B) Colony
formation assay was used to test the proliferative ability of BC
cells. MTT assay was used to assess the proliferation of (C) MCF-7
and (D) MDA-MB-468 cells. (E) Apoptosis of BC cells was evaluated
by flow cytometry. Western blot assay was used to detect the
protein expression levels of Bcl-2, Bax and Cleaved caspase-3 in
(F) MCF-7 and (G) MDA-MB-468 cells. Transwell assays were conducted
to assess the (H) migratory and (I) invasive abilities of BC cells.
*P<0.05 vs. si-NC. Bcl-2, B cell leukemia/lymphoma 2;
Bax, Bcl-2 associated X apoptosis regulator; BC, breast cancer;
circ_0000511, circular RNA 0000511; si, small interfering RNA; NC,
negative control; OD, optical density. |
miR-326 directly interacts with
circ_0000511 in BC cells
Considering the miRNA sponge role of circRNAs
(13), the present study
investigated whether circ_0000511 accelerated the progression of BC
through targeting miRNAs. The complementary binding sites between
circ_0000511 and miR-326 predicted using the StarBase database are
displayed in Fig. 3A. To test if
circ_0000511 interacted with miR-326 through the predicted binding
sites, these sites in circ_0000511 were mutated to 'AAACUCU'
(Fig. 3A). The transfection
efficiencies of miR-326 and anti-miR-326 were both high in BC cells
(Fig. 3B). The partial sequence
in circ_0000511, including the wt or mut binding sites with
miR-326, was cloned into luciferase vectors, named as
circ_0000511-wt or circ_0000511-mut. The luciferase reporter vector
was co-transfected with miR-NC or miR-326 into BC cells. As shown
in Fig. 3C and D, miR-326
overexpression significantly decreased the luciferase activity in
the circ_0000511-wt group compared with in the miR-NC and
circ_0000511-wt co-transfected group. However, miR-326 transfection
had no influence in luciferase activity in the circ_0000511-mut
group compared with in the miR-NC and circ_0000511-mut group
(Fig. 3C and D). The results of
the dual-luciferase reporter assay demonstrated that miR-326 was a
direct target of circ_0000511 in BC cells. Additionally,
circ_0000511 silencing significantly upregulated the expression
levels of miR-326 in BC cells (Fig.
3E), suggesting a negative regulatory association between
miR-326 and circ_0000511 in BC cells. Among three cell lines,
including MCF-10A and two BC cell lines, miR-326 expression was
significantly decreased in BC cell lines compared with in MCF-10A
cells (Fig. 3F). Furthermore,
miR-326 expression was significantly downregulated in BC tissues
compared with that in normal tissues (Fig. 3G). The analysis of Spearman's
correlation coefficient suggested that miR-326 expression was
negatively correlated with circ_0000511 expression in BC tissues
(Fig. 3H). Overall, the present
results indicated that miR-326 was a direct target of circ_0000511
in BC cells.
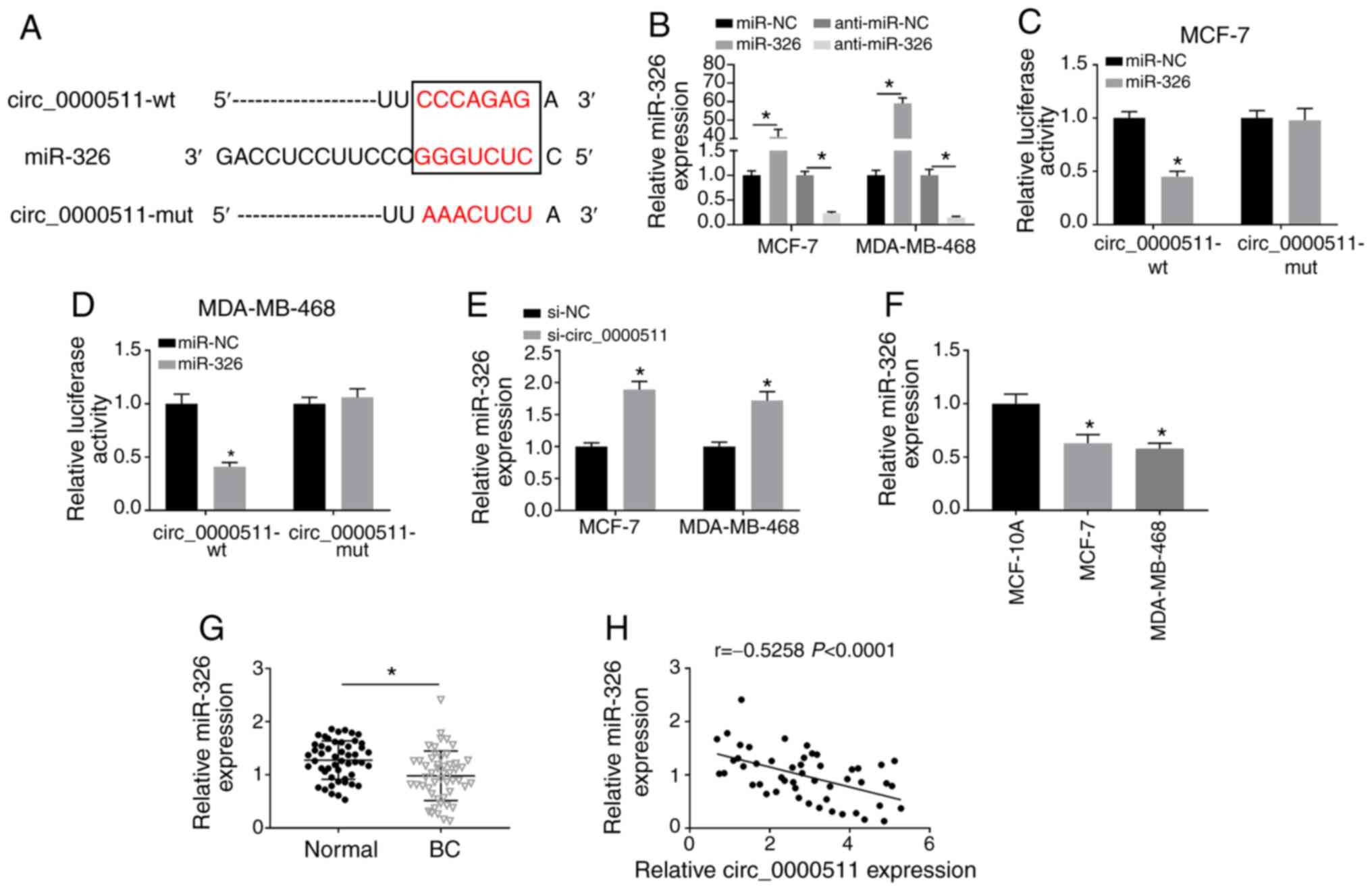 | Figure 3miR-326 directly interacts with
circ_0000511 in BC cells. (A) Circ_0000511-miRNAs interactions were
explored using StarBase 3.0 software. The paring sites between
circ_0000511 and miR-326 are shown, and the complementary site in
circ_0000511 was mutated to 'AAACUCU'. (B) miR-326 expression was
determined in BC cells transfected with miR-NC, miR-326,
anti-miR-NC or anti-miR-326 by RT-qPCR. Dual-luciferase reporter
assay was utilized to verify the target association between miR-326
and circ_0000511 in (C) MCF-7 and (D) MDA-MB-468 cells. (E) miR-326
expression in BC cells transfected with si-NC or si-circ_0000511
was detected by RT-qPCR. miR-326 expression was examined in (F) BC
cell lines and MCF-10A cells, and (G) BC tissues and paired
adjacent normal tissues by RT-qPCR. (H) Linear correlation between
miR-326 and circ_0000511 expression was analyzed by Spearman's
correlation coefficient. *P<0.05 vs. miR-NC, si-NC or
MCF-10A. RT-qPCR, reverse transcription-quantitative PCR;
circ_0000511, circular RNA 0000511; BC, breast cancer; miR,
microRNA; si, small interfering RNA; NC, negative control; wt,
wild-type; mut, mutant. |
Circ_0000511 contributes to BC
progression via targeting miR-326
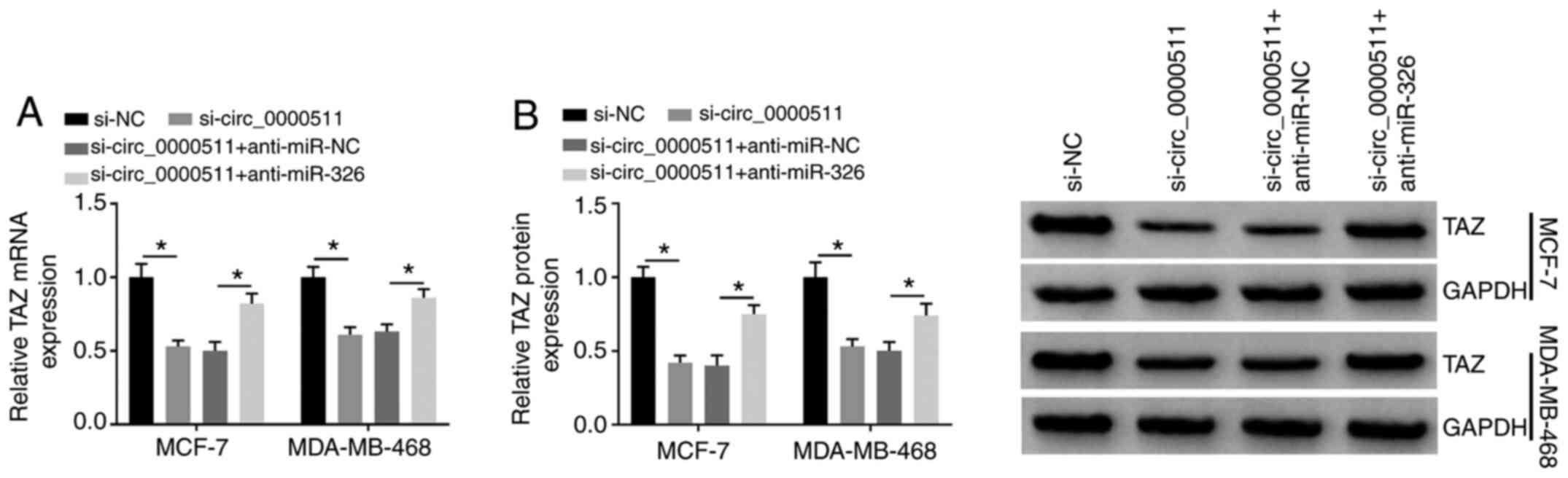
Circ_0000511-knockdown significantly upregulated
miR-326 expression, which was then significantly decreased by the
addition of anti-miR-326 in BC cells (Fig. 4A). Therefore, further rescue
experiments were conducted via transfecting si-circ_0000511 alone
or together with anti-miR-326 into BC cells. The introduction of
anti-miR-326 partly recovered the number of colonies (Figs. 4B and S1A) and the number of viable BC cells
after 3 days of transfection (Fig. 4C
and D), demonstrating that anti-miR-326 addition partly
recovered the proliferative ability of BC cells. Meanwhile, the
apoptosis of these transfected BC cells was assessed by flow
cytometry. Circ_0000511 silencing-induced apoptosis in BC cells was
largely counteracted by the introduction of anti-miR-326 (Figs. 4E and S1B). The protein expression levels of
Bax and Cleaved caspase-3 were significantly upregulated by the
silencing of circ_0000511, while the addition of anti-miR-326
significantly downregulated the expression levels of these two
proteins (Fig. 4F and G). The
abundance of the anti-apoptotic protein Bcl-2 was significantly
downregulated in the si-circ_0000511 group, and the addition of
anti-miR-326 largely recovered Bcl-2 expression (Fig. 4F and G). The present findings
further demonstrated that circ_0000511 restrained the apoptosis of
BC cells via targeting miR-326. Furthermore, migration and invasion
were suppressed with the silencing of circ_0000511, and the
introduction of anti-miR-326 largely rescued the migratory and
invasive abilities of BC cells (Figs.
4H and I, and S1C and D).
Overall, circ_0000511 accelerated the proliferation, migration and
invasion, and inhibited the apoptosis of BC cells via sponging
miR-326.
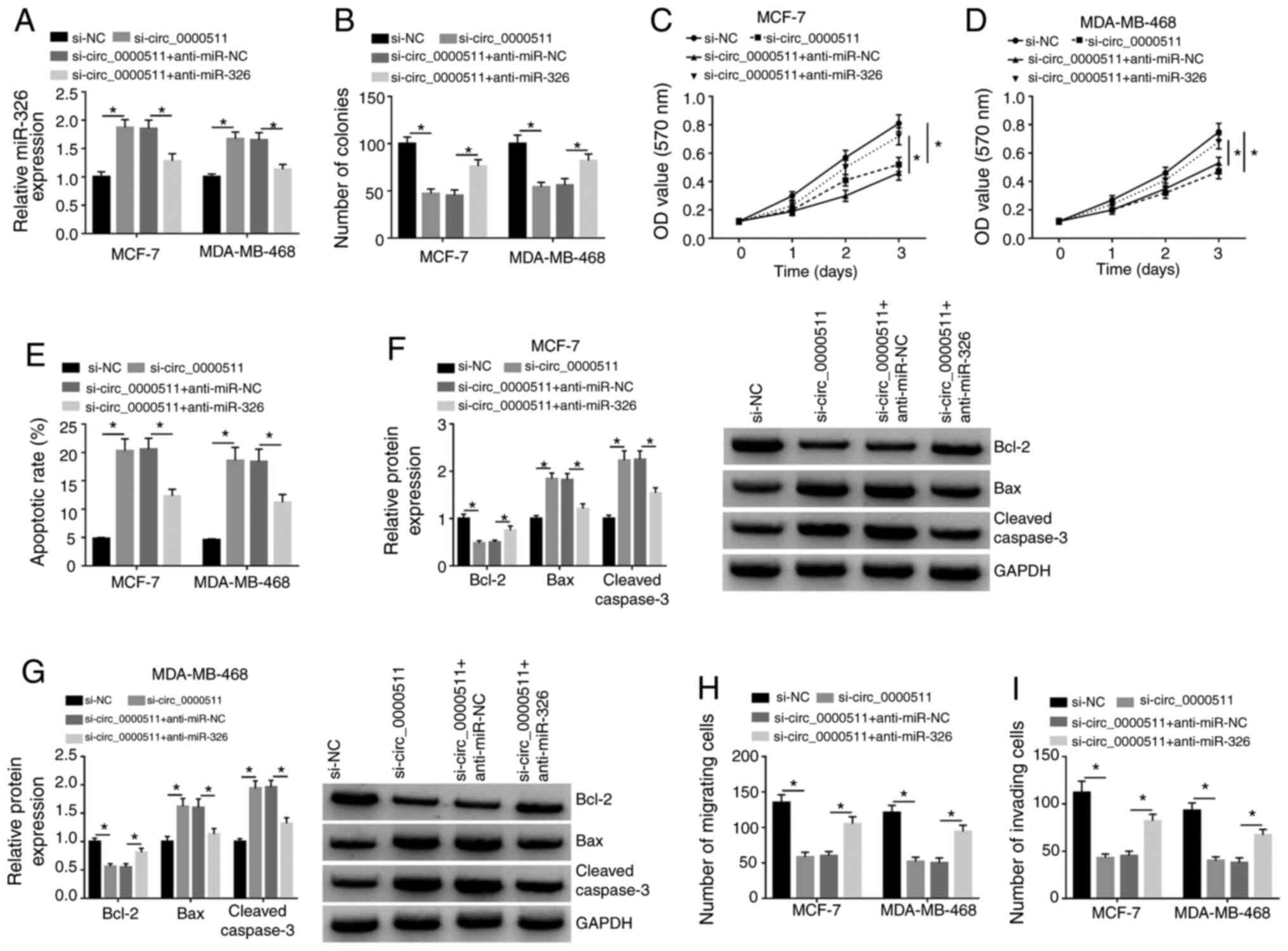 | Figure 4Circ_0000511 contributes to BC
progression via targeting miR-326. MCF-7 and MDA-MB-468 cells were
transfected with the following four groups: si-NC, si-circ_0000511,
si-circ_0000511 + anti-miR-NC or si-circ_0000511 + anti-miR-326.
(A) miR-326 expression in the two transfected BC cell lines was
examined by reverse transcription-quantitative PCR. (B)
Proliferation of BC cells was analyzed using colony formation
assay. MTT assay was utilized to assess the proliferation ability
of (C) MCF-7 and (D) MDA-MB-468 cells. (E) Apoptotic rate in these
four groups was analyzed by flow cytometry. Western blot assay was
utilized to detect the protein expression of Bcl-2, Bax and Cleaved
caspase-3 in (F) MCF-7 and (G) MDA-MB-468 cells. (H) Migration and
(I) invasion of BC cells were analyzed using Transwell assays
(magnification, ×100). *P<0.05. Bcl-2, B cell
leukemia/lymphoma 2; Bax, Bcl-2 associated X apoptosis regulator;
circ_0000511, circular RNA 0000511; BC, breast cancer; miR,
microRNA; si, small interfering RNA; NC, negative control; OD,
optical density. |
miR-326 directly binds to TAZ mRNA in BC
cells
miRNAs are implicated in the pathogenesis of cancer
partly through downregulating the expression levels of target mRNAs
associated with cellular proliferation, apoptosis, migration and
invasion (16,17). miR-326-mRNA interactions were
searched using the StarBase 3.0 database. The complementary
sequence between TAZ mRNA and miR-326 is shown in Fig. 5A. The site-directed mutation in
TAZ is also shown in Fig. 5A.
MCF-7 and MDA-MB-468 cells were co-transfected with miR-NC or
miR-326 and TAZ-wt or TAZ-mut, and the luciferase activities in
these four transfected groups were detected using the
dual-luciferase reporter assay. Compared with the miR-NC and TAZ-wt
co-transfected group, miR-326 transfection significantly decreased
the relative luciferase activity in the TAZ-wt group (Fig. 5B and C). However, miR-NC or
miR-326 transfection did not affect the luciferase activity in the
TAZ-mut groups (Fig. 5B and C),
suggesting a direct association between miR-326 and TAZ mRNA in BC
cells. The present study further explored the regulatory
association between miR-326 and TAZ by detecting the mRNA and
protein expression levels of TAZ in BC cells transfected with
miR-NC, miR-326, anti-miR-NC or anti-miR-326. miR-326
overexpression significantly downregulated the mRNA and protein
expression levels of TAZ, while the inhibition of miR-326
significantly upregulated TAZ mRNA and protein expression (Fig. 5D and E). The current findings
suggested that TAZ was negatively regulated by miR-326 in BC cells.
The expression feature of TAZ and the expression correlation
between TAZ and miR-326 were tested in BC. As shown in Fig. 5F and G, TAZ mRNA and protein
expression was significantly upregulated in BC tissues compared
with in adjacent normal tissues. The results of Spearman's
correlation coefficient analysis revealed that there was a negative
correlation between miR-326 and TAZ mRNA expression in BC tissues
(Fig. 5H). Additionally, TAZ mRNA
and protein expression was significantly upregulated in BC cell
lines compared with in the MCF-10A cell line (Fig. 5I and J). Overall, miR-326
negatively regulated the abundance of TAZ by directly targeting it
in BC cells.
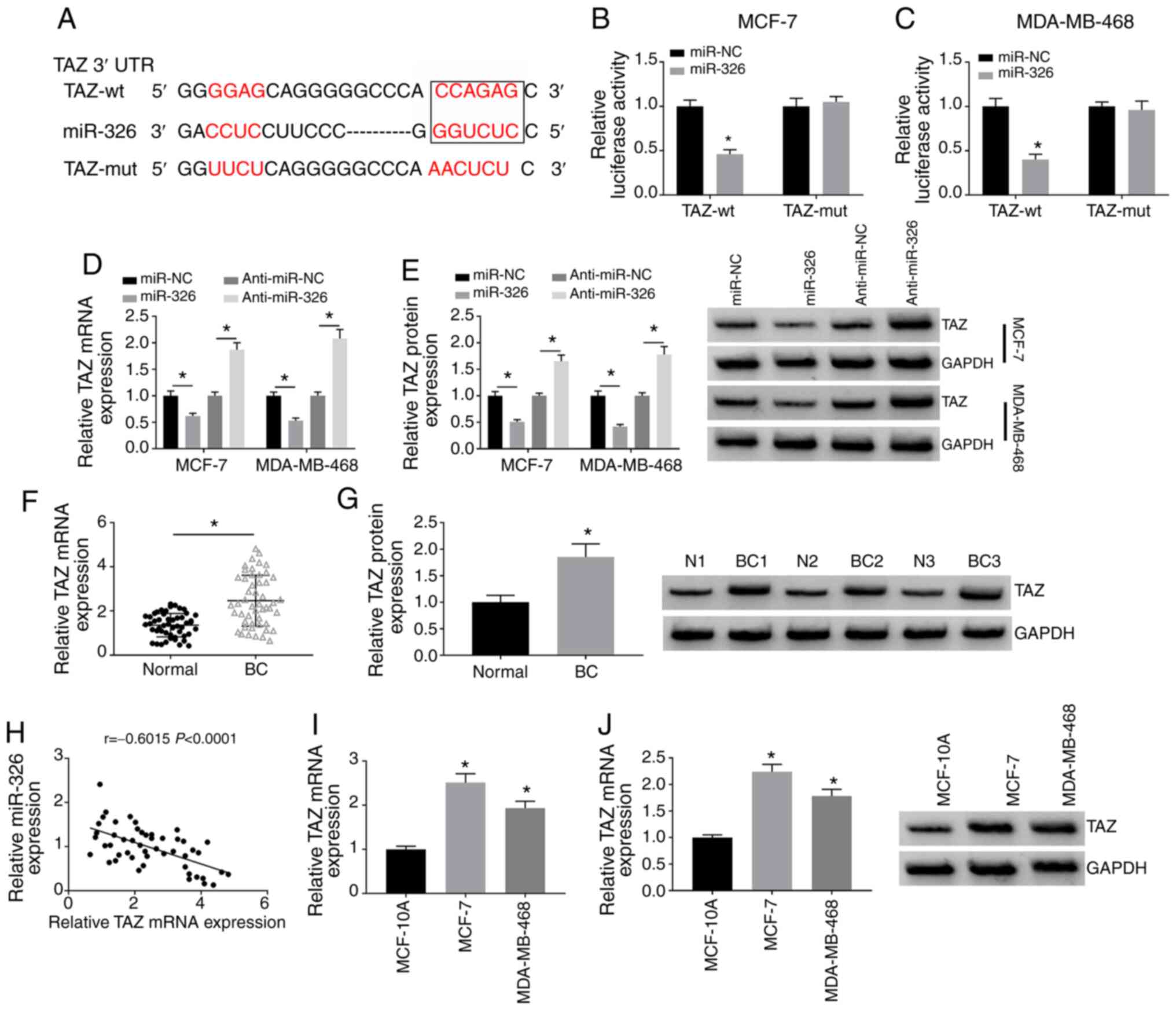 | Figure 5miR-326 directly binds to TAZ mRNA in
BC cells. (A) StarBase software was utilized to search
miR-326-interacted mRNAs, and TAZ was found as a possible target of
miR-326. Luciferase activities in (B) MCF-7 and (C) MDA-MB-468
cells co-transfected with miR-NC or miR-326 and TAZ-wt or TAZ-mut
were detected using dual-luciferase reporter assay. MCF-7 and
MDA-MB-468 cells were transfected with miR-NC, miR-326, anti-miR-NC
or anti-miR-326. TAZ (D) mRNA and (E) protein expression in
transfected BC cells was analyzed by RT-qPCR and western blot
assay, respectively. TAZ (F) mRNA and (G) protein expression in BC
tissues and adjacent normal tissues was detected by RT-qPCR and
western blot assay, respectively. (H) Correlation between miR-326
and TAZ mRNA expression was analyzed using Spearman's correlation
coefficient. (I) RT-qPCR and (J) western blot assay were
implemented to examine the mRNA and protein expression levels of
TAZ, respectively, in BC cell lines and MCF-10A cells.
*P<0.05 vs. miR-NC, normal or MCF-10A. RT-qPCR,
reverse transcription-quantitative PCR; BC, breast cancer; miR,
microRNA; si, small interfering RNA; NC, negative control; wt,
wild-type; mut, mutant; UTR, untranslated region; TAZ,
transcriptional co-activator with PDZ-binding motif; N, normal. |
miR-326-mediated influences are partly
reversed by the overexpression of TAZ in BC cells
The overexpression of TAZ was tested by RT-qPCR
assay and western blot assay. As shown in Fig. S2A and B, the mRNA and protein
expression levels of TAZ were both significantly upregulated
following the transfection of TAZ plasmid in BC cells, suggesting
the high transfection efficiency of the TAZ plasmid. miR-326 over-
expression significantly downregulated the mRNA and protein
expression levels of TAZ in BC cells, and the addition of TAZ
overexpression plasmid rescued the mRNA and protein abundance of
TAZ in BC cells (Fig. 6A and B).
Therefore, further rescue experiments were conducted by
transfecting miR-NC, miR-326, miR-326 + pcDNA or miR-326 + TAZ into
BC cells to test if miR-326 functioned via decreasing TAZ
expression. miR-326 overexpression suppressed cellular
proliferation, migration and invasion, while apoptosis was
increased with the overexpression of miR-326 (Fig. 6C-J), which further demonstrated
that miR-326 exerted an antitumor role in BC cells. The addition of
TAZ overexpression plasmid partly recovered the proliferative
ability of BC cells according to the results of colony formation
assay (Figs. 6C and S3A) and MTT assay (Fig. 6D and E). The results of flow
cytometry (Figs. 6F and S3B) and western blot assay (Fig. 6G and H) both suggested that
miR-326-induced apoptosis in BC cells was partly counteracted by
the overexpression of TAZ. Additionally, the migration and invasion
of BC cells were largely regained in the miR-326 and TAZ
co-transfected group (Figs. 6I and
J, and S3C and D). The
current findings demonstrated that miR-326 suppressed the
progression of BC via downregulating TAZ expression.
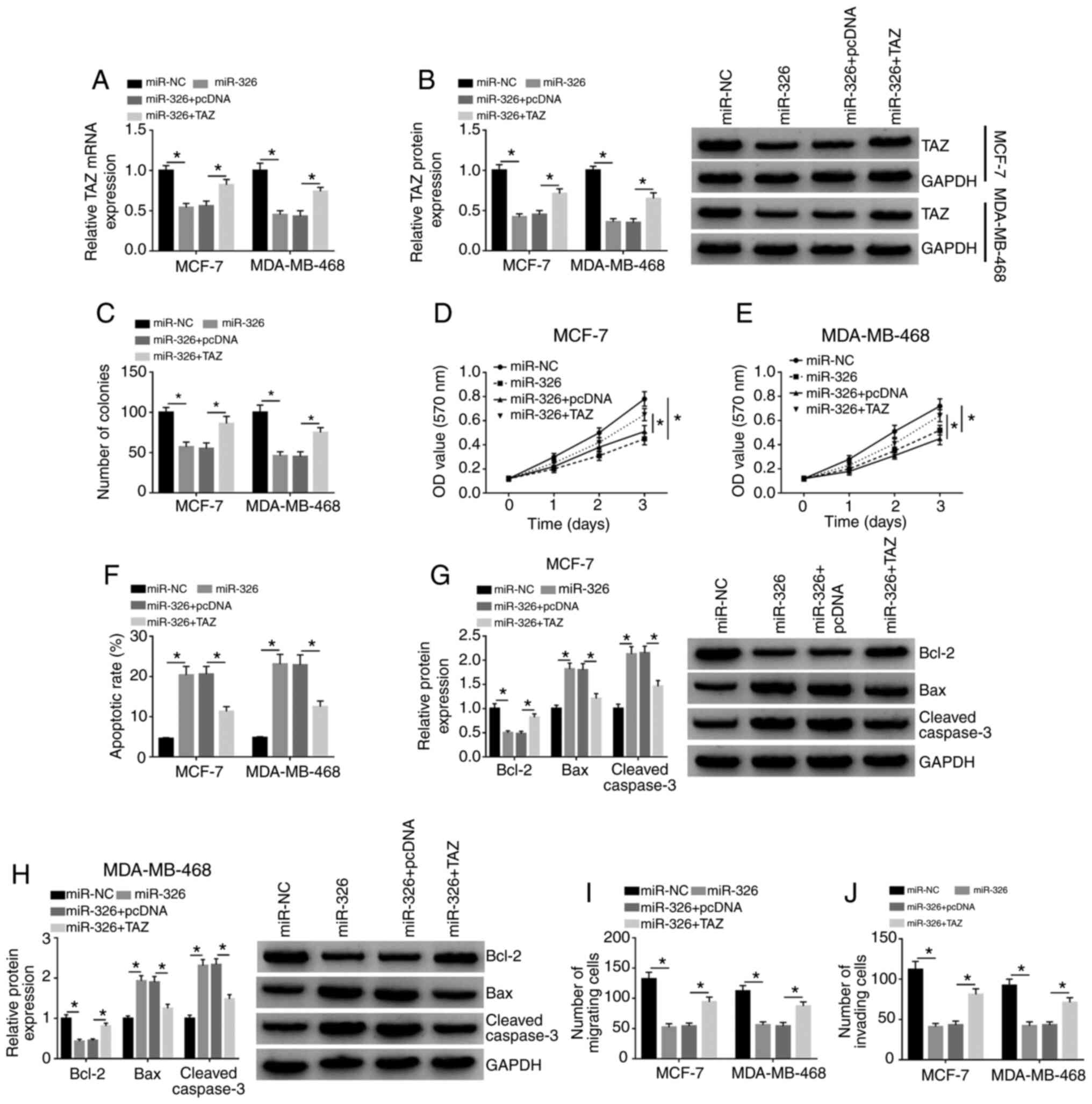 | Figure 6miR-326-mediated influences are
partly reversed by the overexpression of TAZ in BC cells. MCF-7 and
MDA-MB-468 cells were transfected with miR-NC, miR-326, miR-326 +
pcDNA or miR-326 + TAZ. (A) Reverse transcription-quantitative PCR
and (B) western blot assay were used to examine the mRNA and
protein expression levels of TAZ, respectively, in BC cells. (C)
Colony formation assay was performed to measure the proliferative
capacity of BC cells, and the number of colonies in each group was
detected. MTT assay was used to analyze the proliferative ability
of (D) MCF-7 and (E) MDA-MB-468 cells. (F) Flow cytometry was
conducted to assess the apoptosis of BC cells. Protein expression
levels of Bcl-2, Bax and Cleaved caspase-3 were detected in (G)
MCF-7 and (H) MDA-MB-468 cells by western blot assay. Transwell
assays were conducted to assess the (I) migration and (J) invasion
of BC cells. *P<0.05. Bcl-2, B cell leukemia/lymphoma
2; Bax, Bcl-2 associated X apoptosis regulator; BC, breast cancer;
miR, microRNA; NC, negative control; TAZ, transcriptional
co-activator with PDZ-binding motif; OD, optical density. |
Circ_0000511 elevates TAZ mRNA and
protein expression via sponging miR-326 in BC cells
After identifying the negative regulatory
association between miR-326 and circ_0000511 or TAZ, the present
study subsequently explored the association among circ_0000511,
miR-326 and TAZ in BC cells. MCF-7 and MDA-MB-468 cells were
transfected with si-NC, si-circ_0000511, si-circ_0000511 +
anti-miR-NC or si-circ_0000511 + anti-miR-326. The mRNA and protein
expression levels of TAZ were analyzed by RT-qPCR and western blot
assay, respectively. As shown in Fig.
7A and B, circ_0000511 silencing significantly downregulated
the mRNA and protein expression levels of TAZ, and the addition of
anti-miR-326 partly recovered the mRNA and protein abundance of TAZ
in BC cells, suggesting that circ_0000511 enhanced the mRNA and
protein expression levels of TAZ via acting as a sponge of
miR-326.
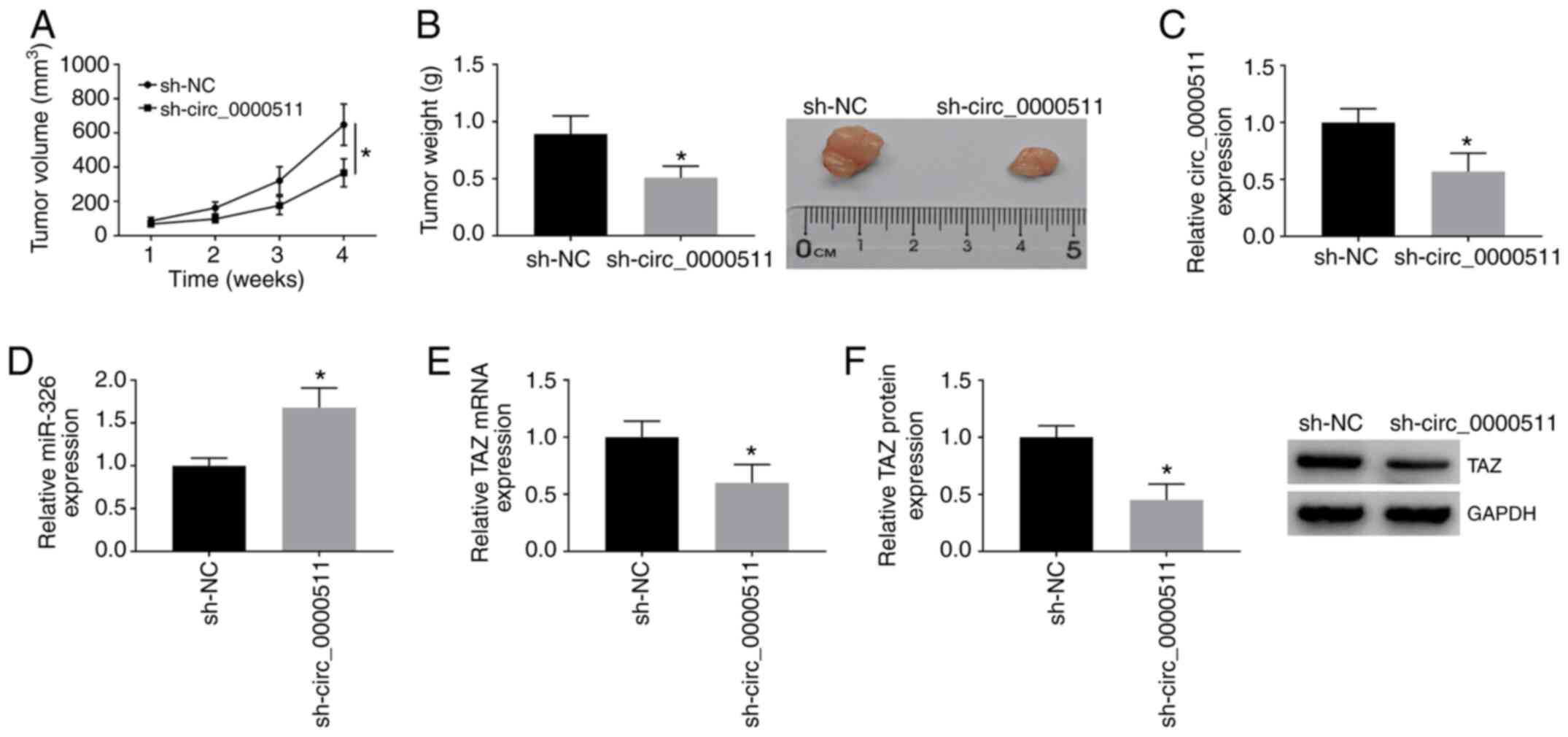
Circ_0000511 promotes BC tumor
progression in vivo
On the basis of the oncogenic role of circ_0000511
in vitro, the present study further assessed the influence
of circ_0000511 silencing on the growth of BC tumors in
vivo. An MCF-7 cell line stably transfected with sh-NC or
sh-circ_0000511 was established. Tumor growth curves were generated
by recording the tumor volume every week for 4 weeks. As shown in
Fig. 8A, tumors in the sh-NC
group were bigger than tumors in the sh-circ_0000511 group.
Additionally, by measuring the tumor weight after resecting tumors
4 weeks after inoculation, it was revealed that circ_0000511
silencing suppressed tumor growth in vivo (Fig. 8B). The mRNA and protein expression
levels of circ_0000511, miR-326 and TAZ were measured by RT-qPCR
and western blot assay, respectively. As shown in Fig. 8C, circ_0000511 abundance was
significantly downregulated in the sh-circ_0000511 group compared
with that in the sh-NC group. Additionally, miR-326 expression was
significantly increased in the sh-circ_0000511 group compared with
that in the sh-NC group (Fig.
8D), and TAZ mRNA and protein expression was significantly
decreased in the sh-circ_0000511 group compared with that in the
sh-NC group (Fig. 8E and F).
Overall, the present results suggested that circ_0000511
accelerated the tumor growth of BC in vivo.
Discussion
CircRNAs have been identified as crucial regulators
in the occurrence and development of different types of cancer. For
instance, circ_0014359 accelerates the development of glioma via
modulating the miR-153/PI3K signaling pathway (24). Circ_0020123 contributes to the
development of non-small cell lung cancer by upregulating ADAM9 via
sponging miR-488-3p (25). In the
present study, circ_0000511 expression was higher in BC tissues
compared with in adjacent normal tissues, which was consistent with
the expression tendency of circ_0000511 in the GSE101124 dataset
(26). By performing
loss-of-function experiments, the current study revealed that
circ_0000511 promoted the proliferation, migration and invasion,
and impaired the apoptosis of BC cells.
miRNA sponge mechanism is an important way by which
circRNAs function. For instance, circ_0008035 accelerates the
proliferation and impedes the apoptosis of gastric cancer cells via
sponging miR-599 to upregulate eukaryotic translation initiation
factor 4A1 (27). Circ_0079593
deteriorates glioma by promoting the proliferation and motility of
glioma cells via sponging miR-182 and miR-433 (28). In the present study, the
interaction between circ_0000511 and miR-326 was predicted using a
bioinformatic software (StarBase) and then verified by
dual-luciferase reporter assay. A previous study has demonstrated
that miR-326 acts as a tumor suppressor in BC cells via regulating
the ErbB/PI3K signaling pathway (18). Furthermore, miR-326 exerts an
antitumor role in a variety of types of cancer by targeting
different downstream mRNAs. For example, Ji et al (29) claimed that miR-326 restrains the
progression of gastric cancer via decreasing the abundance of NIN1
binding protein 1 homolog. Liang et al (30) demonstrated that miR-326 suppresses
the development of prostatic carcinoma via targeting Mucin1. The
results of rescue experiments in the present study demonstrated
that circ_0000511 exhibited an oncogenic role in BC cells partly
via sponging miR-326. The tumor suppressor role of miR-326 was in
agreement with a previous study (18).
The interaction between TAZ mRNA and miR-326 was
also predicted using the StarBase database and was validated by
dual-luciferase reporter assay. TAZ and Yes-associated protein
(YAP) are two closely related transcriptional modulators downstream
of the Hippo signaling pathway that is generally activated in
numerous types of cancer (31-33). The activation of TAZ and YAP
promotes the occurrence and progression of a number of solid tumors
via accelerating the proliferation, motility and drug resistance,
and inducing the attributes of cancer stem cells (31,33). The essential role of YAP/TAZ in
human malignancies suggest the use of YAP/TAZ as potential targets
for anticancer drugs (34,35).
Furthermore, the roles of Hippo signaling and TAZ in BC have been
identified. Hippo signaling exhibits a pivotal role in the
development of mammary cancer and the progression of breast cancer
(36). TAZ has been reported to
accelerate the migration and invasion of BC cells (22). The present study aimed to explore
whether TAZ functioned in a circ_0000511/miR-326 axis-mediated
regulation of BC cells. Rescue experiments were performed by
transfecting miR-326 alone or together with TAZ overexpression
plasmid into BC cells to test if miR-326 functioned via targeting
TAZ. The current results revealed that miR-326 suppressed the
proliferation, migration and invasion, and induced apoptosis of BC
cells via targeting TAZ. Additionally, it was revealed that
circ_0000511 enhanced the abundance of TAZ mRNA and protein via
serving as a miR-326 sponge in BC cells.
According to the current results of in vitro
experiments, it was demonstrated that circ_0000511 accelerated the
proliferation, migration and invasion, and restrained the apoptosis
of BC cells via regulating the miR-326/TAZ axis. The in vivo
role of circ_0000511 was explored by building xenograft mice models
using MCF-7 cells with the stable silencing of circ_0000511 or
control MCF-7 cells. Circ_0000511 interference resulted in the
suppression of BC growth in vivo.
The in vivo influence of circ_0000511 on the
metastasis of BC requires to be further explored in future studies.
Overall, the present findings revealed that circ_0000511 and TAZ
expression was upregulated, while miR-326 expression was
downregulated in BC, and circ_0000511 accelerated the progression
of BC by promoting the proliferation, migration and invasion, and
impeding the apoptosis of BC cells via the miR-326/TAZ axis.
Supplementary Data
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DW and HJ conceived and designed the study. DW, HJ
and ZZ were responsible for data acquisition and interpretation. HJ
and SL performed the statistical analysis. DW and HJ drafted the
manuscript. ZZ and SL critically revised the manuscript for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study involving human samples and animal
experiments was approved by the Ethics Committee of The First
Hospital of Jilin University (Changchun, China) and was conducted
in accordance with the Declaration of Helsinki. All participants
provided written informed consent before surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Tao Z, Shi A, Lu C, Song T, Zhang Z and
Zhao J: Breast cancer: Epidemiology and Etiology. Cell Biochem
Biophys. 72:333–338. 2015. View Article : Google Scholar
|
|
2
|
Baselga J and Swain SM: Novel anticancer
targets: Revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer.
9:463–475. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hynes NE and MacDonald G: ErbB receptors
and signaling pathways in cancer. Curr Opin Cell Biol. 21:177–184.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J,
Chen D, Gu J, He X and Huang S: Circular RNA is enriched and stable
in exosomes: A promising biomarker for cancer diagnosis. Cell Res.
25:981–984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salzman J: Circular RNA expression: Its
potential regulation and function. Trends Genet. 32:309–316. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sekar S and Liang WS: Circular RNA
expression and function in the brain. Noncoding RNA Res. 4:23–29.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Z, Sun B, Huang S and Zhao L: Roles
of circular RNAs in immune regulation and autoimmune diseases. Cell
Death Dis. 10:5032019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Greene J, Baird AM, Brady L, Lim M, Gray
SG, McDermott R and Finn SP: Circular RNAs: Biogenesis, function
and role in human diseases. Front Mol Biosci. 4:382017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han B, Chao J and Yao H: Circular RNA and
its mechanisms in disease: From the bench to the clinic. Pharmacol
Ther. 187:31–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lasda E and Parker R: Circular RNAs:
Diversity of form and function. RNA. 20:1829–1842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Panda AC: Circular RNAs act as miRNA
sponges. Adv Exp Med Biol. 1087:67–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhong Y, Du Y, Yang X, Mo Y, Fan C, Xiong
F, Ren D, Ye X, Li C, Wang Y, et al: Circular RNAs function as
ceRNAs to regulate and control human cancer progression. Mol
Cancer. 17:792018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ding L and Zhang H: Circ-ATP8A2 promotes
cell proliferation and invasion as a ceRNA to target EGFR by
sponging miR-433 in cervical cancer. Gene. 705:103–108. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun H, Xi P, Sun Z, Wang Q, Zhu B, Zhou J,
Jin H, Zheng W, Tang W, Cao H and Cao X: Circ-SFMBT2 promotes the
proliferation of gastric cancer cells through sponging miR-182-5p
to enhance CREB1 expression. Cancer Manag Res. 10:5725–5734. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghaemi Z, Soltani BM and Mowla SJ:
MicroRNA-326 functions as a tumor suppressor in breast cancer by
targeting ErbB/PI3K signaling pathway. Front Oncol. 9:6532019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanai F, Marignani PA, Sarbassova D, Yagi
R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC
and Yaffe MB: TAZ: A novel transcriptional co-activator regulated
by interactions with 14-3-3 and PDZ domain proteins. EMBO J.
19:6778–6791. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mahoney WM Jr, Hong JH, Yaffe MB and
Farrance IK: The transcriptional co-activator TAZ interacts
differentially with transcriptional enhancer factor-1 (TEF-1)
family members. Biochem J. 388:217–225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murakami M, Tominaga J, Makita R, Uchijima
Y, Kurihara Y, Nakagawa O, Asano T and Kurihara H: Transcriptional
activity of Pax3 is co-activated by TAZ. Biochem Biophys Res
Commun. 339:533–539. 2006. View Article : Google Scholar
|
|
22
|
Chan SW, Lim CJ, Guo K, Ng CP, Lee I,
Hunziker W, Zeng Q and Hong W: A role for TAZ in migration,
invasion, and tumorigenesis of breast cancer cells. Cancer Res.
68:2592–2598. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Shi F, Shi Z, Zhao Y and Tian J: CircRNA
hsa-circ-0014359 promotes glioma progression by regulating
miR-153/PI3K signaling. Biochem Biophys Res Commun. 510:614–620.
2019. View Article : Google Scholar
|
|
25
|
Wan J, Hao L, Zheng X and Li Z: Circular
RNA circ_0020123 promotes non-small cell lung cancer progression by
acting as a ceRNA for miR-488-3p to regulate ADAM9 expression.
Biochem Biophys Res Commun. 515:303–309. 2019. View Article : Google Scholar
|
|
26
|
Xu JZ, Shao CC, Wang XJ, Zhao X, Chen JQ,
Ouyang YX, Feng J, Zhang F, Huang WH, Ying Q, et al: circTADA2As
suppress breast cancer progression and metastasis via targeting
miR-203a-3p/SOCS3 axis. Cell Death Dis. 10:1752019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li C, Tian Y, Liang Y and Li Q:
Circ_0008035 contributes to cell proliferation and inhibits
apoptosis and ferroptosis in gastric cancer via miR-599/EIF4A1
axis. Cancer Cell Int. 20:842020. View Article : Google Scholar :
|
|
28
|
Qu Y, Zhu J, Liu J and Qi L: Circular RNA
circ_0079593 indicates a poor prognosis and facilitates cell growth
and invasion by sponging miR-182 and miR-433 in glioma. J Cell
Biochem. 120:18005–18013. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji S, Zhang B, Kong Y, Ma F and Hua Y:
miR-326 Inhibits Gastric Cancer Cell Growth Through Downregulating
NOB1. Oncology Pesearch. 25:853–861. 2017.
|
|
30
|
Liang X, Li Z, Men Q, Li Y, Li H and Chong
T: miR-326 functions as a tumor suppressor in human prostatic
carcinoma by targeting Mucin1. Biomed Pharmacother. 108:574–583.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moroishi T, Hansen CG and Guan KL: The
emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 15:73–79.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nguyen CDK and Yi C: YAP/TAZ signaling and
resistance to cancer therapy. Trends Cancer. 5:283–296. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zanconato F, Cordenonsi M and Piccolo S:
YAP/TAZ at the roots of cancer. Cancer Cell. 29:783–803. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Warren JSA, Xiao Y and Lamar JM: YAP/TAZ
activation as a target for treating metastatic cancer. Cancers
(Basel). 10:1152018. View Article : Google Scholar
|
|
35
|
Zanconato F, Battilana G, Cordenonsi M and
Piccolo S: YAP/TAZ as therapeutic targets in cancer. Curr Opin
Pharmacol. 29:26–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi P, Feng J and Chen C: Hippo pathway in
mammary gland development and breast cancer. Acta Biochim Biophys
Sin (Shanghai). 47:53–59. 2015. View Article : Google Scholar
|